Abstract
Applied to the nasal mucosa in low concentrations, nicotine vapor evokes odorous sensations (mediated by the olfactory system) whereas at higher concentrations nicotine vapor additionally produces burning and stinging sensations in the nose (mediated by the trigeminal system). The objective of this study was to determine whether intranasal stimulation with suprathreshold concentrations of S(−)‐nicotine vapor causes brain activation in olfactory cortical areas or if trigeminal cortical areas are also activated. Individual olfactory detection thresholds for S(−)‐nicotine were determined in 19 healthy occasional smokers using a computer‐controlled air‐dilution olfactometer. Functional magnetic resonance images were acquired using a 1.5T MR scanner with applications of nicotine in concentrations at or just above the individual's olfactory detection threshold. Subjects reliably perceived the stimuli as being odorous. Accordingly, activation of brain areas known to be involved in processing of olfactory stimuli was identified. Although most of the subjects never or only rarely observed a burning or painful sensation in the nose, brain areas associated with the processing of painful stimuli were activated in all subjects. This indicates that the olfactory and trigeminal systems are activated during perception of nicotine and it is not possible to completely separate olfactory from trigeminal effects by lowering the concentration of the applied nicotine. In conclusion, even at low concentrations that do not consistently lead to painful sensations, intranasally applied nicotine activates both the olfactory and the trigeminal system. Hum Brain Mapp, 2009. © 2008 Wiley‐Liss, Inc.
Keywords: olfaction, odor, olfactometer, threshold, olfactory, trigeminal, nociception, fMRI
INTRODUCTION
S(−)‐nicotine is the natural enantiomer in tobacco [Marion,1950], although racemization to R(+)‐nicotine occurs to a small extent during combustion [Zevin et al.,1998]. The pharmacological and physiological properties of nicotine have been extensively studied in the last decades, including its binding sites and action in the nervous system [for review see Aceto and Martin,1982; Domino,1998; Rand,1989; Zevin et al.,1998]. Few studies have, however, focused on the sensory aspects of nicotine, especially its effects on the olfactory and trigeminal systems.
Edwards et al. [1987] were the first to apply S(−)‐nicotine as a vapor to the olfactory epithelium of rats and mice. They demonstrated that the electro‐olfactogram (EOG) generated by nicotine was similar to the EOGs produced by known odorants. The EOG amplitude increased with increasing concentrations of applied nicotine vapor. Thürauf et al. [1995] recorded EOGs from the frog olfactory epithelium after stimulation with different concentrations of S(−)‐ and R(+)‐nicotine. The aim of this study was to determine whether the olfactory system is responsible for the discriminability of the stereoisomers of nicotine. No differences could be found when the responses to the purified stereoisomers were compared, pointing to a similar receptor affinity of S(2)‐ and R(1)‐nicotine within the olfactory system.
Nicotine vapor acts as an odorant with a bimodal percept: intranasally applied to humans in low concentrations nicotine evokes odorous sensations, whereas at higher concentrations burning or even stinging sensations are observed because of activation of nociceptive fibers of the human trigeminal sensory system [Hummel et al.,1992b; Thürauf et al.,1999]. The burning and stinging sensations are probably mediated by nicotinic acetylcholine receptors (nAChRs) on nasal trigeminal nerve endings [Alimohammadi and Silver,2000; Thürauf et al.,1999,2006; Walker et al.,1996]. Thürauf et al. [2006] provided additional evidence that the olfactory chemoreception of nicotine is independent from peripheral nAChRs in the human nasal mucosa. It is therefore assumed that the odor of nicotine is exclusively mediated by binding to G‐protein coupled receptors (GPCRs) of the olfactory receptor family [for review see Mombaerts,1999].
Only a few odorants do not activate the trigeminal system in humans [Doty et al.,1978; Silver et al.,1985]. Thus the olfactory as well as the trigeminal system encodes information contributing significantly to the quality of an odorant [Laska et al.,1997]. Since both the olfactory and the trigeminal system are simultanously activated during odor perception, interactions between the two subsystems might have a powerful influence on the overall perception of chemical stimulants [for review see Brand,2006; Hummel and Livermore,2002]. It has been shown that trigeminal stimulation modulates the olfactory system [Cain and Murphy,1980; Hummel et al.,1992b; Jacquot et al.,2004; Kobal and Hummel,1988; Livermore et al.,1992], and vice versa [Cain and Murphy,1980; Kobal and Hummel,1988; Livermore et al.,1992]. These interactions are likely to take place at a central [Inokuchi et al.,1993; Jacquot et al.,2004] as well as at a peripheral level [Bouvet et al.,1987; Schaefer et al.,2002].
By recording evoked cortical responses (CSERPs) and intensity estimates following nasal stimulation with nicotine vapor in humans, Hummel et al. [1992b] found that the intensity ratings of burning and stinging sensations increased with rising stimulus concentration. In contrast the odorous sensation was highest at medium concentrations indicating an inhibition of the olfactory component by increasing trigeminal activation. After stimulation with low and medium concentrations of nicotine, the largest CSERP amplitudes were obtained in the parietal cortex, at higher concentrations amplitudes peaked at Cz indicating a shift from a distribution known for olfactory event‐related potentials to a distribution known for somatosensory event‐related potentials.
In previous olfactory imaging studies, brain activations in piriform cortex, amygdala, orbitofrontal cortex, cingulate, temporal and frontal cortices, peri‐insular cortex, and cerebellum have been found [for review see Gottfried,2006; Savic,2002; Wiesmann et al.,2004] representing the primary activations of the olfactory system. Following application of painful or mixed olfactory‐trigeminal intranasal stimulation, brain activations have been demonstrated in the thalamus and subcentral gyrus (secondary somatosensory cortex) among other regions [Hari et al.,1997; Savic et al.,2002; Yousem et al.,1997]. Given that nicotine in low concentrations acts as an odorant, it was hypothesized that intranasal stimulation with low concentrations of S(−)‐nicotine would produce activation in brain areas related to olfactory processing.
The objective of this study was to determine whether brain areas activated during suprathreshold olfactory nicotine stimulation correspond to those known to be involved in olfactory processing or if trigeminal cortical areas are also activated. That would provide evidence for a potential interaction between the two systems, even at very low nicotine concentrations.
METHODS
Subjects
Nineteen healthy individuals (9 females, 10 males) aged 22–45 years (mean age 29.0 years, SD 6.1 years) participated in the study. Age did not differ significantly between male and female subjects (male: mean age 27.5 years, SD 5.4 years; female: mean age 30.6 years, SD 6.7 years; t(1,17) = 1.1, P = n.s.). Before the experiment their normal olfactory function was confirmed by means of Sniffin' Sticks [Hummel et al.,1997; Kobal et al.,1996]. All subjects were occasional smokers (≤20 cigarettes per month). They were not taking any medication known to interfere with sensory perception [Doty and Bromley,2004].
All subjects were informed about the aim of the study and gave written informed consent prior to inclusion in the study which was in compliance with the Medical Ethics Committee of the Ludwig‐Maximilians‐University Munich. Subjects were free to end the testing session or their participation in the study at any time.
The study consisted of two parts: (1) an olfactory detection threshold test for nicotine and (2) an fMRI session consisting of two fMRI series and the subjective evaluation of the nicotine stimuli. To avoid adaptation of the olfactory system subjects participated in the two parts of the study on two different days.
Olfactory Stimulation
A computer‐controlled air‐dilution olfactometer (OM6b, Burghart Instruments, Wedel, Germany) was used for repeated chemosensory stimulation without any simultaneous stimulation of mechano‐ or thermoreceptors in the subjects' noses [Kobal,1981,1985; Kobal and Hummel,1988]. This was achieved by interspersing stimuli in a constant air stream (total flow rate 140 mL/s) at a controlled temperature of 36.5°C and relative humidity of 80%.
Specifically designed saturation chambers, as described by Thürauf et al. [1999,2000], were used in this experiment. The chambers were filled with a total of 1.2 mL of 99.9% optically and chemically pure S(−)‐nicotine (Chemische Laboratorien Dr. Christoph Mark, Worms, Germany), which was absorbed on three filter papers (400 μL nicotine on each filter paper), each one shelved on a separate stainless steel screen inside the chamber. The chambers were filled with nicotine under nitrogen atmosphere to avoid oxidation of the S(−)‐nicotine. The chambers were stored at −40°C until installation into the olfactometer. During the experiment, nitrogen was used as a carrier gas to flow through the filter papers resulting in a stream of nitrogen saturated with nicotine vapor. All olfactometer parts which were possibly in contact with nicotine were thermostabilized to prevent condensation inside the tubing system. Additionally, during experiments a specifically designed droplet catcher was used which ensured that only nicotine vapor reached the nose of the subject.
For olfactory stimulation, nicotine vapor was applied by means of a thin teflon tube (outer diameter 4 mm) which was inserted ∼1 cm into the left nostril of the subjects. The duration of each olfactory stimulus was precisely controlled by a vacuum switch, ensuring that only odorless air entered the nose of the subjects during the interstimulus interval. Subjects were trained to breathe through the mouth using velopharyngeal closure [Kobal,1985], ensuring that the nasal cavities are separated from the lower parts of the pharynx by the velum and to avoid respiratory airflow through the nose. Velopharyngeal closure also assured that nicotine did not enter the lungs where it is known to be rapidly absorbed. White noise of ∼80 dB SPL was delivered through earphones to minimize the perception of external auditory stimuli. Nose drops (Xylometazolin, Otriven®, 0.1%) were applied intranasally to the subjects to prevent nasal volume changes due to the nasal cycle, a known phenomenon of alternating congestion and decongestion of the nasal airways [Hasegawa and Kern,1977; Principato and Ozenberger,1970]. Adverse effects of the nose drops on olfactory perception or transduction are not known [Kobal, personal communication].
Olfactory Detection Threshold Determination
For olfactory stimulation nine concentration steps for S(−)‐nicotine (0.1 to 25% v/v of saturated nicotine vapor corresponding to concentrations from 3.8 to 143.0 μg/L, dilution steps 1:2) were established in the olfactometer. S(−)‐nicotine stimuli with a duration of 500 ms were used during threshold determination. Monorhinal detection thresholds were obtained using a single‐staircase, three alternative forced choice (3‐AFC) procedure [Doty,1991; Hummel et al.,1997], which was identical to the threshold testing procedure of the Sniffin' Sticks [Hummel et al.,1997; Kobal et al.,1996]. A newly developed Microsoft Visual Studio 2002 software was utilized (Version 3.0.1759.24863, by Robert J. Maher, Philip Morris, Richmond, VA), which allowed automated threshold testing in combination with the olfactometer. Quantitative analysis of the nicotine concentrations delivered by the olfactometer was done by Michael Czerny and Peter Schieberle (Deutsche Forschungsanstalt für Lebensmittelchemie, Garching, Germany).
Functional Magnetic Resonance Imaging
Subjects were placed in a supine position with their heads resting on a vacuum headrest and fixed with a restraining band drawn across the forehead. The subjects were told to close their eyes during image acquisition [Wiesmann et al.,2006]. Each olfactory fMRI session consisted of two series with a 30 min break between them to avoid adaptation. The stimulation design we used was an events‐in‐blocks design, consisting of 6 stimulation blocks, each with 4 nicotine stimuli (500 ms duration each), followed by an interstimulus interval of 20 s. The nine concentration steps for nicotine established in the olfactometer for threshold determination were also used during imaging sessions. The concentration of nicotine stimuli applied to each subject was equal to that individual's detection threshold if this concentration existed in the olfactometer calibration. If this was not the case the nearest higher concentration step was chosen, i.e., the applied concentration was not higher than two times above the individual detection threshold concentration. After each nicotine block, an auditory block was performed during which subjects heard an auditory stimulus consisting of a beep via MR compatible earphones and were instructed to press a button every time they heard the beep (block length 19.2 s). After each auditory block there was a 80 s rest condition. Given the long stimulation and rest blocks, the auditory condition enabled us to control subjects' alertness. This design allowed the olfactory system to recover from adaptation in between the nicotine blocks, while the total number of stimuli was kept high enough to enable acceptable signal‐to‐noise ratio in the fMRI signal. In addition, the long interval between stimulation blocks prevented the potential build‐up of nicotine at the nasal mucosa or temporal summation at the receptor site, thus minimizing the potential for the occurrence of trigeminal effects [Cometto‐Muniz et al.,2004]. During each fMRI series, a total of 24 nicotine stimuli and 6 auditory stimuli were delivered. The stimulation paradigm applied during imaging sessions is illustrated in Figure 1.
Figure 1.

Stimulation paradigm employed during imaging sessions (Nic, intranasal nicotine stimulation; Beep, auditory stimulation; BL, baseline). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Functional images were acquired on a 1.5 T standard clinical MRI scanner (Siemens Vision, Erlangen, Germany) using echo‐planar imaging (EPI) with a T2*‐weighted gradient echo multi‐slice sequence (echo time (TE) = 60 ms, repetition time (TR) = 3,200 ms, voxel size 3.75 × 3.75 × 6.25 mm3, matrix size 64 × 64). Twenty‐six slices covering the whole brain and the eyes were acquired. The slices were adjusted orthogonally to the bisecting line of an angle between intercommissural line and brainstem line based on a sagittal localizer image to obtain a half‐coronal slice orientation, which is known to reduce susceptibility artifacts at the orbito‐temporal junction [Deichmann et al.,2003]. One scanning session was comprised of two successive series consisting of 366 image volumes each.
Nicotine Stimulus Assessment
Subjective assessment of the nicotine stimuli was obtained after each fMRI series. The subjects were instructed to estimate the perceived intensity of the odorous sensations of nicotine (0 = no odor, 4 = very intense odor) as well as the frequency of burning or stinging sensations in the nose after stimulus delivery (0 = never, 3 = always) to indicate potential stimulation of trigeminal fibers. They rated the perceived affective valence (1 = negative, 9 = positive) and arousal (1 = calm, 9 = aroused) during the fMRI experiment using a 9‐point Self‐Assessment Manikin scale [Bradley and Lang,1994]. In addition, subjects were asked to rate the odor of nicotine as either pleasant, neutral, or unpleasant.
Data Analysis
SPSS (version 15.0 for Windows, SPSS, Chicago, IL) was used for statistical evaluation. For comparison of age and olfactory detection thresholds of female and male subjects t‐tests for independent samples were used. The α‐level was set at 0.05. For statistical evaluation of the nicotine stimulus assessment, all of the ratings were averaged across all subjects. Means are indicated together with standard deviations.
fMRI data were processed using statistical parametric mapping (SPM2) [Friston et al.,1994] implemented in Matlab (Matlab Release 13, Mathworks, Sherborn, MA). The first five volumes of each series were discarded to eliminate spin saturation effects. Motion correction was performed by realigning each volume to the first of each scanning series [Friston et al.,1995]. A correction for field inhomogeneities (unwarping) was applied to the volumes [Andersson et al.,2001; Friston et al.,1996]. Then the image volumes were spatially normalized [Friston et al.,1995] to the Montreal Neurological Institute (MNI) standard template. The resulting voxel size was 2 × 2 × 2 mm3. Next, the data sets were smoothed using an 8‐mm full‐width at half‐maximum (FWHM) isotropic Gaussian kernel to compensate for individual gyral variability and to attenuate high frequency noise in order to improve signal‐to‐noise ratio. For single subject analyses of the data, statistical parametric maps were calculated using the general linear model (GLM) [Friston et al.,1994] with regressors corresponding to the onset times of the nicotine events convolved with the canonical hemodynamic response function. The design matrix was specified in an event‐related design with each nicotine and auditory stimulus modeled as a single event. As primary contrasts, the activations (nicotine stimuli vs. “other times”) and deactivations (“other times” vs. nicotine stimuli) in response to nicotinic chemosensory stimulation were investigated. Utilizing these contrasts assured that only the brain activations due to intranasal nicotine stimulation were considered, while effects during baseline or auditory stimulation were modeled as null variables. A fixed‐effects analysis was done on the individual subject level to collapse repeated measures within a subject. From the resulting contrast images, a random‐effects group analysis was performed.
Subjects were grouped in four different ways according to their sex, the nicotine stimulus assessment questionnaire, and the individual nicotine concentration used for stimulation. Two sample t‐tests were then used to compare the effects of intranasally applied nicotine between the groups “female subjects” vs. “male subjects,” “perceived only olfactory stimulation” vs. “perceived olfactory and trigeminal stimulation,” “perceived nicotine stimuli as pleasant” vs. “perceived nicotine stimuli as unpleasant,” and between the groups “received stimuli at threshold level” vs. “received stimuli above threshold level.”
P‐values of all t‐tests were corrected for false discovery rate (FDR) [Genovese et al.,2002]. For the random‐effects group analyses, P values < 0.05 (FDR‐corrected for whole brain volume) thereby correcting for multiple comparisons across whole‐brain volume were considered significant.
Areas of significant activation at the group level were anatomically categorized using a multitude of techniques. The Montreal Neurological Institute (MNI) coordinates and the parcellation method along with the automated anatomical labeling (AAL) software described by Tzourio‐Mazoyer et al. [2002] were used to define general anatomical activation sites. Because of absence of anatomical labels of some olfactory brain regions in AAL, two experienced neuroradiologists (MW, JL) labeled the olfactory activation clusters. The defined anatomical sites of activation clusters were then verified using an anatomical atlas [Mai et al.,2004]. For identification of cerebellar activation sites, definitions in Schmahmann et al. [2000] were used.
Additionally, we investigated BOLD signal intensity over time in the activation maximum (random‐effects analysis) of a secondary olfactory region (R. piriform cortex (22, 4, −16)) and of a secondary somatosensory region (R. subcentral gyrus (58, −12, 10)). For definition of the VOIs (voxels of interest) and extraction of the signal time courses the Marseille ROI Toolbox (marsbar‐0.38.2‐devel) [Brett et al.,2002] was utilized. To compare signal time courses at the beginning and the end of a block in each VOI, signal time courses were averaged across all first and second events (beginning of a block) or across all third and fourth events (end of a block) of all blocks and all subjects using a custom developed Matlab routine (Matlab Release 13, Mathworks, Sherborn, MA). Nicotine vapor stimulation occurred over the first 500 ms and the average time course was plotted for 14 s after stimulus application. Means and standard error of means of the absolute signal intensities are reported.
RESULTS
Olfactory Detection Thresholds
Individual olfactory detection thresholds of S(−)‐nicotine were determined in the range between 0.1 and 15.9% v/v of saturated nicotine vapor (mean 4.3% v/v, SD 5.1% v/v) corresponding to a nicotine concentration of 3.8–92.1 μg/L (mean 27.3 μg/L, SD 31.9 μg/L) in a bolus of 500 ms duration. No significant difference was found in the detection threshold of S(−)‐nicotine between male and female subjects (male: mean 3.2% v/v, SD 5.1% v/v, female: 5.5% v/v, SD 5.1% v/v, t(1,17) = 1.0, P = n.s.).
Subjective Ratings
Olfactory nicotine vapor stimuli were perceived as medium intense (mean 2.8, SD 0.8). Eleven of the subjects never felt burning or stinging in the nose during stimulation, seven subjects reported that they rarely felt burning or stinging, and only one subject reported frequent burning or stinging in the nose during stimulation. Overall, the nicotine vapor stimuli were perceived as minimally burning or stinging (mean 0.4, SD 0.6). In general, subjects felt emotionally positive (mean 7.1, SD 2.2) and were calm (mean 2.6, SD 2.2) during the experiment. Nine subjects considered the nicotine stimuli to be unpleasant, two subjects rated them as neutral, and eight subjects considered the nicotine stimuli to be pleasant.
Functional Magnetic Resonance Imaging
For eleven out of 19 subjects, the concentration of nicotine stimuli applied during the fMRI sessions was equal to that individual's detection threshold. For eight out of 19 subjects, the detection threshold concentration did not exist in the olfactometer calibration in which case the nearest higher concentration step was chosen. Only one of these subjects corresponded to the eight subjects that perceived trigeminal stimulation during the scanning sessions.
Following intranasal stimulation of all subjects with low concentrations of nicotine, bilateral activation was observed in the superior frontal gyrus (supplementary motor area, SMA), the inferior frontal gyri, the cerebellum, the insula, the supramarginal gyrus, the thalamus, and the inferior parietal lobule. Unilateral activation on the right side was seen in the middle cingulate gyrus, the anterior and the posterior orbital gyrus, the piriform cortex, the middle frontal gyrus, the precuneus, the brainstem, the subcentral gyrus (secondary somatosensory cortex, S II), the superior temporal gyrus, and in the superior parietal lobule. On the left side, unilateral activation of the posterior cingulate cortex, the superior occipital gyrus, the precentral gyrus, the middle temporal gyrus, the calcarine gyrus, the cuneus, and the paracentral lobule was found (Fig. 2). The reported activations were FDR‐corrected for whole brain volume. Detailed results are presented in Table I.
Figure 2.
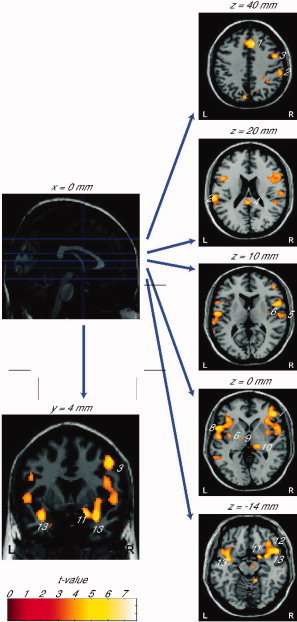
fMRI activation associated with stimulation of the nasal mucosa with S(−)‐nicotine in concentrations just above the individual olfactory detection thresholds. Activation maps showing significant increases in BOLD signal obtained by statistical group analysis for the contrast nicotine using SPM2. The design matrix was specified in an event‐related design with each nicotine and auditory stimulus modeled as a single event. As primary contrasts, the activations (nicotine stimuli vs. “other times”) and deactivations (“other times” vs. nicotine stimuli) in response to nicotinic chemosensory stimulation were investigated. Activations are projected onto a standard template brain (group analysis, n = 19, P < 0.05 FDR‐corrected for whole brain volume, L = left, R = right). Shown are selected sagittal (x = 0 mm), coronal (y = 4 mm) and axial slices (z = ‐14, 0, 10, 20 and 40 mm). Activation is observed in middle cingulate gyrus (1), supramarginal gyri (2), right middle frontal gyrus (3), left posterior cingulate gyrus (4), right subcentral gyrus (secondary somatosensory cortex, S II) (5), bilaterally in the posterior insula (6), right inferior frontal gyrus (triangular part) (7), left inferior frontal gyrus (opercular part) (8), left thalamus (ventrolateral posterior nucleus) (9), right thalamus (lateral pulvinar nucleus) (10), right piriform cortex (11), right posterior orbital gyrus (12), bilaterally in the anterior insula (13).
Table I.
fMRI activation associated with stimulation of the nasal mucosa with S(−)‐nicotine in concentrations just above the individual olfactory detection thresholds (group analysis, n = 19, contrast nicotine, P < 0.05 FDR‐corrected for whole brain volume, R. = right; L. = left, clusters ≤ 6 voxels were disregarded)
| Brain region | MNI coordinates (mm) | No. of activated voxels | Peak Z | ||
|---|---|---|---|---|---|
| x | Y | z | |||
| R. middle cingulate gyrus | 8 | 28 | 38 | 999 | 5.05 |
| R. superior frontal gyrus/R. supplementary motor area | 10 | 20 | 44 | ||
| L. superior frontal gyrus/L. supplementary motor area | −8 | 16 | 50 | ||
| R. inferior frontal gyrus (triangular part)/ R. posterior orbital gyrus | 46 | 38 | 0 | 3,266 | 4.68 |
| R. piriform cortex | 22 | 4 | −16 | ||
| L. cerebellum (lobule 6, declive) | −30 | −60 | −30 | 618 | 4.50 |
| L. inferior frontal gyrus (opercular part) | −54 | 12 | 4 | 2,440 | 4.45 |
| L. anterior insula | −40 | 16 | −8 | ||
| L. supramarginal gyrus | −62 | −28 | 20 | ||
| R. middle frontal gyrus | 42 | 36 | 30 | 133 | 4.24 |
| R. middle frontal gyrus | 50 | 4 | 44 | 266 | 4.09 |
| L. posterior cingulate gyrus | −4 | −34 | 24 | 353 | 3.87 |
| R. precuneus | 24 | −64 | 28 | 78 | 3.86 |
| L. superior occipital gyrus | −6 | −80 | 42 | 79 | 3.81 |
| R. thalamus (lateral pulvinar nucleus) | 20 | −26 | 2 | 291 | 3.79 |
| R. brainstem (red nucleus) | 4 | −30 | −6 | ||
| L. precentral gyrus | −22 | −18 | 56 | 50 | 3.71 |
| R. supramarginal gyrus | 58 | −30 | 38 | 520 | 3.60 |
| R. subcentral gyrus/R. secondary somatosensory cortex | 58 | −12 | 10 | ||
| L. posterior insula | −34 | −4 | 0 | 52 | 3.58 |
| L. middle temporal gyrus | −58 | −50 | −4 | 123 | 3.57 |
| L. cerebellum (lobule 7, crus II) | −8 | −76 | −34 | 107 | 3.49 |
| L. inferior parietal lobule | −38 | −52 | 52 | 66 | 3.44 |
| R. superior temporal gyrus/ R. supramarginal gyrus | 52 | −38 | 22 | 74 | 3.43 |
| L. calcarine gyrus | 2 | −92 | 6 | 36 | 3.42 |
| L. cerebellum (lobule 7b, caudal part of tuber valvulae) | −30 | −40 | −40 | 14 | 3.36 |
| R. inferior parietal lobule | 30 | −38 | 38 | 94 | 3.35 |
| R. superior temporal gyrus | 66 | −32 | 14 | 19 | 3.27 |
| R. cerebellum (lobule 7, crus I) | 26 | −72 | −32 | 84 | 3.23 |
| L. thalamus (ventrolateral posterior nucleus) | −10 | −12 | 4 | 31 | 3.17 |
| R. superior parietal lobule | 50 | −46 | 58 | 34 | 3.16 |
| L. cuneus | −12 | −76 | 28 | 14 | 3.11 |
| R. posterior insula | 34 | −6 | 12 | 17 | 3.05 |
| R. anterior orbital gyrus | 30 | 50 | −8 | 8 | 3.03 |
| R. superior frontal gyrus/R. supplementary motor area | 12 | 8 | 70 | 8 | 3.01 |
| L. paracentral lobule/L. supplementary motor area | −10 | −14 | 62 | 8 | 2.97 |
No deactivations were detected in response to intranasal stimulation with low concentrations of S(−)‐nicotine vapor using an FDR‐corrected P‐value level.
The analyses comparing the effects of intranasally applied nicotine between the groups “female subjects” vs. “male subjects,” “perceived only olfactory stimulation” vs. “perceived olfactory and trigeminal stimulation,” “perceived nicotine stimuli as pleasant” vs. “perceived nicotine stimuli as unpleasant,” and between the groups “received stimuli at threshold level” vs. “received stimuli above threshold level” did not reveal any significant differences at the FDR‐corrected level.
In Figure 3, we show the comparison of means of absolute signal intensity in an olfactory region (R. piriform cortex (VOI 22, 4, −16)) and in a secondary somatosensory region (R. subcentral gyrus (VOI 58, −12, 10)). Signal intensity courses in piriform cortex follow the expected BOLD curve at the beginning as well as at the end of an experimental block. In contrast, in secondary somatosensory cortex the signal intensity course follows the expected BOLD curve at the end of the block but does not show a typical BOLD response at the beginning of a block.
Figure 3.
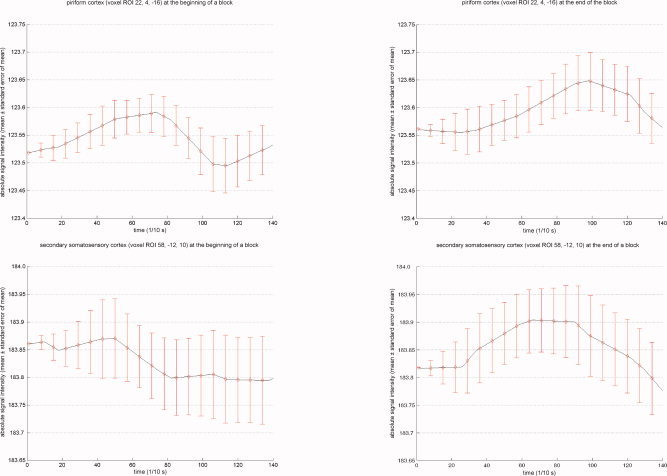
Shown are the means and standard error of means (SEMs) of the absolute signal intensities in secondary olfactory cortex (R. piriform cortex (VOI 22, 4, −16)) and in secondary somatosensory cortex (R. subcentral gyrus (VOI 58, −12, 10)) at the beginning (first two events per block) versus the end (last two events per block) of a block. Nicotine vapor stimulation occurred over the first 500 ms and the time course was plotted for 14 s after stimulus application. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
Since it is known that nicotine has odorous qualities, activation in cortical regions known to be involved in the processing of olfactory stimuli was expected. The activation found in this study is in accordance with both our hypothesis and with previous olfactory studies using a variety of odorants not including nicotine [Anderson et al.,2003; Cerf‐Ducastel and Murphy,2001,2004; Gottfried et al.,2002; Poellinger et al.,2001; Savic,2002; Small et al.,2004; Weismann et al.,2001,2004,2006]. From an evolutionary point of view, the olfactory bulb is not a ganglion but a part of the telencephalon, one of the oldest portions of the brain. Following this line of thought, it has been postulated that the olfactory bulb constitutes the genuine primary olfactory cortex [Albrecht and Wiesmann,2006; Boyle et al.,2007; Cleland and Linster,2003], and accordingly, the piriform cortex constitutes to be a secondary olfactory region. Brain regions detected in response to intranasal stimulation with nicotine (piriform cortex, frontal cortices, cingulate cortices, insulae, supramarginal cortices, Fig. 2, Table I) corresponded to secondary and tertiary olfactory regions and associated cortical areas involved in the processing of odorous stimuli.
In addition to brain areas related to olfactory processing, activation was detected in brain areas known to be involved in the processing of trigeminal stimuli (thalamus, subcentral gyrus (secondary somatosensory cortex, S II), Fig. 2, Table I). These results are in accordance with the results of previous studies examining the processing of painful stimuli, or of mixed olfactory‐trigeminal intranasal stimulation [Boyle et al.,2007; Hari et al.,1997; Hummel et al.,2005; Kettenmann et al.,1996; Savic et al.,2002; Yousem et al.,1997].
The thalamus, which is activated during intranasal stimulation with low concentrations of nicotine, is responsible for processing of trigeminal stimuli. However, it is also thought to be part of the olfactory network. It receives olfactory projections from the piriform cortex and the olfactory tubercle [Price,1985]. It has also been shown that the thalamus is activated in humans in response to olfactory stimuli known to have minimal or no trigeminal effects [Gottfried et al.,2002; Sobel et al.,2000]. Since the thalamus is a very heterogeneous brain area, it is feasible that different subregions of the thalamus responded to trigeminal versus olfactory stimulation.
Surprisingly, intranasally applied nicotine activated both the olfactory and the trigeminal system even at low concentrations and even if subjects felt no or only rarely some burning in the nose. This suggests an interaction between the olfactory and the trigeminal system, which is in line with other studies [Brand,2006; Cain and Murphy,1980; Hummel and Livermore,2002]. The present data show that it is not possible to completely separate olfactory from trigeminal effects by lowering the concentration of the intranasally applied nicotine vapor. Even at the lowest perceivable concentrations, nicotine exerts trigeminal effects.
One possible explanation for brain activation in areas responsible for pain processing although only half of the subjects actually reported a burning in the nose, is that the perceptual threshold for pain is higher than the cortical activation threshold in primary or secondary objective areas [Handwerker and Kobal,1993]. It seems that the lack of conscious awareness during activation of the secondary somatosensory cortex could be explained as an activation threshold required for triggering of consciousness. Unconscious cerebral processes may precede subjective sensory experiences [Libet,2006].
According to Doty and Laing [2003], the signal detection theory [Green and Swets,1966] bases upon signal detection on the relationship between signal and noise. Additionally, both subjects' expectations and rewards influence the detection situation. An olfactory detection threshold test, which is an example of a signal detection situation, provides a measure of olfactory sensitivity and the subject's response criterion. The response criterion is the internal rule used by the subject in deciding whether or not to report detecting the signal [Doty and Laing,2003]. This response criterion might have been different for olfaction and sensory irritation in this study. Since these potential differences were not investigated, this might be considered as a limitation of our study design. It is possible that subjects included in our study underreported the burning, painful sensation in the nose. Bearing in mind that in this study the subjective irritability ratings were recorded post scanning on a three point scale, it cannot be excluded that the delay in evaluating the stimuli and the short range of the scale led to the underreporting of the burning or painful sensation. A more reliable instrument for measuring irritation would have been a bi‐nostril test [Hummel et al.,2003a] but this was not feasible inside the scanner. Further experiments of this sort should use a kind of online evaluation of the intensity of the stimuli using a visual analogue scale during fMRI.
Another potential limitation of this study is that the nicotine stimulation during the detection threshold test was slightly different from that during imaging sessions with respect to number of stimuli and interstimulus interval. During the functional imaging sessions, a stimulus concentration corresponding to the individuals' detection threshold or slightly above it (maximally two times higher in concentration) was used. Since the results of other studies show that the threshold of the burning sensation of nicotine lies between 3.25 and 14.4 times higher than the olfactory detection threshold [Hummel et al.,1992a; Thürauf et al.,1999,2000,2006] it is highly unlikely that subjects in the present study were stimulated with nicotine concentrations above the individuals' trigeminal threshold. Besides this it may be speculated that the olfactory and the trigeminal system follow different time courses of stimulus adaptation, meaning that the olfactory system desensitizes whereas the trigeminal system sensitizes (compare Hummel et al. [2003b], Poellinger et al. [2001] and Edwards et al. [1987]). This could lead to underreporting of burning sensations because of the subjects' focus on what is first and rapidly perceived versus on what starts later with a flat intensity time course.
This suggestion is supported by a figure on the intensity of BOLD response over time in an olfactory versus a trigeminal area (Fig. 3). Comparing the signal time courses of the piriform cortex, one could assume that the olfactory component of nicotine was perceived all over the stimulation block because the signals followed the typical BOLD curve. The signal intensity curve at the beginning of the block in secondary somatosensory cortex did not show a typical BOLD response, whereas at the end of the block it did follow the typical BOLD response. Therefore, it is speculated that the trigeminal sensation is not perceived at the beginning but at the end of a block, whereas the olfactory sensation is perceived all over the block, which could lead to an underreporting of the trigeminal sensation.
Central integration of multisensory cues has already been established for odor‐taste interactions during flavor perception [Dalton et al.,2000; Small et al.,1997] and for mixtures of olfactory and trigeminal compounds [Cain and Murphy,1980]. It is conceivable that the olfactory component of nicotine amplifies its trigeminal component at a central nervous level. This would be similar to the model of mixed sensory adaptation/compensation in the interactions between olfactory and trigeminal system by Frasnelli et al. [2007], which is based on the assumption that the trigeminal activation is reduced on a peripheral level due to adaptation while on the central level it is amplified. Furthermore, it is known that both the olfactory and the trigeminal system activate similar cortical structures [Boyle et al.,2007; Savic et al.,2002] making it hard to distinguish between activations of the two systems based solely on the activation pattern. Nevertheless it is supposed that olfactory stimulation leads to an activation of traditional olfactory regions more than trigeminal stimulation and vice versa.
To minimize or even avoid trigeminal effects, nicotine vapor was applied at or just above detection threshold concentrations. This constitutes a problem in performing fMRI studies because of the intrinsic poor signal‐to‐noise ratio. In other sensory experiments, one would enhance the frequency of the stimuli to increase the BOLD signal and improve the statistical power of the data. This cannot easily be done in olfactory experiments because of rapid desensitization of the olfactory system after repeated stimulation [Poellinger et al.,2001]. Adaptation and habituation occur at the level of the olfactory receptors as well as in the olfactory bulb, and in brain areas involved in processing of olfactory stimuli [Dalton,2000]. According to Edwards et al. [1987], who provided evidence that nicotine acts as an odorant, the time course of adaptation to nicotine stimuli is the same or similar to other chemosensory stimuli.
A problem in studies involving nicotine as an intranasal stimulant is that activation of trigeminal fibers may occur not only as a consequence of a single stimulus of nicotine, but in addition the concentration of nicotine in the nasal mucosa may build up due to repetitive stimulation, resulting in a sensory summation [Cometto‐Muniz et al.,2004]. To avoid or minimize the so called temporal summation of irritation, we stimulated with relatively long interstimulus intervals (20 s) and interblock intervals (99.2 s) [Hummel and Livermore,2002; Hummel et al.,2003b].
In this study, the nicotine stimulus was administered only to the left nostril. Interestingly, activation of olfactory brain areas was found on the right side, although olfactory pathways are ipsilaterally organized. The trigeminal system, on the other hand, utilizes central contralateral projections alluding to the possibility that activation of brain areas observed in the present study was triggered by the trigeminal rather than the olfactory component of nicotine as it has been found in other studies [Boyle et al.,2007; Hummel et al.,2005]. However, our experimental setup renders this unlikely. At their dorsal end, the nostrils are connected via the choanae. Our subjects breathed using velopharyngeal closure causing the constant airflow we applied through the left nostril to leave through the right nostril. Thus, we can assume that the monorhinal stimulation in combination with the breathing technique effectively led to a birhinal chemosensory stimulation.
Because of the air flow of 8 L/min, the applied nicotine had a very short contact time with the nasal mucosa. Because of this and because passage of nicotine to the alveoli of the lungs was prevented by velopharyngeal closure, we can assure that brain activity reported in this study was not triggered or modulated by nicotine absorbed into the blood stream, but was purely due to binding of nicotine to olfactory or trigeminal receptor sites in the nose.
Several experimenters have found activation in the cerebellum following olfactory stimulation [Cerf‐Ducastel and Murphy,2001; Sobel et al.,1998; Weismann et al.,2001; Yousem et al.,1997]. Sniffing plays an important role in the transport of odorous molecules to the olfactory receptors. It is assumed that the cerebellum maintains a feedback mechanism that regulates sniff volume in relation to odor concentration [Sobel et al.,1998]. In this study, cerebellar activation was also found (Fig. 2, Table I) although subjects breathed through the mouth using velopharyngeal closure and thus were not able to sniff during olfactory perception. This indicates that the proposed feedback mechanism between olfactory cortex and cerebellum may be acting anticipatorily even without sniffing being actually performed.
The results of this study confirm and extend previously reported data that nicotine acts as an odorant and sensory irritant. Even with concentrations in which mainly the olfactory component is subjectively perceived, nicotine applied to the nasal mucosa also leads to consistent activation of somatosensory brain areas. Our findings underline the close interaction between the olfactory and the somatosensory system indicating that it may be impossible to visualize effects specific to each system. Future studies should aim at the investigation of both olfactory and trigeminal thresholds of different odorants as a basis for fMRI experiments with concentrations just above the individual olfactory and trigeminal thresholds.
Acknowledgements
The authors wish to thank Haoxian Zhang for her useful advice and training in the handling of nicotine and nicotine application via the olfactometer, and Robert J. Maher for developing the detection threshold testing software. We thank Michael Czerny and Peter Schieberle (Deutsche Forschungsanstalt für Lebensmittelchemie, Garching, Germany) for quantitative analysis of the nicotine flow from the olfactometer. We are grateful to Virginia Flanagin for proofreading the manuscript. Parts of this study were conducted in line with the dissertation of Jessica Albrecht at the Medical Faculty of the Ludwig‐Maximilians‐University of Munich (in preparation).
REFERENCES
- Aceto MD,Martin BR ( 1982): Central actions of nicotine. Med Res Rev 2: 43–62. [DOI] [PubMed] [Google Scholar]
- Albrecht J,Wiesmann M ( 2006): The human olfactory system: Anatomy and physiology. Nervenarzt 77: 931–939. [DOI] [PubMed] [Google Scholar]
- Alimohammadi H,Silver WL. ( 2000): Evidence for nicotinic acetylcholine receptors on nasal trigeminal nerve endings of the rat. Chem Senses 25: 61–66. [DOI] [PubMed] [Google Scholar]
- Anderson AK,Christoff K,Stappen I,Panitz D,Ghahremani DG,Glover G,Gabrieli JD,Sobel N ( 2003): Dissociated neural representations of intensity and valence in human olfaction. Nat Neurosci 6: 196–202. [DOI] [PubMed] [Google Scholar]
- Andersson JL,Hutton C,Ashburner J,Turner R,Friston K ( 2001): Modeling geometric deformations in EPI time series. Neuroimage 13: 903–919. [DOI] [PubMed] [Google Scholar]
- Bouvet JF,Delaleu JC,Holley A ( 1987): Olfactory receptor cell function is affected by trigeminal nerve activity. Neurosci Lett 77: 181–186. [DOI] [PubMed] [Google Scholar]
- Boyle JA,Heinke M,Gerber J,Frasnelli J,Hummel T ( 2007): Cerebral activation to intranasal chemosensory trigeminal stimulation. Chem Senses 32: 343–353. [DOI] [PubMed] [Google Scholar]
- Bradley MM,Lang PJ ( 1994): Measuring emotion: The self‐assessment Manikin and the semantic differential. J Behav Ther Exp Psychiatry 25: 49–59. [DOI] [PubMed] [Google Scholar]
- Brand G ( 2006): Olfactory/trigeminal interactions in nasal chemoreception. Neurosci Biobehav Rev 30: 908–917. [DOI] [PubMed] [Google Scholar]
- Brett M,Anton J,Valabregue R,Poline J ( 2002): Region of interest analysis using an SPM toolbox. (Available on CD‐ROM in Neuroimage 16).
- Cain WS,Murphy CL ( 1980): Interaction between chemoreceptive modalities of odour and irritation. Nature 284: 255–257. [DOI] [PubMed] [Google Scholar]
- Cerf‐Ducastel B,Murphy C ( 2001): fMRI activation in response to odorants orally delivered in aqueous solutions. Chem Senses 26: 625–637. [DOI] [PubMed] [Google Scholar]
- Cerf‐Ducastel B,Murphy C ( 2004): Improvement of fMRI data processing of olfactory responses with a perception‐based template. Neuroimage 22: 603–610. [DOI] [PubMed] [Google Scholar]
- Cleland TA,Linster C ( 2003): Central olfactory structures In: Doty RL, editor. Handbook of Olfaction and Gustation. New York: Marcel Dekker; pp 165–180. [Google Scholar]
- Cometto‐Muniz JE,Cain WS,Abraham MH ( 2004): Chemosensory additivity in trigeminal chemoreception as reflected by detection of mixtures. Exp Brain Res 158: 196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton P ( 2000): Psychophysical and behavioral characteristics of olfactory adaptation. Chem Senses 25: 487–492. [DOI] [PubMed] [Google Scholar]
- Dalton P,Doolittle N,Nagata H,Breslin PA ( 2000): The merging of the senses: Integration of subthreshold taste and smell. Nat Neurosci 3: 431–432. [DOI] [PubMed] [Google Scholar]
- Deichmann R,Gottfried JA,Hutton C,Turner R ( 2003): Optimized EPI for fMRI studies of the orbitofrontal cortex. Neuroimage 19(2 Part 1): 430–441. [DOI] [PubMed] [Google Scholar]
- Domino EF ( 1998): Tobacco smoking and nicotine neuropsychopharmacology: Some future research directions. Neuropsychopharmacology 18: 456–468. [DOI] [PubMed] [Google Scholar]
- Doty RL ( 1991): Olfactory system In: Getchell TV,Doty RL,Bartoshuk LM,Snow JB, Jr, editors. Smell and Taste in Health and Disease. New York: Raven Press; pp 175–199. [Google Scholar]
- Doty RL,Laing DG ( 2003): Psychophysical measurements of human olfactory function, including odorant mixture assessment In: Doty RL, editor. Handbook of Olfaction and Gustation.2nd ed. New York: Marcel Dekker; pp 203–228. [Google Scholar]
- Doty RL,Bromley SM ( 2004): Effects of drugs on olfaction and taste. Otolaryngol Clin North Am 37: 1229–1254. [DOI] [PubMed] [Google Scholar]
- Doty RL,Brugger WE,Jurs PC,Orndorff MA,Snyder PJ,Lowry LD ( 1978): Intranasal trigeminal stimulation from odorous volatiles: Psychometric responses from anosmic and normal humans. Physiol Behav 20: 175–185. [DOI] [PubMed] [Google Scholar]
- Edwards DA,Mather RA,Shirley SG,Dodd GH ( 1987): Evidence for an olfactory receptor which responds to nicotine—nicotine as an odorant. Experientia 43: 868–873. [DOI] [PubMed] [Google Scholar]
- Frasnelli J,Schuster B,Hummel T ( 2007): Subjects with congenital anosmia have larger peripheral but similar central trigeminal responses. Cereb Cortex 17: 370–377. [DOI] [PubMed] [Google Scholar]
- Friston KJ,Holmes AP,Worsley KJ,Poline JP,Frith CD,Frackowiak RSJ ( 1994): Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp 2: 189–210. [Google Scholar]
- Friston KJ,Ashburner J,Frith CD,Poline JB,Heather JD,Frackowiak RSJ ( 1995): Spatial registration and normalization of images. Hum Brain Mapp 3: 165–189. [Google Scholar]
- Friston KJ,Williams S,Howard R,Frackowiak RS,Turner R ( 1996): Movement‐related effects in fMRI time‐series. Magn Reson Med 35: 346–355. [DOI] [PubMed] [Google Scholar]
- Genovese CR,Lazar NA,Nichols T ( 2002): Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15: 870–878. [DOI] [PubMed] [Google Scholar]
- Gottfried JA. ( 2006): Smell: Central nervous processing. Adv Oto‐Rhino‐Laryngol 63: 44–69. [DOI] [PubMed] [Google Scholar]
- Gottfried JA,Deichmann R,Winston JS,Dolan RJ ( 2002): Functional heterogeneity in human olfactory cortex: An event‐related functional magnetic resonance imaging study. J Neurosci 22: 10819–10828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DM,Swets JA ( 1966): Signal Detection Theory and Psychophysics. New York: Wiley. [Google Scholar]
- Handwerker HO,Kobal G ( 1993): Psychophysiology of experimentally induced pain. Physiol Rev 73: 639–671. [DOI] [PubMed] [Google Scholar]
- Hari R,Portin K,Kettenmann B,Jousmaki V,Kobal G ( 1997): Right‐hemisphere preponderance of responses to painful CO2 stimulation of the human nasal mucosa. Pain 72: 145–151. [DOI] [PubMed] [Google Scholar]
- Hasegawa M,Kern EB ( 1977): The human nasal cycle. Mayo Clin Proc 52: 28–34. [PubMed] [Google Scholar]
- Hummel T,Livermore A ( 2002): Intranasal chemosensory function of the trigeminal nerve and aspects of its relation to olfaction. Int Arch Occup Environ Health 75: 305–313. [DOI] [PubMed] [Google Scholar]
- Hummel T,Hummel C,Pauli E,Kobal G ( 1992a): Olfactory discrimination of nicotine‐enantiomers by smokers and non‐smokers. Chem Senses 17: 13–21. [Google Scholar]
- Hummel T,Livermore A,Hummel C,Kobal G ( 1992b): Chemosensory event‐related potentials in man: Relation to olfactory and painful sensations elicited by nicotine. Electroencephalogr Clin Neurophysiol 84: 192–195. [DOI] [PubMed] [Google Scholar]
- Hummel T,Sekinger B,Wolf SR,Pauli E,Kobal G. ( 1997): “Sniffin' sticks”: Olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses 22: 39–52. [DOI] [PubMed] [Google Scholar]
- Hummel T,Futschik T,Frasnelli J,Huttenbrink KB ( 2003a): Effects of olfactory function, age, and gender on trigeminally mediated sensations: A study based on the lateralization of chemosensory stimuli. Toxicol Lett 140/141: 273–280. [DOI] [PubMed] [Google Scholar]
- Hummel T,Mohammadian P,Marchl R,Kobal G,Lotsch J ( 2003b): Pain in the trigeminal system: Irritation of the nasal mucosa using short‐ and long‐lasting stimuli. Int J Psychophysiol 47: 147–158. [DOI] [PubMed] [Google Scholar]
- Hummel T,Doty RL,Yousem DM ( 2005): Functional MRI of intranasal chemosensory trigeminal activation. Chem Senses 30 ( Suppl 1): i205–i206. [DOI] [PubMed] [Google Scholar]
- Inokuchi A,Kimmelman CP,Snow JB Jr ( 1993): Convergence of olfactory and nasotrigeminal inputs and possible trigeminal contributions to olfactory responses in the rat thalamus. Eur Arch Otorhinolaryngol 249: 473–477. [DOI] [PubMed] [Google Scholar]
- Jacquot L,Monnin J,Brand G ( 2004): Influence of nasal trigeminal stimuli on olfactory sensitivity. C R Biol 327: 305–311. [DOI] [PubMed] [Google Scholar]
- Kettenmann B,Jousmaki V,Portin K,Salmelin R,Kobal G,Hari R ( 1996): Odorants activate the human superior temporal sulcus. Neurosci Lett 203: 143–145. [DOI] [PubMed] [Google Scholar]
- Kobal G ( 1981): Elektrophysiologische Untersuchungen des menschlichen Geruchssinns. Stuttgart: Thieme Verlag. [Google Scholar]
- Kobal G ( 1985): Pain‐related electrical potentials of the human nasal mucosa elicited by chemical stimulation. Pain 22: 151–163. [DOI] [PubMed] [Google Scholar]
- Kobal G,Hummel C ( 1988): Cerebral chemosensory evoked potentials elicited by chemical stimulation of the human olfactory and respiratory nasal mucosa. Electroencephalogr Clin Neurophysiol 71: 241–250. [DOI] [PubMed] [Google Scholar]
- Kobal G,Hummel T,Sekinger B,Barz S,Roscher S,Wolf S ( 1996): “Sniffin' sticks”: Screening of olfactory performance. Rhinology 34: 222–226. [PubMed] [Google Scholar]
- Laska M,Distel H,Hudson R ( 1997): Trigeminal perception of odorant quality in congenitally anosmic subjects. Chem Senses 22: 447–456. [DOI] [PubMed] [Google Scholar]
- Libet B ( 2006): Reflections on the interaction of the mind and brain. Prog Neurobiol 78: 322–326. [DOI] [PubMed] [Google Scholar]
- Livermore A,Hummel T,Kobal G ( 1992): Chemosensory event‐related potentials in the investigation of interactions between the olfactory and the somatosensory (trigeminal) systems. Electroencephalogr Clin Neurophysiol 83: 201–210. [DOI] [PubMed] [Google Scholar]
- Mai JK,Assheuer J,Paxinos G ( 2004): Atlas of the Human Brain. Amsterdam: Elsevier Academic Press. [Google Scholar]
- Marion L ( 1950): The pyridine alkaloids In: Manske RH,Holmes HL, editors. The Alkaloids: Chemistry and Physiology. New York: Academic Press; pp 228–269. [Google Scholar]
- Mombaerts P ( 1999): Seven‐transmembrane proteins as odorant and chemosensory receptors. Science 286: 707–711. [DOI] [PubMed] [Google Scholar]
- Poellinger A,Thomas R,Lio P,Lee A,Makris N,Rosen BR,Kwong KK ( 2001): Activation and habituation in olfaction—An fMRI study. Neuroimage 13: 547–560. [DOI] [PubMed] [Google Scholar]
- Price JL ( 1985): Beyond the primary olfactory cortex: Olfactory‐related areas in the neocortex, thalamus and hypothalamus. Chem Senses 10: 239–258. [Google Scholar]
- Principato JJ,Ozenberger JM. ( 1970): Cyclical changes in nasal resistance. Arch Otolaryngol 91: 71–77. [DOI] [PubMed] [Google Scholar]
- Rand MJ ( 1989): Neuropharmacological effects of nicotine in relation to cholinergic mechanisms. Prog Brain Res 79: 3–11. [DOI] [PubMed] [Google Scholar]
- Savic I ( 2002): Imaging of brain activation by odorants in humans. Curr Opin Neurobiol 12: 455–461. [DOI] [PubMed] [Google Scholar]
- Savic I,Gulyas B,Berglund H ( 2002): Odorant differentiated pattern of cerebral activation: comparison of acetone and vanillin. Hum Brain Mapp 17: 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer ML,Bottger B,Silver WL,Finger TE ( 2002): Trigeminal collaterals in the nasal epithelium and olfactory bulb: A potential route for direct modulation of olfactory information by trigeminal stimuli. J Comp Neurol 444: 221–226. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD,Doyon J,Toga AW,Petrides M,Evans AC ( 2000): MRI Atlas of the Human Cerebellum. San Diego: Academic Press. [DOI] [PubMed] [Google Scholar]
- Silver WL,Mason JR,Marshall DA,Maruniak JA ( 1985): Rat trigeminal, olfactory and taste responses after capsaicin desensitization. Brain Res 333: 45–54. [DOI] [PubMed] [Google Scholar]
- Small DM,Jones‐Gotman M,Zatorre RJ,Petrides M,Evans AC ( 1997): Flavor processing: More than the sum of its parts. Neuroreport 8: 3913–3917. [DOI] [PubMed] [Google Scholar]
- Small DM,Voss J,Mak YE,Simmons KB,Parrish T,Gitelman D ( 2004): Experience‐dependent neural integration of taste and smell in the human brain. J Neurophysiol 92: 1892–1903. [DOI] [PubMed] [Google Scholar]
- Sobel N,Prabhakaran V,Hartley CA,Desmond JE,Zhao Z,Glover GH,Gabrieli JD,Sullivan EV ( 1998): Odorant‐induced and sniff‐induced activation in the cerebellum of the human. J Neurosci 18: 8990–9001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel N,Prabhakaran V,Zhao Z,Desmond JE,Glover GH,Sullivan EV,Gabrieli JD ( 2000): Time course of odorant‐induced activation in the human primary olfactory cortex. J Neurophysiol 83: 537–551. [DOI] [PubMed] [Google Scholar]
- Thürauf N,Renner B,Kobal G ( 1995): Responses recorded from the frog olfactory epithelium after stimulation with R(+)‐ and S(−)‐nicotine. Chem Senses 20: 337–344. [DOI] [PubMed] [Google Scholar]
- Thürauf N,Kaegler M,Dietz R,Barocka A,Kobal G ( 1999): Dose‐dependent stereoselective activation of the trigeminal sensory system by nicotine in man. Psychopharmacology (Berl) 142: 236–243. [DOI] [PubMed] [Google Scholar]
- Thürauf N,Kaegler M,Renner B,Barocka A,Kobal G ( 2000): Specific sensory detection, discrimination, and hedonic estimation of nicotine enantiomers in smokers and nonsmokers: Are there limitations in replacing the sensory components of nicotine? J Clin Psychopharmacol 20: 472–478. [DOI] [PubMed] [Google Scholar]
- Thürauf N,Markovic K,Braun G,Bleich S,Reulbach U,Kornhuber J,Lunkenheimer J ( 2006): The influence of mecamylamine on trigeminal and olfactory chemoreception of nicotine. Neuropsychopharmacology 31: 450–461. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N,Landeau B,Papathanassiou D,Crivello F,Etard O,Delcroix N,Mazoyer B,Joliot M ( 2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15: 273–289. [DOI] [PubMed] [Google Scholar]
- Walker JC,Kendal‐Reed M,Keiger CJ,Bencherif M,Silver WL ( 1996): Olfactory and trigeminal response to nicotine. Drug Dev Res 38: 160–168. [Google Scholar]
- Weismann M,Yousry I,Heuberger E,Nolte A,Ilmberger J,Kobal G,Yousry TA,Kettenmann B,Naidich TP ( 2001): Functional magnetic resonance imaging of human olfaction. Neuroimaging Clin N Am 11: 237–250. [PubMed] [Google Scholar]
- Wiesmann M,Kettenmann B,Kobal G. 2004. Functional magnetic resonance imaging of human olfaction. In: Taylor AJ,Roberts DD, editors.Flavor Perception. Oxford: Blackwell; pp 203–227. [Google Scholar]
- Wiesmann M,Kopietz R,Albrecht J,Linn J,Reime U,Kara E,Pollatos O,Sakar V,Anzinger A,Fesl G,Brückmann H,Kobal G,Stephan T( 2006): Eye closure in darkness animates olfactory and gustatory cortical areas. Neuroimage 32: 293–300. [DOI] [PubMed] [Google Scholar]
- Yousem DM,Williams SC,Howard RO,Andrew C,Simmons A,Allin M,Geckle RJ,Suskind D,Bullmore ET,Brammer MJ,Doty RL( 1997): Functional MR imaging during odor stimulation: Preliminary data. Radiology 204: 833–838. [DOI] [PubMed] [Google Scholar]
- Zevin S,Gourlay SG,Benowitz NL. ( 1998): Clinical pharmacology of nicotine. Clin Dermatol 16: 557–564. [DOI] [PubMed] [Google Scholar]


