Abstract
Neurofeedback of functional magnetic resonance imaging (fMRI) can be used to acquire selective control over activation in circumscribed brain areas, potentially inducing behavioral changes, depending on the functional role of the targeted cortical sites. In the present study, we used fMRI‐neurofeedback to train subjects to enhance regional activation in the right inferior frontal gyrus (IFG) to influence speech processing and to modulate language‐related performance. Seven subjects underwent real‐time fMRI‐neurofeedback training and succeeded in achieving voluntary regulation of their right Brodmann's area (BA) 45. To examine short‐term behavioral impact, two linguistic tasks were carried out immediately before and after the training. A significant improvement of accuracy was observed for the identification of emotional prosodic intonations but not for syntactic processing. This evidence supports a role for the right IFG in the processing of emotional information and evaluation of affective salience. The present study confirms the efficacy of fMRI‐biofeedback for noninvasive self‐regulation of circumscribed brain activity. Hum Brain Mapp 2009. © 2008 Wiley‐Liss, Inc.
Keywords: operant conditioning, self‐regulation, real‐time functional magnetic resonance imaging, right inferior frontal gyrus, prosody
INTRODUCTION
Recently, neurofeedback based on functional magnetic resonance imaging (fMRI) has emerged as a promising paradigm in cognitive neuroscience (Sitaram et al., 2007; Weiskopf et al., 2007). Since the 1960s, researchers have applied biofeedback techniques using electrophysiological recordings (EEG). Evidence demonstrating that humans can achieve control over different components of the EEG using operant conditioning paradigms has been collected, and a number of clinical applications reported (Birbaumer et al., 1999; Egner and Gruzelier, 2003; Hammond, 2005; Moore, 2000; Pop‐Jordanova et al., 2005; Schenk et al., 2005; Scott et al., 2005; Thompson and Thompson, 1998). For instance, self‐regulation of slow cortical potentials (SCPs) has been used to suppress epileptic seizures (Kotchoubey et al., 2001) and has been effectively applied to treat clinical impairments such as the attention deficit‐hyperactivity disorder (Fox et al., 2005; Fuchs et al., 2003; Strehl et al., 2006).
By means of fMRI‐biofeedback, humans can learn to control changes of their blood oxygen level‐dependent (BOLD) signal in circumscribed brain areas (Caria et al., 2007; Posse et al., 2003; Weiskopf et al., 2003; Yoo and Jolesz, 2002). This technique is promising as it could be employed to investigate the functional role of brain areas of interest and to induce desirable behavioral effects associated with the controlled cortical sites (Weiskopf et al., 2004). While numerous pieces of evidence have been collected on the applicability and efficacy of EEG‐biofeedback, up to now only little research has been conducted using fMRI (for a review, see Birbaumer, 2006). Nevertheless, it has been shown that it is possible to construct systems that convey individual mental strategies into commands to operate computers or electromechanical hardware. Yoo and co‐workers (2004) developed a brain‐computer interface (BCI) based on fMRI, which allowed subjects to navigate through a 2D maze, solely by their thought processes.
In the present study, we employed fMRI‐neurofeedback to investigate whether healthy persons can learn to self‐regulate the BOLD response in the right IFG (BA 45) and whether a voluntary increase of the signal in this region would modulate specific aspects of language processing.
Evidence has been collected in favor of a central role of Broca's area in syntactic and semantic processing (Friederici and Kotz, 2003; Heim, 2005). While the significance of the left IFG has been highlighted by numerous studies on language processing, the role of its contralateral sites still needs to be clarified. Clinical evidence has suggested that impairments of the left fronto‐temporal network might induce contralateral areas to take over functions previously carried out by the homologues left‐sided brain structures (Chapman et al., 2003; Fernandez et al., 2004; Thulborn et al., 1999; Weiller et al., 1995). Some studies have indicated the recruitment of the right hemisphere during processing of emotional prosody (Friederici and Alter, 2004; Kotz et al., 2003).
Intrigued by these findings, and encouraged by results previously reported (Caria et al., 2007; Weiskopf et al., 2004), we used fMRI‐neurofeedback to train subjects to control the level of activation recorded in the right IFG. We instructed the subjects to up‐regulate this brain region while identifying emotional prosody and performing grammaticality judgments immediately before and after the learning‐phase (i.e., the feedback training). We tested short‐term behavioral effects of up‐regulation upon prosodic and syntactic processing by comparing the accuracy levels and reaction times pre‐ and post‐training.
On the basis of previous evidence (Friederici and Alter, 2004; Kotz et al., 2003), we hypothesized that an improvement of performance would be observed in verbal‐emotional but not in syntactic processing.
The following two hypotheses were tested:
-
1
By means of real‐time fMRI training, participants can learn to control the level of activation in their right IFG and voluntarily regulate it.
-
2
Successful up‐regulation of this brain site facilitates the identification of verbal affective information.
MATERIALS AND METHODS
Participants
Twelve matched males, German native speakers (aged 24–30 years, mean age = 27), right handed according to the Edinburgh Handedness Inventory (Oldfield, 1971), participated in this study. Seven of them were trained to acquire control over the BOLD signal in the right IFG by means of real‐time fMRI feedback. The remaining five were recruited as control subjects. They received the same instructions and underwent the same training as the experimental group, but they could rely on sham‐feedback information only. None of the subjects had a neurological or psychiatric history, hearing impairment, or was on medication. Each of them had normal or corrected‐to‐normal vision. Each participant gave written informed consent and was paid for his participation in the experiment. This study was approved by the Ethics Committee of the Medical Faculty of the University of Tübingen, Germany.
Experimental Procedure and Data Analysis
The fMRI‐neurofeedback system employed was based on a 3‐T whole body scanner with a standard 8‐channel head coil (TIM Trio, Siemens, Erlangen, Germany in combination with the Turbo Brain Voyager software Brain Innovation, Maastricht, The Netherlands; Goebel, 2001) and in‐house written scripts running on Matlab 6.5 (The Math Works, Natick, MA, USA). During the experiment, participants were lying supine in the scanner. To minimize movement, their head was fixed within the head coil by means of foam rubbers. Echo‐planar images (EPIs) sensitive to the BOLD response covering 16 axial slices were acquired using an echo‐planar imaging sequence (EPI; TR = 1.5 s, 64 × 64 matrix size, voxel size = 3.3 × 3.3 × 5 mm3, slice gap = 1 mm, effective echo time (TE) = 30 ms, flip angle = 70°, bandwidth = 1.954 kHz/pixel, slice thickness = 5 mm). The first 10 volumes of each session were excluded from statistical analysis to allow for T1‐equilibration effects. A T1‐weighted structural scan of the whole brain was obtained for each subject and served as anatomical reference (MPRage, matrix size = 256 × 256, 160 partitions, 1 mm3 isotropic voxels, TR = 2300 ms, TE = 3.93 ms, flip angle = 8°).
We chose the pars triangularis of the right IFG as the target region of interest (ROI target) for the experimental group and selected it individually by means of a functional localizer run (details are given in the following section). The ROI target was selected as a square region (6 × 6 voxels, ∼20 × 20 mm) centered on the area of activation. Offline statistical analysis indicated that the ROI placement was accurate and was reproduced across training sessions within subjects with 1–2 voxels of displacement (mean error 1.3 ± 0.4 voxels). The mean location across subjects in MNI (Montreal Neurological Institute templates; Collins et al., 1994) coordinates for the center of the selected ROI target was x = 51, y = 18, z = 6. We used a large reference slice (ROI control) as control area to cancel out global changes of activity and effects due to task‐unspecific activation. During training, the mean BOLD signal was extracted from the ROIs. The differential BOLD response [(ROItarget − ROIcontrol) activation blocks − (ROI target − ROI control) baseline blocks] was transformed into the visual feedback of varying graduations of a thermometer (see Fig. 1) and presented in real‐time to the subjects with video projection.
Figure 1.
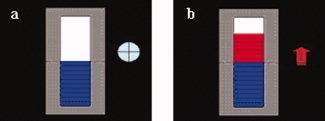
An example of visual feedback displayed to the subjects during training. (a) Feedback provided during baseline blocks. The beginning of each baseline block was signaled by the symbol “+.” (b) Feedback presented during activation blocks. The beginning of each activation block was signaled by the symbol “↑.” [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
As the time interval between the acquisition of new fMRI data and the presentation of its feedback was sufficiently short (about 1.5 s), subjects could monitor in real‐time the efficacy of their self‐regulation. At the end of each training session, subjects were verbally asked to report the cognitive strategies used for both activation and baseline blocks.
The functional data were processed in real‐time with the Turbo Brain Voyager software (Brain Innovation, Maastricht, The Netherlands; Goebel, 2001). Pre‐processing of the EPI data included 3D motion correction and drift removal (for a detailed description of the neurofeedback system in use in the present study, please refer to Weiskopf et al., 2003). Offline data analysis was performed using SPM2 (Wellcome Trust Centre for Neuroimaging, Queens Square, London, UK) within the framework of the General Linear Model (GLM; Friston et al., 1995). Brain Voyager QX 1.9 (Brain Innovation, Maastricht, The Netherlands) was used for ROI analysis. Pre‐processing comprised motion correction and normalization of the EPIs using the MNI templates provided by the Montreal Neurological Institute. The normalized functional images were smoothed with a Gaussian kernel with a full width at half maximum (FWHM) of 12 mm. First level fixed‐effects analyses were computed for every subject. Hemodynamic response amplitudes were estimated using standard regressors, constructed by convolving a boxcar function representing the block duration, with a canonical hemodynamic response function using standard SPM2 parameters. Covariates derived from motion parameters were included in the GLM (Friston et al., 1996) to suppress potential motor artefacts. A group fixed‐effects analysis was performed on all the sessions separately for both experimental and control groups (N = 7 and N = 5, FWE corrected, P < 0.001). Moreover, the increase of signal during activation blocks versus baseline blocks was analyzed for the ROI target by means of a group random effects analysis (ROI analysis, ROI = right BA 45, N = 7, P< 0.05, FDR corrected) performed session by session on the experimental group. Learning effects were investigated by testing whether the BOLD signal increased in the ROItarget across sessions. For both groups, sessions were numbered consecutively (i.e., from 1 to 4), and the session numbers were correlated with the percentage of signal change detected in the ROI target using a linear regression analysis. Moreover, training‐related effects were evaluated by computing an ANOVA for repeated measures for the percentage of signal change in the ROI target across sessions for both groups.
The whole experiment consisted of three types of sessions: one localizer session (refer to the Functional Localizer section) preceding the first training session, four training sessions (refer to the Neurofeedback training section), and two behavioral test sessions (refer to the Behavioral tasks section). Evaluation of attentional skills and assessment of subjective emotional states were measured with the d2 Test (Brickenkamp, 1994) and the Positive and Negative Affect Schedule (PANAS, Watson et al., 1988).
The Functional Localizer
The participants performed a localizer run before the beginning of the training. A precise localization of the ROItarget is crucial to provide subjects with effective neurofeedback‐training. To rely on both anatomical and functional references (Weiskopf et al., 2004), we used a linguistic task that reliably activates the pars triangularis of the right hemisphere (Dogil et al., 2004). This prosody‐unrelated task allowed us to avoid the risk of a facilitation effect, which might have been induced by performing a similar or strictly related task during both the localizer and the performance‐evaluation phases. The localizer session consisted of four activation blocks, separated by five baseline blocks, beginning and ending with a baseline block. Every block lasted for 50 s. The stimuli consisted of 20 balanced German sentences with three syntactic constituents presented visually by means of video projection. During each activation block, 10 sentences (5 s each) were presented without an interstimuli interval. A total of 40 sentences were presented for the whole localizer session (total length of the session = 7.5 min). The subjects were instructed to read each sentence silently, to manipulate its word order and to replace the subject noun phrase (NP) with a hyperonym (for details on this task, see Dogil et al., 2004). This task takes advantage of the fact that German grammar allows for a fairly broad choice with respect to the syntactic constituent that should be followed by the finite verb (Dogil et al., 2004). We instructed the subjects to start each sentence by replacing the initial constituent of the string. An example of this sentence transformation is illustrated below:
Der Schriftsteller wurde über seinen Roman befragt
“The writer was about his novel questioned.”
The writer was questioned about his novel
Substitution
Über seinen Roman wurde der Schriftsteller befragt
“About his novel was the writer questioned.”
Neurofeedback‐Training
The training consisted of four sessions, each of which encompassed six activation blocks (50 s each) separated by five baseline blocks (30 s each), beginning and ending with a baseline block (length of each session = 8.5 min). During activation blocks, the subjects had to increase the level of activation in the right IFG (BA 45). During baseline blocks, they were instructed to relax by performing mental imagery (e.g., imagining themselves being on a beach with a vast expanse of blue water). For both types of blocks, experimental subjects received feedback of the differential BOLD signal detected in the ROI target (BOLD signal in the ROI target minus BOLD signal in the ROI control). Control subjects received sham feedback based on signals taken from other brain areas. Areas that were used to extract sham‐feedback included the parahippocampal place area (PPA) and the posterior cingulate cortex. The symbols “↑” and “+” were presented on the left side of the thermometer to signal the beginning of each activation or baseline block, respectively (see Fig. 1). The same written information explaining the methodology of fMRI‐neurofeedback, with examples of cognitive strategies was provided to experimental and control subjects. Subjects were instructed to monitor online changes of the visual feedback and learn to acquire control over the signal, increasing the level of activation displayed by the thermometer during activation blocks. For the experimental group, achieving this goal implied finding suitable mental strategies that could reliably increase the level of activation in the right pars triangularis.
Behavioral Tasks
Stimuli belonging to two behavioral tasks were divided into two balanced sets (A and B), each of which was performed either before or after the training, in a random but balanced way across subjects. For half of the subjects, the order A‐B was adopted and for the other half the order B‐A. At the end of the training‐phase, subjects were instructed to focus on the cognitive strategies previously successfully adopted and to continuously up‐regulate BA 45 while carrying out the two tasks. No feedback on brain self‐regulation was provided to the subject in this phase.
Identification of emotional prosody was used to test short‐term training effect on prosodic processing. This task consisted of four sets of German sentences belonging to the Tübingen Affect Battery (Breitenstein, 1996), describing happy, sad, angry, or neutral scenarios, for a total of 16 samples. Each sentence was read by a professional actress with sad, happy, angry, and neutral emotional intonations, producing a total of 64 samples. The strings lasted 2 s and were randomly presented. The participants listened to a sentence at once and were instructed to identify its emotional intonation in the shortest time possible, within a 4‐s limit. Prosody judgments were performed by button pressing (i.e., selecting from a four‐button device the key corresponding to the intended emotional intonation). A fixed inter‐trial interval of 12 s was chosen. At the end of the session, subjects were debriefed, and their ability to hear and understand the auditory presented stimuli was confirmed.
Speeded grammaticality judgments were used to test short‐term training effects on syntactic processing. The stimuli consisted of 48 balanced German sentences belonging to three sets: ambiguous, incorrect, and correct sentences. The sets of sentences were approximately the same length and were randomly presented. To control for reading strategies, the strings were provided to the subjects in a segmented and successive manner, and each word was presented visually for 400 ms. The subjects could view just one word at a time and were instructed to perform grammaticality judgments (Meng and Bader, 2000). The critical segment for deciding about grammatical correctness always appeared at the end of each string. After the presentation of the last segment, a question mark signaled to the subjects that they should judge the correctness of the preceding sentence. The judgment task was carried out by button pressing (i.e., right button for correct and left button for incorrect sentences). Subjects were instructed to carry out grammaticality judgment in the shortest time possible, within a 4‐s limit. An intertrial interval of 12 s was chosen.
A previous fMRI investigation indicated that the identification of emotional prosody, as required by the task here adopted, relies on the activation of a bilateral cortical network that includes the right IFG (Rota et al., 2008). On the other hand, no involvement of this brain region was observed for the grammaticality judgment task (Rota, unpublished doctoral dissertation). The tasks were matched for difficulty (Rota, unpublished doctoral dissertation). The mean expected accuracy for the prosody identification task was 73% ± 9% (mean accuracy ± SD); the mean reaction times were 2966.3 ± 353.8 ms (mean RT ± SD), and for the grammaticality judgment task 70% ± 10% (mean accuracy ± SD) and 1307.8 ± 435 ms (mean RT ± SD).
For each task, we measured levels of accuracy and reaction times pre‐ and post‐training. Stimulus presentation and collection of responses were carried out using the E‐prime 1.1 software (Schneider et al., 2002). Statistical analysis of the behavioral data was performed with the statistical package SPSS 14.0 (SPSS Inc., Chicago, IL).
RESULTS
All experimental subjects achieved voluntary differential up‐regulation of the activation‐level recorded in the ROI target, as requested by the task. A progressive increase of the level of activation in the right BA 45 (mean center of activation in MNI coordinates = 51, 18, 6) across training sessions suggests a learning effect (r = 0.98; N = 4 sessions, P = 0.01 one‐tailed; linear regression, see Figs. 2 and 3). A similar but not significant trend was observed for the control group (r = 0.82; N = 4 sessions; P = 0.08 one‐tailed, linear regression).
Figure 2.
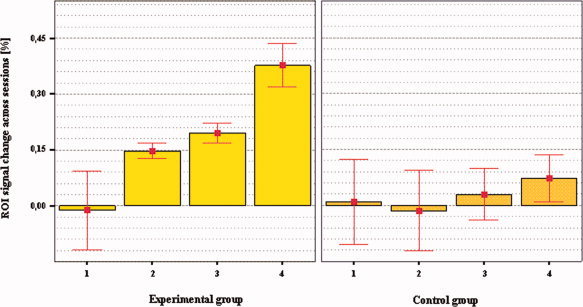
Percentage of signal change recorded in the right IFG (MNI = 51, 18, 6) across training sessions for experimental and control groups. Bars show means and error bars indicate mean ± 1.0 standard error (SE). A significant increase of the BOLD signal in the right IFG (r = 0.98, N = 4 training sessions, P = 0.01 one‐tailed, regression analysis) across sessions was detected for the experimental group only and suggests a learning effect. Differential activation in the ROI target for session 4 versus session 1 was observed for the experimental group only (two‐tailed paired t test, N = 7, P < 0.05). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Figure 3.
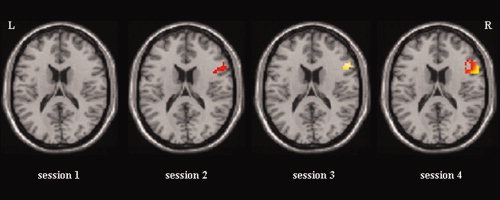
Cortical activation maps of the learning effect obtained by the experimental group across training sessions. Statistical parametric maps are based on a random effects analysis, N = 7, P < 0.05 (uncorrected for visualization). ROI target activation maps are displayed on a single subject T1 template (MNI coordinate z = 18) using a spatial mask (mask = right BA 45) constructed with the WFU‐PickAltas (Maldjian et al., 2004, 2003) implemented in SPM2. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Increased differential activation (calculated for up‐regulation blocks minus rest) in the ROI target across sessions was observed for the experimental group [repeated measures ANOVA, F(3,18) = 6.379, P = 0.004] but not for the control group [repeated measures ANOVA, F(3,12) = 0.216, P = 0.883]. For the experimental group only, fixed‐effects analyses showed an increased level of activation from session 1 (N = 7, FWE corrected, no activation observed, P < 0.001) to session 4 (N = 7, FWE corrected, t = 6.96, P < 0.001). Random effects analyses on the experimental group confirmed the results of the fixed‐effects analyses by showing an increased level of activation for the ROI target from session 1 (ROI analysis, ROI = right BA 45, N = 7, no activation observed, P < 0.05, FDR corrected) to session 4 (ROI analysis, N = 7, t = 3.43, P < 0.05, FDR corrected). A stable activation across sessions was detected in the large reference area for both groups [(experimental group: y = 0.04 (mean ± 0.04% SD of signal‐change across sessions); control group: y = 0.01 (mean ± 0.15% SD of signal‐change across sessions)].
Besides the ROI target (see Fig. 3), a number of coactivated brain loci were observed (N = 7, random effect analysis, P < 0.03, uncorrected). A small activation cluster (voxels = 6) was found on the left Rolandic operculum (t = 2.59, MNI = −42, 6, 15) during the first training session. The insula was activated bilaterally (BA 13, t = 7.05, MNI = 33, 12, 12 and t = 3.69, MNI = −33, 12, 12) during the second session. The superior frontal gyrus (BA 9, t = 4.29, MNI = 57, 18, 27), the supplementary motor area (BA 6, t = 2.33, MNI = −48, 0, 39), and the Putamen (t = 3.04, MNI = 21, 3, −3) were activated during the third session. The supplementary motor area (BA 6), the insula bilaterally, the left superior temporal gyrus (BA 22), the left middle frontal gyrus (BA 9), the anterior cingulate cortex (ACC, BA 32), and the cerebellum were activated during the last training session (Table I).
Table I.
Activation sites under voluntary control as effect of the training process
| Brain site, Brodmann's area | Coordinates, MNI | z value |
|---|---|---|
| IFG (BA 45), R | 51, 18, 6 | 2.87 |
| SMA (BA 6), L | −42, −6, 42 | 4.52 |
| SMA (BA 6), R | 57,−3, 42 | 3.95 |
| MFG, (BA 9), L | −51, 9, 30 | 3.59 |
| ACC (BA 32), R | 3, 21, 42 | 5.06 |
| Cerebellum, L | −6, −51, −33 | 4.49 |
| STG (BA 22), L | −60, −39, 2 | 3.39 |
| Insula, L (BA 13) | −42, 12, 3 | 7.27 |
| Insula, R | 45, 6, 4 | 3.83 |
The table shows brain sites differentially activated during the last session as a result of the real‐time BOLD training. We listed for each brain site the corresponding Brodmann's areas and MNI coordinates. The table depicts z values for each activated brain site observed (random effect analysis over the last session of training, N = 7, P < 0.03, uncorrected). We used the WFU PickAtlas (Tzourio‐Mazoyer et al., 2002) implemented in SPM to perform anatomical labelling of brain activations.
L, left hemisphere; R, right hemisphere; BA, Brodmann's area; IFG, inferior frontal gyrus; ACC, anterior cingulate cortex; SMA, supplementary motor area; MFG, medial frontal gyrus; STG, superior temporal gyrus.
Each of these brain sites was checked for a monotonic increase in activation along sessions of training. For none of these clusters, a significant trend was observed. The absence of a clear monotonic increase of BOLD signal across sessions supports the hypothesis that activation in these areas does not reflect a training‐related effect.
When debriefed, subjects reported strategies connected to speech, such as imagination of lecturing before a class of students, arguing scenes and debates. Other strategies included imagined singing, imagined recitation of poems, and recalling old conversations with friends.
Experimental subjects correctly identified 70% ± 13% (mean ± SD) of the affective prosodic stimuli before the beginning of the training and 84% ± 15% (mean ± SD) after it. Statistical analysis showed a significant difference between the two sets of scores (two‐sided Wilcoxon signed‐rank test, P = 0.01). Pre‐training RTs were 3,455.7 ± 317.2 ms (mean ± SD), and post‐training RTs were 2,926.3 ± 548.5 ms (mean ± SD). Statistical analyses showed no significant difference between the two sets of scores (tested for normality, Shapiro‐Wilks test, two‐sided paired t test, P = 0.05). With respect to the grammaticality judgment task, experimental subjects achieved 49% ± 26% (mean ± SD) of accuracy before the training and 41% ± 29% (mean ± SD) after it. Statistical analysis revealed no significant difference between the two levels of accuracy (two‐sided Wilcoxon signed‐rank test, P = 0.55). Pre‐training RTs were 1547.6 ± 357.4 ms (mean ± SD), and post‐training RTs were 1186.7 ± 558.9 ms (mean ± SD). Statistical analyses showed no significant difference between the two sets of scores (tested for normality, Shapiro‐Wilks test, two‐sided paired t test, P = 0.08). For a summary of the results obtained by the experimental subjects, see Figure 4.
Figure 4.
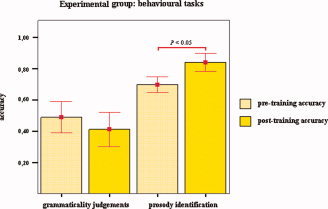
Up‐regulation of the right IFG: behavioral effects for the experimental group. Mean levels of accuracy for grammaticality judgments and prosody identification preceding and following BOLD‐feedback training are shown. A significant improvement was observed for detection of affective intonations only (two‐sided Wilcoxon signed‐rank test, P < 0.05). Bars show means accuracy levels, and error bars indicate mean ± 1.0 SE.
Control subjects identified 74% ± 14% (mean ± SD) of the affective prosodic stimuli before the beginning of the training, and 78% ± 16% (mean ± SD) after it. No significant difference between the two sets of scores was found (two‐sided Wilcoxon signed‐rank test, P = 0.71). Pre‐training RTs were 3613.5 ± 282.2 ms (mean ± SD) and post‐training RTs were 3280 ± 541.2 ms (mean ± SD). Statistical analyses showed no significant difference between the two sets of scores (tested for normality, Shapiro‐Wilks test, two‐sided paired t test, P = 0.15). With respect to the grammaticality judgment task, control subjects correctly identified 66% ± 10% (mean ± SD) of the stimuli before the training and 56% ± 21% (mean ± SD) of the stimuli after the training. Statistical analysis revealed no significant difference between the two sets of scores (two‐sided Wilcoxon signed‐rank test, P = 1). Pre‐training RTs were 1410.1 ± 526.1 ms (mean ± SD) and post‐training RTs were 1057.3 ± 623.8 ms (mean ± SD). No significant difference between the two sets of scores was observed (tested for normality, Shapiro‐Wilks test, two‐sided paired t test, P = 0.26). Details are given in Figure 5.
Figure 5.
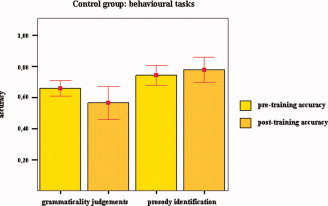
Up‐regulation of the right BA 45: behavioral effects for the control group. Mean levels of accuracy for grammaticality judgments and identification of prosodic intonations preceding and following BOLD‐feedback training are shown. No significant difference was observed for grammaticality judgments (two‐sided Wilcoxon signed‐rank test, P = 1) and for identification of affective prosody (two‐sided Wilcoxon signed‐rank test, P = 0.71). Bars show levels of means accuracy, and error bars mean ± 1.0 SE.
DISCUSSION
In the present study, we tested the hypothesis that humans can learn to volitionally increase the BOLD responses locally in the pars triangularis of the right IFG. In all experimental subjects, the level of activation restricted to the right BA 45 increased across training sessions. This result indicates a progressive learning effect and suggests that real‐time fMRI‐biofeedback is an effective tool for noninvasively manipulating localized brain activity. Even though both experimental and control subjects were provided with the same strategy guidelines, their efficacy in up‐regulating the ROI target greatly differed. The unavailability of genuine feedback information made it impossible for the control group to evaluate the efficacy of the ongoing regulation process, thus presumably impeding the learning process.
The comparison of performances in the two tasks pre‐ and post‐training suggests that up‐regulation of BA 45 correlates to a specific improvement in detecting and identifying emotional prosodic intonations. This effect was not observed for the control group. The findings of the present study suggest that the right IFG plays an important function in mediating the understanding of a speaker's emotional state and intentions.
So far, the role played by the right hemisphere, and specifically by the right BA 45, with respect to language processing has been marginally explored. The predominance of the left hemisphere for a multitude of linguistic aspects has been highlighted by numerous clinical studies, starting with the pioneering works by Broca and Wernicke. Further empirical evidence has been accumulated as neuroimaging tools have been developed (for a review, see Démonet et al., 2005). However, the puzzle posed by the right hemisphere is far from being solved, and conclusions about hemispheric specializations and their role for the processing of distinct linguistic components are far from being drawn.
Important insights have been provided by a number of clinical neurolinguistic studies. Musso and co‐workers (1999) used Positron Emission Tomography (PET) to investigate short‐term cortical changes correlated to recovery from Wernicke's aphasia. A functional reorganization of the brain network and the recruitment of contralateral brain sites were described as key factors for the restoration of lost speech abilities.
Real‐time fMRI feedback offers the possibility of exploring whether the language system can be separately modulated and whether behavioral domain‐specific effects are triggered by this cognitive control. To our knowledge, our study is the first to address these important topics by using BOLD biofeedback.
Diverging conceptualizations exist with respect to the lateralization of prosodic processing in the brain (Gandour, 2000). Coexistence of multiple phonetic cues (such as duration and pitch) and disparate functions (affective, linguistic, etc.) for the speech signal makes it arduous to isolate the neuronal substrate of speech prosody and might be the source of contradictory evidence about its brain mapping. A widely supported conceptualization highlights the dominant engagement of the right hemisphere in assessing emotional prosody regardless of valence (Dogil, 2003; Wildgruber et al., 2005). In a carefully designed experiment, Gandour and colleagues, (2003) dissociated the neural circuits responsible for the processing of emotional and linguistic components of prosodic speech. The researchers took advantage of the fact that in Chinese, both intonation (either statement or question) and emotional cues can be signaled through prosody. Gandour and colleagues reported the exclusive recruitment of prefrontal regions (including the IFG) of the right hemisphere during the processing of emotional prosody. Ross (1981) examined patients affected by focal right hemisphere damage, whose ability to comprehend affective prosody was impaired, and formalized the concept of aprosodic syndromes. Consistent with these observations, further clinical studies have suggested that individuals affected by right hemisphere lesions show “a more pervasive insensitivity to emotive features of prosodic stimuli,” (Pell, 2006) and a reduced decoding capacity for emotional aspects of prosody (Pell, 2007). Winhuisen and colleagues (2005) conducted an experiment on post‐stroke aphasic patients and utilized repetitive Transcranial Magnetic Stimulation (rTMS) to selectively suppress left and right IFG activations during a speech task. By analyzing the patients' responses to both conditions, the authors concluded that for some of the poststroke aphasics examined right IFG activation is essential to carry out residual language functions.
The findings of our experiment support this evidence and are consistent with a number of studies that indicate the involvement of the right IFG in the processing of prosodic features for auditorily presented stimuli (Friederici and Alter, 2004; Kotz et al., 2003). Following these lines, BOLD‐biofeedback training could potentially be used to stimulate and strengthen linguistic abilities connected to emotional processing.
The possibility of facilitating the processing of emotional speech appears to be of particular interest for a number of clinical applications. As reported by Hoekert and colleagues (2007), deficits in the processing of emotional cues conveyed by speech are among the most pervasive disturbances in psychosis. Clinical evidence suggests that schizophrenia correlates with an abnormally reduced cerebral blood volume (Brambilla et al., 2007), and hypofunctioning of the prefrontal cortices (Snitz et al., 2005). Interestingly, the recognition of emotion from prosodic stimuli is also compromised in patient suffering from major depression. Depressed patients are biased in their interpretation of neutral emotions (for both prosody and faces) and tend to attribute a negative valence to them (Kan et al., 2004). de Asis and colleagues (2001) found hypoactivation in hippocampal regions and in the dorsal ACC for patients with unipolar mood disorder. Pieces of evidence have suggested that a reduced blood flow, particularly in the prefrontal regions, could be the source of executive impairments in depression (Fossati et al., 2002; Schlosser et al., 2007).
Presumably, these brain sites could be targeted as loci of self‐regulation, and neurofeedback aids could be employed to normalize hypofunctioning cortical networks. To what extent real‐time fMRI‐biofeedback might benefit the treatment of clinical disorders such as schizophrenia and depression and facilitate the improvement of cognitive and/or behavioral performances in healthy subjects is an important topic of investigation that needs to be clarified by future research.
The comparison of the performances in the two tasks pre‐ and post‐training showed no significant differences for syntactic processing. We registered low accuracy levels for grammaticality judgments for all subjects.
Dual task processing (i.e., simultaneous self‐regulation and performance of a behavioral task) might influence negatively behavioral performances. Weiskopf and colleagues (2004) documented this phenomenon as they investigated the effects of PPA up‐regulation in a memory task. The subjects were trained to differentially up‐regulate the PPA and downregulate the SMA and vice versa. There was a strong interference effect during PPA‐up/SMA‐down but not during SMA‐up/PPA‐down. Contrary to the expectations, the increase of activation in the PPA decreased the performances during a memory task. On the other hand, no interference was observed during the SMA up‐regulation (i.e., the strategies used to up‐regulate the SMA, like motor imagery, were not in conflict with the encoding of information).
The grammatical task used in our experiment required a high working‐memory load: the sentences were presented in a fragmented and successive manner, and subjects needed to remember each word (for a total of ∼14 words) to judge grammaticality. Subjects engaged in the up‐regulation and simultaneously performed this task before and after training. Most subjects used strategies for up‐regulation closely connected to speech and language processing. Presumably, this grammatical task was too resource‐demanding to be performed together with regulation. The results of this task do not allow us to draw any conclusions with respect to the effects of the IFG up‐regulation upon syntactic processing. However, they provide important information for future research with fMRI‐neurofeedback. The present data suggest that while testing behavioral effects of brain regulation, particular effort should be placed on choosing tasks of low complexity (i.e., that require low working‐memory and/or attentional load) to avoid possible interference effects. Testing behavioral performances of regulation during separate sessions should be considered as an alternative option.
CONCLUSIONS
The present study indicated that by using BOLD‐neurofeedback, subjects can achieve voluntary control over activation in the right IFG. A correlation between its exerted local up‐regulation and the improvement of performance during the detection of emotional intonations is suggested.
Acknowledgements
We are indebted to an anonymous reviewer for his comments on the previous version of this manuscript.
REFERENCES
- Birbaumer N ( 2006): Breaking the silence: Brain‐computer interfaces (BCI) for communication and motor control. Psychophysiology 43: 517–532. [DOI] [PubMed] [Google Scholar]
- Birbaumer N,Ghanayim N,Hinterberger T,Iversen I,Kotchoubey B,Kubler A,Perelmouter J,Taub E,Flor H ( 1999): A spelling device for the paralysed. Nature 398: 297–298. [DOI] [PubMed] [Google Scholar]
- Brambilla P,Cerini R,Fabene PF,Andreone N,Rambaldelli G,Farace P,Versace A,Perlini C,Polizza L,Gasparini A,Gatti R,Bellini M,Dusi N,Barbui C,Nose M,Tournikioti K,Sbarbati A,Tansella M ( 2007): Assessment of cerebral blood volume in schizophrenia: A magnetic resonance imaging study. J Psychiatr Res 6: 502–510. [DOI] [PubMed] [Google Scholar]
- Breitenstein C,Daum I,Ackermann H,Lütgehetmann R,Müller E ( 1996): Erfassung der Emotionswahrnehmung bei zentralnervösen Läsionen und Erkrankungen: Psychometrische Gütekriterien der “Tübinger Affekt Batterie”. Neurol Rehab 2: 93–101. [Google Scholar]
- Brickenkamp R ( 1994): Aufmerksamkeits‐Belastungstest d2. Göttingen Hogrefe.
- Caria A,Veit R,Sitaram R,Lotze M,Weiskopf N,Grodd W,Birbaumer N ( 2007): Regulation of anterior insular cortex activity using real‐time fMRI. Neuroimage 15: 1238–1246. [DOI] [PubMed] [Google Scholar]
- Chapman SB,Max JE,Gamino JF,McGlothlin JH,Cliff SN ( 2003): Discourse plasticity in children after stroke: Age at injury and lesion effects. Pediatr Neurol 29: 34–41. [DOI] [PubMed] [Google Scholar]
- Collins DL,Neelin P,Peters TM,Evans AC ( 1994): Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr 18: 192–205. [PubMed] [Google Scholar]
- de Asis JM,Stern E,Alexopoulos GS,Pan H,Van Gorp W,Blumberg H,Kalayam B,Eidelberg D,Kiosses D,Silbersweig DA ( 2001): Hippocampal and anterior cingulate activation deficits in patients with geriatric depression. Am J Psychiatry 158: 1321–1323. [DOI] [PubMed] [Google Scholar]
- Démonet JF,Thierry G,Cardebat D ( 2005): Renewal of the neurophysiology of language: Functional neuroimaging. Physiol Rev 85: 49–95. [DOI] [PubMed] [Google Scholar]
- Dogil G,Frese I,Haider H,Rohm D,Wokurek W ( 2004): Where and how does grammatically geared processing take place and why is Broca's area often involved? A coordinated fMRI/ERBP study of language processing. Brain Lang 89: 337–345. [DOI] [PubMed] [Google Scholar]
- Dogil G ( 2003): Understanding prosody In: Rickheit G,Herrmann T,Deutsch W, editors. Psycholinguistics: An International Handbook. Berlin: Mouton de Gruyter; pp. 544–566. [Google Scholar]
- Egner T,Gruzelier JH ( 2003): Ecological validity of neurofeedback: Modulation of slow wave EEG enhances musical performance. Neuroreport 14: 1221–1224. [DOI] [PubMed] [Google Scholar]
- Fernandez B,Cardebat D,Demonet JF,Joseph PA,Mazaux JM,Barat M,Allard M ( 2004): Functional MRI follow‐up study of language processes in healthy subjects and during recovery in a case of aphasia. Stroke 35: 2171–2176. [DOI] [PubMed] [Google Scholar]
- Fossati P,Ergis AM,Allilaire JF ( 2002): Executive functioning in unipolar depression: A review. Encephale 28: 97–107. [PubMed] [Google Scholar]
- Fox DJ,Tharp DF,Fox LC ( 2005): Neurofeedback: An alternative and efficacious treatment for attention deficit hyperactivity disorder. Appl Psychophysiol Biofeedback 4: 365–373. [DOI] [PubMed] [Google Scholar]
- Friederici AD,Alter K ( 2004): Lateralization of auditory language functions: A dynamic dual pathway model. Brain Lang 89: 267–276. [DOI] [PubMed] [Google Scholar]
- Friederici AD,Kotz SA ( 2003): The brain basis of syntactic processes: Functional imaging and lesion studies. Neuroimage 20: 8–17. [DOI] [PubMed] [Google Scholar]
- Friston KJ,Williams S,Howard R,Frackowiak RS,Turner R ( 1996): Movement‐related effects in fMRI time‐series. Magn Reson Med 35: 346–355. [DOI] [PubMed] [Google Scholar]
- Friston KJ,Holmes A,Worsley KJ,Pline JP,Frith CD,Frackowiak RS ( 1995): Statistical parametric map in functional imaging: A general linear approach. Hum Brain Mapp 2: 189–210. [Google Scholar]
- Fuchs T,Birbaumer N,Lutzenberger W,Gruzelier JH,Kaiser J ( 2003): Neurofeedback treatment for attention‐deficit/hyperactivity disorder in children: A comparison with methylphenidate. Appl Psychophysiol Biofeedback 28: 1–12. [DOI] [PubMed] [Google Scholar]
- Gandour J,Wong D,Dzemidzic M,Lowe M,Tong Y,Li X ( 2003): A cross‐linguistic fMRI study of perception of intonation and emotion in Chinese. Hum Brain Mapp 18: 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandour J ( 2000): Frontiers of brain mapping of speech prosody. Brain Lang 71: 75–77. [DOI] [PubMed] [Google Scholar]
- Goebel R ( 2001): Cortex‐based real‐time fMRI. Neuroimage 13: S129. [Google Scholar]
- Hammond DC ( 2005): Neurofeedback with anxiety and affective disorders. Child Adolesc Psychiatr Clin North Am 14: 105–123. [DOI] [PubMed] [Google Scholar]
- Heim S ( 2005): The structure and dynamics of normal language processing: Insights from neuroimaging. Acta Neurobiol Exp (Wars) 65: 95–116. [DOI] [PubMed] [Google Scholar]
- Hoekert M,Kahn RS,Pijnenborg M,Aleman A ( 2007): Impaired recognition and expression of emotional prosody in schizophrenia: Review and meta‐analysis. Schizophr Res 96: 135–145. [DOI] [PubMed] [Google Scholar]
- Kan Y,Mimura M,Kamijima K,Kawamura M ( 2004): Recognition of emotion from moving facial and prosodic stimuli in depressed patients. J Neurol Neurosurg Psychiatry 75: 667–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotchoubey B,Strehl U,Uhlmann C,Holzapfel S,Konig M,Froscher W,Blankenhorn V,Birbaumer N ( 2001): Modification of slow cortical potentials in patients with refractory epilepsy: A controlled outcome study. Epilepsia 42: 406–416. [DOI] [PubMed] [Google Scholar]
- Kotz SA,Meyer M,Alter K,Besson M,von Cramon DY,Friederici AD ( 2003): On the lateralization of emotional prosody: An event‐related functional MR investigation. Brain Lang 86: 366–376. [DOI] [PubMed] [Google Scholar]
- Maldjian JA,Laurienti PJ,Burdette JH ( 2004): Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage 21: 450–455. [DOI] [PubMed] [Google Scholar]
- Maldjian JA,Laurienti PJ,Burdette JB,Kraft RA ( 2003): An automated method for neuroanatomic and cytoarchitectonic atlas‐based interrogation of fMRI data sets. Neuroimage 19: 1233–1239. [DOI] [PubMed] [Google Scholar]
- Meng M,Bader M ( 2000): Mode of disambiguation and garden path strength: An investigation of subject‐object ambiguities in German. Lang Speech 43: 43–74. [Google Scholar]
- Moore NC ( 2000): A review of EEG biofeedback treatment of anxiety disorders. Clin Electroencephalogr 1: 1–6. [DOI] [PubMed] [Google Scholar]
- Musso M,Weiller C,Kiebel S,Müller SP,Bülau P,Rijntjes M ( 1999): Training‐induced brain plasticity in aphasia. Brain 122: 1781–1790. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Pell MD ( 2007): Reduced sensitivity to prosodic attitudes in adults with focal right hemisphere brain damage. Brain Lang 101: 64–79. [DOI] [PubMed] [Google Scholar]
- Pell MD ( 2006): Cerebral mechanisms for understanding emotional prosody in speech. Brain Lang 96: 221–234. [DOI] [PubMed] [Google Scholar]
- Pop‐Jordanova N,Markovska‐Simoska S,Zorcec T ( 2005): Neurofeedback treatment of children with attention deficit hyperactivity disorder. Prilozi 26: 71–80. [PubMed] [Google Scholar]
- Posse S,Fitzgerald D,Gao K,Habel U,Rosenberg D,Moore GJ,Schneider F ( 2003): Real‐time fMRI of temporolimbic regions detects amygdale activation during single‐trial self‐induces sadness. Neuroimage 18: 760–768. [DOI] [PubMed] [Google Scholar]
- Ross ED ( 1981): The Aprosodias: Functional‐anatomic organization of the affective components of language in the right hemisphere. Arch Neurol 38: 561–569. [DOI] [PubMed] [Google Scholar]
- Rota G,Veit R,Nardo D,Weiskopf N,Birbaumer N,Dogil G ( 2008): Processing of inconsistent emotional information: An fMRI study. Exp Brain Res 186: 401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S,Lamm K,Gundel H,Ladwig KH ( 2005): Neurofeedback‐based EEG alpha and EEG beta training. Effectiveness in patients with chronically decompensated tinnitus. HNO 53: 29–37. [DOI] [PubMed] [Google Scholar]
- Schlosser RG,Nenadic I,Wagner G,Gullmar D,von Consbruch K,Kohler S,Schultz CC,Koch K,Fitzek C,Matthews PM,Reichenbach JR,Sauer H ( 2007): White matter abnormalities and brain activation in schizophrenia: A combined DTI and fMRI study. Schizophr Res 89: 1–11. [DOI] [PubMed] [Google Scholar]
- Schneider W,Eschman A,Zuccolotto A ( 2002): E‐Prime User's Guide. Pittsburgh, PA: Psychology Software Tools Inc. [Google Scholar]
- Scott WC,Kaiser D,Othmer S,Sideroff SI ( 2005): Effects of an EEG biofeedback protocol on a mixed substance abusing population. Am J Drug Alcohol Abuse 31: 455–469. [DOI] [PubMed] [Google Scholar]
- Sitaram R,Caria A,Veit R,Gaber T,Rota G,Kuebler A,Birbaumer N ( 2007): fMRI brain‐computer interface: A tool for neuroscientific research and treatment. Comp Int Neurosci 25487: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snitz BE, MacDonald A,III ,Cohen JD,Cho RY,Becker T,Carter CS ( 2005): Lateral and medial hypofrontality in first‐episode schizophrenia: Functional activity in a medication‐naive state and effects of short‐term atypical antipsychotic treatment. Am J Psychiatr 12: 2322–2329. [DOI] [PubMed] [Google Scholar]
- Strehl U,Leins U,Goth G,Klinger C,Hinterberger T,Birbaumer N ( 2006): Self‐regulation of slow cortical potentials: A new treatment for children with attention‐deficit/hyperactivity disorder. Pediatrics 118: 1530–1540. [DOI] [PubMed] [Google Scholar]
- Thompson L,Thompson M ( 1998): Neurofeedback combined with training in metacognitive strategies: Effectiveness in students with ADD. Appl Psychophysiol Biofeedback 4: 243–263. [DOI] [PubMed] [Google Scholar]
- Thulborn KR,Carpenter PA,Just MA ( 1999): Plasticity of language‐related brain function during recovery from stroke. Stroke 30: 749–754. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N,Landeau B,Papathanassiou D,Crivello F,Etard O,Delcroix N,Mazoyer B,Joliot M ( 2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15: 273–289. [DOI] [PubMed] [Google Scholar]
- Watson D,Clark LA,Tellegen A ( 1988): Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol 54: 1063–1070. [DOI] [PubMed] [Google Scholar]
- Weiller C,Isensee C,Rijntjes M,Huber W,Muller S,Bier D,Dutschka K,Woods RP,Noth J,Diener HC ( 1995): Recovery from Wernicke's aphasia: A positron emission tomographic study. Ann Neurol 37: 723–732. [DOI] [PubMed] [Google Scholar]
- Weiskopf N,Sitaram R,Josephs O,Veit R,Scharnowski F,Goebel R,Birbaumer N,Deichmann R,Mathiak K ( 2007): Real‐time functional magnetic resonance imaging: Methods and applications. Magn Reson Imag 25: 989–1003. [DOI] [PubMed] [Google Scholar]
- Weiskopf N,Scharnowski F,Veit R,Goebel R,Birbaumer N,Mathiak K ( 2004): Self‐regulation of local brain activity using real‐time functional magnetic resonance imaging (fMRI). J Physiol Paris 98: 357–373. [DOI] [PubMed] [Google Scholar]
- Weiskopf N,Veit R,Erb M,Mathiak K,Grodd W,Goebel R,Birbaumer N ( 2003): Physiological self‐regulation of regional brain activity using real‐time functional magnetic resonance imaging (fMRI) methodology and exemplary data. Neuroimage 19: 577–586. [DOI] [PubMed] [Google Scholar]
- Wildgruber D,Riecker A,Hertrich I,Erb M,Grodd W,Ethofer T,Ackermann H ( 2005): Identification of emotional intonation evaluated by fMRI. Neuroimage 24: 1233–1241. [DOI] [PubMed] [Google Scholar]
- Winhuisen L,Thiel A,Schumacher B,Kessler J,Rudolf J,Haupt WF,Heiss WD ( 2005): Role of the contralateral inferior frontal gyrus in recovery of language function in poststroke aphasia: A combined repetitive transcranial magnetic stimulation and positron emission tomography study. Stroke 36: 1759–1763. [DOI] [PubMed] [Google Scholar]
- Yoo SS,Fairneny T,Chen NK,Choo SE,Panych LP,Park HW,Lee SY,Jolesz FA ( 2004): Brain‐computer interface using fMRI: Spatial navigation by thoughts. Neuroreport 15: 1591–1595. [DOI] [PubMed] [Google Scholar]
- Yoo SS,Jolesz FA (2002): Functional MRI for neurofeedback: Feasibility study on hand motor task. Neuroreport 13: 1377–1381. [DOI] [PubMed] [Google Scholar]


