Abstract
Using PET, brain areas representing the use of a well‐learned tool (chopsticks) were investigated in 10 normal common users. The experimental task was to hold the tool in their right hand and use it to pick up and transport a small pin from a table. Data for the same task performed using only the fingers were also obtained as a control. The results showed an extensive overlap in activated areas with and without the use of the tool. The tool‐use prehension, compared to the finger prehension, was associated with higher activities in the caudal‐ventral premotor, dorsal premotor, superior parietal, posterior intraparietal, middle temporal gyrus, and primary sensory, occipital cortices, and the cerebellum. These are thus considered to be the human cortical and subcortical substrates representing the use of the tool studied. The activity of the posterior intraparietal area was negatively correlated with the number of drops of the pin, whereas occipital activity was positively correlated with the same error parameter. The caudal‐ventral premotor and posterior intraparietal areas are together known to be involved in tool use‐related modulation in peripersonal space. The correlation results suggest that this modulation depends on the level of performance. The coactivated left middle temporal gyrus further suggests that familiarity with a tool as well as the knowledge about its usage plays a role in peripersonal space modulation. Superior parietal activation, along with occipital activation, indicates the involvement of visual‐spatial attention in the tool use, possibly reflecting the effect of interaction between the prehension (task) and the tool. Hum Brain Mapp 2009. © 2009 Wiley‐Liss, Inc.
Keywords: PET, chopsticks, tool use, prehension, finger
INTRODUCTION
The central neural structures involved in the control of grasping and manipulating a handheld object in humans and monkeys has been a topic of study for well over two decades. Human neuroimaging studies have identified numerous areas in the frontal (primary motor, dorsal and ventral premotor, supplementary motor, and cingulate motor), parietal (primary and secondary sensory, superior parietal lobule, and inferior parietal lobule), and occipital (primary and secondary visual) cortical areas, as well as the subcortical substrates (basal ganglia, thalamus, and cerebellum) for their significant contribution to various aspects of grasping and/or manipulating objects with the hand [Ehrsson et al.,2000,2001; Grafton et al.,1996; Kinoshita et al.,2000; Rizzolatti et al.,1996].
In human culture, tools such as pincers, pliers, tongs, or chopsticks are often used for grasping and manipulating an object. Observations of ideomotor apraxia patients, as well as recent functional neuroimaging studies in healthy people, have determined that representations of tool‐use skills are functionally dissociable from representations of the sensorimotor transformations involved in prehension and that the distributed brain areas in the left hemisphere most likely play a critical role in everyday tool use skills [Buxbaum,2001; Johnson‐Frey,2004]. Neuroimaging studies of tool use in humans [Beauchamp et al.,2002; Chao et al.,1999; Imazu et al.,2007; Inoue et al.,2001; Johnson‐Frey,2004; Kellenbach et al.,2003; Leiguarda and Marsden,2000; Martin et al.,1996] have demonstrated activation in three distinct regions. These included areas in the left posterior temporal, ventral premotor areas in the inferior frontal, and posterior parietal cortices. The middle temporal gyrus in the posterior temporal cortex is known to play a role in representing semantic information concerning tools and their associated actions [Beauchamp et al.,2002; Chao et al.,1999; Kellenbach et al.,2003; Martin et al.,1996]. This information may be essential to develop appropriate tool use skills.
The ventral premotor areas, together with various regions of the parietal cortex, are known to be involved in multisensori‐motor transformations for manipulating the hand‐held objects. The ventral section of the premotor area has been subdivided into rostral and caudal regions [Binkofski and Buccino,2006; Matelli and Luppino,2000]. The rostral region has a strong connection with the anterior intraparietal area, and these areas together play an essential role in prehension control, while integrating representations of objects' intrinsic spatial properties with properties of the hand and fingers. Certain neurons in this region in monkeys are called “mirror neurons,” which become active both when performing specific goal‐directed hand actions and when observing another monkey or an experimenter performing the same or similar actions [Gallese et al.,1996]. A similar response property has been found in the rostro‐ventral premotor area in humans [Binkofski et al.,2004]. Neurons in the caudal region of the premotor area have firm connections with those in the ventral intraparietal area. In monkeys, most of the neurons in both of these areas are biomodal neurons, responding to somatosensory information from a specific body region and to visual or acoustic information from the space adjacent to it [Colby et al.,1998; Matelli and Luppino,2001; Rizzolatti et al.,1981]. The visual and tactile receptive fields of these neurons are in the spatial register; that is, the visual receptive fields match the location of the tactile receptive fields on the body surface, and extend in depth to the space immediately surrounding the body [Fogassi et al.,1996]. The visual response decreases as the distance between the visual stimulus and the cutaneous receptive fields increases [Graziano et al.,1997]. In addition, Graziano et al. have shown that the visual receptive fields remain anchored to the body part as this is moved. These functional properties indicate that the caudal‐ventral premotor area, together with the posterior parietal cortex, is devoted to the multisensory coding of the peripersonal space centered on the body. Recent brain imaging studies using dummy hands have suggested that areas within this premotor area, the intraparietal sulcus, and in the lateral occipital complex, provide a mechanism for the formation of peripersonal space in humans [Ehrsson et al.,2004; Makin et al.,2007]. Related to the use of a tool, Iriki et al. [1996] have shown that after the monkeys have learned how to use a rake to retrieve a distant food pellet, their visual receptive field of the ventral intraparietal neurons in the intraparietal sulcus increases to cover not only the area around their hand but also the area around the rake. These results suggest that neural representation to process multimodal sensory information changes during the recognition of the movement of body parts with a tool. Using positron emission tomography (PET) and normal human volunteers, Inoue et al. [2001] reported increased activity of the intraparietal area (BA 40) while tongs were being used to manipulate an object. Interestingly, they did not find significant activity in any other cortical or subcortical regions that have been identified as the tool use‐related areas of the brain. More recently, using functional MRI (fMRI), Imazu et al. [2007] showed activity of left intraparietal lobule during pantomime or imagery of the use of chopsticks. However, they found no activation in the same region during the real use of the chopsticks. In another fMRI study, cerebellar activity related to the use of a computer mouse was detected by Imamizu et al. [2000,2003]. The authors stated that the cerebellum plays an essential role in the storage or retrieval of acquired internal models of tools; neural representation can predict how the tools will move, and the sensations that will arise, given a specific motor command.
The present study used PET to investigate the tool use‐related cortical and subcortical areas of the brain in normal human subjects during the simple task of picking up a small object from a table and transporting it to another place. Chopsticks were chosen as the tool of interest, and the subjects were recruited from the native adult Japanese population with more than 20 years of daily use of this tool from an early age. Thus, it is possible to assume that in these subjects, the central neural mechanism representing this tool and its usage or associated skills for its ordinary purposeful use has been well established. In an earlier PET study on the use of tongs as a tool and the fingers as a control for object manipulation, Inoue et al. [2001] were actually less successful in finding significant activation in many of the so‐called tool use‐related cortical and subcortical areas. One major reason for this may be the selection of the tool. Although tongs are simple to use, their daily use is uncommon to most people. It is therefore uncertain whether this less familiar tool had been represented in the central neural system.
METHODS
Subjects
Ten healthy males with a mean age of 22.9 ± 1.9 (mean ± SD) years (range 21–25 years), who gave their written informed consent, served as subjects for the present study. All were right‐handed, as determined by the Edinburgh MRC Handedness Inventory [Oldfield,1971]. The study was approved by the Human Ethics Committee at the School of Human Sciences, Osaka University.
Measurement of Regional Cerebral Blood Flow With PET
A three‐dimensional (3D) PET system (SET‐2400W, Shimazu, Kyoto, Japan) was used in the present study. The inplane and axial resolutions were 4 mm × 4 mm and 5.0 mm full‐width at half maximum (FWHM) at the center of the field of view, respectively. The data were acquired in the 3D mode with septa out. Emission data were acquired in 63 planes without an interslice gap. This enabled us to obtain almost a whole‐brain image, including the cerebellum. Each plane was displayed in a 128 × 128 pixel format with a voxel size of 2.0 mm × 2.0 mm × 3.1 mm. Before the first emission scan, a transmission scan was obtained over 10 min using a 68Ge/68Ga line source rotated around the subject's head; this was used to correct the emission scans for photon attenuation. For each measurement, 7 mCi (259 MBq) of H2 15O in 18 mL of saline was injected intravenously for 36 s by a powered injector when the task began. Approximately 25 s after the injection started, when the H2 15O reached the brain, the emission scan was initiated. Each emission scan lasted for 90 s while the subject carried on the experimental tasks described below. The interval time between the scans was ∼10 min (i.e., approximately four half‐lives of 15O) to allow radioactive decay to attain less than 7% of peak levels.
Procedures
The subjects rested in a supine position on a bed for the PET system. Each subject's head was immobilized with a ready‐made plastic head‐holder tilted at a slight angle (<8°). A 22‐gauge plastic needle was inserted into the left antecubital vein for tracer administration. A wooden table with an adjustable tilting angle was placed above each subject's abdomen so that the right hand, chopsticks, and target objects were within the subject's field of view (see Fig. 1). The right forearm, with the hand pronated ∼90° and flexed ∼30°, was rested on the table surface. To prevent unnecessary proximal muscle contraction, the right upper arm and shoulder were rested on a flexible holder for which the height could be adjusted so that the subjects could carry out the task mentioned below using only slight flexion‐extension at the elbow joint, pronation‐supination at the forearm, flexion‐extension at the wrist joint and finger movement. The left arm was kept resting on the bed throughout the experiment.
Figure 1.
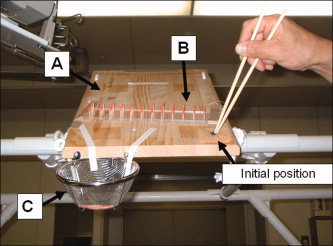
The subject's view of the motor task. (A) Tilted wooden table, (B) target grasping object (pins), (C) basket for dropping the pins. The tips of the chopsticks were placed at the initial position before and at the end of the prehension task. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Experimental Tasks
The experimental tasks for each subject consisted of chopstick and finger tasks, and a complete rest as a baseline task. The chopstick task was the repetitive transport of a small pin using a pair of ordinary wooden chopsticks (length = 22.5 cm, diameter of holding portion = 6 mm, diameter of tips = 3 mm, weight = 3 g). The pins were made using the upper portion of wooden toothpicks (length = 18 mm, diameter = 2 mm, weight = 0.5 g). Forty‐four of these were inserted in shallow holes (diameter = 2.2 mm, depth = 5 mm, 4 lows × 11 lines) drilled on a clear acrylic board (a pin‐holder) fixed on the test table. The pin‐holder was fixed in the middle of the surface of the tilting table so that the center of the pin‐holder came to the middle of the subject's view (see Fig. 1). The subjects held the chopsticks in the right hand, placed the tips at a designated initial position on the right side of the table, and waited for a beep sound that occurred every 3.5 s to initiate the task. The task was to pick up the pin from the pin‐holder using the tips of the chopsticks, transport it above a steel basket (diameter = 150 mm) placed near the end of the test table, drop the pin in the basket, and return the chopstick tips to the initial position. The order in which the pins were to be transported was from the right lowest point of the first column, then the second lowest point of the same column, and so on. The target of the initial position was located in the right hemispace of the subject's view, whereas the basket was placed in the left hemispace of the subject's view. The finger task used the same pin movements as in the chopstick experiment. The subjects were instructed to grip the pins using the tips of the index finger and the thumb. To eliminate a learning effect of these tasks, a few days before the PET scan each subject practiced these tasks under the same experimental environment for ∼30 min. The rest condition was performed as follows: the subjects were instructed to close their eyes, place their index finger and thumb at the starting point, relax the body, and refrain from responding to the beep sound.
The level of luminance in the PET room was controlled with standard room lights. While performing each task, the right forearm and hand were videotaped using a standard video‐tape recorder. Each subject underwent 12 PET scans (four repetitions for each of the three experimental tasks). The order of the tasks was randomized for each subject.
Task Performance Evaluation
At the end of the experiment, the subjects were asked about the difference in difficulty between the two tasks. Video recording of the subjects' right hand was carried out during the entire experiment. From the video, the total number of trials in which the pin was dropped while being lifted from the holder or moved to the basket (performance error) was counted for each subject for each experimental task. The time that elapsed between the onset of movement with the chopsticks or fingers from the starting position and the onset of the lifting of the pin (reach‐lift period), and the time that elapsed between the onset of lifting the pin and the return of the chopsticks to the starting position after dropping the pin in the basket (return period) were also computed from the records.
Image Analysis
A statistical parametric mapping (SPM) program for image analysis and matrix operations (SPM 2; Wellcome Department of Cognitive Neurology, Queens Square, London, UK) in a Matlab environment (Mathworks, Sherborn, MA) was used to analyze the regional cerebral blood flow (rCBF) data on Windows XP. The scans for each subject were first realigned using the first image as a reference, and all images were transformed into a standard stereotaxic space using the Montreal Neurological Institute (MNI) template in the SPM 2 package. The intercommissural (anterior commissural‐posterior commissural: AC‐PC) plane was defined based on a linear regression analysis of the coordinates identified by the locations of the occipital pole, the lower border of the thalamus, and the inferior border of the anterior and posterior corpus callosum. The standardized images were then smoothed with a Gaussian kernel of 12 mm in the x, y, and z axes. Differences in global CBF between subjects and conditions were adjusted by analysis of covariance, with global flow as the confounding variable [Friston et al.,1990]. This process resulted in the generation of a group mean activity for each experimental condition, adjusted to a single overall global CBF of 50 mL/100 mL/min, with an associated residual error variance across subjects for each pixel. The rCBF was analyzed on the planes common to all subjects, covering a region from 52 mm below, to 82 mm above, the AC‐PC plane.
Statistical Tests
Chopstick‐ and finger‐specific group cortical and subcortical activations were assessed by planned comparisons with appropriate linear contrasts with the rest condition. To determine the tool use‐related group cortical and subcortical activation, we used an exclusive masking procedure as implemented in SPM 2. This can identify voxels that were not shared between the two contrasts (the chopstick condition minus the rest condition, and the finger condition minus the rest condition). The resulting set of voxel values for each contrast constituted a statistical parametric map of the t‐statistic (SPM{t}). The SPM {t} was then transformed to a Z distribution (SPM{Z}). The significance of each region was estimated using the probability that the peak height observed could have occurred by chance and/or that the observed number of contiguous voxels could have occurred by chance over the entire volume analyzed. A corrected P value of 0.05 with family‐wise error and an extent threshold of 15 voxels were used as the final threshold for significance for all contrasts.
In addition to the above statistical analyses of peak activation, we also performed a region of interest (ROI) analysis using the MARSBAR tool box installed in the SPM program [Brett et al.,2002]. This was conducted to examine the relationship between the number of performance errors and the magnitude of rCBF, and the tool use‐related brain areas. The mean peak coordinate was used as the central coordinate of the ROI (6 mm × 6 mm × 6 mm). A Pearson's correlation coefficient was computed for each ROI datum. Significance was evaluated at a P value of 0.05.
RESULTS
Task Performance
For the chopstick task, performance errors occurred a total of 30 times (2.9%) in 1,040 trials (26 trials × 4 scans × 10 subjects), while the number of errors that occurred with the fingers was 4 (0.4%). The mean reach‐lift period for the chopsticks for all subjects was 1.08 ± 0.26 s, which was significantly larger than 0.76 ± 0.06 s for the fingers (P < 0.01). The mean return period for the chopstick condition was 1.14 ± 0.19 s, which did not differ from the corresponding period for the finger condition (1.10 ± 0.10 s).
For the subjective rating of task difficulty, eight subjects stated that the finger task was easier than the chopstick task, while two subjects stated that the two tasks were equally easy.
Regional Blood Flow for the Chopstick and Finger Conditions Compared With the Rest Condition
Table I gives the coordinates of the foci and Z‐scores for the regions with significant increases in rCBF with the use of the chopsticks from the baseline rest condition, and those with the use of the fingers from the baseline rest condition. For the chopstick condition, the activated areas were identified in the sensorimotor‐associated cortices (primary motor and sensory, dorsal and ventral premotor, supplementary motor, and cingulate motor areas), parietal cortices (superior parietal lobule, inferior parietal lobule, inferior parietal sulcus, and ventral intraparietal area), middle temporal gyrus, and occipital cortices (primary and secondary visual areas) in the left hemisphere. In the right hemisphere, the fronto‐parietal cortices (dorsal premotor, supplementary motor, cingulate motor, and superior parietal areas) and occipital cortices (primary and secondary visual areas) were also activated. For the subcortical areas, the ventral‐posterior lateral portion of the left thalamus and several areas of the bilateral cerebellum (vermis, paramedian lobule, and lateral hemisphere) were activated for the chopstick condition compared to the baseline rest condition.
Table I.
Foci of significant regional cerebral blood flow (rCBF) increase during chopstick and finger prehension when compared with baseline
| Region of rCBF increase | (BA) | Side | Chopsticks | Z score of peak activation | Fingers | Z score of peak activation | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| MNI image coordinates of peak activation | MNI image coordinates of peak activation | |||||||||
| x | y | z | x | y | z | |||||
| Cortical areas | ||||||||||
| Primary sensory/motor area | (4,3,1,2) | L | — | — | — | — | −36 | −16 | 56 | >8 |
| Primary sensory area | (3,1,2) | L | −40 | −20 | 58 | >8 | — | — | — | — |
| (3,12) | L | −54 | −26 | 42 | >8 | — | — | — | — | |
| Primary motor area | (4) | L | −28 | −22 | 64 | >8 | — | — | — | — |
| Dorsal premotor area | (6) | L | −36 | −16 | 56 | >8 | −26 | −20 | 66 | >8 |
| (6) | R | 20 | −4 | 58 | 5.10 | 44 | −8 | 60 | 5.84 | |
| (6) | R | 12 | −6 | 60 | 4.90 | — | — | — | — | |
| Ventral premotor area | (6) | L | −58 | 2 | 36 | 4.96 | — | — | — | — |
| (6/44) | L | −60 | 4 | 32 | 5.28 | — | — | — | — | |
| Supplementary motor area | (6) | L | −8 | −8 | 56 | 7.54 | −14 | −12 | 60 | >8 |
| Cingulate motor area | (24) | L | −14 | −2 | 46 | 4.93 | −14 | −2 | 46 | 5.29 |
| Secondary sensory area | (2,43) | L | −56 | −22 | 28 | 6.75 | −56 | −24 | 24 | 5.56 |
| Superior parietal lobule | (7) | L | −38 | −58 | 62 | >8 | −24 | −70 | 62 | >8 |
| (7) | L | −18 | −68 | 60 | >8 | −16 | −66 | 62 | 7.59 | |
| (7) | L | −16 | −64 | 60 | >8 | — | — | — | — | |
| (7) | L | −30 | −64 | 58 | 7.73 | — | — | — | — | |
| (7) | L | −22 | −70 | 60 | 7.69 | — | — | — | — | |
| (7) | L | −20 | −68 | 50 | 7.08 | — | — | — | — | |
| (7) | R | 30 | −60 | 58 | 5.85 | — | — | — | — | |
| (7) | R | 12 | −68 | 54 | 5.53 | — | — | — | — | |
| (7) | R | 20 | −66 | 70 | 5.48 | — | — | — | — | |
| Inferior parietal lobule | (40) | L | −40 | −38 | 58 | >8 | −40 | −38 | 58 | >8 |
| Superior/inferior parietal lobule | (7/40) | L | −36 | −50 | 58 | >8 | −26 | −50 | 58 | >8 |
| Inferior parietal sulcus | (40) | L | −40 | −32 | 44 | >8 | −40 | −32 | 44 | >8 |
| Ventral intraparietal | L | −16 | −32 | 42 | 4.88 | −16 | −32 | 46 | 4.89 | |
| Middle temporal gyrus | (37) | L | −40 | −72 | 0 | 4.89 | — | — | — | — |
| Primary visual area | (17) | L | −20 | −82 | 2 | 5.48 | — | — | — | — |
| (17) | R | 28 | −86 | 0 | 6.80 | — | — | — | — | |
| Secondary visual area | (18) | L | −14 | −98 | 0 | 6.31 | — | — | — | — |
| (18) | L | −16 | −100 | −4 | 6.11 | — | — | — | — | |
| (18) | L | −22 | −84 | 6 | 6.03 | — | — | — | — | |
| (18) | L | −20 | −84 | 12 | 5.99 | −20 | −84 | 14 | 6.47 | |
| (18) | L | −6 | −90 | −12 | 4.96 | — | — | — | — | |
| (18) | R | 10 | −90 | 6 | >8 | 26 | −84 | 20 | 4.63 | |
| (18) | R | 22 | −82 | −2 | 6.08 | 10 | −90 | 6 | >8 | |
| (19) | L | −20 | −84 | 32 | >8 | −18 | −84 | 34 | 7.48 | |
| (19) | R | 30 | −88 | 20 | 4.68 | 22 | −82 | 26 | 4.97 | |
| Thalamus | ||||||||||
| Ventral‐posterior lateral portion | L | −16 | −18 | 6 | 6.20 | −18 | −18 | 6 | 5.86 | |
| L | — | — | — | — | −20 | −26 | 2 | 5.58 | ||
| L | — | — | — | — | −18 | −30 | 0 | 5.56 | ||
| Cerebellum | ||||||||||
| Vermis | R | 6 | −70 | −28 | >8 | 4 | −70 | −30 | >8 | |
| Paramedian lobule | R | 10 | −60 | −20 | >8 | 10 | −60 | −18 | >8 | |
| Hemisphere | L | −24 | −58 | −28 | 7.33 | −24 | −60 | −26 | 5.12 | |
| L | −20 | −72 | −22 | 6.79 | — | — | — | — | ||
| L | −26 | −44 | −44 | 5.02 | — | — | — | — | ||
| L | −26 | −42 | −50 | 4.76 | — | — | — | — | ||
| R | 22 | −56 | −24 | >8 | 30 | −50 | −30 | >8 | ||
| R | 28 | −54 | −50 | 5.54 | 28 | −56 | −50 | 5.51 | ||
BA: Brodman's area, X and Y coordinate values are given in millimeters. The X values specify the lateral displacement from the midline (negative values for left hemisphere); Y values give the anteroposterior displacement relative to the anterior commissural (AC) line (negative values for posterior to the AC line); Z values specify the vertical portion relative to the AC‐PC plane (negative values for pixels below this plane). P < 0.05, corrected for multiple comparisons.
For the finger condition, the areas of activation were found in similar regions of the cortical and subcortical structures as those for the chopstick condition. The number of peaks identified was, however, smaller for the finger condition than for the chopstick condition (see Table I).
Difference in Regional Blood Flow in the Chopstick and Finger Tasks
The exclusive masking analysis revealed that compared to the finger condition, the chopstick condition led to a higher activity in the primary sensory area, dorsal and ventral premotor areas, middle temporal gyrus, superior parietal lobule, and the cerebellum in the left hemisphere, and dorsal premotor area, posterior intraparietal area, superior parietal area, and occipital areas in the right hemisphere. Ventral premotor activation occurred in the caudal region. Cerebellum activation was identified in the area of the left dentate nucleus and in the left posterior hemisphere. The opposite comparison revealed that none of the areas showed significantly higher activation for the finger condition than the chopstick condition (see Table II and Fig. 2).
Table II.
Foci of significant rCBF increase during chopstick compared to finger prehension
| Regional of rCBF increase | (BA) | Side | MNI image coordinates of peak activation | Z score of peak activation | r‐value | |||
|---|---|---|---|---|---|---|---|---|
| x | y | z | Performance errors | Reach‐lift period | ||||
| Primary sensory area | (2.3) | L | −56 | −16 | 30 | 5.65 | 0.13 | −0.10 |
| Dorsal premotor area | (6) | L | −32 | −4 | 44 | 5.41 | 0.27 | 0.01 |
| (6) | L | −40 | −6 | 44 | 5.14 | −0.09 | 0.16 | |
| (6) | R | 22 | −6 | 58 | 5.03 | 0.38 | 0.24 | |
| Ventral premotor area | (6) | L | −60 | 4 | 32 | 5.28 | −0.32 | 0.27 |
| (6) | L | −58 | 2 | 36 | 4.95 | −0.13 | 0.17 | |
| Superior parietal lobule | (7) | L | −14 | −70 | 54 | 5.34 | −0.16 | 0.07 |
| (7) | L | −16 | −76 | 52 | 5.00 | −0.17 | −0.26 | |
| (7) | R | 22 | −64 | 70 | 5.38 | −0.06 | −0.29 | |
| (7) | R | 18 | −66 | 70 | 5.35 | 0.01 | −0.63* | |
| Posterior intraparietal area | (7) | R | 32 | −64 | 64 | 5.30 | −0.61* | −0.03 |
| (7) | R | 12 | −70 | 48 | 4.93 | −0.20 | −0.20 | |
| Middle temporal gyrus | (37) | L | −40 | −72 | 0 | 4.89 | 0.43 | 0.02 |
| Secondary visual area | (18) | R | 34 | −88 | −2 | 5.88 | 0.65* | 0.07 |
| (18) | R | 32 | −86 | −6 | 5.43 | 0.71* | 0.18 | |
| (18) | R | 26 | −100 | 0 | 5.36 | −0.16 | 0.38 | |
| (18) | R | 26 | −102 | 4 | 5.34 | 0.15 | 0.41 | |
| (19) | R | 36 | −88 | 4 | 5.01 | −0.11 | −0.35 | |
| Dentate nucleus | L | −18 | −46 | −24 | 5.37 | 0.47 | 0.01 | |
| Cerebellar hemisphere | L | −28 | −58 | −36 | 6.07 | 0.45 | 0.28 | |
| L | −22 | −72 | −28 | 5.05 | 0.33 | 0.29 | ||
| L | −26 | −44 | −44 | 5.02 | 0.42 | −0.17 | ||
The areas of significant activation were determined by the exclusive masking analysis (corrected P < 0.05). The r‐values indicate correlation coefficients computed between the magnitude of rCBF and the number of performance errors, and the rCBF magnitude and reach‐lift period.
P < 0.05.
Figure 2.
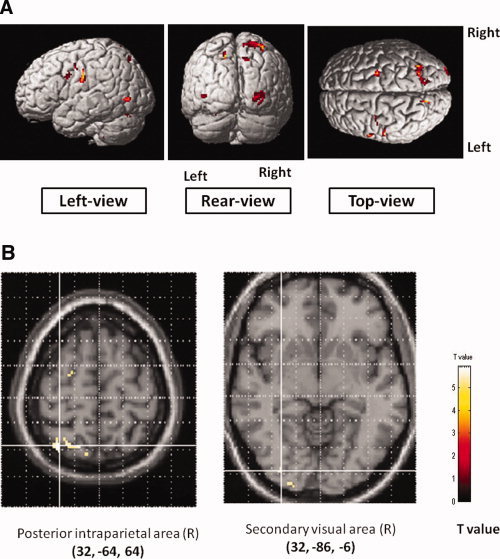
Chopsticks' use‐specific areas of activation. Data (N = 10) overlaid on a 3D brain image for regions significantly activated (A) and axial slices (B).
Results of the ROI Analysis
The correlation coefficient value between the number of performance errors and the magnitude of rCBF at each of the selected tool use‐related areas is given in Table II. A significant relationship was found only in the posterior intraparietal area (upper panel in Fig. 3) and secondary visual area (middle panel in Fig. 3). Significant relationship between the reach‐lift period and the magnitude of rCBF was also found in the right superior parietal cortex (Table II).
Figure 3.
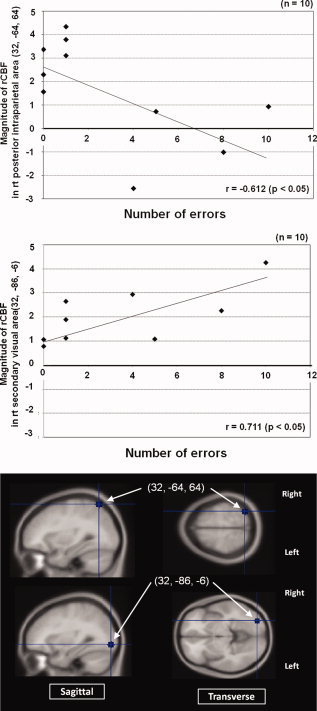
Relationship between the magnitude of rCBF at the right posterior intraparietal area (32, −64, 64) and the number of performance errors (upper panel), and the right secondary visual area (32, −86, −6) and the number of performance errors (middle panel). The lower panel showed placement for the region of interest (ROI). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
The present study demonstrated that brain areas activated during prehension by the use of chopsticks had a large overlap with those activated with the same action goal performed by the thumb and index finger. Relative to the rest condition, the areas activated in common between the two conditions were the primary motor/sensory, dorsal premotor, secondary sensory, superior parietal lobule, ventral intraparietal lobe, inferior parietal lobule and inferior parietal sulcus, and thalamus in the left hemisphere, and the bilateral caudal supplementary motor, cingulated motor, occipital cortices, and cerebellum. This basically agreed with the findings of previous studies that also examined functional brain areas required for visually guided human prehension [Binkofski et al.,1999; Faillenot et al.,1997; Grafton et al.,1996].
A major concern of the present study was to find areas of the brain involved in tool use. As expected, the areas activated during the use of the chopsticks compared to those of the fingers were identified in the ventral and dorsal portions of the premotor area, posterior parietal area including the intraparietal lobule, primary sensory area, inferior occipito‐temporal area at around the middle temporal gyrus, and cerebellum in the left hemisphere. There were also activated areas in the dorsal premotor and secondary occipital areas in the right hemisphere. Thus, these findings supported the notion that the left hemisphere plays a more crucial role in the central neural processing of human tool use [Binkofski et al.,1999; Chao and Martin,2000; Chao et al.,1999 see also review by Johnson‐Frey,2004]. Previous researchers have suggested that areas in the left inferior frontal, parietal, and posterior temporal cortices, as well as the hemisphere of the cerebellum, are of particular importance in representing conceptual knowledge concerning tools and their associated actions and skills.
Ventral premotor activation was identified at its caudal sector (BA 6). Although this area has been hypothesized for its involvement in peripersonal space modulation during actual tool use, none of the previous researchers has confirmed its activation [Holmes et al.,2007; Inoue et al.,2001; Johnson‐Frey,2004]. On the other hand, a localized activation at a similar region has been reported in several fMRI studies in which central representations for feeling of ownership of a limb or peripersonal space were investigated in normal human subjects [Ehrsson et al.,2004; Makin et al.,2007]. Ehrsson et al. [2004] used the “rubber‐hand illusion,” in which the sight of brushing a rubber hand at the same time as brushing the subject's own hidden hand could produce a feeling of ownership of the fake hand. The authors found notable increases in activity of the left ventral premotor area along with the left intraparietal area (BA 7) during the illusion. In addition, the activity of the ventral premotor cortex was correlated to the strength of the perceived illusion (feeling of ownership of the hand), suggesting that multisensory integration in the ventral premotor cortex provided a mechanism for bodily self‐attribution. Makin et al. [2007], who also conducted an experiment to find the brain areas representing peripersonal space, found activation in the ventral premotor cortex and the caudal part of the intraparietal sulcus (BA 7). Behavioral studies performed on neuropsychological patients [Berti and Frassinetti,2000; Farne et al.,2005] and healthy individuals [Holmes et al.,2004; Maravita et al.,2002] have also provided clear evidence that the effective use of a tool to interact with distant objects induces a plastic modulation of peripersonal space representation. The underlying neural mechanism is the presence of tactile‐visual bimodal neurons in the caudal ventral premotor and intraparietal regions [Colby et al.,1998; Iriki et al.,1996; Matelli and Luppino,2001].
Activation in the left ventral premotor area, posterior portion of the intraparietal area, and perhaps also the superior parietal area, may well be explained by the above described mechanisms. That is, the posterior intraparietal and the ventral premotor activations can be attributed to the increased activity of their bimodal neurons in modulating the neural representation of peripersonal space from hand‐centered to tool‐centered. Indeed, our ROI analysis seems to support the above notion by showing that subjects with a smaller number of performance errors (better performers) had a higher level of posterior intraparietal activation, and there was also a similar tendency in the activation of the ventral premotor area. An earlier study by Inoue et al. [2001] failed to find the activation in any of the areas contralateral to the hand used for moving a 19‐mm diameter cylinder using tongs; this is most likely because the nature of their task could not induce strong multisensory interactions. The use of the current tool to pick up a 2‐mm diameter and 0.5‐g pin by their thin tips demands a much higher level of visuo‐tactile‐motor functional processing than the use of the tongs to move a large object. In this regard, a study using other tools similar to ours, such as pincers and precision pliers, may be needed to generalize the above idea of tool use‐related peripersonal space modulation through parieto‐frontal connections. A study on the use of the chopsticks with different lengths to modulate the strength of peripersonal space coding at the ventral premotor and intraparietal cortices may also be of interest since the findings of a behavioral study by Farne et al. [2005] suggest a linear relationship between tool‐length and the magnitude of peripersonal modulation.
The left middle‐temporal gyrus (BA 37) has been considered to be involved in tool identification, since naming as well as answering questions about tools, but not other artifacts, activated this area [Chao et al.,1999; Martin et al.,1996]. More recently, increased activity in the same area has been found when viewing familiar tools in motion [Beauchamp et al.,2002], and during the retrieval of knowledge about actions associated with manipulable tools [Kellenbach et al.,2003]. Activation of this area in this study must reflect the high familiarity of the current tool as well as established knowledge about its associated motor action in our subjects. The factor of familiarity based on long‐term experience with the use of complex tool must have played a role in stronger modulation in the representation of peripersonal space in the present study compared to the previous study that used less familiar tools (e.g., tongs) [Inoue et al.,2001]. This supported the view of Farne et al. [2005] who stated that the elongation of the multisensory areas surround the hand depended on not only purposeful use of a tool as physical extension of the body but also the complexity of action that was required for using the tool.
Activation of the dorsal premotor area can be attributed to its facilitated function in control of the reaching arm with the tool [Johonson‐Frey,2004]. Reaching toward a target object is known to involve transforming a representation of the object's extrinsic spatial properties and knowledge of the limb's position into a motor plan for the moving arm. Studies using monkeys have shown that this process is accomplished by cells in the intraparietal sulcus and the dorsal premotor area, as well as their neural connections [Andersen and Buneo,2002; Culham et al.,2006; Johnson et al.,1996]. A recent fMRI study in normal humans has confirmed an anatomo‐functional resemblance of the dorsal premotor cortex between the humans and monkeys [Amiez et al.,2006]. Since reaching toward a small target pin using the small tips of the 20‐cm long tool requires more precise spatio‐temporal planning of the arm motion, than using the fingers, the activation of the dorsal premotor and intraparietal areas seems to be quite reasonable. The fact that the dorsal premotor activation was not correlated with performance errors further suggests that dorsal premotor activation in the current tool use could be more associated with motor planning than actual motor execution.
The activation in the primary sensory area was observed at a relatively inferior portion in the left hemisphere. According to the well‐known somatotopic map of the primary sensory area, this inferior portion may correspond to the facial areas [Penifield and Boldrey,1937]. However, activation at a similar portion has been reported during sequential finger opposition by the fingers [Gelnar et al.,1999], as well as visually guided prehension of objects with complex shapes [Faillenot et al.,1997]. Thus, the higher activation of this area was probably due to necessary proprioceptive afferents from the fingers for the manipulation of the current tool.
The bilateral superior parietal activation, along with the occipital activation in the right hemisphere, can be argued in relation to the augmented neural processing for visuo‐spatial attention [Culham and Kanwisher,2001; Husain and Nachev,2007]. The findings of a recent behavioral study of crossmodal (visual‐tactile) congruency effects during the use of two hand‐held tools by normal humans demonstrated that multisensory interactions change on a trial‐by‐trial basis, depending upon the predictability of the next movement [Holmes et al.,2004]. Holmes et al., therefore, stated that the effects of tool use on multisensory interactions near hand‐held tools would include strong spatial attention and movement preparation components on top of any proposed modulation of purely sensory representations of hand‐centered space. Related to the latter effect, they have also shown that multisensory interactions were enhanced at the tips, but not the middles, of tools being used. The movement space of the tool's tips thus most likely reflects the side of cortical activation. Since the target pins were always transported to the basket located in the left hemispace, and half of the initial positions of the pins were also in the left hemispace in the current study, a bias toward the left side of the visual space must be present. In addition, unfamiliar spine body posture for tool manipulation caused a greater difficulty of prehension of a small pin with the use of chopsticks. Spatial attention associated with task difficulty, therefore, could have played a strong role in the current tool use. The results of the ROI analysis showed that there were indeed significant correlation between the level of occipital activation and the number of performance errors, and between the superior parietal and the reach‐lift period, which seemed to support this notion.
Activation of the cerebellar cortex contralateral to the moving limb has been discussed in relation to nonmotor or cognitive operations [Allen et al.,1997]. Using fMRI, Allen et al. demonstrated that the posterior lobe of the left cerebellum was activated during a pure visual attention task. When finger action was added to the attention task, this activation was further facilitated in concert with the activation on the side ipsilateral to the moving finger. Allen et al. stated that the neocerebellum's participation in attention arises from the need to predict, prepare for, and adjust to imminent information acquisition, analysis, or action. The cerebellum through its connections via the thalamus to the prefrontal and posterior parietal cortices might enable to the coordination of mental skills in response to a given task with attention. The parallel increase in activity with tool use between the left cerebellum and right superior parietal as well as occipital cortices seems to be consistent with these hypotheses.
The activation of the cerebellum has also been argued in relation to the process of retrieving stored internal models of dynamics and/or kinematics of the tool [Imamizu et al.,2000,1997; Obayashi et al.,2001]. Imamizu et al. [2000] showed that in a task of computer‐mouse use with a novel rotational transformation, a large area of the right cerebellum was activated initially, and then a smaller area remained active after long‐term training. The authors proposed that such local activation spots are the neural correlates of internal models of tools. In another similar study, Imamizu et al. [1997] reported that internal models of different tools would be represented in separated areas in the cerebellum. The hemisphere that is suggested by these authors to be responsible for this function is the right side, and therefore involvement of this role in the current activation may be less conceivable than the former possibility.
In summary, areas of the brain associated with the actual use of a well‐learned tool to grasp an object were identified in normal human subjects. Activation of the caudal ventral premotor area, and posterior intraparietal area were confirmed, which was considered to be involved in the extension of multisensory peripersonal space by tool use. Activation in the middle temporal gyrus suggests that high familiarity of the tool as well as established knowledge about its associated motor action can be an important source of this tool‐centered peripersonal space formation. Superior parietal and occipital activity suggests the requirement of visuo‐spatial attention typical for precision control of motion by the current tool. Cerebellar activation also can be linked to the increased visual attention with the use of the complex tool.
REFERENCES
- Allen G,Buxton RB,Wong EC,Courchesne E ( 1997): Attentional activation of the cerebellum independent of motor involvement. Science 275: 1940–1943. [DOI] [PubMed] [Google Scholar]
- Amiez C,Kostopoulos P,Champod AS,Petrides M ( 2006): Local morphology predicts functional organization of the dorsal premotor region in the human brain. J Neurosci 26: 2724–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen RA,Buneo CA ( 2002): Intentional maps in posterior parietal cortex. Annu Rev Neurosci 25: 189–220. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS,Lee KE,Haxby JV,Martin A ( 2002): Parallel visual motion processing streams for manipulable objects and human movements. Neuron 34: 149–159. [DOI] [PubMed] [Google Scholar]
- Berti A,Frassinetti F ( 2000): When far becomes near: Remapping of space by tool use. J Cogn Neurosci 12: 415–420. [DOI] [PubMed] [Google Scholar]
- Binkofski F,Buccino G ( 2006): The role of ventral premotor cortex in action execution and action understanding. J Physiol Paris 99: 396–405. [DOI] [PubMed] [Google Scholar]
- Binkofski F,Buccino G,Posse S,Seitz RJ,Rizzolatti G,Freund H ( 1999): A fronto‐parietal circuit for object manipulation in man: Evidence from an fMRI‐study. Eur J Neurosci 11: 3276–3286. [DOI] [PubMed] [Google Scholar]
- Binkofski F,Buccino G,Zilles K,Fink GR ( 2004): Supramodal representation of objects and actions in the human inferior temporal and ventral premotor cortex. Cortex 40: 159–161. [DOI] [PubMed] [Google Scholar]
- Brett M,Anton JL,Valabregue R,Poline JB ( 2002): Region of interest analysis using an SPM toolbox. Presented at the 8th international Conference on Functional Mapping of the Human Brain, June 2–6, 2002, Sendai, Japan. Neuroimage 16: 497. [Google Scholar]
- Buxbaum LJ ( 2001): Ideomotor apraxia: A call to action. Neurocase 7: 445–458. [DOI] [PubMed] [Google Scholar]
- Chao LL,Martin A ( 2000): Representation of manipulable man‐made objects in the dorsal stream. Neuroimage 12: 478–484. [DOI] [PubMed] [Google Scholar]
- Chao LL,Haxby JV,Martin A ( 1999): Attribute‐based neural substrates in temporal cortex for perceiving and knowing about objects. Nat Neurosci 10: 913–919. [DOI] [PubMed] [Google Scholar]
- Colby D,Laukkanen HR,Yolton RL ( 1998): Use of the Taylor Visagraph II system to evaluate eye movements made during reading. J Am Optom Assoc 69: 22–32. [PubMed] [Google Scholar]
- Culham JC,Kanwisher NG ( 2001): Neuroimaging of cognitive functions in human parietal cortex. Curr Opin Neurobiol 11: 157–163. [DOI] [PubMed] [Google Scholar]
- Culham JC,Cavina‐Pratesi C,Singhal A ( 2006): The role of parietal cortex in visuomotor control: What have we learned from neuroimaging? Neuropsychologia 44: 2668–2684. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH,Fagergren A,Jonsson T,Westling G,Johansson RS,Forssberg H ( 2000): Cortical activity in precision‐versus power‐grip tasks: An fMRI study. J Neurophysiol 83: 528–536. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH,Fagergren E,Forssberg H ( 2001): Differential fronto‐parietal activation depending on force used in a precision grip task: An fMRI study. J Neurophysiol 85: 2613–2623. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH,Spence C,Passingham RE ( 2004): That's my hand! Activity in premotor cortex reflects feeling of ownership of a limb. Science 305: 875–877. [DOI] [PubMed] [Google Scholar]
- Faillenot I,Toni I,Decety J,Grégoire MC,Jeannerod M ( 1997): Visual pathways for object‐oriented action and object recognition: Functional anatomy with PET. Cereb Cortex 7: 77–85. [DOI] [PubMed] [Google Scholar]
- Farnè A,Demattè ML,Làdavas E ( 2005): Neuropsychological evidence of modular organization of the near peripersonal space. Neurology 65: 1754–1758. [DOI] [PubMed] [Google Scholar]
- Fogassi L,Gallese V,Fadiga L,Luppino G,Matelli M,Rizzolatti G ( 1996): Coding of peripersonal space in inferior premotor cortex (area F4). J Neurophysiol 76: 141–157. [DOI] [PubMed] [Google Scholar]
- Friston KJ,Frith CD,Liddle PF,Dolan RJ,Lammertsma AA,Frackowiak RS ( 1990): The relationship between global and local changes in PET scans. J Cereb Blood Flow Metab 10: 458–466. [DOI] [PubMed] [Google Scholar]
- Gallese V,Fadiga L,Fogassi L,Rizzolatti G ( 1996): Action recognition in the premotor cortex. Brain 119: 593–609. [DOI] [PubMed] [Google Scholar]
- Gelnar PA,Krauss BR,Sheehe PR,Szeverenyi NM,Apkarian AV ( 1999): A comparative fMRI study of cortical representations for thermal painful, vibrotactile, and motor performance tasks. Neuroimage 10: 460–482. [DOI] [PubMed] [Google Scholar]
- Grafton ST,Fagg AH,Woods RP,Arbib MA ( 1996): Functional anatomy of pointing and grasping in humans. Cereb Cortex 6: 226–237. [DOI] [PubMed] [Google Scholar]
- Graziano MS,Hu XT,Gross CG ( 1997): Visuospatial properties of ventral premotor cortex. J Neurophysiol 77: 2268–2292. [DOI] [PubMed] [Google Scholar]
- Holmes NP,Calvert GA,Spence C ( 2004): Extending or projecting peripersonal space with tools? Multisensory interactions highlight only the distal and proximal ends of tools. Neurosci Lett 372: 62–67. [DOI] [PubMed] [Google Scholar]
- Holmes NP,Calvert GA,Spence C ( 2007): Tool use changes multisensory interactions in seconds: Evidence from the crossmodal congruency task. Exp Brain Res 183: 465–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain M,Nachev P ( 2007): Space and the parietal cortex. Trends Cogn Sci 11: 30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamizu H,Miyauchi S,Sasaki Y,Takino R,Putz B,Kawato M ( 1997): Separated modules for visuomotor control and learning in the cerebellum: A functional MRI study In: Toga AW,Frackowiak RSJ, Mazziotta JC, editors.NeuroImage: Third International Conference on Functional Mapping of the Human Brain, Vol. 5 Copenhagen, Denmark: Academic Press; pp. s598. [Google Scholar]
- Imamizu H,Miyauchi S,Tamada T,Sasaki Y,Takino R,Pütz B,Yoshioka T,Kawato M ( 2000): Human cerebellar activity reflecting an acquired internal model of a new tool. Nature 403: 192–195. [DOI] [PubMed] [Google Scholar]
- Imamizu H,Kuroda T,Miyauchi S,Yoshioka T,Kawato M ( 2003): Modular organization of internal models of tools in the human cerebellum. Proc Natl Acad Sci USA 100: 5461–5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imazu S,Sugio T,Tanaka S,Inui T ( 2007): Differences between actual and imagined usage of chopsticks: An fMRI study. Cortex 43: 301–307. [DOI] [PubMed] [Google Scholar]
- Inoue K,Kawashima R,Sugiura M,Ogawa A,Schormann T,Zilles K,Fukuda H ( 2001): Activation in the ipsilateral posterior parietal cortex during tool use: A PET study. Neuroimage 14: 1469–1475. [DOI] [PubMed] [Google Scholar]
- Iriki A,Tanaka M,Iwamura Y ( 1996): Coding of modified body schema during tool use by macaque postcentral neurones. Neuroreport 7: 2325–2330. [DOI] [PubMed] [Google Scholar]
- Johnson PB,Ferraina S,Bianchi L,Caminiti R ( 1996): Cortical networks for visual reaching: Physiological and anatomical organization of frontal and parietal lobe arm regions. Cereb Cortex 6: 102–119. [DOI] [PubMed] [Google Scholar]
- Johnson‐Frey SH ( 2004): The neural bases of complex tool use in humans. Trends Cogn Sci 8: 71–78. [DOI] [PubMed] [Google Scholar]
- Kellenbach ML,Brett M,Patterson K ( 2003): Actions speak louder than functions: The importance of manipulability and action in tool representation. J Cogn Neurosci 15: 30–46. [DOI] [PubMed] [Google Scholar]
- Kinoshita H,Oku N,Hashikawa K,Nishimura T ( 2000): Functional brain areas used for the lifting of objects using a precision grip: A PET study. Brain Res 857: 119–130. [DOI] [PubMed] [Google Scholar]
- Leiguarda RC,Marsden CD ( 2000): Limb apraxias: Higher‐order disorders of sensorimotor integration. Brain 123: 860–879. [DOI] [PubMed] [Google Scholar]
- Makin TR,Holmes NP,Zohary E ( 2007): Is that near my hand? Multisensory representation of peripersonal space in human intraparietal sulcus. J Neurosci 27: 731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maravita A,Spence C,Kennett S,Driver J ( 2002): Tool‐use changes multimodal spatial interactions between vision and touch in normal humans. Cognition 83: 25–34. [DOI] [PubMed] [Google Scholar]
- Martin A,Wiggs CL,Ungerleider LG,Haxby JV ( 1996): Neural correlates of category‐specific knowledge. Nature 379: 649–652. [DOI] [PubMed] [Google Scholar]
- Matelli M,Luppino G ( 2000): Parietofrontal circuits: Parallel channels for sensory‐motor integrations. Adv Neurol 84: 51–61. [PubMed] [Google Scholar]
- Matelli M,Luppino G ( 2001): Parietofrontal circuits for action and space perception in the macaque monkey. Neuroimage 14: 27–32. [DOI] [PubMed] [Google Scholar]
- Obayashi S,Suhara T,Kawabe K,Okauchi T,Maeda J,Akine Y,Onoe H,Iriki A ( 2001): Functional brain mapping of monkey tool use. Neuroimage 14: 853–861. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Penfield W,Boldrey E ( 1937): Somatic motor and sensory representations in the cerebral cortex of man as studied by electrical stimulation. Brain 60: 389–443. [Google Scholar]
- Rizzolatti G,Scandolara C,Matelli M,Gentilucci M ( 1981): Afferent properties of periarcuate neurons in macaque monkeys. II. Visual responses Behav Brain Res 2: 147–163. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G,Fadiga L,Matelli M,Bettinardi V,Paulesu E,Perani D,Fazio F ( 1996): Localization of grasp representations in humans by PET. I. Observation versus execution. Exp Brain Res 111: 246–252. [DOI] [PubMed] [Google Scholar]


