Abstract
Visual attention can be directed either to the global features of a display or to the local elements that make up the display. We investigated whether oscillatory brain responses to globally or locally directed cue stimuli predict behavioral performance in subsequent target processing. Induced alpha band (8–12 Hz) amplitudes in the pre‐stimulus interval were measured separately for the global and the local level, where individual trials were assigned to one of three groups according to the response speed towards incongruent stimuli. Fast responses to local features were associated with high alpha amplitudes in the right centro‐parietal cortex, whereas fast responses to global forms were associated with high alpha in left centro‐parietal cortex. For trials with slower responses, the pattern of hemispheric differences was diminished or even reversed. It is interpreted that the left and the right parietal cortex exert top–down control over hierarchical processing by inhibiting stimulus representations in one hemisphere. Hum Brain Mapp, 2009. © 2008 Wiley‐Liss, Inc.
Keywords: alpha oscillations, attention, EEG, hierarchical processing
INTRODUCTION
Hierarchical visual stimuli are objects or scenes that comprise more than one level of detail. Such stimuli are very common in natural environments. For instance, forests are comprised of different trees, which in turn have leaves and stems. Likewise, in a book store there are many different shelves which in turn hold different books. Ubiquitous to many perceptual tasks, a better understanding of the neural mechanisms underlying hierarchical stimulus processing is essential.
In a typical study on hierarchical processing, the stimuli are compound letters where smaller, local letters are arranged in a way that they form another larger, global letter [Navon,1977] (see Fig. 1). The subjects are usually instructed to attend either to the global or to the local level and to indicate quickly which letter was presented at the target level. Previous studies have indicated that the left and the right cerebral hemispheres are specialized for the processing of each level. In a study with brain‐damaged patients Lamb et al. [1990] found that lesions in the left superior temporal lobe produced a relative response time advantage for processing global forms, whereas lesions in right superior temporal lobe led to an advantage for processing local features [cf. Robertson and Lamb,1991]. Similar results have been reported in brain imaging and electroencephalographic (EEG) studies [Han et al.,2002; Heinze and Münte,1993; Moses et al.,2002]. Typically, the effects occur 250 ms after stimulus onset or even later, and are most prominent in parietal and temporal brain structures associated with mid‐ to high‐level vision. Taken together, the existing data suggest that hierarchical processing is lateralized at later stages of object recognition, where the left and the right hemispheres are specialized for local and global processing, respectively.
Figure 1.
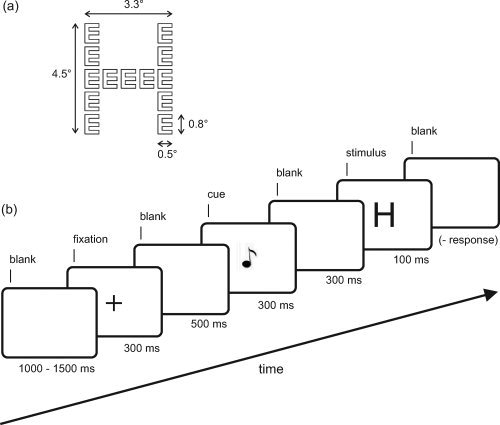
(a) Global H made from local E's. Global letters were constructed from identical local letters in a 5 × 5 grid, where the local letters were drawn as white outlines on a black ground. The size of the stimulus is indicated in degrees of visual angle. (b) Time course of a typical trial is schematically presented as a series of screen displays.
Most of the available studies on global/local processing are concerned with sensory or perceptual processes that take place after the target stimulus is presented. However, global and local selection is not only stimulus driven. To perform goal‐directed behavior with compound stimuli, subjects must be able to bias selection towards the task‐relevant information. There is ample evidence that attention can indeed be voluntarily allocated to one level. For example, it is known that responses towards one target level can be speeded up by increasing the probability for that level [Robertson et al.,1993]. Also, responses are faster if the target level is constant throughout a block of trials compared to a situation with randomized target levels [Hübner,1997]. Thus, there seem to exist specific modules for controlling attention to global or local levels in a top–down manner.
The brain mechanisms associated with global/local attentional orienting were also directly addressed in a recent fMRI study [Weissman and Woldorff,2005; see also Volberg and Hübner,2007; Yamaguchi et al.,2000]. The authors presented an informative pre‐cue (letter “G” for the global level, letter “L” for the local level) and measured the brain activity in the interval between cue and target stimulus presentation. Additionally, the BOLD response in the post‐stimulus interval was investigated. With respect to the latter, the authors found the expected hemispheric differences with more pronounced right‐hemispheric activity for global target levels, whereas left‐hemispheric activity was more pronounced in response to local targets. Interestingly, evidence was also found for hemispheric differences in the pre‐stimulus interval. Cues towards the local level led to enhanced activity in the left compared to the right intraparietal sulcus, and cues towards the global level produced the reversed pattern. That is, cue stimuli directing attention towards global or local levels produced hemispheric differences similar to those observed in response to global and local levels of target stimuli.
One general problem with this finding arises from the fact that cue stimuli typically do not require a direct response. This makes it difficult to control to what extent the cues were actually used for attentional pre‐adjustment towards the levels. It is known that performance is superior in situations where the target level is pre‐cued compared to un‐cued situations [Hübner et al.,2007]. This shows that cue utilization affects selection performance in subsequent target processing. For the investigation of preparatory brain activity, it is desirable to use this selection performance as a correlate of cue utilization.
In this study we explored preparatory brain activity for global/local processing with a novel experimental approach. First, we used alpha band (8–12 Hz) oscillatory brain activity in the scalp EEG as an index of attention. Alpha amplitudes have widely been used as a measure of preparatory brain activity, including spatial attention [Thut et al.,2006; Worden et al.,2000] as well as attention to different sensory modalities [Bastiaansen et al.,2001; for an overview see Ward,2003]. It is commonly assumed that low alpha amplitudes contingent on stimulation reflect excitatory brain processes, whereas increased levels of alpha amplitudes reflect cortical inhibition [Klimesch et al.,2007]. In a second step, we introduced a behavioral correlate of cue utilization. Subjects were presented with incongruent stimuli where information on the target and on the non‐target levels was associated with conflicting responses. In this situation, reaction times critically depend on how efficient the information on the relevant level can be selected [Eriksen and Eriksen,1974]. If attention is correctly allocated towards the target level, concurrent response activations from the irrelevant level are weak and responses should be relatively fast. On the other hand, if the allocation of attention towards the target level is sub‐optimal, concurrent response activations from the irrelevant level are strong which leads to slower responses. Thus, if the hemispheres differ in their preparation, a close relationship between pre‐stimulus alpha amplitudes and behavioral performance can be expected. For the local target level, fast responses where the allocation of attention was optimal should be associated with a higher excitability of the left compared to the right hemisphere, and thus with higher alpha amplitudes in the right compared to the left hemisphere. In contrast, for slower responses (where the allocation of attention is not optimal) hemispheric difference should be absent or at least less pronounced. Analogous predictions can be made for the global level. Fast responses should be associated with a higher excitability of the right compared to the left hemisphere (higher alpha amplitudes in the left compared to the right hemisphere), which should diminish for slower responses.
MATERIALS AND METHODS
Subjects
Sixteen volunteers (12 female, 4 male) aged 19–31 years participated in the experiment. Based on self‐report, all subjects were right‐handed, had no neurological disorders and normal or corrected‐to‐normal vision. The experiment was carried out according to the principles laid down in the Helsinki declaration. Subjects gave written informed consent prior to the experiment.
Stimuli
The target stimuli consisted of 16 hierarchical letters which resulted from the pair‐wise combination of the letters A, S, H, and E (see Fig. 1 for a description). The letters A and S were assigned to one response button, whereas the letters H and E were assigned to another one. Half of the stimuli was incongruent and the other half was congruent, where the latter served for controlling the congruency manipulation. Congruent stimuli contained local and global features with the same response category, whereas for the incongruent stimuli local and global features belonged to different response categories. Each stimulus presentation was preceded by a 300 ms pure tone with a frequency of either 400 or 1600 Hz cueing either the local or the global level. The advantage of auditory cue stimuli is that visual input can be excluded as a possible source of cue‐related changes of alpha activity in visual areas. Thus, possible effects in the visual cortex would be due to the mere cue information and could not be explained by perceptual input [Thut et al.,2006].
Procedure
Subjects were seated in an electrically and acoustically shielded chamber in front of a monitor with externally located power supply, where a chin rest prevented head movements and ensured that the viewing distance remained constant at 57 cm. The stimuli were presented on a 17″ flat screen monitor with a resolution of 1024 × 768 pixels and a vertical refresh rate of 60 Hz.
A trial sequence started with a central presentation of a 300‐ms fixation cross, which indicated the position of the upcoming target stimulus. Participants were instructed to fixate the screen center throughout the experiment and to make as few eye movements as possible. Five hundred milliseconds after the fixation cross, a 300‐ms cue tone was presented. It was delivered through two loudspeakers placed on the left and on the right of the monitor. For half of the subjects, a high tone signaled the global target level, whereas a low tone signaled the local target level. For the other half, the assignment of tones to target levels was reversed. The stimulus onset asynchrony (SOA) between cue and target stimulus presentation was 600 ms (300 ms cue +300 ms cue–stimulus interval). Compared to other studies on preparatory alpha activity, this SOA is relatively short. For example, in their spatial cueing studies Thut et al. [2006] used a 50 ms cue plus 2560 ms cue–stimulus interval (=2610 ms SOA), and Rihs et al. [2007] had a 80 + 1300 = 1380 ms SOA. However, since the focus of this study is on hierarchical processing, the SOA were adopted from other global/local studies [e.g., 600 ms in Volberg and Hübner,2007; 800 ms in Yamaguchi et al.,2000; 900 ms in Volberg and Hübner,2004].
Targets were shown for 100 ms in the center of the screen. The task was to categorize the letter at the cued level by pressing an associated button with the index or middle finger of the same hand. Subjects were told to respond as quickly and accurately as possible. As a response device, two customized response keys were used which were connected to the stimulus presentation computer. A feedback tone of 1000 Hz signaled incorrect responses. After the response, a dark screen was presented until the next trial sequence started. The duration of this interval varied randomly between 1000 and 1500 ms.
Subjects performed 3 blocks with 64 trials each. The factors Congruency (congruent, incongruent) and Target Level (global, local) were randomized. Response hand and the assignment of letters to response buttons were balanced across subjects.
EEG Recording
The EEG was recorded from 62 equidistant electrodes that were mounted in an elastic cap (EasyCap, Herrsching‐Breitbrunn, Germany) and were referenced to FCz during recording. Impedances were kept below 10 kΩ. The signals were amplified between 0.1 and 100 Hz and digitized at a rate of 500 Hz (BrainAmp MR plus, Gilching, Germany). To control for eye movement artifacts, the vertical electro‐oculogram was recorded from an electrode placed below the left eye. After recording, the data were arithmetically re‐referenced to an average reference.
Data Analysis
Behavioral data
Reaction times on trials with correct responses as well as error rates were subjected to a repeated measures ANOVA with the within‐subjects factors Target Level (global, local) and Congruency (congruent, incongruent).
EEG data
The EEG data were analyzed as follows. For each subject, single incongruent trials were ordered according to the behavioral performance. This was done separately for each target level. This resulted in two distributions of individual response times for incongruent trials, one for the global condition and one for the local condition. They were split at the 33rd and 66th percentile, so that three equally sized groups of trials emerged. In either distribution, the first group contained incongruent trials with relatively fast responses, the second group covered incongruent trials with intermediate speed, and in the third group incongruent trials with slow responses were sampled. Trials with wrong behavioral responses (3.89% overall) were excluded. Eye movement and blink artifacts were corrected using individual artifact coefficients and topographies [see Berg and Scherg,1994 for details].
For time–frequency analysis the complex demodulation algorithm as implemented in BESA (MEGIS Software BESA v 5.1.8) was used. BESA employs an FIR filter with a Gaussian window of 130 ms FWHM. The EEG data were filtered in a 2700 ms time window, covering data from 500 ms prior to presentation of the fixation cross to 800 ms after target stimulus onset. As baseline, a 500‐ms interval prior to the onset of the fixation cross was employed. The frequency range was from 4 to 20 Hz. The time resolution of time–frequency decompositions was set to 50 ms. A higher time resolution was not desirable, because the alpha band is rather narrow (8–12 Hz) so that a good frequency resolution is necessary. The 50‐ms filter window allowed for a frequency resolution of 1 Hz. With these settings, BESA smoothes the time–frequency signal to a signal with a time resolution of ±111 ms (50% amplitude drop) relative to a sharp time event and a frequency resolution of ±1.99 Hz (50% amplitude drop) relative to a sharp frequency oscillation.
To examine the differences in event‐related alpha activity, the percentaged increase or decrease in alpha amplitudes relative to the alpha activity in a baseline period was used [see Pfurtscheller and Aranibar,1977]. It was computed as 100 × [A(t, f) − A baseline(f)]/A baseline(f) (in %), where A(t, f) is amplitude at time t and frequency f, and A baseline(f) is mean amplitude at frequency f over the baseline epoch. Prior to the signal change calculation, phase‐locked activity was eliminated by subtracting the averaged evoked signal from the single trials. Thus, the resulting time–frequency matrix contains only induced oscillations that are not phase‐locked to the stimulus onset.
Trial Group (fast, intermediate, slow responses) as well as the Hemisphere of recording (left hemisphere, right hemisphere) served as factors for the statistical analysis. Repeated measures ANOVAs were performed on the mean percentaged signal change in the EEG alpha band amplitudes (8–12 Hz) relative to baseline activity. For the statistical analysis, the percentaged signal change of alpha levels were averaged within four homologous left‐ and right‐hemispheric electrode clusters (temporo‐parietal left: C5/CP5/TP7, temporo‐parietal right: C6/CP6/TP8; centro‐parietal left: CP1/CP3/C3, centro‐parietal right: CP2/CP4/C4; parietal left: P1/P3/P5, parietal right: P2/P4/P6; occipital left: O1/PO3/PO7, occipital right: O2/PO4/PO8). They correspond to those used in Volberg and Hübner [2007] or in Yamaguchi et al. [2000] where four medial and lateral electrode clusters were defined posterior to the central line. Separate analyses were performed for the four homologous clusters. Also, data from trials in the global and in the local condition were analyzed separately.
RESULTS
Behavioral Data
The data showed higher error rates for incongruent stimuli (mean ± SE: 5.42% ± 1.01% errors) as compared to congruent ones (2.36% ± 0.45%). Consequently, the statistical analysis revealed a main effect for Congruency [error rates: F(1, 15) = 5.94, P < 0.05]. A similar trend was also observed for the response times. Responses to incongruent stimuli (809 ± 40 ms) were slower than those to congruent ones (788 ± 46 ms). However, this difference was not significant [F(1, 15) < 1]. There were also no effects involving the factor Target Level (response times: global 787 ± 45 ms, local 810 ± 42 ms; error rates: global 4.12 ± 0.91, local 3.66 ± 0.73, all F < 1).
EEG Data
The alpha amplitude waveforms showed the expected course of a signal decrease, followed by a signal increase at all investigated channels (Figs. 2a and 3a). There was a negative peak with an occipital maximum ∼400 ms after the trial onset, followed by a rebound peaking ∼800–1000 ms (−600 to −400 ms relative to the onset of the stimulus). Gray bars in Figures 2a and 3a indicate the time range of −250 to 0 ms relative to target stimulus onset (350–600 ms relative to cue stimulus onset) where hemispheric differences were most prominent. All statistics were performed on the mean percentaged signal change of alpha amplitudes within this −250 to 0 ms interval. The onset of that time window 350 ms after cue stimulus presentation closely corresponds to the latencies of cue‐related hemispheric differences as observed in ERP studies [∼400 ms in Yamaguchi et al.,2000; ∼ 300 ms in Volberg and Hübner,2007].
Figure 2.
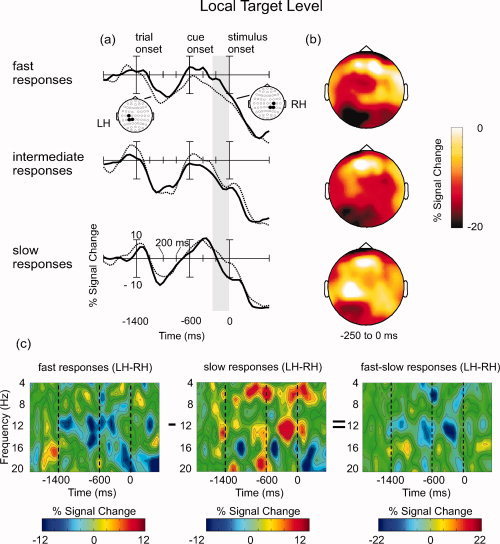
Oscillatory brain activity in the preparation for local target levels, contingent on the behavioral performance (fast, intermediate and slow responses). (a) Waveforms depicting percentaged signal change within the alpha band (8–12 Hz) at centro‐parietal electrodes in the left hemisphere (LH) and in the right hemisphere (RH). The gray vertical column signifies the epoch that yielded the most pronounced differences between hemispheres (−250 to 0 ms). Statistics were performed on the mean amplitudes within this time window. (b) Scalp topographies of percentaged signal change within the alpha band (8–12 Hz), averaged between −250 and 0 ms relative to the stimulus onset. Notice that the scale is unipolar from −20 to 0. (c) Time–frequency plots showing hemispheric differences in the alpha frequency range at centro‐parietal electrodes for fast vs. slow response trials (see text for details).
Figure 3.
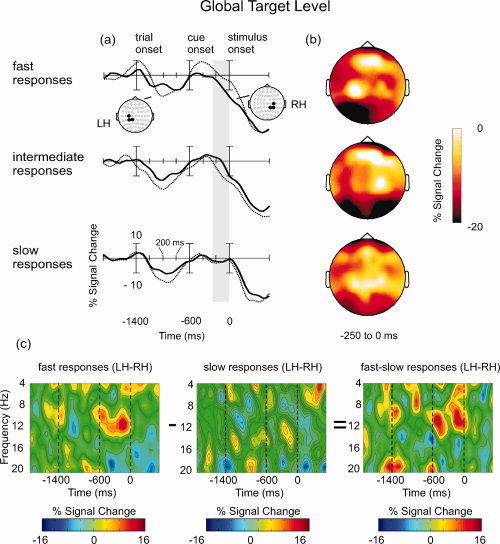
Oscillatory brain activity in the preparation for global target levels, contingent on the behavioral performance (fast, intermediate and slow responses). (a) Waveforms depicting percentaged signal change within the alpha band (8–12 Hz) at centro‐parietal electrodes in the left hemisphere (LH) and in the right hemisphere (RH). The gray vertical column signifies the epoch that yielded the most pronounced differences between hemispheres (−250 to 0 ms). Statistics were performed on the mean amplitudes within this time window. (b) Scalp topographies of percentaged signal change within the alpha band (8–12 Hz), averaged between −250 and 0 ms relative to the stimulus onset. Notice that the scale is unipolar from −20 to 0. (c) Time–frequency plots showing hemispheric differences in the alpha frequency range at centro‐parietal electrodes for fast vs. slow response trials (see text for details).
Local target level
A general effect of Trial Group occurred at centro‐parietal electrodes where alpha amplitudes tended to be larger for slow response trials compared to intermediate and fast response trials, F(2, 30) = 4.67, P < 0.05. This difference can be seen in Figure 2a,b. Notice that, because the signal change relative to baseline activity was mostly negative in the investigated time range, the scale for the scalp topographies (Fig. 2b) is not centered at zero but is unipolar from −20 to 0.
Most important for the present objective, the data also indicate hemispheric differences between trial groups. In the fast response conditions, alpha amplitudes at centro‐parietal electrodes were larger in the right compared to the left hemisphere. By contrast, in slow response trials alpha amplitudes were larger in the left compared to the right hemisphere. Accordingly, there was a two‐way interaction between Hemisphere and Trial Group. It was significant only at centro‐parietal sites, F(2, 30) = 3.33, P < 0.05. The effect can also be illustrated in the corresponding time–frequency plots (see Fig. 2c). Centro‐parietal alpha amplitudes in the right hemisphere were subtracted from those in the left hemisphere. Negative values indicate that centro‐parietal alpha amplitudes were higher in the right than in the left hemisphere, and positive values indicate that the amplitudes were higher in the left compared to the right hemisphere. Thus, the data show right‐lateralized alpha amplitudes in fast response trials and left‐lateralized alpha amplitudes in slow response trials. To highlight this difference, results in the slow response condition were subtracted from those in the fast response condition (Fig. 2c, right panel). In this depiction negative values indicate that alpha amplitudes for fast responses were higher in the right than in the left hemisphere, whereas for slow responses they were higher in the left than in the right hemisphere. The effect was most prominent −250 to 0 ms prior to stimulus onset and was restricted to the alpha frequency range. Because filtering was done with a sliding 50 ms time window, it could be objected that alpha responses within the investigated pre‐stimulus interval also include activity related to the target stimulus presentation. However, it should be noted that the observed effect is maximal (most negative) around −200 ms and decreases afterwards. It is thus unlikely that it reflects alpha activity related to the target stimulus onset.
Global target level
Similar to the results for the local target level, the data in the global condition showed hemispheric differences between trial groups. However, the direction of that difference was reversed to that in the local condition. In fast response trials, alpha amplitudes were larger in the left compared to the right hemisphere. For slow and intermediate responses, this effect was diminished or even reversed (see Fig. 3a,b; as for Fig. 2b, the scale for Fig. 3b is unipolar from −20 to 0.). The interaction of Trial Group and Hemisphere was prominent only at centro‐parietal sites, F(2,30) = 3.61, P < 0.05. The corresponding time–frequency plots are depicted in Fig. 3c. Most informative is the right panel where differences between lateralized brain oscillations in fast and slow response trials are shown, analogously to the local level condition. Positive values indicate that alpha amplitudes for fast responses were higher in the left than in the right hemisphere, whereas for slow responses no such pattern occurred. Again, the differences show up in the alpha band 250 to 0 ms prior to stimulus onset. As in the local condition, the maximum of the effect (most positive value) is around −200 ms and decreases before target stimulus presentation.
Comparison of local and global target levels
To see whether the pattern of hemispheric differences in the global and local conditions differ, the alpha amplitudes at the centro‐parietal electrode cluster were subjected to a three‐way ANOVA with the factors Target Level (global, local), Trial Group (fast, intermediate, slow responses) and Hemisphere of recording (left, right). It revealed a significant interaction of all three factors, F(2,30) = 5.67, P < 0.01. To decompose the interaction, separate ANOVAs with the factors Target Level and Hemisphere were performed for each level of the factor Trial Group. The result was significant for the fast response group only [fast responses: F(1,15) = 7.50, P < 0.05; intermediate responses: F(1,15) = 2.80, P > 0.1; slow responses; F(1,15) = 1.44, P > 0.2)]. These analyses show that the pattern of preparatory alpha activity generally differs between global and local levels. But this is not true for all levels of performance: Reliable differences occurred only in a sub‐sample of trials where the behavioral performance was best. The outcome of the statistical analysis is illustrated in Figure 4. It shows the mean alpha amplitudes (% signal change, averaged −250 to 0 ms) for local and global trials at left and right centro‐parietal sites, as well as difference topographies of alpha amplitudes in the local minus global conditions. Only in fast response trials there is the expected pattern of higher right‐ than left‐hemispheric alpha amplitudes in the local condition, and higher left‐ than right‐hemispheric differences in the global condition (Fig. 4a, upper panel). This is also evident in the difference topographies. For fast response trials, there is a marked negativity (i.e., higher amplitudes in the global compared to the local condition) at left centro‐parietal scalp sites, accompanied by a moderate right‐hemispheric positivity (i.e., higher amplitudes in the local compared to the global condition; see Fig. 4b, upper panel). Figure 4c shows the corresponding bar graphs of the local minus global condition at centro‐parietal sensors. Only in fast response trials, the results show more alpha decrease for local than for global levels in the left hemisphere together with more alpha decrease for global than for local levels in the right hemisphere. By contrast, in intermediate response trials the alpha decrease was larger for the local than for the global level in both hemispheres. In slow response trials there were generally only small differences. Alpha decrease was slightly larger for global than for local levels in the left hemisphere, and larger for local than for global levels in the right hemisphere.
Figure 4.
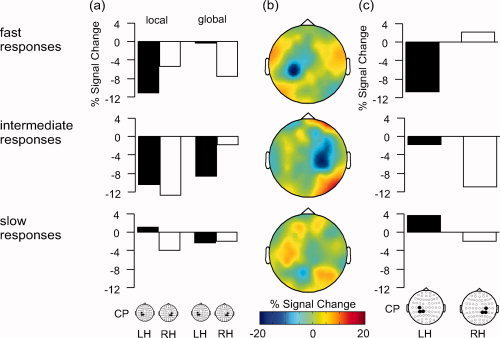
Comparison of alpha (8–12 Hz) amplitudes in the preparation for global vs. local target levels, contingent on the behavioral performance. (a) Mean alpha amplitudes (% signal change) in global and local conditions at left‐hemispheric (LH) and right‐hemispheric (RH) centro‐parietal sensors, averaged −250 to 0 ms prior to target stimulus presentation (b) Scalp topographies showing the difference in alpha amplitudes (% signal change) in local minus global conditions, averaged −250 to 0 ms relative to the target stimulus onset. (c) Bar graphs illustrating hemispheric differences in mean alpha amplitudes for the local minus global condition, averaged −250 to 0 ms prior to target stimulus presentation. The bars correspond to the mean activity at centro‐parietal sensors as shown in (b).
DISCUSSION
We investigated the relationship between alpha oscillations in the attentional preparation for an upcoming stimulus in global or local level conditions and the performance in the subsequent target processing. For each subject, single trials were assigned to one of three groups according to the behavioral performance (fast, intermediate, or slow responses), and alpha amplitudes in the pre‐stimulus interval were measured. Based on previous reports on hemispheric differences during the preparation interval, it was predicted that fast responses to local levels (indicating an optimal preparation) are associated with more pronounced right‐ compared to left‐hemispheric alpha activity. For intermediate and slow speeded responses, the pattern should diminish. For the global condition the opposite pattern was predicted.
For both target levels, the results showed a clear relationship between cue‐elicited hemispheric differences and behavioral performance. On trials with fast responses to local targets, alpha amplitudes were higher in the right compared to the left hemisphere. In contrast, for slow trials the opposite pattern of activation occurred, with higher alpha amplitudes in the left compared to the right hemisphere. A reversed relationship between behavioral performance and hemispheric differences was found in the preparation interval towards global levels. On trials with fast responses, alpha amplitudes were higher in the left compared to the right hemisphere. This difference diminished for slower trials. Taken together, the data show that for both levels alpha amplitudes in centro‐parietal areas predict behavioral performance. Fast responses towards local levels were preceded by high alpha amplitudes in the right hemisphere, whereas fast responses towards global levels were preceded by high alpha amplitudes in the left hemisphere. Additionally, the pattern of left‐ and right‐hemispheric alpha amplitudes was compared between local and global levels. It was found to be significantly different. The effect could be confined to fast response trials where opposing hemispheric differences for global and local levels were observed.
It is generally assumed that alpha oscillations reflect a state of functional inhibition of cortical activity. Increased alpha amplitudes relative to baseline levels reflect a state of inhibition, whereas decreased alpha amplitudes reflect excitation. Unfortunately, it is difficult to interpret the present results in terms of inhibition or excitation. On the one hand, pre‐stimulus alpha amplitudes in left/right parietal cortices were generally lower than those in the baseline period, which suggests excitation. On the other hand, hemispheric differences emerge as the alpha amplitudes rebound to baseline levels after a strong decrease at trial onset. This suggests that left/right cortical activity was modulated by inhibition. In the remainder of this section, we will describe the results in terms of inhibition. This seems legitimate since it is not the major question in this study whether excitation of inhibition serve the pre‐selection of levels. In any case, irrespective of whether there is an absolute decrease of increase of alpha compared to baseline activity, our data indicate that hierarchical selection is accomplished by modulating the relative excitability of left and right parietal visual cortices.
The results for the local condition show that fast responses were preceded by an increase of alpha amplitudes in the right hemisphere, whereas slow responses were preceded by an increase of alpha amplitudes in the left hemisphere. This indicates that local level selection is accomplished by inhibiting right parietal brain regions associated with the processing of global level information. Upon arrival of the target stimulus, such hemispheric pre‐adjustment would act like a filter that suppresses right‐hemispheric neural representations while letting the left‐hemispheric representations pass. As a result, response activations from the local level should be stronger than those from the global level, which leads to better performance (i.e., lower reaction times). In line with this interpretation, the data showed an unfavorable hemispheric pre‐adjustment in situations with slow responses. In these trials, left parietal brain regions associated with local level processing were inhibited to a stronger extent than the homologous right‐hemispheric areas associated with the processing of global level information. Consequently, response activations from the irrelevant global level were relatively pronounced which results in slow responses.
An analogous argumentation can be made for the global level. Fast responses to global stimulus levels were preceded by an increase of alpha amplitudes in the left hemisphere. By inhibiting left parietal brain regions associated with local level processing, interfering neural representations were suppressed so that fast responses to the global level were possible. On contrast, in slower trials pre‐stimulus increase of alpha amplitudes was also observed in the right hemisphere associated with global level processing. Compared to the fast response trials, this results in weaker response activation for the global level information and thus in a prolonged response selection. Therefore, responses are relatively slow.
There are some recent studies where pre‐stimulus alpha activity was related to performance in subsequent perceptual tasks. For example, in an EEG study Hanslmayr et al. [2007] required their subjects to identify four simple letters that were masked shortly after presentation. They found that alpha levels prior to the stimulus presentation distinguished between subjects with good performance (low alpha) and subjects that performed at chance level (high alpha). Analogous relationships between pre‐stimulus alpha levels and task performance were obtained in three magnetoencephalogram studies with spatial attention [Medendorp et al.2007], with somatosensory stimuluation [Linkenkaer‐Hansen et al.,2004] and with a contrast discrimination task [van Dijk et al.,2008]. In an attempt to give a functional interpretation of pre‐stimulus alpha in visual perception, Van Dijk et al. [2008; see also Jokisch and Jensen,2007] speculated that the gain of the dorsal visual stream is reduced with alpha power. The higher the parieto‐occipital alpha, the less information is gated from occipital to dorsal parietal areas. This would reduce the flow of visual information that could interfere at higher processing stages. Possibly, this hypothesis can also be adapted to the present results. One sensory/perceptual difference that distinguishes global from local levels is their spatial frequency content. Fine‐grained local information is mainly carried trough high spatial frequencies, and coarse global information is carried by low spatial frequencies [Sergent,1982]. It is conceivable that our alpha effects reflect a differential gating of low and high spatial frequency channels into the left and right hemispheres. However, whether this speculation is valid can only be answered with further studies where the spatial frequency content of the stimuli is better controlled.
One alternative explanation for our results should be considered. It was recently pointed out that, if hierarchical stimuli are presented at the screen center, global shapes extend over both left and right visual hemifields, whereas single local elements appear laterally in the left as well as in the right hemifield. This implies that local levels can be selected by allocating attention to one side of space [Rose et al.,2006]. Because stimuli are initially projected to the hemisphere contralateral to the attended hemifield, and because the left hemisphere is specialized for local processing, one can expect a superior performance if attention is shifted towards the right hemifield compared to left hemifield attention prior to the stimulus presentation [e.g., Van Kleeck,1989]. Such an outcome for local levels was indeed observed in our study. One could therefore argue that shifts of spatial attention prior to the stimulus presentation produced our results.
However, it is unlikely that this explanation holds. All hierarchical letters had a horizontal middle bar with five local elements and were presented centrally. Accordingly, one local element always appeared at fixation. The best strategy was therefore to fixate the screen center in global as well as in local trials. Self‐reports from subjects did also not indicate that different strategies were used in global and local conditions. Furthermore, from imaging studies it is known that the region of attentional modulation for a cued spatial location corresponds closely to its retinotopic location in early visual areas [Müller et al.,2003; Tootell et al.,1998]. A similar retinotopy was observed for the EEG alpha band response to spatial pre‐cues. For example, Worden et al. [2000] let their subjects detect a visual target in the left/right or upper/lower screen corner, where the locus of the target was pre‐cued by appropriately sized squares. It turned out that, depending on the cued quadrant of visual field, the focus of pre‐stimulus alpha decrease moved between left/right and upper/lower occipital cortex [cf. Thut et al.,2006]. Also modulation in pre‐stimulus alpha increase seems to be retinotopic. This was shown in a recent study by Rihs et al. [2007], where the focus of alpha increase moved in occipital cortex depending on what parts of the display should be ignored. Taken together, these studies show that shifts of spatial attention affect alpha activity in primary (occipital) visual cortex. This was obviously not the case in our study. Thus, our data can not be explained by pre‐stimulus spatial shifts of attention.
Although not directly related to our study aims, the alpha waveforms depicted in Figures 2 and 3 reveal a further interesting result. They show that alpha amplitudes in the left hemisphere were generally lower than those in the right hemisphere within an interval of 200–400 ms after trial onset (i.e., 600–400 ms before the onset of the cue stimulus). Because the left hemisphere is specialized for local processing, this might indicate a general attentional bias towards local levels. This observation fits well with results from behavioral studies where the time course of “zooming in” and “zooming out” of hierarchical patterns was investigated. They generally showed that zooming from local to global is more difficult than zooming from global to local [Stoffer,1993,1994]. It was interpreted that, upon presentation a hierarchical target stimulus, attention is unintentionally focused on the local level. The present data comply with this idea as they suggest a hemispheric pre‐adjustment that favors local attention.
In summary we showed that the pattern of hemispheric alpha activity in the pre‐stimulus interval predicts performance in subsequent hierarchical target processing. For local as well as for global levels, fast responses were preceded by high alpha amplitudes in the parietal cortex of the hemisphere associated with the processing of the distractor level information. For slow responses the pattern of lateralization was diminished (global) or even reversed (local). We suggest that efficient level selection is achieved top–down by suppressing stimulus representations in the hemisphere that is specialized for processing the non‐target level.
Acknowledgements
Statistics were done using the free R language for statistical computing, R Foundation for Statistical Computing, Vienna, Austria (ISBN: 3‐900051‐07‐0, URL: http://www.R-project.org). The University of Regensburg provided start‐up funding for the EEG laboratory.
REFERENCES
- Bastiaansen MC,Böcker KB,Brunia CH,De Munck JC,Spekreijse H ( 2001): Event‐related desynchronization during anticipatory attention for an upcoming stimulus: A comparative EEG/MEG study. Clin Neurophysiol 112: 393–403. [DOI] [PubMed] [Google Scholar]
- Berg P,Scherg M ( 1994): A multiple source approach to the correction of eye artifacts. Electroencephalogr Clin Neurophysiol 90: 229–241. [DOI] [PubMed] [Google Scholar]
- Eriksen B,Eriksen C ( 1974): Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept Psychophys 16: 143–149. [Google Scholar]
- Han S,Weaver JA,Murray SO,Kang X,Yund EW,Woods DL ( 2002): Hemispheric asymmetry in global/local processing: Effects of stimulus position and spatial frequency. NeuroImage 17: 1290–1299. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S,Aslan A,Staudigl T,Klimesch W,Herrmann CS,Bäuml K ( 2007): Prestimulus oscillations predict visual perception performance between and within subjects. NeuroImage 37: 1465–1473. [DOI] [PubMed] [Google Scholar]
- Heinze HJ,Münte TF ( 1993): Electrophysiological correlates of hierarchical stimulus processing: Dissociation between onset and later stages of global and local target processing. Neuropsychologia 31: 841–852. [DOI] [PubMed] [Google Scholar]
- Hübner R ( 1997): The effect of spatial frequency on global precedence and hemispheric differences. Percept Psychophys 59: 187–201. [DOI] [PubMed] [Google Scholar]
- Hübner R,Volberg G,Studer T ( 2007): Hemispheric differences for global/local processing in divided attention tasks: Further evidence for the integration theory. Percept Psychophys 69: 413–421. [DOI] [PubMed] [Google Scholar]
- Jokisch D,Jensen O ( 2007): Modulation of gamma and alpha activity during a working memory task engaging the dorsal or ventral stream. J Neurosci 27: 3244–3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W,Sauseng P,Hanslmayr S ( 2007): EEG alpha oscillations: The inhibition‐timing hypothesis. Brain Res Rev 53: 63–88. [DOI] [PubMed] [Google Scholar]
- Lamb MR,Robertson LC,Knight RT ( 1990): Component mechanisms underlying the processing of hierarchically organized patterns: Inferences from patients with unilateral cortical lesions. J Exp Psychol Learn Mem Cogn 16: 471–483. [DOI] [PubMed] [Google Scholar]
- Linkenkaer‐Hansen K,Nikulin VV,Palva S,Ilmoniemi RJ,Palva JM ( 2004): Prestimulus oscillations enhance psychophysical performance in humans. J Neurosci 24: 10186–10190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medendorp WP,Kramer GFI,Jensen O,Oostenveld R,Schoffelen JM,Fries P ( 2007): Oscillatory activity in human parietal and occipital cortex shows hemispheric lateralization and memory effects in a delayed double‐step saccade task. Cereb Cortex 17: 2364–2374. [DOI] [PubMed] [Google Scholar]
- Moses P,Roe K,Buxton RB,Wong EC,Frank LR,Stiles J ( 2002): Functional MRI of global and local processing in children. NeuroImage 16: 415–424. [DOI] [PubMed] [Google Scholar]
- Müller NG,Bartelt OA,Donner TH,Villringer A,Brandt SA ( 2003): A physiological correlate of the ‘zoom lens’ of visual attention. J Neurosci 23: 3561–3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navon D ( 1977): Forest before the trees: The precedence of global features in visual perception. Cogn Psychol 9: 353–393. [Google Scholar]
- Pfurtscheller G,Aranibar A ( 1977): Event‐related cortical desynchronization detected by power measurements of scalp EEG. Electroencephalogr Clin Neurophysiol 42: 817–826. [DOI] [PubMed] [Google Scholar]
- Rihs TA,Michel CM,Thut G ( 2007): Mechanisms of selective inhibition in visual spatial attention are indexed by alpha‐band EEG synchronization. Eur J Neurosci 25: 603–610. [DOI] [PubMed] [Google Scholar]
- Robertson LC,Lamb MR ( 1991): Neuropsychological contributions to theories of part/whole organization. Cogn Psychol 23: 299–330. [DOI] [PubMed] [Google Scholar]
- Robertson LC,Egly R,Lamb MR,Kerth L ( 1993): Spatial attention and cuing to global and local levels of hierarchical structure. J Exp Psychol Hum Percep Perform 19: 471–487. [DOI] [PubMed] [Google Scholar]
- Rose M,Sommer T,Büchel C ( 2006): Integration of local features into a global percept by neural coupling. Cereb Cortex 16: 1522–1528. [DOI] [PubMed] [Google Scholar]
- Sergent J ( 1982): The cerebral balance of power: Confrontation or cooperation? J Exp Psychol: Hum Percep Perform 8: 253–272. [DOI] [PubMed] [Google Scholar]
- Stoffer TH ( 1993): The time course of attentional zooming: A comparison of voluntary and involuntary allocation of attention to the levels of compound stimuli. Psych Res 56: 14–25. [DOI] [PubMed] [Google Scholar]
- Stoffer TH ( 1994): Attentional zooming and the global‐dominance phenomenon: Effects of level‐specific cueing and abrupt visual onset. Psych Res 56: 83–98. [DOI] [PubMed] [Google Scholar]
- Thut G,Nietzel A,Brandt SA,Pascual‐Leone A ( 2006): Alpha‐band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. J Neurosci 26: 9494–9502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootell RB,Hadjikhani N,Hall EK,Marrett S,Vanduffel W,Vaughan JT,Dale AM ( 1998): The retinotopy of visual spatial attention. Neuron 21: 1409–1422. [DOI] [PubMed] [Google Scholar]
- Van Dijk H,Schoffelen J,Oostenveld R,Jensen O ( 2008): Prestimulus oscillatory activity in the alpha band predicts visual discrimination ability. J Neurosci 28: 1816–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kleeck MH ( 1989): Hemispheric differences in global versus local processing of hierarchical visual stimuli by normal subjects: New data and a meta‐analysis of previous studies. Neuropsychologia 27: 1165–1178. [DOI] [PubMed] [Google Scholar]
- Volberg G,Hübner R ( 2004): On the role of response conflicts and stimulus position for hemispheric differences in global/local processing: An ERP study Neuropsychologia 42: 1805–1813. [DOI] [PubMed] [Google Scholar]
- Volberg G,Hübner R ( 2007): Do the hemispheres differ in their preparation for global/local processing? Exp Brain Res 176: 525–531. [DOI] [PubMed] [Google Scholar]
- Ward, LM ( 2003): Synchronous neural oscillations and cognitive processes. TICS 7: 553–559. [DOI] [PubMed] [Google Scholar]
- Weissman DH,Woldorff MG ( 2005): Hemispheric asymmetries for different components of global/local attention occur in distinct temporo‐parietal loci. Cereb Cortex 15: 870–876. [DOI] [PubMed] [Google Scholar]
- Worden MS,Foxe JJ,Wang N,Simpson GV ( 2000): Anticipatory biasing of visuospatial attention indexed by retinotopically specific alpha‐band electroencephalography increases over occipital cortex. J Neurosci 20: RC63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S,Yamagata S,Kobayashi S ( 2000): Cerebral asymmetry of the top–down allocation of attention to global and local features. J Neurosci 20: 1522–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]


