Abstract
Our current knowledge of pain‐related neuronal responses is largely based on experimental pain studies using contact heat or nontactile laser painful stimulation. Both stimuli evoke pain, yet they differ considerably in their physical and perceptual properties. In sensory cortex, cerebral responses to either stimulus should therefore substantially differ. However, given that both stimuli evoke pain, we hypothesized that at a certain subset of cortical regions the different physical properties of the stimuli become less important and are therefore activated by both stimuli. In contrast, regions with clearly dissociable activity may belong to “lower‐level” pain processing mechanisms depending on the physical properties of the administered stimuli. We used functional magnetic resonance (fMRI) to intraindividually compare pain‐related activation patterns between laser and contact heat stimulation using four different intensities of laser and contact heat stimuli. Common and dissociable neural responses were identified by correlating perceived pain intensities with blood oxygenation level dependent (BOLD) signal changes. Only neuronal responses to stimuli that were perceived as painful were analyzed. Pain‐related BOLD signal increases independent of stimulus modality were detected in the anterior insula, anterior cingulate cortex, medial secondary somatosensory cortex, and the prefrontal cortex. These similarities are likely to reflect higher‐level pain processing, which is largely independent of the single physical parameters that determine the painful nature of the stimuli. Hum Brain Mapp 2008. © 2007 Wiley‐Liss, Inc.
Keywords: contact heat, fMRI, higher level processing, pain, radiant heat
INTRODUCTION
Functional imaging studies have provided considerable progress in our understanding of how the human brain encodes the spatial [Apkarian et al., 2000] and temporal [Casey et al., 2001; Porro et al., 1998] properties of acute [Coghill et al., 2001] and tonic [Ringler et al., 2003] painful stimuli. Most studies of pain‐related activation used either tactile thermal [Becerra et al., 1999; Brooks et al., 2002; Casey et al., 2001; Coghill et al., 2001], electrical [Buchner et al., 2000; Disbrow et al., 1998; Sawamoto et al., 2000; Tran et al., 2003] or nontactile laser stimulation [Bingel et al., 2003, 2004b; Bornhovd et al., 2002; Buchel et al., 2002; Ohara et al., 2004; Ploner et al., 2002, 2004]. Differences of brain activity across these studies are most likely related to several temporal and spatial aspects of the different pain stimuli applied [Apkarian et al., 2000; Ploner et al., 2004; Treede et al., 1999], for example, stimulus duration and size of stimulation site. Moreover, quality and intensity of the elicited pain as well as neural activity differ between purely thermal (e.g., laser) and those pain stimuli, which contain an additional tactile component (e.g., contact heat). However, many pain‐related imaging studies using different painful stimuli revealed quite similar activation patterns in numerous areas of the “pain matrix” [Apkarian et al., 2005]. On the basis of these similarities across studies, we hypothesized that some higher‐level pain processing areas are activated by painful stimuli, but independent of the underlying physical parameters of the stimuli. This can be tested by stimulating the same volunteer with physically distinct pain stimuli and explicitly assess commonalities among the responses [Price and Friston, 1997]. This method has been highly successful in identifying higher‐level perceptional mechanisms in the visual system [Kourtzi and Kanwisher, 2000]. Additional lines of evidence from recent imaging studies, for example, of anticipation [Ploghaus et al., 1999], empathy [Singer et al., 2004], pain imagination [Ogino et al., 2007], or observation of pain [Botvinick et al., 2005] imply that pain perception at some level of cerebral processing not only goes beyond the pure sum of the physical components of peripheral noxious stimuli but also be independent of its presence.
We employed fMRI to compare laser and contact heat stimuli intraindividually to identify common neural pain responses in the human brain in addition to the expected dissociable activity patterns. Because of the different physical properties of the stimuli, we refrained from directly assessing individual stimuli between modalities, but rather investigated stimulus response functions (SRF) across different pain intensity levels for both modalities and then compared the slopes of both SRFs to reveal similarities in pain processing.
MATERIALS AND METHODS
Subjects
Fourteen healthy, men, right‐handed volunteers (age 26 to 45 years; mean age = 31 ± 5.5 years) gave written informed consent to participate in the study, which was conducted in accordance with the declaration of Helsinki and approved by the local Ethics committee. All subjects had normal pain thresholds for laser and thermal contact heat stimuli at the site of stimulus application and no history of neurological or psychiatric disease.
Pain Stimulation
Four different laser (350, 450, 550, or 650 mJ) and thermal contact heat stimuli (40°, 43°, 46°, or 48.5°C) were delivered repetitively in a randomized order (18 repetitions for each laser (duration of 1 ms), and 9 repetitions for each thermal contact heat stimulus (each with a duration of 1 s). Laser stimulation was performed by a Tm:YAG infrared laser (Neurolaser, BAASEL Lasertech, Germany) and contact heat stimuli were delivered by a Peltier‐element (3 × 3 cm2 thermoconducting surface; TSA II, MEDOC, Israel) at the dorsal surface of the left hand (Fig. 1). The Peltier element was fastened on the stimulation area by Velcro strips (MEDOC, Israel). To avoid damage of the epidermis by repetitive application of laser stimuli to the same skin area the laser stimulation focus was moved around the target area by one of the investigators by keeping the angle of the laser beam constant (rectangular to the skin surface) (Fig. 1). The Tm:YAG laser emits near infrared radiation (wavelength 1.96 mm, spot diameter 5 mm) with a penetration depth of 360 μm into the human skin without damaging the skin [Spiegel et al., 2000]. These laser stimuli allow precise restriction of the deposited heat energy to the termination area of the primary nociceptive afferents (20–570 μm), whereas contact heat stimuli activate thermoreceptive afferents and mechanoreceptors. Thus, in contrast to contact heat stimuli, the laser stimuli were delivered without tonic tactile component. The physical stimulus intensities for the laser and contact heat stimuli were chosen to represent a spectrum of psychophysically comparable noxious physical stimuli.
Figure 1.
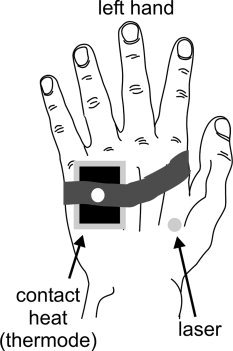
Schematic drawing of the experimental stimulus setup. The thermode with the Peltier element was attached to the ulnar side of the hand while the laser was applied at variable locations at the radial side of the left hand.
Prior to the fMRI sessions, the individual pain threshold of all subjects were obtained psychophysically. First, the pain threshold for laser stimuli and thereafter the thermal pain threshold for the contact heat stimuli were obtained. The pain threshold for laser stimulation was defined as the perception of a stinging sensation [Bingel et al., 2006], while the thermal pain threshold was defined as the first temperature evoking not only a heat but also a slightly painful sensation. Prior to MRI recordings, all volunteers were exposed to all experimental pain stimuli and trained in the rating procedure (see below). All volunteers were informed that the physical stimulus intensities and corresponding stimulation types were presented in a randomized order.
Experimental Protocol
Each volunteer underwent three sessions of MR scanning, each containing a total of 36 noxious and nonnoxious computer‐controlled stimuli applied by the laser and thermode in a randomized order (12 contact heat, 24 laser stimuli) using interstimulus‐intervals (ISI) between 16 and 18 s. Altogether, the experiment consisted of 108 stimuli for each volunteer. Each stimulus was followed by an immediate rating procedure in the scanner (see below). During scanning, two investigators stood inside the scanner room, one to apply the laser stimuli and the other to document the subjects' pain rating of each stimulus. The volunteers were blindfolded by a mask to avoid visual input and could not predict whether the next stimulus was delivered by laser or thermode. The application of both pain stimuli was computer‐controlled and triggered by the Software Presentation (http://www.neurobehavioralsystems.com). About 5.5s after each stimulus, the volunteers were prompted (tap on the volunteer's leg) to rate the perceived pain intensity of each stimulus by giving signs with the right hand. A simple finger scale as described previously [Bornhovd et al., 2002; Buchel et al., 2002; Helmchen et al., 2006; Mohr et al., 2005] covering five perceptional levels ranging from no perception to increasingly painful stimulation (P0–P4). “P0,” indicating that the applied stimulation was not registered at all, was shown by a fist. “P1,” indicating a clear but not painful sensation was shown by one finger. Two fingers (P2) reflected a stimulus that just turned from a perception to a light painful sensation, three (P3) and four fingers (P4) expressed increasing sensations with P4 representing the most intensive pain stimulus used in our experimental setting. The immediate rating of the subjects' perception was important to relate activation patterns to a graded psychophysical response (parametric modulation as SRF). According to psychophysical data prior to fMRI recordings, our stimulus intensities were chosen to (i) elicit clearly distinguishable pain perceptional levels, (ii) compare equally distributed pain ratings, and (iii) allow a parametric fMRI data analysis. The rating with the right hand (contralateral to the left stimulated side) was chosen to prevent combined pain and motor‐related BOLD‐responses contralateral to the stimulation side.
This event related design allowed us to specifically investigate differences and commonalities between the pain‐related activation pattern of the human brain caused by nontactile laser or contact heat stimuli delivered by a thermode. The parametric design was used to determine the stimulus intensity‐related specificity of the effects, whereas BOLD‐responses related to motor activation, tactile components, expectation of painful stimulation, anticipation, and uncertainty of the stimulus onset are not expected to show a parametric modulation [Bornhovd et al., 2002; Buchel et al., 2002; Helmchen et al., 2006; Mohr et al., 2005] and were therefore eliminated during our analysis procedure. Our analysis focuses on activations during stimuli that (i) were perceived as painful in all subjects and (ii) revealed a BOLD‐response increase with increasing pain perception.
Image Acquisition
MRI scanning was performed on a 3 T scanner (Siemens TRIO, Erlangen, Germany; standard head coil). The subjects' head was positioned in a standard head coil with foam pads to avoid head movements. For each subject, 997 volumes (32 contiguous axial slices covering the whole brain, no gap) were acquired during three sessions using a gradient echo, echo‐planar (EPI) T2*‐sensitive sequence [repetition time (TR) 1.984 s, echo time (TE) 30 ms, flip angle 70°, matrix 64 × 64, voxel size 3 × 3 × 3 mm3].
Image Processing and Statistical Analysis
Image processing and statistical analysis were carried out using SPM2 (http://www.fil.ion.ucl.ac.uk/spm, Welcome Department of Imaging Neuroscience, London, UK). All volumes were slice‐time corrected, realigned to the first volume, spatially normalized [Friston et al., 1995] to the standard EPI template of SPM2 and finally smoothed with an 8‐mm isotropic Gaussian kernel. Data analysis was performed using a general linear model (GLM). The GLM was based on the psychophysical parameters, modeling the different laser and contact heat stimuli with the individual pain perception levels derived from each individual stimulus rating (P0, P1, P2, P3, P4) as delta functions (events) convolved with a canonical hemodynamic response function as implemented in SPM2. The movement parameters from the realignment (rotation x, y, z and translation x, y, z), and the rating events were implemented as covariates of no interest. Voxelwise regression coefficients for all regressors were estimated using least squares within SPM2. Effects were then tested with appropriate linear contrasts of the regression coefficients (parameter estimates), resulting in t‐statistics for every voxel.
Our second‐level analysis (random‐effects analysis) of the perceived pain intensity had to account for two assumptions: the activation should (i) reflect pain perception by calculating (P2 + P3 + P4) − P1 and (ii) show a linear increase of the BOLD‐response during pain perception (P2 < P3 < P4) reflecting increasing pain with increasing painful stimulus intensity. Since contact heat stimulation always contained a tactile component, “P0” did not appear in the individual ratings for contact heat stimuli and was therefore excluded for both stimulation types from the random‐effects analysis. To fulfill the first assumption, we performed an ANOVA (within subjects) over the individual contrasts of P1, P2, P3, P4 for laser and contact heat stimulation. Two subjects had to be excluded from the ANOVA due to missing data, that is, they did not indicate ratings over the entire spectrum of the perceptual levels (P1–P4) for laser or for thermal stimulation. In a second step, we computed the contrast: pain > no pain = (P2 + P3 + P4) – P1 for laser and thermode stimulation. This contrast only shows activations related to pain for both stimulation types and was therefore selected as a mask (pain mask) for the subsequent multiple‐regression analysis. To fulfill the second assumption and complete our random‐effects analysis, we performed a multiple‐regression analysis modeling a linear increase from P2 to P4 for both stimulation methods. Each stimulation method was therefore represented by an increasing covariate for P2, P3, and P4 [1 2 3]. This analysis could account for both assumptions and only shows activations, which are pain specific and in addition show an increasing BOLD response to increasing pain, that is, a SRF.
To specify areas showing similar increases in pain perception for both stimulation types, a conjunction analysis for laser and contact heat stimulation [Friston et al., 1997, 2005; Price and Friston 1997] was performed over both regressors from the multiple‐regression analysis. To identify differences in perceived pain‐related activation patterns between laser and contact heat stimulation, we also investigated the interaction between increasing pain intensity and mode of stimulation.
The analysis focused on pain‐related areas for which we had a priori hypotheses for their involvement in pain processing. In these areas, the correction for multiple comparisons was based on a small volume correction [Worsley et al., 1996] with a spherical volume of 4188 mm3 (r = 10 mm). The threshold for the conjunction and interaction analysis was set to P < 0.05 (corrected for multiple comparisons).
The anatomical localizations of the regions are given in MNI coordinates (SPM2) and were compared with the appropriate sagittal and axial sections of the fMRI data set of the human brain [Maldjian et al., 2003]. Inside the cerebellum, we used the appropriate axial sections of the MRI atlas of the human cerebellum [Schmahmann et al., 1999] to determine the anatomical localizations.
RESULTS
Behavioral Data
Both stimulation methods revealed a strong relationship of the physical stimulus intensity (temperature/energy ofthe laser stimulus) and the perceived stimulus intensity levels illustrated by linear regression slopes (laser: y = 119.5x + 308.6 mJ, r = 0.934; thermode: y = 3.4x + 36.3°C, r = 0.844). Imaging data was related to the behavioral data by transforming the different physical stimuli (laser vs. contact heat) into an increasing stimulus hierarchy from stimulus intensity levels 1 to 4. On the basis of the transformation, the regression analysis was repeated to show the relationship between stimulus intensity (1–4) and the perceived stimulus intensity (P1–P4) independent of the different physical properties of both stimulation types. This analysis revealed equal regression slopes with a strong linear relationship of increasing administered stimulus intensity (1–4) to perceived stimulus intensity (P1–P4) for both stimulation methods (laser: y = 1.195x + 0.586, r = 0.845; thermode: y = 1.199x − 0.343, r = 0.934). Both slopes did not differ significantly (P > 0.05).
FMRI Data
Using our specific contrasts, we identified pain‐related regions, which showed linearly increasing BOLD‐responses for increasing perceived pain intensity (see Methods). This approach allowed us to delineate responses evoked by painful from those evoked by nonpainful (e.g., thermal warm) stimuli. Within brain regions showing pain‐related neural responses, we tested for similarities (i.e., using a conjunction, lower part of Table I) and differences (i.e., using interactions) in the SRF between the contact heat and radiant heat condition (see Methods). The upper part of Table I delineates areas, which revealed differences in the activation pattern between laser and thermode stimulation. In the upper part of the table, a higher Z‐score refers to a significantly steeper increase of the SRF for contact heat compared to laser stimulation. In the lower part, the Z‐score refers to the conjunction significance. The different response properties (laser versus thermode) can be illustrated by the relative BOLD response (±standard error of mean, SEM) as parameter estimates of each stimulation type from the multiple regression analysis (see Methods; Figs. 2 and 3).
Table I.
Areas with distinctly different and common (thermode = laser) activation patterns between contact heat (thermode) and laser stimulation (second level conjunction analysis and interaction contrast; multiple regression analysis).
| Contrast | Region | R/L | x | y | z | Z‐score |
|---|---|---|---|---|---|---|
| Interaction Thermode > laser | Posterior ACC | R | 8 | −4 | 42 | 3.29 |
| S II | R | 58 | −18 | 14 | 4.51 | |
| S II | R | 48 | −22 | 16 | 4.16 | |
| S II | L | −56 | −12 | 26 | 3.45 | |
| S II/posterior insula | L | −48 | −12 | 12 | 4.28 | |
| Posterior insula | R | 50 | 4 | 4 | 3.37 | |
| Posterior insula | L | −54 | −20 | 6 | 5.93 | |
| Posterior insula | L | −36 | −16 | 10 | 4.82 | |
| MFC | R/L | 0 | 56 | −16 | 3.79 | |
| MFC | L | −4 | 64 | 4 | 3.63 | |
| MFC | L | −4 | 58 | −10 | 3.28 | |
| SMA | R | 8 | −12 | 54 | 3.83 | |
| M I | R | 20 | −14 | 76 | 3.08 | |
| PA | R | 26 | −44 | 72 | 3.71 | |
| PA | R | 18 | −46 | 66 | 3.43 | |
| Conjunction Thermode = laser | ACC | R | 6 | 16 | 36 | 7.13 |
| ACC | R/L | 0 | 10 | 48 | 6.94 | |
| ACC | L | −8 | 8 | 46 | 6.80 | |
| Anterior insula | R | 32 | 20 | 4 | 6.99 | |
| Anterior insula | R | 34 | 18 | 6 | 6.55 | |
| Anterior insula | R | 40 | 18 | −2 | 6.29 | |
| Anterior insula | R | 46 | 8 | 14 | 5.57 | |
| Anterior insula | L | −32 | 10 | 8 | 7.62 | |
| Anterior insula | L | −38 | 26 | −2 | 6.98 | |
| Anterior insula | L | −36 | 0 | 0 | 6.86 | |
| Insula | R | 36 | 4 | 4 | 6.57 | |
| Insula | R | 40 | −2 | −10 | 7.26 | |
| Insula | R | 40 | 0 | −10 | 7.37 | |
| Insula | R | 36 | 8 | −16 | 7.05 | |
| Insula | R | 32 | 6 | −12 | 6.59 | |
| S II | R | 46 | −18 | 20 | 5.85 | |
| S II | R | 38 | −14 | 18 | 5.84 | |
| S II | R | 62 | −30 | 18 | 5.51 | |
| S II | R | 50 | −14 | 22 | 5.17 | |
| S II | R | 52 | −18 | 18 | 4.96 | |
| S II | R | 52 | −20 | 32 | 5.25 | |
| S II | R | 50 | −24 | 22 | 5.17 | |
| S II | R | 54 | −14 | 14 | 5.95 | |
| S II | R | 34 | −16 | 16 | 5.27 | |
| Thalamus | L | −4 | −10 | −2 | 7.82 | |
| Thalamus | L | −8 | −20 | 6 | 6.15 | |
| Thalamus | R | 6 | −10 | 0 | 7.18 | |
| Thalamus | R | 10 | −10 | −4 | 7.07 | |
| Thalamus | R | 10 | −20 | 8 | 6.89 | |
| Thalamus | R | 10 | −22 | 10 | 6.13 | |
| Nucleus caudatus | R | 10 | 6 | 2 | 4.99 | |
| Nucleus lentiformis | R | 20 | 10 | −12 | 7.53 | |
| Nucleus lentiformis | R | 18 | 6 | 2 | 5.27 | |
| Nucleus lentiformis | L | −12 | 6 | −4 | 6.85 | |
| Nucleus lentiformis | L | −18 | 12 | −8 | 6.66 | |
| Nucleus lentiformis | L | −20 | 8 | 0 | 6.68 | |
| Nucleus lentiformis | L | −20 | 6 | −10 | 6.09 | |
| PFC | R | 28 | −2 | 52 | 6.05 | |
| PFC | R | 28 | −2 | 54 | 6.05 | |
| PFC | L | −28 | −6 | 50 | 4.93 | |
| PFC | L | −30 | −2 | 58 | 5.08 | |
| LPFC | R | 44 | −2 | 56 | 5.27 | |
| LPFC | L | −50 | 4 | 46 | 4.85 | |
| LPFC | R | 36 | 0 | 60 | 4.37 | |
| DLPFC | L | −40 | 48 | 22 | 4.95 | |
| IFC | R | 54 | 6 | 26 | 4.64 | |
| IFC | L | −56 | 6 | 38 | 5.99 | |
| IFC | L | −60 | 12 | 30 | 5.96 | |
| IFC | L | −58 | 8 | 18 | 5.12 | |
| IPC | R | 48 | −32 | 48 | 5.19 | |
| IPC | R | 34 | −40 | 46 | 4.79 | |
| IPC | R | 36 | −48 | 54 | 4.67 | |
| IPC | L | −56 | −38 | 24 | 5.84 | |
| Parahippocampal C | R | 22 | −52 | 0 | 4.91 | |
| Parahippocampal C | R | 16 | −38 | −6 | 4.37 | |
| PAG | L | −6 | −28 | −8 | 7.44 | |
| PAG | L | −4 | −30 | −26 | 5.92 | |
| PAG | R | 8 | −30 | −6 | 7.37 | |
| PAG | R | 6 | −36 | −36 | 6.15 | |
| PAG | R | 4 | −28 | −26 | 5.89 | |
| Cerebellar vermis | L | −4 | −42 | −18 | 4.97 | |
| Cerebellum | R | 14 | −78 | −24 | 4.45 | |
| Cerebellum | L | −10 | −70 | −24 | 4.84 | |
| Cerebellum | L | −44 | −50 | −38 | 5.33 | |
| Cerebellum | L | −36 | −50 | −36 | 5.32 | |
| Cerebellum | L | −36 | −46 | −44 | 5.31 | |
| Cerebellum | L | −20 | −38 | −30 | 4.48 | |
| Cerebellum | L | −14 | −46 | −24 | 4.46 | |
| Cerebellum | L | −28 | −56 | −32 | 4.37 | |
| Motorcortex | L | −58 | −18 | 30 | 5.81 | |
| Motorcortex | L | −22 | −10 | 62 | 4.72 |
The T‐score indicates the significance of interaction (top) or similarity (bottom) for both stimulus conditions (SVC, FWE corrected P < 0.05). No activations were found in the interaction contrast (laser > contact heat stimulation). (R) indicates right, (L) left brain hemisphere.
ACC, anterior cingulated cortex; DLPFC, dorsolateral prefrontal cortex; IFC, inferior frontal cortex; IPC, inferior parietal cortex; LPFC, lateral prefrontal cortex; MFC, mediofrontal cortex; PA, parietal association cortex; PAG, periaqueductal gray; parahippocampal C, parahippocampla cortex; PFC, prefrontal cortex; SI, primary somatosensory cortex; SII, secondary somatosensory cortex.
Figure 2.
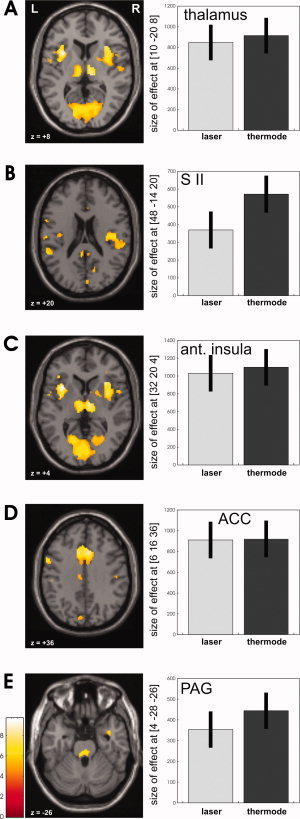
Areas with common response properties, that is, similar increases of the BOLD signal for increasing pain intensity perception for laser and contact heat stimulation (conjunction analysis) are shown on appropriate axial slices for thalamus (A), for the SII (B), anterior insula (C), ACC (D), and the PAG (E) (R = right; L = left). On the right side, the bar plots show the pain‐related parameter estimates corresponding to a linear signal increase from P2 to P4 of the displayed areas.
Figure 3.

Areas with different activation patterns are shown where contact heat elicited a stronger pain‐related increase as compared to laser stimulation (interaction analysis) for the SII (A), posterior insula (B), and posterior ACC (C) on appropriate axial slices (R 5 right; L 5 left). On the right side, the bar plots show the pain‐related parameter estimates corresponding to a linear signal increase from P2 to P4 of the areas.
Areas Showing Similar SRFs
Regions showing common pain‐related linear increases in the BOLD signal (equal for laser and contact heat) as identified by the second‐level conjunction analysis comprised the medial aspects of secondary somatosensory cortex (SII), thalamus (Fig. 2A), secondary somatosensory cortex (SII) (Fig. 2B), anterior insula (Fig. 2C), mid‐anterior cingulate cortex (ACC, Fig. 2D), the basal ganglia, and the periaqueductal gray (PAG, Fig. 2E) (see also Table I).
Areas Showing Different SRF
In general, no area of the cerebral pain network showed a greater increase for painful laser compared to painful contact heat stimulation. Regions showing stronger pain‐related increases for contact heat than radiant heat stimulation as identified by the interaction analysis comprised lateral aspects of SII (Fig. 3A and Fig. 4), posterior insula (Fig. 3B), the posterior part of the ACC (pACC, Fig. 3C), and the middle frontal cortex (MFC), and parietal associative areas (Table I). Since activity reached statistical significance in SI with a SVC with a 10‐mm sphere but not with our prespecified threshold we considered it as trend. Although the pain‐related activation of the anterior insula did not differ intraindividually between laser and contact heat stimulation, the activation of the posterior insula revealed significantly stronger increases with contact heat stimulation (Fig. 3B and Fig. 4).
Figure 4.
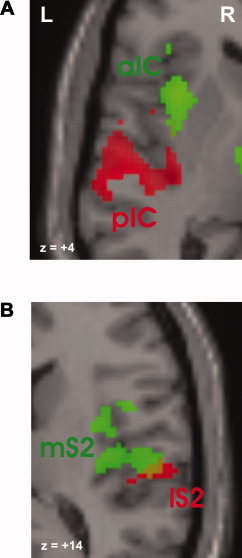
Magnified view on the insula (A) and SII (B), which reveal distinctly different activation sites in the conjunction (green) and interaction (red) analysis. Red symbols indicate stronger increases of activity with contact heat; green symbols reflect not dissociable graded responses between both stimulus modalities. aIC = anterior insula cortex, pIC = posterior insula cortex, mS2 = medial SII, and lS2 = lateral SII. [Color figure can be viewed in the online issue, which is available at www.interscience. wiley.com.]
Activations During Applied But Not Perceived Stimuli (P0)
The most anterior part of the anterior insula was the only region that was activated (P < 0.05 FWE corrected) bilaterally (local maxima: (i) x = 30, y = 20, x = 0, Z = 4.21; (ii) x = −32, y = 26, x = −2, Z = 3.88) during laser stimuli in trials when subjects did not perceive the stimuli, that is, they rated them “P0” (see Fig. 5).
Figure 5.
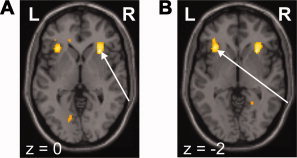
Activation in the right (A) and left (B) anterior insula bilaterally in the absence of stimulus perception during laser stimulation (one‐sample t test over the P0 regressor of each single subject). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
Taking the similar activation pattern in several pain‐related studies conducted with various stimulation techniques into account [Apkarian et al., 2005], we speculated that central pain processing will become at least in part independent of the physical properties that determine the stimuli. Therefore, we identified intraindividual similarities in the SRF between commonly used experimental stimuli in human pain studies: nontactile laser and contact heat stimulation.
As expected, our data show differences between stimulus modalities, but more importantly also similar activation patterns. Taking the substantial differences in the physical properties of both stimulus modalities into account, these common increases of pain‐related activation may indicate higher‐level areas of cerebral pain processing. This implies that pain may be independent of single physical stimulus components that attribute to the perception of pain. Conversely, regions with dissociable neural activity to both stimuli should not belong to higher‐level pain processing.
The integration of interoceptive (body integrity and homeostasis) and exteroceptive signals renders pain a unique perception, clearly distinct from other sensory modalities. This property of pain explains its strong link to emotional and cognitive processes (anticipation, working memory, previous experience, salience, etc.) and its manifestation even in the absence of physical stimuli on one's own body [Botvinick et al., 2005; Ogino et al., 2007; Singer et al., 2004].
Therefore, we hypothesized a higher‐level pain processing, which is active irrespective of the modalities of stimulus delivery, for example, site, duration and spatial extent of stimulation, or stimulated peripheral nerve fibers. Since it appears biologically more meaningful that pain‐related responses covary with intensity, we specifically used graded responses (SRF) to exclude activations related to unspecific effects such as anticipation, attention, fear, or other cognitive or emotional variables.
Pain Intensity‐Related Responses
Both stimulation techniques inherently have considerable different properties with respect to stimulus duration, rise of surface temperature, penetration depth, spatial and temporal summation, habituation, and concomitant stimulation of mechanoreceptors [Iannetti et al., 2006]. On the basis of these considerable differences between the physical properties of laser and contact heat stimulation, our study did not intend to match the physical properties of both stimuli nor did we try to compare different physical properties exerted at the peripheral nociceptor. Accordingly, we did not focus on a categorical comparison (i.e., laser elicited pain versus contact heat pain), but rather designed this study to compare the SRFs in regions of pain‐related activation (see Methods), that is, stimulus‐elicitedBOLD responses with respect to graded painperception levels in the context of a parametric design. Accordingly, areas without pain‐related activations (P0, P1) and without linear increase (from P2 to P4) were excluded from the multiple regression analysis.
In the following, we will provide lines of evidence for higher‐level pain processing by discussing region‐specific commonalities of graded increases of activity.
Activation Sites Without Dissociable Pain‐Related Increase Between Contact Heat and Laser Stimulation
The conjunction analysis delineates areas with similar pain‐related increases (SRF) in the BOLD signal across both stimulus types. In contrast to activation sites with distinctly different activity patterns, confounding factors such as size or tactile component of the stimulus cannot play a role in areas showing a common increase in activity. Thus, a common response, which is independent of the stimulus type, should indicate true pain‐intensity related processing. It is remarkable that such common responses for both stimulus types were observed in several pain‐related brain regions like the anterior insula and the mid‐ACC, which both have previously been implicated in processing the affective components of pain perception [Carlsson et al., 2006; Singer et al., 2004].
Insula and Mid ACC
Despite the different stimulus duration for both stimulus types, the activation increase in the anterior insula did not differ between radiant and contact heat stimulation, which is in line with its pain intensity coding function [Bornhovd et al., 2002; Casey et al., 2001; Coghill et al., 1999; Craig et al., 2000; Peyron et al., 2002]. Several lines of evidence suggest that activity in the anterior insula is largely independent of the sensory quality of the stimuli: first, phasic and tonic painful mechanical stimuli unequivocally activate anterior insula cortex [Ringler et al., 2003]; second, its activity evoked by painful stimuli with [Coghill et al., 2001] and without concomitant tactile components [Bingel et al., 2003] is bilateral and symmetrical. The rostral anterior insula is also consistently activated across various clinical pain conditions irrespective of the underlying pathology [Schweinhardt et al., 2006].
The parametric pain related increases bilaterally in the middle aspects of the ACC supports the concept that it primarily encodes pain intensity independent of the sensory quality of the stimulus [Buchel et al., 2002; Davis et al., 1997; Derbyshire et al., 1998; Gelnar et al., 1999; Kwan et al., 2000; Mohr et al., 2005; Rainville et al., 1997; Tolle et al., 1999]. Attentional aspects [Bantick et al., 2002; Petrovic and Ingvar, 2002] are less likely to account for our common increase of activity in the ACC since expectancy of stimulus intensity and onset (no parametric modulation) was eliminated by our analysis.
The concept of higher level processing of pain in these two areas (anterior insula and ACC) is not only suggested by the commonalities of neural activity in our study but also imaging studies showing pain‐related activation in these sites in the absence of physical stimuli (see below). Moreover, the ACC has also been shown to be engaged in the prolonged salience of painful stimuli since it revealed sustained responses throughout the duration only of a painful but not a nonpainful stimulus [Downar et al., 2003]. This site of higher‐level pain processing could be biologically meaningful since the ACC is also involved in aversive learning [Buchel et al., 1998] and thereby preparing a motivational behavioral response.
Interestingly, the most anterior part of the anterior insula was activated during laser stimulation in the absence of stimulus perception. This activation could be related to the anticipation of pain [Ploghaus et al., 1999; Porro et al., 2002] or its mental representation of the impending sensory stimulation, which might shape the neural processes underlying the actual sensory experience [Koyama et al., 2005]. It is in accord with data showing that the anterior insula is also involved in the cognitive evaluation of pain intensity, high‐level appraisal of emotional material [Kalisch et al., 2006] and the learning about predictions about impending pain even in the absence of any sensory stimulation [Kong et al., 2005; Porro et al., 2002]. The anterior insula and often concomitant the ACC are also activated in the absence of physical stimulation while imaging of one's own pain [Ogino et al., 2007] or viewing of other subjects' pain reflecting human empathy, for example, while viewing facial expressions of other subject's pain [Botvinick et al., 2005] or when pain is delivered to the subjects' beloved partners [Singer et al., 2004]. This activation is distinctly different from the emotion of fear [Ogino et al., 2007] and is in accord with an affective and pain‐intensity coding function of both areas (ACC and anterior insula). Accordingly, both ACC and anterior insula are also activated in our conjunction analysis possibly indicating higher‐level pain processing.
Periaqueductal Gray, Basal Ganglia, and Secondary Somatosensory Cortex
If higher‐level pain processing reflects the unique nature of pain perception irrespective of stimulus delivery, it should be closely linked to antinociceptive mechanisms. Accordingly, the periaqueductal gray (PAG), which is crucially involved in endogenous pain control [Apkarian et al., 2005; Hadjipavlou et al., 2006; Petrovic et al., 2004b], revealed a common site of activity. Similar SRFs for laser and contact heat pain in the PAG might subserve antinociceptive mechanisms, for example, activation of descending inhibitory pathways, irrespective of the stimulus type. Recently, we [Bingel et al., 2006] and others [Petrovic et al., 2002] could show that the PAG is also activated during placebo analgesia. The PAG seems to be particularly active in the initial phase of noxious stimulation [Coghill et al., 2001; Dunckley et al., 2005] and its activity is related to autonomic responses [Petrovic et al., 2004b]. This may indicate a part of or an immediate response to the higher‐level pain processing irrespective of the stimulus properties.
The common bilateral increases in the basal ganglia supports the growing evidence for the role of basal ganglia in human nociception. We have previously shown that behaviorally relevant nociceptive information is represented in the putamen [Bingel et al., 2004a] and might be used for pain related motor responses or learning predictions about pain [Seymour et al., 2004].
The medial SII was commonly activated with contact and radiant heat stimulation. Since both stimuli were applied at the left hand, the somatotopic organization [Bingel et al., 2004b; Disbrow et al., 2000], and lateralization [Bingel et al., 2003; Coghill et al., 2001] cannot account for this common activation. Since SII activity increases with increasing pain intensity [Bornhovd et al., 2002; Coghill et al., 1999; Timmermann et al., 2001], the commonality across both stimulus modalities in this region might also reflect some higher‐level pain processing independent of the physical components of its stimuli.
Activation Sites with Stronger Pain‐Related Increase for Contact Heat Stimulation
According to our initial hypothesis regions with clearly dissociable activity should not belong to higher‐level pain processing mechanisms. Interestingly, despite the deeper penetrance of radiant heat and the much faster and focal rise of temperature at the nociceptor with short‐wavelengths radiant heat [Iannetti et al., 2006], we found no pain‐related areas with a stronger increase of pain‐related activity (SRF) of laser stimulation. There were stronger increases for contact heat stimulation only which might be related to the larger area and duration of stimulation, the additional tactile stimulation of the hand and hence additional stimulation of mechanotactile primary afferents. Our experimental design does not allow and intend to dissect these individual components. One has to keep in mind that similar perceptional levels of pain were compared rather than stimulus intensities to identify commonalities of both stimulation techniques.
The most remarkable difference between both stimulus modalities was found in the lateral aspects of SII and the posterior insula. A comparison of vibrotactile and thermal pain stimuli revealed distinct SII and insula activation being unique to thermal pain perception [Gelnar et al., 1999]. Moreover, the similarity of SII activation with the adjacent posterior insula suggests a function related to thermal discrimination and thermal integration [Craig et al., 2000; Peyron et al., 2002].
In contrast to anterior insula and mid‐ACC, the latter regions may therefore rather be involved in “lower‐level” aspects of pain processing, for example, discriminating thermal and somatosensory parameters of pain.
Somatosensory Cortex and Posterior Insula
Interestingly, there was neither a common increase of nor distinctly different pain‐related activity in SI. One reason might be our conservative threshold. For example, using a lower threshold (10 mm sphere), there was a significantly stronger increase of pain‐related activation in SI with contact heat stimuli, which may be explained by the reciprocal facilitation of nociceptive and mechanoreceptive input to SI [Ploner et al., 2004]. Furthermore, several factors have been implicated to explain controversy of functional imaging results on SI activation among different studies, for example, (i) attention, (ii) degradation of small clustered activations by interindividual anatomical variability, (iii) statistical considerations, and (iv) mixture of parallel excitatory and inhibitory processes [Bushnell et al., 1999].
SII also belongs to the lateral pain system [Peyron et al., 2002; Treede et al., 1999] and contains different subregions subserving different functions with respect to somatotopy [Bingel et al., 2004b; Ferretti et al., 2004] lateralization [Bingel et al., 2003], stimulus localization [Bentley et al., 2004], and different stimulus modalities [Maihofner et al., 2006]. The lateral aspect of SII revealed a significantly greater pain‐related increase during contact heat stimulation. In a recent study, mechanical impact pain elicited greater activations in SII than contact heat stimulation, which has been taken as additional evidence for the strong sensory‐discriminative signal processing in SII [Maihofner et al., 2006]. The combined mechanical and heat stimulation might therefore contribute to the greater SRF increases in lateral SII when compared with our selective radiant heat stimulation of nociceptor afferents.
The distinctly different activation pattern in the anterior and posterior insula are in line with its anatomical and functional heterogeneity [Augustine, 1996]. The posterior insula appears to be preferentially involved in tactile, temperature, and also pain perception [Brooks et al., 2005; Coghill et al., 1999; Craig et al., 2000; Davis et al., 1998] by integrating extero‐ and enteroceptive signals within the limbic sensory cortex [Craig, 2003; Craig et al., 2000]. There is a substantial overlap of innocuous and noxious somaesthetic representations [Ostrowsky et al., 2002]. Stimulation of the human insula and SII elicits not only paresthesia but also temperature and pain sensations [Mazzola et al., 2006]. Thermonociceptive integration might therefore contribute to the larger parametric activation during contact heat compared with laser stimulation since radiant heat does hardly generate the perception of heat.
Prefrontal Cortex
The prefrontal cortex plays a role in the cognitive modulation of pain [Bantick et al., 2002; Petrovic and Ingvar 2002; Rainville et al., 1999], for example, by varying the expectancy and certainty of pain [Ploghaus et al., 1999; Porro et al., 2002; Wager et al., 2004]. Pain‐related activation depends on the degree of uncertainty of the impending stimuli [Ploghaus et al., 2003]. The level of uncertainty for stimulus onset and intensity was balanced between radiant and contact heat stimuli. However, stimulus duration of contact heat was considerably longer than with radiant heat stimulation and the thermode was fixed to the hand. This could indicate different coping mechanisms to regulate subjective distress in this experimental “no‐escape” situation [Petrovic et al., 2004a] and might account for the stronger increase of SRF for contact heat stimulation.
In conclusion, despite several differences in the physical parameters of both modalities, which are widely used in pain‐related studies there were unique similarities of pain‐related increases of activity in some areas (e.g., ACC and anterior insula), which might reflect some higher‐level processing of pain perception, which are probably largely independent of the single physical properties that determine the stimulus modality.
REFERENCES
- Apkarian AV,Gelnar PA,Krauss BR,Szeverenyi NM ( 2000): Cortical responses to thermal pain depend on stimulus size: A functional MRI study. J Neurophysiol 83: 3113–3122. [DOI] [PubMed] [Google Scholar]
- Apkarian AV,Bushnell MC,Treede RD,Zubieta JK ( 2005): Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 9: 463–484. [DOI] [PubMed] [Google Scholar]
- Augustine JR ( 1996): Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev 22: 229–244. [DOI] [PubMed] [Google Scholar]
- Bantick SJ,Wise RG,Ploghaus A,Clare S,Smith SM,Tracey I ( 2002): Imaging how attention modulates pain in humans using functional MRI. Brain 125 (Pt 2): 310–319. [DOI] [PubMed] [Google Scholar]
- Becerra LR,Breiter HC,Stojanovic M,Fishman S,Edwards A,Comite AR,Gonzalez RG,Borsook D ( 1999): Human brain activation under controlled thermal stimulation and habituation to noxious heat: An fMRI study. Magn Reson Med 41: 1044–1057. [DOI] [PubMed] [Google Scholar]
- Bentley DE,Watson A,Treede RD,Barrett G,Youell PD,Kulkarni B,Jones AK ( 2004): Differential effects on the laser evoked potential of selectively attending to pain localisation versus pain unpleasantness. Clin Neurophysiol 115: 1846–1856. [DOI] [PubMed] [Google Scholar]
- Bingel U,Quante M,Knab R,Bromm B,Weiller C,Buchel C ( 2003): Single trial fMRI reveals significant contralateral bias in responses to laser pain within thalamus and somatosensory cortices. Neuroimage 18: 740–748. [DOI] [PubMed] [Google Scholar]
- Bingel U,Glascher J,Weiller C,Buchel C ( 2004a): Somatotopic representation of nociceptive information in the putamen: An event‐related fMRI study. Cereb Cortex 14: 1340–1345. [DOI] [PubMed] [Google Scholar]
- Bingel U,Lorenz J,Glauche V,Knab R,Glascher J,Weiller C,Buchel C ( 2004b): Somatotopic organization of human somatosensory cortices for pain: A single trial fMRI study. Neuroimage 23: 224–232. [DOI] [PubMed] [Google Scholar]
- Bingel U,Lorenz J,Schoell E,Weiller C,Buchel C ( 2006): Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain 120: 8–15. [DOI] [PubMed] [Google Scholar]
- Bornhovd K,Quante M,Glauche V,Bromm B,Weiller C,Buchel C ( 2002): Painful stimuli evoke different stimulus‐response functions in the amygdala, prefrontal, insula and somatosensory cortex: A single‐trial fMRI study. Brain 125 (Pt 6): 326–336. [DOI] [PubMed] [Google Scholar]
- Botvinick M,Jha AP,Bylsma LM,Fabian SA,Solomon PE,Prkachin KM ( 2005): Viewing facial expressions of pain engages cortical areas involved in the direct experience of pain. Neuroimage 25: 312–319. [DOI] [PubMed] [Google Scholar]
- Brooks JC,Nurmikko TJ,Bimson WE,Singh KD,Roberts N ( 2002): fMRI of thermal pain: Effects of stimulus laterality and attention. Neuroimage 15: 293–301. [DOI] [PubMed] [Google Scholar]
- Brooks JC,Zambreanu L,Godinez A,Craig AD,Tracey I ( 2005): Somatotopic organisation of the human insula to painful heat studied with high resolution functional imaging. Neuroimage 27: 201–209. [DOI] [PubMed] [Google Scholar]
- Buchel C,Morris J,Dolan RJ,Friston KJ ( 1998): Brain systems mediating aversive conditioning: An event‐related fMRI study. Neuron 20: 947–957. [DOI] [PubMed] [Google Scholar]
- Buchel C,Bornhovd K,Quante M,Glauche V,Bromm B,Weiller C ( 2002): Dissociable neural responses related to pain intensity, stimulus intensity, and stimulus awareness within the anterior cingulate cortex: A parametric single‐trial laser functional magnetic resonance imaging study. J Neurosci 22: 970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchner H,Richrath P,Grunholz J,Noppeney U,Waberski TD,Gobbele R,Willmes K,Treede RD ( 2000): Differential effects of pain and spatial attention on digit representation in the human primary somatosensory cortex. Neuroreport 11: 1289–1293. [DOI] [PubMed] [Google Scholar]
- Bushnell MC,Duncan GH,Hofbauer RK,Ha B,Chen JI,Carrier B ( 1999): Pain perception: Is there a role for primary somatosensory cortex? Proc Natl Acad Sci USA 96: 7705–7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson K,Andersson J,Petrovic P,Petersson KM,Ohman A,Ingvar M ( 2006): Predictability modulates the affective and sensory‐discriminative neural processing of pain. Neuroimage 32: 1804–1814. [DOI] [PubMed] [Google Scholar]
- Casey KL,Morrow TJ,Lorenz J,Minoshima S ( 2001): Temporal and spatial dynamics of human forebrain activity during heat pain: Analysis by positron emission tomography. J Neurophysiol 85: 951–959. [DOI] [PubMed] [Google Scholar]
- Coghill RC,Sang CN,Maisog JM,Iadarola MJ ( 1999): Pain intensity processing within the human brain: A bilateral, distributed mechanism. J Neurophysiol 82: 1934–1943. [DOI] [PubMed] [Google Scholar]
- Coghill RC,Gilron I,Iadarola MJ ( 2001): Hemispheric lateralization of somatosensory processing. J Neurophysiol 85: 2602–2612. [DOI] [PubMed] [Google Scholar]
- Craig AD ( 2003): Interoception: The sense of the physiological condition of the body. Curr Opin Neurobiol 13: 500–505. [DOI] [PubMed] [Google Scholar]
- Craig AD,Chen K,Bandy D,Reiman EM ( 2000): Thermosensory activation of insular cortex. Nat Neurosci 3: 184–190. [DOI] [PubMed] [Google Scholar]
- Davis KD,Taylor SJ,Crawley AP,Wood ML,Mikulis DJ ( 1997): Functional MRI of pain‐ and attention‐related activations in the human cingulate cortex. J Neurophysiol 77: 3370–3380. [DOI] [PubMed] [Google Scholar]
- Davis KD,Kwan CL,Crawley AP,Mikulis DJ ( 1998): Functional MRI study of thalamic and cortical activations evoked by cutaneous heat, cold, and tactile stimuli. J Neurophysiol 80: 1533–1546. [DOI] [PubMed] [Google Scholar]
- Derbyshire SW,Vogt BA,Jones AK ( 1998): Pain and Stroop interference tasks activate separate processing modules in anterior cingulate cortex. Exp Brain Res 118: 52–60. [DOI] [PubMed] [Google Scholar]
- Disbrow E,Buonocore M,Antognini J,Carstens E,Rowley HA ( 1998): Somatosensory cortex: A comparison of the response to noxious thermal, mechanical, and electrical stimuli using functional magnetic resonance imaging. Hum Brain Mapp 6: 150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disbrow E,Roberts T,Krubitzer L ( 2000): Somatotopic organization of cortical fields in the lateral sulcus of Homo sapiens: Evidence for SII and PV. J Comp Neurol 418: 1–21. [DOI] [PubMed] [Google Scholar]
- Downar J,Mikulis DJ,Davis KD ( 2003): Neural correlates of the prolonged salience of painful stimulation. Neuroimage 20: 1540–1551. [DOI] [PubMed] [Google Scholar]
- Dunckley P,Wise RG,Fairhurst M,Hobden P,Aziz Q,Chang L,Tracey I ( 2005): A comparison of visceral and somatic pain processing in the human brainstem using functional magnetic resonance imaging. J Neurosci 25: 7333–7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti A,Del Gratta C,Babiloni C,Caulo M,Arienzo D,Tartaro A,Rossini PM,Romani GL ( 2004): Functional topography of the secondary somatosensory cortex for nonpainful and painful stimulation of median and tibial nerve: An fMRI study. Neuroimage 23: 1217–1225. [DOI] [PubMed] [Google Scholar]
- Friston KJ,Buechel C,Fink GR,Morris J,Rolls E,Dolan RJ ( 1997): Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 6: 218–229. [DOI] [PubMed] [Google Scholar]
- Friston KJ,Penny WD,Glaser DE ( 2005): Conjunction revisited. Neuroimage 25: 661–667. [DOI] [PubMed] [Google Scholar]
- Gelnar PA,Krauss BR,Sheehe PR,Szeverenyi NM,Apkarian AV ( 1999): A comparative fMRI study of cortical representations for thermal painful, vibrotactile, and motor performance tasks. Neuroimage 10: 460–482. [DOI] [PubMed] [Google Scholar]
- Hadjipavlou G,Dunckley P,Behrens TE,Tracey I ( 2006): Determining anatomical connectivities between cortical and brainstem pain processing regions in humans: A diffusion tensor imaging study in healthy controls. Pain 123: 169–178. [DOI] [PubMed] [Google Scholar]
- Helmchen C,Mohr C,Erdmann C,Binkofski F,Buchel C ( 2006): Neural activity related to self‐ versus externally generated painful stimuli reveals distinct differences in the lateral pain system in a parametric fMRI study. Hum Brain Mapp 27: 755–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannetti GD,Zambreanu L,Tracey I ( 2006): Similar nociceptive afferents mediate psychophysical and electrophysiological responses to heat stimulation of glabrous and hairy skin in humans. J Physiol. 15;577(Pt 1): 235–48. Epub 2006 Sep 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R,Wiech K,Critchley HD,Dolan RJ (2006):Levels of appraisal: A medial prefrontal role in high‐level appraisal of emotional material.Neuroimage 30: 1458–1466. [DOI] [PubMed] [Google Scholar]
- Kong J,White NS,Kwong KK,Vangel MG,Rosman IS,Gracely RH,Gollub RL ( 2005): Using fMRI to dissociate sensory encoding from cognitive evaluation of heat pain intensity. Hum Brain Mapp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourtzi Z,Kanwisher N (2000):Cortical regions involved in perceiving object shape.J Neurosci 20: 3310–3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama T,McHaffie JG,Laurienti PJ,Coghill RC ( 2005): The subjective experience of pain: Where expectations become reality. Proc Natl Acad Sci USA 102: 12950–12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan CL,Crawley AP,Mikulis DJ,Davis KD ( 2000): An fMRI study of the anterior cingulate cortex and surrounding medial wall activations evoked by noxious cutaneous heat and cold stimuli. Pain 85: 359–374. [DOI] [PubMed] [Google Scholar]
- Maihofner C,Herzner B,Otto Handwerker H ( 2006): Secondary somatosensory cortex is important for the sensory‐discriminative dimension of pain: A functional MRI study. Eur J Neurosci 23: 1377–1383. [DOI] [PubMed] [Google Scholar]
- Maldjian JA,Laurienti PJ,Kraft RA,Burdette JH ( 2003): An automated method for neuroanatomic and cytoarchitectonic atlas‐based interrogation of fMRI data sets. Neuroimage 19: 1233–1239. [DOI] [PubMed] [Google Scholar]
- Mazzola L,Isnard J,Mauguiere F ( 2006): Somatosensory and pain responses to stimulation of the second somatosensory area (SII) in humans. A comparison with SI and insular responses. Cereb Cortex 16: 960–968. [DOI] [PubMed] [Google Scholar]
- Mohr C,Binkofski F,Erdmann C,Buchel C,Helmchen C ( 2005): The anterior cingulate cortex contains distinct areas dissociating external from self‐administered painful stimulation: A parametric fMRI study. Pain 114: 347–357. [DOI] [PubMed] [Google Scholar]
- Ogino Y,Nemoto H,Inui K,Saito S,Kakigi R,Goto F ( 2007): Inner experience of pain: Imagination of pain while viewing images showing painful events forms subjective pain representation in human brain. Cereb Cortex 17: 1139–1146. [DOI] [PubMed] [Google Scholar]
- Ohara S,Crone NE,Weiss N,Treede RD,Lenz FA ( 2004): Cutaneous painful laser stimuli evoke responses recorded directly from primary somatosensory cortex in awake humans. J Neurophysiol 91: 2734–2746. [DOI] [PubMed] [Google Scholar]
- Ostrowsky K,Magnin M,Ryvlin P,Isnard J,Guenot M,Mauguiere F ( 2002): Representation of pain and somatic sensation in the human insula: A study of responses to direct electrical cortical stimulation. Cereb Cortex 12: 376–385. [DOI] [PubMed] [Google Scholar]
- Petrovic P,Ingvar M ( 2002): Imaging cognitive modulation of pain processing. Pain 95: 1–5. [DOI] [PubMed] [Google Scholar]
- Petrovic P,Kalso E,Petersson KM,Ingvar M ( 2002): Placebo and opioid analgesia—Imaging a shared neuronal network. Science 295: 1737–1740. [DOI] [PubMed] [Google Scholar]
- Petrovic P,Carlsson K,Petersson KM,Hansson P,Ingvar M ( 2004a): Context‐dependent deactivation of the amygdala during pain. J Cogn Neurosci 16: 1289–1301. [DOI] [PubMed] [Google Scholar]
- Petrovic P,Petersson KM,Hansson P,Ingvar M ( 2004b): Brainstem involvement in the initial response to pain. Neuroimage 22: 995–1005. [DOI] [PubMed] [Google Scholar]
- Peyron R,Frot M,Schneider F,Garcia‐Larrea L,Mertens P,Barral FG,Sindou M,Laurent B,Mauguiere F ( 2002): Role of operculoinsular cortices in human pain processing: Converging evidence from PET, fMRI, dipole modeling, and intracerebral recordings of evoked potentials. Neuroimage 17: 1336–1346. [DOI] [PubMed] [Google Scholar]
- Ploghaus A,Tracey I,Gati JS,Clare S,Menon RS,Matthews PM,Rawlins JN ( 1999): Dissociating pain from its anticipation in the human brain. Science 284: 1979–1981. [DOI] [PubMed] [Google Scholar]
- Ploghaus A,Becerra L,Borras C,Borsook D ( 2003): Neural circuitry underlying pain modulation: Expectation, hypnosis, placebo. Trends Cogn Sci 7: 197–200. [DOI] [PubMed] [Google Scholar]
- Ploner M,Gross J,Timmermann L,Schnitzler A ( 2002): Cortical representation of first and second pain sensation in humans. Proc Natl Acad Sci USA 99: 12444–12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploner M,Pollok B,Schnitzler A ( 2004): Pain facilitates tactile processing in human somatosensory cortices. J Neurophysiol 92: 1825–1829. [DOI] [PubMed] [Google Scholar]
- Porro CA,Cettolo V,Francescato MP,Baraldi P ( 1998): Temporal and intensity coding of pain in human cortex. J Neurophysiol 80: 3312–3320. [DOI] [PubMed] [Google Scholar]
- Porro CA,Baraldi P,Pagnoni G,Serafini M,Facchin P,Maieron M,Nichelli P ( 2002): Does anticipation of pain affect cortical nociceptive systems? J Neurosci 22: 3206–3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ,Friston KJ ( 1997): Cognitive conjunction: A new approach to brain activation experiments. Neuroimage 5,(4, Pt 1): 261–270. [DOI] [PubMed] [Google Scholar]
- Rainville P,Duncan GH,Price DD,Carrier B,Bushnell MC ( 1997): Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science 277: 968–971. [DOI] [PubMed] [Google Scholar]
- Rainville P,Carrier B,Hofbauer RK,Bushnell MC,Duncan GH ( 1999): Dissociation of sensory and affective dimensions of pain using hypnotic modulation. Pain 82: 159–171. [DOI] [PubMed] [Google Scholar]
- Ringler R,Greiner M,Kohlloeffel L,Handwerker HO,Forster C ( 2003): BOLD effects in different areas of the cerebral cortex during painful mechanical stimulation. Pain 105: 445–453. [DOI] [PubMed] [Google Scholar]
- Sawamoto N,Honda M,Okada T,Hanakawa T,Kanda M,Fukuyama H,Konishi J,Shibasaki H ( 2000): Expectation of pain enhances responses to nonpainful somatosensory stimulation in the anterior cingulate cortex and parietal operculum/posterior insula: An event‐related functional magnetic resonance imaging study. J Neurosci 20: 7438–7445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD,Doyon J,McDonald D,Holmes C,Lavoie K,Hurwitz AS,Kabani N,Toga A,Evans A,Petrides M ( 1999): Three‐dimensional MRI atlas of the human cerebellum in proportional stereotaxic space. Neuroimage 10(3, Pt 1): 233–260. [DOI] [PubMed] [Google Scholar]
- Schweinhardt P,Lee M,Tracey I ( 2006): Imaging pain in patients: Is it meaningful? Curr Opin Neurol 19: 392–400. [DOI] [PubMed] [Google Scholar]
- Seymour B,O'Doherty JP,Dayan P,Koltzenburg M,Jones AK,Dolan RJ,Friston KJ,Frackowiak RS ( 2004): Temporal difference models describe higher‐order learning in humans. Nature 429: 664–667. [DOI] [PubMed] [Google Scholar]
- Singer T,Seymour B,O'Doherty J,Kaube H,Dolan RJ,Frith CD ( 2004): Empathy for pain involves the affective but not sensory components of pain. Science 303: 1157–1162. [DOI] [PubMed] [Google Scholar]
- Spiegel J,Hansen C,Treede RD ( 2000): Clinical evaluation criteria for the assessment of impaired pain sensitivity by thulium‐laser evoked potentials. Clin Neurophysiol 111: 725–735. [DOI] [PubMed] [Google Scholar]
- Timmermann L,Ploner M,Haucke K,Schmitz F,Baltissen R,Schnitzler A ( 2001): Differential coding of pain intensity in the human primary and secondary somatosensory cortex. J Neurophysiol 86: 1499–1503. [DOI] [PubMed] [Google Scholar]
- Tolle TR,Kaufmann T,Siessmeier T,Lautenbacher S,Berthele A,Munz F,Zieglgansberger W,Willoch F,Schwaiger M,Conrad B,Willoch F,Schwaiger M,Conrad B,Bartenstein P ( 1999): Region‐specific encoding of sensory and affective components of pain in the human brain: A positron emission tomography correlation analysis. Ann Neurol 45: 40–47. [DOI] [PubMed] [Google Scholar]
- Tran TD,Hoshiyama M,Inui K,Kakigi R ( 2003): Electrical‐induced pain diminishes somatosensory evoked magnetic cortical fields. Clin Neurophysiol 114: 1704–1714. [DOI] [PubMed] [Google Scholar]
- Treede RD,Kenshalo DR,Gracely RH,Jones AK ( 1999): The cortical representation of pain. Pain 79: 105–111. [DOI] [PubMed] [Google Scholar]
- Wager TD,Rilling JK,Smith EE,Sokolik A,Casey KL,Davidson RJ,Kosslyn SM,Rose RM,Cohen JD ( 2004): Placebo‐induced changes in FMRI in the anticipation and experience of pain. Science 303: 1162–1167. [DOI] [PubMed] [Google Scholar]
- Worsley KJ,Marrett S,Neelin P,Vandal AC,Friston KJ,Evans AC ( 1996): A unified statistical approach for determining significant voxels in images of cerebral activation. Human Brain Mapp 4: 58–73. [DOI] [PubMed] [Google Scholar]


