Abstract
It is now widely recognized that cognitive processes are carried out by a distributed network of brain areas, some of which are involved in perceptual processing of a stimulus, whilst others are involved in cognitive control processes required to carry out certain tasks. In this study, differential contributions of higher visual areas and of an area involved in cognitive control processes were investigated in a task requiring participants to simply look at a stimulus or to look with the intention of remembering. Varying the extent to which intentional cognitive processes were required and the stimulus material in this task allowed the analysis of “top‐down” and “bottom‐up” influences on these areas, respectively. Significant increases in the mid‐ventrolateral prefrontal cortex (mid‐VLPFC) were only observed when the stimuli were viewed with an intention in mind, irrespective of the stimulus type. In contrast, activity in the parahippocampal place area and the fusiform face area, was only modulated in conditions requiring intentional control when stimuli were presented that also elicited activity in these regions during passive viewing. These findings help to clarify the complimentary role that the mid‐VLPFC and posterior higher visual areas play in controlled and relatively automatic memory processing. Hum Brain Mapp 2008. © 2007 Wiley‐Liss, Inc.
Keywords: prefrontal, ventrolateral, control, executive, encoding, recognition, fusiform face area, parahippocampal place area, anterior insula, fMRI
INTRODUCTION
The distinction between controlled processes enabling us to carry out actions intentionally, with deliberate conscious control [Norman and Shallice, 1986] and automatic processes not requiring such control has been an important one in major areas of psychology (e.g. memory: [Baddeley and Della Sala, 1996], attention: [Norman and Shallice, 1986], cognitive development: [Flavell, 1976]). It has long been argued that the prefrontal cortex is involved in control processes [Norman and Shallice, 1986]. In functional imaging studies, mid‐ventrolateral prefrontal cortex (mid‐VLPFC), dorsolateral prefrontal cortex and anterior cingulate have consistently shown activity in conditions with control demands [Duncan and Owen, 2000]. However, there is evidence that activity in more posterior areas is also enhanced when control processes are required [Dove et al., 2000]. This could be because frontal and certain posterior regions form part of a network required for “effortful” tasks [Dehaene et al., 1998] in which case it could be difficult to establish differential (i.e. functionally specific) frontal lobe involvement. Alternatively, control processes that are relatively nonspecific with respect to stimulus type, which are predominantly frontal in origin, may have secondary effects on posterior areas that are related to the particular task at hand. Of course both possibilities could apply in different contexts. To look at these issues in the context of a specific task we adapted a previously reported paradigm [Dove et al., 2006] to investigate differential involvement of mid‐VLPFC and higher visual areas in control processes.
The mid‐VLPFC was chosen for the following reasons. This region ‐ spreading from the outer surface along the frontal operculum to become continuous with reported activations in the anterior insula, close to the coordinates −41, 20, 0 and 37, 20, 3, [Duncan and Owen, 2000] ‐ has shown activity in a broad range of tasks requiring control processes, from attention [Duncan and Owen, 2000], memory [Owen, 2000] and task switching [Dove et al., 2000] to less frequently studied tasks such as “choosing a dinner date” [Turk et al., 2004] and “lying” [Langleben et al., 2005], i.e. a range of tasks with different stimuli and contexts. To explain this variety it is necessary either to posit many separate and relatively specific processes in close anatomical proximity within the mid‐VLPFC or a more general process that is required in many tasks. One example of the second view is Petrides' hypothesis that the mid‐VLPFC may be involved in “various executive processes derived from the subject's plans and intended actions and operate on information available in the posterior association cortical areas where perceptual and basic short‐ and long‐term mnemonic processing occurs” ([Petrides, 1994], p 73). Interestingly, he also suggested that “in situations in which incoming or recalled stimuli automatically trigger stored representations, without any explicit conscious control, the mid‐VLPFC will not play a major role” ([Petrides, 1994], p 73). According to this hypothesis, passive viewing of stimuli should elicit little if any mid‐VLPFC activity when compared with a baseline condition. To our knowledge, this hypothesis has not been tested yet.
Unlike the mid‐VLPFC, higher visual areas such as the fusiform face area (FFA) and the parahippocampal place area (PPA) are clearly modulated by stimulus type. Previous literature has shown that FFA and PPA activity is elicited by faces [Kanwisher, 2000; Tong et al., 2000] and scenes [Epstein and Kanwisher, 1998], respectively, even during passive watching of these stimuli [Epstein and Kanwisher, 1998; Kanwisher et al., 1997]. On the other hand, activity in these regions has also been shown to be modulated by instructions to attend to or memorize such stimuli [Vuilleumier et al., 2001; Wojciulik et al., 1998]. This suggests that, in a manner similar to the mid‐VLPFC, activity in these areas may be modulated by “intention.” It is currently unclear whether intentional processing only affects PPA and FFA activity if stimuli are presented that would elicit activity in these areas during passive viewing.
Although it is unclear whether direct anatomical connections exist between the mid‐VLPFC, the PPA and the FFA in humans, in nonhuman primates it is known that visuospatial and object vision areas in the parietal and temporal lobes interact closely with the ventrolateral frontal cortex (Petrides, 1994). Specifically, VLPFC regions have direct long‐range projections to and from posterior visual association cortices, which includes both the FFA and the PPA [Petrides, 1994; Ungerleider et al., 1989]. A recent study by Petrides and Pandya [2002] suggests further that area 47/12, occupying the most ventral part of the ventrolateral prefrontal cortex of the macaque, has similar cytoarchitectonic characteristics to Brodmann area 47 in humans. It is likely, therefore, that Brodmann area 47 in the human has similar direct anatomical links with these inferotemporal regions, similar to those that are known to exist in the macaque. The mid‐VLPFC region of interest in the current study corresponds very closely with Brodmann area 47 in the human, as described by Petrides (1994).
To help clarify the roles of mid‐VLPFC and FFA, and PPA in conditions with high and low demands on intentional control, we utilized a previously reported paradigm that elicited robust activity in mid‐VLPFC [Dove et al., 2006]. Three different types of stimuli, faces, scenes, and colorful abstract paintings (i.e. not representational of faces or scenes) were presented under the following conditions (Fig. 1). In two conditions designed to have low requirement for controlled, intentional processing stimuli were presented with the simple instruction “look at this”. Within this, in the “low intention encoding condition” a picture was shown for the first time. In “low intention reviewing condition” a previously presented picture was shown again. The remaining two conditions were designed to have increased demands on control processes. Here, explicit task instructions (“remember this” and “have you seen this?”) were given. “Remember this” required volunteers to remember a new picture as best they could, and “have you seen this?” required them to indicate whether a given picture had been presented previously or not. Abstract paintings were included partly to replicate the specific effects reported in the previous study and partly as visual stimuli that had a similar salience level to the face and scene pictures, but were less likely to elicit increased activity in the FFA or PPA. We expected that the current experiment would allow us to observe differential involvement of mid‐VLPFC and higher visual areas depending on whether demands to carry out “intentional” processing were high (in the “high intention conditions”) or low (in the “low intention conditions”) and depending on the stimuli that were presented. By including encoding and recognition conditions it was possible to investigate whether similar patterns of activity occur in different types of tasks. More specifically, the following set of clear predictions could be tested:
-
1
Effect of intention. Regardless of stimulus or task type, mid‐VLPFC activity will always show more activity in conditions with high demands on intentional processing (the “high intention conditions”) compared with conditions with low demands on intentional processing (the “low intention conditions”). In contrast, activity in PPA and FFA will only show more activity in conditions with high demands on intentional processing compared with conditions with low demands on intentional processing, if these areas show activity during passive watching of the stimuli in the low intention conditions compared with baseline (blank screen).
-
2
Effect of stimulus type. There will not be an effect of stimulus type on the mid‐VLPFC, whereas PPA and FFA will show most activity whenever their preferred stimulus is presented (i.e. scenes and faces, respectively).
-
3
Activity elicited by passive viewing. There will be little, if any, mid‐VLPFC activity during passive viewing of stimuli (in the “low intention conditions”) compared with baseline (blank screen). On the other hand, passive viewing of their preferred stimulus will elicit activity in the PPA and FFA compared with baseline (blank screen).
Figure 1.
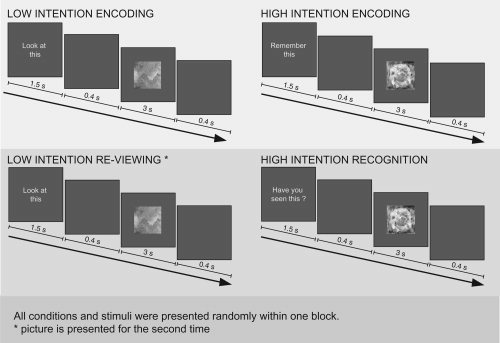
Experimental design. Abstract paintings, faces and scenes were presented shortly after an instruction. In the instruction, volunteers were asked to look at the stimuli (“low intention encoding” and “low intention reviewing”), to deliberately encode the stimuli (“high intention encoding”) or to decide whether a stimulus has been presented previously (“high intention recognition”). See Materials and Methods for details.
MATERIALS AND METHODS
Volunteers
Seventeen right‐handed healthy young adults participated in the imaging study. The data of three volunteers could not be analysed because of excessive movements during scanning. Following the scanning session each volunteer was asked about their performance of the task. One participant said that he had not tried to remember the stimuli in the “remember this” condition, because he thought that this was not necessary for subsequent recognition. His results were therefore excluded from further analysis. Therefore data of 13 volunteers were included in the analyses (nine male, four female, 19–39 years of age). The study received ethical approval from the Central Oxford Research Ethics Committee. Informed written consent was obtained from all volunteers in accordance with the Declaration of Helsinki.
Stimuli and Task Parameters
In the localizer scans, digitized grayscale photographs of faces, common objects, indoor scenes and two other object categories not relevant for this experiment were presented. The length of each scan was 6 min and 15 s. Each scan was divided into 20, 15‐s long picture epochs (four for each of the stimulus categories) interleaved with five epochs during which only a fixation point was presented. In each epoch, 20 photographs from the same category were presented for 300 ms each with an interstimulus interval of 450 ms. The task of the volunteer was to press a button whenever two identical stimuli appeared in a row (1‐back task). There were two such stimulus repetitions per epoch. Epoch order was counterbalanced as described previously [Epstein et al., 1999; Epstein and Kanwisher, 1998].
In the experimental task colorful abstract paintings, faces, and scenes were used as stimuli (Fig. 2). The abstract paintings were collected from web sites of amateur artists. Paintings were chosen that did not contain easily recognizable objects or faces. The face stimuli were color portrait photographs of men and women taken against a white background ([Martinez and Benavente, 1998], http://rvl1.ecn.purdue.edu/~aleix/aleix_face_DB.html). Scenes were color photographs of landscapes. Most scenes did not contain any prominent object (such as a single tree or an animal in the foreground). Since some stimuli may be more easily recognizable than others, stimuli were randomly assigned to the high intention and low intention conditions for each volunteer (for instance a particular stimulus could be presented during low intention encoding for one volunteer but during high intention encoding for another volunteer).
Figure 2.

(A) Beta values observed in the left and right ventrolateral prefrontal cortex, the fusiform face area and the parahippocampal place area during high intention and low intention encoding and recognition (re‐viewing) of paintings (p), faces (f), and scenes (s). The high intention conditions are presented in dark grey and the low intention conditions in light grey. Error bars show the standard error of the mean. (B) Examples of the stimuli used in the experiment (note that the stimuli were presented in color in the experiment).
Before every trial, an instruction was presented for 1.5 s, followed by a 0.4 s delay and the presentation of painting, face or scene for 3 s (Fig. 1). After a further interval of 0.4 s the next trial began. In the low intention encoding condition the instruction was “look at this.” This instruction indicated that the volunteer's task was just to look at the stimulus that followed (72 trials altogether, 24 of each stimulus type). In the high intention encoding condition volunteers received the instruction “remember this.” The task was to remember the stimulus that followed (72 trials altogether, 24 of each stimulus type). In the high intention recognition condition the instruction was “have you seen this?” Here, the volunteer was required to decide whether he or she had seen the stimulus before or not. In some trials, stimuli that had been shown in the high intention encoding condition were presented (72 trials altogether, 24 of each stimulus type). In this case, the correct answer was to indicate that the stimulus had been seen before. In other trials new stimuli were shown (48 trials altogether, 16 of each stimulus type). The correct answer was to indicate that the stimulus had not been seen before. Note that volunteers were only asked to recognize old stimuli that had been shown in the high intention encoding condition to discourage the volunteers from intentionally encoding the stimuli in the low intention encoding condition (contrary to the instruction). A further condition was constructed to control for the fact that stimuli in the high intention recognition condition were being viewed more than once and to examine, as far as is possible, recognition memory in the absence of a specific task instruction. Thus in the low intention reviewing condition, volunteers received the instruction “look at this.” Afterwards stimuli were presented that had previously been shown in the low intention encoding condition (72 trials altogether, 24 of each stimulus type). Additionally, non‐events were presented in which the screen was blank for 5.3 s (72 trials). This condition served to assess BOLD signal intensity during a short resting baseline. The experiment was 36 min long. It was presented in two blocks of 18 min each.
In all conditions, except the non‐events, responses were made. Volunteers were instructed to press two buttons simultaneously with their index and middle fingers during the low intention encoding, high intention encoding and low intention re‐viewing conditions. In the high intention recognition condition volunteers were instructed to only press one button with their index finger to indicate that they had seen the painting before. They were asked to press another button with the middle finger, if they decided that the painting had not been presented before.
All trials were presented randomly with the following constraints: Stimuli that were shown several times were first shown in the low intention or high intention encoding conditions and afterwards in the low intention re‐viewing and high intention recognition conditions, respectively. Stimuli that were repeated re‐occurred within the next 19–26 trials. There were no more than three trials with the same instruction in a row.
Image Acquisition and Analysis
Scanning was carried out at the Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB), Oxford, UK on a 3 Tesla MRI system driven by a Varian Unity Inova console and equipped with an Oxford Magnet Technology magnet, a Siemens body gradient coil and a bird‐cage radio‐frequency head coil built by Enzo Barberi (Robarts Research Institute, Canada).
An In Focus LP1000 projection system (Unicol Engineering, Oxford, UK) was used to project the stimuli onto a white screen located at the foot end of the scanner bed. Subjects could view this screen by wearing a pair of prism spectacles (Wardray‐Premise Engineering, Surrey, UK) during scanning. Subjects' responses were made using two specified buttons (“left” and “right”) on a four‐button response box held in the right hand. Foam padding was utilised to reduce participant movements in the scanner within the MRI head coil.
For functional data, an echo planar imaging (EPI) pulse sequence was implemented to acquire T2*‐weighted image volumes with blood oxygen level dependent (BOLD) contrast. Each volume consisted of 21 slices with a voxel size of 3 × 4 × 6 mm3 (TR = 3 s, TE = 30 ms, flip angle of 90, FOV: 256 × 256, matrix size: 64 × 64). A map of the magnetic field was acquired and then used to correct for distortion to the EPIs resulting from inhomogeneities in the field [Cusack and Papadakis, 2002; Jezzard and Balaban, 1995]. This procedure has been shown to improve anatomical localization and increase the power of group studies by achieving better spatial registration between the data from different subjects [Cusack et al., 2003]. The field map was always acquired directly before or after the acquisition of the functional data to ensure that the head position of the volunteer was maximally comparable. A high resolution T1 structural scan was acquired (voxel size: 1 × 1 × 3 mm3) either in the same scanning session or on a different day.
SPM99 software was used for preprocessing and statistical analyses (http://www.fil.ion.ucl.ac.uk/spm). The first five volumes of the EPIs were discarded due to T1 saturation effects. After realignment of the data slice timing correction was carried out. The field map information was used to correct for distortions in the phase‐encode directions of the EPIs using an SPM [Cusack and Papadakis, 2002; Cusack et al., 2003]. The EPIs were normalized by using a masked EPI to EPI template normalization [Brett et al., 2001] and smoothed with an 8 mm Gaussian kernel.
A region of interest (ROI) approach was used to analyse the data. To define the ROI in the PPA and FFA, we followed the approach developed by Kanwisher and others [Kanwisher et al., 1997] and Epstein and Kanwisher [Epstein and Kanwisher, 1998]. ROI were defined individually for each volunteer using data from the localizer scans, which were analyzed using a general linear model as implemented in SPM99. For each scan, the response at each voxel was modeled using an 11‐regressor model in which the first five regressors modeled the response to each of the five stimulus types as boxcar function convolved with a canonical hemodynamic response function, and the next six regressors modeled motion‐specific effects. Data was temporally filtered before analysis to remove low‐frequency confounds. Linear contrasts were used to identify clusters of contiguous voxels in the occipital temporal region that responded more to (1) scenes compared with objects (candidate PPA voxels) and (2) faces compared with objects (candidate FFA voxels). As we wished to distinguish between the functional response in regions that were spatially very proximate, we used a particularly stringent definition of the PPA and FFA, which we defined for each volunteer by either choosing the voxel with peak activity or, if there were several peak voxels that were similarly active, the voxel that was closest to the mean MNI coordinates of all volunteers in that region. Note that because of the spatial smoothing of the functional data of the main experiment, each peak voxel translates into an 8‐mm‐wide FWHM Gaussian ROI.
For the mid‐VLPFC, a different approach had to be taken because no established localizer task exists to define participant‐specific ROIs in these areas. To this end, ROIs were defined using data from a previous study [Dove et al., 2006]. A random effects group analysis was carried out contrasting conditions in which participants were asked to intentionally encode or recognize abstract paintings with conditions in which the viewing of abstract paintings was passive – i.e. the identical manipulation to that used in the current study albeit with only abstract painting stimuli. The results showed increased mid‐VLPFC activation in the high‐intention condition. ROIs for the current study were generated by drawing 7 mm spheres around the peaks using the MARSBAR tool [Brett et al., 2002]. These spheres were somewhat smaller than the whole activation cluster, to ensure that only voxels with relatively high t‐values would be chosen in the current experiment. Note that we choose a sphere here rather than a single voxel because the ROI could not be defined for each volunteer individually, as in the FFA and PPA. Choosing a larger ROI makes it more likely that the ROI of an individual volunteer contains voxels that show activity in the experimental conditions. The MNI coordinates of the peak activation were 36, 22, −4 in the right mid‐VLPFC and −36, 16, −4 in the left mid‐VLPFC (Fig. 3).
Figure 3.
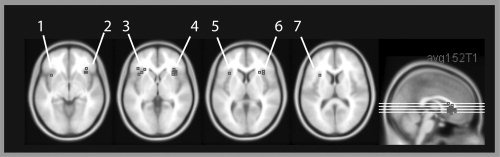
Peak activity in the mid‐ventrolateral prefrontal cortex or anatomically close to the mid‐ventrolateral prefrontal cortex in studies with different cognitive demands, overlaid onto a normalized and averaged structural image in MNI space (MNI 152 image from the SPM distribution). The figure was created using MRIcro by Prof. Chris Rorden. References 1–7 refer to Table I.
A general linear model was applied to the functional data of each volunteer [Friston et al., 1995]. The model included covariates for sustained neuronal responses elicited during high intention encoding of paintings, faces and scenes. Similarly there were separate covariates for the three stimulus types in the conditions “high intention recognition of previously presented stimuli,” “high intention recognition of new stimuli,” “low intention encoding” and “low intention re‐viewing of stimuli shown for the second time”. The onsets of events were at the time the instruction was shown. The duration of the events was the whole trial duration (5.3 s). A boxcar function convolved with a canonical hemodynamic response was used to model these events. Additionally six motion parameters derived during realignment were used to correct for residual movement artefacts. The non‐event condition was modeled implicitly. This had the advantage that the signal intensity in the nonevent condition was set to “0”. A high‐pass filter with a cut‐off of 250 s was employed to correct for low frequency drifts in BOLD‐signal. No global scaling was used. Parameter estimates for each covariate were calculated from the least mean squares fit of the model to the data.
The beta values of all covariates were extracted for each ROI for each volunteer using the MARSBAR tool [Brett et al., 2002]. Only the beta values of the covariates for all high intention and low intention encoding conditions, the covariates for high intention recognition of previously seen pictures and the covariates for low intention re‐viewing were analysed further. Data were entered into SPSS.
RESULTS
Behavioral Results
In the task participants viewed abstract pictures, faces or scenes with either the intent to remember them or to simply view them. They also saw again stimuli from both of these conditions but were only asked to make an explicit judgement concerning their recognition of stimuli shown in the high‐intention (“remember this”) conditon. In this respect, the accuracy of their responses were transformed into d'‐measures for each volunteer [Macmillan and Creelman, 1991]. This gives a measure of performance that is independent of response bias. Data were then averaged over participants. The resulting values in the high intention recognition condition were 2.73 (paintings), 2.86 (faces) and 2.97 (scenes). One‐tailed t‐tests testing against “0” showed that performance was significantly greater than chance in all three conditions (paintings: t(12) = 12.06, P < 0.0001; faces: t(12) = 15.72, P < 0.0001; scenes: t(12) = 13.09, P < 0.0001). A repeated measures analysis of variance was conducted with the factor “stimulus material.” This test showed that there was no significant effect of material type (F(2,24) = 1.18, P = 0.32).
By definition no behavioral data from the low intention memory conditions were available from the fMRI study. However, an initial pilot experiment using abstract paintings only [Dove et al., 2006] showed that, as predicted, high intention encoding leads to significantly better subsequent recognition than low intention encoding. Recognition for pictures was however well above chance even if the stimuli were presented once in the low intention encoding condition suggesting—as might be expected—that some encoding was taking place.
FMRI Results
Localizer task
PPA could be identified in 13 out of 13 volunteers in the right hemisphere and FFA could be identified in 11 out of 13 volunteers in the right hemisphere.1 The mean MNI coordinates in the right PPA were 30, −46, −10 and in the right FFA 42, −52, −20. In addition, the PPA could be identified in the left hemisphere in 13 out of 13 volunteers. The mean coordinates in the left PPA were −26, −49, −10. Note that our finding that PPA could be identified bilaterally and FFA in the right hemisphere only is consistent with the literature [Kanwisher et al., 2001].
Main experiment
For each ROI an ANOVA was computed with the factors “intention,” “stimulus type,” and “task.” The significance level was 0.05. Furthermore we examined whether the β‐values in the six low intention conditions differed significantly from “0”. Since the baseline level of activity was set to “0”, these tests could establish whether there was any activity in a given low intention condition compared with the baseline task (looking at a blank screen). Because 6 tests were carried out, the corrected significance level of 0.008 was used (Bonferroni correction) for all ROIs except the left and right mid‐VLPFC. In this area we expected no significant activity in the low intention conditions compared with baseline, and therefore wished to use the more liberal significance level of 0.05.
Left mid‐VLPFC
As can be seen in Figure 2, signal intensity in the left mid‐VLPFC was higher when participants viewed stimuli with the intention of remembering and with the intention of recognizing compared with parallel conditions in which they were asked to passively view the stimuli. Accordingly, the main effect of the factor “intention” reached significance (F(1,12) = 35.32, P < 0.05) in the ANOVA. There were no other significant main effects or interactions. Notably, therefore, activity in this region was not sensitive to stimulus type. In line with our predictions, there was no significant activity in the low intention conditions compared with baseline at the 0.05 significance level (t(12) < 1.608, P > 0.05) with the exception of the low‐intention re‐viewing of scenes (t(12) = 2.721, P < 0.05).
A t‐test against “0” for all low‐intention conditions combined was not significant at the 0.05 significance level (t(12) = 1.79, P = 0.099 (two‐tailed)).
Right mid‐VLPFC
The results in the right mid‐VLPFC were very similar (Fig. 2). Again signal intensity was higher in the high intention condition compared with the low intention conditions. In the ANOVA there was a main effect of intention (F(1,12) = 27.92, P < 0.05). Again activity in this region was not sensitive to stimulus type. There was no activity in any of the six low intention conditions compared with baseline (t(12) < 1.627, P > 0.05).
A t‐test against “0” for all low‐intention conditions combined was not significant (t(12) = 0.55, P = 0.59 (two‐tailed)).
Right FFA
As expected, the main effect of stimulus type in the ANOVA was significant (F(2,20) = 41.36; P < 0.05, Fig. 2). Faces elicited more activity in this area than paintings and scenes. Two other main effects reached significance: intention (F(1,10) = 7.35, P < 0.05) and task (F(1,10) = 10.88; P < 0.05): There was more activity in the FFA in the high intention conditions compared with the low intention conditions, and more activity in the encoding conditions compared with the recognition/re‐viewing conditions. A significant interaction of intention and task was observed (F(1,10) = 7.36, P < 0.05). To explain this interaction, data of the stimulus type conditions were combined. Two post‐hoc t‐tests were carried out, Bonferroni corrected for multiple comparisons at 0.025 (two‐tailed). High intention encoding elicited more activity than low intention encoding (t(10) = 2.87; P < 0.025), whereas activity during high intention recognition did not differ from low intention re‐viewing (t(10) = 1.90; P > 0.025).
There was activity in each of the six low intention conditions compared with looking at a blank screen (t(10) > 3.280; P < 0.0083), Fig. 2). Thus, FFA showed activity during low intention encoding and re‐viewing of faces, as predicted, but also during low intention encoding and re‐viewing of paintings and scenes.
Left PPA
Figure 2 shows that, as predicted, signal intensity in the left PPA was highest whenever scenes were presented. Thus, there was a main effect of stimulus type (F(2,24) = 39.56, P < 0.05) in the left PPA. In addition the factor “task” reached significance (F(1,12) = 7.31, P < 0.05) – activity during encoding was on the whole higher than during recognition/re‐viewing. There were two interactions: intention and stimulus type (F(2,24) = 7.94, P < 0.05) and stimulus type and task (F(2,24) = 5.85, P < 0.05). Three post‐hoc t‐tests were carried out to explain the intention and stimulus type interaction. Data from the high intention encoding and recognition conditions and the low intention encoding and re‐viewing conditions were combined for each stimulus type and task condition. Only when scenes were shown, signal intensity in the high intention conditions (high intention encoding and recognition combined) was higher than in the low intention conditions (low intention encoding and re‐viewing combined), as predicted (t(12) = 4.09; P < 0.017 one‐tailed). Left PPA signal intensities during high intention and low intention processing of faces (t(12) = 1.01, P > 0.017) and high intention and low intention processing of paintings (t(12) = 0.49; P > 0.017) did not differ significantly. Three further post‐hoc tests were carried out to explain the stimulus type and task interaction. Data of high and low intention encoding and high and low re‐viewing/recognition were combined for each stimulus type and task condition. Activity during encoding and recognition of scenes differed significantly from each other (t(12) = 3.39; P < 0.017); but this was not the case for paintings (t(12) = 2.26; P > 0.017) and faces (t(12) = 0.68; P > 0.017).
All low intention conditions in which scenes were presented differed significantly from baseline (t(12) > 4.469; P < 0.008 (one‐tailed), Fig. 2), as predicted. None of the other low intention conditions reached significance in the one‐sample t‐tests (t(12) < 1.621; P > 0.008).
Right PPA
The results of the right PPA were very similar to the results in the left PPA (Fig. 2). There was a main effect of stimulus type (F(2,24) = 65.28; P < 0.05) and task (F(1,12) = 9.75; P < 0.05), an intention * stimulus type interaction (F(2,24) = 7.30, P < 0.05) and a task * stimulus type interaction (F(2,24) = 9.26; P < 0.05). The intention * stimulus type interaction can be explained by a significant difference in activity between high intention and low intention processing of scenes, as predicted (t(12) = 3.22, P < 0.017 (one‐tailed)), and no difference between high and low intention processing for faces (t(12) = 0.28; P > 0.017) or paintings (t(12) = 0.78; P > 0.017). Regarding the task * stimulus interaction, it was again the case that signal intensity in the right PPA was significantly higher during encoding of scenes compared with recognition of scenes (t(12) = 4.33; P < 0.017). There was no such effect for faces (t(12) = 0.90; P > 0.017) and paintings (t(12) = 1.76; P > 0.017).
As predicted, low intention encoding and re‐viewing of scenes elicited significant activity compared with baseline (t(12) > 5.79; P < 0.008 (one‐sided)). Furthermore there was activity during low intention encoding of paintings compared to baseline (t(12) = 3.64; P < 0.008). None of the other conditions reached significance in the one‐sample t‐test (t(12) < 2.68; P > 0.008).
DISCUSSION
Summary
In a region of interest analysis, the pattern of activity in the mid‐VLPFC region was clearly distinguishable from the patterns of activity in the analysed posterior brain regions. Firstly, this brain area was only significantly active in the high intention conditions compared with the low intention conditions, whereas the PPA and FFA showed significant activity in low intention conditions in which volunteers were simply asked to “just look,” compared with baseline. Secondly, the task instruction was the only factor that modulated activity in this area, whereas other factors such as stimulus type, task type or stimulus repetition did not show any effect.
In contrast to the pattern of activity in the mid‐VLPFC, FFA and PPA activity was not only modulated by the task instruction but also by stimulus and task type. FFA and PPA showed most activity whenever faces and scenes were presented respectively. Both FFA and PPA showed less activity when stimuli were shown during recognition compared with encoding. FFA and PPA activity was only modulated in conditions requiring intentional control if the stimuli presented were of a type that elicited activity in these regions during passive viewing.
Mid‐VLPFC Activity
In functional imaging, it is statistically difficult to demonstrate convincingly that an area is not active and, perhaps as a result of this, research on prefrontal areas has focussed on conditions that activate these regions rather than those that do not. However, the conditions under which a region is not active are potentially as informative in considering its function(s) as those in which it is. Therefore we wished to investigate whether there is mid‐VLPFC activity in the low intention conditions compared with baseline. In a whole brain analysis there may be insufficient power to detect real activity in an area, due to corrected thresholds for multiple comparisons. Thus it can always be argued that there may be sub‐threshold activity. To maximize the possible sensitivity of this study in this respect, we employed an ROI approach.
The results suggest that the mid‐VLPFC was no more engaged by the presentation of faces, scenes or abstract paintings than simply looking at a blank screen ‐ despite other regions being highly activated by these stimuli (see below) ‐ when there was no specific instruction to do something with those stimuli. This is consistent with the argument that this mid‐VLPFC region is not involved in situations in which stimuli automatically trigger stored representations, without the necessity for explicit conscious control processes ([Petrides, 1994, p 73). A similar point has been made more recently by Bunge [2004]. According to this framework VLPFC is involved in processing rules that guide our behavior and is not needed whenever well‐learned rules can be retrieved automatically ([Bunge, 2004], p 575).
The mid‐VLPFC showed higher signal intensity in the high intention conditions compared with the low intention conditions. Whilst this finding could be due to differences in response selection during recognition (volunteers have to select a response in the high intention recognition condition, but press two buttons during the low intention re‐viewing condition), this hypothesis cannot explain the results during encoding. Therefore, we suggest that this pattern of activity reflects differences in cognitive control processes between the conditions. In addition to the work of Petrides [1994] and others, this result ties in well with the view that the mid‐VLPFC is activated under a wide variety of conditions that require control processes (Fig. 3 and Table I; [Braver et al., 2003; Bunge et al., 2001; Cadoret et al., 2001; Cools et al., 2002; Dove et al., 2000, 2006; Duncan and Owen, 2000; Houde et al., 2000; Jenkins et al., 1994; Kostopoulos and Petrides, 2003; Langleben et al., 2005; Turk et al., 2004]). Note that the coordinates reported in these studies are anatomically very close to the centre of our regions of interest in the mid‐VLPFC (Fig. 3 and Table I). As pointed out in the introduction, “mid‐VLPFC” activity as defined in this paper spreads from the outer surface of the frontal operculum to become continuous with reported activity in the anterior insula [Duncan and Owen, 2000]. One of our ROI, in the right mid‐VLPFC is more clearly located in the frontal operculum, whereas the other ROI is somewhat closer to the anterior insula. Both ROIs, however, show the same pattern of results. One account is that activation that may appear to be in the anterior insula could in fact be slightly misplaced frontal operculum activity. This would conform to “mid‐VLPFC,” according to Duncan and Owen's definition [Duncan and Owen, 2000]. An alternative account is that the activity stems from the anterior insula, which is showing the same modulatory pattern as the “mid‐VLPFC.” It would be interesting to clarify this question in future studies.
Table I.
Studies with peak activity in the mid‐VLPFC or anatomically close to the mid‐VLPFC. The table includes a task description, the coordinates in Talairach and Tournoux (1988) or MNI space and a reference number referring to Figure 3
| Task | Study | Coordinates | References |
|---|---|---|---|
| Intention | |||
| High intention encoding and retrieval | Based on Dove et al. 2006, centre of ROIs in current study | −36, 16, −4 (MNI) | 1 |
| High intention encoding and retrieval | 36, 22, −4 (MNI) | 2 | |
| Encoding | |||
| Working memory load | Bunge et al. 2001 | 36, 30, −4 (TAL) | 2 |
| Working memory load | Bunge et al. 2001 | −34, 20, 4 (TAL) | 5 |
| Working memory load | Bunge et al. 2001 | 36, 24, 4 (TAL) | 6 |
| Array of two spatial positions followed by recognition probe | Smith et al 1995a | 32, 18, −1 (TAL) | 4 |
| Retrieval | |||
| Retrieval | Cadoret et al. 2001 | 34, 28, 1 (TAL) | 4 |
| Retrieval phase | Kostopoulos and Petrides 2003 | 32, 22, 2 (TAL) | 4 |
| Reasoning | |||
| Logical reasoning/overcome perceptual matching bias | Houde et al. 2000 | −30, 16, 12 (MNI) | 7 |
| Decision making/personal choice | |||
| Choosing dinner date | Turk et al. 2004 | −32, 23, −1 (TAL) | 3 |
| Choosing dinner date | Turk et al. 2004 | 36, 23, −1 (TAL) | 4 |
| Lying | |||
| Concealing the identity of a card | Langleben et al. 2005 | 34, 22, −8 (TAL) | 2 |
| Concealing the identity of a card | Langleben et al. 2005 | 37, 19, −1 (TAL) | 4 |
| Novelty | |||
| Eight‐movement finger sequence | Jenkins et al. 1994, a | 36, 20, 4 (TAL) | 6 |
| Response conflict | |||
| Respond to letter with its own or different letter name | Taylor et al. 1994a | 37, 17, −2 (TAL) | 4 |
| Taylor et al. 1994a | −39, 17, 2 (TAL) | 3 | |
| Task switching | |||
| Task switching/response reversal | Dove et al. 2000 | 28, 23, 8 (TAL) | 6 |
| Final reversal error | Cools et al. 2002 | 38, 24, −2 (MNI) | 4 |
| Task switching | Braver et al. 2003 | −40, 30, 0 (TAL) | 3 |
Studies cited after Duncan and Owen (2000).
In contrast to findings in the FFA and PPA, mid‐VLPFC activity was not modulated by stimulus type or task. This observation is consistent with findings in the literature (see reviews by [Bunge, 2004; Duncan and Owen, 2000; Owen, 2000]) and with the view that the VLPFC subserves rather general control functions that may be needed in quite different task contexts [Bunge, 2004; Duncan and Owen, 2000; Petrides, 1994; Thompson‐Schill et al., 2005].
In all conditions in which the mid‐VLPFC was active – the high intention conditions ‐ there was also a modulation of activity in the FFA or PPA, if the stimulus was of a type to which that region was particularly sensitive. The act of paying particular attention or attempting to encode appears therefore to be reflected in ‐ or possibly partly enacted by ‐ increased activity in regions tuned to those stimuli even under “passive” viewing conditions. This is consistent with the view that the VLPFC may modulate activity in posterior regions [Petrides, 1994; Bunge, 2004]. Whilst it is difficult to specify these functions any more clearly at present, it seems likely that the mid‐VLPFC acts by biasing or “tuning” attentional processing between competing representations in modality‐specific posterior regions in order to maintain their relevance to current behavioral goals [Dove et al., 2006]. Activity in posterior regions may either be enhanced in conditions requiring intentional control, or, as suggested by Houde and colleagues [Houde et al., 2000], it may be necessary to inhibit perceptual biases originating in posterior cortex in order to activate intentional control processes.
FFA and PPA Activity
As expected, FFA showed most activity when faces were presented [Kanwisher et al., 1997] and PPA when scenes were shown [Epstein et al., 1999]. This was the case when participants had a particular activity to perform with the stimulus and, as would be predicted from previous work, under more passive viewing conditions [Epstein and Kanwisher, 1998; Kanwisher et al., 1997].
PPA showed a significant increase in signal intensity compared with baseline for scenes (and during low intention encoding of paintings compared with baseline in the right PPA) but not for faces, whereas the FFA exhibited above‐baseline response for all stimuli. PPA activity has mostly been reported for scenes or houses, with weaker but reliable response for objects [Epstein, 2005]. The fact that PPA response to abstract paintings was lower than its response to scenes is interesting, as it supports the hypothesis, proposed by Epstein and Kanwisher [1998], that the PPA processes information about the geometric layout of local space. Although the abstract paintings are as complex and interesting as the scenes and cover the same section of the visual field, they do not depict realistic three‐dimensional environments and thus would be predicted by the spatial layout hypothesis to engage the PPA less strongly than the scenes, as we observed. The FFA, in line with previous findings [Gauthier et al., 1999; Tarr and Gauthier, 2000; Tong et al., 2000; Xu, 2005] while responding most strongly to faces, also responded more to other nonface stimuli than to the blank‐screen baseline.
FFA and PPA activity was also modulated by intentional task instructions, suggesting that activity in these areas can reflect both the “executive” demands of a condition as well as the stimulus characteristics. However, in contrast to patterns of activity in the mid‐VLPFC, it was not the case that all high intention conditions elicited more activity than low intention conditions. As discussed, FFA and PPA activity was only modulated by intention, if stimuli the stimuli were of a type “favored” by that region. In the PPA, this modulation was only observed for scenes. The FFA, which was activated by all stimuli during the passive condition relative to baseline, showed increased activity in the high‐intention conditions for all stimulus categories. Several studies in the memory literature suggest that PPA and FFA activity may be modulated by executive demands of the task [Druzgal and D'Esposito, 2003; Jha et al., 2004; Ranganath et al., 2004]. For instance, Ranganath and others [Ranganath et al., 2004] showed that FFA and PPA exhibited greater encoding‐related activity when their preferred stimulus was relevant to the recognition task. Other studies have demonstrated increased FFA and PPA activity when attention is directed towards their preferred stimuli [Vuilleumier et al., 2001; Wojciulik et al., 1998]. Taken together these findings suggest that, similar to findings in the mid‐VLPFC, activity in posterior regions such as the FFA and PPA can be modulated by control processes required in different cognitive domains, rather than by specific “attentional” or “mnemonic” processes.
Both FFA and PPA showed less activity when stimuli where shown during recognition compared with encoding (main effect of “task”). These findings could be explained by stimulus repetition effects. Such effects have been observed in the PPA [Epstein et al., 1999, 2005; Menon et al., 2000] and the FFA [Eger et al., 2004; Ishai et al., 2004; Soon et al., 2003; Vuilleumier et al., 2003], although the opposite finding has also been observed [Henson, 2003]. Interestingly, these repetition effects were specific to scenes in the PPA, but were not specific to faces in the FFA. The specificity of the repetition effect in the PPA contrasts with an earlier study by Avidan and colleagues [Avidan et al., 2002], in which repetition reductions were found for both houses and faces in this region. Differences between the experimental paradigms may explain these discrepant results; for example, the earlier study measured signal reductions caused by presentation of a stimulus twelve times within a block, while here we measured signal reductions caused by presentation of a stimulus twice in different trials separated by intervening items.
In the current study the FFA and PPA were localized using a “1‐back” task. This could have led to the selection of regions within the FFA and PPA involved in memory as well as perceptual processes ‐ although there are other findings which suggest that the 1‐back technique produces similar results to passive viewing [Kanwisher et al., 2001].
CONCLUSION
The mid‐VLPFC was differentially activated under conditions during which volunteers were asked to have a particular intention towards the stimuli. This result is consistent with the idea that certain areas within the prefrontal cortex are involved in control processes, i.e. processes that enable us to carry out actions intentionally, with deliberate conscious control [Duncan and Owen, 2000; Norman and Shallice, 1986].
Novel aspects of our approach include the testing of specific hypotheses about a specific area within the mid‐VLPFC [Duncan and Owen, 2000], instead of focusing on the prefrontal cortex or VLPFC as a whole, as in many previous studies. Furthermore we aimed to differentiate patterns of activity in the mid‐VLPFC and in higher visual areas.
The results showed that not only the mid‐VLPFC, but in fact all investigated areas were differentially activated under conditions during which volunteers were asked to have an intention towards the stimulus. Thus all areas were sometimes involved in or at least activated by intentional instructions‐ or, more broadly speaking, some control process. The results are certainly consistent with the idea of modulation of activity in posterior areas by the mid‐VLPFC [Petrides, 1994] although other routes leading to coactivation are possible. Another finding is consistent with this idea: FFA and PPA activity was only modulated in conditions requiring intentional control, if stimuli were presented that elicited activity in these regions during passive viewing compared with baseline. Perhaps intentional processing leads to a modulation of activity in those posterior regions that process a stimulus during passive viewing.
Furthermore the results suggest that the mid‐VLPFC was the area that was the least influenced by the actual stimuli and most clearly influenced by the task goal or by what was intended to be done with the stimulus. In fact, just presenting stimuli under passive viewing conditions, in the absence of a particular intention what to do with them, elicited as much activity as looking at a blank screen for the same amount of time. In contrast to the mid‐VLPFC, FFA, and PPA were active during passive viewing conditions compared with baseline, if the stimulus was the right kind for that area.
Acknowledgements
The authors thank the staff at the Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB), Oxford, UK, for their assistance with the present study. We thank all the volunteers who took part. We thank Matthew Brett for assistance with the data analysis and Tom Nielsen and Rhodri Cusack for programming the task. We thank Simon Lewis for helpful discussions on this work.
Footnotes
The MNI‐coordinates of the ROIs of each volunteer were as follows: 38 −46 −24, 38 −52 −20, 40 −48 −18, 40 −50 −18, 40 −52 −16, 40 −54 −24, 40 −62 −16, 44 −62 −22, 46 −50 −16, 46 −52 −32, 46 −54 −24 in the right FFA, 24 −52 −10, 24 −54 −10, 24 −54 −8, 26 −44 −8, 28 −48 −6, 30 −44 −10, 30 −46 −8, 30 −50 −10, 32 −42 −10, 32 −46 −8, 32 −48 −12, 34 −48 −10, 38 −44 −12 in the right PPA and −20 −52 −14, −22 −46 −12, −22 −48 −6, −24 −46 −8, −24 −50 −10, −24 −52 −10, −26 −44 −12, −26 −46 −6, −28 −48 −10, −28 −48 −6, −28 −60 −8, −32 −52 −14, −34 −48 −8 in the left PPA.
REFERENCES
- Avidan G,Hasson U,Hendler T,Zohary E,Malach R ( 2002): Analysis of neural selectivity underlying low fMRI signals. Curr Biol 12: 964–972. [DOI] [PubMed] [Google Scholar]
- Baddeley A,Della Sala S ( 1996): Working memory and executive control. Philos Trans R Soc Lond B 351: 1397–1404. [DOI] [PubMed] [Google Scholar]
- Braver TS,Reynolds JR,Donaldson DI ( 2003): Neural mechanisms of transient and sustained cognitive control during task switching. Neuron 39: 713–726. [DOI] [PubMed] [Google Scholar]
- Brett M,Leff AP,Rorden C,Ashburner J ( 2001): Spatial normalization of brain images with focal lesions using cost function masking. Neuroimage 14: 486–500. [DOI] [PubMed] [Google Scholar]
- Brett M,Anton J‐L,Valabregue R,Poline J‐B ( 2002): Region of interest analysis using an SPM toolbox. Presented at the Eighth International Conference on Functional Mapping of the Human Brain,Sendai, Japan. Available on CD‐ROM in Neuroimage.
- Bunge SA ( 2004): How we use rules to select actions: A review of evidence from cognitive neuroscience. Cogn Affect Behav Neurosci 4: 564–579. [DOI] [PubMed] [Google Scholar]
- Bunge SA,Ochsner KN,Desmond JE,Glover GH,Gabrieli JDE ( 2001): Prefrontal regions involved in keeping information in and out of mind. Brain 124: 2074–2086. [DOI] [PubMed] [Google Scholar]
- Cadoret G,Pike B,Petrides M ( 2001): Selective activation of the ventrolateral prefrontal cortex in the human brain during active retrieval processing. Eur J Neurosci 14: 1164–1170. [DOI] [PubMed] [Google Scholar]
- Cools R,Clark L,Owen AM,Robbins TW ( 2002): Defining the neural mechanisms of probabilistic reversal learning using event‐related functional magnetic resonance imaging. J Neurosci 22: 4563–4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusack R,Papadakis N ( 2002): New robust 3D phase unwrapping algorithms: Application to magnetic field mapping and undistorting echo‐planar images. Neuroimage 16: 754–764. [DOI] [PubMed] [Google Scholar]
- Cusack R,Brett M,Osswald K ( 2003): An evaluation of the use of magnetic field maps to undistort echo‐planar images. Neuroimage 18: 127–142. [DOI] [PubMed] [Google Scholar]
- Dehaene S,Kerszberg M,Changeux J‐P ( 1998): A neuronal model of a global workspace in effortful cognitive tasks. Proc Nat Acad Sci USA 95: 14529–14534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove A,Pollmann S,Schubert T,Wiggins CJ,von Cramon DY ( 2000): Prefrontal cortex activation in task switching: An event‐related fMRI study. Cogn Brain Res 9: 103–109. [DOI] [PubMed] [Google Scholar]
- Dove A,Brett M,Cusack R,Owen AM ( 2006): Dissociable contributions of the mid‐ventrolateral frontal cortex and the medial temporal lobe system to human memory. Neuroimage 31: 1790–1801. [DOI] [PubMed] [Google Scholar]
- Druzgal TJ,D'Esposito M ( 2003): Dissecting contributions of prefrontal cortex and fusiform face area to face working memory. J Cogn Neurosci 15: 771–784. [DOI] [PubMed] [Google Scholar]
- Duncan J,Owen AM ( 2000): Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci 23: 475–483. [DOI] [PubMed] [Google Scholar]
- Eger E,Schyns PG,Kleinschmidt A ( 2004): Scale invariant adaptation in fusiform face‐responsive regions. Neuroimage 22: 232–242. [DOI] [PubMed] [Google Scholar]
- Epstein RA ( 2005): The cortical basis of visual scene processing. Vis Cogn 12: 954–978. [Google Scholar]
- Epstein R,Kanwisher N ( 1998): A cortical representation of the local visual environment. Nature 392: 598–601. [DOI] [PubMed] [Google Scholar]
- Epstein R,Harris A,Stanley D,Kanwisher N ( 1999): The parahippocampal place area: Recognition, navigation, or encoding? Neuron 23: 115–125. [DOI] [PubMed] [Google Scholar]
- Epstein RA,Higgins JS,Thompson‐Schill SL ( 2005): Learning places from views: Variation in scene processing as a function of experience and navigational ability. J Cogn Neurosci 17: 73–83. [DOI] [PubMed] [Google Scholar]
- Flavell J ( 1976): Metacognitive aspects of problem solving In: Resnick L,editor. The Nature of Intelligence. Hillsdale, NJ: Erlbaum Assoc; pp 231–235. [Google Scholar]
- Friston KJ,Holmes AP,Worsley KJ,Poline J‐P,Frith CD,Frackowiak RSJ ( 1995): Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp 2: 189–210. [Google Scholar]
- Gauthier I,Tarr MJ,Anderson AW,Skudlarski P,Gore JC ( 1999): Activation of the middle fusiform ‘face area’ increases with expertise in recognizing novel objects. Nat Neurosci 2: 568–573. [DOI] [PubMed] [Google Scholar]
- Henson RNA ( 2003): Neuroimaging studies of priming. Prog Neurobiol 70: 53–81. [DOI] [PubMed] [Google Scholar]
- Houde O,Zago L,Mellet E,Moutier S,Pineau A,Mazoyer B,Tzourio‐Mazoyer N ( 2000): Shifting from the perceptual brain to the logical brain: The neural impact of cognitive inhibition training. J Cogn Neurosci 12: 721–728. [DOI] [PubMed] [Google Scholar]
- Ishai A,Pessoa L,Bikle PC,Ungerleider LG ( 2004): Repetition suppression of faces is modulated by emotion. Proc Natl Acad Sci USA 101; 9827–9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins IH,Brooks DJ,Nixon PD,Frackowiak RS,Passingham RE ( 1994): Motor sequence learning: A study with positron emission tomography. J Neurosci 14: 3775–3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezzard P,Balaban RS ( 1995): Correction for geometric distortion in echo planar images from B0 field variations. Magn Reson Med 34: 65–73. [DOI] [PubMed] [Google Scholar]
- Jha AP,Fabian SA,Aguirre GK ( 2004): The role of prefrontal cortex in resolving distractor interference. Cogn Affect Behav Neurosci 4: 517–527. [DOI] [PubMed] [Google Scholar]
- Kanwisher N ( 2000): Domain specificity in face perception. Nat Neurosci 3: 759–763. [DOI] [PubMed] [Google Scholar]
- Kanwisher N,McDermott J,Chun MM ( 1997): The fusiform face area: A module in human extrastriate cortex specialized for face perception. J Neurosci 17: 4302–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N,Downing P,Epstein R,Kourtzi Z ( 2001): Functional neuroimaging of visual recognition In: Cabeza R,Kingstone A, editors. Handbook of Functional Neuroimaging of Cognition. Cambridge: MIT Press; pp 109–151. [Google Scholar]
- Kostopoulos P,Petrides M ( 2003): The mid‐ventrolateral prefrontal cortex: Insights into its role in memory retrieval. Eur J Neurosci 17: 1489–1497. [DOI] [PubMed] [Google Scholar]
- Langleben DD,Loughead JW,Bilker WB,Ruparel K,Childress AR,Busch SI,Gur RC ( 2005): Telling truth from lie in individual subjects with fast event‐related fMRI. Hum Brain Mapp 26: 262–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmillan NA,Creelman CD. 1991. Detection Theory: A User's Guide. New York: Cambridge University Press. [Google Scholar]
- Martinez AM,Benavente R. 1998. The AR face database. CVC Technical Report No. 24. Barcelona, Spain: Computer Vision Center.
- Menon V,White CD,Eliez S,Glover GH,Reiss AL ( 2000): Analysis of a distributed neural system involved in spatial information, novelty, and memory processing. Hum Brain Mapp 11: 117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman DA,Shallice T ( 1986): Attention to Action. Willed and automatic control of behavior In: Davidson RJ,Schwartz GE, Shapiro D, editors. Consciousness and Self‐Regulation. Advances in Research and Theory. New York: Premium Press; pp 1–19. [Google Scholar]
- Owen AM ( 2000): The role of the lateral frontal cortex in mnemonic processing: The contribution of functional imaging. Exp Brain Res 133: 33–43. [DOI] [PubMed] [Google Scholar]
- Petrides M ( 1994): Frontal lobes and working memory: Evidence from investigations of the effects of cortical excisions in nonhuman primates In: Boller F,Grafman J, editors. Handbook of Neuropsychology, Vol. 9 Amsterdam: Elsevier; pp 59–82. [Google Scholar]
- Ranganath C,DeGutis J,D'Esposito M ( 2004): Category‐specific modulation of inferior temporal activity during working memory encoding and maintenance. Cogn Brain Res 20: 37–45. [DOI] [PubMed] [Google Scholar]
- Smith EE,Jonides J,Koeppe RA,Awh E,Schumacher EH,Minoshima S ( 1995): Spatial vs object working memory: PET investigations. J Cogn Neurosci 7: 337–356. [DOI] [PubMed] [Google Scholar]
- Soon C‐S,Venkrataman V,Chee MWL ( 2003): Stimulus repetition and hemodynamic response refractoriness in event‐related fMRI. Hum Brain Mapp 20: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J,Tournoux P ( 1988): Co‐planar stereotaxic atlas of the human brain. New York: Thieme. [Google Scholar]
- Tarr MJ,Gauthier I ( 2000): FFA: A flexible fusiform area for subordinate‐level visual processing automatized by expertise. Nat Neurosci 3: 764–769. [DOI] [PubMed] [Google Scholar]
- Thompson‐Schill SL,Bedny M,Goldberg RF ( 2005): The frontal lobes and the regulation of mental activity. Curr Opin Neurobiol 15: 219–224. [DOI] [PubMed] [Google Scholar]
- Tong F,Nakayama K,Moscovitch M,Weinrib O,Kanwisher N ( 2000): Response properties of the human fusiform face area. Cogn Neuropsychol 17: 257–279. [DOI] [PubMed] [Google Scholar]
- Turk DJ,Banfield JF,Walling BR,Heatherton TF,Grafton ST,Handy TC,Gazzaniga MS,Macrae CN ( 2004): From facial cue to dinner for two: The neural substrates of personal choice. Neuroimage 22: 1281–1290. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P,Armony JL,Driver J,Dolan RJ ( 2001): Effects of attention and emotion on face processing in the human brain: An event‐related fMRI study. Neuron 30: 829–841. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P,Armony JL,Driver J,Dolan RJ ( 2003): Distinct spatial frequency sensitivities for processing faces and emotional expressions. Nat Neurosci 6: 624–631. [DOI] [PubMed] [Google Scholar]
- Wojciulik E,Kanwisher N,Driver J ( 1998): Covert visual attention modulates face‐specific activity in the human fusiform gyrus: fMRI study. J Neurophysiol 79: 1574–1578. [DOI] [PubMed] [Google Scholar]
- Xu Y ( 2005): Revisiting the role of the fusiform face area in visual expertise. Cereb Cortex 15: 1234–1242. [DOI] [PubMed] [Google Scholar]


