Abstract
The perspective from where the world is perceived is an important aspect of the bodily self and may break down in neurological conditions such as out‐of‐body experiences (OBEs). These striking disturbances are characterized by disembodiment, an external perspective and have been observed after temporoparietal damage. Using mental own body imagery, recent neuroimaging work has linked perspectival changes to the temporoparietal cortex. Because the disembodied perspective during OBEs is elevated in the majority of cases, we tested whether an elevated perspective will interfere with such temporoparietal mechanisms mental own body imagery. We designed stimuli of life‐sized humans rotated around the vertical axis and rendered as if viewed from three different perspectives: elevated, lowered, and normal. Reaction times (RTs) in an own body transformation task, but not the control condition, were dependent on the rotation angle. Furthermore, RTs were shorter for the elevated as compared with the normal or lowered perspective. Using high‐density EEG and evoked potential (EP) mapping, we found a bilateral temporoparietal and frontal activation at ≈330–420 ms after stimulus onset that was dependent on the rotation angle, but not on the perspective. This activation was also found in response‐locked EPs. In the time period ≈210–330 ms we found a temporally distinct posterior temporal activation with its duration being dependent on the perspective, but not the rotation angle. Collectively, the present findings suggest that temporoparietal and frontal as well as posterior temporal activations and their timing are crucial neuronal correlates of the bodily self as studied by mental imagery. Hum Brain Mapp, 2009. © 2009 Wiley‐Liss, Inc.
Keywords: mental perspective taking, imagery, first person perspective, electrical brain imaging, event‐related, response‐locked, LAURA
INTRODUCTION
The perception and cognition of the positions, actions, and intentions of other people as well as those of oneself are important skills and enable humans to distinguish self from other. Recent years have seen a renewed interest of neurobiological research into the self and how it is grounded in mechanisms of bodily perception and cognition [Gallagher, 2000; Jeannerod, 2003; Vogeley and Fink, 2003]. The human body (of others as well as one's own) provides a particularly rich source of perceptual information and recent neuroimaging research has unraveled several brain mechanisms involved in the visual perception and recognition of human bodies [Allison et al., 2000; Downing et al., 2001; Thierry et al., 2006; Urgesi et al., 2007], either of one's own or of others [Ehrsson et al., 2004; Keenan et al., 2001; Sugiura et al., 2005]. These studies revealed the existence of an extended cortical network encompassing extrastriate areas, temporoparietal and parietal cortex, as well as medial and lateral frontal regions.
Another line of research has studied mental imagery with respect to human bodies. Mental imagery or mental transformation is the ability to imagine the transformation of an object (or a human body) in space and is essential in many everyday cognitive tasks such as spatial reasoning, action planning and problem solving [Corballis, 1997; Shepard and Hurwitz, 1984]. Concerning human bodies, Parsons [ 1987] showed that the time required to mentally simulate a change from one's actual position and perspective to that of another depicted human body is similar to the time taken to actually perform that position and perspective change. Only few studies have investigated the neural mechanisms underlying the mental transformation of human bodies [Blanke et al., 2005; Parsons, 1987; Zacks, 2002; Zacks et al., 1999], although many have studied mental transformation of nonbodily objects [i.e. Harris et al., 2000; Jordan et al., 2001; Kosslyn et al., 1994; Pegna et al., 1997; Podzebenko et al., 2002]. Using schematic line drawings of human bodies and performing functional magnetic resonance imaging (fMRI) during a mental own body transformation task, Zacks et al. [ 1999] revealed increased activation in temporo‐occipital and temporoparietal cortex, with a left hemisphere predominance. Using EP mapping and electrical neuroimaging, Blanke et al. [ 2005] showed that such own body transformations activate temporoparietal cortex during a time period ≈330–400 ms after stimulus onset, and that this activation was not found during control tasks not requiring mental own body transformations [Blanke et al., 2005].
Other neuroimaging studies focused on the manipulation of the perspective during mental imagery and revealed activations within a network of brain areas including precuneus, prefrontal cortex, somatosensory cortex [Ruby and Decety, 2001], as well as right premotor cortex, superior temporal cortex, and cingulate cortex [David et al., 2006; Vogeley et al., 2004]. It is likely that subjects imagined changes in perspective and position jointly during most aforementioned studies, independently of whether investigators focused on body transformations or perspective changes. Arzy et al. [ 2006], however, manipulated the mental transformation of body position and perspective separately, asking subjects to image a perspective change from their actual body position (“embodied” condition with only an imagined perspective change) or from the position of another human body (“disembodied” condition with an imagined body position and perspective change). They found that activation in temporoparietal cortex at ≈367 ms was associated with an imagined perspective and position change in the disembodied condition, whereas an earlier activation at ≈318 ms in temporo‐occiptal cortex was associated with the imagined perspective change in the embodied condition.
Data from autobiographical memory studies also show that during recall of distant and emotionally loaded memories an external and disembodied perspective is frequently used [Nigro and Neisser, 1983]. Interestingly, data from neurological patients with OBEs show that in most instances of such disembodiment an elevated perspective is experienced visually, whereas a lowered or eye‐level perspective is only reported in extremely rare cases [Blanke and Mohr, 2005; Blanke et al., 2004; Brugger et al., 1997; Devinsky et al., 1989]. Based on these data, we hypothesize a preference for an elevated perspective in certain disembodied conditions (like neurological OBEs or disembodied mental imagery) that is open to experimental control and can be characterized using electrical neuroimaging. Since mental imagery performance can be accessed at the behavioural level in terms of differences in RTs, which are also reflected in differences in brain activation [Arzy et al., 2006; Blanke et al., 2005], here we use high‐density electrical neuroimaging (the method of choice for investigating the dynamics of neuronal processing) and focus on own body transformations. We use disembodied conditions and characterize the effects of the perspective (normal eye‐level, elevated, and lowered) from which the imaged perspective and position change is performed.
MATERIALS AND METHODS
Participants
Eleven healthy volunteers (five males; aged 18–24 years; mean ± SD, 21.18 ± 2.27 years) participated in the experiment. All participants were right‐handed, and had normal or corrected‐to‐normal vision. Written informed consent was provided to participate in the experiment, which was approved by the Ethical Committee of the University Hospital of Lausanne (conform to the Declaration of Helsinki).
Stimuli and Procedure
We generated images of human figures that were rotated around their vertical (yaw) axis and depicted from three different points of view (Fig. 1), corresponding to the perspectives from which the own body transformations had to be performed. The figures were always shown as if seen from a position that was 3 m behind the figure (fixation point was at the height of the belly button). The point of view was changed by manipulating the pitch angle from which the participant had the impression of looking at the figure. This was either (1) as if looking down at the figure from an elevated perspective (rotated 60° upwards, “elevated”), (2) as if looking up at the figure from a lowered perspective (rotated 60° downwards, “lowered”) or from the normal, “eye‐level”, perspective. The stimuli were presented in a circular aperture (Fig. 1) and in life‐size (as if the figure was actually viewed from a 3‐m distance).
Figure 1.
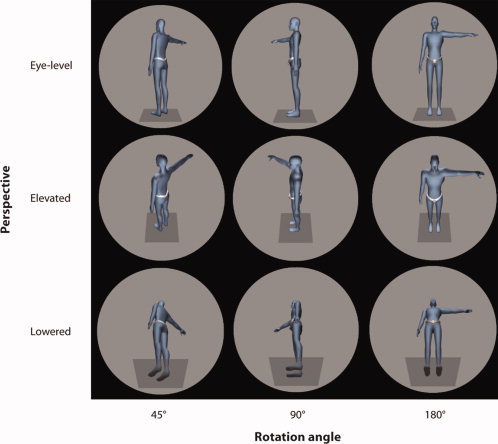
Stimuli and experimental setup. Examples of visual stimuli (always flashed for 200 ms) used in both the OBT and LAT with varying rotation angles (from left to right: 45°, 90°, 180°) and different perspectives (from top to bottom: eye‐level, elevated, lowered). The stimuli were presented on a large back‐projection screen (3 m × 2.5 m) in ”life size“, i.e. as if viewed from a distance of 3 m.
Participants were seated 2 m in front of the screen in a dimmed room. Each trial started with a fixation point shown in the center of the aperture for a random duration of 500–1,500 ms. Then, a stimulus was presented for 200 ms [Arzy et al., 2006; Blanke et al., 2005]. In the own body transformation task (OBT) the participants were asked to indicate, as fast and accurate as possible, whether the extended arm is the left or right arm of the figure, after having imagined themselves to be at the location of the shown figure, as if the shown body were their body. We also tested a control condition, based on previous data [Blanke et al., 2005]. In this lateralization control task (LAT) the subjects were asked as to whether the extended arm is to the left or right of the midline of the screen (aligned with their own midline). After 200 ms the stimulus disappeared and only the empty circular aperture was shown. The participants responded by pressing either the left or the right button on a response box with their digit and middle finger. Five participants used the left hand, while six used the right hand. After the response the next trial started with the presentation of the fixation point.
There were eight different rotation angles (rotation angles: 0°, ±45°, ±90°, ±135°, 180°) and three different visual perspectives (perspective; elevated, lowered, normal). In order to calculate the mean EP we combined clockwise and counter‐clockwise rotations, which resulted in 5 angles. Therefore each of these 3 × 5 conditions was repeated 90 times in the OBT, randomized within a given block keeping only the perspective constant. For the rotation angles 45°–135° we showed both clockwise and counter‐clockwise rotated stimuli. In the LAT, however, each condition was shown only 30 times, because based on behavioural pilot studies we expected the perspective to affect the RTs in the OBT, but not in the LAT. Hence, for the LAT an EP could be computed for each rotation angle, but averaged over all perspectives. However, while this allowed for comparing EPs between the OBT and LAT for different rotation angles (Fig. 3a), effects specific for the perspective could only be investigated within the OBT (Fig. 4a). We also made this compromise, because otherwise the duration of the whole experiment would have been too long (>1.5 hours). After an initial training, we presented the stimuli to the subjects in blocks in the following sequential order: 2 × OBT, 1 × LAT, 2 × OBT, 1 × LAT, 2 × OBT, 1 × LAT. The perspectives in the blocks were held constant, but were randomized across the blocks.
Figure 3.
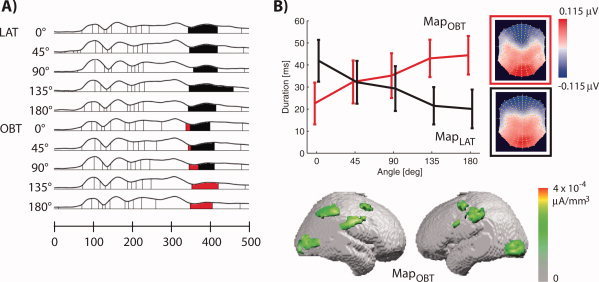
EP mapping of OBT and LAT. (a) Segments of stable map topographies in the LAT (rows 1–5) and OBT (rows 6–10) for different rotation angles under the GFP curve (see Methods). Two of these map topographies, MapLAT and MapOBT, are shown as flattened scalp topographies in (b). (b) The duration of MapLAT and MapOBT in the time‐window 350–410 ms in the OBT condition (LAT condition not shown). The duration of MapOBT was correlated with the rotation angle in the OBT condition, but not in the LAT.
Figure 4.
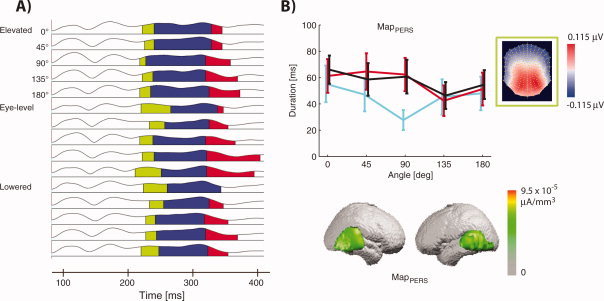
EP mapping of the OBT for the three different perspectives. (a) Segments of stable map topographies with a dependence of their duration of the perspective and rotation angle [see (b) for a flattened view of the EP maps] in the OBT for all rotation angles and perspectives. These are the results of the second segmentation, which confirms that at 330 ms brain activity specific to OBT depends on the rotation angle, but also allows for an investigation of the effects of the visuo‐spatial perspective. (b) The durations of MapPERS, see green segment in panel (a), in the time‐window 214–330 ms for the elevated (cyan), eye‐level (red) and lowered perspective (black). MapPERS is shorter in the elevated perspective for humanoid figures as seen from behind.
EEG Recording
The continuous EEG was acquired with an ActiveTwo system (Biosemi, The Netherlands) from 256 electrodes (recorded at 2,048 Hz and downsampled to 512 Hz for further processing, bandpass‐filtered from 0.1 to 40 Hz). Only the EEG epochs of correct responses were used to calculate stimulus‐ and response‐locked EPs. Each epoch was visually inspected, and artifacts due to, for example, eye‐blinks or movements of the participant were rejected. This yielded an average of 85 (LAT) and 180 (OBT) accepted epochs per subject for each rotation angle (when averaged over the different visuospatial perspectives, see Figs. 3 and 5) and an average of 60 accepted epochs per subject for each rotation angle and perspective in the OBT (see Fig. 4). Electrodes yielding unreliable or too noisy signals (“bad electrodes”) were interpolated using the signals form the neighboring electrodes (on average 31 ± 12.6 electrodes per subject, corresponding to 12% or all electrodes). The stimulus‐locked EPs were calculated against the average reference for −200 to 1,000 ms relative to the stimulus onset and for −1,000 to 500 ms for the response‐locked EPs. Both the stimulus‐ and response‐locked EPs were baseline corrected using the time‐averaged signals in the 200 ms preceding the stimulus. The analysis was performed using the Cartool software by Denis Brunet (http://brainmapping.unige.ch/Cartool.htm) and the MeanMachine software by Pär Halje and Lars Schwabe (http://meanmachine.sourceforge.net).
Figure 5.
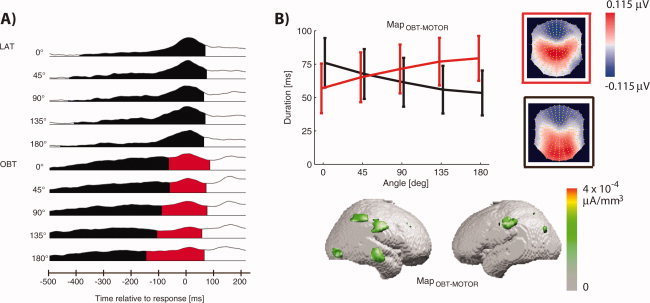
EP mapping of response‐locked EEG signals in the OBT and LAT. (a) Segments of stable map topographies in the OBT with EPs computed locked to the response of the participants and averaged over all perspectives. This segmentation yielded a MapOBT‐MOTOR only in the OBT, but not in the LAT, and hence confirms that brain activity after the initial stimulus‐locked EPs (see Fig. 3a) differs between the OBT and LAT. (b) Similar to MapOBT the duration of MapOBT‐MOTOR was longer for higher rotation angles, which suggests the presence of at least one more mental transformation process in addition to the activity we described at 330 ms.
Choosing the average reference heavily influences the waveforms at single electrodes compared with other choices of the reference [for a recent discussion see Murray et al., 2008]. Thus, one should be cautious when comparing waveforms at the same electrode between recordings with different references. The question of the correct reference electrode has been intensively debated in the literature [Desmedt and Tomberg, 1990; Gencer et al., 1996; Junghofer et al., 1999; Pascual‐Marqui and Lehmann, 1993; Tomberg et al., 1990]. And the reference‐problem has been taken as a major disadvantage of EEG versus MEG [Pataraia et al., 2002; Wikswo et al., 1993; Williamson et al., 1991]. Yet, many studies have shown that—while the choice of the reference heavily influences waveform analysis—it is actually irrelevant for the analysis of topographic maps (as done in the present study) and for source localization (as done in the present study) as long as the reference is correctly included in the model. This is because the configuration of the scalp topography is independent of the reference electrode [Fender, 1997; Geselowitz, 1998; Lehmann, 1987] in the sense that at each time step (1/512 seconds (2 ms) the shape of the activation pattern remains constant, although the zero line may be shifted. This is comparable to the shape of a chain of mountains, which remains constant even if the altitude of the sea level were to change [Murray et al., 2008; Lehmann, 1987].
EP Mapping and Distributed Linear Inverse Solution
We applied EP mapping [Michel, 2004; Michel et al., 2001; Murray et al., 2008], which searches for time segments of stable map topographies presumably representing stable microstates of the brain like, for example, the common activation of a set of cortical areas. Recording activity from many different sites simultaneously over the scalp allows the construction of topographic maps evolving over time. These can be used to determine when map configurations change and/or differ between experimental conditions. The underlying assumption is that whenever spatial configurations of the electric field on the scalp differ, different neuronal populations are active in the brain. For this we used a spatiotemporal segmentation algorithm derived from spatial cluster analysis, as described previously [Pascual‐Marqui et al., 1995]. This method applies a k‐means cluster analysis for identifying the most dominant scalp topographies appearing in the group‐averaged EPs of each condition over time. This spatial pattern analysis summarizes the whole EP data by a limited number of scalp potential configurations. The number of maps that explains the whole data set is determined by a cross‐validation criterion which optimizes between the degrees of freedom and the explained variance [Pascual‐Marqui et al., 1995]. The clustering algorithm considers only the spatial pattern of the scalp activations and is independent of the strength of the signal, which is reflected by the global field power (GFP, [Lehmann and Skrandies, 1980]). The colors used to show the scalp maps in this article were scaled to the same range order to ease the visual comparison of these different patterns.
Even though this cluster analysis does not rely on any temporal characteristics of the data, it leads to a segmentation of the EPs into discrete successive time periods. This is due to the fact that map topographies do not randomly change over time but remain stable in a certain configuration for certain time periods. In other words, the EP is decomposed into a sequence of stable map topographies and we analyzed those segments in greater detail that showed differences in their duration between the experimental conditions. In all figures in this article (depicting results of the segmentation procedure), we show the time‐dependent GFP as a quantification of the signal strength. The area below the GFP curve is separated into the segments found by the clustering procedure and those segments are colored that show statistically significant dependencies of their duration on experimental conditions,.
The dominant scalp topographies (identified in the group‐averaged data and shown as segments below the GFP curves) are then fitted to the EPs of each individual subject using spatial fitting procedures. For each time point of the individual subject's EP, the scalp topography was compared by means of normalized spatial correlation to each segmentation map and was labeled according to the one with which the correlations was maximal. From this fitting procedure, we determined the duration that a given segmentation map was observed for a given condition across subjects. In particular, we always choose the time‐window from the earliest onset until the latest offset of a map as the time window to be considered, and selected all maps present in the group‐averaged EP as candidates to describe the EP of the participants. The durations for the individual maps were then subjected to statistical analysis. Consequently, the error bars shown in Figs. 3b, 4b and 5b reflect the variability over subjects.
The result of a segmentation (in terms of the segments representing stable microstates of the brain) depends on the EP used as an input to the segmentation algorithm. For example, in order to find similarities or differences in the brain activity between experimental conditions (in terms of the timing or duration of such segments), the corresponding EPs should be analyzed within a single segmentation procedure. Since we were interested in the similarities and differences between the OBT and LAT as well as possible differences within the OBT as a function of the visuospatial perspective, we performed two separate segmentations to investigate the related brain mechanisms. We also performed a third segmentation for the motor‐locked EP analysis. In particular, in the first segmentation we segmented the EPs in the LAT for all rotation angles together with the EPs in the OBT (Fig. 3a). Both sets of EPs were computed by averaging the correct and artifact‐free epochs for all three perspectives. In the second segmentation, we segmented the EPs of only the OBT, where we computed an EP for each rotation angle and perspective (Fig. 4a). In the third segmentation we computed the EPs based on the same epochs as in the first segmentation, but relative to the time of the manual response (button press) (Fig. 5a). Note that for each epoch, and before averaging, we applied the same baseline correction relative to the stimulus onset.
The neural generators for a given mean EP map were estimated by applying a distributed linear inverse solution, based on a local autoregressive average [LAURA, [Grave de Peralta Menendez et al., 2001, 2004], see [Michel, 2004; Murray et al., 2008] for an in‐depth discussion of this method as well as references therein]. Before we applied the LAURA source localization, and only for this processing step, we downsampled (by means of a 3D spline interpolation) the map topography from 256 to 111 electrodes. The LAURA algorithm needs to be parameterized with, for example, regularization and spatial smoothing parameters. Our own preliminary evaluations showed that increasing the number of electrodes from 111 to 256 affects the choice of the range of valid parameter values, but since the only recent availability of very high‐density brain imaging data, systematic studies and experimental tests for the determination of these parameter values are lacking. Consequently, we here used the established 111 configuration, because the LAURA algorithm we used has been tested extensively for this configuration.
RESULTS
Behavioral Performance
We compared the RTs of the participants in the OBT and LAT (repeated measures ANOVA with the effects task, rotation angle, and perspective) and found significant shorter RTs in the LAT than in the OBT (Fig. 2a,b, main effect of task P < 0.001) as well as an interaction of the task and the rotation angle (P < 0.001) with the rotation angle strongly affecting RTs in the OBT (Fig. 2a, main effect of rotation angle, P < 0.001), but not in the LAT (Fig. 2a, P = 0.10). This is probably due to the fact that the participants indeed performed the OBT as requested, which is cognitively more demanding than the LAT and depends on the rotation angle. Note that, for example, for the 0° rotation angle the OBT and the LAT required the same stimulus–response mapping. However, the RTs for 0° rotation were 150 ms longer in the OBT than in the LAT (Fig. 2a), which suggests that the participants performed a mental transformation as compared with the more simple stimulus‐response mapping in the LAT. As predicted, we also found that the perspective affected RTs (Fig. 2b) in the OBT (repeated measures ANOVA with effects rotation angle and perspective, main effect of perspective, P = 0.042), but not in the LAT (P = 0.83; repeated measures ANOVA: tendency for an interaction of task and perspective, P = 0.083). In particular, the RTs in the OBT were faster for the elevated compared with the eye‐level and lowered perspective. All RTs and results of the posthoc paired t‐tests are shown in the Supporting Information Table S1.
Figure 2.
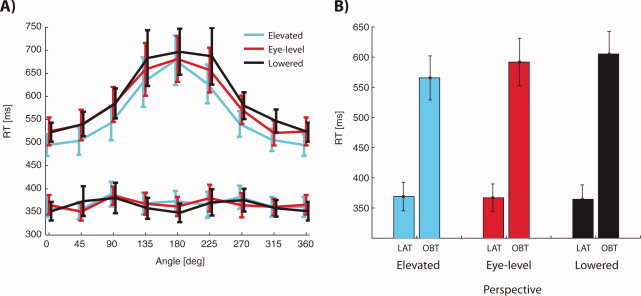
Behavioral results. (a) RTs for the OBT (upper curves) and LAT (lower curves) for the elevated (cyan), eye‐level (red) and lowered (black) perspective. RTs were dependent on the rotation angle in the OBT, but not in the LAT. In the OBT we found a perspective x rotation angle interaction in the OBT, but not in the LAT. All error bars show the standard error of the mean (SEM). (b) When averaged over all angles, RTs were shortest for the elevated perspective in the OBT (566 ± 36 ms, 592 ± 39 ms and 605 ± 37 ms for elevated, eye‐level and lowered perspectives, respectively), but almost identical in the LAT (369 ± 24 ms, 367 ± 23 ms and 364 ± 24 ms). Error bars show the SEM. All RTs and results of posthoc paired t‐tests are shown in the Supporting Information Figure S1.
Stimulus‐Locked EP Mapping: Duration of Brain Activity Related to Own Body Transformations Depends on Rotation Angle
To characterize the neural correlates of mental own body transformations, we segmented the group‐averaged EPs in the OBT and the LAT into stable map topographies (see Material and methods, first segmentation). This yielded two EP maps MapLAT and MapOBT at 350–410 ms, which distinguished between the OBT and LAT conditions. While MapLAT was present in the LAT for all rotation angles, it was present in the OBT only for the less demanding rotation angles 0°–90° (Fig. 3a). The segmentation also revealed that MapOBT was present only in the OBT (Fig. 3a). Statistical analysis showed that the duration of MapOBT—between 350 and 410 ms after stimulus onset—was correlated positively with the rotation angle of the bodily stimulus in the OBT (Fig. 3b, repeated measurements ANOVA with the effects map and rotation angle, interaction of map and rotation angle, P = 0.014). This was not the case in the LAT (P = 0.28) suggesting that MapOBT is likely to reflect brain activity related to mental transformation. The linear inverse solution [LAURA, [Grave de Peralta Menendez et al., 2001, 2004]] showed occipital activations in both MapLAT and MapOBT. Additional activations were seen for MapOBT in right posterior parietal cortex and bilateral frontal cortex (presumably in premotor cortex) and consistent with previous reports [Arzy et al., 2006; Blanke et al., 2005] in inferior parietal cortex extending toward the temporal lobe in the right hemisphere (for the linear inverse solution of MapOBT see Fig. 3b).
Stimulus‐Locked EP Mapping: Brain Activity in Own Body Transformations Depends on the Perspective
We now consider the differences in stimulus‐locked brain activity for the different perspectives in the OBT (where our behavioral analysis revealed perspective‐dependant RT differences). For this, we performed a second segmentation with all 15 (three perspectives × five rotation angles) conditions (see Material and methods, second segmentation). This yielded MapPERS (Fig. 4a, green segment) with its earliest occurrence at 214 ms as well as a MapOBT2 (Fig. 4a, red segment, see below), which first occurred at 330 ms. When using MapPERS and the subsequent map (Fig. 4a, blue segment) in order to describe the EPs between 214 and 330 ms, we found that MapPERS showed a perspective effect depending on the rotation angle (Fig. 4b, significant interaction between perspective and rotation angle; repeated measures ANOVA (rotation angle × perspective, P < 0.01). Thus, MapPERS was shorter for the elevated compared with the other two perspectives for the rotation angles 0°–90° (posthoc paired t‐tests, P < 0.01) but not for the larger rotation angles (130° and 180° rotation; P > 0.1). The linear inverse solution localized MapPERS to the bilateral posterior temporal lobe (Fig. 4b).
Finally, the second segmentation yielded the map MapOBT2 at 330 ms (Fig. 4a, red segment), which was similar to map MapOBT (cf. Fig. 3b) and its duration from 330 to 420 ms also dependent on the rotation angle (interaction of map × rotation angle in a repeated measures ANOVA with the effects perspective, map and rotation angle, P = 0.02, similar to MapOBT for 350–410 ms) but did not distinguish between the perspectives (interaction of map and perspective, P = 0.38). It is worthwhile to notice the appearance and similarity of MapOBT2 with MapOBT in the second segmentation. This is because these data show that the segmentation procedure produces reliable and reproducible results, even when different sets of EPs are compared (segmentation 1 vs. 2) with the clustering procedure. The k‐means segmentation [Pascual‐Marqui et al., 1995] proceeds by grouping similar time frames in the group‐averaged EP into stable segments and aims to minimize the reconstruction error of the original EPs. It is conceivable that MapOBT appeared in the first segmentation of the clustering method due to the fact that the EPs in the OBT were segmented together with the EPs for the control condition (LAT). In particular, the emergence of MapLAT could be due to processes due to the planning or execution of the motor response in the LAT. Accordingly, MapOBT may have emerged due to these motor differences and not related to the mental transformation per se. However, the timing of MapOBT is congruent with many previous EP studies on metal transformations using a large variety of control conditions in EP work [i.e. Arzy et al., 2006] and coincides temporally and anatomically with effects of a transcranial stimulation study [Blanke et al., 2005]. Most importantly, the presence of both maps (MapOBT, MapOBT2) in the time period from 330 to 420 ms (350–410 and 330–420 ms, respectively), the dependence of their durations on the rotation angle, and the appearance of a related map during our response‐locked analysis (see next section), suggest that a process related to the mental transformation, independent of the perspective, takes place during this time period.
Response‐Locked EP Mapping: Duration of Brain Activity Related to Own Body Transformations Depends on Rotation Angle
The differences in RT in the OBT between the 0° and 180° mental transformation conditions (Fig. 2a) are almost an order of magnitude larger than the differences in the duration of the MapOBT or MapOBT2 (Fig. 3b). Thus, the processes reflected by these maps do not fully account for the RT differences between these different rotation angles. Moreover, other maps occurring earlier or later than MapOBT (or MapOBT2) obtained by the first or second segmentation did not show any significant dependence on the rotation angle. This could be due to the fact that the mental own body transformations are carried out in the brain by means of multiple brain areas, also at different latencies, such that the stable map topographies we described at 330–420 ms reflect only the first step in a series of processing steps, which may also involve other cortical structures at different times after stimulus onset such as, for example, the motor cortex [Georgopoulos et al., 1989; Wexler et al., 1998]. It is conceivable that different analysis techniques could reveal such later activations accounting more fully for our participants' behavior.
As a first step toward such an analysis we computed response‐locked EPs (Fig. 5a). As in the first segmentation (see Fig. 3a), we here averaged over the different perspectives and consider the differences in brain activity between the OBT and LAT. This analysis revealed a map, MapOBT‐MOTOR (red segment), that appeared only in the OBT, but not in the LAT. The duration of MapOBT‐MOTOR was dependent on the task and the rotation angle (interaction of map, task, and rotation angle in a repeated measures ANOVA, P = 0.003) and increased in duration only in the OBT with the rotation angle (as did MapOBT, Fig. 5a). MapOBT‐MOTOR appeared ∼150 ms before the response was given, started earliest in conditions with large rotation angles and continued through the motor response. The map topography of MapOBT‐MOTOR showed a pronounced fontal negativity, comparable to MapOBT. However, areas of positive EPs were observed over parietal and central as compared with occipital electrodes (compare Figs. 3b and 5b). MapOBT‐MOTOR was localized to the right posterior parietal cortex (as stimulus‐locked MapOBT) and the inferior parietal cortex (as stimulus‐locked MapOBT, although the right‐sided activation did not extend into the temporal lobe, but towards frontal cortex). There was an additional right midtemporal activation and in comparison to the stimulus‐locked MapOBT, MapOBT‐MOTOR showed less or no occipital and no premotor involvement.
DISCUSSION
We have investigated the behavioral and neurophysiological correlates of mental own body transformation (OBT). First, we were interested in describing the brain activity related to OBT processing in greater detail by testing a large range of rotation angles between the depicted and the subject's body. Second, we were in particular interested in the effects of an elevated perspective on brain activity in such transformations. Reports of spontaneous OBEs [Blanke and Mohr, 2005; Blanke et al., 2004; Brugger et al., 1997; Devinsky et al., 1989] suggest a preference for an elevated perspective in disembodied mental states. Thus we tested if this preference expands to OBT which mentally mimic such states. Extending our previous behavioral and electrical neuroimaging work we find that RTs were dependent on the rotation angle and fastest when an OBT was done from an elevated perspective which was also reflected in stimulus‐locked brain activation.
Mental Own Body Transformations Depend on the Rotation Angle
The time period of stimulus‐locked brain activity associated with the rotation angle during OBT in the time period ≈330–420 ms is compatible with previous EP data for mental transformation of nonbodily objects (300–600 ms, [Pegna et al., 1997; Harris and Miniussi, 2003]), mental transformations of body parts (280–400 ms, [Overney et al., 2005]) and full human bodies (330–450 ms, [Arzy et al., 2006; Blanke et al., 2005]). The present data extend the latter by showing that—when tested with many rotation angles—the duration of this brain activation increases with the rotation angle reflecting the behavioural mental transformation curves. Accordingly, we suggest that brain activity in posterior parietal, temporoparietal and frontal cortex represents aspects of the mental body transformations encoding the rotation angle of the body, independent of the perspective. Our finding of temporoparietal activation is compatible with the results of earlier EP and electrical neuroimaging studies [Arzy et al., 2006, 2007; Blanke et al., 2005] and fMRI [Zacks et al., 1999]. Activations in the present study also included the right posterior parietal cortex, an area, whose implication has been found in most studies testing mental transformations, especially of nonbodily objects [i.e. Harris et al., 2000; Jordan et al., 2001; Podzebenko et al., 2002; Richter et al., 2000]. Finally, frontal activations found in the present study have also been revealed in previous mental transformation studies, especially when using stimuli that depict graspable nonbodily objects or human bodies [Georgopoulos et al., 1989; Kosslyn et al., 1994]. Future EP work will have to show whether some of these activations during this time period reflect activations related to general mental transformation processes (i.e. active for nonbodily objects, body parts, and full human bodies) or to more specialized processes (i.e. only for full human bodies).
Elevation of Perspective is Associated With Activation in Posterior Temporal Cortex
The RT differences in our experiment suggest that own body transformations are facilitated when performed from an elevated perspective. In particular, we found that RTs were fastest in OBTs from an elevated perspective, which was also reflected in stimulus‐locked bilateral posterior temporal activation at 210 ms that was significantly shorter for OBTs from the elevated perspective (as compared with the lowered perspective, but only for less demanding rotation angles). This was even found compared with the eye‐level perspective and thus for the perspective from which we mostly see in everyday life, suggesting that under some conditions brain processing is facilitated for the elevated perspective. This is compatible with a preference for an elevated perspective in disembodied mental states. While the distinction between egocentric (”embodied“) and allocentric (”disembodied“) perspectives has attracted much interest in the context of memory and spatial representation [Burgess, 2006], surprisingly few studies [i.e. Vogeley et al., 2004] have investigated such distinctions in the context of mental imagery. The present data suggest that the elevated perspective (“bird's eye” view), may not only play an important role in spatial navigation, path integration, and map reading [Burgess, 2006; Vidal et al., 2004], but also in own body transformations and the bodily self as well as spatial navigation [Arzy et al., 2006; Blanke et al., 2005; Ruby and Decety, 2001].
Vogeley et al. [ 2004] investigated the effects of an elevated perspective in comparison with an eye‐level perspective or vantage point, but failed to find significant differences in RTs and brain activation as measured by fMRI between both conditions. The RT difference in our study were significant, but of the order of ≈30 ms. Hence, subtle task differences could explain the discrepancy between our behavioral data and those of Vogeley et al. [ 2004]: While we used a body‐related decision, Vogeley et al. [ 2004] used an environment‐related decision (balls as seen from the imagined position and perspective). Moreover an effect of perspective could have been masked since Vogeley et al. [ 2004] averaged over different rotation angels while we have shown that the perspectival effects are strongest for the less demanding rotation angle. In addition, effects of early and task‐independent visual processing related to perspective needs to be considered, especially since the perspectival neural differences were found relatively early (≈210 ms) as compared with the mental transformation processes (≈330 ms). However, since the RTs depend only in the OBT (and not in the LAT) on the perspective it is unlikely that this accounts for the shortened RTs and neural changes for the elevated perspective.
Response‐Locked Data Analysis
We also analyzed the response‐locked EPs in order to use a new methodological approach to further characterize the neural correlates of the mental transformation process in the OBT. We performed such an analysis, because behavioural [Sirigu and Duhamel, 2001; Wexler et al., 1998], brain imaging [Porro et al., 1996] and data from monkey physiology [Georgopoulos et al., 1989] suggest an involvement of motor cortex in the mental transformation of objects or bodily imagery. A methodological motivation for this response‐locked analysis was the fact that the differences in the RTs between the least and most demanding rotation angles were almost a magnitude larger than the differences in the duration of MapOBT. Hence, it is likely that the brain processes reflected by MapOBT may represent only one step in a longer series of processing steps related to the own body transformation. The response‐locked EP mapping revealed a distinct brain activation, MapOBT‐MOTOR, which was only present in the OBT, but not in the LAT and that was correlated with the rotation angle in the same way as MapOBT, i. e., longer duration corresponded to larger rotation angles. This supports the idea that brain activity at ∼330 ms is only an initial step in the mental transformation process (MapOBT), followed by subsequent processing steps (like MapOBT‐MOTOR) ending at the motor response (∼750 ms, Fig. 2a). Since we did not observe differences in brain activity between LAT and OBT before ∼330 ms, but only after ∼330 ms as well as before the button press, we argue that the participants in our experiment were in a mentally embodied state at the beginning of each trial in both tasks, but changed into a mentally disembodied state at ∼330 ms after stimulus onset and remained in this state until the motor response.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supplement Figure 1
Acknowledgements
The Cartool software (http://brainmapping.unige.ch/Cartool.htm) has been programmed by Denis Brunet, from the Functional Brain Mapping Laboratory, Geneva, Switzerland, and is supported by the Center for Biomedical Imaging (CIBM) of Geneva and Lausanne.
REFERENCES
- Allison T,Puce A,McCarthy G ( 2000): Social perception from visual cues: Role of the STS region. Trends Cogn Sci 4: 267–278. [DOI] [PubMed] [Google Scholar]
- Arzy S,Mohr C,Michel CM,Blanke O ( 2007): Duration and not strength of activation in temporo‐parietal cortex positively correlates with schizotypy. Neuroimage 35: 326–333. [DOI] [PubMed] [Google Scholar]
- Arzy S,Thut G,Mohr C,Michel C,Blanke O ( 2006): Neural basis of embodiment: Distinct contributions of temporoparietal junction and extrastriate body area. J Neurosci 26: 8074–8081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanke O,Landis T,Spinelli L,Seeck M ( 2004): Out‐of‐body experience and autoscopy of neurological origin. Brain 127: 243–258. [DOI] [PubMed] [Google Scholar]
- Blanke O,Mohr C ( 2005): Out‐of‐body experience, heautoscopy, and autoscopic hallucination of neurological origin Implications for neurocognitive mechanisms of corporeal awareness and self‐consciousness. Brain Res Brain Res Rev 50: 184–199. [DOI] [PubMed] [Google Scholar]
- Blanke O,Mohr C,Michel C,Pascual‐Leone A,Brugger P,Seeck M,Landis T,Thut G ( 2005): Linking out‐of‐body experience and self processing to mental own‐body imagery at the temporoparietal junction. J Neurosci 25: 550–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugger P,Regard M,Landis T ( 1997): Illusory reduplification of one's own body: Phenomenology and classification of autoscopic phenomena. Cogn Neuropsychiatr 2: 19–38. [DOI] [PubMed] [Google Scholar]
- Corballis MC ( 1997): Mental rotation and the right hemisphere. Brain Lang 57: 100–121. [DOI] [PubMed] [Google Scholar]
- David N,Bewernick BH,Cohen MX,Newen A,Lux S,Fink GR,Shah NJ,Vogeley K ( 2006): Neural representations of self versus other: Visual‐spatial perspective taking and agency in a virtual ball‐tossing game. J Cogn Neurosci 18: 898–910. [DOI] [PubMed] [Google Scholar]
- Desmedt JE,Tomberg C ( 1990): Topographic analysis in brain mapping can be compromised by the average reference. Brain Topogr 3: 35–42. [DOI] [PubMed] [Google Scholar]
- Devinsky O,Feldmann E,Burrowes K,Bromfield E ( 1989): Autoscopic phenomena with seizures. Arch Neurol 46: 1080–1088. [DOI] [PubMed] [Google Scholar]
- Downing PE,Jiang Y,Shuman M,Kanwisher N ( 2001): A cortical area selective for visual processing of the human body. Science 293: 2470–2473. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH,Spence C,Passingham RE ( 2004): That's my hand! Activity in premotor cortex reflects feeling of ownership of a limb. Science 305: 875–877. [DOI] [PubMed] [Google Scholar]
- Fender DH ( 1997): Source localization of brain electrical activity In: Gevins AS, Remond A, editors. Handbook of Electroencephalography and Clinical Neurophysiology, Methods of Analysis of Brain Electrical and Magnetic Signals. Amsterdam: Elsevier; pp 355–399. [Google Scholar]
- Gallagher II ( 2000): Philosophical conceptions of the self: Implications for cognitive science. Trends Cogn Sci 4: 14–21. [DOI] [PubMed] [Google Scholar]
- Gencer NG,Williamson SJ,Gueziec A,Hummel R ( 1996): Optimal reference electrode selection for electric source imaging. Electroencephalogr Clin Neurophysiol 99: 163–173. [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP,Lurito JT,Petrides M,Schwartz AB,Massey JT ( 1989): Mental rotation of the neuronal population vector. Science 243: 234–236. [DOI] [PubMed] [Google Scholar]
- Geselowitz DB ( 1998): The zero of potential. IEEE Eng Med Biol Mag 1: 128–132. [DOI] [PubMed] [Google Scholar]
- Grave de Peralta Menendez R,Gonzalez Andino S,Lantz G,Michel CM,Landis T ( 2001): Noninvasive localization of electromagnetic epileptic activity. I. Method descriptions and simulations. Brain Topogr 14: 131–137. [DOI] [PubMed] [Google Scholar]
- Grave de Peralta Menendez R,Murray MM,Michel CM,Martuzzi R,Gonzalez Andino SL ( 2004): Electrical neuroimaging based on biophysical constraints. Neuroimage 21: 527–539. [DOI] [PubMed] [Google Scholar]
- Harris IM,Egan GF,Sonkkila C,Tochon‐Danguy HJ,Paxinos G,Watson JD ( 2000): Selective right parietal lobe activation during mental rotation: A parametric PET study. Brain 123: 65–73. [DOI] [PubMed] [Google Scholar]
- Harris IM,Miniussi C ( 2003): Parietal lobe contribution to mental rotation demonstrated with rTMS. J Cogn Neurosci 15: 315–323. [DOI] [PubMed] [Google Scholar]
- Jeannerod M ( 2003): The mechanism of self‐recognition in humans. Behav Brain Res 142: 1–15. [DOI] [PubMed] [Google Scholar]
- Jordan K,Heinze HJ,Lutz K,Kanowski M,Jancke L ( 2001): Cortical activations during the mental rotation of different visual objects. Neuroimage 13: 143–152. [DOI] [PubMed] [Google Scholar]
- Junghofer M,Elbert T,Tucker DM,Braun C ( 1999): The polar average reference effect: A bias in estimating the head surface integral in EEG recording. Clin Neurophysiol 110: 1149–1155. [DOI] [PubMed] [Google Scholar]
- Keenan JP,Nelson A,O'Connor M,Pascual‐Leone A ( 2001): Self‐recognition and the right hemisphere. Nature 409: 305. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM,Alpert NM,Thompson WL,Chabris CF,Rauch SL,Anderson AK ( 1994): Identifying objects seen from different viewpoints. A PET investigation. Brain 117: 1055–1071. [DOI] [PubMed] [Google Scholar]
- Lehmann D ( 1987): Principles of spatial analysis In: Gevins AS, Remond A, editors. Handbook of Electroencephalography and Clinical Neurophysiology. Amsterdam: Elsevier; pp 309–354. [Google Scholar]
- Lehmann D,Skrandies W ( 1980): Reference‐free identification of components of checkerboard‐evoked multichannel potential fields. Electroencephalogr Clin Neurophysiol 48: 609–621. [DOI] [PubMed] [Google Scholar]
- Michel C ( 2004): EEG source imaging. Clin Neurophysiol 115: 2195–2222. [DOI] [PubMed] [Google Scholar]
- Michel CM,Thut G,Morand S,Khateb A,Pegna AJ,Grave de Peralta R,Gonzalez S,Seeck M,Landis T ( 2001): Electric source imaging of human brain functions. Brain Res Brain Res Rev 36: 108–118. [DOI] [PubMed] [Google Scholar]
- Murray MM,Brunet D,Michel CM ( 2008): Topographic ERP analyses: A step‐by‐step tutorial review. Brain Topogr 20: 249–264. [DOI] [PubMed] [Google Scholar]
- Nigro G,Neisser U ( 1983): Point of view in personal memories. Cogn Psychol 15: 467–482. [Google Scholar]
- Overney LS,Michel CM,Harris IM,Pegna AJ ( 2005): Cerebral processes in mental transformations of body parts: Recognition prior to rotation. Brain Res Cogn Brain Res 25: 722–734. [DOI] [PubMed] [Google Scholar]
- Parsons L ( 1987): Imagined spatial transformation of one's body. J Exp Psychol Gen 116: 172–191. [DOI] [PubMed] [Google Scholar]
- Pascual‐Marqui RD,Lehmann D ( 1993): Topographic maps, source localization inference, and the reference electrode: Comments on a paper by Desmedt et al. Electroencephalogr Clin Neurophysiol 88: 532–536. [DOI] [PubMed] [Google Scholar]
- Pascual‐Marqui RD,Michel CM,Lehmann D ( 1995): Segmentation of brain electrical activity into microstates: Model estimation and validation. IEEE Trans Biomed Eng 42: 658–665. [DOI] [PubMed] [Google Scholar]
- Pataraia E,Baumgartner C,Lindinger G,Deecke L ( 2002): Magnetoencephalography in presurgical epilepsy evaluation. Neurosurg Rev 25: 141–159; discussion 160–161. [DOI] [PubMed] [Google Scholar]
- Pegna AJ,Khateb A,Spinelli L,Seeck M,Landis T,Michel CM ( 1997): Unraveling the cerebral dynamics of mental imagery. Human Brain Mapp 5: 410–421. [DOI] [PubMed] [Google Scholar]
- Podzebenko K,Egan GF,Watson JD ( 2002): Widespread dorsal stream activation during a parametric mental rotation task, revealed with functional magnetic resonance imaging. Neuroimage 15: 547–558. [DOI] [PubMed] [Google Scholar]
- Porro CA,Francescato MP,Cettolo V,Diamond ME,Baraldi P,Zuiani C,Bazzocchi M,di Prampero PE ( 1996): Primary motor and sensory cortex activation during motor performance and motor imagery: A functional magnetic resonance imaging study. J Neurosci 16: 7688–7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter W,Somorjai R,Summers R,Jarmasz M,Menon RS,Gati JS,Georgopoulos AP,Tegeler C,Ugurbil K,Kim SG ( 2000): Motor area activity during mental rotation studied by time‐resolved single‐trial fMRI. J Cogn Neurosci 12: 310–320. [DOI] [PubMed] [Google Scholar]
- Ruby P,Decety J ( 2001): Effect of subjective perspective taking during simulation of action: A PET investigation of agency. Nat Neurosci 4: 546–550. [DOI] [PubMed] [Google Scholar]
- Shepard RN,Hurwitz S ( 1984): Upward direction, mental rotation, and discrimination of left and right turns in maps. Cognition 18: 161–193. [DOI] [PubMed] [Google Scholar]
- Sirigu A,Duhamel JR ( 2001): Motor and visual imagery as two complementary but neurally dissociable mental processes. J Cogn Neurosci 13: 910–919. [DOI] [PubMed] [Google Scholar]
- Sugiura M,Watanabe J,Maeda Y,Matsue Y,Fukuda H,Kawashima R ( 2005): Cortical mechanisms of visual self‐recognition. Neuroimage 24: 143–149. [DOI] [PubMed] [Google Scholar]
- Thierry G,Pegna AJ,Dodds C,Roberts M,Basan S,Downing P ( 2006): An event‐related potential component sensitive to images of the human body. Neuroimage 32: 871–879. [DOI] [PubMed] [Google Scholar]
- Tomberg C,Noel P,Ozaki I,Desmedt JE ( 1990): Inadequacy of the average reference for the topographic mapping of focal enhancements of brain potentials. Electroencephalogr Clin Neurophysiol 77: 259–265. [DOI] [PubMed] [Google Scholar]
- Urgesi C,Candidi M,Ionta S,Aglioti SM ( 2007): Representation of body identity and body actions in extrastriate body area and ventral premotor cortex. Nat Neurosci 10: 30–31. [DOI] [PubMed] [Google Scholar]
- Vidal M,Amorim MA,Berthoz A ( 2004): Navigating in a virtual three‐dimensional maze: How do egocentric and allocentric reference frames interact? Brain Res Cogn Brain Res 19: 244–258. [DOI] [PubMed] [Google Scholar]
- Vogeley K,Fink GR ( 2003): Neural correlates of the first‐person‐perspective. Trends Cogn Sci 7: 38–42. [DOI] [PubMed] [Google Scholar]
- Vogeley K,May M,Ritzl A,Falkai P,Zilles K,Fink GR ( 2004): Neural correlates of first‐person perspective as one constituent of human self‐consciousness. J Cogn Neurosci 16: 817–827. [DOI] [PubMed] [Google Scholar]
- Wexler M,Kosslyn SM,Berthoz A ( 1998): Motor processes in mental rotation. Cognition 68: 77–94. [DOI] [PubMed] [Google Scholar]
- Wikswo JP Jr,Gevins A,Williamson SJ ( 1993): The future of the EEG and MEG. Electroencephalogr Clin Neurophysiol 87: 1–9. [DOI] [PubMed] [Google Scholar]
- Williamson SJ,Lu ZL,Karron D,Kaufman L ( 1991): Advantages and limitations of magnetic source imaging. Brain Topogr 4: 169–180. [DOI] [PubMed] [Google Scholar]
- Zacks J ( 2002): A parametric study of mental spatial transformations of bodies. Neuroimage 16: 857–872. [DOI] [PubMed] [Google Scholar]
- Zacks J,Rypma B,Gabrieli JD,Tversky B,Glover GH ( 1999): Imagined transformations of bodies: An fMRI investigation. Neuropsychologia 37: 1029–1040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supplement Figure 1


