Abstract
The arcuate fasciculus is a major white matter tract involved in language processing that has also been repeatedly implicated in intelligence and reasoning tasks. Language in the human brain is lateralized in terms of both function and structure, and while the arcuate fasciculus reflects this asymmetry, its pattern of lateralization is poorly understood in children and adolescents. We used diffusion tensor imaging (DTI) and tractography to examine arcuate fasciculus lateralization in a large (n = 183) group of healthy right‐handed volunteers aged 5–30 years; a subset of 68 children aged 5–13 years also underwent cognitive assessments. Fractional anisotropy and number of streamlines of the arcuate fasciculus were both significantly higher in the left hemisphere than the right hemisphere in most subjects, although some subjects (10%) were right lateralized. Age and gender effects on lateralization were not significant. Children receiving cognitive assessments were divided into three groups: a “left‐only” group in whom only the left side of the arcuate fasciculus could be tracked, a left‐lateralized group, and a right‐lateralized group. Scores on the Peabody Picture Vocabulary Test (PPVT) and NEPSY Phonological Processing task differed significantly among groups, with left‐only subjects outperforming the right‐lateralized group on the PPVT, and the left‐lateralized children scoring significantly better than the right‐lateralized group on phonological processing. In summary, DTI tractography demonstrates leftward arcuate fasciculus lateralization in children, adolescents, and young adults, and reveals a relationship between structural white matter lateralization and specific cognitive abilities in children. Hum Brain Mapp, 2009. © 2009 Wiley‐Liss, Inc.
Keywords: diffusion tensor imaging, tractography, superior longitudinal fasciculus, asymmetry
INTRODUCTION
It has long been known that language function in the human brain is lateralized, ever since Broca in 1861 and Wernicke in 1874 demonstrated that brain lesions associated with loss of language function are most often located in the left hemisphere [Broca,1861; Wernicke,1874]. Many recent studies have reported functional language lateralization in adults [Binder et al.,2000; Wood et al.,2004], children [Balsamo et al.,2002; Gaillard et al.,2001; Spironelli et al.,2008], and infants [Dehaene‐Lambertz et al.,2002]. Structural lateralization of language areas, although not as well characterized as functional lateralization, also exists in the human brain. Several consistent structural asymmetries have been reported, including leftward lateralization of the planum temporale and the auditory cortex [Galaburda et al.,1978; Galuske et al.,2000; Geschwind and Levitsky,1968; Good et al.,2001; Penhune et al.,1996; Pujol et al.,2002], and white matter volume asymmetries in the frontal, parietal and temporal lobes [Galaburda et al.,1978; Good et al.,2001; Gur et al.,1980; Pujol et al.,2002]. Lesion, tumor, and epilepsy studies provide further evidence of left‐lateralized language function and structure in humans [Davtian et al.,2008; Dronkers et al.,2007; Gazzaniga,1995; Matsumoto et al.,2008; Rodrigo et al.,2008; Toga and Thompson,2003].
The arcuate fasciculus, a subdivision of the superior longitudinal fasciculus, is a major white matter tract that is one of the primary fiber bundles involved in human language processing [Dejerine,1895; Geschwind,1970]. This tract connects Broca's area in the frontal lobe, a region mainly involved in speech production [Broca,1861], with Wernicke's area in the temporal lobe, a region related to speech comprehension [Wernicke,1874]. The arcuate fasciculus is not only important in language function [Ashtari et al.,2007; Breier et al.,2008; Marslen‐Wilson and Tyler,2007], but it is also part of a network that has been repeatedly implicated in reasoning and intelligence tasks [Hoeft et al.,2007; Jung and Haier,2007; Jung et al.,2005; Schmithorst et al.,2005; Turken et al.,2008]. Structural asymmetry of the arcuate fasciculus has been reported in children, with the left arcuate demonstrating higher “white matter density” than the right side, based on T1‐weighted MRI scans [Paus et al.,1999]. Furthermore, the volumetric white matter asymmetries observed in adults [Galaburda et al.,1978; Good et al.,2001; Gur et al.,1980; Pujol et al.,2002] suggest volumetric asymmetries of underlying white matter fiber bundles such as the arcuate fasciculus.
Diffusion tensor imaging (DTI) is an advanced MRI technique that is more sensitive to tissue microstructure than conventional imaging and is especially adept at virtually reconstructing white matter pathways in vivo, via tractography [Basser et al.,2000; Conturo et al.,1999; Jones et al.,1999; Mori et al.,1999]. Using tractography, DTI parameters such as fractional anisotropy (FA), an indirect measure of myelination and/or axonal density within white matter [Beaulieu,2002], can be measured, along specific white matter tracts, including the arcuate fasciculus [Catani et al.,2002]. DTI studies of the arcuate fasciculus have demonstrated leftward asymmetry in most adults for both structure [Glasser and Rilling,2008; Hagmann et al.,2006; Parker et al.,2005] and diffusion parameters [Buchel et al.,2004; Nucifora et al.,2005; Powell et al.,2006; Upadhyay et al.,2008; Vernooij et al.,2007]; however, these studies were generally small in size (n = 4–43), and focused solely on adults. One DTI study of 31 children aged 6–17 years reported overall leftward asymmetry of FA values across the group, and absence of the right arcuate in 29% of subjects [Eluvathingal et al.,2007]. Further studies of children and adolescents are needed to get a full picture of lateralization during development and to investigate possible age or gender differences. Furthermore, a relationship between arcuate fasciculus asymmetry and language ability was reported in 40 young adults [Catani et al.,2007], but it is not known if a similar relationship exists in children. A thorough understanding of arcuate fasciculus asymmetry throughout childhood, adolescence, and young adulthood would help address the question of whether the structural asymmetry seen in adults develops over time or is present early in life, as well as providing further information about the relationship between lateralization and cognition.
Here we use DTI and tractography to explore arcuate fasciculus asymmetry in a large group of healthy subjects (n = 183) ranging from 5 to 30 years of age. The goal of this study was to characterize lateralization of the arcuate fasciculus in children, adolescents and young adults, including an investigation of age‐ and gender‐related changes, and to explore the relationship between arcuate fasciculus asymmetry and cognitive ability in a subgroup of children aged 5–13 years (n = 68).
METHODS
Subjects
Subjects were 183 healthy, right‐handed individuals (86 females/97 males) aged 5–30 years (mean ± standard deviation: 16.3 ± 6.8 years). Health was verified by asking participants a series of questions to ensure there was no history of neurological or psychiatric disease or brain injury. Subjects with a wide range of reading ability were recruited, as a broad spectrum of abilities was desired in order to better detect relationships between reading skill and brain structure. Subjects were not formally screening for reading disabilities, but were administered cognitive assessments associated with reading ability (Woodcock Word ID and Word Attack). Informed consent was obtained from all adult volunteers; child assent and parent/guardian consent were obtained for each subject under 18 years.
Cognitive Assessment
Cognitive assessments were performed on a subset of 68 children aged 5–13 years (mean ± standard deviation: 9.4 ± 2.0 years, 30 females/38 males). NEPSY Phonological Processing, Word Identification and Word Attack (subtests of the Woodcock Reading Mastery Test‐Revised), the Peabody Picture Vocabulary Test (PPVT‐III), and the Test of Nonverbal Intelligence (TONI) were used, although not all subjects completed all tests (see Table I).
Table I.
Age‐standardized score means, standard deviations, and ranges for cognitive assessments performed on a subset of 68 children aged 5–13 years
| Cognitive assessment | n | Mean score | Standard deviation | Range |
|---|---|---|---|---|
| Phonological processing | 66 | 10.8 | 2.7 | 3–18 |
| PPVT | 67 | 116 | 13 | 90–154 |
| TONI | 65 | 112 | 14 | 82–142 |
| Word Attack | 66 | 107 | 13 | 74–134 |
| Word ID | 67 | 107 | 14 | 71–145 |
Image Acquisition
All imaging data were acquired on the exact same 1.5 T Siemens Sonata MRI scanner (Siemens, Erlangen, Germany) using identical methods. Total acquisition time was approximately 26 minutes and included anatomical imaging and DTI. DTI was acquired using a dual spin‐echo, single shot echo‐planar imaging sequence with the following parameters: forty 3 mm thick slices with no interslice gap, TR = 6,400 ms, TE = 88 ms, six noncollinear diffusion sensitizing gradient directions with b = 1,000 s/mm2, eight averages, field‐of‐view 220 × 220 mm2, matrix of 128 × 128 with 75% phase partial Fourier, zero‐filled to 256 × 256. Total DTI acquisition time was 6:06 minutes. Although anisotropic voxel size may be of some concern, a comparison of this sequence with an isotropic voxel size acquisition (2 × 2 × 2 mm3 with the same six directions and eight averages) in four volunteers demonstrated no differences of lateralization grouping and only minimal variation in key parameters derived from tractography of the arcuate fasciculus (standard deviation of FA = 0.016 for each hemisphere, standard deviation of lateralization index = 0.08). The SNR of b0 images in this study was quite high (average SNR = 76, range 53–93), which should help mitigate concerns about the use of six directions as opposed to more.
Fiber Tracking Diffusion Measurements
Fiber tracking was performed in ExploreDTI (A. Leemans, Antwerp, Belgium), using a deterministic streamline method. FA thresholds were set to 0.25 to initiate and continue tracking, while the angle threshold was set to 60°. An FA threshold of 0.25 was chosen to avoid voxels that are not part of white matter tracts (cortex has FA ∼ 0.2), minimize the inclusion of voxels with a high degree of partial volume contamination, and limit the presence of spurious tracts. The arcuate fasciculus was delineated manually in each hemisphere for each subject. Semiautomated tracking, as we used in a previous study to delineate the superior longitudinal fasciculus [Lebel et al.,2008], was not appropriate for this study due to the difficulty of isolating the frontotemporal arcuate fasciculus from the frontoparietal fibers of the superior longitudinal fasciculus. Superior longitudinal fibers (both frontotemporal and frontoparietal) can be obtained using seeding and target regions on coronal slices, a method well suited to semiautomated tractography. However, obtaining only the arcuate fibers requires a target region on an axial slice in an area much more sensitive to small differences of region placement. Therefore, manual tracking was used to ensure accurate delineation of the entire arcuate fasciculus alone. The operator was not blind to left/right, but was blind to cognitive scores. To ensure accurate and unbiased tracking, a specific set of predefined rules was followed in the same way for each hemisphere. One seeding region was selected in each hemisphere on a coronal slice on which the arcuate fasciculus—appearing as a green (indicating anterior/posterior orientation) triangular shape—was seen to be largest (see Fig. 1). Great care was taken to ensure that the seeding region was large enough in each hemisphere to encompass all possible fibers belonging to the arcuate fasciculus, and a margin of at least 5 mm around the tract on all sides was included. A target region was used on an axial slice through which the arcuate fasciculus passes in the inferior/superior direction (appears in blue/purple); again, this target region was drawn larger than the visible region corresponding to the tract to ensure that no fibers were missed. The seeding region was used to initiate tracking, while the target region selected only fibers passing through both areas. Exclusion regions were used as necessary to eliminate spurious tracts, and were often required in the region of the internal capsules. Only individuals with more than five streamlines (total across hemispheres) were included in further analysis. Originally, 207 right‐handed subjects were included in the study; 24 were excluded due to either motion artifacts or having too few streamlines. Average fractional anisotropy (FA) and number of streamlines were calculated for the arcuate fasciculus in each hemisphere for each subject. FA values were calculated by averaging across all voxels in the tract; it is important to note that each voxel was counted only once. Note that FA values were not calculated in hemispheres with no streamlines (63 individuals had no streamlines on the right; six had no streamlines on the left).
Figure 1.
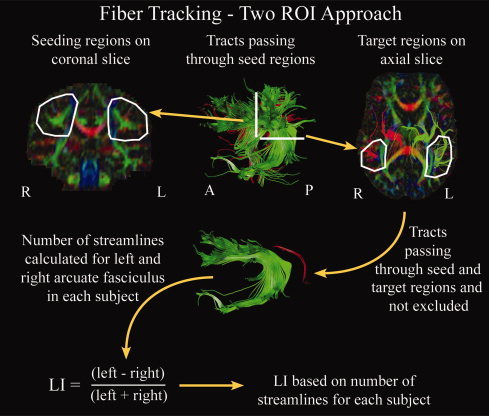
Schematic of tracking methods used to delineate the arcuate fasciculus. A manual two region‐of‐interest approach was used in which a seeding region was drawn on a coronal slice and a target region outlined on an axial slice in each hemisphere for each subject. Exclusion regions were used as needed to eliminate spurious streamlines, and were often needed in the area of the internal capsule. Tracts passing through both the seeding and target regions, without passing through exclusion regions, were retained for calculation of the lateralization index (LI). LI was calculated for each subject based on the number of streamlines in each hemisphere. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Statistical Analysis
Parametric tests were used for FA values; nonparametric tests were used for number of streamlines. Differences of FA values between hemispheres were tested in individuals having streamlines bilaterally (n = 114) using a paired t‐test; hemispheric differences of number of streamlines were tested across the population (n = 183) using a Wilcoxon signed ranks test for two related samples (equivalent to a paired t‐test for nonparametric data). To further characterize asymmetry, a lateralization index, LI = (left − right)/(left + right), was calculated for the number of streamlines for each subject. Correlations of age with LI were calculated using Spearman's rho; sex differences were examined using an independent samples Mann‐Whitney test. LI was compared with zero using a one‐ sample Kolmogorow‐Smirnov test.
To analyze the relationship between cognitive ability and lateralization, the 68 children receiving cognitive assessments were divided into groups based on their lateralization scores. This was important to allow detection of differences that were not simply linear correlations. Children were divided into three groups: left only (LI = 1), left lateralized (0 < LI < 1), and right lateralized (LI < 0). Subjects were divided in this manner to produce logical groups with reasonably close sample sizes (although the left‐lateralized group was larger). Age‐standardized test scores were compared among groups using a series of ANOVAs. Where the ANOVA test was significant, post hoc tests (Tukey's honestly significantly different test) were used to determine where differences existed among groups, and Spearman's rho was used to test for correlations between LI and cognitive scores. Age and FA differences among these groups were tested using a series of ANOVAs; LI and number of streamline differences were assessed using the Kruskal‐Wallis test, and Mann‐Whitney tests with Bonferroni correction for multiple comparisons were used for post hoc testing.
RESULTS
Lateralization Indices
Across the entire group (n = 183), individuals had a significantly higher number of streamlines in the left hemisphere than the right (P < 0.001), and LI values were significantly different from zero (P < 0.001), indicating overall leftward asymmetry in this group. Amongst individuals with streamlines bilaterally (n = 114), FA values were significantly higher in the left arcuate fasciculus than the right (mean ± standard deviation: FAleft = 0.52 ± 0.03, FAright = 0.50 ± 0.04, P < 0.001). Most individuals (n = 153) were left lateralized for number of streamlines (LI > 0), while some subjects (n = 30) were right lateralized (LI < 0); the median LI was 0.78. Across the group, the left arcuate was tracked in 177 individuals (out of 183), and the right was tracked in 120 subjects. In total, 34% of individuals had no detectable right arcuate, 3% showed no detectable left, and in the remaining subjects, both sides were tracked, to varying degrees. If symmetry is defined as −0.2 < LI < 0.2, 81% of subjects are considered left lateralized for number of streamlines, 9% are symmetric, and 10% are right lateralized. Figure 2 shows examples of arcuate fasciculus arrangements for several subjects with a range of LI values.
Figure 2.
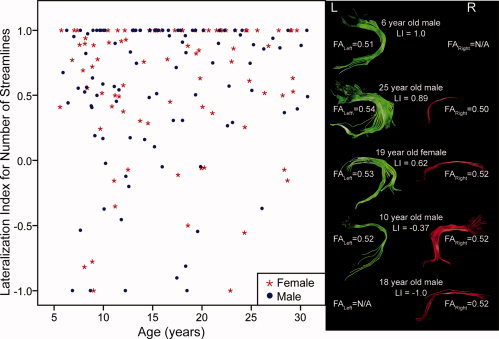
Lateralization index based on number of streamlines (LI) versus age graph for 183 right‐handed subjects, as well as sample arrangements of the arcuate fasciculus with various values of LI. Most individuals are left lateralized (LI > 0), although there are some right‐lateralized (LI < 0) subjects. LI was not significantly correlated with age nor was it significantly different between males and females. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
The 68 children receiving cognitive assessments were divided into three groups according to their LI scores: a “left‐only” group containing subjects in whom only the left side of the arcuate fasciculus could be tracked (LI = 1); a left‐lateralized group containing subjects with streamlines bilaterally (0 < LI < 1), and a right‐lateralized group (LI < 0). Subjects were divided in this way in order to produce logical groups relatively similar in size; there were not enough symmetric subjects to form a group. It is important to note that the left‐lateralized group contains some individuals who are nearly symmetric (two individuals with 0 < LI < 0.2), while the right‐lateralized group contains subjects ranging from those with only the right segment (four subjects) to those who are nearly symmetric (five subjects with 0 > LI > −0.2). In total, 15 children (22%) were classified as right lateralized (median LI = −0.45); 36 children (53%) were left lateralized (median LI = 0.58), and 17 children (25%) were left only (LI = 1). See Table II for group characteristics.
Table II.
Marginal group means and significance values for ANOVAs of group characteristics and standard test scores
| Right lateralized (n = 15, 6 f/9 m) | Left lateralized (n = 36, 17 f/19 m) | Left only (n = 17, 7 f/10 m) | ANOVAa P value | |
|---|---|---|---|---|
| Age | 10.2 | 9.1 | 9.3 | 0.180 |
| StreamlinesLeft | 15 | 47** | 31** | 0.000 |
| StreamlinesRight | 34 | 11** | 0**†† | 0.000 |
| LI | −0.45 | 0.58** | 1.0**† | 0.000 |
| FAleft | 0.50b | 0.50 | 0.50 | 0.823 |
| FAright | 0.48 | 0.48 | N/Ac | 0.717 |
| Cognitive assessments | ||||
| Phonological processing | 9.2 | 11.5* | 10.7 | 0.016 |
| PPVT | 111 | 115 | 123* | 0.030 |
| TONI | 108 | 114 | 114 | 0.298 |
| Word ID | 100 | 108 | 110 | 0.089 |
| Word attack | 104 | 109 | 107 | 0.215 |
Non‐parametric Kruskal‐Wallis test used for number of streamlines and LI.
Mean of FA values for individuals with streamlines on both sides (11 subjects).
No FA values obtained for right side.
Significantly different compared with right‐lateralized group: *P corrected < 0.05, **P corrected < 0.001.
Significantly different compared with left‐lateralized group: †P corrected < 0.05, ††P corrected < 0.001.
Age and Gender Differences
Lateralization index (LI) was not significantly correlated with age (R = 0.09, P = 0.214), nor was it significantly different between males and females (P = 0.456). See Figure 2 for a plot of LI versus age. Testing a similar lateralization index for tract volume also produced no significant differences of age or gender (data not shown).
Cognitive Assessments and Lateralization
Summary results for the cognitive assessments are shown in Table I and results of the ANOVA tests relating DTI‐based lateralization to the age‐standardized cognitive scores are presented in Table II. Age did not differ significantly amongst groups and each group had approximately the same proportion of males and females. Scores on the PPVT and the NEPSY Phonological Processing were significantly related to lateralization groupings (P = 0.030, P = 0.016, respectively; see Table II and Fig. 3). Post hoc tests revealed that the left‐only group significantly outperformed the right‐lateralized group on the PPVT (P corrected = 0.028) and PPVT scores were significantly linearly correlated with LI (R = 0.32, P = 0.009) across all children (see Fig. 4A). For the NEPSY Phonological Processing task, the left‐lateralized group performed significantly better than the right‐lateralized group (P corrected = 0.012), but the linear correlation with LI was not significant (R = 0.13, P = 0.286, Fig. 4B). The same results were observed when a lateralization index calculated based on tract volume was compared across groups (data not shown).
Figure 3.
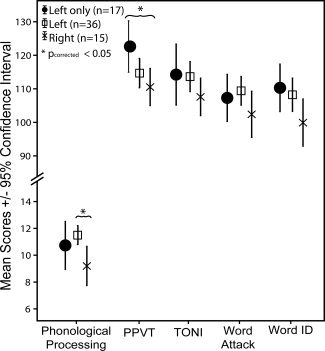
Mean scores (±95% confidence intervals) for each test for different lateralization groups of 68 children aged 5–13 years. The Peabody Picture Vocabulary Test (PPVT) and NEPSY Phonological Processing scores differed significantly among groups, with the left‐only group scoring significantly better than the right‐lateralized group for the PPVT, and the left‐lateralized group significantly outperforming the right‐lateralized group for phonological processing.
Figure 4.
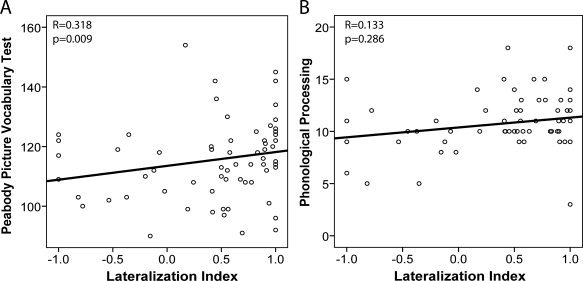
Plots of lateralization index (LI) versus age‐standardized scores for (A) Peabody Picture Vocabulary Test (PPVT) and (B) NEPSY Phonological Processing for the entire group of 68 children with cognitive assessments. There was a significant linear correlation between PPVT and LI, whereas the linear correlation between Phonological Processing and LI was not significant. Note the significant lateralization group findings shown in Figure 3.
DISCUSSION
Using DTI and tractography, we have characterized the lateralization of the arcuate fasciculus in children, adolescents and young adults, and have also demonstrated a significant relationship between this asymmetry and specific cognitive abilities in children. Across the entire group, number of streamlines and FA values were significantly higher in the left hemisphere than the right hemisphere, and most individuals were left lateralized, in agreement with many previous DTI studies in healthy adult populations [Catani et al.,2007; Makris et al.,2005; Nucifora et al.,2005; Parker et al.,2005; Powell et al.,2006; Vernooij et al.,2007], healthy children [Eluvathingal et al.,2007], and adult patients [Matsumoto et al.,2008]. Approximately 10% of individuals demonstrated rightward lateralization for number of streamlines (LI < −0.2), while 34% had extreme leftward lateralization with no detectable streamlines in the right hemisphere. Previous adult studies have observed up to 4% of subjects to be right‐lateralized, and anywhere from 0% to 62% to have streamlines in only the left hemisphere [Catani et al.,2007; Nucifora et al.,2005; Vernooij et al.,2007]. The number of left‐only arrangements observed in this study is well within the range of previous findings and is closest to observations in children, specifically that 29% of children have no detectable right streamlines [Eluvathingal et al.,2007]. We observed more right‐lateralized individuals than other studies; however, our sample size was considerably larger (n = 183 as opposed to n = 20–40), and therefore may be expected to contain a wider range of individuals. Interestingly, our findings are consistent with volumetric studies of the planum temporale, which have shown that while the majority of the population is left lateralized, approximately 9%–12% of right‐handers exhibit rightward asymmetry of planum temporale volume [Dos Santos Sequeira et al.,2006; Pujol et al.,2002; Steinmetz,1996].
There were no significant age or gender effects on lateralization. The consistent pattern of asymmetry observed suggests that arcuate fasciculus lateralization is present in early childhood and remains constant throughout adolescence into adulthood. Significant development of the arcuate and superior longitudinal fasciculi occurs during childhood and adolescence, as evidenced by increasing FA values [Barnea‐Goraly et al.,2005; Lebel et al.,2008; Schmithorst et al.,2002] and “white matter density” [Paus et al.,1999]; age‐related changes of FA within this population were also significant (data not shown). Despite this development, however, lateralization does not change with age in this cross‐sectional cohort, suggesting that the relationship between hemispheres is maintained even as the brain develops. Similar observations of consistent lateralization have been made with regards to leftward asymmetry of the planum temporale, which exists in adults and is also observed in fetal and neonate brains [Chi et al.,1977; Witelson and Pallie,1973]. Increases of functional lateralization of language have been shown during childhood and adolescence [Everts et al.,2009; Holland et al.,2007; Ressel et al.,2008]. However, these functional changes are believed to correspond to skill acquisition rather than more generalized brain development [Holland et al.,2007], a hypothesis supported by the lack of age‐related structural lateralization changes in our study. Most previous DTI studies of the arcuate fasciculus do not comment on gender effects with respect to lateralization; however, one study reported gender differences of arcuate fiber density lateralization, with females having a more symmetric arrangement [Catani et al.,2007]. Findings of gender differences in functional language lateralization and asymmetry of other brain structures have been mixed [Dos Santos Sequeira et al.,2006; Good et al.,2001; Kansaku and Kitazawa,2001; Pujol et al.,2002; Shaywitz et al.,1995; Sommer et al.,2004; Vikingstad et al.,2000].
Children with a greater number of arcuate fasciculus streamlines on the left performed significantly better on certain cognitive tasks than those with more on the right. The left‐only group outperformed the right‐lateralized group on the PPVT, and the left‐lateralized group scored significantly better than the right‐lateralized group on the NEPSY Phonological Processing task. Rightward lateralization was not associated with better scores on either task. Both the PPVT, a test of receptive vocabulary, and phonological processing tasks involve frontal and temporal brain areas [Burton et al.,2000; Dronkers et al.,2004; Shalom and Poeppel,2008; Temple,2002; Wells et al.,2008]. Therefore, it is not surprising that the arcuate fasciculus, connecting the frontal and temporal regions, seems to play a role in both of these tasks. However, the Word ID and Word Attack tasks also involve frontal and temporal regions, yet performance on these tasks was not observed to be significantly different among lateralization groups. The relationship between lateralization of the arcuate fasciculus and cognitive skills is still unclear. Future studies on different populations will help to further elucidate the relationship between cognitive skills such as these and lateralization of white matter tracts.
One previous study found a significant correlation between arcuate fasciculus lateralization and a measure of word encoding and list retrieval in adults, with the more bilateral individuals performing best [Catani et al.,2007]. Although these differ from our results, the tasks involved were quite different, as were the populations. Furthermore, the arcuate fasciculus is a complex fiber bundle, and is not the only pathway involved in language. Another, more ventral route has been implicated in language processing [Frey et al.,2008; Parker et al.,2005], and may play an additional role in cognitive tasks such as these. Future studies looking at lateralization of this and other brain pathways and their relationship to cognitive abilities may further elucidate the complex interplay between brain structure and language.
The arcuate fasciculus is part of a large, distributed network of brain systems implicated in intelligence tasks [Jung and Haier,2007; Shaw,2007]. Based on changes in DTI parameters, this tract matures relatively slowly compared with other white matter fiber bundles [Lebel et al.,2008; Zhang et al.,2007], and even appears to be absent in some children with global developmental delay [Sundaram et al.,2008]. Furthermore, the arcuate fasciculus is much smaller or completely absent in other, nonhuman primates [Rilling et al.,2008], pointing to its advanced role in human cognition. FA values in the arcuate fasciculus correlate with various cognitive scores [Ashtari et al.,2007; Breier et al.,2008; Schmithorst et al.,2005; Turken et al.,2008], and arcuate lateralization is related to verbal recall in adults [Catani et al.,2007]. Here, we provide further evidence of a key role for the arcuate fasciculus by demonstrating a correlation with verbal intelligence and phonological processing tasks.
For this study, a six‐direction diffusion encoding scheme with multiple averages was used for DTI acquisition. Although using more directions is advantageous for robust estimates of anisotropy [Jones,2004], particularly at lower SNR values (such as 15 as presented in that article), SNR values were very high for our DTI scans (average SNR = 76 on b0 images). It has been shown that the effects of different encoding schemes may be less of an issue compared with intrasession reliability and the impact of SNR, and that with SNR levels greater than ∼ 30, a six‐direction acquisition scheme is comparable to those with more encoding directions in terms of FA estimates and power to detect group differences [Landman et al.,2007].
Deterministic tractography, as used in this study, aims to delineate white matter fiber tracts passing through specific regions, essentially creating a three‐dimensional region of interest containing one specific tract. However, it is prone to errors in areas of crossing fibers where artificially low FA values are often obtained, such as the region where the arcuate fasciculus descends into the temporal lobe. To minimize errors in this study, a multiple region‐of‐interest approach was used with seeding and target regions for each tract placed according to a priori knowledge of tract location and trajectory [Makris et al.,2005; Wakana et al.,2004]. Furthermore, all tracts were visually inspected to ensure that they conformed to known anatomical trajectories and did not contain spurious fibers. Crossing fibers, however, remain a limitation of deterministic tractography studies. Probabilistic tractography, on the other hand, takes into account the uncertainty in each voxel along the tract [Behrens et al.,2003; Jones and Pierpaoli,2005; Lazar and Alexander,2005] and helps overcome problems in crossing fiber regions by estimating all possible connections from a seeding region and their likelihood. Since the trajectory of the arcuate fasciculus is well known [Catani et al.,2007; Makris et al.,2005; Wakana et al.,2004], and measurements of number of streamlines and FA along the entire length of the tract were desired, deterministic tractography was the appropriate method for this study, despite its limitations. Furthermore, previous studies of the arcuate fasciculus using both deterministic [Nucifora et al.,2005; Vernooij et al.,2007] and probabilistic [Powell et al.,2006] tractography are in agreement. Overall, diffusion tractography is a powerful technique able to virtually extract many of the major white matter connections of the brain, and evidence suggests that the trajectories of many major white matter tracts obtained via tractography agree with the results of postmortem studies [Catani et al.,2002; Wakana et al.,2007].
CONCLUSION
DTI tractography has revealed a pattern of leftward asymmetry of the arcuate fasciculus in children, adolescents, and young adults that is consistent across both age and gender. In a subset of children aged 5–13 years, DTI has demonstrated a relationship between arcuate fasciculus lateralization and cognitive ability; extreme leftward lateralization was associated with better receptive vocabulary scores, while a phonological processing task was performed best by those with more moderate leftward lateralization. These findings suggest that the left‐lateralized arrangement of the arcuate fasciculus is already present in childhood, and that this lateralization plays an important role in certain cognitive tasks. Future studies exploring the relationship between structural and functional lateralization and cognitive ability may further elucidate the interplay of brain lateralization and cognitive function.
Acknowledgements
The authors thank Lindsay Walker and Dawne Roy, who scanned and administered cognitive assessments to many of the subjects, respectively. MRI infrastructure was provided by the Canada Foundation for Innovation, Alberta Science and Research Authority, AHFMR, and the University Hospital Foundation.
REFERENCES
- Ashtari M,Cervellione KL,Hasan KM,Wu J,McIlree C,Kester H,Ardekani BA,Roofeh D,Szeszko PR,Kumra S ( 2007): White matter development during late adolescence in healthy males: A cross‐sectional diffusion tensor imaging study. Neuroimage 35: 501–510. [DOI] [PubMed] [Google Scholar]
- Balsamo LM,Xu B,Grandin CB,Petrella JR,Braniecki SH,Elliott TK,Gaillard WD ( 2002): A functional magnetic resonance imaging study of left hemisphere language dominance in children. Arch Neurol 59: 1168–1174. [DOI] [PubMed] [Google Scholar]
- Barnea‐Goraly N,Menon V,Eckert M,Tamm L,Bammer R,Karchemskiy A,Dant CC,Reiss AL ( 2005): White matter development during childhood and adolescence: A cross‐sectional diffusion tensor imaging study. Cereb Cortex 15: 1848–1854. [DOI] [PubMed] [Google Scholar]
- Basser PJ,Pajevic S,Pierpaoli C,Duda J,Aldroubi A ( 2000): In vivo fiber tractography using DT‐MRI data. Magn Reson Med 44: 625–632. [DOI] [PubMed] [Google Scholar]
- Beaulieu C ( 2002): The basis of anisotropic water diffusion in the nervous system—A technical review. NMR Biomed 15: 435–455. [DOI] [PubMed] [Google Scholar]
- Behrens TE,Woolrich MW,Jenkinson M,Johansen‐Berg H,Nunes RG,Clare S,Matthews PM,Brady JM,Smith SM ( 2003): Characterization and propagation of uncertainty in diffusion‐weighted MR imaging. Magn Reson Med 50: 1077–1088. [DOI] [PubMed] [Google Scholar]
- Binder JR,Frost JA,Hammeke TA,Bellgowan PS,Springer JA,Kaufman JN,Possing ET ( 2000): Human temporal lobe activation by speech and nonspeech sounds. Cereb Cortex 10: 512–528. [DOI] [PubMed] [Google Scholar]
- Breier JI,Hasan KM,Zhang W,Men D,Papanicolaou AC ( 2008): Language dysfunction after stroke and damage to white matter tracts evaluated using diffusion tensor imaging. AJNR Am J Neuroradiol 29: 483–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broca P ( 1861): Remarques sur le siege de la faculte du langage articule, suivies d'une observation d'aphemie (perte de la parole). Bull Soc Anthropol 6: 330–357. [Google Scholar]
- Buchel C,Raedler T,Sommer M,Sach M,Weiller C,Koch MA ( 2004): White matter asymmetry in the human brain: A diffusion tensor MRI study. Cereb Cortex 14: 945–951. [DOI] [PubMed] [Google Scholar]
- Burton MW,Small SL,Blumstein SE ( 2000): The role of segmentation in phonological processing: An fMRI investigation. J Cogn Neurosci 12: 679–690. [DOI] [PubMed] [Google Scholar]
- Catani M,Allin MP,Husain M,Pugliese L,Mesulam MM,Murray RM,Jones DK ( 2007): Symmetries in human brain language pathways correlate with verbal recall. Proc Natl Acad Sci U S A 104: 17163–17168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M,Howard RJ,Pajevic S,Jones DK ( 2002): Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage 17: 77–94. [DOI] [PubMed] [Google Scholar]
- Chi JG,Dooling EC,Gilles FH ( 1977): Left‐right asymmetries of the temporal speech areas of the human fetus. Arch Neurol 34: 346–348. [DOI] [PubMed] [Google Scholar]
- Conturo TE,Lori NF,Cull TS,Akbudak E,Snyder AZ,Shimony JS,McKinstry RC,Burton H,Raichle ME ( 1999): Tracking neuronal fiber pathways in the living human brain. Proc Natl Acad Sci USA 96: 10422–10427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davtian M,Ulmer JL,Mueller WM,Gaggl W,Mulane MP,Krouwer HG ( 2008): The superior longitudinal fasciculus and speech arrest. J Comput Assist Tomogr 32: 410–414. [DOI] [PubMed] [Google Scholar]
- Dehaene‐Lambertz G,Dehaene S,Hertz‐Pannier L ( 2002): Functional neuroimaging of speech perception in infants. Science 298: 2013–2015. [DOI] [PubMed] [Google Scholar]
- Dejerine J ( 1895): Anatomie des Centres Nerveux. Paris: Masson. [Google Scholar]
- Dos Santos Sequeira S,Woerner W,Walter C,Kreuder F,Lueken U,Westerhausen R,Wittling RA,Schweiger E,Wittling W ( 2006): Handedness, dichotic‐listening ear advantage, and gender effects on planum temporale asymmetry–A volumetric investigation using structural magnetic resonance imaging. Neuropsychologia 44: 622–636. [DOI] [PubMed] [Google Scholar]
- Dronkers NF,Plaisant O,Iba‐Zizen MT,Cabanis EA ( 2007): Paul Broca's historic cases: High resolution MR imaging of the brains of Leborgne and Lelong. Brain 130: 1432–1441. [DOI] [PubMed] [Google Scholar]
- Dronkers NF,Wilkins DP,Van Valin RD Jr,Redfern BB,Jaeger JJ ( 2004): Lesion analysis of the brain areas involved in language comprehension. Cognition 92: 145–177. [DOI] [PubMed] [Google Scholar]
- Eluvathingal TJ,Hasan KM,Kramer L,Fletcher JM,Ewing‐Cobbs L ( 2007): Quantitative diffusion tensor tractography of association and projection fibers in normally developing children and adolescents. Cereb Cortex 17: 2760–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everts R,Lidzba K,Wilke M,Kiefer C,Mordasini M,Schroth G,Perrig W,Steinlin M ( 2009): Strengthening of laterality of verbal and visuospatial functions during childhood and adolescence. Hum Brain Mapp 30: 473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey S,Campbell JS,Pike GB,Petrides M ( 2008): Dissociating the human language pathways with high angular resolution diffusion fiber tractography. J Neurosci 28: 11435–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard WD,Pugliese M,Grandin CB,Braniecki SH,Kondapaneni P,Hunter K,Xu B,Petrella JR,Balsamo L,Basso G ( 2001): Cortical localization of reading in normal children: An fMRI language study. Neurology 57: 47–54. [DOI] [PubMed] [Google Scholar]
- Galaburda AM,LeMay M,Kemper TL,Geschwind N ( 1978): Right‐left asymmetrics in the brain. Science 199: 852–856. [DOI] [PubMed] [Google Scholar]
- Galuske RA,Schlote W,Bratzke H,Singer W ( 2000): Interhemispheric asymmetries of the modular structure in human temporal cortex. Science 289: 1946–1949. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS ( 1995): Principles of human brain organization derived from split‐brain studies. Neuron 14: 217–228. [DOI] [PubMed] [Google Scholar]
- Geschwind N ( 1970): The organization of language and the brain. Science 170: 940–944. [DOI] [PubMed] [Google Scholar]
- Geschwind N,Levitsky W ( 1968): Human brain: Left‐right asymmetries in temporal speech region. Science 161: 186–187. [DOI] [PubMed] [Google Scholar]
- Glasser MF,Rilling JK ( 2008): DTI tractography of the human brain's language pathways. Cereb Cortex 18: 2471–2482. [DOI] [PubMed] [Google Scholar]
- Good CD,Johnsrude I,Ashburner J,Henson RN,Friston KJ,Frackowiak RS ( 2001): Cerebral asymmetry and the effects of sex and handedness on brain structure: A voxel‐based morphometric analysis of 465 normal adult human brains. Neuroimage 14: 685–700. [DOI] [PubMed] [Google Scholar]
- Gur RC,Packer IK,Hungerbuhler JP,Reivich M,Obrist WD,Amarnek WS,Sackeim HA ( 1980): Differences in the distribution of gray and white matter in human cerebral hemispheres. Science 207: 1226–1228. [DOI] [PubMed] [Google Scholar]
- Hagmann P,Cammoun L,Martuzzi R,Maeder P,Clarke S,Thiran JP,Meuli R ( 2006): Hand preference and sex shape the architecture of language networks. Hum Brain Mapp 27: 828–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F,Barnea‐Goraly N,Haas BW,Golarai G,Ng D,Mills D,Korenberg J,Bellugi U,Galaburda A,Reiss AL ( 2007): More is not always better: Increased fractional anisotropy of superior longitudinal fasciculus associated with poor visuospatial abilities in Williams syndrome. J Neurosci 27: 11960–11965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland SK,Vannest J,Mecoli M,Jacola LM,Tillema JM,Karunanayaka PR,Schmithorst VJ,Yuan W,Plante E,Byars AW ( 2007): Functional MRI of language lateralization during development in children. Int J Audiol 46: 533–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK ( 2004): The effect of gradient sampling schemes on measures derived from diffusion tensor MRI: A Monte Carlo study. Magn Reson Med 51: 807–815. [DOI] [PubMed] [Google Scholar]
- Jones DK,Pierpaoli C ( 2005): Confidence mapping in diffusion tensor magnetic resonance imaging tractography using a bootstrap approach. Magn Reson Med 53: 143–149. [DOI] [PubMed] [Google Scholar]
- Jones DK,Simmons A,Williams SC,Horsfield MA ( 1999): Non‐invasive assessment of axonal fiber connectivity in the human brain via diffusion tensor MRI. Magn Reson Med 42: 37–41. [DOI] [PubMed] [Google Scholar]
- Jung RE,Haier RJ ( 2007): The parieto‐frontal integration theory (P‐FIT) of intelligence: Converging neuroimaging evidence. Behav Brain Sci 30: 135–154; discussion 154‐187. [DOI] [PubMed] [Google Scholar]
- Jung RE,Haier RJ,Yeo RA,Rowland LM,Petropoulos H,Levine AS,Sibbitt WL,Brooks WM ( 2005): Sex differences in N‐acetylaspartate correlates of general intelligence: An 1H‐MRS study of normal human brain. Neuroimage 26: 965–972. [DOI] [PubMed] [Google Scholar]
- Kansaku K,Kitazawa S ( 2001): Imaging studies on sex differences in the lateralization of language. Neurosci Res 41: 333–337. [DOI] [PubMed] [Google Scholar]
- Landman BA,Farrell JA,Jones CK,Smith SA,Prince JL,Mori S ( 2007): Effects of diffusion weighting schemes on the reproducibility of DTI‐derived fractional anisotropy, mean diffusivity, and principal eigenvector measurements at 1.5T. Neuroimage 36: 1123–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar M,Alexander AL ( 2005): Bootstrap white matter tractography (BOOT‐TRAC). Neuroimage 24: 524–532. [DOI] [PubMed] [Google Scholar]
- Lebel C,Walker L,Leemans A,Phillips L,Beaulieu C ( 2008): Microstructural maturation of the human brain from childhood to adulthood. Neuroimage 40: 1044–1055. [DOI] [PubMed] [Google Scholar]
- Makris N,Kennedy DN,McInerney S,Sorensen AG,Wang R,Caviness VS Jr,Pandya DN ( 2005): Segmentation of subcomponents within the superior longitudinal fascicle in humans: A quantitative, in vivo, DT‐MRI study Cereb Cortex 15: 854–869. [DOI] [PubMed] [Google Scholar]
- Marslen‐Wilson WD,Tyler LK ( 2007): Morphology, language and the brain: The decompositional substrate for language comprehension. Philos Trans R Soc Lond B Biol Sci 362: 823–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto R,Okada T,Mikuni N,Mitsueda‐Ono T,Taki J,Sawamoto N,Hanakawa T,Miki Y,Hashimoto N,Fukuyama H,Takahashi R,Ikeda A ( 2008): Hemispheric asymmetry of the arcuate fasciculus : A preliminary diffusion tensor tractography study in patients with unilateral language dominance defined by Wada test. J Neurol 255: 1703–1711. [DOI] [PubMed] [Google Scholar]
- Mori S,Crain BJ,Chacko VP,van Zijl PC ( 1999): Three‐dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol 45: 265–269. [DOI] [PubMed] [Google Scholar]
- Nucifora PG,Verma R,Melhem ER,Gur RE,Gur RC ( 2005): Leftward asymmetry in relative fiber density of the arcuate fasciculus. Neuroreport 16: 791–794. [DOI] [PubMed] [Google Scholar]
- Parker GJ,Luzzi S,Alexander DC,Wheeler‐Kingshott CA,Ciccarelli O,Lambon Ralph MA ( 2005): Lateralization of ventral and dorsal auditory‐language pathways in the human brain. Neuroimage 24: 656–666. [DOI] [PubMed] [Google Scholar]
- Paus T,Zijdenbos A,Worsley K,Collins DL,Blumenthal J,Giedd JN,Rapoport JL,Evans AC ( 1999): Structural maturation of neural pathways in children and adolescents: In vivo study. Science 283: 1908–1911. [DOI] [PubMed] [Google Scholar]
- Penhune VB,Zatorre RJ,MacDonald JD,Evans AC ( 1996): Interhemispheric anatomical differences in human primary auditory cortex: Probabilistic mapping and volume measurement from magnetic resonance scans. Cereb Cortex 6: 661–672. [DOI] [PubMed] [Google Scholar]
- Powell HW,Parker GJ,Alexander DC,Symms MR,Boulby PA,Wheeler‐Kingshott CA,Barker GJ,Noppeney U,Koepp MJ,Duncan JS ( 2006): Hemispheric asymmetries in language‐related pathways: A combined functional MRI and tractography study. Neuroimage 32: 388–399. [DOI] [PubMed] [Google Scholar]
- Pujol J,Lopez‐Sala A,Deus J,Cardoner N,Sebastian‐Galles N,Conesa G,Capdevila A ( 2002): The lateral asymmetry of the human brain studied by volumetric magnetic resonance imaging. Neuroimage 17: 670–679. [PubMed] [Google Scholar]
- Ressel V,Wilke M,Lidzba K,Lutzenberger W,Krageloh‐Mann I ( 2008): Increases in language lateralization in normal children as observed using magnetoencephalography. Brain Lang 106: 167–176. [DOI] [PubMed] [Google Scholar]
- Rilling JK,Glasser MF,Preuss TM,Ma X,Zhao T,Hu X,Behrens TE ( 2008): The evolution of the arcuate fasciculus revealed with comparative DTI. Nat Neurosci 11: 426–428. [DOI] [PubMed] [Google Scholar]
- Rodrigo S,Oppenheim C,Chassoux F,Hodel J,de Vanssay A,Baudoin‐Chial S,Devaux B,Meder JF ( 2008): Language lateralization in temporal lobe epilepsy using functional MRI and probabilistic tractography. Epilepsia 49: 1367–1376. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ,Wilke M,Dardzinski BJ,Holland SK ( 2002): Correlation of white matter diffusivity and anisotropy with age during childhood and adolescence: A cross‐sectional diffusion‐tensor MR imaging study. Radiology 222: 212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ,Wilke M,Dardzinski BJ,Holland SK ( 2005): Cognitive functions correlate with white matter architecture in a normal pediatric population: A diffusion tensor MRI study. Hum Brain Mapp 26: 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalom DB,Poeppel D ( 2008): Functional anatomic models of language: Assembling the pieces. Neuroscientist 14: 119–127. [DOI] [PubMed] [Google Scholar]
- Shaw P ( 2007): Intelligence and the developing human brain. Bioessays 29: 962–973. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA,Shaywitz SE,Pugh KR,Constable RT,Skudlarski P,Fulbright RK,Bronen RA,Fletcher JM,Shankweiler DP,Katz L,Gore JC ( 1995): Sex differences in the functional organization of the brain for language. Nature 373: 607–609. [DOI] [PubMed] [Google Scholar]
- Sommer IE,Aleman A,Bouma A,Kahn RS ( 2004): Do women really have more bilateral language representation than men? A meta‐analysis of functional imaging studies. Brain 127: 1845–1852. [DOI] [PubMed] [Google Scholar]
- Spironelli C,Penolazzi B,Angrilli A ( 2008): Dysfunctional hemispheric asymmetry of theta and beta EEG activity during linguistic tasks in developmental dyslexia. Biol Psychol 77: 123–131. [DOI] [PubMed] [Google Scholar]
- Steinmetz H ( 1996): Structure, functional and cerebral asymmetry: In vivo morphometry of the planum temporale. Neurosci Biobehav Rev 20: 587–591. [DOI] [PubMed] [Google Scholar]
- Sundaram SK,Sivaswamy L,Makki MI,Behen ME,Chugani HT ( 2008): Absence of arcuate fasciculus in children with global developmental delay of unknown etiology: A diffusion tensor imaging study. J Pediatr 152: 250–255. [DOI] [PubMed] [Google Scholar]
- Temple E ( 2002): Brain mechanisms in normal and dyslexic readers. Curr Opin Neurobiol 12: 178–183. [DOI] [PubMed] [Google Scholar]
- Toga AW,Thompson PM ( 2003): Mapping brain asymmetry. Nat Rev Neurosci 4: 37–48. [DOI] [PubMed] [Google Scholar]
- Turken AU,Whitfield‐Gabrieli S,Bammer R,Baldo JV,Dronkers NF,Gabrieli JD ( 2008): Cognitive processing speed and the structure of white matter pathways: Convergent evidence from normal variation and lesion studies. Neuroimage 42: 1032–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay J,Hallock K,Ducros M,Kim DS,Ronen I ( 2008): Diffusion tensor spectroscopy and imaging of the arcuate fasciculus. Neuroimage 39: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernooij MW,Smits M,Wielopolski PA,Houston GC,Krestin GP,van der Lugt A ( 2007): Fiber density asymmetry of the arcuate fasciculus in relation to functional hemispheric language lateralization in both right‐ and left‐handed healthy subjects: A combined fMRI and DTI study. Neuroimage 35: 1064–1076. [DOI] [PubMed] [Google Scholar]
- Vikingstad EM,George KP,Johnson AF,Cao Y ( 2000): Cortical language lateralization in right handed normal subjects using functional magnetic resonance imaging. J Neurol Sci 175: 17–27. [DOI] [PubMed] [Google Scholar]
- Wakana S,Caprihan A,Panzenboeck MM,Fallon JH,Perry M,Gollub RL,Hua K,Zhang J,Jiang H,Dubey P,Blitz A,van Zijl P,Mori S ( 2007): Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage 36: 630–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S,Jiang H,Nagae‐Poetscher LM,van Zijl PC,Mori S ( 2004): Fiber tract‐based atlas of human white matter anatomy. Radiology 230: 77–87. [DOI] [PubMed] [Google Scholar]
- Wells CT,Mahone EM,Matson MA,Kates WR,Hay T,Horska A ( 2008): Relationship of temporal lobe volumes to neuropsychological test performance in healthy children. Brain Cogn 68: 171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernicke C ( 1874): Der aphasische Symptomenkomplex: Eine Psychologische Studie auf Anatomischer Basis. Breslau: Cohn und Welgert. [Google Scholar]
- Witelson SF,Pallie W ( 1973): Left hemisphere specialization for language in the newborn. Neuroanatomical evidence of asymmetry. Brain 96: 641–646. [DOI] [PubMed] [Google Scholar]
- Wood AG,Harvey AS,Wellard RM,Abbott DF,Anderson V,Kean M,Saling MM,Jackson GD ( 2004): Language cortex activation in normal children. Neurology 63: 1035–1044. [DOI] [PubMed] [Google Scholar]
- Zhang J,Evans A,Hermoye L,Lee SK,Wakana S,Zhang W,Donohue P,Miller MI,Huang H,Wang X,van Zijl PC,Mori S ( 2007): Evidence of slow maturation of the superior longitudinal fasciculus in early childhood by diffusion tensor imaging. Neuroimage 38: 239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]


