Abstract
Pre‐attentive registration of aberrations in predictable sound patterns is attributed to the temporal cortex. However, electrophysiology suggests that frontal areas become more important when deviance complexity increases. To play an instrument in an ensemble, professional musicians have to rely on the ability to detect even slight deviances from expected musical patterns and therefore have highly trained aural skills. Here, we aimed to identify the neural correlates of experience‐driven plasticity related to the processing of complex sound features. We used functional magnetic resonance imaging in combination with an event‐related oddball paradigm and compared brain activity in professional musicians and non‐musicians during pre‐attentive processing of melodic contour variations. The melodic pattern consisted of a sequence of five tones each lasting 50 ms interrupted by silent interstimulus intervals of 50 ms. Compared to non‐musicians, the professional musicians showed enhanced activity in the left middle and superior temporal gyri, the left inferior frontal gyrus and in the right ventromedial prefrontal cortex in response to pattern deviation. This differential brain activity pattern was correlated with behaviorally tested musical aptitude. Our results thus support an experience‐related role of the left temporal cortex in fast melodic contour processing and suggest involvement of the prefrontal cortex. Hum Brain Mapp, 2009. © 2009 Wiley‐Liss, Inc.
Keywords: fMRI, musicians, deviance detection, auditory system, prefrontal cortex
INTRODUCTION
Music and speech are among the most complex achievements of human species. Similarly to speech, complexity of music builds up on rule‐based permutations of a limited number of elements like time and pitch to achieve meaningful structures like melodies. Perceptually, a melody consists of two aspects, contour and interval [Dowling,1978]. Contour represents the information about the dynamic up‐ and downward pattern of pitch changes, while interval represents the exact ratio of pitch between successive tones. The observation that both infants and musically untrained adults are able to process contour information but have difficulties to encode the pitch intervals of unfamiliar melodies leads to the conclusion that contour processing is more fundamental than interval processing [Bartlett and Dowling,1980; Cuddy and Cohen,1976; Dowling,1978; Trehub et al.,1993]. Studies concerning the processing of melodies suggest that melody processing involves primary and secondary auditory cortex [Patterson et al.,2002]. However, not only the temporal lobes but also the prefrontal cortex is involved in melody processing [Janata et al.,2002]. According to the neurocognitive model of music perception proposed by Kölsch and Siebel [2005], fundamental auditory features such as pitch height and intensity are extracted in the primary auditory cortex before acoustic information enters the second stage of music perception, the auditory sensory memory. To detect the up‐ and downward pattern of a melody, a comparison between successive pitches is essential.
The operations of the auditory sensory memory are reflected in the mismatch negativity (MMN) potential [Näätänen et al.,2001]. The MMN is an electrophysiological signature of the pre‐attentive processing of changes in a regular sequence of auditory stimuli [Kölsch et al.,1999; Näätänen et al.,2001] and is elicited by infrequent deviant stimuli among frequent standard stimuli. The repetitive “standard” sound forms a memory trace in the auditory system, and if a new deviant sound does not match this memory trace‐dependent template the MMN is elicited (for more details about MMN see Kujala et al.,2007]. Results from electro‐encephalography (EEG), magnet‐encephalography (MEG) and event‐related potentials (ERP) studies supported the view that MMN is generated in the primary and secondary auditory cortex [Alho et al.,1993; Javitt et al.,1994]. However, an additional frontal generator of the MMN has been described [Giard et al.,1990]. Accordingly, Opitz et al. [2002] reported right inferior frontal gyrus activation related to changes in pitch in a functional magnetic resonance imaging (fMRI) study. According to this finding the authors proposed a contrast enhancement view concerning the deviance detection. This view claims that the prefrontal cortex is active in situations, when the temporal lobe deviance detection system fails to discriminate stimuli. Further evidence of frontal involvement in acoustic deviance detection was presented in a positron emission tomography (PET) study, in which complex novel sounds activated the left superior temporal gyrus and the left and right inferior frontal gyrus [Müller et al.,2002]. However, as Deouell [2007] states in his recent review about the frontal generators of MMN, the exact cortical localization and the conditions under which the frontal generators of MMN are activated remain still elusive.
Since MMN is associated with auditory discrimination accuracy, improvement of this accuracy achieved by longlasting musical training should enhance the MMN. Tervaniemi et al. [1997] showed that subjects who scored higher in a musicality score had a larger MMN than non‐musical subjects when confronted with temporal deviance in a simple repetitive sound pattern. This study demonstrated that the musicians' brain is adapted to cope with the complex tasks of professional musicians. Further studies showed structural differences in modality‐specific cortical areas like Heschl`s gyrus [Schneider et al.,2002], corpus callosum [Schlaug et al.,1995a] and pyramidal tracts [Bengtsson et al.,2005] between musicians and non‐musicians (for review see Münte et al. [2002]). MEG [Vuust et al.,2005] and fMRI [Bangert et al.,2006] added further evidence to functional adaptation of the musicians' brain. This effect was even stronger when musical training started at an early age [Elbert et al.,1995].
The prefrontal cortex together with the anterior cingulate plays a crucial role in acquisition and performance of cognitive tasks [Fincham and Anderson,2006]. Frontal cortex is involved in both melody processing and deviance detection. Furthermore, inferior frontal gyri have previously been suggested to be involved in pre‐attentive processing of deviance [Doeller et al.,2003; Downar et al.,2001, 2002; Maess et al.,2001; Molholm et al.,2005; Müller et al.,2002]. The impact of musical expertise on attentive processing of irregular chords in frontal regions was shown by Kölsch et al. [2005]. In the latter fMRI study musical training was related to stronger bilateral activation in the pars opercularis of the inferior frontolateral cortex.
On the basis of these results, we hypothesized that professional musicians show functional differences in response to pre‐attentive processing of melodic pattern deviations. More specifically, we aimed to test whether potential functional differences in melodic pattern processing in musical experts, which could be regarded as a functional correlate of experience‐driven plasticity, are represented in auditory or frontal brain regions, or both. Thus, we used fMRI in combination with an event‐related oddball paradigm to investigate differential blood oxygenation level‐dependent (BOLD) responses to melodic pattern deviance between professional musicians and non‐musicians.
MATERIALS AND METHODS
Subjects
We investigated two groups of healthy subjects (Table I) with normal vision and audition. The first group consisted of eight professional musicians with a mean (± standard deviation [SD]) age of 44.5 ± 9.9 y. Seven of them were male and one subject was female. All were professional musicians in the field of classical music. Two musicians stated to have an absolute pitch (AP), three a quasi absolute pitch (QAP) and three subjects stated to rely on relative pitch (RP) according to their individual experience in music classes. Information about the AP status was based on self‐reports of the subjects. Since several groups have reported a high agreement between self report of AP and performance in standard AP tests [Deutsch,1982; Gregersen et al.,1999; Keenan et al.,2001], we adopted these reports in our study. The musicians began to take music lessons at a mean age of 6.5 ± 2 years and spent weekly about 17.5 ± 20.8 h on musical training during the last 2 years. Additionally, they devoted 6.2 ± 7.4 h per week to mental training concerning music and 10.4 ± 10.1 h on focused listening to music in the last 2 years. They received early childhood musical education at the age of 3.1 ± 2.9 years and learned to play an instrument at the age of 7.5 ± 0.9 years. The eight control subjects were matched for age and sex and spent 4.3 ± 10.4 h per week focused on listening to music in the last 2 years. They did not take music lessons or have early childhood musical education and did not learn to play any instruments during their life.
Table 1.
Characterization of the groups (N = 8) with normal vision and audition
| Musicians Mean (± SD) | Non‐musicians Mean (± SD) | T‐test | |
|---|---|---|---|
| Age | 44.5 (± 9.9) | 42.9 (± 10.7) | t(14) = 0.1 P = 0.9 |
| Gender (male/females) | 7/1 | 7/1 | |
| Absolute pitch status (AP = absolute pitch; QAP = quasi absolute pitch, RP = relative pitch) | AP = 2; QAP = 3; RP = 3 | N = 0 | |
| Music experience | |||
| Beginning to take music lesions at age | 6.5 (± 2.0) | – | |
| Average numbers of hours per week spent on musical training. (over the past 2 years) | 17.5 (± 20.8) | – | |
| Mental training (in the heads, without music) over the past 2 years, hours per week | 6.2 (± 7.4) | – | |
| Focused listening over the past two years, hours per week | 10.4 (± 10.1) | 4.3 (± 10.4) | t(14) = 0.7, P = 0.5 |
| Early childhood musical education, at age | 3.1 (± 2.9) | – | |
| Learning to play an instrument, at age | 7.5 (0.9) | – | |
| Measurement of musical aptitude (AMMA) | |||
| AMMA, tonal test, raw score | 34.5 (± 2.6) | 27.9 (± 4.3) | t(14) = 3.5, P < 0.005 |
| AMMA, rhythm test, raw score | 34.9 (± 2.4) | 29.5 (± 2.9) | t(14) = 3.7, P < 0.005 |
SD, standard deviation.
Stimulation and Paradigm
The pattern deviance paradigm was adapted from an EEG paradigm by Tervaniemi et al. [2001], which consists of a five‐tone melody as standard pattern (see Fig. 1). In abstract terms, the pattern follows the rule ABCED. For pattern deviance the penultimate tone was replaced by the first one (ABCAD). The pattern consisted of sinusoidal tones (duration 50 ms, 5 ms rise and fall times, 50‐ms interstimulus interval). There were five different baseline frequencies of the standard pattern and five corresponding deviant patterns starting at the same baseline frequencies. The lowest base frequency was 660 Hz, corresponding to tone ‘A’ in the standard pattern. The patterns were presented with an ‘inter‐melody‐interval’ of 1,200 ms. Each standard pattern is repeated sixty times and each deviant pattern was presented four times in a pseudorandomized order with an average interval of approximately 18 s (16.8–19.2 s) between two deviants. This way the ratio between standard and deviant patterns was 15/1. During the recordings the stimulation was delivered by MR‐compatible headphones (Commander XG, Resonance Technology, Northridge, CA) while the participants were instructed to watch a silent cartoon movie (La Linea 1, Osvaldo Cavandoli, Monitorpop, Germany, 2003) without paying attention to the presented sounds.
Figure 1.
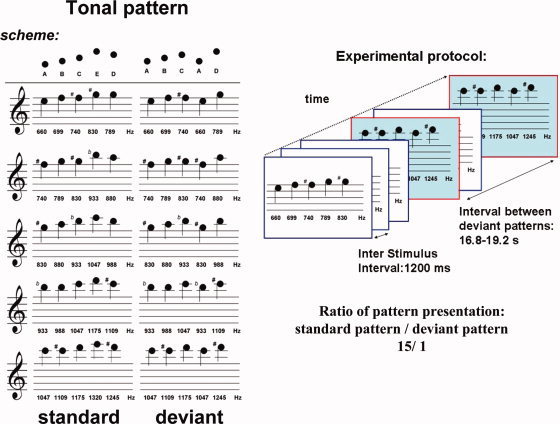
The contour deviance paradigm adapted from Tervaniemi et al. [2001] consists of a five‐tone melody as standard pattern. In abstract terms, the contour follows the rule ABCED. For contour deviance, the penultimate tone was replaced by the first one (ABCAD). There were five different baseline frequencies of the standard pattern and five corresponding deviant patterns starting at the same baseline frequencies. The lowest base frequency was 660 Hz, corresponding to tone ‘A’ in the standard pattern. The patterns were presented with an ‘inter‐melody‐interval’ of 1,200 ms. Each standard pattern is repeated sixty times and each deviant pattern was presented four times in a pseudorandomized order with an average interval of approximately 18 s (16.8–19.2 s) between two deviants. This way the ration between standard and deviant patterns was 15/1.
Data Acquisition
The fMRI data were acquired on a 1.5 T MRI scanner (Sonata, Siemens, Erlangen, Germany) equipped with a Sonata gradient system and a circularly polarized radio frequency headcoil. The subjects' head was fixated with foam pads to minimize movement during the experiment. A T1‐weighted high‐resolution data set covering the whole brain was collected for each subject with a three‐dimensional magnetization‐prepared rapid acquisition gradient echo with 1.2 (1 × 1 × 1.2) mm3 cubic voxels. To reduce perceptual and physiological interactions of the BOLD signal due to the acoustic noise produced by switching magnetic field gradients in fMRI with activity induced by experimental acoustic stimulation, we used a recently developed low impact noise acquisition fMRI sequence to increase the dynamic range of BOLD signal [Seifritz et al.,2006]. This sequence emits continuous rather than pulsed gradient sound by implementing a novel quasi‐continuous gradient switch pattern. Compared to conventional fMRI, continuous‐sound fMRI reduced auditory cortex BOLD baseline and increased BOLD amplitude with graded sound stimuli, short sound events, and sounds as complex as orchestra music with preserved temporal resolution. The functional volumes were positioned parallel to the lateral sulcus and consisted of a gradient‐recalled echoplanar imaging sequence with 16 image slices having a thickness of 5 mm and a volume repetition time (TR) of 1,850 ms (field of view, 1802 mm2; matrix, 642 pixels; echo time, 61 ms; flip angle, 90°; bandwidth, 1,280 Hz/pixel; slice acquisition time, 116 ms).
Image Preprocessing and Statistical Analysis
Image time‐series were processed using the software package Brain Voyager QX 1.6 (Brain Innovation, Maastricht, The Netherlands). For each subject, the first two echoplanar images were discarded to allow for magnetization signal full saturation and all the remaining scans were re‐aligned to the first included volume scan using a Levenberg‐Marquardt‐algorithm optimizing three translation and three rotation parameters on a resampled version of each image. The resulting head motion‐corrected time‐series were then corrected for the different slice scan times using an interpolation procedure and then filtered in the temporal domain. For temporal filtering a high‐pass filter with cut‐off to six cycles (114 s) was used to reduce linear and non‐linear trends in the time‐courses. Using the results of the image registration with anatomical scans, the functional image‐time series were then warped into Talairach space and resampled into 3 mm isotropic voxel time‐series. Finally, to perform a group‐level analysis, the resampled volume time‐series were spatially filtered (smoothing) using an isotropic 6 mm full‐width‐at‐half‐maximum gaussian kernel.
According to the illustrated paradigm, there were a much higher number of standard stimuli compared to the deviant pattern stimuli. To calculate a balanced contrast between the responses to standard and deviant patterns, we randomly extracted from the series of stimuli a number of standard patterns equal to the number of deviant patterns and built the general linear model (GLM) design matrix [Friston et al.,1995] upon them separately for each subject. Using this strategy it was possible not only to investigate the main effects of the deviant pattern but also contrast them against an equalized set of standard stimuli, randomly extracted from the series of the many frequent standard stimuli. The matrix had two predictors of interest, standard (i.e., randomly extracted standard pattern) and deviant (i.e., deviant pattern), both of them obtained as linear convolution of the series of events with a standard double‐gamma hemodynamic impulse response function [Friston et al.,1998]. The GLM was estimated voxel‐wise and a correction of serial correlations was performed using a fit‐re‐fit strategy [Bullmore et al.,1996]. The resulting estimates were statistically evaluated and compared using one‐ (whole‐group) and two‐ (musicians vs. non‐musicians) sample random effects t‐tests. The main effects of standard and deviant predictors as well as their differential contrast were evaluated respectively at the statistical thresholds of P = 0.005 and P = 0.01 (uncorrected). To correct the t‐maps for the multiple voxel‐level comparisons, we employed a cluster‐level threshold correction procedure based on Montecarlo simulations [Forman et al.,1995] and accepted a cluster‐level corrected significance of 5%.
Behavioral Musical Testing
To explore whether there were any differences at the behavioral level in task involving discrimination of melody patterns we used the Advanced Measures of Music Audition (AMMA), [Gordon,1997] in all subjects after the fMRI scan session. The AMMA is considered to reflect musical aptitude, i.e., the ability to use mental imagery to hear music and to discriminate differences between musical pieces in the absence of auditory stimuli. It starts with three practical trials after which there are 30 trials. Subjects have then to decide whether two melodies they hear are the same or different and when considered to be different whether they differ in tonality or rhythm.
RESULTS
Functional Imaging
The standard pattern and the deviant pattern evaluated as main effects compared to silent baseline produced a widely distributed significant signal change in bilateral regions of the auditory cortices in both groups. The standard pattern showed in contrast to silent baseline not only activity in the right and left superior temporal gyrus but also in the left superior occipital gyrus. The deviant stimulus as contrasted to silence baseline elicited a slightly different pattern with an additional activation of the left anterior cingulate and the middle temporal gyri bilaterally (Table II). However, contrasting deviant against random standard over all 16 subjects showed no statistically significant difference. In the group comparison of the main effect of the deviant pattern compared to silent baseline between the musicians and the non‐musicians, we found a significant differential response to the deviant stimuli (Fig. 2A, Table II). In musicians, the deviant stimulus elicited an enhanced activation in the right ventromedial prefrontal cortex near to the opercular part of the inferior frontal gyrus and the anterior cingulate, and in left‐hemispheric inferior frontal gyrus, middle temporal gyrus and the superior temporal gyrus, corresponding to the lateral part of the anterior Heschl`s gyrus, compared to non‐musicians.
Table 2.
Talairach coordinates of activation due to standard and deviant stimuli for all subjects and the group contrast
| Brain region | Center of mass Talairach coordinates: | Number of Voxels | P | |||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Main effect: All subjects: | ||||||
| Deviant | R superior temporal gyrus | 51 | −15 | 7 | 7,016 | < 0.005 |
| R middle temoral gyrus | 35 | −8 | −13 | 855 | ||
| L anterior cingulate gyrus | −4 | 3 | 26 | 1,487 | ||
| L parietal lobe | −13 | −51 | 27 | 1,616 | ||
| L middle frontal gyrus | −30 | 38 | 14 | 833 | ||
| L superior temporal gyrus | −44 | −25 | 7 | 5,900 | ||
| L middle frontal lobe | −37 | 21 | 21 | 905 | ||
| L superior occipital gyrus | −43 | −76 | 27 | 1,040 | ||
| Random standard | R superior temporal gyrus | 47 | −6 | −5 | 893 | < 0.005 |
| L superior occipital gyrus | −30 | −71 | 26 | 863 | ||
| L superior temporal gyrus | −47 | −14 | 4 | 1534 | ||
| Group effect: Musicians vs non‐musicians: | ||||||
| Deviant | R VMPFC | 17 | 28 | −7 | 899 | < 0.005 |
| L inferior frontal gyrus | −42 | 29 | −8 | 350 | ||
| L middle temporal gyrus | −53 | −14 | −12 | 442 | ||
| L superior temporal gyrus | −58 | −18 | 14 | 352 | ||
R = right, L = left, ventromedial prefrontal cortex = VMPFL.
Figure 2.
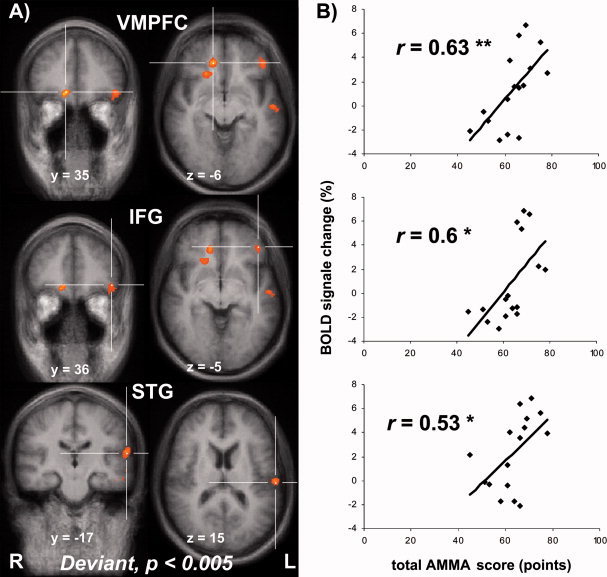
A: Significant difference (P < 0.005, corrected) in brain response to the deviant condition between musicians and non‐musicians is displayed (R = right, L = left). For display purposes, the results are superimposed on the mean structural scan of all 16 participants. VMPFC = ventromedial prefrontal cortex; IFG = inferior frontal gyrus; STG = superior temporal gyrus, corresponding to the lateral part of the anterior Heschl`s gyrus. B: The correlation between total AMMA‐score (points) and BOLD signal change (%) in the regions in which a significant (P < 0.005) group effect was found is shown with the correlation coefficient (r) and the level of significance **P < 0.01, *P < 0.05.
Musicality Testing
In the AMMA, musicians showed in the tonal test section a mean raw score of 34.5 ± 2.6 score points whereas the non‐musicians scored 27.9 ± 4.3 score points. These scores differed significantly (P < 0.005). In the rhythm section of the AMMA, the musicians scored 34.9 ± 2.4 score points and the non‐musicians 29.5 ± 2.9 score points. These scores were also significantly different (P < 0.005; Table I).
A linear correlation analysis between the AMMA scores of all subjects and the fMRI responses in the regions of significant BOLD signal change to deviant stimuli in the group contrast were calculated (Fig. 2B). We found a significant positive correlation of the AMMA subscores for tonality, rhythm and the total AMMA score and the BOLD signal in all regions that showed a differential activation in the group contrast.
DISCUSSION
We used fMRI in combination with an event‐related oddball paradigm to evaluate the neural correlates of pre‐attentive processing of melodic contour deviance in musicians and non‐musicians. The main effect of the whole group for the standard and the deviant stimuli (contrast: standard > silent baseline and contrast: deviant > silent baseline) showed an activation of superior temporal gyrus bilaterally without a significant contrast between standard and deviant conditions. This, however, did not apply for the group comparison, in which we found a clearly different activation pattern in musicians. Contrasting the deviant condition against the silent baseline, showed in musicians enhanced activation in response to the deviant melodic pattern in the right ventromedial prefrontal cortex, which spreads to the opercular part of the right inferior frontal gyrus and activation in the left inferior frontal gyrus and superior temporal gyrus, corresponding to the lateral part of the anterior Heschls`s gyrus (Fig. 2A). The presence of a significant differential activation due to deviance only in the group contrast might explain why the contrast random standard against deviant did not reach significance over all subjects.
The melodic pattern stimulus that we used here was similar to the one proposed by Tervaniemi et al. [2001]. In their combined EEG‐MEG study musicians showed superior pre‐attentive encoding of patterns after a first attentive training session. Fujioka et al. [2004] confirmed superiority of musicians regarding to melodic pattern processing in their MEG study even without an attentive training session.
Selective attention to a sound stimulus has been reported to modulate neural response in human auditory cortex [Jäncke et al.,1999, 2001; Petkov et al.,2004]. Furthermore attention to a special sound feature, in particular spatial localization and spectral pattern, has shown to result in increased selectivity for this feature [Ahveninen et al.,2006]. However, a recent EEG study of Baumann et al. [2008] showed that the enhancement of auditory evoked potentials in musicians reflects rather an influence of expertise and not of selective attention. Our fMRI data add further evidence to this notion, since in professional musicians deviant stimuli induced a different activation pattern when sound were presented outside the focus of attention. This pattern includes the opercular part of the right medial frontal gyrus near to anterior cingulate and inferior frontal gyrus, left inferior frontal gyrus and superior temporal gyrus, which all have previously been suggested to be involved in pre‐attentive processing of deviance [Doeller et al.,2003; Downar et al.,2001, 2002; Maess et al.,2001; Molholm et al.,2005; Müller et al.,2002].
Pantev et al. [2003] used MEG and found that deviation of melodic contour elicited a significantly larger MMN in musicians compared to non‐musicians, while simple deviance detection of a single sine‐wave tone showed no difference between groups. The notion that in our study musicians showed enhanced BOLD responses to complex deviance in sound sequences is consistent with previous studies [Fujioka et al.,2004; Kölsch et al.,1999; Tervaniemi et al.,1997, 2001, 2006; van Zuijen et al.,2004, 2005]. The results of our study confirmed our hypothesis that musicians would show a different response to deviant patterns than non‐musicians. In addition, our data fit with previous findings and extend them by localizing high‐level deviance detection in highly trained musicians to left temporal cortex and frontal regions. We suggest that the group difference in pre‐attentive deviance detection points to neuroplastic changes induced by long lasting training. Näätänen et al. [1993] showed that even a short‐term training leads to development of MMN enhancement in other aspects of sound processing. The same effect on the pre‐attentive processing was found after long lasting training [Kölsch et al.,1999]. Using fMRI we collected information about the localization of pattern processing in musicians. One disadvantage of this method, however, is the sluggishness of the hemodynamic response measured. A combined fMRI and EEG approach could help to clarify the relation between BOLD activity and the potential link to MMN, which would allow a better understanding of the cognitive processes associated with tone, pitch and pattern processing.
Earlier studies already suggested that there are correlations between musicality test scores and pre‐attentive neural sound processing [Tervaniemi et al.,1997]. They found that subjects who learned to differentiate a contour in an attending condition developed an MMN also in the pre‐attentive task and were more accurate in the behavioral task. In our study, the AMMA was performed in both groups to evaluate the discrimination of complex sounds between musicians and non‐musicians at a behavioral level. The AMMA is considered to probe musical aptitude, i.e., the ability to discriminate rhythmic or tonal variations in sound patterns [Gordon,1997]. As expected from earlier studies [Schneider et al.,2002] the musicians show significantly higher scores in this test. However, the tonal results of the AMMA‐test are quite high even for professional musicians (score of 34/40) being four points higher as compared to the expected mean value of 30/40 for professional musicians given by Gordon [1997] and therefore corroborate an outstanding musical aptitude of the test subjects recruited from the Scola Cantorum Basiliensis and the Music Academy, Basel, Switzerland. Here we found a significant positive correlation between the BOLD signal change and the AMMA score (Fig. 2B) in the brain regions identified in the group contrast. This suggests the enhanced responsivness of left temporal and frontal cortices observed in our study represents a functional correlate of the superior musical aptitude in musicians. According to the literature there is a close relation between early musical education and the development of AP [Baharloo et al.,1998]. The musicians in our sample started at mean age of 6.5 (± 2.0) years to take music lesions. Both subjects who stated to have AP started much earlier with musical education as the rest of the group, namely at the age of 4 years. This is of special interest as results of other studies indicate that there is a sensitive period for the development of AP between the 2 and 6 years [Wilson et al.,2009]. Within the group of musicians these two subjects might be considered as even more specialized by AP status and early music training. Three subjects stated to have a QAP. QAP subjects are able to label only single tones—often their tuning note—but fail to show the automatic and rapid identification found in subjects with true AP [Levitin and Rogers,2005]. As the total number of AP subjects is only two, it is unlikely that our group comparison results are driven by these subjects. Generally, prevalence of AP in musicians is about 15% [Baharloo et al.,1998; Gregersen et al.,2001]. However prevalence shows a significant association between type of institution or music program. In a conservatory setting a prevalence of 24.6% was reported [Gregersen et al.,1999]. Thus prevalence in our study group would reflect the previously reported prevalence of AP in the setting from which subjects were recruited. Having this in mind one might assume that a small number of AP subjects among a group of professional musicians represents a rather normal distribution in this group. Since early studies showed that behavioral and functional differences between musicians and non‐musicians are stronger when musical training started early [Elbert et al.,1995], most subsequent studies about behavioral or structural differences between musicians and non‐musicians have been conducted with highly trained musicians from conservatory level musical programs, which usually tend to have had early and intensive musical training. Due to this approach AP subjects probably have been included in many other studies implicitly comparing musicians and non‐musicians. As far as reported, AP status was not tested in many of the studies that focus on the difference between musicians and non‐musicians [Elbert et al.,1995; Kölsch et al.,1999; Kölsch and Siebel,2005; Tervaniemi et al.,1997, 2000, 2001, 2006].
A recent fMRI study by Wilson et al. [2009] showed that not only structural but also functional differences in a pitch naming task between AP, QAP and RP subjects could be found. So it seems to be questionable to consider QAP as similar condition as AP. There is still an ongoing discussion whether AP lies on a tail of a continuous perceptual spectrum from AP over QAP to RP or rather defines a distinct perceptual trait. A first important answer to this question comes from Athos et al., [2007]. This study showed that AP is a dichotomous trait, e.g. there is no continuum but rather an AP status or a non‐AP status, which seems to include QAP and RP. A small subset of anatomical studies about musicians addressed AP. One finding has been replicated in different studies. Apparently there is a leftward asymmetry in size of planum temporale in AP possessor as compared to control musicians and non‐musicians [Keenan et al.,2001; Schlaug et al.,1995b]. Functional data by Ohnishi et al. [2001] showed a positive correlation between BOLD signal in left planum temporale and AP proficiency and data from Zatorre et al., [1998] showed a positive correlation between planum temporale volume and AP. In contrast to earlier studies, which failed to show differences outside the temporal lobe, a recent voxel‐based morphometric study revealed a thinner cortex in the posterior dorsal frontal regions (rostral area six, caudal area eight) in AP subjects as compared to musicians without AP [Bermudez et al.,2008]. Functionally these areas are critically involved in aspects of conditional associative memory [Lepage et al.,2003]. This activity reflects the ability to retrieve an arbitrary conditional association between a stimulus attribute and a verbal label. This notion was confirmed as musically naïve subjects who learned to discriminate and label acoustic stimuli also showed activity in these areas [Bermudez and Zatorre,2005]. The main result of our current study was that professional musicians showed enhanced activity in the left middle and superior temporal gyri, the left inferior frontal gyrus and in the right ventromedial prefrontal cortex in response to pattern deviation. We did not find enhanced activity in the planum temporale or the posterior dorsal frontal cortex as one would expect if results would be driven by AP status.
On a functional level, the detection of deviance can be considered as a pre‐condition to a music performance to play in tune with other musicians. This is reflected by the finding that also amateur band musicians, which, in contrast to professional musicians, have a limited amount of systematical training and rely specifically on spatial encoding when playing live on a stage, extract local information more accurately than non‐musicians [Tervaniemi et al.,2006].
Previous findings indicating a crucial involvement of the left temporal cortex in the deviance detection in the frequency domain [Celsis et al.,1999]. The lateralization of tonal pattern deviance to the left superior temporal gyrus in our study is however an interesting finding as it seems to be contrary to the neuropsychological model of pattern processing [Peretz,1990]. This model suggests that the global pattern of ups and downs of melodic patterns are evaluated in the right superior temporal gyrus while the local patter, e.g. the precise intervals, are proceeded in the left hemisphere. This model is sustained by brain lesion data [Peretz,1990]. However brain lesions are heterogeneous and rarely circumscribed. Apart from the above mentioned lesion studies, Johnsrude et al. [2000] showed in a group of 14 neurologically normal subjects and 31 patients who had undergone surgical resection of either the right or left temporal lobe that, on a behavioral level, subjects with a lesion of he right Heschl`s gyrus performed worse in a pitch direction task. Not only pitch direction but also fine pitch resolution is attributed to the right auditory cortex [Hyde et al.,2008]. Zartorre et al. [1994] used PET to show that the right superior temporal gyrus is involved in perceptual analysis of melodies, while pitch comparisons were effected via networks that include the right prefrontal cortex. In addition, active pitch retention was found to involve an interaction of right temporal and frontal cortices. Thus, our data seems to be in conflict with the above suggested model claiming a right hemisphere specialization for pitch processing. Recently published fMRI data challenged this model and showed that processing of pattern deviance led to a more pronounced left hemispheric activation of the superior temporal sulcus [Stewart et al.,2008]. The latter observation is supported by our data. In our study design we used a fast sequence of tones 50 ms each interrupted by 50 ms silence. Earlier studies also indicated a lateralization to the left auditory cortex in response to stimuli with rapidly changing temporal patterns [Zatorre and Belin,2001], a finding which is corrobated by our results. Furthermore, Boemio et al. [2005] proposed a model in which sounds are analyzed on two distinct timescales of 25–50 and 200–300 ms. According to this model stimuli with slower (200–300 ms) modulated temporal characteristics were preferentially processed in the right hemisphere while faster modulated stimuli (25–50 ms) were processed more in the left hemisphere.
Inferior frontal cortex plays a role in music processing and integration of sequential information over time [Tillmann et al.,2006]. In musicians, it is necessary to ensure an optimal performance and ongoing control of the music they play or perceive to allow behavioral adjustment if unexpected deviances are encountered. As mentioned above, long lasting training leads to improved and more accurate pre‐attentive processing of deviance. Our data support the notion that prefrontal areas like inferior frontal gyrus are involved in deviance detection. The prefrontal cortex, according to the contrast enhancement view [Opitz et al.,2002], is active in situations, when the temporal lobe deviance detection system gets in difficulty in discriminating stimuli. Further evidence for this concept was found by Müller et al., [2002] in a PET study, in which complex novel sounds activated the left superior temporal gyrus, left inferior frontal gyrus and right inferior frontal gyrus. They proposed a two‐stage model of auditory deviance detection. According to this model smaller and therefore less salient stimulus changes are detected in superior temporal gyrus and inferior frontal gyrus whereas larger stimulus changes additionally involve mid‐dorsolateral prefrontal cortex. Nonetheless, data are still inconsistent with respect to the lateralization of the frontal activity. Doeller et al. [2003] used fMRI and EEG and showed that the right inferior frontal gyrus mediates auditory deviance detection in case of low discriminability between a sensory memory trace and an auditory input. Maess et al. [2001] showed in an MEG study that harmonic incongruities occurring within a major‐minor tonal context elicited an early negativity similar to the MMN in the opercular part of the inferior frontal gyrus whereas right inferior frontal gyrus was activated by visual and auditory deviant stimuli regardless of task relevance in two different studies [Downar et al.,2001, 2002]. Molholm et al. [2005] found in a frequency‐specific condition an activation of right and in a duration‐specific condition an activation of the left inferior frontal gyrus. Kölsch and Siebel [2005] showed that in musically trained subjects activation due to irregular cords was stronger and involved the frontal operculum bilateraly in an attentively tested task. Activation of the right opercular part of the inferior frontal gyrus related to pre‐attentive deviance detection in the musicians is a new finding since previous studies did not show such activation in this anatomical region. Janata et al. [2002] showed that ventromedial prefrontal cortex is involved in tracking activation in tonal space. According to their data, the ventromedial prefrontal cortex maintains a distributed topographic representation of the tonality surface. This region has been implicated in the assessing of musical consonance or dissonance [Blood et al.,1999]. The concept that cognitive structures maintaining tonal contexts are not limited to the auditory cortices is supported by the finding that even with bilateral auditory cortex ablations the ability to generate expectancies based on tonal contexts remains intact [Tramo et al.,1990]. Janata [2002] concluded that that ventromedial prefrontal cortex is a crucial node for binding musical elements with memories within a broader melody‐responsive network. This notion fits PET studies in which ventromedial prefrontal cortex was active in evaluation of consonance/dissonance in chords accompanying melodies [Blood et al.,1999] or in familarity judgments of sets of acoustic stimuli [Platel et al.,2003]. The activation related to pattern deviance in professional musicians in our study supports the hypothesis of tonal binding in ventromedial frontal cortex.
CONCLUSIONS
In summary, professional musicians showed differential brain responses in response to tonal pattern variation in the right ventromedial prefrontal cortex, left inferior frontal gyrus and left superior temporal gyrus when compared to musical laypersons. In these regions, the BOLD signal was correlated with behaviorally tested musical aptitude. These results support the hypothesis that musical experience leads to specific changes of the neural mechanisms for processing melodic information and that long‐term training improves the decoding of pitch relation between successive notes in temporal and frontal brain regions. However, to dissociate experience‐ from disposition‐related effects in differential musicians' brain acitivity, longterm, and repeated within‐subjects studies are required.
Acknowledgements
The authors thank Elke Hofmann (Music Academy, Basel, Switzerland) and Hans Peter Weber (Schola Cantorum Basiliensis, Basel, Switzerland) for their help in recruting the professional musicians.
REFERENCES
- Ahveninen J, Jaaskelainen IP, Raij T, Bonmassar G, Devore S, Hamalainen M, Levanen S, Lin FH, Sams M, Shinn‐Cunningham BG, Witzel T, Belliveau JW ( 2006): Task‐modulated “what” and “where” pathways in human auditory cortex. Proc Natl Acad Sci U S A 103: 14608–14613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alho K, Huotilainen M, Tiitinen H, Ilmoniemi RJ, Knuutila J, Naatanen R ( 1993): Memory‐related processing of complex sound patterns in human auditory cortex: A MEG study. Neuroreport 4: 391–394. [DOI] [PubMed] [Google Scholar]
- Athos EA, Levinson B, Kistler A, Zemansky J, Bostrom A, Freimer N, Gitschier J ( 2007): Dichotomy and perceptual distortions in absolute pitch ability. Proc Natl Acad Sci U S A 104: 14795–14800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baharloo S, Johnston PA, Service SK, Gitschier J, Freimer NB ( 1998): Absolute pitch: An approach for identification of genetic and nongenetic components. Am J Hum Genet 62: 224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangert M, Peschel T, Schlaug G, Rotte M, Drescher D, Hinrichs H, Heinze HJ, Altenmuller E ( 2006): Shared networks for auditory and motor processing in professional pianists: Evidence from fMRI conjunction. Neuroimage 30: 917–926. [DOI] [PubMed] [Google Scholar]
- Bartlett JC, Dowling WJ ( 1980): Recognition of transposed melodies: A key‐distance effect in developmental perspective. J Exp Psychol Hum Percept Perform 6: 501–515. [DOI] [PubMed] [Google Scholar]
- Baumann S, Meyer M, Jancke L ( 2008): Enhancement of auditory‐evoked potentials in musicians reflects an influence of expertise but not selective attention. J Cogn Neurosci 20: 2238–2249. [DOI] [PubMed] [Google Scholar]
- Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullen F ( 2005): Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci 8: 1148–1150. [DOI] [PubMed] [Google Scholar]
- Bermudez P, Zatorre RJ ( 2005): Conditional associative memory for musical stimuli in nonmusicians: Implications for absolute pitch. J Neurosci 25: 7718–7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez P, Lerch JP, Evans AC, Zatorre RJ ( 2008): Neuroanatomical correlates of musicianship as revealed by cortical thickness and voxel‐based morphometry. Cereb Cortex [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Blood AJ, Zatorre RJ, Bermudez P, Evans AC ( 1999): Emotional responses to pleasant and unpleasant music correlate with activity in paralimbic brain regions. Nat Neurosci 2: 382–387. [DOI] [PubMed] [Google Scholar]
- Boemio A, Fromm S, Braun A, Poeppel D ( 2005): Hierarchical and asymmetric temporal sensitivity in human auditory cortices. Nat Neurosci 8: 389–395. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Brammer M, Williams SC, Rabe‐Hesketh S, Janot N, David A, Mellers J, Howard R, Sham P ( 1996): Statistical methods of estimation and inference for functional MR image analysis. Magn Reson Med 35: 261–277. [DOI] [PubMed] [Google Scholar]
- Celsis P, Boulanouar K, Doyon B, Ranjeva JP, Berry I, Nespoulous JL, Chollet F ( 1999): Differential fMRI responses in the left posterior superior temporal gyrus and left supramarginal gyrus to habituation and change detection in syllables and tones. Neuroimage 9: 135–144. [DOI] [PubMed] [Google Scholar]
- Cuddy LL, Cohen AJ ( 1976): Recognition of transposed melodic sequences. Q J Exp Psychol 28: 255–270. [Google Scholar]
- Deouell L ( 2007): The frontal generator of the mismatch negativity revisited. J Psychophysiol 21: 188–203. [Google Scholar]
- Deutsch D ( 1982): The Psychology of Music. New York: Academic Press. [Google Scholar]
- Doeller CF, Opitz B, Mecklinger A, Krick C, Reith W, Schroger E ( 2003): Prefrontal cortex involvement in preattentive auditory deviance detection: Neuroimaging and electrophysiological evidence. Neuroimage 20: 1270–1282. [DOI] [PubMed] [Google Scholar]
- Dowling WJ ( 1978): Scale and contour: Two components of a theory of memory for melodies. Psychol Rev 85: 341–354. [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD ( 2001): The effect of task relevance on the cortical response to changes in visual and auditory stimuli: An event‐related fMRI study. Neuroimage 14: 1256–1267. [DOI] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD ( 2002): A cortical network sensitive to stimulus salience in a neutral behavioral context across multiple sensory modalities. J Neurophysiol 87: 615–620. [DOI] [PubMed] [Google Scholar]
- Elbert T, Pantev C, Wienbruch C, Rockstroh B, Taub E ( 1995): Increased cortical representation of the fingers of the left hand in string players. Science 270: 305–307. [DOI] [PubMed] [Google Scholar]
- Fincham JM, Anderson JR ( 2006): Distinct roles of the anterior cingulate and prefrontal cortex in the acquisition and performance of a cognitive skill. Proc Natl Acad Sci U S A 103: 12941–12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC ( 1995): Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster‐size threshold. Magn Reson Med 33: 636–647. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, Turner R ( 1995): Analysis of fMRI time‐series revisited. Neuroimage 2: 45–53. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Josephs O, Rees G, Turner R ( 1998): Nonlinear event‐related responses in fMRI. Magn Reson Med 39: 41–52. [DOI] [PubMed] [Google Scholar]
- Fujioka T, Trainor LJ, Ross B, Kakigi R, Pantev C ( 2004): Musical training enhances automatic encoding of melodic contour and interval structure. J Cogn Neurosci 16: 1010–1021. [DOI] [PubMed] [Google Scholar]
- Giard MH, Perrin F, Pernier J, Bouchet P ( 1990): Brain generators implicated in the processing of auditory stimulus deviance: A topographic event‐related potential study. Psychophysiology 27: 627–640. [DOI] [PubMed] [Google Scholar]
- Gordon E ( 1997): Learning Sequences in Music. Chicago, Illinois: GIA. [Google Scholar]
- Gregersen PK, Kowalsky E, Kohn N, Marvin EW ( 1999): Absolute pitch: Prevalence, ethnic variation, and estimation of the genetic component. Am J Hum Genet 65: 911–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregersen PK, Kowalsky E, Kohn N, Marvin EW ( 2001): Early childhood music education and predisposition to absolute pitch: Teasing apart genes and environment. Am J Med Genet 98: 280–282. [DOI] [PubMed] [Google Scholar]
- Hyde KL, Peretz I, Zatorre RJ ( 2008): Evidence for the role of the right auditory cortex in fine pitch resolution. Neuropsychologia 46: 632–639. [DOI] [PubMed] [Google Scholar]
- Janata P, Birk JL, Van Horn JD, Leman M, Tillmann B, Bharucha JJ ( 2002): The cortical topography of tonal structures underlying Western music. Science 298: 2167–2170. [DOI] [PubMed] [Google Scholar]
- Jäncke L, Mirzazade S, Shah NJ ( 1999): Attention modulates activity in the primary and the secondary auditory cortex: A functional magnetic resonance imaging study in human subjects. Neurosci Lett 266: 125–128. [DOI] [PubMed] [Google Scholar]
- Jäncke L, Buchanan TW, Lutz K, Shah NJ ( 2001): Focused and nonfocused attention in verbal and emotional dichotic listening: an FMRI study. Brain Lang 78: 349–363. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Steinschneider M, Schroeder CE, Vaughan HG Jr, Arezzo JC ( 1994): Detection of stimulus deviance within primate primary auditory cortex: Intracortical mechanisms of mismatch negativity (MMN) generation. Brain Res 667: 192–200. [DOI] [PubMed] [Google Scholar]
- Johnsrude IS, Penhune VB, Zatorre RJ ( 2000): Functional specificity in the right human auditory cortex for perceiving pitch direction. Brain 123 ( Part 1): 155–163. [DOI] [PubMed] [Google Scholar]
- Keenan JP, Thangaraj V, Halpern AR, Schlaug G ( 2001): Absolute pitch and planum temporale. Neuroimage 14: 1402–1408. [DOI] [PubMed] [Google Scholar]
- Kölsch S, Siebel WA ( 2005): Towards a neural basis of music perception. Trends Cogn Sci 9: 578–584. [DOI] [PubMed] [Google Scholar]
- Kölsch S, Schroger E, Tervaniemi M ( 1999): Superior pre‐attentive auditory processing in musicians. Neuroreport 10: 1309–1313. [DOI] [PubMed] [Google Scholar]
- Kölsch S, Fritz T, Schulze K, Alsop D, Schlaug G ( 2005): Adults and children processing music: An fMRI study. Neuroimage 25: 1068–1076. [DOI] [PubMed] [Google Scholar]
- Kujala T, Tervaniemi M, Schroger E ( 2007): The mismatch negativity in cognitive and clinical neuroscience: Theoretical and methodological considerations. Biol Psychol 74: 1–19. [DOI] [PubMed] [Google Scholar]
- Lepage M, Brodeur M, Bourgouin P ( 2003): Prefrontal cortex contribution to associative recognition memory in humans: An event‐related functional magnetic resonance imaging study. Neurosci Lett 346: 73–76. [DOI] [PubMed] [Google Scholar]
- Levitin DJ, Rogers SE ( 2005): Absolute pitch: Perception, coding, and controversies. Trends Cogn Sci 9: 26–33. [DOI] [PubMed] [Google Scholar]
- Maess B, Koelsch S, Gunter TC, Friederici AD ( 2001): Musical syntax is processed in Broca's area: An MEG study. Nat Neurosci 4: 540–545. [DOI] [PubMed] [Google Scholar]
- Molholm S, Martinez A, Ritter W, Javitt DC, Foxe JJ ( 2005): The neural circuitry of pre‐attentive auditory change‐detection: An fMRI study of pitch and duration mismatch negativity generators. Cereb Cortex 15: 545–551. [DOI] [PubMed] [Google Scholar]
- Müller BW, Juptner M, Jentzen W, Müller SP ( 2002): Cortical activation to auditory mismatch elicited by frequency deviant and complex novel sounds: A PET study. Neuroimage 17: 231–239. [DOI] [PubMed] [Google Scholar]
- Münte TF, Altenmuller E, Jancke L ( 2002): The musician's brain as a model of neuroplasticity. Nat Rev Neurosci 3: 473–478. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Schroger E, Karakas S, Tervaniemi M, Paavilainen P ( 1993): Development of a memory trace for a complex sound in the human brain. Neuroreport 4: 503–506. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Tervaniemi M, Sussman E, Paavilainen P, Winkler I ( 2001): “Primitive intelligence” in the auditory cortex. Trends Neurosci 24: 283–288. [DOI] [PubMed] [Google Scholar]
- Ohnishi T, Matsuda H, Asada T, Aruga M, Hirakata M, Nishikawa M, Katoh A, Imabayashi E ( 2001): Functional anatomy of musical perception in musicians. Cereb Cortex 11: 754–760. [DOI] [PubMed] [Google Scholar]
- Opitz B, Rinne T, Mecklinger A, von Cramon DY, Schroger E ( 2002): Differential contribution of frontal and temporal cortices to auditory change detection: fMRI and ERP results. Neuroimage 15: 167–174. [DOI] [PubMed] [Google Scholar]
- Pantev C, Ross B, Fujioka T, Trainor LJ, Schulte M, Schulz M ( 2003): Music and learning‐induced cortical plasticity. Ann N Y Acad Sci 999: 438–450. [DOI] [PubMed] [Google Scholar]
- Patterson RD, Uppenkamp S, Johnsrude IS, Griffiths TD ( 2002): The processing of temporal pitch and melody information in auditory cortex. Neuron 36: 767–776. [DOI] [PubMed] [Google Scholar]
- Peretz I ( 1990): Processing of local and global musical information by unilateral brain‐damaged patients. Brain 113 ( Part 4): 1185–1205. [DOI] [PubMed] [Google Scholar]
- Petkov CI, Kang X, Alho K, Bertrand O, Yund EW, Woods DL ( 2004): Attentional modulation of human auditory cortex. Nat Neurosci 7: 658–663. [DOI] [PubMed] [Google Scholar]
- Platel H, Baron JC, Desgranges B, Bernard F, Eustache F ( 2003): Semantic and episodic memory of music are subserved by distinct neural networks. Neuroimage 20: 244–256. [DOI] [PubMed] [Google Scholar]
- Schlaug G, Jancke L, Huang Y, Staiger JF, Steinmetz H ( 1995a): Increased corpus callosum size in musicians. Neuropsychologia 33: 1047–1055. [DOI] [PubMed] [Google Scholar]
- Schlaug G, Jancke L, Huang Y, Steinmetz H ( 1995b): In vivo evidence of structural brain asymmetry in musicians. Science 267: 699–701. [DOI] [PubMed] [Google Scholar]
- Schneider P, Scherg M, Dosch HG, Specht HJ, Gutschalk A, Rupp A ( 2002): Morphology of Heschl's gyrus reflects enhanced activation in the auditory cortex of musicians. Nat Neurosci 5: 688–694. [DOI] [PubMed] [Google Scholar]
- Seifritz E, Di Salle F, Esposito F, Herdener M, Neuhoff JG, Scheffler K ( 2006): Enhancing BOLD response in the auditory system by neurophysiologically tuned fMRI sequence. Neuroimage 29: 1013–1022. [DOI] [PubMed] [Google Scholar]
- Stewart L, Overath T, Warren JD, Foxton JM, Griffiths TD ( 2008): fMRI evidence for a cortical hierarchy of pitch pattern processing. PLoS ONE 3: e1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervaniemi M, Ilvonen T, Karma K, Alho K, Naatanen R ( 1997): The musical brain: Brain waves reveal the neurophysiological basis of musicality in human subjects. Neurosci Lett 226: 1–4. [DOI] [PubMed] [Google Scholar]
- Tervaniemi M, Medvedev SV, Alho K, Pakhomov SV, Roudas MS, Van Zuijen TL, Naatanen R ( 2000): Lateralized automatic auditory processing of phonetic versus musical information: A PET study. Hum Brain Mapp 10: 74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervaniemi M, Rytkonen M, Schroger E, Ilmoniemi RJ, Naatanen R ( 2001): Superior formation of cortical memory traces for melodic patterns in musicians. Learn Mem 8: 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervaniemi M, Castaneda A, Knoll M, Uther M ( 2006): Sound processing in amateur musicians and nonmusicians: Event‐related potential and behavioral indices. Neuroreport 17: 1225–1228. [DOI] [PubMed] [Google Scholar]
- Tillmann B, Koelsch S, Escoffier N, Bigand E, Lalitte P, Friederici AD, von Cramon DY ( 2006): Cognitive priming in sung and instrumental music: Activation of inferior frontal cortex. Neuroimage 31: 1771–1782. [DOI] [PubMed] [Google Scholar]
- Tramo MJ, Bharucha JJ, Musiek FE ( 1990): Music perception and cognition following bilateral lessions of auditory cortex. J Cogn Neurosci 2: 195–212. [DOI] [PubMed] [Google Scholar]
- Trehub SE, Trainor LJ, Unyk AM ( 1993): Music and speech perception in the first year of life In: Reese HW, Lipsitt LP, editors. Advances in Child Development and Behavior. New York: Academic Press; pp 1–35. [DOI] [PubMed] [Google Scholar]
- van Zuijen TL, Sussman E, Winkler I, Naatanen R, Tervaniemi M ( 2004): Grouping of sequential sounds‐‐an event‐related potential study comparing musicians and nonmusicians. J Cogn Neurosci 16: 331–338. [DOI] [PubMed] [Google Scholar]
- van Zuijen TL, Sussman E, Winkler I, Naatanen R, Tervaniemi M ( 2005): Auditory organization of sound sequences by a temporal or numerical regularity—A mismatch negativity study comparing musicians and non‐musicians. Brain Res Cogn Brain Res 23: 270–276. [DOI] [PubMed] [Google Scholar]
- Vuust P, Pallesen KJ, Bailey C, van Zuijen TL, Gjedde A, Roepstorff A, Ostergaard L ( 2005): To musicians, the message is in the meter pre‐attentive neuronal responses to incongruent rhythm are left‐lateralized in musicians. Neuroimage 24: 560–564. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Lusher D, Wan CY, Dudgeon P, Reutens DC ( 2009): The neurocognitive components of pitch processing: Insights from absolute pitch. Cereb Cortex 19: 724–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ, Belin P ( 2001): Spectral and temporal processing in human auditory cortex. Cereb Cortex 11: 946–953. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Evans AC, Meyer E ( 1994): Neural mechanisms underlying melodic perception and memory for pitch. J Neurosci 14: 1908–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ, Perry DW, Beckett CA, Westbury CF, Evans AC ( 1998): Functional anatomy of musical processing in listeners with absolute pitch and relative pitch. Proc Natl Acad Sci U S A 95: 3172–3177. [DOI] [PMC free article] [PubMed] [Google Scholar]


