Abstract
The questions of whether and how indiscriminate drug‐related stimuli could influence drug‐users are important to our understanding of addictive behavior, but the answers are still inconclusive. In the present preliminary functional magnetic resonance imaging study using a backward masking paradigm, the effect of indiscriminate smoking‐related stimuli on 10 smokers and 10 nonsmokers was examined. The BOLD response showed a significant reduction (P = 0.001) in the right amygdala of smokers when they viewed but did not perceive masked smoking‐related stimuli, while no significant differences were found in the nonsmoker group. More voxels in anterior cingulate cortex were negatively correlated with the amygdala during the masked smoking‐related picture condition in smokers but not in nonsmokers, whereas more positively correlated voxels were observed during the masked neutral condition. The BOLD response in drug‐users indicates the amygdala responds to drug‐related stimuli that are below the perceptual threshold. The functional connectivity data suggest a functional interaction between the amygdala and the anterior cingulate cortex when drug users view 33ms back‐masked drug‐related stimuli. This observation suggests that the amygdala plays an important role in the indiscriminate drug‐related cue process. Hum Brain Mapp, 2009. © 2008 Wiley‐Liss, Inc.
Keywords: unawareness, smoking‐related cue, functional magnetic resonance imaging (fMRI), amygdala, addiction
INTRODUCTION
Nicotine addiction is the leading preventable cause of death in developed countries [Graul and Prous, 2005]. Millions of nicotine users are willing to stop but few of them are able to do so [Graul and Prous, 2005] because addiction is a compulsive pattern of drug‐related behaviors including drug‐seeking and drug‐taking [Everitt and Robbins, 2005; Koob and Le, 2001; Robinson and Berridge, 1993, 2003; Tiffany, 1990; Tiffany and Conklin, 2000; Volkow and Li, 2004]. The reason for the compulsivity of addictive behaviors and the difficulty for drug‐users to stop using drugs are central questions in addiction [Edwards et al., 1981; Robinson and Berridge, 2003; Volkow and Li, 2005].
Behaviors related to drug seeking or taking tend to occur relatively fast, often without awareness [Robinson and Berridge, 2003; Tiffany, 1990]. Therefore, the unawareness process is the important addiction characteristics [Tiffany, 1990] and involved in smoking initiation [Kremers et al., 2004]. However, to date, the effects of unawareness processing of drug‐related visual input remain controversial. For example, it was found that in abstinent crack cocaine‐dependent men, visual scanning of the preattentive (indiscriminate) cocaine‐related visual cue was modulated by their cocaine craving scores [Rosse et al., 1997]. In another study, Ingjaldsson et al., [2003] also reported that alcoholics showed heart rate deceleration after exposure to masked and invisible alcohol‐related pictures. In comparison, other studies reported contradictory results [Bradley et al., 2004; Franken et al., 2000; Mogg and Bradley, 2002], including the lack of attentional bias in smokers for indiscriminate smoking‐related pictures followed by a visual probe [Bradley et al., 2004] and the absence of an indiscriminate attentional effect for drug‐related words in a modified Stroop task, in which the drug‐related words were presented instead of the color words [Franken et al., 2000; Mogg and Bradley, 2002]. This discrepancy may be attributed mainly to the difference in measurements used in the above studies. To help resolve this discrepancy, it is important to study the potential cortical and subcortical responses to the masked drug‐related visual input.
To date, no published neuroimaging studies have specifically examined the effect of drug‐related stimulus in the absence of input awareness. Existing studies of the neural basis of processing indiscriminate visual stimuli are limited exclusively to emotional stimuli [Pessoa, 2005]. Among the structures identified, the amygdala is perhaps the most important brain area in the unawareness processing of emotional information [Etkin et al., 2004; Killgore and Yurgelun‐Todd, 2004; LeDoux, 1996, 2000; Whalen et al., 1998, 2004] along with the anterior cingulate cortex (ACC) [David et al., 2005; Gallagher and Frith, 2003; Killgore and Yurgelun‐Todd, 2004; LeDoux, 2000]. Additionally, the amygdala and the ACC are also the most commonly reported loci of activation induced by drug‐related cues in brain‐imaging studies [Brody et al., 2004; David et al., 2005; Franklin et al., 2007; Lee et al., 2005; Lim et al., 2005; McBride et al., 2006; Wilson et al., 2004, 2005]. For example, Due et al., [2002] reported that neural substrates are modulated by explicit visual smoking‐related cues in smokers. They observed stronger BOLD signals in several brain areas (e.g., amygdala, ACC, DLPFC, etc.) when smokers were exposed to smoking‐related images than when they were exposed to neutral images and that these conscious smoking‐related images also affected the smokers' craving levels [Due et al., 2002].
In this study, we investigated the neural processing of indiscriminate smoking‐related stimulus with functional magnetic resonance imaging (fMRI). A traditional backward masking paradigm was used to render the stimulus indiscriminate, and an fMRI paradigm was used to ascertain which brain locations generated significant indiscriminate smoking‐related stimuli activation in a smoker group and a control (nonsmoker) group. On the basis of the above discussion, we hypothesize that the indiscriminate smoking‐related cue would influence the BOLD response in some brain areas (e.g., the amygdala).
Additionally, there is evidence [Bokde et al., 2006; Buchel et al., 1999; Williams et al., 2006a] showing that the BOLD response and the connectivity may reveal different aspects of the brain mechanisms of a cognitive function. Functional connectivity between brain regions has been defined as the temporal correlation between spatially remote neurophysiological events [Friston et al., 1993]. Previous research [Jacobsen et al., 2004, 2007] indicates that nicotine‐abuse may be dependent on a distributed neuronal network consisting of cortical and limbic regions rather than on the activity of a discrete brain region. Therefore, functional brain abnormalities in smokers may be present in the functional connectivity between brain regions as well as within discrete brain regions. Particularly, ACC is found to have direct neural connections to the amygdala [LeDoux, 1996, 2000]. It is predicted that, in this study, the correlation between different regions (e.g., the amygdala and the ACC) for the assessment of functional connectivity in smokers will be influenced by indiscriminate smoking‐related cues.
METHODS AND MATERIALS
Participants
Twenty right‐handed healthy adults participated in this study after providing informed consent as approved by the Anhui Medical University review board. Half of the participants (mean age: 25.10 ± 1.07, range: 18–29) were smokers who had a history of smoking for at least 1.5 years (mean: 4.85 ± 1.02 years, range: 1.5–10) and consumed >10 cigarettes/day (consistent with the criterion in Bradley's [2003] study). The other half were nonsmokers (mean age: 23.80 ± 0.81, range: 21–28) who never smoked during their lifetime. Groups did not significantly differ on age (t = 0.967, ns). None of the participants had a history of any neuropsychiatric disorders or other drug dependence. Only male participants were selected as gender effect was not a focus of this study. Smokers also went through the Fagerstrom test for nicotine dependence [Heatherton et al., 1991; Moolchan et al., 2002] and scored an average of 5.00 (SD:1.63, range:3–7). All participants confirmed verbally that they abstained from nicotine, alcohol, coffee, and tea starting from the preceding midnight. The participant's abstinent status was monitored by experimenters for 2 h prior to scanning.
Paradigm and Procedure
On the basis of our pilot experiments and previous studies of unconscious drug‐related cues [Bradley et al., 2003; Franken et al., 2000; Mogg and Bradley, 2002] and emotional cues [Killgore and Yurgelun‐Todd, 2004; Whalen et al., 1998], the signal associated with the indiscriminate stimulus was expected to be very small. To ensure that we could detect this effect, the block design was used as it provides robust results [Loubinoux et al., 2001; Machielsen et al., 2000; Rombouts et al., 1997, 1998], a relatively high statistical power [Friston et al., 1999] and a relatively large BOLD signal change related to baseline [Buxton et al., 1998]. Additionally, in our pilot experiment, some participants reported they were able to see the masked stimuli, especially when the indiscriminate condition was presented following the discriminate condition. Therefore, in this study, all indiscriminate scanning runs were conducted before the discriminate scanning runs.
All participants attended one scan session including six passive viewing scanning runs for the indiscriminate condition. Because the signal associated with the subliminal stimulus was expected to be very small, we tried to collect subliminal data as much as possible during the limited scanner time. Therefore, only fourteen of them (seven smokers and seven nonsmokers) completed another two passive viewing scanning runs for the discriminate condition following the indiscriminate scanning runs. In each scanning run, there were 13 blocks consisting of six stimulation blocks and seven baseline blocks. The stimulation blocks were separated by the baseline blocks, in which a fixation cross (1° × 1° visual angle) was displayed on the screen. Each block lasted 20 s, except for the first and the last baseline blocks, which were 10‐s long for equaling the number of time points between the baseline and the cognitive task.
In indiscriminate scanning runs (i.e., the indiscriminate condition), there were two categories of stimulation blocks: indiscriminate (unconscious) smoking‐related (US, three blocks) and indiscriminate (unconscious) neutral (UN, three blocks). The order of the US and UN blocks was counterbalanced across the participants. Each stimulation (i.e., US or UN) block consisted of 10 trials. Each trial in the indiscriminate scanning runs was a traditional backward masking presentation trial as used in previous studies [Killgore and Yurgelun‐Todd, 2004; Morris et al., 1998, 1999; Sheline et al., 2001; Whalen et al., 1998]. The trial started with a 33‐ms presentation of a stimulus picture, followed by a masking picture displayed for 167 ms and an 1,800‐ms fixation cross (see Fig. 1). There were 10 smoking‐related stimulus pictures (6.5° × 5° in visual angle) showing a man smoking a cigarette with different poses. Ten paired neutral pictures showed the same man holding a microphone with the same pose as that of the corresponding smoking‐related pictures (see Fig. 1). The background in all pictures was discarded to decrease the interference from other information. The mask picture was the result of randomization of pieces in the stimulus pictures such that it did not have any perceivable object but shared the color and texture of the stimulus pictures (same to the previous studies [Ingjaldsson et al., 2003; Ohman and Soares, 1993]). The stimuli were presented on a screen through a projector controlled by a computer.
Figure 1.
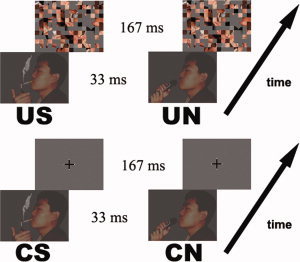
Sample stimuli and experimental parameters in the four tasks (US/UN/CS/CN). There were two kinds of stimulus pictures, i.e., smoking‐related and neutral. One representative pair was showed above.
According to the previous study [Williams et al., 2004], stimulus detection remains possible and masking interferes only with the subsequent ability to discriminate it at the 33 ms presentation of stimuli. Therefore, the indiscriminate condition in this study is at the discrimination but not the detection level [Williams et al., 2004]. The criterion of “indiscriminate” in this study is determined by the ability of the participants to observe the key items (i.e., cigarette, microphone).
The trials in the discriminate condition were similar to those in the indiscriminate condition. The only difference was that the fixation was displayed instead of the masking picture after the task picture so that the participants were able to perceive the stimulus consciously (see Fig. 1). Similar to the indiscriminate condition, there were two types of task blocks: discriminate (conscious) smoking‐related (CS) and discriminate (conscious) neutral (CN).
Data Acquisition
Images were obtained with a GE 1.5T MR scanner (GE Medical Systems, Milwaukee, WI). A circularly polarized head coil was used, with foam padding to restrict head motion. Functional images were acquired with a T2*‐weighted echo‐planar imaging sequence (TE = 45ms, TR = 2s, FOV = 24 cm) with 22 axial slices (no slice gap, one voxel: 3.75 × 3.75 × 4 mm3). Corresponding high‐resolution T1‐weighted spin‐echo (for anatomical overlay) images and three‐dimensional gradient‐echo (for stereotaxic transformation) images were also collected. Each functional run lasted 4 min 8 s (124 images/slice). The first four time points in each run were discarded to account for the approach to steady state in the BOLD signal.
Data Analysis
The imaging data were analyzed with analysis of functional neuroimages (AFNI), [Cox, 1996]. As detailed below, data analysis included the BOLD response and the functional connectivity.
Analysis of BOLD response
Activation map generation
The raw data were motion corrected and normalized. Then, the six functional scanning runs for the indiscriminate condition and the two scanning runs for the discriminate condition were concatenated, respectively, to obtain a combined data set for each condition. Subsequently, correlation analysis was performed on these two data sets based on the contrasts between the tasks and the baseline to generate corresponding activation maps (threshold: P < 0.05) for each participant. A spatial cluster size threshold of four connected voxels was applied to the activation maps to eliminate isolated sporadic active voxels. With the spatial clustering, the false positive level, corrected for multiple comparison in the entire imaged volume, was <0.001 (Monte Carlo simulation conducted with AFNI). An example of the activation map in one representative participant is showed in the first row in Figure 2.
Figure 2.
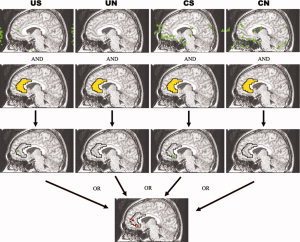
The definition of ROI. There is a schematic diagram for definition of ROI of the right ACC in one representative participant. The green area is the activation map (after clustered) in each task (US/UN/CS/CN). The yellow area is the right ACC based on the anatomical structure and stereotaxic coordinates [Talairach and Tournoux, 1988]. The red area is the ROI of the right ACC in this participant. The definition of the ROIs in the OTC, OVC, DLPFC, and IFG are same as that of the right ACC.
Regions of interest definition
Several brain regions were selected as regions of interest (ROI) if there are one or more activation clusters within an anatomically delineated area in at least eight out of 10 participants and one or more activation clusters within the same anatomical area in every participant during at least one task. These areas included bilateral occipital/temporal cortex (OTC, BA37), bilateral occipital visual cortex (OVC, BA17/18/19), bilateral dorsolateral prefrontal cortex (DLPFC, BA9/46), bilateral inferior frontal gyrus (IFG, BA44/45) and right ACC (BA24/32). A ROI is defined for each of these areas in each participant based on the union of activations for any of the tasks (US\UN\CS\CN) within an anatomically delineated area (see Fig. 2). The size of these ROIs averages 23–131 voxels.
In addition to the areas selected based on activation, bilateral amygdala, bilateral caudate and bilateral pulvinar were also considered because they have been suggested to be involved in unconscious processing of emotional and drug‐related visual cues [Etkin et al., 2004; Killgore and Yurgelun‐Todd, 2004; LeDoux, 1996, 2000; Morris et al., 1999; Wilson et al., 2004]. Since these ROIs did not consistently show significant activation in the activation maps, they were defined based on anatomical structures and stereotaxic coordinates [Talairach and Tournoux, 1988]. The ROIs in these regions contained 29 voxels for amygdala (1.6–1.7 cm3, which is consistent with previous studies [Brierley et al., 2002; Robinson et al., 2004]; see details in the next paragraph and Fig. 4A), 47 voxels for pulvinar, and 96 voxels for caudate. No significant differences were found between the smoker group and the nonsmoker group in the volume of these ROIs [the amygdala (left: t = 0.107, ns; right: t = 0.788, ns), the pulvinar (left: t = 0.551, ns; right: t = 0.973, ns) and the caudate (left: t = 0.081, ns; t = 0.788, ns)].
Figure 4.
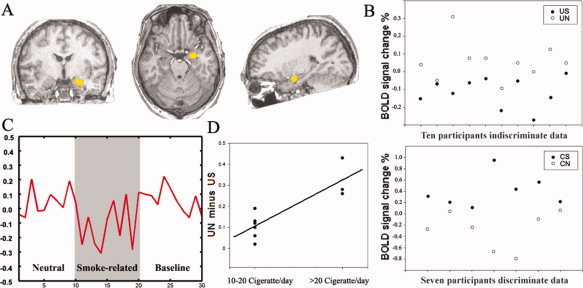
Position of the amygdala and the BOLD responses under different tasks. (A) The boundaries for the right amygdala in this study. The boundaries for the right amygdala are displayed in Talairach space in this figure only for presentation; however, we calculated the BOLD response in individual original space. (B) A graphical display of individual BOLD response of the right amygdala in the US, UN, CS, and the CN in smokers. All smokers showed the BOLD response of the US was significantly lower than that of the UN, while the BOLD response of the CS was significantly higher than that of the CN. (C) The averaged time‐course of the right amygdala from the smokers in indiscriminate tasks. (D) A graphical display of the difference in BOLD response between the UN and the US in the right amygdala. The smokers who consume over 20 cigarettes per day showed larger difference BOLD response between the UN and the US than the persons who smoke 10–20 cigarettes per day.
All three planes were used to determine the boundaries of the amygdala following the previous studies [Sheline et al., 2001; Gur et al., 2000, 2004]. Visualized in the coronal section, the anterior boundary of the amygdala was the first section in which the white matter connecting the frontal and temporal lobes became continuous. The posterior boundary was drawn adjacent to the anterior boundary of the hippocampus. The dorsal boundary was determined first by the coronal slice cutting through the most inferior‐anterior point of the temporal horn of the lateral ventricle (chosen from the sagittal plane). Ventrally, visualized in the sagittal section, the amygdala was bounded by a horizontal line connecting to the ventral edge of the hippocampus. Medially, seen in the coronal section, the amygdala was bounded by subarachnoid space. Laterally, seen in the coronal section, the amygdala was bounded by white matter.
Characterization of BOLD response
The time courses of the BOLD signal were segmented into blocks for each task and the first time point of each block was removed to minimize the effects of hemodynamic delay. The time courses for each task were then averaged across all the voxels in each ROI. The amplitude of BOLD response was obtained by the average BOLD signal within the duration of each task (US/UN/CS/CN).
Sub‐group analysis
To test whether the BOLD response difference between the US and the UN conditions (i.e., UN minus US) in the smoker group is related to the addiction level, a correlation analysis between the number of cigarettes smoked per day and the BOLD response to the indiscriminate tasks (UN minus US) was performed in the ROIs where the BOLD difference (UN vs. US) was significant in smokers.
Analysis of functional connectivity
Preprocessing
For the purpose of performing functional connectivity analysis, four preprocessing steps (see Fig. 3) were applied to the raw functional data, following the procedure described in previous studies [Hashimoto and Sakai, 2004; Homae et al., 2003]. Specifically, the preprocessing steps include: (a) correction of temporal shifts between slices, (b) motion correction and normalization, (c) separation of fMRI time courses into blocks for each task with the first time point removed to minimize the effect of the hemodynamic response delay, and (d) construction of task‐specific (US/UN/CS/CN) time courses by concatenating the time courses of individual task blocks in each run and averaging across the repeated runs. Thus, each of the resultant task‐specific time courses contained 27 time points.
Figure 3.
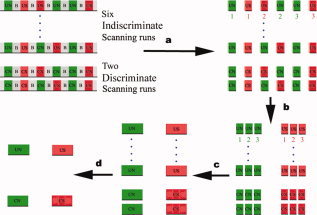
The preprocess of functional connectivity analysis. (a) The time courses of BOLD signals were segmented into blocks for each task (smoking‐related and neutral, respectively), with the first time point removed to minimize the effect of hemodynamic changes from its preceding task block. (b,c) Task‐specific (US, UN, CS, and CN) time courses were constructed by merging all the time courses of individual task blocks into one scan. (d) The resultant task‐specific time courses were averaged across the scans, respectively. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Functional connectivity calculation
The right amygdala, the only area exhibited significant differences in BOLD response between the US and the UN in smokers, was selected as the seed ROI in this study. The other relevant brain areas (i.e., the bilateral OTC, OVC, DLPFC, IFG, caudate, pulvinar, and the right ACC) in the BOLD response analysis were selected as the target ROIs. Functional connectivity between the seed ROI (the right amygdala) and the target ROIs was calculated. Functional connectivity was evaluated by examining the volume of the voxels with significant positive or negative correlation coefficient (threshold: r > 0.5 or r < −0.5, P < 0.0093, uncorrected) within each target ROI. The analysis protocol for functional connectivity analysis in this study was same to that in previous published studies [Homae et al., 2003; Stein et al., 2000].
RESULTS
Debriefing Participants
After entering the scanner but before starting the fMRI data collection, the participants were examined individually to assess the effect of backward masking using the same set of pictures later used in the fMRI runs. To eliminate the subjective bias as much as possible, the participants were required not only to report whether they could see the pictures or not but also to describe what they saw in detail. Once the word “cigarette” or “microphone” appeared in the participant's report, the luminance and the contrast of the stimulus pictures were reduced until the participant did not report any meaningful scenes. Two participants (one smoker and one nonsmoker) were found to need such reduction. The reduction was applied in both indiscriminate and discriminate conditions. After the fMRI runs were completed, the participants were examined in the same manner again to ensure the effectiveness of the masking and to make sure that the masked stimulus remained invisible. All participants reported that none of pictures with the mask but all of pictures without the mask were perceived. Similar to Morris' [1998] study, some participants reported awareness of flickering occurring during the unconscious presentations, but did not report the perception of any meaningful scenes, especially the images of the cigarette or the microphone.
BOLD Responses
Because of the different number scanning runs and fixed order between the discriminate and the indiscriminate conditions and only seven smokers and seven nonsmokers were collected the discriminate data, we focused on the difference of the patterns of BOLD responses (i.e., the result of the smoking‐related task vs. the neutral task) between the discriminate and the indiscriminate conditions instead of the direct comparison between these two conditions.
Indiscriminate Condition
The repeated‐measures analysis of variance with a between‐subjects factor comparing the addiction group (smoker and nonsmoker) and a within‐subjects factor comparing the stimulus (smoking‐related and neutral) revealed there was a significant interaction (threshold: P < 0.05) between the addiction group and the stimulus type in the right amygdala [F(1,18) = 7.135, P = 0.016; no main effect on the addiction group, F(1,18) = 0.457, ns; and significant main effect on the stimulus, F(1,18) = 12.616, P = 0.002]. A planned contrast t‐test (paired t‐test, threshold P < 0.05) in the smoker group in this ROI (t = 4.506, P = 0.001) showed that the BOLD response during the presentation of indiscriminate smoking‐related pictures was lower than during the presentation of indiscriminate neutral pictures, while no significant difference (t = 0.609, ns) was found in the nonsmoker group (Table I, Fig. 4).
Table I.
The BOLD response of each ROI (%), threshold P < 0.05
| Smoker | Nonsmokers | ANOVA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Smoking ‐related | Neutral | t | P | Smoking ‐related | Neutral | t | P | F | P | |
| Indiscriminate | ||||||||||
| Left | ||||||||||
| Amygdala | 0.08 | 0.05 | 0.386 | — | 0.02 | −0.02 | 1.347 | — | 0.046 | — |
| Pulvinar | 0.04 | −0.04 | 1.985 | — | −0.01 | 0.01 | 0.626 | — | 3.778 | — |
| DLPFC | 0.21 | 0.05 | 1.689 | — | 0.17 | 0.07 | 1.148 | — | 0.293 | — |
| IFG | 0.40 | −0.01 | 1.247 | — | 0.13 | 0.12 | 0.170 | — | 1.353 | — |
| OVC | 0.35 | 0.31 | 0.455 | — | 0.37 | 0.32 | 0.790 | — | 0.004 | — |
| OTC | 0.25 | 0.019 | 0.575 | — | 0.28 | 0.31 | 0.302 | — | 0.403 | — |
| Caudate | 0.00 | 0.04 | 0.898 | — | 0.02 | −0.01 | 1.087 | — | 1.897 | — |
| Right | ||||||||||
| Amygdala | −0.11 | 0.06 | 4.506 | 0.001 | −0.02 | 0.01 | 0.609 | — | 7.135 | 0.016 |
| Pulvinar | 0.00 | −0.01 | 0.122 | — | 0.00 | −0.04 | 0.901 | — | 0.240 | — |
| ACC | 0.20 | −0.15 | 1.112 | — | 0.13 | 0.10 | 0.443 | — | 0.972 | — |
| DLPFC | 0.12 | 0.11 | 0.050 | — | 0.54 | 0.11 | 1.087 | — | 0.959 | — |
| IFG | 0.11 | −0.00 | 0.993 | — | 0.28 | −0.00 | 2.067 | — | 1.007 | — |
| OVC | 0.31 | 0.36 | 0.555 | — | 0.41 | 0.36 | 1.032 | — | 0.975 | — |
| OTC | 0.23 | 0.29 | 0.708 | — | 0.34 | 0.25 | 1.530 | — | 2.073 | — |
| Caudate | 0.02 | 0.00 | 0.515 | — | 0.01 | 0.02 | 0.290 | — | 0.346 | — |
| Discriminate | — | |||||||||
| Left | — | |||||||||
| Amygdala | 0.13 | 0.03 | 0.416 | — | −0.06 | 0.09 | 1.293 | — | 0.884 | — |
| Pulvinar | −0.05 | 0.04 | 0.886 | — | 0.07 | −0.01 | 0.995 | — | 1.735 | — |
| DLPFC | 0.78 | −0.02 | 4.698 | 0.005 | 0.15 | 0.50 | 1.133 | — | 10.752 | 0.008 |
| IFG | 0.40 | 0.76 | 0.454 | — | 0.71 | 0.29 | 1.857 | — | 0.896 | — |
| OVC | 0.65 | 0.53 | 0.389 | — | 0.61 | 0.34 | 1.622 | — | 0.198 | — |
| OTC | 0.70 | 0.72 | 0.079 | — | 0.84 | 0.74 | 1.018 | — | 0.177 | — |
| Caudate | 0.16 | −0.06 | 2.959 | 0.025 | 0.04 | −0.02 | 1.294 | — | 2.892 | — |
| Right | — | |||||||||
| Amygdala | 0.40 | −0.28 | 3.517 | 0.013 | −0.01 | −0.02 | 0.116 | — | 8.177 | 0.014 |
| Pulvinar | −0.03 | 0.06 | 0.553 | — | 0.04 | 0.03 | 0.322 | — | 0.392 | — |
| ACC | −0.10 | 1.13 | 0.973 | — | 0.28 | 0.25 | 1.352 | — | 0.940 | — |
| DLPFC | 0.64 | 0.018 | 1.466 | — | 0.55 | 0.27 | 1.272 | — | 0.227 | — |
| IFG | 0.92 | 0.46 | 2.110 | — | 0.43 | 0.68 | 0.822 | — | 3.528 | — |
| OVC | 0.66 | 0.60 | 0.248 | — | 0.71 | 0.44 | 2.379 | — | 0.514 | — |
| OTC | 1.31 | 0.09 | 2.746 | 0.033 | 0.74 | 0.59 | 1.001 | — | 5.206 | 0.042 |
| Caudate | 0.05 | −0.02 | 1.676 | — | 0.06 | −0.04 | 1.602 | — | 0.105 | — |
In particular, the significant decrease of BOLD response in the UN than in the US in the right amygdala was influenced significantly by the number of cigarettes consumed by the smokers per day (r = 0.858, P = 0.001, Fig. 4). This result suggests that the BOLD response difference between the US and the UN may be functionally related to the level of nicotine use.
Discriminate Condition
The repeated‐measures analysis of variance revealed there was a significant interaction between the addiction group and the stimulus in the right amygdala [F(1,12) = 8.177, P = 0.014; no main effect on the addiction group, F(1,12) = 1.251, ns; and significant main effect on the stimulus, F(1,12) = 8.760, P = 0.012], the left DLPFC [F(1,12) = 10.752, P = 0.008; no main effect on the addiction group, F(1,12) = 0.125, ns; and no main effect on the stimulus, F(1,12) = 1.693, ns] and the right OTC [F(1,12) = 5.206, P = 0.042; no main effect on the addiction group, F(1,12) = 0.074, ns; and significant main effect on the stimulus, F(1,12) = 8.535, P = 0.013] in the discriminate conditions. A planned contrast t‐test (paired t‐test) in the smoker group in these ROIs [the right amygdala (t = 3.224, P = 0.018), the left DLFPC (t = 4.698, P = 0.005), the right OTC (t = 2.746, P = 0.033)] showed that the BOLD response during the presentation of discriminate smoking‐related pictures was higher than during the presentation of neutral pictures, while no significant difference was found in the nonsmoker group [the right amygdala (t = 0.116, ns), the left DLFPC (t = 1.583, ns), the right OTC (t = 1.001, ns)] (Table I, Fig. 4).
Functional Connectivity
Indiscriminate condition
The repeated‐measures analysis of variance with a between‐participants factor comparing the addiction group (smoker and nonsmoker), a within‐participants factor comparing the stimulus (smoking‐related and neutral) and a within‐participants factor comparing the correlation coefficient (r > 0.5 and r < −0.5) revealed there was significant interaction between the addiction group, the stimulus type, and the coefficient [F(1,18) = 10.600, P = 0.004; no main effect on the addiction group, F(1,18) = 0.395, ns; no main effect on the stimulus, F(1,18) = 0.543, ns and no main effect on the coefficient, F(1,18) = 0.182, ns] in the right ACC. In addition, the results also showed a significant interaction between the stimulus type and the correlation coefficient [F(1,18) = 8.325, P = 0.010] in the right ACC. A planned contrast t‐test (paired t‐test) showed that compared with the UN, the volume of the positively correlated (r > 0.5) voxels between the right amygdala and the right ACC during the US task was significantly smaller (t = 3.178, P = 0.011) and the volume of the negatively correlated (r < −0.5) voxels was significantly larger (t = 6.274, P < 0.001) in the smokers (Table II and Fig. 5). In contrast, no significant difference in functional connectivity was seen in the nonsmokers in this ROI.
Table II.
Mean volume (mm3) of the voxels in each ROI exceeding a significance criterion (r > 0.5 or <−0.5) in functional connectivity analysis
| ROI | Unconscious | Conscious | Unconscious | Conscious | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r > 0.5 | r < −0.5 | r > 0.5 | r < −0.5 | r > 0.5 | r < −0.5 | r > 0.5 | r < −0.5 | |||||||||
| S | N | S | N | S | N | S | N | S | N | S | N | S | N | S | N | |
| Left | ||||||||||||||||
| Pulvinar | 84 | 141 | 39 | 73 | 64 | 64 | 88 | 96 | 79 | 68 | 62 | 56 | 32 | 129 | 40 | 48 |
| IFG | 383 | 433 | 287 | 287 | 410 | 707 | 273 | 426 | 377 | 366 | 422 | 411 | 297 | 321 | 329 | 354 |
| Caudate | 146 | 231 | 129 | 124 | 257 | 394 | 177 | 121 | 191 | 219 | 163 | 180 | 145 | 169 | 177 | 145 |
| OVC | 917 | 1266 | 872 | 1103 | 1213 | 972 | 1069 | 1117 | 1142 | 1035 | 984 | 1204 | 956 | 1037 | 1004 | 1109 |
| OTC | 225 | 315 | 191 | 186 | 257 | 362 | 104 | 225 | 293 | 253 | 236 | 236 | 233 | 193 | 153 | 233 |
| DLPFC | 294 | 188 | 150 | 294 | 309 | 309 | 178 | 366 | 406 | 294 | 406 | 337 | 291 | 413 | 366 | 291 |
| Right | ||||||||||||||||
| ACC | 304 | 681 | 636 | 360 | 611 | 1069 | 370 | 579 | 551 | 579 | 495 | 563 | 281 | 378 | 402 | 410 |
| Pulvinar | 84 | 113 | 96 | 113 | 129 | 96 | 104 | 137 | 101 | 96 | 51 | 90 | 24 | 96 | 104 | 80 |
| IFG | 388 | 461 | 383 | 366 | 771 | 812 | 394 | 329 | 467 | 422 | 388 | 354 | 346 | 321 | 338 | 378 |
| Caudate | 152 | 191 | 113 | 158 | 217 | 305 | 177 | 113 | 152 | 158 | 197 | 203 | 137 | 177 | 96 | 193 |
| OVC | 1182 | 1496 | 928 | 1305 | 1021 | 1655 | 996 | 1310 | 1249 | 962 | 883 | 1176 | 1029 | 844 | 852 | 1013 |
| OTC | 186 | 298 | 124 | 304 | 490 | 378 | 241 | 321 | 326 | 270 | 214 | 231 | 249 | 233 | 153 | 233 |
| DLPFC | 169 | 275 | 131 | 194 | 553 | 375 | 291 | 272 | 275 | 344 | 350 | 269 | 309 | 300 | 291 | 281 |
Figure 5.
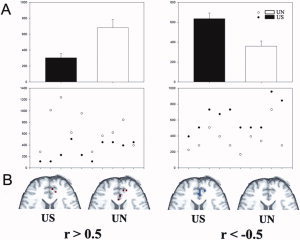
Functional connectivity result between the right ACC and the right amygdale. (A) A graphical display of the averaged (up row) and individual (below row) volume of the voxels in which r value exceeded the threshold in the right ACC. The volume of the positively correlated voxels (r > 0.5) of the US was less than the UN (left), while the volume of the negatively correlated voxels (r < −0.5) of the US was more than the UN (right). Bars represent standard errors. (B) The significant positively (r > 0.5, red voxels) and negatively (r < −0.5, blue voxels) correlated voxels in the right ACC in one smoker.
Discriminate condition
The repeated‐measures analysis of variance, same to that of the indiscriminate condition, revealed there was a significant interaction between the addiction group, the stimulus type, and the correlation coefficient in the right Caudate [F(1,12) = 4.830, P = 0.048; no main effect on the addiction group, F(1,12) = 0.981, ns; no main effect on the stimulus, F(1,12) = 0.603, ns and no main effect on the coefficient, F(1,12) = 0.106, ns] and the right Pulvinar [F(1,12) = 6.818, P = 0.023; no main effect on the addiction group, F(1,12) = 1.671, ns; no main effect on the stimulus, F(1,12) = 1.042, ns and no main effect on the coefficient, F(1,12) = 0.372, ns]. However, there was no significant interaction between the stimulus type and the coefficient in these two ROIs [the right caudate: F(1,12) = 0.017, ns; the right pulvinar: F(1,12) = 0.273, ns].
DISCUSSION
In the right amygdala, the response to the US was significantly lower than that to the UN in the smoker group, while no significant difference was found in the nonsmokers (Fig. 4, Table I). Additionally, the sub‐group result suggests that the difference in BOLD signal response between the US and the UN may be functionally relevant to the abuse of nicotine in smokers. Our results provide the evidence that the amygdala response to drug‐related stimuli does not rely on the participant's discrimination of the stimuli. This result is consistent with previous findings that the amygdala (especially the right amygdala) is a key brain area for classical unconscious emotional learning [Etkin et al., 2004; Killgore and Yurgelun‐Todd, 2004; LeDoux, 1996, 2000; Whalen et al., 1998, 2004].
The activation level in the amygdala is related to the drug craving in drug‐abusers [Wilson et al., 2004]. The smoking‐related visual cue can increase the amygdala activation [Due et al., 2002] and the cigarette craving [Drummond, 2001] at the same time. In this study, an amygdala activity decrease was found when smokers see masked smoking‐related pictures. A recent study [McClernon et al., 2007] showed a selectively activation reduce response to smoking cue in the amygdala following a smoking cessation treatment. Therefore, the result in this study might display some possibilities in the strategies to addiction treatment.
Besides drug‐related information, for the nicotine abstinent increases the smoker's anxiety level [reviewed by Morissette et al., 2007] and the smokers were restricted from smoking at least for 2 h in this study, the anxiety may be play a role in producing current results. However, in the recent studies about unconscious fearful stimuli [Bryant et al., 2007; Etkin et al., 2004], increased anxiety is generally associated with increased amygdala activity. Therefore, it is unlikely that the reduction in amygdala activity in response to US stimuli was contributed by smokers' increased anxiety during the current study.
In the existing behavioral studies, differential functional characteristics of the drug‐related stimulus at the discriminate and indiscriminate levels have also been reported. For example, Rosse et al., [1997] found that drug craving scores were correlated negatively with the number of unconscious fixations and were correlated positively with the number of conscious fixations during visual scanning. In another study, drug‐users' heart rates were decelerated only when the drug‐related pictures were presented at the indiscriminate level [Ingjaldsson et al., 2003]. When the images were perceived by the drug‐users, their heart rates were either not decelerated [Ingjaldsson et al., 2003] or even increased [Sinha et al., 2000]. The present results demonstrated the same smoking‐related stimulus generated either increased or decreased BOLD signals in the right amygdala in the discriminate and indiscriminate conditions, respectively, and are consistent with these prior behavioral results and provide convergent neural evidence supporting the hypothesis that the discriminate and indiscriminate drug‐related stimulus processes are not the same. It is also similar to the subliminal and supraliminal fear process that are dissociated in the amygdala‐MPFC/ACC system [Jiang and He, 2006; Williams et al., 2006b]. However, for bradycardia can be associated with either increased or decreased amygdala activity [Henderson et al., 2002; Wu et al., 1999], the functional relationship between the amygdala activity and the heart rate in this study is still to be elucidated in the future study.
This study indicates the functional connectivity between the right amygdala and the right ACC is more negative in the smoking‐related indiscriminate task than in the neutral task. In previous studies, ACC was found to have direct neural connections to the amygdala [LeDoux, 1996, 2000] inhibition is an important function of ACC in normal population [Fan et al., 2003]. Additionally, a path analysis study [Stein et al., 2007] found the activity in the amygdala was suppressed by the ACC during an emotional face matching paradigm. Therefore, one possible explanation for our result is that the ACC inhibits the indiscriminate drug‐related stimulus activity in the amygdala, i.e., there may be an inhibition process when drug‐users view the indiscriminate drug‐related stimulus. Additionally, Brody et al. [2007] found that the activation of ACC is associated to self‐suppressing cigarette craving. Therefore, the observation in this study may provide some clues for finding treatment strategies for drug abuse. However, the functional connectivity analysis in our study is based on temporal correlation, which did not allow us to determine the full extent of the pathway. Therefore, further examination using effective connectivity analysis will be needed.
Our functional connectivity result is seemly inconsistence with some recent findings (e.g. [Williams et al., 2006a]) about indiscriminate process. Williams found activation in the bilateral amygdala covaried positively with the rostral portion of the ACC, while we found significant negative correlation between the right amygdala and the right ACC. There are several apparent differences between Williams' and our studies in the experiment design. The most salient one is that we used smoking‐related cue while William focused on the fearful stimulus. Additionally, the presentation of stimuli in this study (33 ms) is significantly longer than that in Williams' study (20 ms). According to Williams' viewpoint [Williams et al., 2004], the duration of about 30 ms may not provide an exhaustive test for amygdala responses to nonconscious perception, and detection without recognition may lead to uncertainty in participants and may be sufficient to engage cortical inhibitory influences on the amygdala. Therefore, the inhibition process during the indiscriminate condition in this study might be from some discriminate influence or uncertainty. However, the absence of significant correlation during the discriminate condition in smokers and during both the discriminate and indiscriminate condition in nonsmokers may mean that there is only a minimal impact of the stimulus detection.
The present fMRI study not only provides evidence supporting the neural processing of indiscriminate drug‐related stimuli but also helps to resolve seemingly conflicting reports in the literature, (i.e., the effects of unawareness processing of drug‐related visual input via different measurements remain controversial). In this study, the indiscriminate drug‐related stimulus affected smokers' amygdala significantly, but no such effect was observed in the DLPFC and the IFG (Table I). As an important structure in the limbic system, the amygdala plays a critical role in autonomic functions (e.g., heart rate) [Henderson et al., 2004]. Therefore, the present result is consistent with the previous report [Ingjaldsson et al., 2003] that the processing of indiscriminate drug‐related stimuli was closely related to autonomic functions. Conversely, the lack of indiscriminate modulation in DLPFC and IFG by indiscriminate drug‐related information, combined with many previous neuroimaging studies [Blackwood et al., 2000; Kincade et al., 2005] that showed the frontal area's important role in attentional bias, provides a reasonable account for the failure to observe high‐level cognitive effects from indiscriminate drug‐related stimuli in previous behavior studies [Bradley et al., 2004; Franken et al., 2000; Mogg and Bradley, 2002].
There are several limitations of this preliminary study. Drug cues would normally induce a series of activities or processes including rapid physiological/autonomic responses [Carter and Tiffany, 1999; Chiamulera, 2005] and some sustained reactions, e.g. craving and mood/emotion [Franken et al., 1999; Sinha et al., 2000]. However, because this study used a blocked design with relatively low temporal resolution, it was difficult to identify the relationships between each process and the dynamic activities in the amygdala. In the future, a mixed blocked/event‐related fMRI design [Scheibe et al., 2006] or combined fMRI and EEG/MEG design [Babiloni et al., 2004] with some physiological and behavioral measurements [Breiter et al., 1997; McClernon et al., 2005; Wexler et al., 2001] will be used to address this open question. Another limitation is the exclusive use of the stimuli showing male smoking. Smoking has a strong social component and male smoking might be associated with a particular image. Future experiment should also include different smoking‐related cues (e.g., female smoking, smoking‐related materials, etc). The third one is some participants reported awareness of flickering occurring during the indiscriminate presentations. The “smidgen of consciousness” may cause a degree of subjective uncertainty about the stimulus and thereby interfere with the brain activity from indiscriminate stimuli [Williams et al., 2004]. Therefore, we cannot exclude the function of stimulus duration as one potential reason for the finding of reduced activity in the right amygdala in our study. However, we did not find same reduced activity in nonsmokers. It suggests that this subjective uncertainty effect should be minimal in this study. The fourth limitation is the difference between the indiscriminate and discriminate conditions is that the discriminate condition lacks of the mask. Many previous studies [Etkin et al., 2004; Ingjaldsson et al., 2003; Kubota et al., 2000] about unconscious processes employed unmasked stimuli as the conscious condition. Although no participants recognized any meaningful scene in the mask during the study, the potential bias due to an imbalance between the indiscriminate and discriminate stimuli should be paid attention in future. The fifth limitation is all indiscriminate scanning runs were conducted before the discriminate scanning runs in this study. Although it can prevent perceiving indiscriminate stimulus by participants, a potential order effect may be produced. The last limitation is the relatively small sample size [i.e., 10 participants (only three smokers smoked 21–30 cigarette per day) per group and only seven participants were collected discriminate data]. Therefore, the present findings need to be confirmed by large sample study in the future. However, despite the limitation that the data were collected from a small group, the difference between smoking‐related cue and the neutral cue in the key brain area (e.g., the amygdala) reached the threshold (i.e., P < 0.05).
CONCLUSIONS
As limited number of participants, interpretations and conclusions of the this study should be treat as preliminary. We found the activity in the amygdala was modulated by the indiscriminate smoking‐related stimulus in smokers. Specifically, the same smoking‐related stimulus generated either increased or decreased BOLD signal in the smoker's right amygdala in the discriminate and indiscriminate conditions, respectively. This observation suggests that the amygdala plays an important role in the indiscriminate drug‐related process and different neural circuits may be involved in processing discriminate and indiscriminate drug‐related stimuli. Furthermore, functional connectivity results indicate the correlation between the right amygdala and the right ACC is more negative in the smoking‐related indiscriminate task than in the neutral task and give a potential possibility to the existence of inhibition processes in the unawareness drug‐related pathway.
Acknowledgements
We thank Cheng Zhang for technical assistance and Mary Pfeiffer and Pradeep Kurup for correcting English.
REFERENCES
- Babiloni F,Mattia D,Babiloni C,Astolfi L,Salinari S,Basilisco A,Rossini PM,Marciani MG,Cincotti F ( 2004): Multimodal integration of EEG, MEG and fMRI data for the solution of the neuroimage puzzle Magn Reson Imaging 22: 1471–1476. [DOI] [PubMed] [Google Scholar]
- Blackwood NJ,Howard RJ,ffytche DH,Simmons A,Bentall RP,Murray RM ( 2000): Imaging attentional and attributional bias: An fMRI approach to the paranoid delusion. Psychol Med 30: 873–883. [DOI] [PubMed] [Google Scholar]
- Bokde AL,Lopez‐Bayo P,Meindl T,Pechler S,Born C,Faltraco F,Teipel SJ,Moller HJ,Hampel H ( 2006): Functional connectivity of the fusiform gyrus during a face‐matching task in subjects with mild cognitive impairment. Brain 129: 1113–1124. [DOI] [PubMed] [Google Scholar]
- Bradley B,Field M,Mogg K,De Houwer J ( 2004): Attentional and evaluative biases for smoking cues in nicotine dependence: Component processes of biases in visual orienting. Behav Pharmacol 15: 29–36. [DOI] [PubMed] [Google Scholar]
- Bradley BP,Mogg K,Wright T,Field M ( 2003): Attentional bias in drug dependence: Vigilance for cigarette‐related cues in smokers. Psychol Addict Behav 17: 66–72. [DOI] [PubMed] [Google Scholar]
- Breiter HC,Gollub RL,Weisskoff RM,Kennedy DN,Makris N,Berke JD,Goodman JM,Kantor HL,Gastfriend DR,Riorden JP,Mathew RT,Rosen BR,Hyman SE ( 1997): Acute effects of cocaine on human brain activity and emotion. Neuron 19: 591–611. [DOI] [PubMed] [Google Scholar]
- Brierley B,Shaw P,David AS ( 2002): The human amygdala: A systematic review and meta‐analysis of volumetric magnetic resonance imaging. Brain Res Brain Res Rev 39: 84–105. [DOI] [PubMed] [Google Scholar]
- Brody AL,Mandelkern MA,Lee G,Smith E,Sadeghi M,Saxena S,Jarvik ME,London ED ( 2004): Attenuation of cue‐induced cigarette craving and anterior cingulate cortex activation in bupropion‐treated smokers: A preliminary study. Psychiatry Res 130: 269–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL,Mandelkern MA,Olmstead RE,Jou J,Tiongson E,Allen V,Scheibal D,London ED,Monterosso JR,Tiffany ST,Korb A,Gan JJ,Cohen MS ( 2007): Neural substrates of resisting craving during cigarette cue exposure. Biol Psychiatry 62: 642–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant RA,Kemp AH,Felmingham KL,Liddell B,Olivieri G,Peduto A,Gordon E,Williams LM ( 2007): Enhanced amygdala and medial prefrontal activation during nonconscious processing of fear in posttraumatic stress disorder: An fMRI study. Hum Brain Mapp; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchel C,Coull JT,Friston KJ ( 1999): The predictive value of changes in effective connectivity for human learning. Science 283: 1538–1541. [DOI] [PubMed] [Google Scholar]
- Buxton RB,Wong EC,Frank LR ( 1998): Dynamics of blood flow and oxygenation changes during brain activation: The balloon model. Magn Reson Med 39: 855–864. [DOI] [PubMed] [Google Scholar]
- Carter BL,Tiffany ST ( 1999): Meta‐analysis of cue‐reactivity in addiction research. Addiction 94: 327–340. [PubMed] [Google Scholar]
- Chiamulera C ( 2005): Cue reactivity in nicotine and tobacco dependence: A “multiple‐action” model of nicotine as a primary reinforcement and as an enhancer of the effects of smoking‐associated stimuli. Brain Res Brain Res Rev 48: 74–97. [DOI] [PubMed] [Google Scholar]
- Cox RW ( 1996): AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29: 162–173. [DOI] [PubMed] [Google Scholar]
- David SP,Munafo MR,Johansen‐Berg H,Smith SM,Rogers RD,Matthews PM,Walton RT ( 2005): Ventral striatum/nucleus accumbens activation to smoking‐related pictorial cues in smokers and nonsmokers: A functional magnetic resonance imaging study. Biol Psychiatry 58: 488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond DC ( 2001): Theories of drug craving, ancient and modern. Addiction 96: 33–46. [DOI] [PubMed] [Google Scholar]
- Due DL,Huettel SA,Hall WG,Rubin DC ( 2002): Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: Evidence from functional magnetic resonance imaging. Am J Psychiatry 159: 954–960. [DOI] [PubMed] [Google Scholar]
- Edwards G,Arif A,Hadgson R ( 1981): Nomenclature and classification of drug‐ and alcohol‐related problems: A WHO Memorandum. Bull World Health Organ 59: 225–242. [PMC free article] [PubMed] [Google Scholar]
- Etkin A,Klemenhagen KC,Dudman JT,Rogan MT,Hen R,Kandel ER,Hirsch J ( 2004): Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron 44: 1043–1055. [DOI] [PubMed] [Google Scholar]
- Everitt BJ,Robbins TW ( 2005): Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nat Neurosci 8: 1481–1489. [DOI] [PubMed] [Google Scholar]
- Fan J,Flombaum JI,McCandliss BD,Thomas KM,Posner MI ( 2003): Cognitive and brain consequences of conflict. Neuroimage 18: 42–57. [DOI] [PubMed] [Google Scholar]
- Franken IH,de Haan HA,van der Meer CW,Haffmans PM,Hendriks VM ( 1999): Cue reactivity and effects of cue exposure in abstinent posttreatment drug users. J Subst Abuse Treat 16: 81–85. [DOI] [PubMed] [Google Scholar]
- Franken IH,Kroon LY,Wiers RW,Jansen A ( 2000): Selective cognitive processing of drug cues in heroin dependence. J Psychopharmacol 14: 395–400. [DOI] [PubMed] [Google Scholar]
- Franklin TR,Wang Z,Wang J,Sciortino N,Harper D,Li Y,Ehrman R,Kampman K,O brien CP,Detre JA,Childress AR ( 2007): Limbic activation to cigarette smoking cues independent of nicotine withdrawal: A perfusion fMRI study. Neuropsychopharmacology 32: 2301–2309. [DOI] [PubMed] [Google Scholar]
- Friston KJ,Frith CD,Liddle PF,Frackowiak RS ( 1993): Functional connectivity: The principal‐component analysis of large (PET) data sets. J Cereb Blood Flow Metab 13: 5–14. [DOI] [PubMed] [Google Scholar]
- Friston KJ,Zarahn E,Josephs O,Henson RN,Dale AM ( 1999): Stochastic designs in event‐related fMRI. Neuroimage 10: 607– 619. [DOI] [PubMed] [Google Scholar]
- Gallagher HL,Frith CD ( 2003): Functional imaging of “theory of mind.” Trends Cogn Sci 7: 77–83. [DOI] [PubMed] [Google Scholar]
- Graul AI,Prous JR ( 2005): Executive summary: Nicotine addiction. Drugs Today (Barc ) 41: 419–425. [DOI] [PubMed] [Google Scholar]
- Gur RE,Cowell PE,Latshaw A,Turetsky BI,Grossman RI,Arnold SE,Bilker WB,Gur RC ( 2000): Reduced dorsal and orbital prefrontal gray matter volumes in schizophrenia. Arch Gen Psychiatry 57: 761–768. [DOI] [PubMed] [Google Scholar]
- Gur RE,Kohler C,Turetsky BI,Siegel SJ,Kanes SJ,Bilker WB,Brennan AR,Gur RC ( 2004): A sexually dimorphic ratio of orbitofrontal to amygdala volume is altered in schizophrenia. Biol Psychiatry 55: 512–517. [DOI] [PubMed] [Google Scholar]
- Hashimoto R,Sakai KL ( 2004): Learning letters in adulthood: Direct visualization of cortical plasticity for forming a new link between orthography and phonology. Neuron 42: 311–322. [DOI] [PubMed] [Google Scholar]
- Heatherton TF,Kozlowski LT,Frecker RC,Fagerstrom KO ( 1991): The Fagerstrom test for nicotine dependence: A revision of the Fagerstrom tolerance questionnaire. Br J Addict 86: 1119–1127. [DOI] [PubMed] [Google Scholar]
- Henderson LA,Yu PL,Frysinger RC,Galons JP,Bandler R,Harper RM ( 2002): Neural responses to intravenous serotonin revealed by functional magnetic resonance imaging. J Appl Physiol 92: 331–342. [DOI] [PubMed] [Google Scholar]
- Henderson LA,Richard CA,Macey PM,Runquist ML,Yu PL,Galons JP,Harper RM ( 2004): Functional magnetic resonance signal changes in neural structures to baroreceptor reflex activation. J Appl Physiol 96: 693–703. [DOI] [PubMed] [Google Scholar]
- Homae F,Yahata N,Sakai KL ( 2003): Selective enhancement of functional connectivity in the left prefrontal cortex during sentence processing. Neuroimage 20: 578–586. [DOI] [PubMed] [Google Scholar]
- Ingjaldsson JT,Thayer JF,Laberg JC ( 2003): Craving for alcohol and pre‐attentive processing of alcohol stimuli. Int J Psychophysiol 49: 29–39. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK,D'souza DC,Mencl WE,Pugh KR,Skudlarski P,Krystal JH ( 2004): Nicotine effects on brain function and functional connectivity in schizophrenia. Biol Psychiatry 55: 850–858. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK,Mencl WE,Constable RT,Westerveld M,Pugh KR ( 2007): Impact of smoking abstinence on working memory neurocircuitry in adolescent daily tobacco smokers. Psychopharmacology (Berl) 193: 557–566. [DOI] [PubMed] [Google Scholar]
- Jiang Y,He S ( 2006): Cortical responses to invisible faces: Dissociating subsystems for facial‐information processing. Curr Biol 16: 2023–2029. [DOI] [PubMed] [Google Scholar]
- Killgore WD,Yurgelun‐Todd DA ( 2004): Activation of the amygdala and anterior cingulate during nonconscious processing of sad versus happy faces. Neuroimage 21: 1215–1223. [DOI] [PubMed] [Google Scholar]
- Kincade JM,Abrams RA,Astafiev SV,Shulman GL,Corbetta M ( 2005): An event‐related functional magnetic resonance imaging study of voluntary and stimulus‐driven orienting of attention. J Neurosci 25: 4593–4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF,Le MM ( 2001): Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 24: 97–129. [DOI] [PubMed] [Google Scholar]
- Kremers SP,Mudde AN,de Vries NK,Brug J,de VH ( 2004): Unplanned smoking initiation: new insights and implications for interventions. Patient Educ Couns 55: 345–352. [DOI] [PubMed] [Google Scholar]
- Kubota Y,Sato W,Murai T,Toichi M,Ikeda A,Sengoku A ( 2000): Emotional cognition without awareness after unilateral temporal lobectomy in humans. J Neurosci 20: RC97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE ( 1996): The emotional brain: The Mysterious Underpinnings of Emotional Life. New York: Simon & Schuster. [Google Scholar]
- LeDoux JE ( 2000): Emotion circuits in the brain. Annu Rev Neurosci 23: 155–184. [DOI] [PubMed] [Google Scholar]
- Lee JH,Lim Y,Wiederhold BK,Graham SJ ( 2005): A functional magnetic resonance imaging (FMRI) study of cue‐induced smoking craving in virtual environments. Appl Psychophysiol Biofeedback 30: 195–204. [DOI] [PubMed] [Google Scholar]
- Lim HK,Pae CU,Joo RH,Yoo SS,Choi BG,Kim DJ,Lee C,Lee CU ( 2005): fMRI investigation on cue‐induced smoking craving. J Psychiatr Res 39: 333–335. [DOI] [PubMed] [Google Scholar]
- Loubinoux I,Carel C,Alary F,Boulanouar K,Viallard G,Manelfe C,Rascol O,Celsis P,Chollet F ( 2001): Within‐session and between‐session reproducibility of cerebral sensorimotor activation: A test–retest effect evidenced with functional magnetic resonance imaging. J Cereb Blood Flow Metab 21: 592–607. [DOI] [PubMed] [Google Scholar]
- Machielsen WC,Rombouts SA,Barkhof F,Scheltens P,Witter MP ( 2000): FMRI of visual encoding: Reproducibility of activation. Hum Brain Mapp 9: 156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride D,Barrett SP,Kelly JT,Aw A,Dagher A ( 2006): Effects of expectancy and abstinence on the neural response to smoking cues in cigarette smokers: An fMRI study. Neuropsychopharmacology 31: 2728–2738. [DOI] [PubMed] [Google Scholar]
- McClernon FJ,Hiott FB,Huettel SA,Rose JE ( 2005): Abstinence‐induced changes in self‐report craving correlate with event‐related FMRI responses to smoking cues. Neuropsychopharmacology 30: 1940–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ,Hiott FB,Liu J,Salley AN,Behm FM,Rose JE ( 2007): Selectively reduced responses to smoking cues in amygdala following extinction‐based smoking cessation: Results of a preliminary functional magnetic resonance imaging study. Addict Biol 12: 503–512. [DOI] [PubMed] [Google Scholar]
- Mogg K,Bradley BP ( 2002): Selective processing of smoking‐related cues in smokers: Manipulation of deprivation level and comparison of three measures of processing bias. J Psychopharmacol 16: 385–392. [DOI] [PubMed] [Google Scholar]
- Moolchan ET,Radzius A,Epstein DH,Uhl G,Gorelick DA,Cadet JL,Henningfield JE ( 2002): The Fagerstrom test for nicotine dependence and the diagnostic interview schedule: Do they diagnose the same smokers? Addict Behav 27: 101–113. [DOI] [PubMed] [Google Scholar]
- Morissette SB,Tull MT,Gulliver SB,Kamholz BW,Zimering RT ( 2007): Anxiety, anxiety disorders, tobacco use, and nicotine: A critical review of interrelationships. Psychol Bull 133: 245–272. [DOI] [PubMed] [Google Scholar]
- Morris JS,Ohman A,Dolan RJ ( 1998): Conscious and unconscious emotional learning in the human amygdala. Nature 393: 467–470. [DOI] [PubMed] [Google Scholar]
- Morris JS,Ohman A,Dolan RJ ( 1999): A subcortical pathway to the right amygdala mediating “unseen” fear. Proc Natl Acad Sci USA 96: 1680–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohman A,Soares JJ ( 1993): On the automatic nature of phobic fear: Conditioned electrodermal responses to masked fear‐relevant stimuli. J Abnorm Psychol 102: 121–132. [DOI] [PubMed] [Google Scholar]
- Pessoa L ( 2005): To what extent are emotional visual stimuli processed without attention and awareness? Curr Opin Neurobiol 15: 188–196. [DOI] [PubMed] [Google Scholar]
- Robinson S,Windischberger C,Rauscher A,Moser E ( 2004): Optimized 3 T EPI of the amygdalae. Neuroimage 22: 203–210. [DOI] [PubMed] [Google Scholar]
- Robinson TE,Berridge KC ( 1993): The neural basis of drug craving: An incentive‐sensitization theory of addiction. Brain Res Brain Res Rev 18: 247–291. [DOI] [PubMed] [Google Scholar]
- Robinson TE,Berridge KC ( 2003): Addiction Annu Rev Psychol 54: 25–53. [DOI] [PubMed] [Google Scholar]
- Rombouts SA,Barkhof F,Hoogenraad FG,Sprenger M,Valk J,Scheltens P ( 1997): Test‐retest analysis with functional MR of the activated area in the human visual cortex. AJNR Am J Neuroradiol 18: 1317–1322. [PMC free article] [PubMed] [Google Scholar]
- Rombouts SA,Barkhof F,Hoogenraad FG,Sprenger M,Scheltens P ( 1998): Within‐subject reproducibility of visual activation patterns with functional magnetic resonance imaging using multislice echo planar imaging. Magn Reson Imaging 16: 105–113. [DOI] [PubMed] [Google Scholar]
- Rosse RB,Johri S,Kendrick K,Hess AL,Alim TN,Miller M,Deutsch SI ( 1997): Preattentive and attentive eye movements during visual scanning of a cocaine cue: Correlation with intensity of cocaine cravings. J Neuropsychiatry Clin Neurosci 9: 91–93. [DOI] [PubMed] [Google Scholar]
- Scheibe C,Wartenburger I,Wustenberg T,Kathmann N,Villringer A,Heekeren HR ( 2006): Neural correlates of the interaction between transient and sustained processes: A mixed blocked/event‐related fMRI study. Hum Brain Mapp 27: 545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI,Barch DM,Donnelly JM,Ollinger JM,Snyder AZ,Mintun MA ( 2001): Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: An fMRI study. Biol Psychiatry 50: 651–658. [DOI] [PubMed] [Google Scholar]
- Sinha R,Fuse T,Aubin LR,O'Malley SS ( 2000): Psychological stress, drug‐related cues and cocaine craving. Psychopharmacology (Berl) 152: 140–148. [DOI] [PubMed] [Google Scholar]
- Stein JL,Wiedholz LM,Bassett DS,Weinberger DR,Zink CF,Mattay VS,Meyer‐Lindenberg A ( 2007): A validated network of effective amygdala connectivity. Neuroimage 36: 736–745. [DOI] [PubMed] [Google Scholar]
- Stein T,Moritz C,Quigley M,Cordes D,Haughton V,Meyerand E ( 2000): Functional connectivity in the thalamus and hippocampus studied with functional MR imaging. AJNR Am J Neuroradiol 21: 1397–1401. [PMC free article] [PubMed] [Google Scholar]
- Talairach J,Tournoux P ( 1988): Co‐planar Stereotaxic Atlas of the Human Brain. New York: Thieme. [Google Scholar]
- Tiffany ST ( 1990): A cognitive model of drug urges and drug‐use behavior: Role of automatic and nonautomatic processes. Psychol Rev 97: 147–168. [DOI] [PubMed] [Google Scholar]
- Tiffany ST,Conklin CA ( 2000): A cognitive processing model of alcohol craving and compulsive alcohol use. Addiction 95 ( Suppl 2): S145–S153. [DOI] [PubMed] [Google Scholar]
- Volkow N,Li TK ( 2005): The neuroscience of addiction. Nat Neurosci 8: 1429–1430. [DOI] [PubMed] [Google Scholar]
- Volkow ND,Li TK ( 2004): Drug addiction: The neurobiology of behaviour gone awry. Nat Rev Neurosci 5: 963–970. [DOI] [PubMed] [Google Scholar]
- Wexler BE,Gottschalk CH,Fulbright RK,Prohovnik I,Lacadie CM,Rounsaville BJ,Gore JC ( 2001): Functional magnetic resonance imaging of cocaine craving. Am J Psychiatry 158: 86–95. [DOI] [PubMed] [Google Scholar]
- Whalen PJ,Rauch SL,Etcoff NL,McInerney SC,Lee MB,Jenike MA ( 1998): Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci 18: 411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ,Kagan J,Cook RG,Davis FC,Kim H,Polis S,McLaren DG,Somerville LH,McLean AA,Maxwell JS,Johnstone T ( 2004): Human amygdala responsivity to masked fearful eye whites. Science 306: 2061. [DOI] [PubMed] [Google Scholar]
- Williams LM,Liddell BJ,Rathjen J,Brown KJ,Gray J,Phillips M,Young A,Gordon E ( 2004): Mapping the time course of nonconscious and conscious perception of fear: An integration of central and peripheral measures. Hum Brain Mapp 21: 64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM,Das P,Liddell BJ,Kemp AH,Rennie CJ,Gordon E ( 2006a) Mode of functional connectivity in amygdala pathways dissociates level of awareness for signals of fear. J Neurosci 26: 9264–9271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM,Liddell BJ,Kemp AH,Bryant RA,Meares RA,Peduto AS,Gordon E ( 2006b) Amygdala‐prefrontal dissociation of subliminal and supraliminal fear. Hum Brain Mapp 27: 652–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ,Sayette MA,Fiez JA ( 2004): Prefrontal responses to drug cues: A neurocognitive analysis. Nat Neurosci 7: 211–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ,Sayette MA,Delgado MR,Fiez JA ( 2005): Instructed smoking expectancy modulates cue‐elicited neural activity: A preliminary study. Nicotine Tob Res 7: 637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MT,Hsieh JC,Xiong J,Yang CF,Pan HB,Chen YC,Tsai G,Rosen BR,Kwong KK ( 1999): Central nervous pathway for acupuncture stimulation: Localization of processing with functional MR imaging of the brain–preliminary experience. Radiology 212: 133–141. [DOI] [PubMed] [Google Scholar]


