Abstract
Objective:
To assess the early cortical changes following an acute motor relapse secondary to a pseudotumoral lesion in MS patients, the longitudinal cortical functional correlates of clinical recovery, and the evolution over time of cortical reorganization.
Methods:
FMRI during the performance of a simple motor task were obtained from 12 MS patients (after a clinical attack involving the motor system secondary to a pseudotumoral lesion) and 15 matched controls. In six patients and five controls, a longitudinal fMRI study was also performed.
Results:
In patients, at baseline, the primary sensorimotor cortex (SMC) of the ipsilateral (contralesional) hemisphere was significantly more active during task performance with the impaired than the unimpaired hand. During task performance with the unimpaired hand, the ipsilateral cerebellum and several motor areas in the contralateral hemisphere were significantly more active. Pseudotumoral lesion volume was correlated with activation of the primary SMC bilaterally (r = −0.86 and −0.85) and the nine‐hole peg test score with activation of the primary SMC of the affected hemisphere (r = 0.88). A recovery of function of the primary SMC of the affected hemisphere was found in the four patients with clinical improvement. In the two patients without clinical recovery, there was a persistent recruitment of the primary SMC of the unaffected hemisphere.
Conclusions:
Pseudotumoral MS lesions affecting the motor system can determine short‐term cortical changes characterized by the recruitment of pathways in the unaffected hemisphere. The regain of function of motor areas of the affected hemisphere seems to be a critical factor for a favorable recovery. Hum Brain Mapp 2008. © 2007 Wiley‐Liss, Inc.
Keywords: multiple sclerosis, functional magnetic resonance imaging, pseudotumoral lesions, relapse, clinical recovery
INTRODUCTION
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system (CNS) causing a progressive accumulation of tissue damage and worsening of clinical disability in most of the patients [Noseworthy et al.,2000]. However, the clinical patterns of disease evolution and recoveryof symptoms are highly variable and scarcely correlated with structural CNS damage, as measured using MRI [Filippi and Grossman,2002]. Among the reasons for such a clinical/MRI paradox, the efficacy of reparative mechanisms and interindividual variations in cortical reorganization are likely to play a role [Filippi and Rocca,2004].
The application of functional MRI (fMRI) for the assessment of the motor system in clinically stable MS patients has demonstrated abnormal patterns of brain activations, which have been interpreted as reflecting adaptive mechanisms contributing to limit the impact of disease‐related structural damage on clinical progression and disability. Different movement‐associated patterns of activations throughout the course of the disease have been described, mainly characterized by an expanded representation of cortical motor areas and unmasking of latent motor pathways (such as activation of the areas of the hemisphere ipsilateral to the movement) early in the disease course [Filippi et al.,2004a; Rocca et al.,2003a] and functional recruitment of additional motor and “extra‐motor” areas (involving networks for multimodal integration) in the progressive phases [Filippi et al.,2002a; Rocca et al.,2003b]. These results have been mainly obtained from cross‐sectional studies conducted on selected and homogeneous groups of patients. However, a key feature of MS is the presence of lesions with different temporal evolutions. The assessment of patients with acute relapses secondary to the involvement of a specific system as well as the acquisition of longitudinal data would allow to better define the functional correlates of clinical recovery in the disease. In this context, patients with pseudotumoral acute MS lesions represent an ideal model to be studied, since the pseudotumoral lesions are typically associated with only a few additional brain lesions and they are also related to the onset of a specific, acute symptom. Against this background, the aims of this study were: (1) to assess the short‐term cortical changes following an acute motor relapse secondary to a pseudotumoral lesion using fMRI; (2) to evaluate the functional correlates of clinical recovery over time and, finally, (3) to investigate the evolution over time of cortical reorganization in a subgroup of these patients.
METHODS
Patients
We recruited consecutively patients attending the Emergency Unit of our Institute with MS or clinically isolated syndromes (CIS) suggestive of MS [McDonald et al.,2001; Polman et al.,2005] according to the following criteria: (a) the occurrence of a clinical attack involving the motor system; (b) motor impairment entirely ascribable to a single large demyelinating lesion affecting the corticospinal tracts in the brain; (c) absence of clinical disability documented at the expanded disability status scale (EDSS) [Kurtzke,1983] assessed during neurological examination prior to the attack in relapsing remitting (RR) MS patients or of preexisting subjective neurological deficits in CIS patients; (d) strong right‐handedness according to the Edinburgh Handedness Inventory scale (i.e., use of the right hand for 8 or more of the 10 activities) [Oldfield,1971]; (e) ability to perform a flexion‐extension task with the affected arm fingers. To be included, CIS patients also had to have a demonstration of disease dissemination over space according to the revised McDonald criteria [Polman et al.,2005]. RRMS patients had to have no previous clinical involvement of the upper limbs. Patients with neurological symptoms or clinical deficits that could be related to involvement of other brain areas were excluded. Fifteen sex‐ and age‐matched right‐handed (handedness was defined as indicated for patients) healthy volunteers (nine women and six men, mean age = 38.6 years, range = 21–54 years) with no previous history of neurological dysfunction and a normal neurological exam served as controls for baseline examination. Five of them underwent a second fMRI acquisition after a mean time interval of 6 months (±1 week). Local Ethics Committee approval and written informed consent from all subjects were obtained prior to study initiation.
Functional Assessment
Motor functional assessment of the upper limbs was performed for all subjects at the time of MRI acquisition and after 6 months (±1 week), using the nine‐hole peg test (NHPT) [Herndon,1997]. The mean of two trials for both upper limbs for each subject was computed. In each patient, we obtained an index of performance [normalised ratio score (NHPTr)] corrected for the unimpaired hand performance, calculated as follows:
| (1) |
where u = unaffected and a = affected hand. This index is equal to 0 when the two limbs have identical performance, while it results = −1 when the affected limb is completely unable to perform the task [Ward et al.,2006]. At follow‐up, clinical recovery was measured as follows: good if NHPTr ≥ −0.06 (within 2 SD from the mean value of healthy controls), poor/absent if NHPTr remained stable or was <−0.06. The same formula was applied to calculate the motor performance of healthy controls, taking into account their left hemispheric dominance. In this case, we considered as “u” the right hand and as “a” the left hand. Baseline NHPTr was significantly different between healthy controls (mean = −0.008, SD = 0.03) and patients (mean = −0.41, SD = 0.37) (P = 0.003).
Experimental Design
Using a block design (ABAB), where five periods of activation were alternated with six periods of rest (each period of activation and rest consisting of five measurements), the subjects were scanned while performing a simple motor task consisting of repetitive flexion‐extension of the last four fingers of the hand moving together. The movements were paced by a metronome at a 1‐Hz frequency. The subjects were trained before performing the experiments and were instructed to keep their eyes closed during fMRI acquisition. They were monitored visually during scanning to ensure accurate task performance and to check for additional (e.g., mirror) movements. In healthy controls, the task was performed only with the right hand, while patients performed the task with both the affected and the unaffected hands, during two different fMRI runs.
fMRI Acquisition
Brain MRI scans were obtained on a magnet operating at 1.5 T (Vision, Siemens, Erlangen, Germany). Sagittal T1‐weighted images were acquired to define the anterior–posterior commissural (AC–PC) plane. Functional MR images were acquired using a T2*‐weighted echo‐planar imaging (EPI) sequence (repetition time [TR] = 5.5 s, echo time [TE] = 66 ms, flip angle = 90°, matrix size = 128 × 128, field of view [FOV] = 256 × 256 mm2). Twenty‐four axial slices, parallel to the AC–PC plane, with a thickness of 5 mm, covering the whole brain were acquired during each measurement. Shimmimg was performed for the entire brain using an autoshim routine, which yielded satisfactory magnetic field homogeneity. During each fMRI session, which lasted for 5 min and 30 sec, 60 measurements were acquired. The first five measurements of each run were not considered in the analysis to minimize spin saturation effects.
Conventional MRI Acquisition
During the same imaging session, the following additional sequences of the brain were acquired to image the brain: (a) dual‐echo turbo spin‐echo (SE) (TR = 3,300 ms, first echo TE = 16 ms, second echo TE = 98 ms, echo train length = 5); (b) T1‐weighted conventional SE (TR = 768 ms; TE = 15 ms) before and after the administration of 0.1 mmol/Kg of Gadolinium (Gd). For all the scans, 24 contiguous axial slices were acquired with 5‐mm slice thickness, 256 × 256 matrix, and 250 × 250 mm2 FOV. The slices were positioned to run parallel to a line that joins the most inferoanterior and inferoposterior parts of the corpus callosum [Miller et al.,1991].
fMRI Analysis
All image postprocessing was performed on an independent computer workstation (Sun Sparcstation, Sun Microsystems, Mountain View, CA) by a single observer unaware of subjects' identity and structural MRI findings. fMRI data were analyzed using the statistical parametric mapping (SPM99) software developed by Friston et al. [1995]. Prior to statistical analysis, all images were realigned to the first one to correct for subject motion, spatially normalized into the stereotaxic space of SPM provided by Montreal Neurological Institute (MNI), and smoothed with a 10‐mm, 3D Gaussian filter. To have on the same side the hemisphere contralateral to movement, scans obtained when motor task was performed with the left hand were flipped. With this approach, all patients were assumed to have left hemispheric lesions and right hemiparesis.
Conventional MRI Analysis
Conventional MRI postprocessing was performed by a single observer, unaware to whom the scans belonged and blinded to fMRI results. Lesions were identified on the proton‐density (PD)‐weighted and post‐contrast T1‐weighted scans. The T2‐weighted and the pre‐contrast T1‐weighted images were always used to increase confidence in lesion identification. Then, PD lesion volumes were measured using a segmentation technique based on local thresholding, as previously described [Filippi et al.,2001].
Statistical Analysis
Changes in blood oxygenation level dependent (BOLD) contrast associated with the performance of the motor task were assessed on a pixel‐by‐pixel basis, using the general linear model and the theory of Gaussian fields [Worsley and Friston,1995]. Specific effects were tested by applying appropriate linear contrasts. Significant hemodynamic changes for each contrast were assessed using t statistical parametric maps (SPMt). The within‐group activations and between‐groups (hands) comparisons were investigated with a random‐effect analysis [Friston et al.,1999]. A one‐sample t test was used to assess within‐group activations during left and right hand movements. A paired t test was used to compare activations between left and right hand movements in patients and changes of activation over time in healthy controls. A two‐sample t test was used to compare activations between healthy controls and patients and between patients with good and poor motor recovery. Cluster of voxels with a height threshold P < 0.001 (uncorrected) were considered as significant. In areas where an a priori hypothesis was available, the cut‐off value for significance was set at P < 0.05, applying a small volume correction (SVC) for multiple comparisons by using a 10‐mm radius. A linear regression model was used to assess the relation between fMRI changes and NHPT, pseudotumoral lesion volume, disease duration, and time of examination. For this analysis, we report activation at a threshold of P < 0.001, uncorrected for multiple comparisons.
RESULTS
Clinical Characteristics
We studied 12 patients (5 men; mean age 35.1 years, range = 18–63 years) in the acute‐subacute phase (mean time 20 days; range 6–45 days) of the clinical attack. Nine of them had RR MS (median disease duration = 3 years, range = 1–12 years), while the remaining three patients had a CIS suggestive of MS with spatial dissemination of lesions [Polman et al.,2005]. The clinical attack, entirely ascribable to a single large demyelinating lesion, consisted of left (eight patients) or right (four patients) hemiparesis with a sudden onset and a mild worsening over the next few days. All patients were treated with 1 g methylprednisolone i.v./day for 5 days at symptom onset. In Table I, the main clinical and conventional MRI characteristics of the patients studied at the time of the acute relapse are summarized. In two of the CIS patients, a stereotactic biopsy of the lesion was performed because of the tumor‐like appearance. After the analysis of the biopsed material revealed pathological findings consistent with a demyelinating disease, they were included into the study. Seven patients had a good recovery of the motor deficits after a 6 months relapse‐free follow‐up, while the remaining five patients had poor or absent motor recovery. None of the patients had any further clinical attack during the follow‐up period. Monthly serial MRI examinations (mean follow up, relapse‐free, period = 180 days, range = 173–187 days) were obtained from six patients (3 CIS and 3 RR MS patients). Only in four of them there was a good motor recovery. Temporal dissemination of lesions was detected during follow‐up in all the three CIS patients [Polman et al.,2005].
Table I.
Main clinical and MRI characteristics of the 12 patients studied
| Patient | Age/sex | MS type | Disease duration (years) | Number of previous attacks | CSF analysis | Number and site of brain lesions | Time since symptom onset (days) | Nine hole peg test ratio | Baseline EDSS | 6 month EDSS | Baseline pseudotumoral lesion volume (ml) | 6 month pseudotumoral lesion volume (ml) | Brain T2 lesion load (ml) | Clinical recovery |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 28/F | RR | 12 | 1 | OB | 3PV, 1 GD | 30 | −0.91 | 3.0 | 1.5 | 50.8 | 5.1 | 53.6 | Yes |
| 2 | 29/M | RR | 3 | 2 | OB | 3PV, 1 IC, 1 IF, 1 GD | 45 | −0.21 | 3.0 | 2.5 | 51.7 | n.d. | 71.1 | No |
| 3 | 37/F | RR | 1 | 1 | n.d. | 5PV, 2 IC, 1 GD | 15 | 0.01 | 2.0 | 1.0 | 2.2 | n.d. | 10.1 | Yes |
| 4 | 63/M | RR | 3 | 1 | OB | 1 ST, 1 GD | 7 | −0.91 | 4.5 | 4.0 | 3.6 | 3.5 | 9.6 | No |
| 5 | 30/M | CIS | — | 0 | OB | 1 IC, 1 GD, 1 IF | 28 | −0.19 | 3.5 | 3.0 | 31.6 | 11.1 | 32.1 | No |
| 6 | 43/F | RR | 2 | 3 | OB | 3 PV, 1 IC, 1 GD | 9 | −0.25 | 3.0 | 1.0 | 122.9 | 20.2 | 133.4 | Yes |
| 7 | 18/F | CIS | — | 0 | OB | 4 PV, 2 IC, 1 GD | 35 | −0.91 | 2.0 | 1.0 | 53.1 | 23.4 | 54.3 | Yes |
| 8 | 27/M | RR | 3 | 1 | OB | 1 PV, 1 IC, 1 GD | 6 | −0.17 | 2.0 | 1.0 | 2.4 | n.d. | 3.2 | Yes |
| 9 | 20/F | RR | 1 | 2 | OB | 3 PV, 1 IC, 1 GD | 45 | −0.21 | 2.0 | 1.5 | 23.4 | n.d. | 25.5 | Yes |
| 10 | 31/F | RR | 2 | 2 | OB | 1 IC, 1 IF, 1 GD | 6 | −0.34 | 3.0 | 1.0 | 6.7 | n.d. | 9.9 | Yes |
| 11 | 41/F | CIS | — | 0 | OB | 3 PV, 1 GD, 1 IC | 12 | −0.08 | 2.0 | 1.5 | 2.5 | 1.2 | 2.7 | Yes |
| 12 | 55/M | RR | 3 | 2 | OB | 5 PV, 2 IC, 1 GD | 6 | −0.91 | 3.5 | 3.5 | 75.27 | n.d. | 93.3 | No |
F = female; M = male; RR = relapsing remitting; EDSS = Expanded Disability Status Scale; CIS = clinically isolated syndrome; n.d. = not done; OB = oligoclonal bands; PV = periventricular; GD = gadolinium enhancing; IC = juxtacortical; IF = infratentorial.
Structural MRI of Pseudotumoral Lesions
The pseudotumoral lesion was located in the right hemisphere in eight patients and in the left hemisphere in the remaining four. Lesions were located along the corticospinal tracts, mostly in the centrum semiovale; two patients had also involvement of the internal capsule. In all the cases, lesion enhancement was detected on T1‐weighted images after Gd administration. The median T2‐weighted lesion volume was 35.5 ml (SD = 37.3 ml). In the six patients who underwent serial MRI examination, a progressive reduction of pseudotumoral lesion volume was observed (mean pseudotumoral lesion volumes at follow‐up = 10.8; SD = 9.2; P vs. baseline lesion volumes = n.s.) (see Fig. 1). No correlation was found between pseudotumoral lesion volume and motor performance evaluated with the NHPT (both at baseline and follow‐up). Whole brain PD‐weighted lesion load was 41.5 ml (SD = 41.1).
Figure 1.
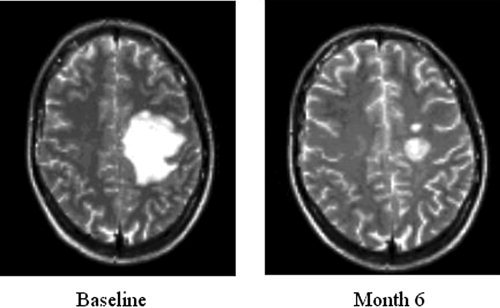
Evolution over time (baseline and month 6) of a pseudotumoral MS lesion in one patient followed up over 6 months. On T2‐weighted images, a progressive reduction of lesion volumes over time is visible.
fMRI Results: Cross‐Sectional Evaluation
During fMRI acquisition, all patients were able to perform the tasks adequately, although with lower velocity with the impaired hand, and no additional movements were noted. During tasks performance, all subjects had a pattern of cortical activations which involved areas known to be associated with motor planning and performance [Fink et al.,1997] (Table II). Figure 2 shows intragroup activations during task performance in healthy subjects (right hand) and patients (unimpaired and impaired hand).
Table II.
Brain areas activated in healthy controls during task performance with the right hand and in patients during task performance with impaired and unimpaired hands
| Activation sites | Healthy controls | Patients impaired hand | Patients unimpaired hand | |||
|---|---|---|---|---|---|---|
| SPM coordinate (X, Y, Z) | t | SPM coordinates (X, Y, Z) | t | SPM coordinate (X, Y, Z) | t | |
| L primary SMC | −40, −16, −58 | 15.8 | −42, −34, 62 | 6.1 | −32, −22, 54 | 14.0 |
| R primary SMC | 50, −32, 50 | 9.5 | 52, 4, 34 | 6.4 | 54, 2, 36 | 5.1 |
| SMA | −2, −6, 50 | 9.8 | 6, −6, 48 | 6.4 | 4, −4, 60 | 7.1 |
| L SII | −58, −22, 20 | 8.8 | −56, −30, 30 | 8.1 | −58, −26, 22 | 6.9 |
| R SII | 64, −22, 24 | 8.9 | 64, −20, 22 | 5.2 | 60, −28, 32 | 6.9 |
| L IFG | −56, 4, 28 | 7.0 | −56, 12, 4 | 6.2 | −52, 12, 2 | 8.4 |
| R IFG | 58, 6, 34 | 6.4 | — | — | 50, 4, 4 | 10.9 |
| L thalamus | −12, −18, 4 | 6.6 | — | — | −16, −22, 6 | 6.7 |
| L MFG | — | — | —32, 40, 22 | 5.8 | — | — |
| CMA | 2, 6, 40 | 9.8 | 8, −4, 40 | 6.2 | −10, −16, 44 | 6.2 |
| L cerebellum | −24, −60, −26 | 5.1 | −34, −48, −28 | 5.0 | — | — |
| R cerebellum | 18, −52, −24 | 18.4 | — | — | 24, −52, −26 | 8.7 |
R: right; L: left; SMC: sensorimotor cortex; SMA: supplementary motor area; SII: secondary sensorimotor cortex; IFG: inferior frontal gyrus; MFG: middle frontal gyrus; CMA: cingulate motor area. See text for further details.
Figure 2.
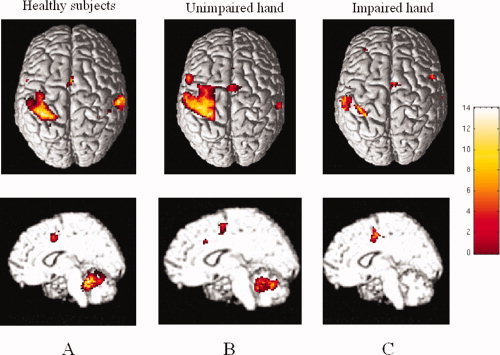
Cortical activations during right hand movement in healthy subjects (A) and during movement of unimpaired (B) and impaired (C) hand in patients in the acute‐subacute phase of a clinical relapse. In patients, scans obtained during task performance with the left hand have been flipped in order to keep the left hemisphere contralateral to movement. Images are in neurological convention. See text for further details.
Between‐group comparisons: Patients vs. controls
During task performance with the unimpaired hand, when contrasted to controls, patients had more significant activations (P < 0.001 uncorrected, P < 0.05 after SVC) of several regions located in the contralateral (contralesional) cerebral hemisphere, including the primary sensorimotor cortex (SMC) (SPM space coordinates: −38, −24, 52), supplementary motor area (SMA) (SPM space coordinates: −14, −14, 48), inferior frontal gyrus (IFG) (SPM space coordinates: −48, 14, 4), infraparietal sulcus (IPS) (SPM space coordinates: −22, −66, 44), and secondary sensorimotor cortex (SII) (SPM space coordinates: −50, −24, 28). During task performance with the impaired hand, when contrasted to controls, patients had more significant activations (P < 0.001 uncorrected, P < 0.05 after SVC) of the ipsilateral primary SMC (SPM space coordinates: 26, −12, 48), cingulate motor area (CMA) (SPM space coordinates: 12, −28, 38), ipsilateral SII (SPM space coordinates: 56, −44, 14), contralateral IFG (SPM space coordinates: −50, 16, 8), and IPS, bilaterally (SPM space coordinates: right 28, 58, 54, left −22, −76, 40). Compared with controls, patients also had significantly reduced activations (P < 0.001 uncorrected, P < 0.05 after SVC) of the contralateral thalamus (SPM space coordinates: −6, −16, 8), and ipsilateral cerebellar hemisphere (SPM space coordinates: 20, −50, −20). The use of a lower threshold (t = 2) in this latter analysis showed also a reduced activation of the contralateral primary SMC (SPM space coordinates: −40, −16, 58; t = 2.47, P < 0.05 after SVC) in patients compared with controls.
Within‐group comparison (patients): Unimpaired vs. impaired hand
In comparison with task performance with the unimpaired hand, during task performance with the impaired hand the primary SMC of the ipsilateral (contralesional) hemisphere (SPM space coordinates: 42, −10, 56; P < 0.05 after SVC) was significantly more activated (see Fig. 3). In contrast, during task performance with the unimpaired hand, the ipsilateral cerebellum (SPM space coordinates: 24, −58, −20; P < 0.05 after SVC), and several motor areas in the contralateral hemisphere, including the primary SMC (SPM space coordinates: −42, −28, 52; P < 0.05 corrected), SII (SPM space coordinates: −50, −22, 16; P < 0.05 after SVC), and thalamus (SPM space coordinates: −14, −14, 20; P < 0.05 after SVC) were significantly more active (see Fig. 3).
Figure 3.
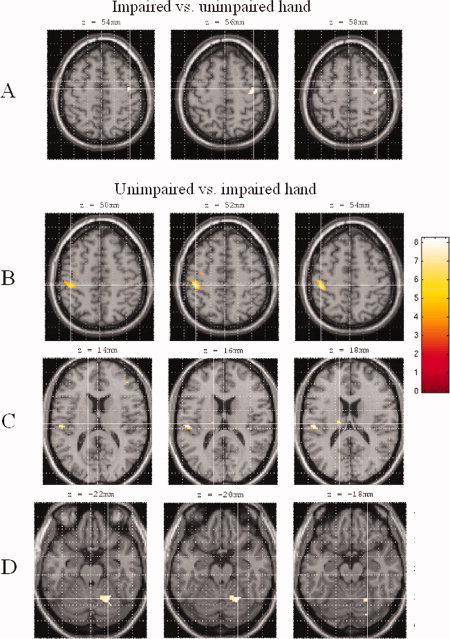
Comparison of cortical activations between task performance with unimpaired and impaired hands. Scans obtained during task performance with the left hand have been flipped in order to keep the left hemisphere contralateral to movement. (A) During task performance with the impaired hand, the primary sensorimotor cortex (SMC) of the ipsilateral hemisphere was significantly more activated than during the unimpaired hand task. (B–D) During task performance with the unimpaired hand, several motor areas in contralateral hemisphere, including the primary SMC (B), secondary sensorimotor area, thalamus (C), and ipsilateral cerebellum (D) were significantly more active than during impaired hand task. Images are in neurological convention. See text for further details.
During task performance with the impaired hand, the volume of the pseudotumoral lesion was significantly correlated with relative activations of the contralateral primary SMC (SPM space coordinates: −48, −18, 54; t = 5.25; r = −0.86), and ipsilateral primary SMC (SPM space coordinates: 32, −18, 58; t = 5.08; r = −0.85) (see Fig. 4). During task performance with the impaired hand, the motor performance, assessed by NHPTr, was significantly correlated with relative activation in the contralateral primary SMC (SPM space coordinates: −32, −16, 46; t = 6.03; r = 0.88) (see Fig. 4). During both tasks, no correlation was found between the extent of movement‐associated cortical activations and disease duration.
Figure 4.
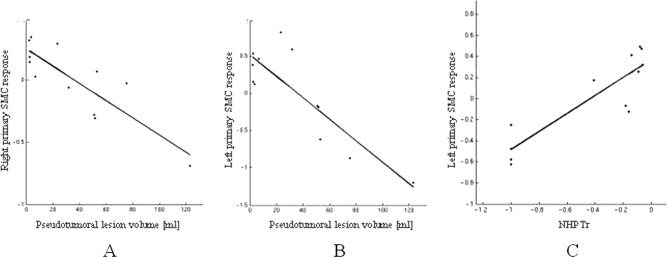
Scatterplots of the correlations between volume of the pseudotumoral lesions and the relative activations of the ipsilateral (A) and controlateral (B) primary SMC and between NHPTr and relative activation of the contralateral primary SMC (C) in patients during task performance with the impaired hand.
Within‐group comparison: Patients with good vs. patients with poor/absent recovery
The comparison of the baseline movement‐associated brain patterns of cortical activations during task performance with the impaired hand between patients with good recovery (seven) and those with poor/absent recovery (five) at 6 months follow‐up showed increased activations (P < 0.001 uncorrected, P < 0.05 after SVC) of the contralateral CMA (SPM space coordinates: −16, −6, 42), contralateral primary SMC (SPM space coordinates: −14, −22, 62), ipsilateral SMA (SPM space coordinates: 10, −16, 48), and SII, bilaterally (SPM space coordinates: right 40, −40, 12, left −48, −44, 10) in the former group (see Fig. 5). Conversely, when contrasted to patients with good recovery, patients with poor/absent recovery at follow‐up had an increased recruitment (P < 0.001 uncorrected, P < 0.05 after SVC) of several regions located in the frontal and parietal lobes, bilaterally, including the superior frontal sulcus (SPM space coordinates: 36, 28, 28 and −36, 30, 40), IFG (SPM space coordinates: −40, 50, −12), CMA (SPM space coordinates: 2, 22, 38 and 8, −42, 56), and IPS (SPM space coordinates: −14, −76, 50).
Figure 5.
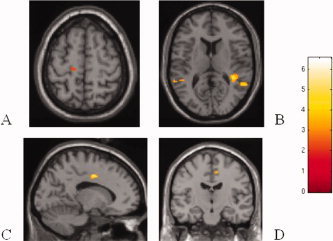
SPMt maps superimposed on high‐resolution T1‐weighted images showing relative cortical activations during task performance with the impaired hand between patients with good recovery and those with poor/absent recovery: (A) Contralateral primary SMC; (B) Bilateral SII; (C) Contralateral CMA; (D) Ipsilateral SMA.
fMRI Results: Longitudinal Evaluation
Longitudinal fMRI study was performed in five healthy controls, four patients with good recovery and two patients with poor/absent recovery (patients 4 and 5 from Table I). During the follow up, no change in the patterns of cortical activations was observed in healthy controls. In patients, the analysis of unimpaired hand movement showed reduced activations over time of the ipsilateral primary SMC (SPM space coordinates: 42, −24, 58; t = 16.74), SMA (SPM space coordinates: 6, −10, 66; t = 15.63), and contralateral SII (SPM space coordinates: −56, −30, 24; t = 34.35). No areas with a significantly increased activation over time were observed. The analysis of impaired hand movement was performed considering the clinical recovery of the patients. In the four patients with a clinical improvement, a progressive recovery of function of the primary SMC of the affected hemisphere together with reduction of ipsilateral primary SMC recruitment were detected (see Fig. 6). On the contrary, in the two patients who showed a poor/absent clinical recovery, there was a persistent recruitment of the ipsilateral primary SMC in the unaffected hemisphere (see Fig. 6).
Figure 6.
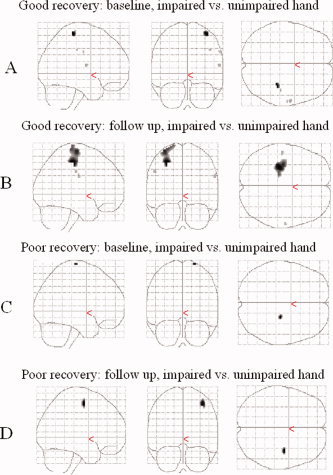
Longitudinal evolution of cortical activations in the primary SMC, bilaterally, during task performance with impaired hand compared to unimpaired hand in one patient with good clinical recovery during follow up (A and B) and in one patient with poor/absent clinical recovery (C and D). Scans obtained during left hand motor task have been flipped in order to keep the left hemisphere contralateral to movement. Activations have been superimposed on a glass brain. At baseline, both patients showed an increased activation of the primary SMC of the unaffected (ipsilateral) hemisphere (A and C). During follow up, the patient with good clinical recovery showed an increased functionality of the primary SMC of the affected hemisphere (B), while the patient with poor/absent clinical recovery continued to show an recruitment of the primary SMC of the unaffected hemisphere (D).
DISCUSSION
Despite the extensive literature describing the movement‐associated patterns of cortical activations in patients with MS, only a single study, performed on an individual patient, has assessed the short‐term fMRI changes following an acute motor relapse [Reddy et al.,2000]. The main problem in planning such studies is that they require a rigorous subject selection based upon clinical and MRI criteria, because MS patients typically have a multifocal symptomathology and multiple T2‐visible lesions with different temporal evolutions, which, in addition, are poorly interrelated. A central aspect of this study is the selection of patients without previous overt neurological symptoms/signs, in the acute‐subacute phase of a new attack completely ascribable to a single pseudotumoral lesion, with similar clinical manifestations at the time of the onset of the symptoms (except for the side affected). The majority of the patients had a previous diagnosis of RR MS, while two of the three patients at their first clinical manifestation had a biopsy‐proven diagnosis of demyelinating disease, compatible with MS. Furthermore, they had a disease dissemination over space and time according to the revised McDonald criteria [Polman et al.,2005]. The use of such stringent inclusion criteria is likely to reduce the ability to generalize our finding to the entire MS population. However, this was an unavoidable requisite to assess whether motor system plasticity follows the same strategies in case of acute damage, independently of its aetiology.
In patients with acute CNS insults of different aetiologies, it is well established that spontaneous clinical recovery may occur. In addition to resolution of the primary insult, cortical adaptive reorganization is one of the mechanisms responsible for recovery after brain injury. In this context, neurophysiologic and neuroimaging studies have provided convincing evidence that the adult human cerebral cortex is capable of significant functional plasticity, which may be due to different substrates, such as an increased axonal expression of sodium channels, synaptic changes, increased recruitment of parallel existing pathways or “latent” connections, and reorganization of distant sites [Calautti and Baron,2003; Cifelli and Matthews,2002].
In the case of MS, fMRI studies of the motor system in clinically stable, uncompromised, patients with RR MS [Filippi et al.,2002b,2004b; Rocca et al.,2002,2005] and CIS [Filippi et al.,2004a; Rocca et al.,2003a,2005] have essentially demonstrated an increased recruitment of “classical” motor areas (including the primary SMC, the SII, and the SMA) of the dominant cerebral hemisphere, which might represent a compensatory mechanism contributing to the maintenance of a normal level of function after tissue damage. A study conducted in clinically stable RR MS patients with a previous hemiparesis [Pantano et al.,2002] showed a marked recruitment of motor areas of the ipsilateral cerebral hemisphere in these patients when contrasted to healthy controls and patients with previous optic neuritis, suggesting that recruitment of parallel existing pathways might contribute to motor recovery. However, fMRI acquisition was performed in these patients at a mean interval of 24 months after the acute episode, therefore no conclusion can be driven regarding the short‐term changes of fMRI patterns of activations and their relation with the clinical outcome. More recently, the follow‐up of these patients demonstrated changes in the recruitment of motor areas over time (characterized by the reduction of the activation of the right primary SMC and left cerebellum) [Pantano et al.,2005]. However, the precise relation between motor system structural damage and the corresponding functional changes remains unclear, because all the patients were studied outside the acute phase and the location and characteristics of the lesions responsible for the motor symptomathology were not evaluated.
In this study, we found early changes in the movement‐associated brain pattern of cortical activations in MS patients following an acute motor relapse when studying both the affected and the unaffected upper limbs. The analysis of the movement‐associated brain pattern of cortical activations during task performance with the unimpaired hand showed, in agreement with previous findings in patients with RR MS and no clinical disability [Rocca et al.,2002] and in CIS patients without motor impairment [Filippi et al.,2004a; Rocca et al.,2003a,2005], an increased activation of several regions mainly located in the contralateral cerebral hemisphere, including the primary SMC, the SMA, the IFG, and the IPS. In line with previous studies on patients with acute/subacute stroke [Ward,2004] and on a single patient with a large demyelinating lesion [Reddy et al.,2000], we also detected significant increased activation of the contralesional (ipsilateral) primary SMC during task performance with the impaired hand. Although EMG monitoring was not performed, patients were carefully monitored during fMRI acquisition and none of them showed mirror movements during tasks performance. Therefore, we can reasonably rule out that the activation of this region reflects additional, involuntary movements, in the resting side. Conversely, the overrecruitment of this region might reflect disinhibition of parallel existing pathways and bilateral representation of motor function. In addition, studies of healthy individuals have shown that ipsilateral primary SMC activity increases with increasing task complexity [Ehrsson et al.,2000; Wexler et al.,1997]. Therefore, it is tempting to speculate that, after an acute motor deficit related to MS, the “recovering” brain might consider a simple task as a complex task, thus using alternative strategies for performing it.
By comparing task performance with the affected and unaffected hand, and by considering the results obtained from the comparisons of tasks performance between patients and controls, our analysis also showed that during task performance with the impaired hand patients had an increased activation of the ipsilateral SII and a reduction of the activation of the ipsilateral cerebellum and the contralateral primary SMC and thalamus. SII is activated by attention, tactile recognition, tactile learning, and memory [Mima et al.,1998]. In addition, neurons from SII project directly to the spinal cord [Dobkin,2003; Galea and Darian‐Smith,1994], indicating that this region might provide alternative pathways for motor controls in case of primary SMC damage. The decreased activation of the contralateral primary SMC might reflect damage of this area due to the large demyelinating lesion, as suggested by the correlation found between the activity of this region and both pseudotumoral lesion volume and motor performance. The decreased activation of the thalamus and cerebellum might instead be the consequence of a functional disconnection and/or diaschisis of anatomical pathways connecting these two regions with the primary SMC.
One of the main topic in MS research is based on the attempt to define objective markers useful for identifying patients at high risk for an unfavorable clinical evolution. In this context, the identification of patients with a reduced reserve of functional recovery after an acute attack might be an important step forward. To study this aspect, we compared, during task performance with the affected hand, the movement‐associated brain patterns of cortical activations in the acute stage of the relapse between patients with and those without clinical recovery at 6‐month follow‐up. Patients with good clinical recovery had a baseline pattern of movement‐associated activations which involved almost exclusively the “classical” areas devoted to simple motor task performance, such as the primary SMC, the SII, the SMA, and the CMA. Conversely, patients with poor/absent recovery showed a widespread recruitment of several regions located in the frontal and parietal lobes, which are involved in different stages and with different roles in movement performance [Picard and Strick,1996; Rizzolatti and Luppino,2001; Rizzolatti et al.,1997]. These results are in line with studies on stroke patients, where an overactivation and overrecruitment of a widespread cortical network has been related to an unfavorable clinical outcome [Calautti and Baron,2003].
Albeit longitudinal fMRI studies were performed only on a limited number of subjects, this analysis gave important insights into the mechanisms of recovery after acute damage in MS. In the four patients with a good clinical recovery, we found a normalization of inter‐hemispheric balance, mainly characterized by a progressive recovery of function of the primary SMC of the affected hemisphere and a reduction of activity of ipsilateral primary SMC. These results are in agreement with the study of a single patients by Reddy et al. [2000] and with several reports on patients with acute and chronic strokes [Calautti and Baron,2003], in whom the recovery of function of the lesioned hemisphere has been associated with a better clinical recovery. These results also suggest that unaffected (ipsilateral) SMC activation may not be necessary for recovery, and, rather, might represent a phenomenon of maladaptive cortical reorganization due to lack/damage of transcallosal inhibition. This hypothesis is supported, on the one hand, by the demonstration of poor or absent clinical improvement in the two patients who continued to show increased activation of the undamaged primary SMC, and, on the other, by the negative correlation found, at baseline, between the activity of this region and the volume of the pseudotumoral lesion, which suggests an abnormal transcallosal inhibition or a functional disconnection (diaschisis) between the primary SMC of two hemispheres. Obviously, we can not completely rule out a possible role of lesion resolution and improvement of the severity of intrinsic lesion damage on the observed clinical recovery. However, considering the absence of significant pseudotumoral lesion volume changes over time and the lack of correlation between pseudotumoral lesion volume and NHPT performance, the previous two factors are likely to play limited contribution in comparison with that of cortical functional changes. Combined with the results of previous studies obtained in patients with acute stroke [Calautti and Baron,2003], these results would suggest a common behavior of mechanisms of brain plasticity in case of acute motor system affections, independent of the nature of the damage.
It is worth noting that, in agreement with the study of Pantano et al. [2005], we found longitudinal changes of the movement‐associated brain pattern of cortical activations also during the analysis of unimpaired hand movement. In addition to practice effect, which has been shown also during the repetition of simple motor sequences in healthy individuals [Dirnberger et al.,2004; Karni et al.,1995; Morgen et al.,2004], we can not exclude that other factors, including the severity of intrinsic lesion damage and the extent of normal‐appearing brain and cord tissues involvements, might have influenced these findings [Filippi and Grossman,2002; Filippi and Rocca,2004].
Clearly, our study is not without limitations. First, even if a good reproducibility of longitudinal fMRI studies has been demonstrated in healthy subjects [Loubinoux et al.,2001; Mattay et al.,1996; Yetkin et al.,1996], caution must be exercised when interpreting fMRI results obtained from pathology because various factors, including abnormalities of the BOLD effect related to inflammatory lesions, could affect the reliability of the data across sessions. Second, in line with several studies performed in patients with stroke we included patients with both left or right hemiparesis and combined their findings. Although, theoretically, a simple motor task should not be affected a great deal by hemispheric dominance, some reports describe a cortical asymmetry, with greater extension of motor areas activations during nondominant hand task [Ziemann and Hallett,2001]. Third, the range of interval between the onset of the attack and the first MRI acquisition is quite heterogeneous, thus not allowing us to completely exclude a different degree of motor recovery among patients at the time of fMRI acquisition. Finally, patients with pseudotumoral lesions and with the inclusion criteria of the present study represent only a small percentage of MS patients, therefore our results need to be replicated on patients with more typical disease forms, in order to be generalized.
REFERENCES
- Barkhof F, Filippi M, Miller DH, Scheltens P, Campi A, Polman CH, Comi G, Ader HJ, Losseff N, Valk J ( 1997): Comparison of MRI criteria at first presentation to predict conversion to clinically definite multiple sclerosis. Brain 120: 2059–2069. [DOI] [PubMed] [Google Scholar]
- Calautti C, Baron JC ( 2003): Functional neuroimaging studies of motor recovery after stroke in adults: A review. Stroke 34: 1553–1566; Review. [DOI] [PubMed] [Google Scholar]
- Cifelli A, Matthews PM ( 2002): Cerebral plasticity in multiple sclerosis: Insights from fMRI. Mult Scler 8: 193–199; Review. [DOI] [PubMed] [Google Scholar]
- Dirnberger G, Duregger C, Lindinger G, Lang W ( 2004): Habituation in a simple repetitive motor task: A study with movement‐related cortical potentials. Clin Neurophysiol 115: 378–384. [DOI] [PubMed] [Google Scholar]
- Dobkin BH ( 2003): Functional MRI: A potential physiologic indicator for stroke rehabilitation interventions. Stroke 34: 23–28. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH, Fagergren A, Jonsson T, Westling G, Johansson RS, Forssberg H ( 2000): Cortical activity in precision‐ versus power‐grip tasks: An fMRI study. J Neurophysiol 83: 528–536. [DOI] [PubMed] [Google Scholar]
- Filippi M, Grossman RI ( 2002): MRI techniques to monitor MS evolution: The present and the future. Neurology 58: 1147–1153; Review. [DOI] [PubMed] [Google Scholar]
- Filippi M, Rocca MA ( 2004): Cortical reorganisation in patients with MS. J Neurol Neurosurg Psychiatry 75: 1087–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi M, Cercignani M, Inglese M, Horsfield MA, Comi G ( 2001): Diffusion tensor magnetic resonance imaging in multiple sclerosis. Neurology 56: 304–311. [DOI] [PubMed] [Google Scholar]
- Filippi M, Rocca MA, Falini A, Caputo D, Ghezzi A, Colombo B, Scotti G, Comi G ( 2002a): Correlations between structural CNS damage and functional MRI changes in primary progressive MS. Neuroimage 15: 537–546. [DOI] [PubMed] [Google Scholar]
- Filippi M, Rocca MA, Colombo B, Falini A, Codella M, Scotti G, Comi G ( 2002b): Functional magnetic resonance imaging correlates of fatigue in multiple sclerosis. Neuroimage 15: 559–567. [DOI] [PubMed] [Google Scholar]
- Filippi M, Rocca MA, Mezzapesa DM, Ghezzi A, Falini A, Martinelli V, Scotti G, Comi G ( 2004a): Simple and complex movement‐associated functional MRI changes in patients at presentation with clinically isolated syndromes suggestive of multiple sclerosis. Hum Brain Mapp 21: 108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi M, Rocca MA, Mezzapesa DM, Falini A, Colombo B, Scotti G, Como G ( 2004b): A functional MRI study of cortical activations associated with object manipulation in patients with MS. Neuroimage 21: 1147–1154. [DOI] [PubMed] [Google Scholar]
- Fink GR, Frackowiak RS, Pietrzyk U, Passingham RE ( 1997): Multiple nonprimary motor areas in the human cortex. J Neurophysiol 77: 2164–2174. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, Turner R ( 1995): Analysis of fMRI time‐series revisited. Neuroimage 2: 45–53. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Price CJ, Buchel C, Worsley KJ ( 1999): Multisubject fMRI studies and conjunction analyses. Neuroimage 10: 385–396. [DOI] [PubMed] [Google Scholar]
- Galea MP, Darian‐Smith I ( 1994): Multiple corticospinal neuron populations in the macaque monkey are specified by their unique cortical origins, spinal terminations, and connections. Cereb Cortex 4: 166–194. [DOI] [PubMed] [Google Scholar]
- Herndon RM ( 1997): Handbook of Neurologic Rating Scales. New York: Demos Vermande. [Google Scholar]
- Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG ( 1995): Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature 377: 155–158. [DOI] [PubMed] [Google Scholar]
- Kurtzke JF ( 1983): Rating neurological impairment in multiple sclerosis: En expanded disability status scale (EDSS). Neurology 33: 1444–1452. [DOI] [PubMed] [Google Scholar]
- Loubinoux I, Carel C, Alary F, Boulanouar K, Viallard G, Manelfe C, Rascol O, Celsis P, Chollet F ( 2001): Within‐session and between‐session reproducibility of cerebral sensorimotor activation: A test–retest effect evidenced with functional magnetic resonance imaging. J Cereb Blood Flow Metab 21: 592–607. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Frank JA, Santha AK, Pekar JJ, Duyn JH, McLaughlin AC, Weinberger DR ( 1996): Whole‐brain functional mapping with isotropic MR imaging. Radiology 201: 399–404. [DOI] [PubMed] [Google Scholar]
- McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, McFarland HF, Paty DW, Polman CH, Reingold SC, Sandberg‐Wollheim M, Sibley W, Thompson A, van den Noort S, Weinshenker BY, Wolinsky JS ( 2001): Recommended diagnostic criteria for multiple sclerosis: Guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol 50: 121–127. [DOI] [PubMed] [Google Scholar]
- Miller DH, Barkhof F, Berry I, Kappos L, Scotti G, Thompson AJ ( 1991): Magnetic resonance imaging in monitoring the treatment of multiple sclerosis: Concerted action guidelines. J Neurol Neurosurg Psychiatry 54: 683–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mima T, Nagamine T, Nakamura K, Shibasaki H ( 1998): Attention modulates both primary and second somatosensory cortical activities in humans: A magnetoencephalographic study. J Neurophysiol 80: 2215–2221. [DOI] [PubMed] [Google Scholar]
- Morgen K, Kadom N, Sawaki L, Tessitore A, Ohayon J, Frank J, McFarland H, Martin R, Cohen LG ( 2004): Kinematic specificity of cortical reorganization associated with motor training. Neuroimage 21: 1182–1187. [DOI] [PubMed] [Google Scholar]
- Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG ( 2000): Multiple sclerosis. N Engl J Med 343: 938–952. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Pantano P, Mainero C, Iannetti GD, Caramia F, Di Legge S, Piattella MC, Pozzilli C, Bozzao L, Lenzi GL ( 2002): Contribution of corticospinal tract damage to cortical motor reorganization after a single clinical attack of multiple sclerosis. Neuroimage 17: 1837–1843. [DOI] [PubMed] [Google Scholar]
- Pantano P, Mainero C, Lenzi D, Caramia F, Iannetti GD, Piattella MC, Pestalozza I, Di Legge S, Bozzao L, Pozzilli C ( 2005): A longitudinal fMRI study on motor activity in patients with multiple sclerosis. Brain 128: 2146–2153. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL ( 1996): Motor areas of the medial wall: A review of their location and functional activation. Cereb Cortex 6: 342–353; Review. [DOI] [PubMed] [Google Scholar]
- Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, Lublin FD, Metz LM, McFarland HF, O'Connor PW, Sandberg‐Wollheim M, Thompson AJ, Weinshenker BG, Wolinsky JS ( 2005): Diagnostic criteria for multiple sclerosis: 2005 Revisions to the “McDonald Criteria.” Ann Neurol 58: 840–846. [DOI] [PubMed] [Google Scholar]
- Reddy H, Narayanan S, Matthews PM, Hoge RD, Pike GB, Duquette P, Antel J, Arnold DL ( 2000): Relating axonal injury to functional recovery in MS. Neurology 54: 236–239. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G ( 2001): The cortical motor system. Neuron 31: 889–901; Review. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V ( 1997): Parietal cortex: From sight to action. Curr Opin Neurobiol 7: 562–567; Review. [DOI] [PubMed] [Google Scholar]
- Rocca MA, Falini A, Colombo B, Scotti G, Comi G, Filippi M ( 2002): Adaptive functional changes in the cerebral cortex of patients with non‐disabling MS correlate with the extent of brain structural damage. Ann Neurol 51: 330–339. [DOI] [PubMed] [Google Scholar]
- Rocca MA, Mezzapesa DM, Falini A, Ghezzi A, Martinelli V, Scotti G, Comi G, Filippi M ( 2003a): Evidence for axonal pathology and adaptive cortical reorganization in patients at presentation with clinically isolated syndromes suggestive of multiple sclerosis. Neuroimage 18: 847–855. [DOI] [PubMed] [Google Scholar]
- Rocca MA, Gavazzi C, Mezzapesa DM, Falini A, Colombo B, Mascalchi M, Scotti G, Comi G, Filippi M ( 2003b): A functional magnetic resonance imaging study of patients with secondary progressive multiple sclerosis. Neuroimage 19: 1770–1777. [DOI] [PubMed] [Google Scholar]
- Rocca MA, Colombo B, Falini A, Ghezzi A, Martinelli V, Scotti G, Comi G, Filippi M ( 2005): Cortical adaptation in patients with MS: A cross‐sectional functional MRI study of disease phenotypes. Lancet Neurol 4: 618–626. [DOI] [PubMed] [Google Scholar]
- Tintoré M, Rovira A, Martinez MJ, Rio J, Diaz‐Villoslada P, Brieva L, Borras C, Grivé E, Capellades J, Montalban X( 2000): Isolated demyelinating syndromes: Comparison of different MRI criteria to predict conversion to clinically definite multiple sclerosis. AJNR Am J Neuroradiol 21: 702–706. [PMC free article] [PubMed] [Google Scholar]
- Yetkin FZ, McAuliffe TL, Cox R, Haughton VM ( 1996): Test‐retest precision of functional MR in sensory and motor task activation. AJNR Am J Neuroradiol 17: 95–98. [PMC free article] [PubMed] [Google Scholar]
- Ward NS ( 2004): Functional reorganization of the cerebral motor system after stroke. Curr Opin Neurol 17: 725–730; Review. [DOI] [PubMed] [Google Scholar]
- Ward NS, Newton JM, Swayne OB, Lee L, Thompson AJ, Greenwood RJ, Rothwell JC, Frackowiak RS ( 2006): Motor system activation after subcortical stroke depends on corticospinal system integrity. Brain 129: 809–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler BE, Fulbright RK, Lacadie CM, Skudlarski P, Kelz MB, Constable RT, Gore JC ( 1997): An fMRI study of the human cortical motor system response to increasing functional demands. Magn Reson Imaging 15: 385–396. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ ( 1995): Analysis of fMRI time‐series revisited—Again. Neuroimage 2: 173–181. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Hallett M ( 2001): Hemispheric asymmetry of ipsilateral motor cortex activation during unimanual motor tasks: Further evidence for motor dominance. Clin Neurophysiol 112: 107–113. [DOI] [PubMed] [Google Scholar]


