Abstract
We reasoned that if an area is devoted to processing only the visual features of objects, then transcranial magnetic stimulation (TMS) applied to this area in either hemisphere would affect the naming of objects presented in contralateral but not ipsilateral space. In contrast, if an area is involved in language, then one might expect to see effects of TMS when applied over the left but not the right hemisphere, regardless whether objects are in contralateral or ipsilateral space. Our experiments reveal two important findings. First, TMS delivered to the lateral‐occipital complex (LOC), a visual‐form area, affected the naming of objects presented in contralateral but not ipsilateral space, independent of which hemisphere was stimulated. In two additional experiments, when participants named the color of objects or made judgments about the size of stimuli as shown physically on a computer screen, TMS over the contralateral LOC did not affect color naming but did affect the participants' ability to make size judgments. Second, TMS delivered to the left but not the right posterior inferior‐frontal gyrus (pIFG) affected the naming of objects irrespective of whether objects were presented in contralateral or ipsilateral space. In a separate experiment, when participants were asked to either read or categorize words, TMS over the left but not the right pIFG affected word categorization but not word reading. On the basis of these findings, we propose that when people name visually‐presented objects, LOC processes the visual form of objects while the left pIFG processes the semantics of objects. Hum Brain Mapp, 2009. © 2009 Wiley‐Liss, Inc.
Keywords: visual perception, semantic processing, object naming, lateral‐occipital complex, Broca's area, transcranial magnetic stimulation
INTRODUCTION
Functional neuroimaging studies have revealed that the lateral‐occipital complex (LOC) and the posterior inferior‐frontal gyrus (pIFG) are frequently activated when people name visually‐presented objects [for review, Price et al.,2005]. On the basis of these results, it has been suggested that LOCs role is largely visual [e.g., Chouinard et al.,2008; Kanwisher et al.,1996; Malach et al.,1995] whereas pIFG is more involved in language processing, including a possible role in the retrieval and/or selection of semantics [e.g., Barde and Thompson‐Schill,2002; Chouinard et al.,2008; Gold and Buckner,2002; for review, see Bookheimer,2002]. Yet, these observations remain largely correlational. Functional neuroimaging does not allow one to infer causal relationships between brain and behavior. In contrast, transcranial magnetic stimulation (TMS) allows researchers to manipulate brain activity and then examine the consequences that these manipulations have on behavior. Although earlier TMS studies have looked at the effects of stimulating either LOC or pIFG, ours is the first to use a paradigm to differentiate the roles that LOC and pIFG might play in visual and semantic processing in the same tasks. In designing our experiments, we took advantage of the following facts. First, visual areas have a preference for contralateral stimuli with a stage‐wise decrease in this preference as one moves from earlier to later visual areas. Second, the neural processes related to language tend to be lateralized to the left hemisphere.
We reasoned that if an area is devoted to processing only the visual features of objects, then TMS applied to this area in either hemisphere would affect the naming of objects presented in contralateral but not ipsilateral space. Because ventral‐stream visual areas, such as LOC, have been shown to be driven more by stimuli in contralateral than ipsilateral space [McKyton and Zohary,2007; Niemeier et al.,2005], one would predict that TMS‐induced disruption of this area would be observed for objects presented in contralateral but not ipsilateral space. Furthermore, if LOC processes only the visual features of objects, then this contralateral effect should be the same when TMS is applied over LOC in the left and right hemispheres. But if processing in LOC is also affected by the semantics of objects, then one would expect greater effects of TMS when applied over the left than the right hemisphere. Moreover, this effect might be evident in both contralateral as well as ipsilateral space. This is because, in right‐handed people, neural processes related to language are, for the most part, lateralized to the left hemisphere [Hickok and Poeppel,2007], although some mechanisms in the right hemisphere do play some kind of role in language [Lindell,2006].
In our first experiment, we demonstrate that stimulating LOC in either hemisphere disrupted the naming of objects presented in contralateral but not ipsilateral space and that stimulating pIFG in the left but not the right hemisphere disrupted the naming of objects in both contralateral and ipsilateral space. Although we can infer from this experiment that LOC is more concerned with visual processing than it is with language and that the left pIFG is more concerned with language than it is with visual processing, this experiment alone does not allow us to make more specific conclusions. We therefore performed three additional experiments to examine more specifically the contributions of LOC and pIFG in naming objects.
In a second experiment, participants named the colors of objects, a task in which we already know that LOC has no role to play. Functional neuroimaging studies have revealed that LOC is specialized for processing the form of objects rather than their color [e.g., Cant and Goodale,2007; Cant et al.,2009]. Color and other surface cues like texture appear to be processed much more medially in areas that would not have been affected by the TMS applied in our experiments [for review, see Gegenfurtner and Kiper,2003]. As expected, we found that stimulating LOC in either hemisphere had no effect on color naming. This shows that the effects of stimulating LOC in the first experiment were not due to a disruption of attentional mechanisms or the induction of scotomas in the contralateral hemifield. Otherwise, color naming would have been just as susceptible to the effects of stimulation as object naming.
In a third experiment, participants made decisions about the size of stimuli as shown physically on a computer screen. Stimulating LOC in either hemisphere disrupted size discrimination for objects presented in contralateral but not ipsilateral space, whereas stimulating the pIFG in either hemisphere had no effect. These findings reaffirmed that the left pIFG had a verbal role to play in the first experiment and that the effects of stimulating LOC in the first experiment were the direct result of having disrupted the processing of physical features of objects. Finally, in a fourth experiment, we demonstrated that TMS over the left pIFG affected the categorization but not the reading of words. Thus, the effects of stimulating the left pIFG in the first experiment were likely the result of having disrupted semantic processing and not simply the production of speech.
We think that this study makes two important contributions. First, functional neuroimaging has so far not been able to fully disambiguate brain areas that process visual features of objects from those that are involved in semantic processing. With TMS, we were able to show that LOC in both hemispheres plays a role in processing the visual but not the semantic features of objects whereas the left pIFG is more concerned with processing the semantic features of objects. Second, this is the first TMS study to our knowledge that shows that LOC preferentially processes objects in contralateral space. This finding complements the findings of earlier single‐unit recording studies in the macaque monkey [Op De Beeck and Vogels,2000] and the results of earlier fMRI work in humans [McKyton and Zohary,2007; Niemeier et al.,2005]. In summary, our experiments provide compelling evidence that when people name visually‐presented objects, LOC processes the visual form of objects while the left pIFG processes the semantics of objects.
METHODS
Participants
Twelve volunteers (five females, age range = 21–29 years, mean = 26.5) participated in the first experiment, which we will refer to as the “Object Naming” experiment, and 12 volunteers (four females, age range = 22–30 years, mean = 27.2) participated in the other three experiments, which we will refer to as the “Color Naming,” “Size Discrimination,” and “Reading versus Categorization” experiments. Five months had elapsed between the time we had finished collecting data for the first experiment and the time we had began collecting data for the other experiments. Nevertheless, nine of the 12 volunteers who participated in the Object Naming experiment also participated in the other experiments. All participants had a right‐hand preference as determined by a questionnaire [Oldfield,1971] and provided informed written consent. Participants spoke English as their first language were not color blind, had corrected‐to‐normal visual acuity, and did not have any history of neurological impairments. The Research Ethics Board of the University of Western Ontario (London, ON, Canada) approved all procedures. We selected participants for whom we had acquired anatomical magnetic‐resonance images (MRIs) in earlier studies [1–2 mm thick contiguous horizontal slices that covered the whole brain; 4‐T Varian/Siemens (Erlangen, Germany) MRI system]. We also selected participants who had resting motor‐thresholds (rMTs) of lower than 65% of the maximum output of our transcranial magnetic stimulator. This was done to ensure that the TMS coil would not overheat during a block of trials and to reduce the possibility of discomfort during TMS.
Overview of the Four Experiments
For the Object Naming experiment, an object appeared briefly to either the left or the right of a fixation dot followed by a mask (Fig. 1A). Participants named the objects while trains of high‐frequency repetitive TMS were delivered to either LOC or pIFG in either the left or the right hemisphere. Sham stimulation was also applied with the edge of the coil placed over the posterior parietal cortex in either hemisphere. Verbal responses were recorded and analyzed off‐line to assess whether or not there were differences in reaction times and errors between conditions. We counter‐balanced across participants which objects were presented either to the left or to the right of fixation and which objects were presented during stimulation to a particular site of TMS or of Sham stimulation. This counter‐balancing ensured that naming difficulty, word frequency in everyday speech, and delays in voice reaction times due to initial phonemes of object names were matched across all conditions. Thirty‐two objects were presented in a block of trials for a total of 192 objects.
Figure 1.
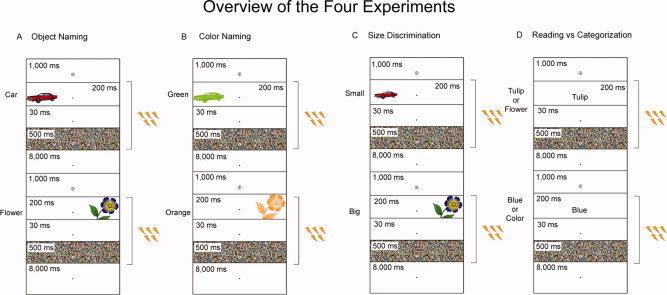
Overview of the four experiments. A trial began with an alerting cue that was shown for 1,000 ms. This was followed by the presentation of a stimulus for 200 ms. After the stimulus disappeared, a blank screen was shown for 30 ms followed by a mask for 500 ms. Five pulses of 10 Hz TMS at 90% rMT was applied for 400 ms starting at the onset of stimulus presentation. (A) In the Object Naming experiment, participants named objects presented to either the left or the right of fixation. (B) In the Color Naming experiment, participants named the color of objects presented to either the left or the right of fixation. (C) In the Size Discrimination experiment, participants had to indicate whether a stimulus presented to either the left or the right of fixation was “small” or “big.” (D) In the Reading versus Categorization experiment, participants had to either read or classify words that were presented at fixation.
We did not apply Sham stimulation in subsequent experiments. This is because we did not find any differences in either reaction times or errors when TMS was applied to the right pIFG, which subsequently served as a control site for this study, compared to the Sham conditions in the Object Naming experiment. Furthermore, it should be pointed out that Sham stimulation does not control for all peripheral effects of real stimulation of the brain. It releases magnetic fields around the head (not in the brain) and it has been our observation that it feels quite different to subjects than real TMS. Thus, it is our view that real TMS applied to regions of interest (i.e., left pIFG, left LOC, and right LOC) and real TMS applied to a control brain region (i.e., right pIFG) provides a much more robust experimental design for a TMS study than one that only uses Sham stimulation to control for peripheral effects of TMS.
For the Color Naming experiment, a colored object appeared briefly to either the left or the right of a fixation dot followed by a mask (Fig. 1B). Participants named the color of the objects while trains of high‐frequency repetitive TMS were delivered to either LOC or pIFG in either the left or the right hemisphere. Verbal responses were recorded and analyzed off‐line to assess whether or not there were differences in reaction times and errors between conditions. For the Size Discrimination experiment, a “small” object or a big “object” appeared briefly to either the left or the right of a fixation dot followed by a mask (Fig. 1C). Participants responded with button pressing as to whether the physical size of the stimuli were “small” or “big” while trains of high‐frequency repetitive TMS were delivered to either LOC or pIFG in either the left or the right hemisphere. The same objects presented in the Object Naming experiment were presented again in the Color Naming and Size Discrimination experiments. We counter‐balanced across participants which objects were presented to the left or to the right of fixation, which objects were presented during stimulation to a particular site of TMS, and which objects were presented during the Color Naming and Size Discrimination experiments. All objects were counterbalanced across conditions, and therefore, although some form of learning could have been maintained over the intervening 5 months, this would have been matched across conditions.
For the Reading versus Categorization experiment, words were presented briefly at the center of the computer screen followed by a mask (Fig. 1D). Participants had to either read or classify the words into categories while trains of high‐frequency repetitive TMS were delivered to pIFG in either the left or the right hemisphere. We counter‐balanced across participants which words had to be read or classified into categories and which words were presented during stimulation to a particular site of TMS.
Stimuli, Voice Recordings, and Button Responses
Object stimuli were taken from Michael Tarr's collection [Rossion and Pourtois,2004; available at: http://titan.cog.brown.edu:8080/TarrLab/stimuli/objects/svlo.zip/view]. For the Object Naming and Color Naming experiments, we resized images to 200 × 200 pixels at 150 dpi. After resizing all images for the Color Naming experiment, we gray‐scaled the images and then converted them into images with a uniform color of either gray, gold, purple, blue, green, orange, red, or pink. For the Size Discrimination experiment, we resized images to 150 × 150 pixels at 150 dpi to create a set of “small” objects and 200 × 200 pixels at 150 dpi to create a set of “big” objects. All image manipulations were performed in Adobe Photoshop (Adobe Systems, San Jose, CA).
We used E‐Prime (Psychology Software Tools, Pittsburg, PA) to present stimuli and an RB Series Response Pad to collect button responses (Cedrus, San Pedro, CA). Matlab (MathWorks, Natick, MA) was used to collect verbal responses from a Shure PG58 microphone (Shure, Nile, IL) at a sample rate of 8,000 Hz. Figure 1A–D illustrates the time course for each of the four experiments. In the Object Naming, Color Naming, and Size Discrimination experiments, participants maintained fixation on a small circle that was always present in the center of the computer screen (Fig. 1A–C). A trial began when an alerting cue was displayed for 1,000 ms. This event was followed by an object that was presented 6° either to the left or to the right of fixation for only 200 ms (a duration too short for a saccade to be initiated before stimulus offset). After the object disappeared, a blank screen was shown for 30 ms and then a mask was shown for 500 ms. In the Object Naming experiment, participants were asked to name the objects as quickly and accurately as possible. In the Color Naming experiment, participants were asked to name the color of the objects as quickly as possible. They were told before the experiment that they could choose between gray, gold, purple, blue, green, orange, red, or pink. In the Size Discrimination experiment, participants responded with either their right index finger whenever a “small” object appeared or their right middle finger whenever a “big” object appeared. The reason why we recorded button responses in the Size Discrimination experiment as opposed to requiring verbal responses was to test the contribution of LOC and pIFG during a task in which verbal processing was minimized.
In the Reading versus Categorization experiment, participants maintained fixation on a small circle that was present in the center of the computer screen (Fig. 1D). A trial began when an alerting cue was displayed for 1,000 ms. This event was followed by a word presented foveally at the center of the computer screen. Words were presented in black “Tahoma Bold” font with a font size of 18. In Table I, we present a list of these words and their expected categorical responses. After the word disappeared, a blank screen was shown for 30 ms and then a mask was shown for 500 ms. Participants were asked either to read or to classify the words into categories.
Table I.
Words presented during the reading versus categorization experiment
| Set 1 | Set 2 | Expected responses for categorization | Variations made during categorization |
|---|---|---|---|
| Trout | Salmon | Fish | 0 |
| Peas | Carrot | Vegetable | 0 |
| Honda | Mazda | Car | 0 |
| Peach | Apple | Fruit | 0 |
| Drum | Flute | Instrument | Musical instrument |
| Spain | Greece | Country | 0 |
| Mars | Venus | Planet | 0 |
| Four | Nine | Number | 0 |
| Chair | Table | Furniture | 0 |
| Oak | Maple | Tree | 0 |
| Golf | Tennis | Sport | Game |
| Red | Blue | Color | 0 |
| Daisy | Tulip | Flower | 0 |
| Fly | Ant | Insect | Bug |
| Coke | Pepsi | Drink | Beverage, pop, and cola |
| Wolf | Lion | Animal | 0 |
The Table lists the words that were presented during the reading versus categorization experiment. We counterbalanced across participants which words were presented during reading and categorization (within a set) and which words (sets 1 and 2) were presented to a particular site of TMS.
Resting Motor‐Thresholds
We determined resting motor‐thresholds (rMTs) for the right first dorsal interosseus muscle by first determining the optimal position for activating the muscle and then by reducing the intensity from a supra‐threshold level until we found the lowest intensity to induce five motor‐evoked potentials (MEPs) of ≥50 μV in a series of 10 stimuli. We recorded MEPs using Ag/AgCl electrodes fixed on the skin with a belly‐tendon montage. We sampled the electromyographic signal using a Grass 15A54 quad amplifier (Astro‐Med, West Warwick, RI) with a bandwidth of 10–1,000 Hz and a sampling rate of 2,000 Hz.
Transcranial Magnetic Stimulation
We used a four step probabilistic procedure [Paus et al.,1997] to place the TMS coil over LOC and pIFG. First, we transformed the subject's MRI into standardized space using an automated feature‐matching algorithm and the average‐305 brain from the Montreal Neurological Institute (MNI) as a template (both available at http://www.bic.mni.mcgill.ca/software/distribution). Second, we derived probabilistic locations for LOC (left: X = –52, Y = –62, Z = –12; right: X = 40, Y = –76, Z = –16) and pIFG (X = ±46, Y = 14, Z = 28) based on an fMRI study that examined the neural correlates of naming visually‐presented objects [Chouinard et al.,2008]. Third, we transformed these sites to the subject's brain coordinate space. Fourth, we used frameless stereotaxy to position the coil over the location of these sites marked on the subject's MRI (Brainsight Software, Rogue Research, Montreal, QC, Canada; Polaris System, Northern Digital, Waterloo, ON, Canada).
TMS was performed using a Magstim rapid‐rate stimulator and figure‐of‐eight coil (Magstim Company, Spring Gardens, United Kingdom; diameter of each coil = 7 cm). We applied five pulses of 10 Hz TMS for 400 ms starting at the moment the objects were presented. TMS was delivered at a subthreshold intensity of 90% rMT. We chose this intensity because pilot testing revealed that it minimized discomfort (as well as peripheral muscle stimulation) when TMS was applied to either pIFG and LOC, and eye blinking when TMS was applied to pIFG compared to higher levels of stimulation. For LOC and pIFG stimulation, we held the coil tangentially to the scalp with the short axis angled 45° relative to the inter‐hemispheric fissure and perpendicular to the central sulcus. The induced current in the brain flowed in a posterior‐to‐anterior and lateral‐to‐medial direction. Sham stimulation was applied with the edge of the coil placed over the posterior parietal cortex such that the induced magnetic field was directed away from the brain.
After guiding the TMS coil to a target location, we then locked the TMS coil into place and saved in Brainsight the location of where the TMS coil touched the scalp in the participant's MRI. Using a procedure described in detail elsewhere [Paus and Wolforth,1998], we used this information to create images of virtual trajectories (diameter = 6 mm) that projected from the TMS coil into the head perpendicularly to the plane of the coil from where it touched the scalp. Images of these virtual trajectories were then transformed into standardized space. This end product served to provide rough estimations for the location of induced currents in the brain. Overall, the location of projected coil trajectories for LOC and pIFG in each of the two hemispheres were consistent across participants (see Fig. 2).
Figure 2.
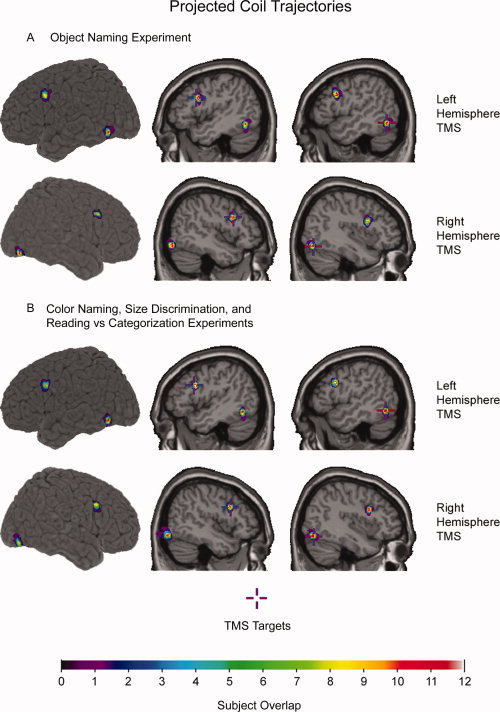
Projected coil trajectories superimposed on an anatomical MRI in standardized space. These projected coil trajectories (diameter = 6 mm) provide rough estimations for induced currents in the brain during TMS over pIFG and LOC during (A) the Object Naming experiment, and (B) the Color Naming, Size Discrimination, and Reading versus Categorization experiments. Cooler colors (purple = 1) indicate less overlap among subjects than warmer colors (white = 12). Target locations for pIFG (X = ±46, Y = 14, Z = 28) and LOC (left: X = –52, Y = –62, Z = −12; right: X = 40, Y = –76, Z = –16) were based on an fMRI‐adaptation study that examined neural correlates of naming visually‐presented objects [Chouinard et al.,2008].
Data Analyses
Voice data were analyzed off‐line. Voice reaction times were computed in Matlab as the first time point of “voice” that was higher than 5% of the maximum peak in voice. The TMS‐induced artifacts in the recordings never overlapped with the actual naming of objects and colors or with the actual reading or categorization of words. This is because the last pulse of TMS was delivered 400 ms after object onset, which is earlier in time than any of our participants could respond verbally. Responses were classified as errors by the second author of this study (R.L.W.) who was blind to which responses corresponded to which condition. Responses were classified as errors when participants either did not produce a response or falsely named an object (e.g., saying pencil instead of trumpet) or falsely named the color of an object (e.g., saying red instead of blue). Variations (e.g., saying horn instead of trumpet) were not classified as errors, but were also tabulated. We then calculated the proportion of errors and variations made for each condition [e.g., (number of errors/number of trials) × 100]. Separate ANOVAs were performed on the reaction times and on the proportion of errors and variations made by participants. For the Object Naming, Color Naming, and Size Discrimination experiments, Side of Presentation, Site of Stimulation, and Stimulated Hemisphere were used as within‐subject factors. For the Reading versus Categorization experiment, in which we only stimulated pIFG in either of the two hemispheres, Task and Stimulated Hemisphere were used as within‐subject factors. Simple effect tests, which corrected for multiple comparisons, were used to make comparisons between conditions.
RESULTS
None of the participants reported significant discomfort when asked how the TMS or Sham stimulation felt after each block of trials. We also did not observe any eye blinking during either TMS or Sham stimulation. In the Object‐Naming experiment, rMTs ranged between 42 and 64% of the maximum output of the stimulator (mean ± SD: 55.0% ± 7.2%). In the three other experiments, rMTs ranged between 43 and 64% of the maximum output of the stimulator (mean ± SD: 54.8% ± 6.9%).
Object Naming Experiment
Stimulating LOC in either hemisphere resulted in slower reaction times for naming objects in contralateral but not ipsilateral space. ANOVA revealed a Side of Presentation × Site of Stimulation × Stimulated Hemisphere interaction (F (2,22) = 5.25; P = 0.014). This interaction was found to be driven by effects of TMS on LOC that were present only when participants named objects in contralateral space, irrespective of which of the two hemispheres was stimulated (Fig. 3A). TMS over the left LOC prolonged reaction times when objects were presented in right compared to left space (F (1,66) = 7.84; P = 0.007), whereas TMS over the right LOC prolonged reaction times when objects were presented in left compared to right space (F (1,66) = 4.47; P = 0.039). A paired t‐test found that reaction times to naming objects in contralateral space did not differ when LOC was stimulated in the left compared to the right hemisphere (T (11) = 0.92; P = 0.379).
Figure 3.
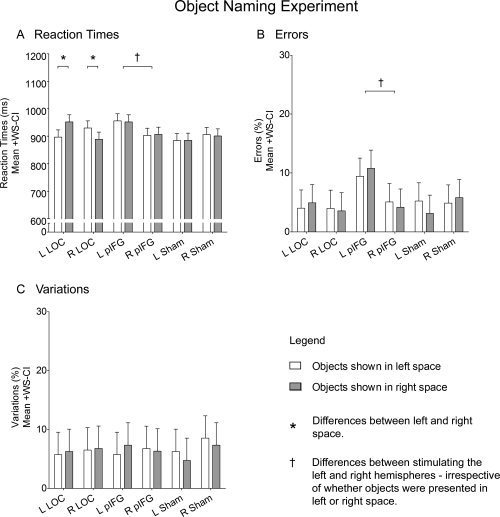
Object Naming experiment. The graphs plot mean reaction times (A), errors (B), and variations (C) to naming objects presented in either left (white) or right (gray) space when TMS was applied over the left LOC, the right LOC, the left pIFG, or the right pIFG, as well as when Sham was applied with the edge of the TMS coil over the left or the right PPC. Responses were classified as errors when participants either did not produce a response or falsely named an object (e.g., saying pencil instead of trumpet) and responses were classified as variations when participants produced a response that was semantically correct but that was not expected (e.g., saying horn instead of trumpet). Asterisks (*) denote differences between objects in left versus right space. Daggers (†) denote differences when TMS was applied to the left compared to the right hemisphere, irrespective of whether objects were presented in left or right space. Error bars represent 95% confidence intervals for within‐subject contrasts [Loftus and Masson,1994].
Stimulating the left pIFG resulted in slower reaction times for naming objects in both contralateral and ipsilateral space. ANOVA revealed a Site of Stimulation × Stimulated Hemisphere interaction (F (2,22) = 3.56; P = 0.046). This interaction was found to be driven by effects of TMS on pIFG that were present only when stimulation was applied to the left hemisphere (Fig. 3A). TMS over the left pIFG prolonged reaction times compared to the right pIFG, irrespective of whether objects were presented in contralateral or ipsilateral space (F (1,33) = 6.72; P = 0.014). In contrast, TMS over the left LOC did not induce an overall prolongation in reaction times compared to TMS over the right LOC (F (1,33) = 0.63; P = 0.434) and Sham stimulation over the left PPC did not induce an overall prolongation in reaction times compared to Sham stimulation over the right PPC (F (1,33) = 1.04; P = 0.315).
Stimulating the left pIFG also resulted in a greater number of errors for naming objects in both contralateral and ipsilateral space compared to stimulating the right pIFG. ANOVA revealed a Site of Stimulation × Stimulated Hemisphere interaction (F (2,22) = 3.49; P = 0.048). This interaction was found to be driven by effects of TMS on pIFG that were present only when stimulation was applied to the left hemisphere (Fig. 3B). TMS over the left pIFG resulted in more errors compared to TMS over the right pIFG, irrespective of whether objects were presented in contralateral or ipsilateral space (F (1,33) = 6.55; P = 0.016). TMS over the left LOC did not result in more errors compared to TMS over the right LOC (F (1,33) = 0.10; P = 0.750) and Sham stimulation over the left PPC did not result in more errors compared to Sham stimulation over the right PPC (F (1,33) = 0.29; P = 0.596). ANOVA on variations did not reveal any main effects or interactions (Fig. 3C).
Color Naming Experiment
Stimulating the left pIFG resulted in slower reaction times for naming the color of objects presented in both contralateral and ipsilateral space. ANOVA revealed a Site of Stimulation × Stimulated Hemisphere interaction (F (1,11) = 6.92; P = 0.023). This interaction was found to be driven by effects of TMS on pIFG that were present only when stimulation was applied to the left hemisphere (Fig. 4A). TMS over the left pIFG prolonged reaction times compared to the right pIFG, irrespective of whether objects were presented in contralateral or ipsilateral space (F (1,22) = 6.75; P = 0.020). In contrast, TMS over the left LOC did not induce an overall prolongation in reaction times compared to TMS over the right LOC (F (1,22) = 0.16; P = 0.700). Moreover, stimulating LOC in either hemisphere had no differential effect on reaction time for color naming in contralateral versus ipsilateral space (left LOC: F (1,44) = 0.32, P = 0.575; right LOC: F (1,44) = 2.23, P = 0.143). ANOVA on errors did not reveal any main effects or interactions (Fig. 4B). Participants did not make any variations in their responses given that they were informed prior to the experiment of all the possible choices that they could make for color naming.
Figure 4.
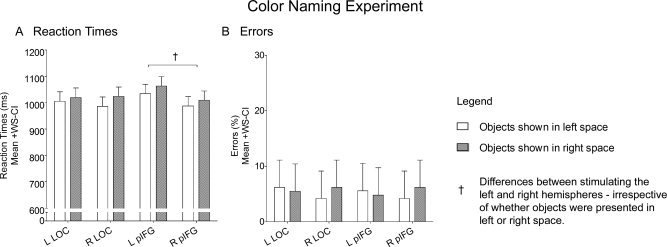
Color naming experiment. The graphs plot mean reaction times (A) and errors (B) while participants named the color of objects presented in either left (white) or right (gray) space when TMS was applied over the left LOC, the right LOC, the left pIFG, or the right pIFG. Daggers (†) denote differences when TMS was applied to the left compared to the right hemisphere, irrespective of whether objects were presented in left or right space. Error bars represent 95% confidence intervals for within‐subject contrasts [Loftus and Masson,1994].
Size Discrimination Experiment
Stimulating LOC in either hemisphere resulted in slower reaction times for discriminating the size of objects when objects were presented in contralateral but not ipsilateral space. ANOVA on reaction times revealed a Side of Presentation × Site of Stimulation × Stimulated Hemisphere interaction (F (1,11) = 4.86; P < 0.050). This interaction was found to be driven by effects of TMS on LOC that were present only when participants discriminated the size of objects in contralateral space, irrespective of which of the two hemispheres was stimulated (Fig. 5A). TMS over the left LOC prolonged reaction times when objects were presented in right compared to left space (F (1,44) = 12.51; P = 0.001), whereas TMS over the right LOC prolonged reaction times when objects were presented in left compared to right space (F (1,44) = 8.11; P = 0.008). A paired t‐test found that reaction times for discriminating the size of objects in contralateral space did not differ when LOC was stimulated in the left compared to the right hemisphere (T(11) = 1.38; P = 0.193). ANOVA on errors did not reveal any main effects or interactions (Fig. 5B). Participants did not make any variations in their responses given that the nature of this task was to make a forced choice between two possible button presses.
Figure 5.
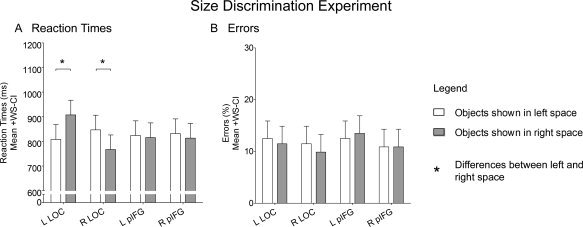
Size Discrimination experiment. The graphs plot mean reaction times (A) and errors (B) while participants responded with button pressing as to whether the physical size of the stimuli as shown on the computer screen were “small” or “big” for objects presented in either left (white) or right (gray) space when TMS was applied over the left LOC, the right LOC, the left pIFG, or the right pIFG. Asterisks (*) denote differences between objects in left versus right space. Error bars represent 95% confidence intervals for within‐subject contrasts [Loftus and Masson,1994].
Reading Versus Categorization experiment
Stimulating the left pIFG resulted in slower reaction times for categorizing but not for reading words. ANOVA revealed a Task × Stimulated Hemisphere interaction (F (1,11) = 7.56; P = 0.019). This interaction was found to be driven by effects of TMS on pIFG that were present only when stimulation was applied to the left hemisphere when participants categorized words but not when they read words (Fig. 6A). Namely, participants were slower to categorize words when TMS was applied to the left compared to the right pIFG (F (1,22) = 12.07; P = 0.022) but were not slower to read words when TMS was applied to the left compared to the right pIFG (F (1,22) = 0.25; P = 0.625). ANOVA on errors and on variations did not reveal any main effects or interactions (Fig. 6B,C).
Figure 6.
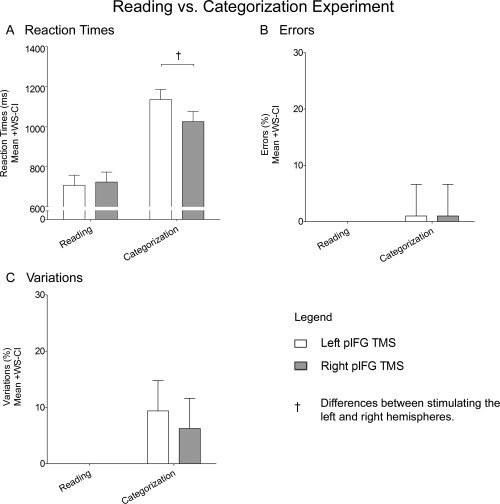
Reading vs. Categorization experiment. The graphs plot mean reaction times (A), errors (B), and variations (C) for reading and categorizing words when TMS was applied over the left pIFG (white) or the right pIFG (gray), Daggers (†) denote differences when TMS was applied to the left compared to the right hemisphere. Error bars represent 95% confidence intervals for within‐subject contrasts [Loftus and Masson,1994].
DISCUSSION
We reasoned that if an area is devoted to processing only the visual features of objects, then transcranial magnetic stimulation (TMS) applied to this area in either hemisphere would affect the naming of objects presented in contralateral but not ipsilateral space. In contrast, if an area is involved in language, then one might expect to see effects of TMS when applied over the left but not the right hemisphere, regardless whether objects are in contralateral or ipsilateral space. Our experiments reveal two important findings. First, TMS delivered to LOC in either hemisphere affected the naming of objects presented in contralateral but not ipsilateral space (see Fig. 3). In two additional experiments, when participants named the color of objects or made judgments about the size of stimuli as shown physically on a computer screen, TMS over the contralateral LOC did not affect color naming (see Fig. 4) but instead affected the participants' ability to make size judgments (see Fig. 5). Second, TMS delivered to the left but not the right pIFG affected the naming of objects irrespective of whether objects were presented in contralateral or ipsilateral space (see Fig. 3). In a separate experiment, when participants were asked to either read or categorize words, TMS over the left but not the right pIFG affected word categorization but not word reading (see Fig. 6). Taken together, these findings strongly suggest that when people name visually‐presented objects, LOC processes the visual form of objects while the left pIFG processes the semantics of objects.
Role of the Left and Right LOC in Naming Visually Presented Objects
The fact that stimulating LOC slowed down the naming (Fig. 3A) and the size discrimination (Fig. 4A) of objects but only when those objects were presented in contralateral space suggests that LOC is more concerned with processing visual rather than the semantic features of objects. Although an eye tracker was not used in our experiments to confirm that subjects fixated their eyes on the center of the screen, stimuli were presented too briefly for a saccade to be initiated before stimulus offset. Moreover even if subjects had managed to make a saccade, or had not fixated properly, then the differences we observed for stimuli presented in the contralateral and ipsilateral space would have actually been reduced or even washed out. In other words, eye movements would have weakened rather than strengthened the effects we observed in our experiments.
Mapping of visual areas in the human cortex shows that both striate and extrastriate regions show a preference for visual stimuli presented in contralateral space [Engel et al.,1994]. Thus, the contralateral effect of LOC stimulation on object naming and size discrimination is consistent with the idea that LOC is a visual area devoted to dealing with the physical features of objects. Further evidence for this claim comes from the observation that the contralateral effect of TMS on naming was no larger for left LOC stimulation than it was for right LOC stimulation. If processing in LOC were modulated by semantics, particularly the semantics associated with naming, then one might expect greater disruption when stimulation was applied over LOC in the left (language dominant) than the right hemisphere.
In macaque monkeys, neurons in the inferior‐temporal (IT) area, which is thought to be homologous to the human LOC, not only fire to the visual presentation of objects [Tanaka,1993], but also show a stronger preference for objects presented in contralateral versus ipsilateral space [Op De Beeck and Vogels,2000]. This effect has largely been replicated in humans using fMRI [McKyton and Zohary,2007; Niemeier et al.,2005; but see Grill‐Spector et al.,1998] provided that images are displayed a sufficient distance from fixation. Although our study is the first TMS study to our knowledge that shows that LOC has a preference for objects in contralateral space, an earlier TMS study has shown that area MT, another extra‐striate visual area, also has a preference for moving stimuli in contralateral space [Beckers and Hömberg,1992].
So, what visual features of objects does LOC process? Our results show that stimulating LOC in either hemisphere had no effect on color naming (see Fig. 4). This finding rules out the possibility that the TMS‐induced effects over LOC in the Object Naming experiment were related to color processing. More importantly, this finding also reveals that the effects of stimulating LOC in the first experiment could not have been the result of having disrupted attentional mechanisms or from having induced scotomas in the contralateral hemifield. In two fMRI studies, Cant and Goodale [2007] and Cant et al. [2009] demonstrate that LOC does not process information about the color of objects but processes instead information about their form. In contrast, the primary visual area and other medial areas in the cuneus cortex (areas which could not have been affected by TMS in our study; see Fig. 2) processed the color of objects as opposed to the form of objects. Based on these findings, these authors suggested that the extraction of an object's color occurs earlier during visual analysis than the extraction of object form, perhaps because the latter requires more complex computations. In support of this view, Lerner et al. [2001] showed that as an object is more and more scrambled, higher‐order extra‐striate visual areas, such as LOC, show less and less fMRI activity, once more underscoring the fact that LOC and other higher‐order extra‐striate visual areas process more complex features of objects. Similarly, our study reveals that LOC has no role to play in naming colors (see Fig. 4), but is required in making decisions about more complex features of objects, such as their size (see Fig. 5).
Many investigators have argued that LOC's role in object recognition is that of a general purpose shape analyzer and it is not in the business of processing conceptual information [e.g., Chouinard et al.,2008; Eger et al.,2008; Grill‐Spector et al.,2001; Tyler et al.,2004]. In fact, some of the earliest fMRI studies to describe LOC's role in object recognition noted that activation in LOC was the same for the visual presentation of both familiar and nonsense objects [Kanwisher et al.,1996; Malach et al.,1995]. In our study, if LOC were concerned with the semantics of objects, then one might have expected to see an increase in naming errors with TMS. As it turns out, only TMS applied to the left pIFG produced an increase in errors when participants named objects (Fig. 3A). Similar to our results, an earlier TMS study has shown that stimulating the left posterior portion of Brodmann's area (BA) 37, an area which appeared to be located at a location rostral to what we would call LOC, prolonged reaction times but did not affect accuracy when people named pictures of objects [Stewart et al.,2001]. The same study also showed that TMS over the same area did not interfere with people's ability to read words or name colors, which again suggests that the deficits induced by TMS when people named the objects was not the result of having interfered with semantic processing. This led Stewart et al. [2001] to conclude that the disruption of picture naming that accompanied stimulation of the left BA 37 was due to an interference with object recognition rather than semantic processing.
Role of the Left pIFG in Naming Visually Presented Objects
It seems likely that a language area would not be concerned about the location of objects in space in a naming task. As predicted, TMS over the left pIFG disrupted participants' ability to name objects or the color of objects irrespective of whether objects were presented in contralateral or ipsilateral space (Figs. 3A,B and 4). In contrast, stimulation over the right pIFG did not interfere with the participants' ability to name objects or the color of objects. The question then arises as to whether or not this TMS‐induced disruption was the result of having induced speech arrest or other types of processes related to language. Although it already seems unlikely that speech arrest could account for the low number of errors (mean = 9.4%, SEM = 3.6%) made by participants in the Object Naming experiment (Fig. 3B), we nonetheless conducted an additional experiment in which we had participants either read or categorize words presented at the center of the computer screen while TMS was applied to the pIFG in either hemisphere. As it turns out, participants were slower to categorize but not read words when TMS was applied to pIFG in the left compared to the right hemisphere (Fig. 6A). Thus, the effects of stimulating the left pIFG in the Object Naming experiment were not the result of having disrupted speech production.
TMS over the left inferior frontal cortex at much higher levels of stimulation than those used in this study have been shown to induce forms of speech arrest [e.g., Flitman et al.,1998; Jennum et al.,1994; Michelucci et al.,1994; Pascual‐Leone et al.,1991; for review, see Devlin and Watkins,2007]. However, it is unclear from many of these studies as to whether the observed speech arrest was the direct result of having disrupted speech processing or the indirect result of having induced discomfort. TMS applied to the inferior frontal cortex at high levels of stimulation can cause great discomfort or even pain, which in turn has been shown to influence a person's ability to name objects [Epstein et al.,1996]. To ensure that stimulation was more comfortable for participants, we excluded participants with high rMTs and used a subthreshold intensity of TMS. As such, all participants reported after each block of trials that the TMS was within a range of comfort that was tolerable and not painful. Moreover, the fact that TMS over the left pIFG prolonged reaction times and increased errors while the same levels of stimulation applied over the right pIFG had no effect on object naming, supports our claim that the effects observed in this study from stimulating the left pIFG was the direct result of having disrupted neural processing to this area.
So, what specific effects did stimulating the left pIFG have on language? We cannot rule out the possibility that TMS over the left pIFG disrupted phonological processing [Gough et al.,2005; Nixon et al.,2004]. This was not formally tested. Nevertheless, we can conclude that stimulating the left pIFG disrupted semantic processing. In the fourth experiment, both the reading aloud task and the categorization task involved accessing a word's phonological code, but only the categorization task put extra demands on semantic processing. Given that stimulating the left pIFG influenced performance only in the categorization task, it follows that this manipulation affected the accessing of semantic meaning rather than phonology. This result is consistent with findings from functional neuroimaging studies showing that the left pIFG plays a role in semantic processing [Barde and Thompson‐Schill,2002; Chouinard et al.,2008; Gold and Buckner,2002; for review, see Bookheimer,2002]. We could have conducted yet a fifth experiment to determine whether or not we may have also disrupted phonological processing with TMS to the left pIFG, but this would have added only a nicety to our study and would have gone well beyond the scope of what we had originally set out to do: namely, to examine the differential contributions of LOC and pIFG in visual and semantic processing when people name visually‐presented objects.
The location that we used to stimulate the inferior frontal cortex was more dorsal and more posterior compared to earlier TMS studies that disrupted semantic processing with TMS in the context of memory [e.g., Devlin et al.,2003; Gough et al.,2005; Köhler et al.,2004], which is quite a different context from having people simply name objects that they see on a computer screen. It is conceivable that naming, which is less taxing and more automatic than other types of semantic tasks, could involve more posterior parts of the inferior frontal cortex. In support of this view, Kostopoulos and Petrides [2008] have shown that as demands for retrieving semantic information increases, fMRI activation to semantic processing shifts from more posterior to more anterior areas in the inferior frontal cortex. In other words, more anterior areas in the inferior frontal cortex are engaged in what they call “active” retrieval whereas more posterior areas are engaged in more automatic forms of retrieval, such as when people have to provide names for objects or colors that they see.
Nevertheless, the question arises as to whether or not our effects of TMS was the result of a spread of current to more anterior parts of the inferior frontal cortex. We argue against this possibility for two reasons. First, our earlier work has shown dissociable effects of repetitive TMS when applied at 90% rMT (the same intensity of TMS used for this study) at two adjacent cortical sites that were separated by only ∼2.5 cm on both cerebral activity as measured with positron emission tomography [Chouinard et al.,2003] and behavior [Chouinard et al.,2005]. Second, we applied TMS over the part of the inferior frontal cortex in which we had previously found repetition suppression in fMRI to be greatest for semantics in the context of naming objects [Chouinard et al.,2008]. In this fMRI‐adaptation study, repetition suppression for naming semantically‐related objects was present in the posterior and not anterior part of the inferior frontal cortex. Thus, we think it is justifiable to conclude that that we disrupted semantic processing from stimulating the left pIFG.
Acknowledgements
We thank Dr. Marc Joanisse for his comments during our revisions to this paper.
REFERENCES
- Barde LH, Thompson‐Schill SL ( 2002): Models of functional organization of the lateral prefrontal cortex in verbal working memory: Evidence in favor of the process model. J Cognit Neurosci 14: 1054– 1063. [DOI] [PubMed] [Google Scholar]
- Beckers G, Hömberg V ( 1992): Cerebral visual motion blindness: Transitory akinetopsia induced by transcranial magnetic stimulation of human area V5. Proc Biol Sci 249: 173– 178. [DOI] [PubMed] [Google Scholar]
- Bookheimer S ( 2002): Functional MRI of language: New approaches to understanding the cortical organization of semantic processing. Annu Rev Neurosci 25: 151– 188. [DOI] [PubMed] [Google Scholar]
- Cant JS, Goodale MA ( 2007): Attention to form or surface properties modulates different regions of human occipitotemporal cortex. Cereb Cortex 17: 713– 731. [DOI] [PubMed] [Google Scholar]
- Cant JS, Arnott SR, Goodale MA ( 2009): fMR‐adaptation reveals separate processing regions for the perception of form and texture in the human ventral stream. Exp Brain Res 192: 391– 405. [DOI] [PubMed] [Google Scholar]
- Chouinard PA, Van Der Werf YD, Leonard G, Paus T ( 2003): Modulating neural networks with transcranial magnetic stimulation applied over the dorsal premotor and primary motor cortices. J Neurophysiol 90: 1071– 1083. [DOI] [PubMed] [Google Scholar]
- Chouinard PA, Leonard G, Paus T ( 2005): Role of the primary motor and dorsal premotor cortices in the anticipation of forces during object lifting. J Neurosci 25: 2277– 2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouinard PA, Morrissey BF, Kohler S, Goodale MA ( 2008): Repetition suppression in occipital‐temporal visual areas is modulated by physical rather than semantic features of objects. Neuroimage 41: 130– 144. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Watkins KE ( 2007): Stimulating language: Insights from TMS. Brain 130: 610– 622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin JT, Matthews PM, Rushworth MF ( 2003): Semantic processing in the left inferior prefrontal cortex: A combined functional magnetic resonance imaging and transcranial magnetic stimulation study. J Cognit Neurosci 15: 71– 84. [DOI] [PubMed] [Google Scholar]
- Eger E, Ashburner J, Haynes JD, Dolan RJ, Rees G ( 2008): fMRI activity patterns in human LOC carry information about object exemplars within category. J Cognit Neurosci 20: 356– 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SA, Rumelhart DE, Wandell BA, Lee AT, Glover GH, Chichilnisky EJ, Shadlen MN ( 1994): fMRI of human visual cortex. Nature 369: 525. [DOI] [PubMed] [Google Scholar]
- Epstein CM, Lah JJ, Meador K, Weissman JD, Gaitan LE, Dihenia B ( 1996): Optimum stimulus parameters for lateralized suppression of speech with magnetic brain stimulation. Neurology 47: 1590– 1593. [DOI] [PubMed] [Google Scholar]
- Flitman SS, Grafman J, Wassermann EM, Cooper V, O'Grady J, Pascual‐Leone A, Hallett M ( 1998): Linguistic processing during repetitive transcranial magnetic stimulation. Neurology 50: 175– 181. [DOI] [PubMed] [Google Scholar]
- Gegenfurtner KR, Kiper DC ( 2003): Color vision. Annu Rev Neurosci 26: 181– 206. [DOI] [PubMed] [Google Scholar]
- Gold BT, Buckner RL ( 2002): Common prefrontal regions coactivate with dissociable posterior regions during controlled semantic and phonological tasks. Neuron 35: 803– 812. [DOI] [PubMed] [Google Scholar]
- Gough PM, Nobre AC, Devlin JT ( 2005): Dissociating linguistic processes in the left inferior frontal cortex with transcranial magnetic stimulation. J Neurosci 25: 8010– 8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill‐Spector K, Kushnir T, Hendler T, Edelman S, Itzchak Y, Malach R ( 1998): A sequence of object‐processing stages revealed by fMRI in the human occipital lobe. Hum Brain Mapp 6: 316– 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill‐Spector K, Kourtzi Z, Kanwisher N ( 2001): The lateral occipital complex and its role in object recognition. Vision Res 41: 1409– 1422. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D ( 2007): The cortical organization of speech processing. Nat Rev Neurosci 8: 393– 402. [DOI] [PubMed] [Google Scholar]
- Jennum P, Friberg L, Fuglsang‐Frederiksen A, Dam M ( 1994): Speech localization using repetitive transcranial magnetic stimulation. Neurology 44: 269– 273. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, Chun MM, McDermott J, Ledden PJ ( 1996): Functional imaging of human visual recognition. Brain Res Cogn Brain Res 5: 55– 67. [DOI] [PubMed] [Google Scholar]
- Kohler S, Paus T, Buckner RL, Milner B ( 2004): Effects of left inferior prefrontal stimulation on episodic memory formation: A two‐stage fMRI‐rTMS study. J Cognit Neurosci 16: 178– 188. [DOI] [PubMed] [Google Scholar]
- Kostopoulos P, Petrides M ( 2008): Left mid‐ventrolateral prefrontal cortex: Underlying principles of function. Eur J Neurosci 27: 1037– 1049. [DOI] [PubMed] [Google Scholar]
- Lerner Y, Hendler T, Ben‐Bashat D, Harel M, Malach R ( 2001): A hierarchical axis of object processing stages in the human visual cortex. Cereb Cortex 11: 287– 297. [DOI] [PubMed] [Google Scholar]
- Lindell AK ( 2006): In your right mind: Right hemisphere contributions to language processing and production. Neuropsychol Rev 16: 131– 148. [DOI] [PubMed] [Google Scholar]
- Loftus GR, Masson MEJ ( 1994): Using confidence intervals in within‐subjects designs. Psychonom Bull Rev 1: 476– 490. [DOI] [PubMed] [Google Scholar]
- Malach R, Reppas JB, Benson RR, Kwong KK, Jiang H, Kennedy WA, Ledden PJ, Brady TJ, Rosen BR, Tootell RB ( 1995): Object‐related activity revealed by functional magnetic resonance imaging in human occipital cortex. Proc Natl Acad Sci USA 92: 8135– 8139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKyton A, Zohary E ( 2007): Beyond retinotopic mapping: The spatial representation of objects in the human lateral occipital complex. Cereb Cortex 17: 1164– 1172. [DOI] [PubMed] [Google Scholar]
- Michelucci R, Valzania F, Passarelli D, Santangelo M, Rizzi R, Buzzi AM, Tempestini A, Tassinari CA ( 1994): Rapid‐rate transcranial magnetic stimulation and hemispheric language dominance: Usefulness and safety in epilepsy. Neurology 44: 1697– 1700. [DOI] [PubMed] [Google Scholar]
- Niemeier M, Goltz HC, Kuchinad A, Tweed DB, Vilis T ( 2005): A contralateral preference in the lateral occipital area: Sensory and attentional mechanisms. Cereb Cortex 15: 325– 331. [DOI] [PubMed] [Google Scholar]
- Nixon P, Lazarova J, Hodinott‐Hill I, Gough P, Passingham R ( 2004): The inferior frontal gyrus and phonological processing: An investigation using rTMS. J Cognit Neurosci 16: 289– 300. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97– 113. [DOI] [PubMed] [Google Scholar]
- Op De Beeck H, Vogels R ( 2000): Spatial sensitivity of macaque inferior temporal neurons. J Comp Neurol 426: 505– 518. [DOI] [PubMed] [Google Scholar]
- Pascual‐Leone A, Gates JR, Dhuna A ( 1991): Induction of speech arrest and counting errors with rapid‐rate transcranial magnetic stimulation. Neurology 41: 697– 702. [DOI] [PubMed] [Google Scholar]
- Paus T, Wolforth M ( 1998): Transcranial magnetic stimulation during PET: Reaching and verifying the target site. Hum Brain Mapp 6: 399– 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Jech R, Thompson CJ, Comeau R, Peters T, Evans AC ( 1997): Transcranial magnetic stimulation during positron emission tomography: A new method for studying connectivity of the human cerebral cortex. J Neurosci 17: 3178– 3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Devlin JT, Moore CJ, Morton C, Laird AR ( 2005): Meta‐analyses of object naming: Effect of baseline. Hum Brain Mapp 25: 70– 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossion B, Pourtois G ( 2004): Revisiting Snodgrass and Vanderwart's object pictorial set: The role of surface detail in basic‐level object recognition. Perception 33: 217– 236. [DOI] [PubMed] [Google Scholar]
- Stewart L, Meyer B, Frith U, Rothwell J ( 2001): Left posterior BA37 is involved in object recognition: A TMS study. Neuropsychologia 39: 1– 6. [DOI] [PubMed] [Google Scholar]
- Tanaka K ( 1993): Neuronal mechanisms of object recognition. Science 262: 685– 688. [DOI] [PubMed] [Google Scholar]
- Tyler LK, Stamatakis EA, Bright P, Acres K, Abdallah S, Rodd JM, Moss HE ( 2004): Processing objects at different levels of specificity. J Cognit Neurosci 16: 351– 362. [DOI] [PubMed] [Google Scholar]


