Abstract
Current evidence suggests that attention deficit hyperactivity disorder (ADHD) involves dysfunction in wide functional networks of brain areas associated with attention and cognition. This study examines the structural integrity of white‐matter neural pathways, which underpin these functional networks, connecting fronto‐striatal and fronto‐parietal circuits, in children with ADHD. Fifteen right‐handed 8 to 18‐year‐old males with ADHD‐combined type and 15 right‐handed, age, verbal, and performance IQ‐matched, healthy males underwent diffusion tensor imaging. A recent method of tract‐based spatial statistics was used to examine fractional anisotropy (FA) and mean diffusivity within major white‐matter pathways throughout the whole‐brain. White‐matter abnormalities were found in several distinct clusters within left fronto‐temporal regions and right parietal‐occipital regions. Specifically, participants with ADHD showed greater FA in white‐matter regions underlying inferior parietal, occipito‐parietal, inferior frontal, and inferior temporal cortex. Secondly, eigenvalue analysis suggests that the difference in FA in ADHD may relate to a lesser degree of neural branching within key white‐matter pathways. Tractography methods showed these regions to generally form part of white‐matter pathways connecting prefrontal and parieto‐occipital areas with the striatum and the cerebellum. Our findings demonstrate anomalous white‐matter development in ADHD in distinct cortical regions that have previously been shown to be dysfunctional or hypoactive in fMRI studies of ADHD. These data add to an emerging picture of abnormal development within fronto‐parietal cortical networks that may underpin the cognitive and attentional disturbances associated with ADHD. Hum Brain Mapp, 2009. © 2008 Wiley‐Liss, Inc.
Keywords: ADHD, fractional anisotropy, TBSS, diffusion tensor imaging
INTRODUCTION
Attention deficit hyperactivity disorder (ADHD) is defined as a persistent pattern of inattention and/or hyperactivity/impulsivity that interferes with aspects of children's academic, professional, and/or social lives. Neuroimaging studies have revealed abnormalities in brain structure and function in ADHD, although the precise etiology and pathogenesis of the disorder is still unclear.
Structural imaging studies have reported abnormal development of several key brain regions in ADHD, including the prefrontal cortex, cerebellum, striatum and basal ganglia, corpus callosum, and the parietal cortex (Castellanos et al., 1994, 1996, 2002; Filipek et al., 1997; Giedd et al., 1994; Hynd et al., 1990; Mostofsky et al., 1998; Sowell et al., 2003). Functional imaging studies have also highlighted a number of distinct brain regions that appear to function abnormally in ADHD, the most prominent of these being the prefrontal cortex and striatum (fronto‐striatal circuits) and the parietal cortex (King et al., 2003; Konrad et al., 2006; Rubia et al., 2006; Schulz et al., 2005; Silk et al., 2005).
As understanding of the neuropathology of ADHD has developed, the early frontal‐lobe hypotheses have extended to consider ADHD as involving dysfunction within wider functional networks subserving attention, cognition, and self‐regulation (Booth et al., 2005; Vaidya et al., 2005). Our previous research (Silk et al., 2005; Vance et al., 2007) has shown dysfunction of a larger, more extensive attentional, cognitive and visuo‐spatial network that is centered on foci within frontal, striatal, and parietal areas. This network is of fundamental importance for attentional and cognitive control (Mesulam, 1990).
While functional brain imaging studies have revealed specific regions of dysfunction in ADHD, and it is now emerging that ADHD may be a disorder of abnormal fronto‐striatal and fronto‐parietal circuits; it is particularly important to know how the nodes within this network are structurally connected. Conventional structural MRI can give valuable information on the macrostructure and volume of gray and white‐matter.
Diffusion tensor imaging (DTI) is a relatively new method, which gives an indication of the microstructure of white‐matter. DTI is sensitive to gross differences in the structure of the cellular matrix within major white‐matter fiber pathways, and can provide a richer understanding of white‐matter integrity and connectivity of the brain (Mori and Zhang, 2006). This technique can potentially be important in allowing us to examine the nature and extent of neuronal disruption associated with ADHD that conventional MRI cannot identify, and in the future, provide new avenues to help reveal the relationships between the clinical symptoms and the affected brain regions.
Fundamentally, DTI is sensitive to the direction and extent of the diffusion of water in the brain. Such diffusion is typically quantified by two derived measures: Mean Diffusivity (MD), representing apparent mobility of water, is simply the magnitude of diffusion in each measured voxel (in mm2/s); Fractional Anisotropy (FA), which is an index of the ‘directionality’ of diffusion in each voxel (a normalized measure with values of 0 to 1, with 0 representing isotropic diffusion). In general, white‐matter regions with parallel myelinated axon fibers will have high values of FA, since water diffusion is constrained by the cellular structure to be aligned primarily along the direction of the myelinated fiber tracts. However, interpretation of the diffusion parameters is not always straightforward (Beaulieu, 2002). Higher FA values could signify either an increase in fiber bundle density or myelination, or a decrease in regional branching and crossing of fibers.
In this study, we examined the structure of white‐matter fiber pathways in children with ADHD compared with age‐matched controls. We applied a recent method of tract‐based spatial statistics (TBSS) (Smith et al., 2006) to examine FA and MD within major white‐matter pathways throughout the whole‐brain.
Currently, there are very few studies reporting DTI changes in brain structure in ADHD. Ashtari et al. (2005) used a whole‐brain voxel‐based morphology (VBM) analysis technique and reported decreased FA in children with ADHD, in regions suggesting a circuit of ‘corticopontocerebellar’ anomalies. Casey et al. (2007) found that the prefrontal regions of functional deficit, using fMRI in ADHD, were positively correlated with FA.
While previous studies have often used VBM methods for whole‐brain analysis, this approach has several limitations when applied to DTI data (Smith et al., 2006). Methodologically, limitations relate to difficulties in accurate intersubject image registration, which is crucial for DTI data in which large image intensity boundaries exist between white‐matter tracts (very high FA values) and cortical gray‐matter or subcortical nuclei (low FA values). White‐matter is inherently highly variable between individuals and therefore registration for white‐matter between individuals on a voxel bases is critical.
TBSS is a recent analysis method developed specifically for DTI data that restricts analysis to just the center of major white‐matter tracts, rather than indiscriminately across the whole‐brain volume (Smith et al., 2006). TBSS minimizes intersubject registration problems and problems of multiple comparisons by first determining a mean FA ‘skeleton,’ representing only the center of major white‐matter fiber tracts, then mapping each participant's DTI data directly onto that skeleton. Analysis of FA differences is then restricted to regions, which, with high confidence, represent only the center of equivalent white‐matter tracts in each individual. TBSS methods are therefore highly sensitive to changes in microstructure within the major white‐matter fiber pathways of the brain.
The aim of the current study was to examine white‐matter brain microstructure, in terms of FA and MD, in equivalent white‐matter pathways in ADHD and control children. Areas of difference between the groups were then examined with probabilistic tractography methods to fully characterize the origins and pathways of the white‐matter tracts in which abnormalities were detected. Given our previous findings of fronto‐striatial and parietal dysfunction in ADHD (Silk et al., 2005; Vance et al., 2007), we expected that children with ADHD would have different diffusion properties, representing abnormalities in the development of white‐matter microstructure, within the underlying white‐matter tracts involved in fronto‐parietal and fronto‐striatal networks.
MATERIALS AND METHODS
Participants
Fifteen males aged 8–18 years (mean 12.6 ± 2.4 years) with ADHD‐combined type (ADHD‐CT) fulfilling the DSM‐IV criteria were identified in a specialized clinic for ADHD based at the Royal Children's Hospital, Melbourne, Australia. The Anxiety Disorders Interview Schedule for Children (A‐DISC) (Silverman and Albano, 1996), a semistructured clinical interview with the participant's parent(s); and the Conners' Global Index (CGI)(Conners, 1985), a parent and/or teacher report (teacher and parent, n = 13; parent only, n = 2) were used to assess the core symptom domains of ADHD‐CT. CGI subscale scores greater than 1.5 standard deviations above the mean for a given participant's age and gender were required as inclusion criteria for the ADHD‐CT group. The A‐DISC was used to exclude children with comorbid depressive, anxiety, and conduct disorders. All participants had a full scale IQ above 80, according to an age‐appropriate Weschler test (2004: mean performance IQ: 104.9 ± 11.5; mean verbal IQ: 102.9 ± 14.7); and there were no known neurological or endocrine disease, psychotic symptoms, reading/spelling/arithmetic learning disorders, developmental coordination disorder, or alcohol/substance abuse/dependence disorders. The participants were all medication naïve or medication free for 24 h before scanning (medication naïve, n = 12; medication withdrawn, n = 3), and met the inclusion criteria of living in a family home (and not in an institution) and attending normal primary schools.
Fifteen healthy male control participants were matched in age (8–18 years; mean 12.9 ± 2.6 years) and IQ (mean performance IQ: 111.6 ± 9.2; mean verbal IQ: 111.9 ± 9.7) to the ADHD group. Normal behavioral functioning was determined in the control participants through the A‐DISC, the parent form of the Child Behaviour Checklist and the CGI specifically to screen for characteristics of ADHD. Only right‐handers participated. All participants (and parents/guardians) gave written informed consent, and all procedures were approved by the Human Experimentation Ethics Committee of the Royal Children's Hospital, Melbourne, Australia.
Data Acquisition and Analysis
Data were acquired on a 3‐Tesla GE Signa MR scanner (GE, Medical Systems, USA), at the Brain Research Institute, Austin Health, Melbourne. Participants lay supine with their head supported in a volume coil. Diffusion‐weighted echoplanar images (EPI) were acquired along 28 diffusion gradient directions for acquisition of 50 slices through the whole brain (TR = 5,800 ms, TE = 16 ms, FOV = 240 mm2, 96 × 96 matrix, 2.5 mm in‐plane resolution, b value of 1,100 s/mm2, slice thickness = 2.5 mm).
Analyses of diffusion‐weighted images were done using FSL software (Oxford, UK): FMRIB's Diffusion Toolbox (FDT). Initially, eddy current correction was run to correct for gradient‐coil distortions and small head motions, using affine registration to a reference volume. Maps of FA, MD, and the primary, secondary, and tertiary eigenvalues were calculated from the diffusion‐weighted images using DTIFit, which fits a diffusion tensor model to each voxel, estimating the principle directions of diffusion.
Correlation Analysis
To determine whether FA values increase with age in our sample, as previously reported in healthy control adolescents (Ashtari et al., 2007; Barnea‐Goraly et al., 2005; Schmithorst et al., 2005; Snook et al., 2005), linear regression using Spearman's correlation was calculated on mean FA values for white and gray‐matter combined across the whole brain compared with age for both the ADHD and control groups (significance threshold P < 0.05, one‐tailed). Mean FA values were also correlated against symptom severity for the ADHD group using Conners Parent Rating Scale scores.
Tract‐Based Spatial Statistics
Values of FA and MD derived from the DTI images in ADHD and control groups were statistically compared for regional differences using the TBSS method in FSL software (Oxford, UK). The TBSS method minimizes the potential misalignment problems of other voxel‐based whole‐brain analysis methods by determining a white‐matter “skeleton” restricted only to the center of major white‐matter tracts, and mapping FA and MD values from each individual directly onto this standard skeleton for group comparison.
Firstly, images from all individuals were aligned to each other using nonlinear registration (IRTK local matrix deformation) to determine the most representative individual (i.e., the closest to the mean of the group) to be defined as the target image. This target image was then aligned to the standard stereotactic co‐ordinate space using affine registration to the MNI brain template. Using these realignment parameters, the FA images from each individual were then registered to the target image of the most representative individual and a group mean FA image was calculated. A white‐matter skeleton was then generated, thresholding the mean FA map (FA > 1.5) and representing a single line running down the centers of all the common white‐matter fibers. This is achieved by searching for all local voxels along tracts in the perpendicular direction, and the voxel with the highest FA is identified as the center of the tract.
Group statistical analysis was then conducted only on voxels within the white‐matter skeleton mask, therefore restricting the voxel‐wise analysis only to voxels with high confidence of lying within equivalent major white‐matter pathways in each individual. At this stage, MD and eigenvalue maps, which were generated using DTIfit, were incorporated into the group analysis. Differences in FA and MD between ADHD and control groups were assessed using voxelwise independent t‐tests. To calculate probabilities corrected for multiple comparisons, nonparametric permutation tests were conducted, generating cluster‐size statistics based on 5,000 random permutations. We used a cluster‐defining threshold (voxel‐level t > 3.0), and only report clusters with a corrected P‐value <0.05.
Absolute Magnitudes of Diffusion
For each participant, the principal three directions of diffusion were determined within each voxel, described by primary, secondary, and tertiary eigenvectors and eigenvalues. The absolute magnitudes of diffusion along each of the three principal directions (the eigenvalues λ1, λ2, and λ3) were obtained and used to further investigate how changes in FA were related to differential changes in the magnitudes of diffusion along each of the three principal diffusion directions.
A region of interest (ROI) mask was manually generated for each of the significant clusters identified in the TBSS analysis. This was done by including only the voxels that showed a significant difference in FA (P corrected < 0.05). These ROI masks were then back‐projected to the original images of each individual and mean values of diffusion parameters were obtained. Independent t‐tests were conducted (SPSS v12.0.1, USA) to compare the mean absolute magnitudes of diffusion in the three principal directions within each ROI between ADHD participants and controls. Differences significant at P < 0.05 were reported.
Probabilistic Tractography
To further characterize the origins and pathways of the white‐matter tracts in which abnormalities in FA were found, the probabilistic tractography method implemented within FSL software (Oxford, UK) was used to track likely paths extending from the clusters identified by the TBSS analysis. ROI masks were generated for each cluster showing significant differences in FA in ADHD compared with control groups from the TBSS analysis. These ROI masks were then back‐projected to the original images of the most representative individual (the registration target for the TBSS analysis). Probabilistic fiber‐tracking, as implemented within FSL, was then conducted using each of these ROI masks as “seed” regions, with a step length of 0.5 mm, a maximum of 2,000 steps, and a curvature threshold of 0.2. Using this method, we obtained probabilistic connectivity maps representing the most likely paths extending from the clusters in which abnormalities in FA had been found. The cortical origins and end points of these connectivity paths were reported based on anatomical labels of the Automated Anatomic Labelling template (Tzourio‐Mazoyer et al., 2002).
RESULTS
Correlation Analysis
Mean FA values over the whole‐brain white and gray‐matter showed a significant increase with age in both ADHD and control groups (Fig. 1; ADHD: r s = 0.64, N = 15, P < 0.01; control: r s = 0.46, N = 15, P < 0.05). While the ADHD group showed slightly higher mean FA values overall, there was no significant difference between the means of the two groups (P = 0.22) or between the slopes of the correlations over age (Z = 1.15). There was no significant correlation between mean FA and symptom severity (Conners Parent Rating Scale scores) in the ADHD group.
Figure 1.
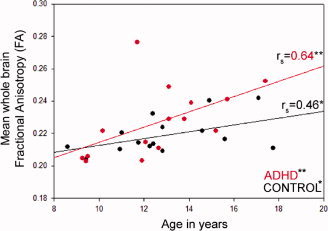
Correlation relationship between mean fractional anisotropy and age in the ADHD and control groups. Correlation coefficients are shown on the right‐hand side of the graph. *Significant correlation, P < 0.05; **Significant correlation, P < 0.01. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
TBSS Analysis of Regional White‐Matter Differences
TBSS analysis identified three main clusters showing significantly increased FA in ADHD children compared to controls (see Table I; Fig. 2). These clusters were located in the white‐matter underlying right occipito‐parietal cortex, left inferior frontal cortex/striatum, and left inferior temporal regions. Other two clusters, with a trend toward significance (P corrected < 0.8, P uncorrected < 0.001), lay in the white‐matter underlying right inferior parietal and left inferior frontal regions. There were no regions showing significantly higher FA in controls compared to the ADHD group, and there were no significant differences between ADHD and control participants for MD measures.
Table I.
Regions that showed significantly greater fractional anisotropy in the ADHD group compared to the control group
| Cluster | Anatomic definition [closest gray‐matter] (White matter tract) | Coordinates (mm) | Cluster size | P value |
|---|---|---|---|---|
| 1 | R Occipito‐parietal region (Cingulum) | 8, −76, 10 | 245 | P corrected < 0.01 |
| 2 | L Inf. frontal/striatum (Uncinate fasciculus) | −21, 10, −24 | 168 | P corrected < 0.03 |
| 3 | L Inf. temporal region (Inf. longitudinal fasciculus) | −40, −21, −35 | 156 | P corrected < 0.04 |
| 4a | R Inf. parietal region (Sup. longitudinal fasciculus) | 38, −60, 27 | 132 | P corrected < 0.06 (P uncorrected < 0.001) |
| 5a | L Inf. frontal region (Uncinate fasciculus) | −46, 29, −18 | 113 | P corrected < 0.08 (P uncorrected < 0.001) |
Note: Coordinates expressed in MNI stereotactic x, y, and z axes; the coordinate reported was the peak voxel in the cluster.
Trend to significance.
Figure 2.
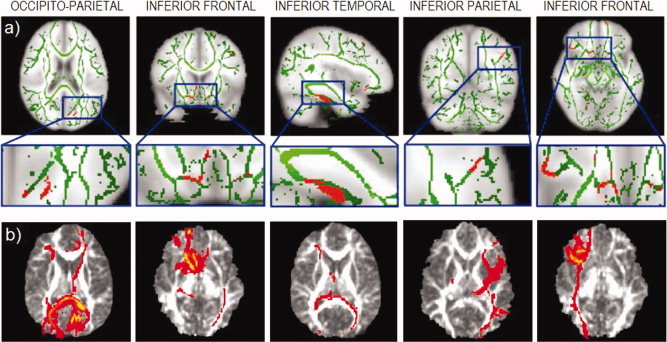
(a) TBSS analysis showing the mean FA skeleton (green) and regions showing significant differences between ADHD and control groups (red) displayed on the MNI152 brain (neurological view, R = R). (b) Axial slices showing the connectivity distribution of fibers originating from each of the areas of FA difference shown immediately above. Connectivity maps are displayed on the representative target FA image to which all other images are registered. Note: Clusters 4 and 5 showed only a trend toward significance.
Absolute Magnitudes of Diffusion
For the five clusters identified in the TBSS analysis, absolute magnitudes of diffusion in the three principal diffusion directions were evaluated and are summarized in Table II. In general, increased FA in the ADHD group was associated with both increased diffusion in the primary direction (λ1) and decreased diffusion in secondary (λ2) and tertiary (λ3) directions. The two clusters in the occipital/parietal regions (Clusters 1 and 4) showed significant increases in λ1 and significant decreases in both λ2 and λ3. Cluster 3 (temporal region) showed a significant increase in λ1 and a significant decrease only in λ3, Cluster 2 (inferior frontal/striatum) showed only a significant increase inλ1, while Cluster 5 (inferior frontal region) showed only a significant decrease in λ3. In general, however, these findings suggest that the greater FA values in the ADHD group resulted not only from an increase in diffusion along the primary direction, but also by a decrease in diffusion along the secondary and tertiary directions.
Table II.
Absolute magnitudes of diffusion along primary, secondary, and tertiary directions within the regions showing significant FA differences in the ADHD group compared to the control group
| Cluster | Anatomic definition | ADHD (n = 15) | Controls (n = 15) | P value (corrected) | |
|---|---|---|---|---|---|
| 1 | R Occipito‐parietal region | λ1 | 1241.3 ± 70.7 | 1188.2 ± 63.2 | P < 0.05 |
| λ2 | 820.5 ± 48.1 | 887.3 ± 47.0 | P < 0.001 | ||
| λ3 | 646.1 ± 64.1 | 749.3 ± 48.5 | P < 0.001 | ||
| 2 | L Inf. frontal/striatum | λ1 | 1213.5 ± 69.3 | 1134.6 ± 33.5 | P < 0.001 |
| λ2 | 941.9 ± 48.9 | 923.5 ± 27.8 | NS | ||
| λ3 | 725.4 ± 64.6 | 740.3 ± 41.3 | NS | ||
| 3 | L Inf. temporal region | λ1 | 1187.5 ± 80.6 | 1112 ± 47.0 | P < 0.005 |
| λ2 | 882.8 ± 60.9 | 991.7 ± 40.7 | NS (P = 0.08) | ||
| λ3 | 668.5 ± 75.0 | 738.6 ± 82.5 | P < 0.05 | ||
| 4a | R Inf. parietal region | λ1 | 1295.8 ± 133.1 | 1166.4 ± 57.0 | P < 0.005 |
| λ2 | 801.9 ± 61.3 | 870.3 ± 57.1 | P < 0.005 | ||
| λ3 | 536.7 ± 63.0 | 680.4 ± 92.5 | P < 0.001 | ||
| 5a | L Inf. frontal region | λ1 | 1151.6 ± 83.6 | 1106.9 ± 76.9 | NS |
| λ2 | 890.6 ± 60.6 | 925.7 ± 64.0 | NS | ||
| λ3 | 716.9 ± 87.4 | 779.2 ± 77.9 | P < 0.05 |
Note: Mean ± standard deviation (×10−6 mm2/s). NS, nonsignificant. Primary (λ1), secondary (λ2), and tertiary (λ3) eigenvalues.
Trend to significance.
Additionally, to investigate any effect of age on the differences in eigenvalues, each off the ROI clusters that demonstrated a significant difference between the ADHD group and controls were subjected to a correlation analysis. There were no significant age correlations in either the ADHD or the control group for the individual eigenvalues.
Probabilistic Tractography
Figure 2b shows the probabilistic connectivity maps representing the most likely paths extending to and from each cluster identified in the TBSS analysis. Anatomic descriptions of the cortical origins and endpoints of these paths were obtained by reference to the Automated Anatomical Labeling template of Tzourio‐Mazoyer et al. (2002), and white‐matter tracts were obtained by reference to the MRI atlas of human white‐matter of Mori et al. (2005).
Right Occipito‐Parietal
The cluster in the right occipito‐parietal region formed part of white‐matter pathways interconnecting occipital, temporal, and prefrontal brain regions. Pathways extended along the posterior thalamic radiation to the ipsilateral cuneus, calcarine, superior occipital, and inferior temporal gyri, as well as extending anteriorly along the anterior thalamic radiation on the medial surface of the thalamus along the anterior limb of the internal capsule, ending in the right superior frontal gyrus and anterior cingulate. Pathways also looped around via the splenium of the corpus callosum to the left calcarine, superior and middle occipital gyri, and inferior temporal gyrus, from where they also extended anteriorally via the superior longitudinal fasciculus to the left inferior and middle frontal gyri. Pathways also appeared to travel via the middle cerebellar peduncle to left lobule VIII of the vermis in the cerebellum.
Left Inferior Frontal/Striatum
The cluster in the left inferior frontal/striatum region formed part of pathways connecting the caudate nucleus and prefrontal and orbitofrontal regions, as well as extending posteriorly to temporal and occipital regions. Pathways extended via uncinate fasciculus to ipsilateral inferior and superior frontal gyri, middle and medial orbital gyri, and the anterior cingulate, as well as extending into the left inferior temporal gyrus and, via the genu of the corpus callosum, to the right medial orbital and superior frontal gyri. Pathways appeared to surround both caudate heads, then run along the right inferior longitudinal fasciculus to the calcarine and middle temporal gyrus.
Left Inferior Temporal
The left inferior temporal cluster formed part of pathways connecting temporal, occipital, and prefrontal regions in the left hemisphere. Pathways extended to the left inferior temporal lobe running along the inferior longitudinal fasciculus to left calcarine, inferior and middle occipital gyri, and via the genu of the corpus callosum, to the contralateral calcarine and cuneus. Pathways also ran along the left anterior thalamic radiation and ended in the left inferior frontal and right middle frontal gyri.
Right Inferior Parietal
The right inferior parietal cluster showed extensive projections, mainly in the right hemisphere, to occipital, parietal, temporal, and prefrontal regions, as well as to the cerebellum. Pathways extended to the postcentral gyrus, angular gyrus, superior and inferior parietal gyri, and inferiorly to the cuneus and middle occipital gyrus, all in the right hemisphere. Pathways also appeared to travel via the middle cerebellar peduncle to right lobules VIII and IX of the vermis in the cerebellum. Numerous tracts traveled along the inferior longitudinal fasciculus to inferior, middle, and superior temporal gyri, and along the superior longitudinal fasciculus and external capsule via the lateral edge of the head of caudate to inferior, middle, and superior frontal gyri.
Left Inferior Frontal
The cluster in the left inferior frontal region formed part of white‐matter pathways connecting prefrontal, temporal, and occipital regions mostly in the left hemisphere. Pathways extended to the inferior frontal gyrus and along the inferior fronto‐occipital fasciculus to the medial orbital gyrus. Pathways also traveled posteriorly to inferior and middle temporal gyri, and to the calcarine, inferior and middle occipital gyri, as well as crossing over to right calcarine and cuneus.
DISCUSSION
Previous DTI studies have shown that FA increases with maturation over adolescence, thought to represent ongoing myelination throughout adolescent brain development (Ashtari et al., 2007; Barnea‐Goraly et al., 2005; Schmithorst et al., 2005; Snook et al., 2005). Our correlation analysis of mean FA values shows the same increase in FA with age, consistent with these previous findings, and revealed no differences between control and ADHD groups, although the ADHD group showed a slightly higher whole‐brain white and gray‐matter FA value, particularly into adolescence.
Children with ADHD showed abnormal white‐matter structure in several distinct clusters within two main regions: prefrontal‐temporal regions of the left hemisphere and parietal‐occipital regions of the right hemisphere. These areas of abnormal white‐matter structure were generally associated with neural pathways connecting prefrontal and occipito‐parietal regions with the striatum and the cerebellum. Specifically, using the recent method of TBSS to restrict comparisons to the center of major white‐matter pathways revealed greater FA in children with ADHD in white‐matter regions underlying right inferior parietal and occipito‐parietal areas, and left inferior frontal and inferior temporal areas.
Studies of other neurodevelopmental disorders have also reported and increase in FA in certain brain regions in bipolar disorder (Yurgelun‐Todd et al., 2007) and Williams Syndrome (Hoeft et al., 2007). Hoeft et al., using the same TBSS method on a cohort with Williams Syndrome, found high FA in the right superior longitudinal fasciculus and additionally found a correlation of increased FA with an increase in cognitive function deficit (visual–spatial).
While our results clearly indicate an abnormality in the development of these specific white‐matter pathways in children with ADHD, it is still not clear at a cellular level what changes in FA represent. DTI uses water diffusion characteristics to indicate cellular organization through the derived measures of diffusion directionality (FA) and magnitude (MD). Values of FA can be affected by many variations in the white‐matter structure, including changes in intra‐ and extra‐cellular volume, permeability of cell membranes, fiber coherence, and axonal loss (Basser and Pierpaoli, 1996). DTI studies commonly attribute an increase in FA to an increase in density, more coherent organization, and greater myelination of fibers (Mori and Zhang, 2006).
The current study's second finding resulted from the examination of the magnitudes of diffusion along the three major diffusion directions. Rather than solely an increase in density and fiber organization affecting the strength of diffusion along the primary direction, the increase in FA observed in the ADHD group was also due to a decrease in diffusion along secondary and tertiary directions. This reduction in diffusion along secondary and tertiary directions is also associated with maturation in childhood development (Mukherjee et al., 2001; Snook et al., 2005; Suzuki et al., 2003). It is suggested that, in areas with significant crossing and branching of neuronal fibers, FA may be relatively low given that diffusion may be relatively strong across multiple directions aligned with the crossing and branching fibers. If the degree of neuronal branching is less, the magnitude of diffusion across secondary and tertiary directions is reduced and greater FA values are observed (Suzuki et al., 2003). We therefore suggest that the greater FA values observed in the ADHD group in our study may represent an abnormal reduction in the degree of neuronal branching within these key white‐matter pathways underlying fronto‐temporal and occipito‐parietal regions. The lack of a significant decrease in the secondary and tertiary directions in Cluster 2 may be representative of the clusters location. The location of the cluster is in a region of a high density of crossing fibers, which are continuing to strengthen over development. Thus, particularly at the age of the cohort, perhaps changes might be seen later in development.
A long standing debate exists as to whether ADHD is simple a delay of neural development, a maturation lag, given that ADHD symptoms can improve over time during development (for review, see El‐Sayed et al., 2003). Our results show an overall significant and similar increase in FA with age in both ADHD and control groups, but specifically localized regions of difference in the ADHD group. This appears less consistent with a developmental delay in children with ADHD; however, further research would be needed to specifically address this issue.
While the etiology and the cognitive and behavioral implications of these structural abnormalities are unclear, it is clear that these regional FA anomalies in children with ADHD correspond to the white‐matter underlying regions that have been reported to be functionally anomalous in ADHD (King et al., 2003; Konrad et al., 2006; Schulz et al., 2005). The results are also concordant with the regions that our previous functional imaging research has reported to be under‐active in children and adolescents with ADHD (Silk et al., 2005; Vance et al., 2007). Further, reduced integrity of white‐matter underlying fronto‐parietal regions is consistent with deficits of attention, working memory and inhibition that are prominent in ADHD (Booth et al., 2005; Vaidya et al., 2005).
As discussed earlier, previous studies have shown that FA increases with age during childhood and adolescent development. These previous studies have generally been conducted using a limited number of ROIs, but have consistently reported age‐related increases in FA in regions including the prefrontal cortex, internal capsule, corpus callosum, ventral visual stream, head of the caudate nucleus, and in major tracts including corticospinal or thalamocortical tracts and the arcuate fasciculus (Ashtari et al., 2007; Barnea‐Goraly et al., 2005; Klingberg et al., 1999; Mukherjee et al., 2001; Schmithorst et al., 2002, 2005; Schneider et al., 2004; Snook et al., 2005). Our results show a similar increase in FA across age in both healthy control and ADHD groups, concordant with previous DTI studies on normal white‐matter development. The TBSS analysis of regional white‐matter changes in the ADHD group, however, does not replicate the findings of Ashtari et al. (2005). The key difference between our study and that of Ashtari et al. (2005) is our application of the TBSS method to restrict comparisons to only the center of major white‐matter pathways. While we cannot comment on regions outside the centers of white‐matter pathways, it is possible that regions toward the borders of white‐matter may show a reduction in FA in children with ADHD similar to the change in FA reported by Ashtari et al. (2005). Our results are not incompatible with the findings of Ashtari et al. (2005) and extend importantly on their results. We can definitively conclude that, at least in the center of major white‐matter pathways, FA values are significantly greater in children and adolescents with ADHD compared with controls in regions underlying right occipito‐parietal cortex and left fronto‐temporal cortex. Further, this increase in FA is generally associated with reduced diffusion along secondary and tertiary directions, which we suggest may represent an abnormal reduction in the degree of neuronal branching and crossing of fibers within these regions in children with ADHD.
In an attempt to reduce the possible confounding effects of long‐term medication use, twelve participants with ADHD were medication naïve. While the remaining three participants were taken off their medication 24 h before the scan, the medication wash‐out period cannot safeguard against any potential long‐term effects of the stimulant medication on brain function. Notably, Castellanos et al. (2002) reported that the decrease of white‐matter volume in ADHD compared to controls was less in medicated patients. Therefore, it is possible that medication may have long‐term effects on brain development. While this phenomenon was not addressed in the current study, it will be important for future research to investigate.
In the future, FA measures could potentially serve as an important biomarker or endophenotype for ADHD. Potential applications include differentiating ADHD from its key comorbid disorders, investigating the stability of FA measures across key developmental stages, determining the strength of the association between FA measures and the response of ADHD sufferers to medication or other treatments, and examining possible genetic factors, which may influence the development of white‐matter pathways.
The present study has used the relatively new method of TBSS to specifically examine localized differences in FA at the centers of major white‐matter pathways in children with ADHD. DTI has an important role in revealing the relationships between clinical disorders and affected brain regions, and in this study has provided further insight into the organization and the structure of white‐matter pathways in ADHD. We have identified key areas of FA difference in fronto‐parietal regions, and suggest that these differences may result from a lesser degree of neuronal branching as indicated in the secondary and tertiary eigenvalues. These key regions have been previously associated with hypo‐activation and implicated in the cognitive and attentional disturbances of the disorder.
REFERENCES
- Ashtari M,Kumra S,Bhaskar SL,Clarke T,Thaden E,Cervellione KL,Rhinewine J,Kane JM,Adesman A,Milanaik R,Maytal J,Diamond A,Szeszko P,Ardekani BA ( 2005): Attention‐deficit/hyperactivity disorder: A preliminary diffusion tensor imaging study. Biol Psychiatry 57: 448–455. [DOI] [PubMed] [Google Scholar]
- Ashtari M,Cervellione KL,Hasan KM,Wu J,McIlree C,Kester H,Ardekani BA,Roofeh D,Szeszko PR,Kumra S ( 2007): White matter development during late adolescence in healthy males: A cross‐sectional diffusion tensor imaging study. Neuroimage 35: 501–510. [DOI] [PubMed] [Google Scholar]
- Barnea‐Goraly N,Menon V,Eckert M,Tamm L,Bammer R,Karchemskiy A,Dant CC,Reiss AL ( 2005): White matter development during childhood and adolescence: A cross‐sectional diffusion tensor imaging study. Cereb Cortex 15: 1848–1854. [DOI] [PubMed] [Google Scholar]
- Basser PJ,Pierpaoli C ( 1996): Microstructural and physiological features of tissues elucidated by quantitative‐diffusion‐tensor MRI. J Magn Reson B 111: 209–219. [DOI] [PubMed] [Google Scholar]
- Beaulieu C ( 2002): The basis of anisotropic water diffusion in the nervous system—A technical review. NMR Biomed 15(7/8): 435–455. [DOI] [PubMed] [Google Scholar]
- Booth JR,Burman DD,Meyer JR,Lei Z,Trommer BL,Davenport ND,Li W,Parrish TB,Gitelman DR,Mesulam MM ( 2005): Larger deficits in brain networks for response inhibition than for visual selective attention in attention deficit hyperactivity disorder (ADHD). J Child Psychol Psychiatry 46: 94–111. [DOI] [PubMed] [Google Scholar]
- Casey BJ,Epstein JN,Buhle J,Liston C,Davidson MC,Tonev ST,Spicer J,Niogi S,Millner AJ,Reiss A,Garrett A,Hinshaw SP,Greenhill LL,Shafritz KM,Vitolo A,Kotler LA,Jarrett MA,Glover G ( 2007): Frontostriatal connectivity and its role in cognitive control in parent–child dyads with ADHD. Am J Psychiatry 164: 1729–1736. [DOI] [PubMed] [Google Scholar]
- Castellanos FX,Giedd JN,Eckburg P,Marsh WL,Vaituzis AC,Kaysen D,Hamburger SD,Rapoport JL ( 1994): Quantitative morphology of the caudate nucleus in attention deficit hyperactivity disorder. Am J Psychiatry 151: 1791–1796. [DOI] [PubMed] [Google Scholar]
- Castellanos FX,Giedd JN,Marsh WL,Hamburger SD,Vaituzis AC,Dickstein DP,Sarfatti SE,Vauss YC,Snell JW,Lange N,Kaysen D,Krain AL,Ritchie GF,Rajapakse JC,Rapoport JL ( 1996): Quantitative brain magnetic resonance imaging in attention‐deficit hyperactivity disorder. Arch Gen Psychiatry 53: 607–616. [DOI] [PubMed] [Google Scholar]
- Castellanos FX,Lee PP,Sharp W,Jeffries NO,Greenstein DK,Clasen LS,Blumenthal JD,James RS,Ebens CL,Walter JM,Zijdenbos A,Evans AC,Giedd JN,Rapoport JL ( 2002): Developmental trajectories of brain volume abnormalities in children and adolescents with attention‐deficit/hyperactivity disorder. JAMA 288: 1740–1748. [DOI] [PubMed] [Google Scholar]
- Conners CK ( 1985): Parent symptom questionnaire. Psychopharmacol Bull 21: 816–822. [Google Scholar]
- El‐Sayed E,Larsson JO,Persson HE,Santosh PJ,Rydelius PA ( 2003): “Maturational lag” hypothesis of attention deficit hyperactivity disorder: An update. Acta Paediatr 92: 776–784. [PubMed] [Google Scholar]
- Filipek PA,Semrud‐Clikeman M,Steingard RJ,Renshaw PF,Kennedy DN,Biederman J ( 1997): Volumetric MRI analysis comparing subjects having attention‐deficit hyperactivity disorder with normal controls. Neurology 48: 589–601. [DOI] [PubMed] [Google Scholar]
- Giedd JN,Castellanos FX,Casey BJ,Kozuch P,King AC,Hamburger SD,Rapoport JL ( 1994): Quantitative morphology of the corpus callosum in attention deficit hyperactivity disorder [see comment]. Am J Psychiatry 151: 665–669. [DOI] [PubMed] [Google Scholar]
- Hoeft F,Barnea‐Goraly N,Haas BW,Golarai G,Ng D,Mills D,Korenberg J,Bellugi U,Galaburda A,Reiss AL ( 2007): More is not always better: Increased fractional anisotropy of superior longitudinal fasciculus associated with poor visuospatial abilities in Williams syndrome. J Neurosci 27: 11960–11965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynd GW,Semrud‐Clikeman M,Lorys AR,Novey ES,Eliopulos D ( 1990): Brain morphology in developmental dyslexia and attention deficit disorder/hyperactivity. Arch Neurol 47: 919–926. [DOI] [PubMed] [Google Scholar]
- King JA,Tenney J,Rossi V,Colamussi L,Burdick S ( 2003): Neural substrates underlying impulsivity. Ann N Y Acad Sci 1008: 160–169. [DOI] [PubMed] [Google Scholar]
- Klingberg T,Vaidya CJ,Gabrieli JD,Moseley ME,Hedehus M ( 1999): Myelination and organization of the frontal white matter in children: A diffusion tensor MRI study. Neuroreport 10: 2817–2821. [DOI] [PubMed] [Google Scholar]
- Konrad K,Neufang S,Hanisch C,Fink GR,Herpertz‐Dahlmann B ( 2006): Dysfunctional attentional networks in children with attention deficit/hyperactivity disorder: Evidence from an event‐related functional magnetic resonance imaging study. Biol Psychiatry 59: 643–651. [DOI] [PubMed] [Google Scholar]
- Mesulam MM ( 1990): Large‐scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol 28: 597–613. [DOI] [PubMed] [Google Scholar]
- Mori S,Zhang J ( 2006): Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron 51: 527–539. [DOI] [PubMed] [Google Scholar]
- Mori S,Wakana S,Van Zijl PCM ( 2005): MRI Atlas of Human White Matter. Amsterdam: Elsevier. [Google Scholar]
- Mostofsky SH,Reiss AL,Lockhart P,Denckla MB ( 1998): Evaluation of cerebellar size in attention‐deficit hyperactivity disorder. J Child Neurol 13: 434–439. [DOI] [PubMed] [Google Scholar]
- Mukherjee P,Miller JH,Shimony JS,Conturo TE,Lee BC,Almli CR,McKinstry RC ( 2001): Normal brain maturation during childhood: Developmental trends characterized with diffusion‐tensor MR imaging. Radiology 221: 349–358. [DOI] [PubMed] [Google Scholar]
- Rubia K,Smith AB,Woolley J,Nosarti C,Heyman I,Taylor E,Brammer M ( 2006): Progressive increase of frontostriatal brain activation from childhood to adulthood during event‐related tasks of cognitive control. Hum Brain Mapp 27: 973–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ,Wilke M,Dardzinski BJ,Holland SK ( 2002): Correlation of white matter diffusivity and anisotropy with age during childhood and adolescence: A cross‐sectional diffusion‐tensor MR imaging study. Radiology 222: 212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ,Wilke M,Dardzinski BJ,Holland SK ( 2005): Cognitive functions correlate with white matter architecture in a normal pediatric population: A diffusion tensor MRI study. Hum Brain Mapp 26: 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JFL,Il'yasov KA,Hennig J,Martin E ( 2004): Fast quantitative diffusion‐tensor imaging of cerebral white matter from the neonatal period to adolescence. Neuroradiology 46: 258–266. [DOI] [PubMed] [Google Scholar]
- Schulz KP,Newcorn JH,Fan J,Tang CY,Halperin JM ( 2005): Brain activation gradients in ventrolateral prefrontal cortex related to persistence of ADHD in adolescent boys. J Am Acad Child Adolesc Psychiatry 44: 47–54. [DOI] [PubMed] [Google Scholar]
- Silk T,Vance A,Rinehart N,Egan G,O'Boyle M,Bradshaw JL,Cunnington R ( 2005): Decreased fronto‐parietal activation in Attention Deficit Hyperactivity Disorder, combined type (ADHD‐CT): An fMRI study. Br J Psychiatry 187: 282–283. [DOI] [PubMed] [Google Scholar]
- Silverman WK,Albano AM. 1996. Anxiety Disorders Interview Schedule for DSM‐IV. San Antonio, TX: Graywind. [Google Scholar]
- Smith SM,Jenkinson M,Johansen‐Berg H,Rueckert D,Nichols TE,Mackay CE,Watkins KE,Ciccarelli O,Cader MZ,Matthews PM,Behrens TE ( 2006): Tract‐based spatial statistics: Voxelwise analysis of multi‐subject diffusion data. Neuroimage 31: 1487–1505. [DOI] [PubMed] [Google Scholar]
- Snook L,Paulson LA,Roy D,Phillips L,Beaulieu C ( 2005): Diffusion tensor imaging of neurodevelopment in children and young adults. Neuroimage 26: 1164–1173. [DOI] [PubMed] [Google Scholar]
- Sowell ER,Thompson PM,Welcome SE,Henkenius AL,Toga AW,Peterson BS ( 2003): Cortical abnormalities in children and adolescents with attention‐deficit hyperactivity disorder. Lancet 362: 1699–1707. [DOI] [PubMed] [Google Scholar]
- Suzuki Y,Matsuzawa H,Kwee IL,Nakada T ( 2003): Absolute eigenvalue diffusion tensor analysis for human brain maturation. NMR Biomed 16: 257–260. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N,Landeau B,Papathanassiou D,Crivello F,Etard O,Delcroix N,Mazoyer B,Joliot M ( 2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15: 273–289. [DOI] [PubMed] [Google Scholar]
- Vaidya CJ,Bunge SA,Dudukovic NM,Zalecki CA,Elliott GR,Gabrieli JD ( 2005): Altered neural substrates of cognitive control in childhood ADHD: Evidence from functional magnetic resonance imaging. Am J Psychiatry 162: 1605–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance A,Silk T,Casey M,Rinehart N,Bradshaw JL,Prakash C,Bellgrove MA,Cunnington R ( 2007): Right parietal dysfunction in children with attention deficit hyperactivity disorder, combined type: A functional MRI study. Mol Psychiatry 12: 826–832. [DOI] [PubMed] [Google Scholar]
- Yurgelun‐Todd DA,Silveri MM,Gruber SA,Rohan ML,Pimentel PJ ( 2007): White matter abnormalities observed in bipolar disorder: A diffusion tensor imaging study. Bipolar Disord 9: 504–512. [DOI] [PubMed] [Google Scholar]


