Abstract
The functions of human alpha oscillations (∼10 Hz) were related to cognitive processes such as memory and top‐down control. Recent models suggest that alpha phase serves as a mechanism especially relevant for the timing of neural activity, whereas alpha amplitude is important for the inhibition of task‐irrelevant brain areas. This study investigates directly the influence of top‐down modulation on phase‐locked and nonphase‐locked alpha rhythms. We conducted an EEG experiment where subjects performed a working memory task. In the encoding phase of the task subjects had to learn presented pictures of nonliving objects that could later be asked to be retrieved. We varied the top‐down modulation by including cues indicating either to remember or to forget (not to remember) the next following item. Spectral analyses showed that nonremember cues elicited pronounced alpha amplitude increase compared to remember cues. Furthermore, phase‐locking in low frequencies, especially in the alpha range (7–12 Hz), was stronger for remember as opposed to not‐to‐remember items. In conclusion, we propose that alpha amplitude reflects top‐down modulated inhibition and that alpha phase is important for the exact timing of neural activity and can be related to binding processes. Hum Brain Mapp, 2009. © 2009 Wiley‐Liss, Inc.
Keywords: alpha, oscillations, top‐down, phase‐locking
INTRODUCTION
The meaning of brain oscillations for different cognitive processes encounters increasing interest. In this study, the influence of top‐down processing on brain oscillations such as the human alpha rhythm (∼10 Hz) was investigated. The functional significance of alpha synchronization (increase in amplitude) is not yet clear and has been debated under several aspects such as idling [e.g. Pfurtscheller, 2001] or inhibition. As proposed by Klimesch et al. [2007b], alpha event‐related synchronization (ERS) [Pfurtscheller, 2001] most likely reflects inhibition as derived from several studies including, for example, working memory tasks [Busch and Herrmann, 2003; Jensen et al., 2002; Klimesch et al., 1999, 2000; Sauseng et al., 2005a] and motor memory tasks [e.g. Hummel et al., 2002]. The inhibition‐timing hypothesis comprises of two main aspects in regard to alpha oscillations: (i) the amplitude increase reflecting inhibition and (ii) the phase of the alpha rhythm that was considered as relevant for the timing of neural information processing. This means that oscillations enable time frames in which neurons are most likely to fire and, as a consequence, target cells receive their inputs synchronously which is thought to be important for neuronal communication [Varela et al., 2001]. The exact timing of neural activity would result in efficient behavioral task‐performance.
With respect to the phase of alpha oscillations, some studies reported evidence for the importance of alpha (phase) coupling in top‐down control and binding of memory processes [Bäuml et al., 2008; Sauseng et al., 2005a; Von Stein et al., 2000]. Further, increased alpha phase‐locking (measured with the PLI: phase‐locking index) has been shown to be relevant for correct categorization/identification to enable good performance [Hanslmayr et al., 2005; Klimesch et al., 2004]. Palva and Palva [2007] argue that alpha phase and not amplitude is of primary importance for cognitive functions such as attention and consciousness. For instance, it was shown that consciously perceived as opposed to unperceived stimuli were characterized by stronger alpha phase‐locking [Palva et al., 2005]. Phase‐concentration or high phase‐locking at around stimulus onset probably mirrors a phase‐reset [cf. Makeig et al., 2004]. Klimesch et al. [2007b] proposed that early processing of a stimulus is accompanied by top‐down processing, for example expectancy or attention. Top‐down activation most likely leads to a phase‐reset of an oscillation during an early poststimulus time‐window. Because the P1 component of the ERP was found to be the earliest manifestation of top‐down control, this seems a likely time‐window for a phase‐reset. In addition, in a very recent study, Mazaheri and Jensen [2008] could nicely demonstrate that also slow ERP components are probably generated by alpha amplitude variations.
Considering alpha ERS, it was found in several experiments that upper alpha increases with memory load and task demands, this has been interpreted as inhibition or disengagement of task‐irrelevant areas [Cooper et al., 2003; Jensen et al., 2002; Klimesch et al., 1999; Tuladhar et al., 2007]. In particular, Tuladhar et al. [2007] questioned whether the load‐dependent increase in upper alpha is related directly to working memory maintenance or to the inhibition of task‐irrelevant brain areas. If the alpha increase was directly related to working memory maintenance it would show up in specific areas relevant for working memory. Otherwise, if the increase was found in other areas not related to working memory it could be interpreted as inhibition of nontask relevant regions. They found strongest alpha effects in parieto‐occipital areas speaking in favor of the inhibition hypothesis. Similar results were obtained in a visual discrimination task relating the alpha increase to inhibition of the dorsal stream [Vanni et al., 1997]. Vanni et al. briefly presented line drawings of real and distorted objects. They found the strongest increase in alpha amplitude for nonobjects. This was interpreted as inhibition or disengagement of neural systems relevant for object recognition.
In our study, we used a modified version of the traditional Sternberg paradigm similar to a task by Mainy et al. [2007] while recording the EEG. The main idea of this task was to vary the encoding phase while holding constant the memory load. Each trial consisted of a set of 16 items (photographs) where each item was preceded by a cue indicating either to remember or to inhibit (not to remember) the following item. Several predictions can be derived from this design: (i) when alpha is related to inhibitory processing not‐to‐be‐remembered items should be preceded by alpha synchronization and (ii) this synchronization is most likely located to parieto‐occipital regions where access to relevant memory regions is blocked for further processing of the nonrelevant items, and thus, hinders efficient encoding of not‐to‐be‐remembered items [see Jokisch and Jensen, 2007; Tuladhar et al., 2007; Vanni et al., 1997].
To reveal effects concerning the timing aspect of the inhibition‐timing hypothesis, we included phase‐locking measures (PLI, phase‐locking index) in our analyses. The PLI provides a measure for the degree of phase‐locking for a specific oscillation to a stimulus event. An increase in PLI can be interpreted as a phase‐reorganization or phase resetting of an oscillation. For instance, it has been shown that during the time‐window of the P1‐N1 enhanced phase‐locking in the theta (4–6 Hz) and alpha range is apparent [Klimesch et al., 2004]. Most interestingly, the stronger phase‐locking was also associated with better memory performance during recognition. This is well in line with a recent study where enhanced alpha phase‐locking was related to good perception performance [Hanslmayr et al., 2005]. These findings show that oscillations reorganize their phases, as reflected in high PLI values, due to external events to establish good cognitive performance during task execution, and it also shows that phase‐locked oscillations do probably modulate or even generate ERP components. As outlined earlier, alpha phase‐coupling was associated with, for example, binding of memories [see Bäuml et al., 2008], thus we expected to find increased alpha phase‐locking for to‐be‐remembered as opposed to not‐to‐be‐remembered items. In several studies [e.g. Gruber et al., 2005; Klimesch et al., 2004, 2007b; Makeig et al., 2002], phase‐locked alpha was found to possibly modulate P1 component, thus, we also analyzed this component with special interest on similarities to alpha amplitude and/or phase‐locking. We expected to find enhanced alpha phase locking over areas where the P1 component is most pronounced and shows task‐related effects.
METHODS
Subjects
Thirty healthy university students (20 female, 10 male) with a mean age of 22.4 years (SD = 4.0) participated in this experiment. All except three were right‐handed. None of the subjects reported psychological or neurological disorders. Subjects had to sign an informed consent form, and the experiment was conducted according to the code of ethics [World Medical Association, 1996]. Seven subjects had to be excluded due to abundant eye or muscle artifacts resulting in a final pool of 23 subjects for data analyses.
Procedure
We used a modified version of the traditional Sternberg paradigm [Sternberg, 1966]. Subjects were seated 1 m in front of a CRT screen with a frame rate of 75 Hz. Each trial (a single trial is depicted in Fig. 1) started with a fixation screen followed by a train of 16 items of nonliving objects (e.g. ashtrays, suitcases, etc.) that were randomly cued either to remember (thumb up) or not‐to‐remember (thumb down). In each trial 8 items were cued as “remember” and 8 as “not‐to‐be‐remembered.” Cues were presented for 800 ms followed by a short fixation interval of 600 ms and the items were then presented for 1,000 ms. The interval between item‐offset and cue‐onset was 500 ms. Each object covered a visual angle of 4.3° × 6.0°. Subjects were explicitly instructed to only encode remember items and to ignore (or inhibit to encode) not‐to‐remember items. After the last object a retention interval of 1,800 ms was included and the probe items were presented for 1,000 ms. The subjects had to respond to “old” or “new” items by pressing a respective button. “New” items were objects not already presented in remember or not‐to‐be‐remembered trials but the stimulus‐category remained the same. For the whole experiment 8 “old” and 8 “new” probe items were presented. Items that were cued as not‐to‐be‐remembered were never presented as probes. In sum, the whole experiment involved 64 items (8 NonRem‐items per trial × 8 trials) for the to‐be‐remembered and 64 (8 Rem‐items per trial × 8 trials) items for the not‐to‐be‐remembered condition.
Figure 1.
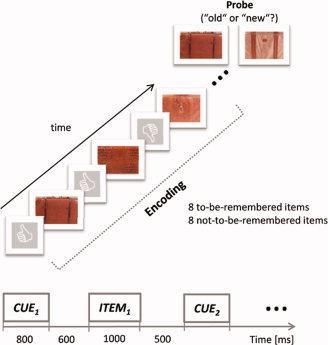
Experimental procedure of the modified Sternberg task is depicted. Each item was preceded by a cue either indicating to remember (thumb) or to not remember (thumb down) the item. In every trial eight items were presented, four to‐be‐remembered items and four not‐to‐be‐remembered items. Items were presented for 1,000 ms, cues for 800 ms, followed by a short interval of 600 ms between cue and item. After the presentation of the last item in the encoding list a retention interval of a duration of 1,800 ms was included followed by the presentation of a probe item that had to be judged as “old” or “new.” [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
EEG Recordings
EEG‐signals were recorded using a BrainAmp system (BrainProducts) referenced to a nose electrode and subsequently rereferenced to digitally linked earlobes. Band‐pass filters were set from 0.15 to 100 Hz with a notch filter at 50 Hz. Signals were digitized at a sampling rate of 500 Hz. Thirty‐one Ag‐AgCl‐electrodes were mounted on the following positions using an EasyCap: Fp1, Fp2, F7, F3, Fz, F4, F8, Fc3, Fcz, Fc4, T3, C3, Cz, C4, T4, Cp5, Cp1, Cp2, Cp6, T5, P3, Pz, P4, T6, Po7, Po3, Po4, Po8, O1, Oz, O2. Impedances were kept below 8 kΩ. To control for vertical and horizontal eye movements, two bipolar EOG‐channels were mounted. After rereferencing the data were transformed into laplacian current source density values (μA/m2). Current source density transformation enables to overcome possible influences of volume conduction and referencing problems [Nunez et al., 2001]. Epochs containing eye or muscle artifacts were rejected. Data analyses were performed using BrainVision Analyzer (BrainProducts) and Matlab® 7.6. (The MathWorks).
P1‐Amplitude
For ERP analyses the data were filtered within 1 and 30 Hz and segmented for remember and nonremember items separately from −1,000 to 1,000 ms in respect to onset of set items. Within this time‐window, we performed a semiautomatic peak detection as implemented in BrainVision Analyzer 1.05.
Total Power
For time‐frequency estimations, the segmented data (−1,900 to 600 ms in respect to onset of individual items) were wavelet‐transformed and subsequently averaged. To establish an adequate time‐frequency resolution, for lower frequencies from 1 to 25 Hz, we used a 9‐cycle complex Morlet wavelet and for higher frequencies from 26 to 70 Hz, a 7‐cycle wavelet. For power analyses six frequency bands were selected: theta = 4–6 Hz, lower alpha = 7–9 Hz, upper alpha = 10–12 Hz, beta = 15–25 Hz, lower gamma = 26–45 Hz, higher gamma = 55–70 Hz.
Phase‐Locking Index
The phase‐locking index (PLI) reflects the extent of intertrial phase‐variability for a given frequency across time [Tallon‐Baudry et al., 1996]. PLI values range from 0 (high‐phase variability) to 1 (no phase‐variability). For PLI analyses we first filtered the data with the Gabor expansion from 1 to 20 Hz and within −1,000 to 1,000 ms around item appearance. For every time and frequency bin PLI values were calculated resulting in a time‐by‐frequency matrix. For later statistical analyses PLI bins were averaged for three frequency bands: theta (4–6 Hz), lower alpha (7–9 Hz), and upper alpha (10–12 Hz). These bands were selected because of findings showing functional similarities between ERPs and oscillations [e.g. Freunberger et al., 2007]. Posterior electrodes (P3, Pz, P4, Po7, Po3, Po4, Po8, O1, Oz, O2) were selected for PLI analyses, given that in these regions alpha shows most pronounced amplitudes.
Statistical Designs
Visual inspection of ERPs showed effects for the P1 component on posterior‐electrodes only. We therefore selected electrodes Po7 and Po8 for ERP analyses given that P1 was most pronounced on these locations and calculated an ANOVA with factors ELECTRODE (Po7, Po8) and CONDITION (to‐be‐remembered, not‐to‐be‐remembered).
For analyses of power estimates the wavelet‐transformed data were averaged for four time‐windows and for the six frequency bands. The time‐windows were defined as two preitem intervals from −400 to −200 ms and from −200 to 0 ms, and two postitem intervals from 0 to 200 ms and from 200 to 400 ms. We calculated two‐way ANOVAS with factors CONDITION (to‐be‐remembered, not‐to‐be‐remembered) and ELECTRODE (all 31 electrodes) for each time‐window separately.
PLI was analyzed by application of two‐way ANOVAs for four time‐windows (t1 = −400 to −200 ms, t2 = −200 to 0 ms, t3 = 0–200 ms, t4 = 200–400 ms) with factors ELECTRODE (P3, Pz, P4, Po7, Po3, Po4, Po8, O1, Oz, O2) and CONDITION (to‐be‐remembered, not‐to‐be‐remembered). Greenhouse‐Geisser correction was applied where necessary and the significance level was set to P < 0.05. For significant interactions Scheffé post‐hoc comparisons were calculated.
For the evaluation of possible dependencies between phase‐locking of oscillations and ERP components, we calculated Spearman's correlation coefficients.
RESULTS
Behavioral Data
Subjects responded correctly to old items with a mean percentage hit rate of 82.1% (SD = 13.0) and to new items with 91.8% (SD = 9.0), respectively. Mean reaction times for old items were 1046.0 ms (SD = 252.4) and 984.1 ms (SD = 191.5) for new items. Hit rates and reaction times showed no significant differences between old and new items as revealed by t‐tests.
P1‐Amplitude
The two‐way ANOVA yielded significant main effects for ELECTRODE (F 1/22 = 4.64, P = 0.042) and CONDITION (F 1/22 = 5.88, P = 0.024) indicating that P1 amplitude is generally higher for to‐be‐remembered as compared to not‐to‐be‐remembered items and that this effect is more pronounced on Po8 as compared to Po7 (cf. Fig. 2a,b).
Figure 2.
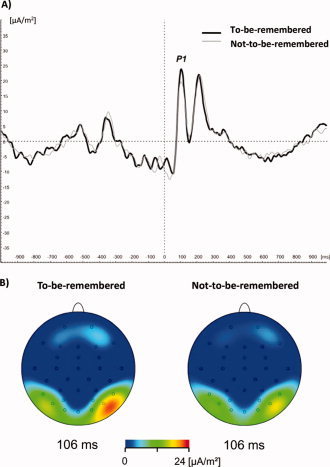
ERP curves and topographical maps for electrode Po8 is shown. (A) Bold black lines indicate to‐be‐remembered and thin gray lines not‐to‐be‐remembered items. Time zero denotes item presentation. Note the stronger P1 at around 100 ms for to‐be‐remembered as compared to not‐to‐be‐remembered items. (B) Topographical maps of P1 component at 106 ms for to‐be‐remembered and not‐to‐be‐remembered items. Strongest task‐related effects were found on the right hemisphere. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Total Power
Time‐frequency plots for a representative electrode (Po8) are plotted in Figure 3. Not‐to‐be‐remembered compared to to‐be‐remembered items showed enhanced upper alpha power over time‐windows from −200 to 400 ms as indicated by the main effects for CONDITION (t2: F 1/22 = 4.53, P = 0.045; t3: F 1/22 = 9.54, P = 0.005; t4: F 1/22 = 11.34, P = 0.003). We also found the usual effects for factor ELECTRODE (t1: F 30,660 = 12.80, P < 0.001; t2: F 30,660 = 15.12, P < 0.001; t3: F 30,660 = 16.25, P < 0.001; t4: F 30,660 = 7.80, P < 0.001) and two CONDITION × ELECTRODE interactions for the time‐windows from 0 to 200 ms and from 200 to 400 ms (t3: F 60/660 = 5.61, P = 0.001; t4: F 60/660 = 5.61, P < 0.001, respectively). For t3, post‐hoc comparisons (Scheffé test, diffcrit = 10.911; P < 0.01) revealed that alpha power was more pronounced for not‐to‐be‐remembered items compared to to‐be‐remembered items on electrodes T6, Po7, Po4, Po8, O2 and for t4 on electrodes T6 and Po7 (cf. Fig. 4). The mean alpha amplitudes averaged over the significant posterior electrode positions amounted to 98.38 μA/m2 (SD = 41.86) for t3 and 70.32 μA/m2 (SD = 23.71) for t4 for to‐be‐remembered items; for not‐to‐be‐remembered items the mean values were 113.48 μA/m2 (SD = 56.84) for t3 and 79.42 μA/m2 (SD = 28.95) for t4.
Figure 3.
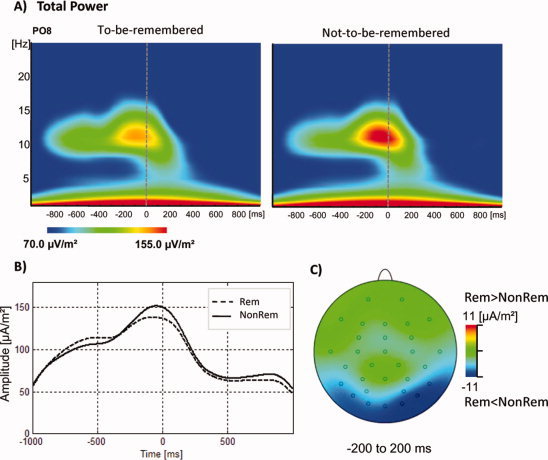
Total power plots as revealed by wavelet transformation for electrode Po8. (A) Time‐frequency plots for to‐be‐remembered items (time = 0 ms) and for not‐to‐be‐remembered items. Warm colors denote power increases. There is a stronger increase at frequencies at around 12 Hz for not‐to‐be‐remembered compared to to‐be‐remembered items. (B) 12 Hz power is plotted as a function of time. Dotted lines denote to‐be‐remembered and black lines not‐to‐be‐remembered items. Not‐to‐be‐remembered items showed a stronger increase in 12 Hz power at around item‐onset when compared to to‐be‐remembered items. (C) Topographical plots of 12 Hz power for to‐be‐remembered minus not‐to‐be‐remembered items for the time‐range from −200 to 200 ms. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Figure 4.
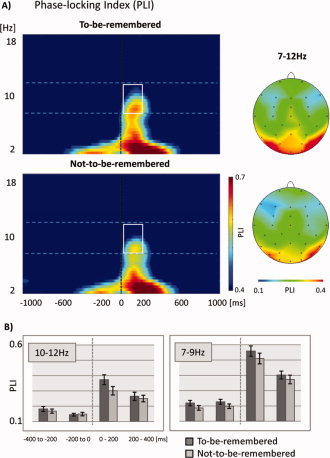
Phase‐locking as revealed by the phase‐locking index (PLI) of frequencies from 1–20 Hz over time for electrode Po8. (A) Time‐frequency plots of PLI values for to‐be‐remembered and not‐to‐be‐remembered items. Topographical distributions are provided for the 7–12 Hz range (denoted by the white squares within the time‐frequency plots). Warm colors indicate strong phase‐locking, cold colors no phase‐locking. (B) Two frequency bands extracted from the time‐frequency plots. Strongest differences between to‐be‐remembered (red lines) and not‐to‐be‐remembered (black lines) items were found in the range from 10–12 Hz. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
For lower alpha, theta, and beta bands, as well as for higher gamma frequencies the two‐way ANOVAs yielded no task‐specific effects for the factor CONDITION and/or for the interaction ELECTRODE × CONDITION.
PLI
PLI results are shown as time‐frequency plots in Figure 4a, the results for each of the three frequency bands are plotted in Figure 4b, respectively. For upper alpha the ANOVAs yielded significant main effects for factor CONDITION (F 1/22 = 11.97, P = 0.002) and ELECTRODE (F 1/22 = 3.24, P = 0.012) within the time‐window from 0 to 200 ms. In the lower alpha band ANOVAs reached significance for the time‐windows ranging from −200 to 0 ms (CONDITION: F 1/22 = 11.02, P = 0.003; ELECTRODE: F 1/22 = 5.17, P < 0.001), from 0 to 200 ms (CONDITION: F 1/22 = 11.00, P < 0.001; ELECTRODE: F 1/22 = 5.90, P < 0.001), and from 200 to 400 ms (CONDITION: F 1/22 = 6.00, P = 0.023; ELECTRODE: F 1/22 = 4.09, P = 0.004). All of the effects indicate a higher phase‐locking for to‐be‐remembered as compared to not‐to‐be‐remembered items. For the theta band no significant effects were revealed for factor CONDITION or the interaction CONDITION × ELECTRODE.
Correlation Between PLI and P1 Component
For estimation of Spearman's correlation coefficients, we first calculated the ratio of to‐be‐remembered versus not‐to‐be‐remembered items for PLIs from theta, lower, upper alpha, and the P1 amplitude over electrode Po8. For data normalization the resulting quotients were logarithmically transformed. We found a positive correlation between P1 and PLI ratios for lower alpha (r = 0.438, P = 0.037) and upper alpha (r = 0.617, P = 0.002) but not for theta. This finding indicates a relationship between alpha phase‐locking and P1 modulation.
DISCUSSION
As predicted we found an increase in upper alpha power when subjects were cued not to remember the following item in a time‐period around onset of item‐presentation. This is in line with a variety of studies providing strong evidence that the human alpha rhythm plays a crucial role for top‐down processing and inhibition during visual working memory [e.g. Herrmann et al., 2004; Klimesch et al., 1999; Jensen et al., 2002; Jokisch and Jensen, 2007; Sauseng et al., 2005a; Tuladhar et al., 2007], auditory working memory [e.g. Kaiser et al., 2007], object detection [e.g. Vanni et al., 1997], and attention [Freunberger et al., 2008; Sauseng et al., 2005b; Thut et al., 2006]. In this study, alpha power increased steadily in response to both types of cues, probably reflecting the initiation of top‐down control. Most importantly, however, we found a functional relationship between phase‐locked alpha and the P1 amplitude as well as dissociation between phase‐locked and non‐phase‐locked alpha activity. The P1 and phase‐locked alpha showed similar reactivity to task‐variations, i.e. we found higher P1 amplitudes and a larger alpha PLI for to‐be‐remembered as compared to not‐to‐be‐remembered items (see Figs. 2 and 4, respectively). This functional relationship is also indicated by the correlation analysis. On the other hand, non‐phase‐locked alpha activity in the preitem interval increased stronger for not‐to‐be‐remembered items (Fig. 3) when compared with to‐be‐remembered items showing a functional dissociation between phase‐locked and nonphase‐locked alpha.
The obtained effects can be interpreted as alpha reflecting top‐down control, which would be relevant in the present task for attentional and memory processing. Phase‐locking of alpha oscillations across electrodes [i.e. phase‐coupling indexed by PLV, phase‐locking values, Lachaux et al., 1999] were recently discussed as a mechanism associated with binding of memories in a directed forgetting task [Bäuml et al., 2008]. In this task subjects were presented with two lists of 20 words. Between the word lists a cue was given indicating either to remember or to forget the List‐1 items. List‐2 items were always cued to remember. After a distracter condition, subjects were tested to recall items from List‐1 and List‐2 separately. The behavioral data showed a specific effect in a way that forget cues induced memory enhancement of List‐2 items and forgetting of List‐1 items. Most interestingly, phase‐coupling was generally lower in the forget condition as compared to the remember condition. Noteworthy, this phase‐coupling decrease was apparent during the whole trial already in prestimulus intervals. The authors conclude that this decrease of upper alpha phase‐coupling can be associated with “unbinding” of previously learned List‐1 items. In addition, they showed that increased alpha power was a correlate of memory enhancement of List‐2 items in the forget condition. This can be interpreted as a top‐down process to inhibit interfering and distracting information. A similar interpretation of alpha oscillations as a relevant top‐down mechanism important for memorization has been shown in an auditory Sternberg task [Leiberg et al., 2006].
The study by Bäuml et al. [2008] can be well linked to the results of the presented study. We also suggest that the upper alpha power increase after not‐to‐be‐remembered cues reflects top‐down regulations to inhibit processing of the following item. The stronger phase‐locking most likely can be associated with some sort of binding of information during memory storage. Rizzuto et al. [2003] found a stronger phase‐locking to probe items in a verbal working memory task that they interpreted as a correlate of phase reset. In their study, phase‐locking of oscillations at 8 Hz was negatively correlated to power in the same range. Interestingly, they also found enhanced phase‐locking to list items in specific areas mainly located in the right occipital and temporal cortex. These findings fit to the data given that the effects in our study were also located in posterior regions more pronounced on the right hemisphere (cf. Figs. 2b and 3c.) and, thus, can well be linked to other studies showing memory‐related top‐down effects within posterior regions [e.g. Tuladhar et al., 2007; Vanni et al., 1997]. Evidence for the assumption that alpha phase‐locking reflects processes relevant for memory encoding also comes from a study showing that increased alpha PLI is associated with better memory performance and that the enhanced phase‐locking correlates with early ERP components such as the P1 and N1 [Klimesch et al., 2004]. In our study, we found a strong correlation between upper alpha phase‐locking and P1 modulation speaking in favor of similar functional processes.
When interpreting the reported findings, we have to keep in mind that oscillations can influence the generation of an ERP component in at least two different ways, by (i) the degree of phase locking and (ii) a change in amplitude. The influence of these two factors can be estimated by calculating alpha phase locking (e.g., by the phase locking index, PLI as we have done in the present experiment) and alpha power. Here, we have associated the increased P1 with increased phase locking. But it is important to emphasize that an increase in the amplitude of an ERP component (in one condition as compared to another) can be obtained also when an oscillation increases in amplitude in one condition (with no difference in PLI between two conditions). As a consequence, a functional relationship between ERPs and oscillations can be investigated with respect to phase‐locking and/or power variations of a certain oscillation. Later, we report findings, where an increase in P1 amplitude was associated with an increase in alpha power. In these cases an increase in amplitude appears functionally related to inhibition. For instance, Freunberger et al. [2008] reported higher P1 amplitudes associated with higher alpha power in a spatial cueing task. In this study subjects had to direct their attention towards a cued position, either on the right or left visual hemifield. It could be observed that during the cue‐target interval upper alpha increased stronger on ipsilateral compared to the contralateral sites leading to the assumption that alpha inhibits task‐irrelevant sites [see also Rihs et al., 2007; Sauseng et al., 2005b for similar results]. In addition, similar to alpha power, P1 amplitude was also stronger on task‐irrelevant ipsilateral sites. These and also the present results provide evidence that P1 and alpha show a covariation. In previous studies the ERP‐P1 component was typically found to be stronger on contralateral as compared to ipsilateral sites [e.g. Heinze et al., 1994; Mangun and Hillyard, 1991; for reviews see Hillyard and Anllo‐Vento, 1998; Posner and Dehaene, 1994]. The enhancement of P1 in spatial attention tasks was thought to reflect top‐down modulated amplification of neural networks relevant for target processing.
To conclude, our findings provide evidence that alpha is an important top‐down control mechanism for inhibitory processing within areas being task‐irrelevant and that P1 shows a functional relationship to alpha oscillations.
Acknowledgements
P.S. is recipient of an APART fellowship of the Austrian Academy of Sciences. The authors want to thank the students from the laboratory course “ES Biologische Psychologie” for their help in data acquisition and recruitment of subjects. Their names are in alphabetical order: J. Acker, F. Dicke, M. Fillinger, S. Frank, M. Glennon, A. Heidecke, V. Karl, S. Kohl, L. König, C. Mühlberger, A. Narr, Y. Pees, M. Peters, A.‐T. Saliger, M. Schroffenegger, D. Udovicic. We also thank Mark Glennon for language editing and two anonymous reviewers for their helpful comments on a previous version of this manuscript.
REFERENCES
- Bäuml KH,Hanslmayr S,Pastötter B,Klimesch W ( 2008): Oscillatory correlates of intentional updating in episodic memory. Neuroimage 41: 596–604. [DOI] [PubMed] [Google Scholar]
- Busch NA,Herrmann CS ( 2003): Object‐load and feature‐load modulate EEG in a short‐term memory task. NeuroReport 14: 1721–1724. [DOI] [PubMed] [Google Scholar]
- Cooper NR,Croft RJ,Dominey SJJ,Burgess AP,Gruzelier JH ( 2003): Paradox lost? Exploring the role of alpha oscillations during externally vs. internally directed attention and the implications for idling and inhibition hypotheses. Int J Psychophysiol 47: 65–74. [DOI] [PubMed] [Google Scholar]
- Freunberger R,Klimesch W,Doppelmayr M,Höller Y ( 2007): Visual P2 component is related to theta phase‐locking. Neurosci Lett 426: 181–186. [DOI] [PubMed] [Google Scholar]
- Freunberger R,Höller Y,Griesmayr B,Gruber W,Sauseng P,Klimesch W ( 2008): Functional similarities between the P1 component and alpha oscillations. Eur J Neurosci 27: 2330–2340. [DOI] [PubMed] [Google Scholar]
- Gruber WR,Klimesch W,Sauseng P,Doppelmayr M ( 2005): Alpha phase synchronization predicts P1 end N1 latency and amplitude size. Cereb Cortex 15: 371–377. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S,Klimesch W,Sauseng P,Gruber W,Doppelmayr M,Freunberger R,Pecherstorfer T ( 2005): Visual discrimination performance is related to decreased alpha amplitude but increased phase locking. Neurosci Lett 375: 64–68. [DOI] [PubMed] [Google Scholar]
- Heinze HJ,Mangun GR,Burchert W,Hinrichs H,Scholz M,Münte TF,Gös A,Scherg M,Johannes S,Hundeshagen H,Gazzaniga MS,Hillyard SA ( 1994): Combined spatial and temporal imaging of brain activity during visual selective attention in humans. Nature 372: 543–546. [DOI] [PubMed] [Google Scholar]
- Herrmann CS,Senkowski D,Röttger S ( 2004): Phase‐locking and amplitude modulations of EEG alpha: Two measures reflect different cognitive processes in a working memory task. Exp Psychol 51: 311–318. [DOI] [PubMed] [Google Scholar]
- Hillyard SA,Anllo‐Vento L ( 1998): Event‐related brain potentials in the study of visual selective attention. Proc Natl Acad Sci USA 95: 781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel F,Andres F,Altenmüller E,Dichgans J,Gerloff C ( 2002): Inhibitory control of acquired motor programmes in the human brain. Brain 125: 404–420. [DOI] [PubMed] [Google Scholar]
- Jensen O,Gelfand J,Kounios J,Lisman JE ( 2002): Oscillations in the alpha band (9‐12 Hz) increase with memory load during retention in a short‐term memory task. Cereb Cortex 12: 877–882. [DOI] [PubMed] [Google Scholar]
- Jokisch D,Jensen O ( 2007): Modulation of gamma and alpha activity during a working memory task engaging the dorsal or ventral stream. J Neurosci 27: 3244–3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser J,Heidegger T,Wibral M,Altmann CF,Lutzenberger W ( 2007): Alpha synchronization during auditory spatial short‐term memory. NeuroReport 18: 1129–1132. [DOI] [PubMed] [Google Scholar]
- Klimesch W,Doppelmayr M,Schwaiger J,Auinger P,Winkler T ( 1999): 'Paradoxical' alpha synchronization in a memory task. Cognit Brain Res 7: 493–501. [DOI] [PubMed] [Google Scholar]
- Klimesch W,Doppelmayr M,Röhm D,Pöllhuber D,Stadler W ( 2000): Simultaneous desynchronization and synchronization of different alpha responses in the human electroencephalograph: A neglected paradox? Neurosci Lett 284: 97–100. [DOI] [PubMed] [Google Scholar]
- Klimesch W,Schack B,Schabus M,Doppelmayr M,Gruber W,Sauseng P ( 2004): Phase‐locked alpha and theta oscillations generate the P1‐N1 complex and are related to memory performance. Cognit Brain Res 19: 302–316. [DOI] [PubMed] [Google Scholar]
- Klimesch W,Sauseng P,Hanslmayr S,Gruber W,Freunberger R ( 2007b): Event‐related phase reorganization may explain evoked neural dynamics. Neurosci Biobehav Rev 31: 1003–1016. [DOI] [PubMed] [Google Scholar]
- Lachaux JP,Rodriguez E,Martinerie J,Varela FJ ( 1999): Measuring phase synchrony in brain signals. Hum Brain Mapp 8: 194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiberg S,Lutzenberger W,Kaiser J ( 2006): Effects of memory load on cortical oscillatory activity during auditory pattern working memory. Brain Res 1120: 131–140. [DOI] [PubMed] [Google Scholar]
- Mainy N,Kahane P,Minotti L,Hoffmann D,Bertrand O,Lachaux JP ( 2007): Neural correlates of consolidation in working memory. Hum Brain Mapp 28: 183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeig S,Westerfield M,Jung TP,Enghoff S,Townsend J,Courchesne E,Sejnowski TJ ( 2002): Dynamic brain sources of visual evoked responses. Science 295: 690–694. [DOI] [PubMed] [Google Scholar]
- Makeig S,Debener S,Onton J,Delorme A ( 2004): Mining event‐related brain dynamics. Trends Cognit Sci 8: 204–210. [DOI] [PubMed] [Google Scholar]
- Mangun GR,Hillyard SA ( 1991): Modulations of sensory‐evoked brain potentials indicate changes in perceptual processing during visual‐spatial priming. J Exp Psychol: Hum Percept Perform 17: 1057–1074. [DOI] [PubMed] [Google Scholar]
- Mazaheri A,Jensen O ( 2008): Asymmetric amplitude modulations of brain oscillations generate slow evoked responses. J Neurosci 28: 7781–7787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez PL,Wingeier BM,Silberstein RB ( 2001): Spatial‐temporal structures of human alpha rhythms: Theory, microcurrent sources, multiscale measurements, and global binding of local networks. Hum Brain Mapp 13: 125–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva S,Palva JM ( 2007): New vistas for alpha‐frequency band oscillations. Trends Neurosci 30: 150–158. [DOI] [PubMed] [Google Scholar]
- Palva S,Linkenkaer‐Hansen K,Nätäänen R,Palva JM ( 2005): Early neural correlates of conscious somatosensory perception. J Neurosci 25: 5248–5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G ( 2001): Functional brain imaging based on ERD/ERS. Vision Res 41: 1257–1260. [DOI] [PubMed] [Google Scholar]
- Posner MI,Dehaene S ( 1994): Attentional networks. Trends Neurosci 17: 75–79. [DOI] [PubMed] [Google Scholar]
- Rihs TA,Michel CM,Thut G ( 2007): Mechanisms of selective inhibition in visual spatial attention are indexed by alpha‐band EEG synchronization. Eur J Neurosci 25: 603–610. [DOI] [PubMed] [Google Scholar]
- Rizzuto DS,Madsen JR,Bromfield EB,Schulze‐Bonhage A,Seelig D,Aschenbrenner‐Scheibe R,Kahana MJ ( 2003): Reset of human neocortical oscillations during a working memory task. Proc Natl Acad Sci USA 100: 7931–7936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauseng P,Klimesch W,Doppelmayr M,Pecherstorfer T,Freunberger R,Hanslmayr S ( 2005a): EEG alpha synchronization and functional coupling during top‐down processing in a working memory task. Hum Brain Mapp 26: 148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauseng P,Klimesch W,Stadler W,Schabus M,Doppelmayr M,Hanslmayr S,Gruber WR,Birbaumer N ( 2005b): A shift of visual spatial attention is selectively associated with human EEG alpha activity. Eur J Neurosci 22: 2917–2926. [DOI] [PubMed] [Google Scholar]
- Sternberg S ( 1966): High‐speed scanning in human memory. Science 153: 652–654. [DOI] [PubMed] [Google Scholar]
- Tallon‐Baudry C,Bertrand O,Delpuech C,Pernier J ( 1996): Stimulus specificity of phase‐locked and non‐phase‐locked 40 Hz visual responses in human. J Neurosci 16: 4240–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut G,Nietzel A,Brandt SA,Pascual‐Leone A ( 2006): Alpha‐band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. J Neurosci 26: 9494–9502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuladhar AM,Ter Huurne N,Schoffelen JM,Maris E,Oostenveld R,Jensen O ( 2007): Parieto‐occipital sources account for the increase in alpha activity with working memory load. Hum Brain Mapp 28: 785–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanni S,Revonsuo A,Hari R ( 1997): Modulation of the parieto‐occipital alpha rhythm during object detection. J Neurosci 17: 7141–7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela FJ,Lachaux JP,Rodriguez E,Martinerie J ( 2001): The brainweb: Phase synchronization and large‐scale integration. Nat Rev Neurosci 2: 229–239. [DOI] [PubMed] [Google Scholar]
- Von Stein A,Chiang C,König P ( 2000): Top‐down processing mediated by interareal synchronization. Proc Natl Acad Sci USA 97: 14748–14753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Medical Association ( 1996): Declaration of Helsinki. Br Med J 313: 1448–1449. [Google Scholar]


