Abstract
While cognitive impairments are well documented for the acute episode of major depressive disorder (MDD), less is known about cognitive functioning in the euthymic state. For working memory, dysfunctional activation of lateral prefrontal and cingulate cortex has been reported in the acute episode. This study investigates working‐memory function and its neurobiological correlate in euthymic MDD patients, particularly whether dysfunctional activation persists when depressive symptoms improve. We investigated 56 subjects with functional magnetic resonance imaging (fMRI) at 3 Tesla. To challenge working‐memory function, a classical verbal n‐back task (0‐, 1‐, and 2‐back) was used in 28 well‐characterized, euthymic, unipolar MDD patients and 28 healthy control subjects matched according to age, sex, and educational level. Data were analyzed using SPM5. In the absence of significant behavioral differences, we observed comparable overall patterns of brain activation in both groups. As expected, both groups showed stronger activation of the typical working‐memory network with increasing memory load. However, significant hyperactivation of the cingulate cortex was observed in euthymic patients, while lateral prefrontal activation was comparable between patients and controls. Working‐memory challenge in the euthymic state of MDD revealed a dissociation of lateral prefrontal and cingulate brain function. Cingulate function, which is important for both emotional and cognitive processing and their integration, is still abnormal when mood is restored. This could reflect a different speed of normalization in prefrontal and limbic cortices, persistent systematic changes in neuronal networks after an episode of MDD, or a compensatory mechanism to maintain working‐memory performance. Hum Brain Mapp, 2009. © 2008 Wiley‐Liss, Inc.
Keywords: major depression, remission, cognitive deficits, functional magnetic resonance imaging, working memory, n‐back
INTRODUCTION
Major depressive disorder (MDD) is one of the most prevalent psychiatric disorders leading to a dramatic reduction of quality of life, increased mortality risk, (Alonso and Lepine, 2007; Cuijpers and Smit, 2002) and causing a significant individual and economic burden as the most costly brain disorder in Europe (Sobocki et al., 2006; von Knorring et al., 2006).
Neuropsychological deficits of different functional domains are well documented for the acute phase of a depressive episode (Airaksinen et al., 2004; Burt et al., 1995; Castaneda et al., 2008; Landro et al., 2001; Ravnkilde et al., 2002; Veiel, 1997; Zakzanis et al., 1998). However, the nature of these deficits, the cognitive domains affected, as well as the severity of cognitive impairments is still a matter of ongoing debate. Compared with the acute phase of major depression, even less is known about the neurocognitive profile of patients who recovered from depression. Several studies reported lasting deficits in some cognitive domains (Austin et al., 2001; Kessing, 1998; Marcos et al., 1994; Paelecke‐Habermann et al., 2005; Paradiso et al., 1997) such as executive functions and attention (Paelecke‐Habermann et al., 2005; Smith et al., 2006; Trichard et al., 1995). The influence of clinical depression on working‐memory function is still under debate (Channon et al., 1993; Christopher and MacDonald, 2005; Harvey et al., 2004; Landro et al., 2001; Rose and Ebmeier, 2006; Zakzanis et al., 1998).
As we know from clinical experience, MDD patients often complain about problems with thinking and concentration (Nair et al., 1999). While impairments of working memory in the acute phase of MDD have been reported previously, studies focusing on this crucial cognitive function in remitted depression are rare. Subtle deficits were reported for strategic aspects of a spatial working‐memory task (Weiland‐Fiedler et al., 2004).
Working memory is an extensively researched psychological concept dealing with the temporary storage and processing of information (Baddeley, 1992; Baddeley, 2003). Intact working memory is essential for every day functioning. Working‐memory tasks require several cognitive processes, such as online monitoring, continuous updating, manipulating stored information, and decision making, which all might be affected by MDD. The neuronal processes underlying working‐memory processes have been widely investigated with neuroimaging techniques (Owen et al., 2005; Wager and Smith, 2003). In healthy subjects, the verbal n‐back task activated a bilateral network consisting of dorsolateral and ventrolateral prefrontal cortex, lateral premotor cortex, dorsal cingulate and medial premotor cortex, frontal poles, and medial and lateral posterior parietal cortex (Owen et al., 2005). Task‐related activity was shown to be correlated with working‐memory load. Especially dorsolateral and left inferior regions of the prefrontal cortex show a linear relationship between activity and task complexity (Braver et al., 2001).
To date, only a few imaging studies investigated working memory in major depression, almost exclusively focusing on the acute phase (Fitzgerald et al., 2008; Harvey et al., 2005; Matsuo et al., 2007; Rose et al., 2006; Walter et al., 2007a; Walter et al., 2007b). These studies revealed abnormalities in cortico‐limbic networks fundamentally involved in the pathophysiology of major depression (Dougherty and Rauch, 2007; Mayberg, 1997). Compared with healthy control subjects, a stronger activation was observed in the limbic system and lateral prefrontal cortex of MDD patients, in the absence of significant behavioral differences (Fitzgerald et al., 2008; Matsuo et al., 2007). For example, Matsuo et al. reported stronger left dorsolateral and anterior cingulate cortex (ACC) activation in 15 MDD patients performing a visuo‐spatial task, while healthy controls failed to show cingulate activation (Matsuo et al., 2007). Harvey et al. used a verbal variant of the n‐back task and compared 10 MDD patients with 10 controls (Harvey et al., 2005). Both groups showed similar activation, but the lateral prefrontal cortex and the anterior cingulate were activated more strongly in MDD patients. Rose et al. investigated 10 MDD patients and 10 healthy controls with an n‐back task and also reported anterior cingulate differences in load‐dependent activation between patients and controls (Rose et al., 2006). Using a longitudinal design, Walsh et al. reported greater load‐response in the verbal working‐memory network of patients (Walsh et al., 2007). Taken together, previous studies indicate that an acute episode of MDD is associated with abnormal cortico‐limbic activation in working‐memory, mainly characterized by hyperactivation of lateral prefrontal and cingulate areas. Almost nothing is known as to whether this hyperactivation observed in the acute phase is a state‐dependent phenomenon and whether or not brain activation normalizes when depressive symptoms are no longer predominant.
Although the above studies often failed to find differences on behavioral measures, Walter et al. found behavioral differences between 12 partially remitted patients (mean Hamilton depression rating scale [HDRS] score of 18.2) and controls in a delayed match‐to‐sample working‐memory task (Hamilton, 1960; Walter et al., 2007b). The authors also reported stronger activation in the dorsolateral prefrontal cortex (DLPFC) for the highest cognitive load condition, and in the ventromedial prefrontal cortex for the control condition.
To the best of our knowledge, no functional magnetic resonance imaging (fMRI) study has yet investigated working‐memory function in a large group of completely euthymic unipolar depressed patients. Thus, the goal of this study was to investigate working‐memory function, in particular prefrontal and cingulate activation during working‐memory performance, in euthymic MDD patients. We hypothesized that behavioral working‐memory performance of euthymic MDD patients is almost equal to healthy controls. We expected neurobiological differences in brain regions such as cingulate gyrus and prefrontal areas between euthymic patients with MDD and controls.
MATERIALS AND METHODS
Subjects
In total, 56 subjects were recruited for this study. Twenty‐eight inpatients from the Department of Psychiatry of the University of Muenster or the LWL‐Clinic Muenster (16 female, 12 male subjects), fulfilling DSM‐IV criteria for a MDD, participated in this study (for details see Table I). A diagnosis of either first (n = 9) or recurrent episode (n = 19) of unipolar depression was verified using the standardized SCID‐I‐ Interview (German version) (Wittchen et al., 1997), in addition to clinical assessment by two board‐certified specialists in Psychiatry. MDD patients with psychotic depression or axis‐II disorders were excluded. Twenty‐three patients had MDD alone; three patients had comorbid dysthymia (“double depression”). Further comorbid axis‐I disorders were excluded if symptoms of the comorbid disorder required current treatment. One patient was additionally diagnosed with social phobia, and one patient with panic disorder with agoraphobia. This was, however, not relevant for current hospitalization. Patients participated just before discharge from the hospital after achieving a stable euthymic state characterized by HDRS (HDRS ≤8) and confirmed by two board‐certified specialists in Psychiatry. The following additional inclusion criteria were applied: age between 18 and 55 years, no treatment with electroconvulsive therapy during the previous depressive episode, no history of any other serious medical or neurological disease, no serious head injury, no suicidal tendency, no benzodiazepine treatment 3 days before scanning, and no MRI contraindications. All patients were right‐handed, as assessed by the Edinburgh Handedness Inventory (Oldfield, 1971) and had more than 12 years of education. Twenty‐seven patients were treated according to current treatment guidelines in a stable dosage and one patient did not receive any medication. The following antidepressants were prescribed as antidepressive monotherapy (13), combined antidepressive therapy (3), or combined antidepressive/antipsychotic therapy (11): citalopram (2), escitalopram (8), mirtazapine (11), venlafaxine (11), reboxetine (1), duloxetine (1), trancylpromine (1); none were taking tricyclic antidepressants. To rule out any negative effects on memory function antipsychotics were used instead of benzodiazepines for treatment of agitation and nervousness in some patients: quetiapine (9), risperidone (2), pipamperone (1). None of the patients was taking benzodiazepines at the time of testing.
Table I.
Mean and standard deviation (SD) for age, intelligence, BDI, HDRS assessed at time of testing, number of depressive episodes and hospitalizations, and days of current hospitalization
| Controls (N = 28) | Patients (N = 28) | Significance test | |
|---|---|---|---|
| Gender ratio (f/m) | 16 f/12 m | 16 f/12 m | χ2 = 0, df = 1, P = 1 |
| Age | 33.42 ± 9.62 | 34.18 ± 10.62 | t = −0.26, df = 54, P > 0.05 |
| Intelligence, MWT‐B‐score | 32.14 ± 2.27 | 31.04 ± 2.85 | t = 1.61, df = 54, P > 0.05 |
| Beck depression inventory (BDI) | 2.54 ± 3.12 | 8.92 ± 6.24 | t = −4.85, df = 39.71, P < 0.001 |
| Hamilton depression rating scale (HDRS) | — | 3.64 ± 2.63 | — |
| Number of depressive episodes | — | 2.54 ± 1.75 | — |
| Number of hospitalization | — | 1.54 ± 0.70 | — |
| Days of current hospitalization | — | 75.61 ± 34.60 | — |
Twenty‐eight healthy, right‐handed control subjects, recruited by advertisement in the local newspaper, were 1:1 matched to the patients according to sex and age (±3 years). Education level, both in terms of years of education and highest graduation level, was also balanced between groups. All control subjects underwent an initial telephone screening to ensure matching criteria, to exclude medical and neurological diseases, or MRI contraindications. The standardized SCID‐I‐Interview was performed to exclude any current or previous psychiatric disorders (Wittchen et al., 1997). In healthy controls no psychiatric disorders in first degree relatives were reported.
All procedures were approved by the local Institutional Ethical Review Board. The ethical standards of the Declaration of Helsinki were met and all participants provided written informed consent.
Materials and Procedures
The working‐memory task was the first part of a larger fMRI and neuropsychological study of memory processes in euthymic MDD. We used a classical letter variant of the n‐back task (Braver et al., 1997). Before entering the scanner, a detailed task instruction was given and participants were familiarized with the n‐back task until they succeeded in the training trials. A standardized brief instruction announced the start of the task in the scanner. Working‐memory load was manipulated in three levels (0‐2‐back), presented in a block design. During the 0‐back condition, subjects had to press the response button of a MRI‐compatible response box if the target letter “X” appeared on the screen. In the 1‐back condition subjects had to decide if the actual letter on the screen was identical to the previous letter. During the 2‐back condition, subjects had to decide if the actual letter was identical to the letter presented two trials before. Subjects responded with their right hand, using the index finger for targets and middle finger for nontargets.
Each active n‐back condition lasted 36 s and n‐back blocks were presented in a fixed order (1‐0‐2‐0‐1‐2) to each subject. Subjects completed two blocks of each n‐back condition. White letters were presented in the centre of a black screen for 500 ms, with an interstimulus interval of 2500 ms (Presentation Software®, Version 0.81, 2004, Neurobehavioral Systems, Albany, CA). Only orthographically distinct uppercase consonants were used (B, C, D, F, G, H, J, K, M, Q, R, S, T, V, X, Z). Each letter sequence consisted of 12 consonants, including one‐third targets. During fMRI scanning, a short instruction announced the n‐back type. All n‐back conditions were separated by a pause of 21 s during which participants had to look at a white fixation cross on a black screen.
As part of the larger study protocol, all patients and control subjects underwent neuropsychological testing, such as the Mehrfachwahlwortschatz‐Test (MWT‐B) as an estimate of verbal intelligence (Lehrl et al., 1995) and the Beck Depression Inventory (BDI) (Beck et al., 1961).
Scanning Procedures
MRI data acquisition was performed in a 3 Tesla whole‐body scanner (Intera T 3.0, Philips, Best, NL), equipped with master gradients (nominal gradient strength 30mT/m, maximal slew rate 150mT/m/ms). A circularly polarized transmit/receive birdcage head coil with an HF reflecting screen at the cranial end was used for spin excitation and resonance signal acquisition. Functional images were acquired using a T2* weighted single shot echo planar (EPI) sequence (whole brain coverage, TE = 38, TR = 3000ms, flip angle 90°, slice thickness 3.6 mm without gap, matrix 64 × 64, FOV 230 mm, in‐plane resolution 3.6 × 3.6). 36 transversal slices orientated to the AC‐PC line were acquired.
Behavioral Data Analysis
During fMRI scanning, responses and response latencies (in ms) for the n‐back performance were recorded. Behavioral results were acquired from all 28 patients. Data from three control subjects were omitted because of technical difficulties. Performance is reported as accuracy rate (percentage of correct answers) for each n‐back condition. Repeated‐measures analyses of variance (ANOVAs), with one between‐subject factor (group: two levels) and one within‐subject factor (working‐memory load: three levels), were performed for accuracy rate and response latency.
Functional Data Analysis
Functional MRI data were analyzed using SPM5 standard routines and templates (http://www.fil.ion.ucl.ac.uk/spm). The first 10 images of each session (30 s prestimulus interval) were discarded to allow for saturation effects of the BOLD signal. The remaining images were realigned, normalized, and resliced to a voxel size of 2 mm × 2 mm × 2 mm. Gaussian smoothing was performed using a 9 mm kernel. Data were filtered with a high‐pass filter (cut‐off period of 128 s). A boxcar function convolved with the canonical hemodynamic response function implemented in SPM5 was used to model BOLD‐responses for the working‐memory task. In a first‐level fixed‐effects analysis, the conditions 0‐back, 1‐back, 2‐back, and visual instruction were modeled. Contrast images for 0‐, 1‐, and 2‐back conditions to general baseline and for 2‐back versus other activation conditions (2vs0‐back and 2vs1‐back) were derived. The individual contrast images were entered into a second‐level random‐effects analysis to obtain activation maps across subjects. To display the different contrasts in each group, one‐sample t‐tests were performed (P < 0.05, corrected for false discovery rate [FDR], contiguity threshold ≥15 voxels). On the basis of previous findings (Harvey et al., 2005; Matsuo et al., 2007) and our hypotheses of higher cingulate and prefrontal activation, differences between patients and controls were calculated in the cingulate gyrus and in the inferior, middle, and superior DLPFC, using two‐sample t‐tests (P < 0.05, corrected for FDR, contiguity threshold ≥15 voxels). The regions of interest in the bilateral cingulate cortex (anterior, medial and posterior part) as well as the inferior, middle, and superior dorsolateral frontal gyrus were defined according to the automated anatomical labeling (AAL) atlas (Tzourio‐Mazoyer et al., 2002) as implemented in the WFU PickAtlas Toolbox (Maldjian et al., 2003) (ROI names: ACIN, MCIN, PCIN, F1, F2, F3OP/T). To verify that this approach does not overlook important effects outside the ROIs, a whole‐brain analysis was performed at a more liberal threshold (P < 0.0005, uncorrected for multiple comparisons, contiguity threshold ≥15 voxels). Within each group, a correlation analysis between behavioral data (response latency and accuracy) and task‐related activity was performed across all voxels and all conditions. Clinical variables (HDRS, days of hospitalization, and number of depressive episodes) were additional variables for the patient group.
RESULTS
Behavioral Results
No significant differences between groups were observed. Analysis of variance on accuracy and response latency revealed a significant main effect of working‐memory load (F(2,102) = 12.64, P < 0.001) and (F(2,102) = 32.65, P < 0.001), respectively. As expected, accuracy decreased and response latency increased from 0‐back to 2‐back condition (Figs. 1 and 2). However, no main effects of group (F(1,51)<1, P > 0.05) or interactions between group and working‐memory load (F(2,102)<1, P > 0.05) emerged for accuracy or response latency. Furthermore, no significant differences were observed as a function of verbal intelligence (MWT‐B) (two‐sample t‐test, T = 1.61, df = 56, P > 0.05).
Figure 1.

Behavioral data for accuracy rate (percentage of correct answers, mean ± standard error) with varying working memory load in patients and control subjects reveal a significant effect of working memory load, but not of group or interaction.
Figure 2.
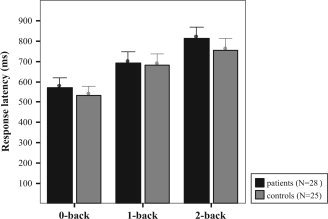
Behavioral data for response latency (mean ± standard error) in all load conditions in patients and control subjects. A main effect of working memory load condition was observed, but no effect of group or interaction.
Activation Patterns Across Load Conditions and Groups
For each group, activation was investigated for each load condition of the working‐memory task separately. Healthy controls and patients activated the brain areas relevant for a verbal working‐memory task, as expected from the literature (Owen et al., 2005; Wager and Smith, 2003). Activation was found in the medial frontal and inferior frontal gyrus, insula, pre‐ and postcentral gyrus, inferior parietal lobule, and cerebellum in both groups. In both groups, we observed an increase of brain activation from the 0‐back to 2‐back condition (see Supporting Information). Activation increased with working‐memory demand, in particular, with respect to the bilateral activation of the inferior and middle frontal cortex.
Regions Activated With Increasing Working‐Memory Load
Activation increases from 0‐back to 2‐back (2vs0‐back contrast)
Common to both groups were the following effects for the 2vs0‐back contrast (P < 0.05, corrected for FDR, contiguity threshold ≥15 voxels). First, we observed extended activation clusters of the inferior, middle, superior, and medial frontal cortex, including typical verbal working‐memory regions, such as parts of the medial frontal cortex, dorsolateral and ventrolateral prefrontal cortex (BA 9, 46, 45, 47) (Fig. 3). Next, we found activation of the insula, supplementary motor area, temporal lobe, and cerebellum. Finally, there was strong activation in the parietal lobe, in the inferior and superior parietal lobule (BA 7, 40), the angular and supramarginal gyrus extending to the superior and middle occipital gyrus (BA 19, 18). However, while healthy controls showed only few activated clusters in the cingulate cortex, parahippocampal gyrus, and hippocampus, patients activated large parts of the cingulate cortex (BA 24, 32, 33), and parahippocampal gyrus (BA 27, 28, 35, 36) in the 2vs0‐back contrast (for details see also Supporting Information).
Figure 3.
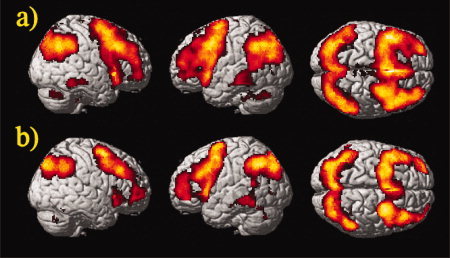
Group activation in (a) patients and (b) controls for the 2vs0‐back contrast (one sample t‐test, P < 0.05, corrected for FDR, contiguity threshold ≥15 voxels). Random‐effects analysis rendered on the surface of the canonical template image used by SPM5. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Activation increases from 1‐back to 2‐back (2vs1‐back contrast)
For the 2vs1‐back contrast (see Fig. 4), healthy controls activated few and small clusters in the inferior frontal cortex and the superior frontal cortex (BA6) (P < 0.05, corrected for FDR, contiguity threshold ≥15 voxels). Activation was also found in the precuneus, inferior and superior parietal lobule. In patients, we observed the following: there were large activated clusters in the inferior, middle, medial, and superior frontal gyrus (BA 6, 8, 9, 44‐47); parallel to the 2vs0‐back contrast, the cingulate cortex (BA 24, 32, 33), parahippocampal gyrus (BA 35, 36), and hippocampus were significantly activated; significant activations were also observed in the insula, pre‐ and postcentral gyrus, temporal and occipital lobe, and cerebellum; and finally, in the parietal lobe, the angular and supramarginal gyrus, the inferior and superior parietal lobule, and precuneus were bilaterally activated (BA 7, 39, 40) (for details see also Supporting Information).
Figure 4.
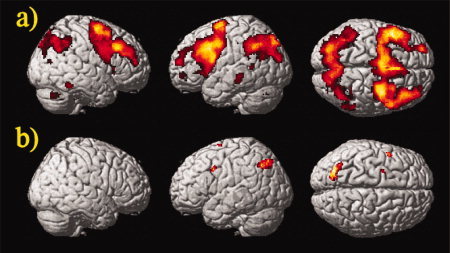
Group activation in (a) patients and (b) controls for the 2vs1‐back contrast (one sample t‐test, P < 0.05, corrected for FDR, contiguity threshold ≥15 voxels). Random‐effects analysis rendered on the surface of the canonical template image used by SPM5. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Analysis of additional factors
No significant correlations (P < 0.05, corrected for FDR, contiguity threshold ≥15 voxels) between brain activation and behavioral measures (accuracy and response latency) were observed, neither in patients nor in controls. One exception concerned a small correlation of accuracy with the right inferior frontal lobe in patients for the 2‐back condition (MNI coordinate 32/34/12) (Fig. not shown). Moreover, no significant correlations between brain activation and clinical variables such as Hamilton scores, days of hospitalization, or number of depressive episodes were found in patients (P < 0.05, corrected for FDR, contiguity threshold ≥15 voxels).
Further analysis in the patient group revealed no significant differences between the 13 patients treated with antidepressive monotherapy and the 14 patients treated with a combination therapy of antidepressants or antipsychotics for the 2vs0‐back and 2vs1‐back contrast (two‐sample t‐test, P < 0.05, corrected for FDR, contiguity threshold ≥15 voxels). An analysis of variance showed no effect of gender (P < 0.05, corrected for FDR, contiguity threshold ≥15 voxels).
Between‐Group Comparisons
As we expected group differences in specialized working‐memory areas, particularly prefrontal areas and the cingulate cortex, a ROI‐analysis was performed between groups (two‐sample t‐test, P < 0.05, corrected for FDR, contiguity threshold ≥15). In the cingulate cortex, both the 2vs0‐back and the 2vs1‐back contrast revealed stronger activation of the anterior and posterior cingulate cortex (BA 24, 32, 23, 31) for patients than healthy controls. Unlike patients, healthy controls showed no increased cingulate activation (Figs. 5 and 6). In the prefrontal cortex, especially dorsolateral (BA 9, 46) and ventrolateral (BA 45, 47) PFC, no significant differences between patients and controls were found (Fig. not shown).
Figure 5.
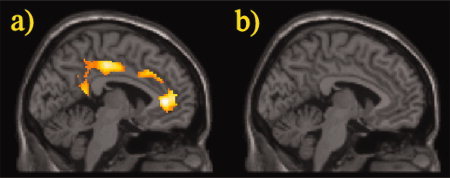
Differences in cingulate brain activation between (a) patients versus controls and (b) controls versus patients in the 2vs0‐back contrast. ROI analysis, two sample t‐test, P < 0.05, corrected for FDR, contiguity threshold ≥15 voxels, projected on the canonical template image used by SPM5. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Figure 6.
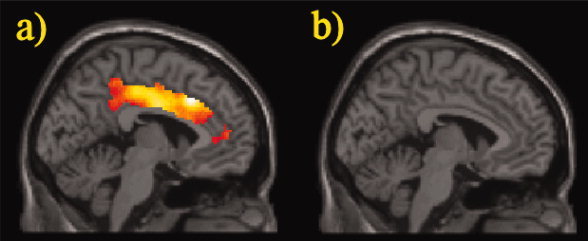
Differences in cingulate brain activation between (a) patients versus controls and (b) controls versus patients in the 2vs1‐back contrast. ROI analysis, two sample t‐test, P < 0.05, corrected for FDR, contiguity threshold ≥15 voxels, projected on the canonical template image used by SPM5. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
To verify that this approach does not overlook important effects outside the ROIs, a whole‐brain analysis was performed at a more liberal threshold (P < 0.0005, uncorrected for multiple comparisons, contiguity threshold ≥15). The cingulate difference between groups for both the 2vs0‐back and the 2vs1‐back contrast was corroborated and no other relevant activations outside the ROIs were detected (see Supporting Information).
DISCUSSION
Cognitive impairments are an important characteristic of major depression. Modern neuroimaging methods indicate that dysfunction of cortico‐limbic networks plays an important role in the pathophysiology of both affective and cognitive symptoms in MDD (Dougherty and Rauch, 2007). In the acute episode of depression, brain metabolism is significantly altered, with pathological changes in the dorsolateral prefrontal and limbic cortex at rest and during cognitive activation (Drevets, 2001; Ebmeier et al., 2006; Fitzgerald et al., 2006; Greicius et al., 2007). Much less is known about brain function when depressed patients reach the euthymic mood state. Neuropsychological data suggest that cognitive deficits persist in certain domains, and thus might represent more a trait than a state characteristic (Paelecke‐Habermann et al., 2005). This study investigated networks involved in working‐memory function in recently remitted patients with major depression. We explored whether dysfunctional activation of the lateral prefrontal and cingulate cortex would still be present in the euthymic phase of major depression, as had been previously reported for the acute episode of major depression (Harvey et al., 2005; Matsuo et al., 2007; Rose et al., 2006; Walter et al., 2007b).
In line with previous reports, we found the classic working‐memory network activated in the n‐back task (Owen et al., 2005; Wager and Smith, 2003). With increasing working‐memory demand, strong activation was observed in both patients and controls, in the dorsolateral and ventrolateral prefrontal cortex, middle frontal cortex, and precentral gyrus. Both groups also showed activation in the parietal cortex, of the angular and supramarginal gyrus, inferior and superior parietal lobule, precuneus, and superior occipital gyrus. Activation was also observed in the temporal cortex, whose role for working‐memory processes is as yet poorly understood, and subject of current research (Axmacher et al., 2007; Picchioni et al., 2007).
A novel and interesting finding is that our data point to a deviance of the working‐memory network in patients with MDD even in the euthymic state. So far, altered prefrontal and cingulate activity during working‐memory tasks has only been reported in severely depressed patients, mainly in the acute phase of major depression. The majority of these studies did not find behavioral deficits between patients and controls (Harvey et al., 2005; Matsuo et al., 2007; Rose et al., 2006). Patients in the acute phase performing working‐memory tasks showed hyperactivation of the DLPFC (Harvey et al., 2005; Matsuo et al., 2007) and ACC (Harvey et al., 2005; Matsuo et al., 2007; Rose et al., 2006). These findings were taken as evidence for the recruitment of additional resources to fulfill the cognitive demands of a given task.
In this study, patients in the euthymic state showed hyperactivation of the cingulate cortex, a region involved in both emotional and cognitive processing, while lateral prefrontal hyperactivation was not observed relative to healthy controls. Both of these areas on the lateral and medial surface of the prefrontal cortex are known to play a central role in the pathophysiology of depression. Baseline functional‐imaging studies demonstrated metabolic and regional blood flow abnormalities in major depression, in particular a decreased metabolism in DLPFC and increased metabolism in orbitofrontal cortex (Dougherty and Rauch, 2007). This cortico‐limbic network also reveals abnormal function when challenged by cognitive tasks such as working memory, most prominently evident as an increase of lateral prefrontal and limbic activity (Harvey et al., 2005; Matsuo et al., 2007; Rose et al., 2006). As a major result, our data indicate that metabolic abnormalities in the cingulate persist even in the euthymic state of MDD, while lateral cortical abnormalities normalize. Our results might reflect an earlier normalization of lateral prefrontal function occurring prior to possible similar changes in anterior cingulate areas in the course of remission.
The role of the ACC has been controversially discussed in depression and recovery, playing an important role in both cognitive and emotional processing. The dorsal subdivision of the ACC subserves many cognitive functions, including working memory, and is highly interconnected with other regions involved in working memory, such as the above‐mentioned DLPFC (Bush et al., 2000; Devinsky et al., 1995). This dorsal ACC region is involved in task complexity, mental effort or attentional processes (Mulert et al., 2007; Mulert et al., 2005), conflict monitoring, and error processing (Bioulac et al., 2005; Botvinick et al., 2004; Carter et al., 1999; Carter et al., 1998; Kerns et al., 2004; Michelet et al., 2007; Sohn et al., 2007; van Veen and Carter, 2006). On the other hand, the rostral part of the ACC subserves emotional processing, especially for the assessment of emotional information and the regulation of emotional responses (Whalen et al., 1998). This part is highly interconnected with the amygdala, hippocampus, hypothalamus, nucleus accumbens, and orbitofrontal cortex. Alterations of (rostral) ACC metabolism have been associated with depressive symptoms, their severity, and treatment response in MDD patients (Chen et al., 2007; Konarski et al., 2007; Mayberg et al., 1997; Milak et al., 2005). Moreover, brain imaging studies revealed altered brain activation of the rostral part of the ACC for emotional tasks in depressed patients (Frodl et al., 2007; Mitterschiffthaler et al., 2008).
In this study, we observed an activation increase of the ACC with increasing working‐memory load in patients, which seemed to involve both the dorsal and the rostral part. Our findings corroborate cingulate cortex hyperactivation observed in patients in the acute depressive episode (Harvey et al., 2005; Matsuo et al., 2007). Here, we demonstrate that cingulate hyperactivation during working‐memory performance is still present when affective symptoms such as depressed mood or reduced drive are much relieved or have even subsided. As in acute depression, we might now hypothesize that enhanced recruitment of these cerebral resources is necessary to fulfill the cognitive demands of the given task. Enhanced recruitment might as well be necessary as baseline metabolism is decreased in the ACC even after recovery from depression (Holthoff et al., 2004). Our finding of hyperactivation of the ACC in an affective disorder during a cognitive task underlines the importance of this region for both emotional and cognitive processing. A clear allocation of the hyperactivation to either limbic or cognitive circuits based on neuroanatomy (Bush et al., 2000) is not warranted by our findings.
As mentioned above, behavioral performance was not significantly different between euthymic MDD patients and healthy controls. This was expected on the basis of results from fMRI studies with acute depressed patients (Harvey et al., 2005; Matsuo et al., 2007). It is thus unlikely that behavioral differences between patients and healthy controls are responsible for the observed differential activation pattern. However, our study used a block design and only two levels of task difficulty, which might be not sensitive enough to detect subtle disturbances of working‐memory capacity. Some other limitations of our study also need to be mentioned. We cannot exclude medication effects, since our patients did receive psychiatric treatment to modern standards of care. Previous studies on the effects of antidepressant medication revealed that pharmacological treatment leads to an attenuation or decrease of limbic activation in response to emotional stimuli rather than to an increase of ACC activation, as observed in the present study (Arce et al., 2008; Fu et al., 2004; Harmer et al., 2006; Sheline et al., 2001). Although the above evidence points towards an attenuating effect of antidepressants on brain activation, we cannot completely exclude an opposite effect in a working‐memory task, but this seems rather unlikely. This study does not claim to investigate medication effects on working memory performance in depression. Additional studies will have to tackle this problem. Furthermore, patients were only included when depressive symptoms were considerably reduced. We did not assess the time course of brain activation during the course of recovery, so additional studies need to clarify if further changes occur when more time elapses after the acute depressive episode. A strength of our study is the high number of well‐characterized patients, for whom strict exclusion criteria were met.
To summarize, we demonstrated that even after clinical improvement of affective symptoms, abnormal cingulate activation was associated with a classical working‐memory task in patients compared with healthy controls. In contrast to patients in the acute depressive episode, ACC hyperactivation, but no lateral prefrontal hyperactivation, occurred in patients in the euthymic state. Our data might reflect a different lateral prefrontal and cingulate pace of normalization, a trait marker of changes in neuronal networks after an episode of MDD, or a compensatory mechanism to maintain adequate working‐memory performance.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information Figure 1. Group activation in a) patients and b) controls for the 0‐, 1‐, and 2‐back condition (one sample t‐test, p<0.05, corrected for family wise error (FWE), contiguity threshold ≥ 15 voxels). Random‐effects analysis rendered on the surface of the canonical template image used by SPM5. Supporting Information Figure 2. Comparison of working memory activation in a) patients versus healthy controls and b) healthy controls versus patients for the 2vs0‐back contrast (whole brain analysis, p < 0.0005, uncorrected for multiple comparisons, contiguity threshold ≥ 15 voxels), projected on the canonical template image used by SPM5. Significant activation in a) were observed in the cingulate cortex (BA 24, 31, 32) extending into the medial frontal gyrus (BA 6), parahippocampal gyrus (BA 35), middle temporal (BA 21) and paracentral gyrus (BA 2, 3, 4). Healthy controls did not show any significant activation relative to patients. Supporting Information Figure 3. Comparison of working memory activation in a) patients versus healthy controls and b) healthy controls versus patients for the 2vs1‐back contrast (whole brain analysis, p < 0.0005, uncorrected for multiple comparisons, contiguity threshold ≥ 15 voxels), projected on the canonical template image used by SPM5. Significant activation in a) were observed in the cingulate cortex (BA 24, 31) extending into the inferior parietal lobule and supramarginal gyrus (BA 40) and angular gyrus (BA 39) and medial frontal gyrus (BA 6), and pre‐ and postcentral gyrus (BA 2, 3, 4). Healthy controls did not show any significant activation relative to patients. Supporting Information Table 1. Cortical activation pattern for the 2‐0‐back and 2‐1‐back contrast for a) patients b) healthy control subjects, as obtained by a random effect analysis (puncorr.<0.05, FDR corrected, contiguity threshold ≥ 15 voxels). Supporting Information Table 2. Cortical activation pattern for the 2‐0‐back and 2‐1‐back contrast for a) patients vs. controls b) healthy control subjects vs. patients, as obtained by a random effect analysis (whole brain analysis, p < 0.0005, uncorrected, contiguity threshold ≥ 15 voxels).
REFERENCES
- Airaksinen E,Larsson M,Lundberg I,Forsell Y ( 2004): Cognitive functions in depressive disorders: Evidence from a population‐based study. Psychol Med 34: 83–91. [DOI] [PubMed] [Google Scholar]
- Alonso J,Lepine JP ( 2007): Overview of key data from the European Study of the Epidemiology of Mental Disorders (ESEMeD). J Clin Psychiatry 68( Suppl 2): 3–9. [PubMed] [Google Scholar]
- Arce E,Simmons AN,Lovero KL,Stein MB,Paulus MP ( 2008): Escitalopram effects on insula and amygdala BOLD activation during emotional processing. Psychopharmacologia 196: 661–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin MP,Mitchell P,Goodwin GM ( 2001): Cognitive deficits in depression: Possible implications for functional neuropathology. Br J Psychiatry 178: 200–206. [DOI] [PubMed] [Google Scholar]
- Axmacher N,Mormann F,Fernandez G,Cohen MX,Elger CE,Fell J ( 2007): Sustained neural activity patterns during working memory in the human medial temporal lobe. J Neurosci 27: 7807–7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A ( 1992): Working memory. Science 255: 556–559. [DOI] [PubMed] [Google Scholar]
- Baddeley A ( 2003): Working memory: Looking back and looking forward. Nat Rev Neurosci 4: 829–839. [DOI] [PubMed] [Google Scholar]
- Beck AT,Ward CH,Mendelson M,Mock J,Erbaugh J ( 1961): An inventory for measuring depression. Arch Gen Psychiatry 4: 561–571. [DOI] [PubMed] [Google Scholar]
- Bioulac B,Michelet T,Guehl D,Aouizerate B,Burbaud P ( 2005): [The anterior cingulate cortex in error detection and conflict monitoring. Unitary neuronal activity in monkeys]. Bull Acad Natl Med 189: 1529–1538; discussion1538–1540. [PubMed] [Google Scholar]
- Botvinick MM,Cohen JD,Carter CS ( 2004): Conflict monitoring and anterior cingulate cortex: An update. Trends Cogn Sci 8: 539–546. [DOI] [PubMed] [Google Scholar]
- Braver TS,Barch DM,Kelley WM,Buckner RL,Cohen NJ,Miezin FM,Snyder AZ,Ollinger JM,Akbudak E,Conturo TE,Petersen SE ( 2001): Direct comparison of prefrontal cortex regions engaged by working and long‐term memory tasks. Neuroimage 14(1 Pt 1): 48–59. [DOI] [PubMed] [Google Scholar]
- Braver TS,Cohen JD,Nystrom LE,Jonides J,Smith EE,Noll DC ( 1997): A parametric study of prefrontal cortex involvement in human working memory. Neuroimage 5: 49–62. [DOI] [PubMed] [Google Scholar]
- Burt DB,Zembar MJ,Niederehe G ( 1995): Depression and memory impairment: A meta‐analysis of the association, its pattern, and specificity. Psychol Bull 117: 285–305. [DOI] [PubMed] [Google Scholar]
- Bush G,Luu P,Posner MI ( 2000): Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4: 215–222. [DOI] [PubMed] [Google Scholar]
- Carter CS,Botvinick MM,Cohen JD ( 1999): The contribution of the anterior cingulate cortex to executive processes in cognition. Rev Neurosci 10: 49–57. [DOI] [PubMed] [Google Scholar]
- Carter CS,Braver TS,Barch DM,Botvinick MM,Noll D,Cohen JD ( 1998): Anterior cingulate cortex, error detection, and the online monitoring of performance. Science 280: 747–749. [DOI] [PubMed] [Google Scholar]
- Castaneda AE,Tuulio‐Henriksson A,Marttunen M,Suvisaari J,Lonnqvist J ( 2008): A review on cognitive impairments in depressive and anxiety disorders with a focus on young adults. J Affect Disord 106: 1–27. [DOI] [PubMed] [Google Scholar]
- Channon S,Baker JE,Robertson MM ( 1993): Working memory in clinical depression: An experimental study. Psychol Med 23: 87–91. [DOI] [PubMed] [Google Scholar]
- Chen CH,Ridler K,Suckling J,Williams S,Fu CH,Merlo‐Pich E,Bullmore E ( 2007): Brain imaging correlates of depressive symptom severity and predictors of symptom improvement after antidepressant treatment. Biol Psychiatry 62: 407–414. [DOI] [PubMed] [Google Scholar]
- Christopher G,MacDonald J ( 2005): The impact of clinical depression on working memory. Cognit Neuropsychiatry 10: 379–399. [DOI] [PubMed] [Google Scholar]
- Cuijpers P,Smit F ( 2002): Excess mortality in depression: A meta‐analysis of community studies. J Affect Disord 72: 227–236. [DOI] [PubMed] [Google Scholar]
- Devinsky O,Morrell MJ,Vogt BA. ( 1995): Contributions of anterior cingulate cortex to behavior. Brain 118 (Pt 1): 279–306. [DOI] [PubMed] [Google Scholar]
- Dougherty DD,Rauch SL ( 2007): Brain correlates of antidepressant treatment outcome from neuroimaging studies in depression. Psychiatr Clin North Am 30: 91–103. [DOI] [PubMed] [Google Scholar]
- Drevets WC ( 2001): Neuroimaging and neuropathological studies of depression: Implications for the cognitive‐emotional features of mood disorders. Curr Opin Neurobiol 11: 240–249. [DOI] [PubMed] [Google Scholar]
- Ebmeier K,Rose E,Steele D ( 2006): Cognitive impairment and fMRI in major depression. Neurotox Res 10: 87–92. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB,Oxley TJ,Laird AR,Kulkarni J,Egan GF,Daskalakis ZJ ( 2006): An analysis of functional neuroimaging studies of dorsolateral prefrontal cortical activity in depression. Psychiatry Res 148: 33–45. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB,Srithiran A,Benitez J,Daskalakis ZZ,Oxley TJ,Kulkarni J,Egan GF ( 2008): An fMRI study of prefrontal brain activation during multiple tasks in patients with major depressive disorder. Hum Brain Mapp 29: 490–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T,Scheuerecker J,Albrecht J,Kleemann AM,Muller‐Schunk S,Koutsouleris N,Moller HJ,Bruckmann H,Wiesmann M,Meisenzahl E. ( 2007): Neuronal correlates of emotional processing in patients with major depression. World J Biol Psychiatry: 1–7. [DOI] [PubMed] [Google Scholar]
- Fu CH,Williams SC,Cleare AJ,Brammer MJ,Walsh ND,Kim J,Andrew CM,Pich EM,Williams PM,Reed LJ,Mitterschiffthaler MT,Suckling J,Bullmore ET ( 2004): Attenuation of the neural response to sad faces in major depression by antidepressant treatment: A prospective, event‐related functional magnetic resonance imaging study. Arch Gen Psychiatry 61: 877–889. [DOI] [PubMed] [Google Scholar]
- Greicius MD,Flores BH,Menon V,Glover GH,Solvason HB,Kenna H,Reiss AL,Schatzberg AF ( 2007): Resting‐state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry 62: 429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M ( 1960): A rating scale for depression. J Neurol Neurosurg Psychiatry 23: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer CJ,Mackay CE,Reid CB,Cowen PJ,Goodwin GM ( 2006): Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biol Psychiatry 59: 816–820. [DOI] [PubMed] [Google Scholar]
- Harvey PO,Fossati P,Pochon JB,Levy R,Lebastard G,Lehericy S,Allilaire JF,Dubois B ( 2005): Cognitive control and brain resources in major depression: An fMRI study using the n‐back task. Neuroimage 26: 860–869. [DOI] [PubMed] [Google Scholar]
- Harvey PO,Le Bastard G,Pochon JB,Levy R,Allilaire JF,Dubois B,Fossati P ( 2004): Executive functions and updating of the contents of working memory in unipolar depression. J Psychiatr Res 38: 567–576. [DOI] [PubMed] [Google Scholar]
- Holthoff VA,Beuthien‐Baumann B,Zundorf G,Triemer A,Ludecke S,Winiecki P,Koch R,Fuchtner F,Herholz K ( 2004): Changes in brain metabolism associated with remission in unipolar major depression. Acta Psychiatr Scand 110: 184–194. [DOI] [PubMed] [Google Scholar]
- Kerns JG,Cohen JD,MacDonald AW 3rd,Cho RY,Stenger VA,Carter CS ( 2004): Anterior cingulate conflict monitoring and adjustments in control. Science 303: 1023–1026. [DOI] [PubMed] [Google Scholar]
- Kessing LV ( 1998): Cognitive impairment in the euthymic phase of affective disorder. Psychol Med 28: 1027–1038. [DOI] [PubMed] [Google Scholar]
- Konarski JZ,Kennedy SH,McIntyre RS,Rafi‐Tari S,Soczynska JK,Mayberg HS ( 2007): Relationship between regional brain metabolism, illness severity and age in depressed subjects. Psychiatry Res 155: 203–210. [DOI] [PubMed] [Google Scholar]
- Landro NI,Stiles TC,Sletvold H ( 2001): Neuropsychological function in nonpsychotic unipolar major depression. Neuropsychiatry Neuropsychol Behav Neurol 14: 233–240. [PubMed] [Google Scholar]
- Lehrl S,Triebig G,Fischer B ( 1995): Multiple choice vocabulary test MWT as a valid and short test to estimate premorbid intelligence. Acta Neurol Scand 91: 335–345. [DOI] [PubMed] [Google Scholar]
- Maldjian JA,Laurienti PJ,Kraft RA,Burdette JH ( 2003): An automated method for neuroanatomic and cytoarchitectonic atlas‐based interrogation of fMRI data sets. Neuroimage 19: 1233–1239. [DOI] [PubMed] [Google Scholar]
- Marcos T,Salamero M,Gutierrez F,Catalan R,Gasto C,Lazaro L ( 1994): Cognitive dysfunctions in recovered melancholic patients. J Affect Disord 32: 133–137. [DOI] [PubMed] [Google Scholar]
- Matsuo K,Glahn DC,Peluso MA,Hatch JP,Monkul ES,Najt P,Sanches M,Zamarripa F,Li J,Lancaster JL,Fox PT,Gao JH,Soares JC ( 2007): Prefrontal hyperactivation during working memory task in untreated individuals with major depressive disorder. Mol Psychiatry 12: 158–166. [DOI] [PubMed] [Google Scholar]
- Mayberg HS ( 1997): Limbic‐cortical dysregulation: A proposed model of depression. J Neuropsychiatry Clin Neurosci 9: 471–481. [DOI] [PubMed] [Google Scholar]
- Mayberg HS,Brannan SK,Mahurin RK,Jerabek PA,Brickman JS,Tekell JL,Silva JA,McGinnis S,Glass TG,Martin CC,Fox PT ( 1997): Cingulate function in depression: A potential predictor of treatment response. Neuroreport 8: 1057–1061. [DOI] [PubMed] [Google Scholar]
- Michelet T,Bioulac B,Guehl D,Escola L,Burbaud P ( 2007): Impact of commitment on performance evaluation in the rostral cingulate motor area. J Neurosci 27: 7482–7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milak MS,Parsey RV,Keilp J,Oquendo MA,Malone KM,Mann JJ ( 2005): Neuroanatomic correlates of psychopathologic components of major depressive disorder. Arch Gen Psychiatry 62: 397–408. [DOI] [PubMed] [Google Scholar]
- Mitterschiffthaler MT,Williams SC,Walsh ND,Cleare AJ,Donaldson C,Scott J,Fu CH ( 2008): Neural basis of the emotional Stroop interference effect in major depression. Psychol Med 38: 247–256. [DOI] [PubMed] [Google Scholar]
- Mulert C,Leicht G,Pogarell O,Mergl R,Karch S,Juckel G,Moller HJ,Hegerl U ( 2007): Auditory cortex and anterior cingulate cortex sources of the early evoked gamma‐band response: Relationship to task difficulty and mental effort. Neuropsychologia 45: 2294–2306. [DOI] [PubMed] [Google Scholar]
- Mulert C,Menzinger E,Leicht G,Pogarell O,Hegerl U ( 2005): Evidence for a close relationship between conscious effort and anterior cingulate cortex activity. Int J Psychophysiol 56: 65–80. [DOI] [PubMed] [Google Scholar]
- Nair J,Nair SS,Kashani JH,Reid JC,Mistry SI,Vargas VG ( 1999): Analysis of the symptoms of depression—A neural network approach. Psychiatry Res 87: 193–201. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Owen AM,McMillan KM,Laird AR,Bullmore E ( 2005): N‐back working memory paradigm: A meta‐analysis of normative functional neuroimaging studies. Hum Brain Mapp 25: 46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paelecke‐Habermann Y,Pohl J,Leplow B ( 2005): Attention and executive functions in remitted major depression patients. J Affect Disord 89: 125–135. [DOI] [PubMed] [Google Scholar]
- Paradiso S,Lamberty GJ,Garvey MJ,Robinson RG ( 1997): Cognitive impairment in the euthymic phase of chronic unipolar depression. J Nerv Ment Dis 185: 748–754. [DOI] [PubMed] [Google Scholar]
- Picchioni M,Matthiasson P,Broome M,Giampietro V,Brammer M,Mathes B,Fletcher P,Williams S,McGuire P ( 2007): Medial temporal lobe activity at recognition increases with the duration of mnemonic delay during an object working memory task. Hum Brain Mapp 28: 1235–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravnkilde B,Videbech P,Clemmensen K,Egander A,Rasmussen NA,Rosenberg R ( 2002): Cognitive deficits in major depression. Scand J Psychol 43: 239–251. [DOI] [PubMed] [Google Scholar]
- Rose EJ,Ebmeier KP ( 2006): Pattern of impaired working memory during major depression. J Affect Disord 90: 149–161. [DOI] [PubMed] [Google Scholar]
- Rose EJ,Simonotto E,Ebmeier KP ( 2006): Limbic over‐activity in depression during preserved performance on the n‐back task. Neuroimage 29: 203–215. [DOI] [PubMed] [Google Scholar]
- Sheline YI,Barch DM,Donnelly JM,Ollinger JM,Snyder AZ,Mintun MA ( 2001): Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry 50: 651–658. [DOI] [PubMed] [Google Scholar]
- Smith DJ,Muir WJ,Blackwood DH ( 2006): Neurocognitive impairment in euthymic young adults with bipolar spectrum disorder and recurrent major depressive disorder. Bipolar Disord 8: 40–46. [DOI] [PubMed] [Google Scholar]
- Sobocki P,Jonsson B,Angst J,Rehnberg C ( 2006): Cost of depression in Europe. J Ment Health Policy Econ 9: 87–98. [PubMed] [Google Scholar]
- Sohn MH,Albert MV,Jung K,Carter CS,Anderson JR ( 2007): Anticipation of conflict monitoring in the anterior cingulate cortex and the prefrontal cortex. Proc Natl Acad Sci USA 104: 10330–10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trichard C,Martinot JL,Alagille M,Masure MC,Hardy P,Ginestet D,Feline A ( 1995): Time course of prefrontal lobe dysfunction in severely depressed in‐patients: A longitudinal neuropsychological study. Psychol Med 25: 79–85. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N,Landeau B,Papathanassiou D,Crivello F,Etard O,Delcroix N,Mazoyer B,Joliot M ( 2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15: 273–289. [DOI] [PubMed] [Google Scholar]
- van Veen V,Carter CS ( 2006): Error detection, correction, and prevention in the brain: A brief review of data and theories. Clin EEG Neurosci 37: 330–335. [DOI] [PubMed] [Google Scholar]
- Veiel HO ( 1997): A preliminary profile of neuropsychological deficits associated with major depression. J Clin Exp Neuropsychol 19: 587–603. [DOI] [PubMed] [Google Scholar]
- von Knorring L,Akerblad AC,Bengtsson F,Carlsson A,Ekselius L ( 2006): Cost of depression: Effect of adherence and treatment response. Eur Psychiatry 21: 349–354. [DOI] [PubMed] [Google Scholar]
- Wager TD,Smith EE ( 2003): Neuroimaging studies of working memory: A meta‐analysis. Cogn Affect Behav Neurosci 3: 255–274. [DOI] [PubMed] [Google Scholar]
- Walsh ND,Williams SC,Brammer MJ,Bullmore ET,Kim J,Suckling J,Mitterschiffthaler MT,Cleare AJ,Pich EM,Mehta MA,Fu CH ( 2007): A longitudinal functional magnetic resonance imaging study of verbal working memory in depression after antidepressant therapy. Biol Psychiatry 62: 1236–1243. [DOI] [PubMed] [Google Scholar]
- Walter H,Vasic N,Hose A,Spitzer M,Wolf RC ( 2007a): Working memory dysfunction in schizophrenia compared to healthy controls and patients with depression: Evidence from event‐related fMRI. Neuroimage 35: 1551–1561. [DOI] [PubMed] [Google Scholar]
- Walter H,Wolf RC,Spitzer M,Vasic N ( 2007b): Increased left prefrontal activation in patients with unipolar depression: An event‐related, parametric, performance‐controlled fMRI study. J Affect Disord 101: 175–185. [DOI] [PubMed] [Google Scholar]
- Weiland‐Fiedler P,Erickson K,Waldeck T,Luckenbaugh DA,Pike D,Bonne O,Charney DS,Neumeister A ( 2004): Evidence for continuing neuropsychological impairments in depression. J Affect Disord 82: 253–258. [DOI] [PubMed] [Google Scholar]
- Whalen PJ,Bush G,McNally RJ,Wilhelm S,McInerney SC,Jenike MA,Rauch SL ( 1998): The emotional counting Stroop paradigm: A functional magnetic resonance imaging probe of the anterior cingulate affective division. Biol Psychiatry 44: 1219–1228. [DOI] [PubMed] [Google Scholar]
- Wittchen H,Wunderlich U,Gruschwitz S,Zaudig M ( 1997): SKID‐I,Strukturiertes Klinisches Interview für DSM‐IV. Hogrefe Göttingen, Germany. [Google Scholar]
- Zakzanis KK,Leach L,Kaplan E ( 1998): On the nature and pattern of neurocognitive function in major depressive disorder. Neuropsychiatry Neuropsychol Behav Neurol 11: 111–119. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information Figure 1. Group activation in a) patients and b) controls for the 0‐, 1‐, and 2‐back condition (one sample t‐test, p<0.05, corrected for family wise error (FWE), contiguity threshold ≥ 15 voxels). Random‐effects analysis rendered on the surface of the canonical template image used by SPM5. Supporting Information Figure 2. Comparison of working memory activation in a) patients versus healthy controls and b) healthy controls versus patients for the 2vs0‐back contrast (whole brain analysis, p < 0.0005, uncorrected for multiple comparisons, contiguity threshold ≥ 15 voxels), projected on the canonical template image used by SPM5. Significant activation in a) were observed in the cingulate cortex (BA 24, 31, 32) extending into the medial frontal gyrus (BA 6), parahippocampal gyrus (BA 35), middle temporal (BA 21) and paracentral gyrus (BA 2, 3, 4). Healthy controls did not show any significant activation relative to patients. Supporting Information Figure 3. Comparison of working memory activation in a) patients versus healthy controls and b) healthy controls versus patients for the 2vs1‐back contrast (whole brain analysis, p < 0.0005, uncorrected for multiple comparisons, contiguity threshold ≥ 15 voxels), projected on the canonical template image used by SPM5. Significant activation in a) were observed in the cingulate cortex (BA 24, 31) extending into the inferior parietal lobule and supramarginal gyrus (BA 40) and angular gyrus (BA 39) and medial frontal gyrus (BA 6), and pre‐ and postcentral gyrus (BA 2, 3, 4). Healthy controls did not show any significant activation relative to patients. Supporting Information Table 1. Cortical activation pattern for the 2‐0‐back and 2‐1‐back contrast for a) patients b) healthy control subjects, as obtained by a random effect analysis (puncorr.<0.05, FDR corrected, contiguity threshold ≥ 15 voxels). Supporting Information Table 2. Cortical activation pattern for the 2‐0‐back and 2‐1‐back contrast for a) patients vs. controls b) healthy control subjects vs. patients, as obtained by a random effect analysis (whole brain analysis, p < 0.0005, uncorrected, contiguity threshold ≥ 15 voxels).


