Abstract
A coherent and meaningful percept of the world is essential for human nature. Consequently, much speculation has focused on how this is achieved in the brain. It is thought that all conscious experiences have reference to the self. Self‐reference may either be minimal or extended, i.e., autonoetic. In minimal self‐reference subjective experiences are self‐aware in the weak sense that there is something it feels like for the subject to experience something. In autonoetic consciousness, consciousness emerges, by definition, by retrieval of memories of personally experienced events (episodic memory). It has been shown with transcranial magnetic stimulation (TMS) that a medial paralimbic circuitry is critical for self‐reference. This circuitry includes anterior cingulate/medial prefrontal and posterior cingulate/medial parietal cortices, connected directly and via thalamus. We here hypothesized that interaction in the circuitry may bind conscious experiences with widely different degrees of self‐reference through synchrony of high frequency oscillations as a common neural event. This hypothesis was confirmed with magneto‐encephalography (MEG). The observed coupling between the neural events in conscious experience may explain the sense of unity of consciousness and the severe symptoms associated with paralimbic dysfunction. Hum Brain Mapp, 2010. © 2009 Wiley‐Liss, Inc.
Keywords: self‐awareness, consciousness, paralimbic circuitry, asperger syndrome, vegetative state, MEG
INTRODUCTION
When we are awake we may be engaged in an activity demanding retrieval of memories and estimation of the future, such as writing, which is at the focus of our attention. Still we continue to receive a number of unattended sensory stimuli, like hearing the rain outside the window, a bird singing, feeling the comfort or discomfort of the chair, etc. Such sensory input, like our memories, feelings, and emotions, can be conscious when they are attended to, and preconscious when not attended to [Kircher and Leube,2003]. Together, it is all experienced as a single scene, coherent in space and time. Even when someone enters the room, or the phone rings, and the scene and our focus of attention shifts completely, we usually experience the changed scene as continuous with the preceding [Edelman,2004]. The self offers a possibility to understand this coherence: per definition, we cannot have free‐floating sensations with no self to experience them, and we cannot have a self completely devoid of qualia, i.e. sensory experiences, memories or feelings. In other words: qualia and the self are complementary [Ramachandran,2004]. If each of the different, ever‐changing qualia is attached to the same coherent self‐structure across space and time, qualia will be bound to each other via the self. We have previously argued that a circuitry of paralimbic prefrontal and parietal regions might qualify for such binding. This was based on the finding that the circuitry is integrated as a common feature in the different patterns of regional hemodynamic interaction in different mental states and experiences [Cabeza and Nyberg,2000; Greene et al.,2004; Kjaer and Lou,2000; Kjaer et al.,2002; Lou et al.,2005; Northoff and Bermpohl,2004]. The concept is further supported by studies with transcranial magnetic stimulation, showing that activity in this circuitry is critical for self‐awareness and retrieval of memories of personal experiences, i.e. episodic memory [Kwan et al.,2007; Lou et al.,2004]. Episodic memory retrieval is by definition an indispensable component of the more complex forms of self‐awareness and consciousness with a coherent self across time, which are described in various terms such as extended self [Neisser,1988], autonoetic consciousness [Gardiner,2001; Tulving,1985], or narrative self [Gallagher,2000]. In fact, “episodic memory is identified with autonoetic, or self‐knowing, consciousness” [Gardiner,2001]. In contrast to autonoetic self‐awareness, the minimal self is prereflexive, immediate, and normally infallible. It involves the sense of ownership of experiences [Gallagher,2000]. For the minimal self, data on the neural organization are scarcer, but indicate that the same paralimbic structures are active here [Kjaer et al.,2001; Vogeley et al.,2004]. A critical role in consciousness for the medial parietal cortex and its connections with medial prefrontal cortex, directly and via thalamus, is also suggested by the fact that their default is closely correlated with the vegetative state, and their recovery with recovery of consciousness [Laureys et al.,2006].
The focus of the present study is to investigate how these three structures are functionally integrated in a paralimbic circuitry. Theoretically, tight functional integration would be reflected in synchrony in the high frequency range [Jensen et al.,2007; Rodriguez et al.,1999; Tononi and Edelman,2000]. To test this hypothesis, we examined synchronization in the gamma range (30–100 Hz) during three tasks with widely different degrees of self‐reference, assuming that the degree of self‐reference would be reflected in the degree of gamma synchronization. The beta frequency band (15–30 Hz) was used for comparison. Our hypotheses were tested with MEG and dynamic imaging of coherent sources (DICS) [Gross et al.,2001].
SUBJECTS AND METHODS
Subjects
Recordings were obtained from 12 healthy, right‐handed, gender‐matched German subjects with the local ethics committee's approval. All subjects gave their informed consent.
Experimental Procedures
Three conditions were used. Two of these were examples of autonoetic consciousness [Gallagher,2000; Gardiner,2001], requiring episodic retrieval of the subjects' previous personal judgment on two subjects: one‐self (“Self”‐condition), and a well‐known public figure in Germany, the football star Franz Beckenbauer (“Franz”‐condition). The third condition, “Syl”, required calculation of syllables with minimal self‐reference and memory. The three conditions were chosen to represent maximal self‐reference (“Self”), and minimal self‐reference (“Syl”), as well as a condition of intermediate self‐reference (“Franz”).
In each trial a series of adjectives were presented sequentially on a back‐projection screen at a distance of 1.2 m from the subject [Anderson,1968]. Sensory input and motor output were similar in all three conditions. For Self, an initial task required encoding of personal judgment of one‐self by recording how well each adjective of a series fitted one‐self. For Franz, word presentation was preceded by encoding of personal judgment of Franz Beckenbauer, preceded by a 500‐ms long presentation of a fixation cross and a 500‐ms presentation of a blank screen. The presentation time for each word varied randomly between 2,000 and 2,500 ms to make it less straightforward for the subject to predict when to respond. Immediately after word offset, a response screen was presented consisting of the four choices for the judgment condition [— (meaning: does not match at all), − (matches rather not), + (matches reasonably well), +++ (matches absolutely)]. The subject was allowed to take as long as he wished up till 5 s for the decision. Then the series was shown again in the same order, and again with up to 5 s to make the response. Each word was presented again randomly for 2,000–2,500 ms, and up to 5,000 ms allowed for the response. The subjects were here required to respond as to whether the adjective had previously been judged to be rather fitting or not. For retrieval, each word appeared again in the same sequence as encoding. The response was done on a two‐point scale (yes/no). Each response elicited immediate presentation of the next adjective. The approximate time interval between judgment and retrieval of judgment for each adjective was 4 min. This delay, the fact that in the meantime 40 different adjectives had been presented, and the requirement to choose between two options vs. four for encoding, ensured that “retrieval response” was dependent on episodic memory. This procedure required explicit introspection and was closely related to the one described earlier for evaluating episodic memory retrieval of words [Gardiner and Java1991; Tulving,1985]. The encoding process was excluded from analysis, as autonoetic consciousness specifically involves retrieval [Gardiner and Java,1991; Tulving,1985].
In contrast, the third condition required only minimal self‐awareness. It involved the same steps as the Self and Franz conditions: fixation, blank screen, stimulus presentation (adjectives), and response. Here the subject was required to report on a yes/no keyboard response as to whether each of a different set of adjectives similarly presented had an even or odd number of syllables. No encoding or retrieval tasks were applied and analysis was done during determination of whether the adjective had an even or uneven number of syllables took place.
Time‐lines of the tasks of episodic memory retrieval and the syllable task are shown in Figure 1. For the first subject in the study, judgment and retrieval of judgment of one‐self was followed by judgment and subsequent retrieval of judgment of Franz Beckenbauer, and, finally the syllable‐counting task with minimal self‐reference, each evaluating a series of 40 adjectives. This sequence was repeated twice. For the next subject, the order was reversed, and reversed again for the third subject, etc. to avoid any order effect.
Figure 1.
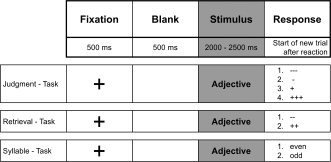
Time lines of experimental tasks. Upper line: Judgment of presented adjectives for fitting one‐self, or, alternatively, Franz Beckenbauer, preceded by fixation cross. Middle line: Autonoetic consciousness by retrieval of previously judged fitting of each adjective: “Self” and “Franz” conditions. Lower line: cognitive task, requiring subject to calculate if presented adjectives have an even or odd number of syllables. This task is without intended memory component and only minimal self‐awareness. “Syl” condition.
MEG
Neural activity was recorded with a Neuromag (Helsinki) 122 whole‐scalp neuromagnetometer [Ahonen,1993] in a magnetically shielded room. MEG signals were recorded with a passband of 0.03–333 Hz and digitized with 1,000 Hz. High‐resolution T1‐weighted magnetic resonance images were obtained for each subject for anatomical coregistration. For each condition, trials were extracted time‐locked to the onset of the word and visually inspected for artifacts. Trials with artifacts were rejected. Three midline regions were analyzed. These were situated between the left and right of the medial paralimbic regions of interest (ROIs) identified in a previous study with positron emission tomography (PET): anterior cingulate/medial‐prefrontal 0, 59, 40; pulvinar thalami: (0, −38, 8); and posterior cingulate/medial parietal region (0, −50, 28). [Talairach coordinates; Lou et al.,2004]. For each ROI, Talairach coordinates were transformed into individual coordinates using the deformation toolbox in SPM2 (http://www.fil.ion.ucl.ac.uk/spm). Time courses of activity were extracted individually for each subject, ROI and condition using a spatial filter [Gross et al.,2001; van Veen et al.,1997; Sekihara et al.,2002]. Conservatively estimated, this method has a spatial resolution of ∼10 mm for the cortical regions, and 20 mm for thalamus [Gross et al.2003]. The time courses were subjected to spectral analysis as a function of time based on multitaper using the fieldtrip toolbox (F. C. Donders Centre for Cognitive Neuroimaging, http://www.ru.nl/fcdonders/fieldtrip). The phase synchronization index (SI) [Varela et al.,2001] was computed for all one by one combinations of the three sites (anterior cingulate/medial prefrontal, posterior cingulate/medial parietal, and thalamus) in the paralimbic loop. SI was not computed on the original time‐series with millisecond resolution. Instead we used the Fourier transformed data (Time‐frequency representation). The Fourier coefficients were used to compute either power or SI. Thus the 400‐ms window and 20‐ms spacing refer to the computation of time‐frequency representation containing the Fourier coefficients. We chose this approach to keep power and SI results comparable. Increases in SI were observed with all three conditions in all one by one sets of interactions between the three regions in the loop during the 2‐s observation periods (Fig. 2, P < 0.001, Wilcoxon). SI quantifies the phase coupling between different regions. It is computed as the absolute value of the sum of the complex phase differences of both regions divided by the number of epochs and is bordered between 0 (indicating no phase locking at all, and 1 (indicating complete phase locking). Synchronization index was computed during a 2‐s stimulus presentation and the preceding 500‐ms prestimulus baseline 2 to 100 Hz in 400‐ms‐long windows with a space of 20 ms between windows. A 400‐ms time window was chosen to allow a multitaper frequency smoothing of ±5 Hz [Gross et al.,2001]. Computations of gamma synchrony with shorter windows of 200 ms were also done as a control of interaction between window length and gamma synchrony. Identical results were obtained. The interdigitating and balanced design of the experiment should preclude any difference between prestimulus baseline for the synchronization indices of the three conditions: Self, Franz, and Syl. Computation confirmed this assumption (P > 0.25, Wilcoxon). Phase synchronization and power results (normalized to per cent change with respect to baseline) were separately subjected to ANOVA analysis. Values were averaged separately for each subject and experimental condition from 0 to 2 s during stimulus presentation in gamma and beta bands. A two‐way ANOVA was computed with factors frequency (gamma, beta) and condition (Self, Franz, Syl). The multicompare function in Matlab (http://www.mathworks.com) was used with multiple comparison correction (Tukey‐Kramer correction) to compute population marginal means.
Figure 2.
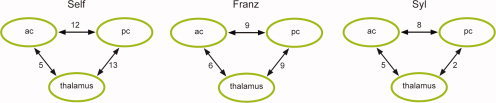
Synchronization between individual paralimbic regions. Increase in synchronization indices between each set of two of the three paralimbic regions in each of the three conditions (P < 0.001 for all values). The numbers close to the arrows indicate percent increase in synchronization in each set of two regions connected by the arrow (means of 12 subjects) to illustrate the effect of stimulus presentation on neural synchrony. Normalization to percent increase are chosen to facilitate comparisons of synchronization effects. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
RESULTS
Behavioral Analysis
In the Self‐condition, mean correct episodic retrieval rate was 94.9% (range 88.3–100%), and in the Franz condition a mean correct response of 90.4 (range 76.7–96.7%). The syllable task produced a mean correct response rate for even or odd number of syllables of 94.7% (range 86.6–99.2%). These high and quite similar numbers indicate that the experimental procedure was adequate. This allowed us to be confident that the subjects did their best to comply with the requirements of the tasks.
MEG
The ANOVA results for phase synchronization (SI) revealed a significant effect of frequency (P < 0.001) and condition (P = 0.028), but no significant interaction. Population marginal means revealed a significantly stronger increase of phase synchronization with respect to baseline for gamma band than for beta band. Marginal means for condition factor showed a gradual increase of SI change from low values of self‐reference in the Syl condition, through medium values for the Franz condition, to the highest values for the Self‐condition. SI increased significantly more for Self than Syl. However, SI increased significantly in all three conditions.
For power, there was a significant effect of frequency (P < 0.001), no significant effect of condition, and no significant interaction between frequency and condition. Further inspection of the frequency factor showed a significant suppression of beta power with respect to baseline and no significant change in gamma power (Figs. 3 and 4). Depression of power in the low frequency range has also been observed in other mental tasks [Neuper, et al.,2006].
Figure 3.
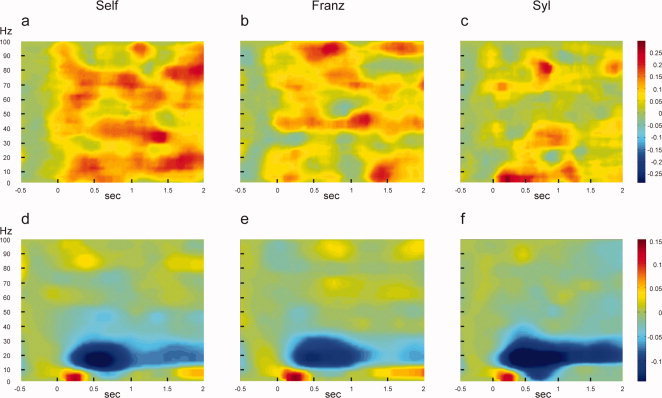
Overall changes in paralimbic synchronization and power in conscious experiences. MEG signals were sampled from midline between bilateral paralimbic regions defined previously in a regional cerebral blood flow study of self‐reference: anterior cingulate/medial‐prefrontal 0, 59, 40; pulvinar thalami: (0, −38, 8); and posterior cingulate/medial parietal region (0, −50, 28) [Talairach coordinates; Lou et al.,2004]. (a–c) Time‐frequency maps, showing increased synchrony in gamma frequency band (30–100 Hz) in all three conditions compared to baseline, a 500‐ms period of rest with open eyes before word onset. The increase depends on the degree of self‐reference (In decreasing order: Self = Retrieval of self‐judgment, Franz = Retrieval of personal judgments of Franz Beckenbauer and Syl = calculation of number of syllables in presented adjectives). (d–f) Time‐frequency maps of power showing low power in beta frequency band for all conditions. Time‐frequency plots are grand‐averages over all subjects and the three pair‐wise connections between paralimbic regions Maps are compilations of 12 subjects. Color scales to the right indicate changes as fraction of 1.
Figure 4.
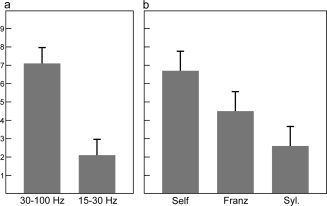
Increase in synchronization index during conscious experience depends on frequency band and condition. Figure 4 shows results from ANOVA analysis of population marginal means, i.e., means after removing the main effects of all other factors in the ANOVA model. The ANOVA factors frequency and condition are computed with multicompare function in Matlab (Mathworks). (a) SI increases (%, error bars: standard error of the mean) predominantly in gamma band (P = 0.0001, ANOVA). (b) SI increases (%, error bars: standard errors of the mean) in all conditions. The increase depends on the degree of self‐reference (Self > Franz > Syl; P = 0.028, ANOVA).
DISCUSSION
Thus the increase in synchronization occurred in all conditions and in both frequency bands. The power analysis suggests that these changes are not the effect of volume conduction [Gross et al.,2001]. The gamma band synchronization occurred preferentially as a function of self‐reference. This preference suggests a role for paralimbic gamma synchrony in self‐reference and is in agreement with the specificity of the paralimbic circuitry for self‐reference documented elsewhere with transcranial magnetic stimulation (TMS) [Kwan et al.,2007; Lou et al.,2004].
As the study exclusively attempted to test the hypothesis of interaction of three paralimbic regions, the results do not exclude that other interactions occur. This seems, in fact very plausible, considering the hemodynamic interaction of several other regions shown previously [Lou et al.,1999, 2004]. It would be in agreement with the influential “global workspace” theory of conscious experience [Baars,2002; Dehaene et al.,2003].
Anatomically, the medial prefrontal/anterior cingulate and medial parietal/posterior cingulate regions are connected directly via the band of white matter in the cingulate gyrus called cingulum, and indirectly, through their rich connections via the “limbic” and intralaminar thalamic nuclei, located centrally at the base of the forebrain [Parvizi et al.,2006]. The connections are reciprocal (i.e. “re‐entrant”), forming a limbic/paralimbic loop [Tononi and Edelman,2000]. We here confirm our hypothesis that the circuitry is preferential for extended self‐awareness in the gamma range. Relatively weak paralimbic gamma synchronization is, however, also seen during a condition with minimal self‐awareness. Being preferential for self and a common neural path for conscious experiences with widely differing degrees of self‐orientation, paralimbic gamma synchronization may be instrumental in coherence of consciousness through self‐reference. Previous hemodynamic studies indicate that this paralimbic circuitry interacts with different cortical regions depending on the contribution of the environment to the content of consciousness [Lou et al.,2004].
The duration of several hundred milliseconds of task‐elicited changes in gamma frequency synchrony and power was a general feature in our experiments (see Fig. 3). They were independent of the duration of the windows (400 and 200 ms), being considerably shorter than the effect on gamma synchrony. The long duration of gamma synchrony is in agreement with global workspace models for consciousness based on sustained activity in long‐range neuronal interaction, and is consistent with the prediction by Dehaene and Naccache [2001], providing sufficient time for consciousness to emerge [Libet et al.,1991].
Each structure in the paralimbic circuitry contributes fundamental properties of extended self‐awareness [Northoff and Bermpohl,2004]: the medial prefrontal cortex is a classical region involved in self‐reference: the orbito‐medial prefrontal region at the base of the frontal lobes is a site for convergence of intero‐ceptive and extero‐ceptive stimuli. It has been called the “entrance door to self‐awareness” based on EEG and MEG studies showing comparatively early engagement after onset of emotional stimuli [Northoff and Bermpohl,2004]. Self‐referential stimuli are then monitored in the dorsal anterior cingulate cortex in the sense that stimuli are selected here among competing stimuli for access to consciousness. The supplementary motor area is closely functionally related to the anterior cingulate cortex in preparation for action, in particular when there are no external cues to tell the subject what to do. Self‐referential stimuli are evaluated in the dorso‐medial prefrontal cortex in the sense that stimuli are here judged to be pertaining to one‐self or other persons [Amodio and Frith,2006]. “Theory of the mind,” or attributing mental states to others, is also associated with activity in the region, which therefore may constitute a link between introspection and understanding others [Amodio and Frith,2006].
These frontal monitoring and evaluative functions of self‐awareness are complemented by functions in the posterior midline establishing spatial and diachronic unity of self and of consciousness: spatial organization in a first person framework involves posterior cingulate/medial parietal cortex [Vogeley et al.,2004] which is also active in linking new information with prior knowledge on the subject matter [Maguire et al.,1999]. In autonoetic consciousness, the region is active in retrieval of episodic memory for autobiographical self‐consciousness [Cabeza and Nyberg,2000]. The functional integrity of medial parietal cortex is critical for extended self‐awareness. This has been shown by TMS, targeting precuneus, to transiently disrupt the normal function of this region [Kwan et al.,2007; Lou et al.,2004]. Thalamus, including intralaminar nuclei, medial pulvinar, and the dorso‐medial nuclei, is connected with paralimbic cortical regions by reciprocal, reentrant connections [Tononi and Edelman,2000]. The central role of the intralaminar nuclei for consciousness is illustrated by the fact that even minute lesions here may cause loss of consciousness [Mesulam,2000]. Activity in the limbic circuit with associated neocortical regions is modulated by loops through the striatum and through the cerebellum. One function of the striatum is dopaminergic sensory gating [Horvitz,2002], and the cerebellum is participating in attention, monitoring and sequencing of events [Allen et al.,1997].
We may now ask: what happens if the paralimbic circuitry is impaired in disease? The present study alone does not allow us to answer this question, but recent studies of pathological conditions have indicated that impairment of coherence of consciousness and self‐awareness are indeed associated with dysfunctional paralimbic circuitry, for instance in autism, a relatively common multigenetic disorder, and in particular in its high‐functioning variant Asperger syndrome. Individuals with Asperger syndrome clearly have a problem with reflecting upon themselves and gaining a coherent overview of their world. In particular they have difficulty identifying and reflecting on their own emotional states. They also have peculiar concrete thought patterns and a tendency to focus on external events rather than inner experiences [Hill and Frith,2003]. In other words: introspection, self‐awareness, and coherence of consciousness are deficient. In autism, low activity and underconnectivity are seen in the medial paralimbic regions [Just et al.,2004; McAlonan et al.,2005]. This finding is in agreement with evidence for volume reduction and reduction in glucose metabolism in the entire cingulate gyrus in autism [Haznedar et al.,2000] and neuropathological studies [Palmen et al.,2004].
Even more pervasive disturbances of consciousness may, of course, be seen in brain damage with coma. If and when coma resides, the patient may attain the vegetative state where he is awake but without awareness of self or environment. The hallmark of the vegetative state is a dysfunction of the polymodal association cortices with posterior cingulate/medial parietal—medial prefrontal disconnection, and intralaminar thalamo‐cortical disconnection. Of special interest is that it has been shown that recovery of consciousness in vegetative patients is linked to restoration of impaired metabolism in paralimbic medial prefrontal and parietal cortices. More specifically, low metabolic activity in medial parietal cortex is the best indicator separating the minimal conscious state from the vegetative state [Laureys et al.,2006].
CONCLUSION AND PERSPECTIVES
It is concluded that widely different conscious experiences are linked by increased synchrony in a paralimbic circuitry which has previously been shown to be critical for self‐reference [Kwan et al.,2007; Lou et al.,2004]. The synchronization is preferential for self‐reference in the gamma range.
In pathology, its dysfunction is linked to fractionation and impairment of consciousness, which may be ameliorated by thalamic stimulation [Schiff et al.,2007]. At term‐equivalent age functional magnetic resonance imaging (fMRI) studies show nascent synchroneous hemodynamic paralimbic activity independent on external stimuli in these regions [Fransson et al.,2007], and the connections are anatomically available at around the 24th week of gestation [Lee et al.,2005]. Our discovery that interacting paralimbic regions provide a neural basis of coherence in consciousness therefore may become useful in defining the time of transition for the fetus into a viable, self‐aware individual [Lagercrantz,2007].
Acknowledgements
The authors thank Michael Posner and Klaus Linkenkaer‐Hansen for invaluable comments, Stephanie Franzkowiak for assistance in preparation of MEG studies, Henriette Vuust for assistance in preparation of illustrations.
REFERENCES
- Ahonen A ( 1993): 122‐channel squid instrument for investigating the magnetic signals from the human brain. Phys Script T49: 198–205. [Google Scholar]
- Allen G, Buxton RB, Wong EC, Courchesne E ( 1997): Attentional activation of the cerebellum independent of motor involvement. Science 275: 1940–1943. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD ( 2006): Meeting of minds: The medial frontal cortex and social cognition. Nat Rev Neurosci 7: 268–277. [DOI] [PubMed] [Google Scholar]
- Anderson NH ( 1968): Likableness ratings of 555 personality‐trait words. J Pers Soc Psychol 9: 272–279. [DOI] [PubMed] [Google Scholar]
- Baars BJ ( 2002): The conscious access hypothesis: Origins and recent evidence. Trends Cogn Sci 6: 47–51. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L ( 2000): Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci 12: 1–47. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Naccache L ( 2001): Towards a cognitive neuroscience of consciousness: Basic evidence and a workspace framework. Cognition 79: 1–37. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Sergent C, Changeux JP ( 2003): A neural network model linking subjective reports and objective physiological data during conscious perception. Proc Natl Acad Sci USA 100: 8520–8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman GM 2004. Wider than the Sky. New Haven: Yale University Press; 7 p. [Google Scholar]
- Fransson P, Skiöld B, Horsch S, Nordell A, Blennow M, Lagercrantz H, Aden U ( 2007): Resting‐state networks in the infant brain. Proc Natl Acad Sci USA 140: 15531–15536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher S ( 2000): Philosophical conceptions of the self: Implications for cognitive science. Trends Cogn Sci 4: 14–21. [DOI] [PubMed] [Google Scholar]
- Gardiner JM ( 2001): Episodic memory and autonoetic consciousness: A first person approach. Philos Trans R Soc Lond B Biol Sci 356: 1351–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner JM, Java RI ( 1991): Forgetting in recognition memory with and without recollective experience. 19: 617–623. [DOI] [PubMed] [Google Scholar]
- Greene JD, Nystrom LE, Engell AD, Darley JM, Cohen JD ( 2004): The neural bases of cognitive conflict and control in moral judgment. Neuron 44: 389–400. [DOI] [PubMed] [Google Scholar]
- Gross J, Kujala J, Hamalainen M, Timmermann L, Schnitzler A, Salmelin R ( 2001): Dynamic imaging of coherent sources: Studying neural interactions in the human brain. Proc Natl Acad Sci USA 98: 694–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J, Timmermann L, Kujala J, Salmelin R, Schnitzler A ( 2003): Properties of MEG tomographic maps obtained with spatial filtering. Neuroimage 19: 1329–1336. [DOI] [PubMed] [Google Scholar]
- Haznedar MM, Buchsbaum MS, Wei OR, Cartwright C, Bienstock CA, Hollander E ( 2000): Limbic circuitry in patients with autism spectrum disorders studied with positron emission tomography and magnetic resonance imaging. Am J Psychiatry 157: 1994–2001. [DOI] [PubMed] [Google Scholar]
- Hill EL, Frith U ( 2003): Understanding autism: Insights from mind and brain. Philos Trans R Soc Lond B Biol Sci 358: 281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz JC ( 2002): Dopamine gating of glutaminergic sensori‐motor and incentive motivational input signals to the striatum. Behav Brain Res 137: 65–74. [DOI] [PubMed] [Google Scholar]
- Jensen O, Kaiser J, Lachaux J‐P ( 2007): Human gamma‐frequency oscillations associated with attention and memory. Trends Neurol Sci 30: 317–324. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassy VL, Keller TA, Minshew NJ ( 2004): Cortical activation and synchronization during sentence comprehension in high‐functioning autism: Evidence of under‐connectivity. Brain 127: 1811–1821. [DOI] [PubMed] [Google Scholar]
- Kircher TT, Leube DT ( 2003): Self‐consciousness, self‐agency, and schizophrenia. Consc Cogn 12: 656–669. [DOI] [PubMed] [Google Scholar]
- Kjaer TW, Lou HC ( 2000): Interaction between precuneus and dorsolateral prefrontal cortex may play a unitary role in consciousness. Consciousness Cogn 9: S59 (abstract). [Google Scholar]
- Kjaer TW, Nowak M, Lou AR, Lou HC ( 2001): Precuneus‐prefrontal activity during awareness of visual verbal stimuli. Consciousness Cogn 10: 356–365. [DOI] [PubMed] [Google Scholar]
- Kjaer TW, Nowak M, Lou HC ( 2002): Reflective self‐awareness and conscious states: PET evidence for a common midline parietofrontal core. Neuroimage 17: 1080–1086. [PubMed] [Google Scholar]
- Kwan VS, Barrios V, Ganis G, Gorman J, Lange C, Kumar M, Shepard A, Keenan JP ( 2007): Assessing the neural correlates of self‐enhancement bias: A transcranial magnetic stimulation study. Exp Brain Res 182: 379–385. [DOI] [PubMed] [Google Scholar]
- Lagercrantz H ( 2007): The emergence of the mind—A borderline of human viability? Acta Paediat 96: 327–328. [DOI] [PubMed] [Google Scholar]
- Laureys S, Boly M, Maquet P ( 2006): Tracking the recovery of consciousness from coma. J Clin Invest 116: 1823–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Ralston HJP, Drey EA, Partridge JC, Rosen MA ( 2005): Fetal pain. A systematic multi‐disciplinary review of the evidence. J Am Med Ass 244: 947–954. [DOI] [PubMed] [Google Scholar]
- Libet B, Pearl DK, Morledge DE, Gleason CA, Hosobuchi Y, Barbaro NM ( 1991): Control of the transition from sensory detection to sensory awareness in man by the duration of a thalamic stimulus. Brain 114: 2505–2520. [DOI] [PubMed] [Google Scholar]
- Lou HC, Kjaer TW, Friberg L, Wildschiodtz G, Holm S, Nowak M ( 1999): A 15O‐H2O PET study of meditation and the resting state of normal consciousness. Hum Brain Mapp 7: 98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou HC, Luber B, Crupain M, Keenan JP, Nowak M, Kjaer TW, Sackeim HA, Lisanby SH ( 2004): Parietal cortex and representation of the mental self. Proc Natl Acad Sci USA 101: 6827–6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou HC, Nowak M, Kjaer TW ( 2005): The mental self. Prog Brain Res 150: 197–204. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Frith C, Morris RG ( 1999): The functional neuroanatomy of comprehension and memory: The importance of prior knowledge. Brain 122: 1839–1850. [DOI] [PubMed] [Google Scholar]
- McAlonan GM, Cheung V, Cheung C, Suckling J, Lam GY, Tai KS, Murphy DG, Chua SE ( 2005): Mapping the brain in autism. A voxel‐based MRI study of volumetric differences and intercorrelations in autism. Brain 128: 268–276. [DOI] [PubMed] [Google Scholar]
- Mesulam MM ( 2000): Principles of Behavioral and Cognitive Neurology. New York: Oxford University Books; p 73. [Google Scholar]
- Neisser U ( 1988): Five kinds of self‐knowledge. Philos Psychol 1: 35–59. [Google Scholar]
- Neuper C, Wörtz M, Pfurtscheller G ( 2006): ERD/ERS patterns reflecting sensorimotor activation and deactivation. Prog Brain Res 159: 211–222. [DOI] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F ( 2004): Cortical midline structures and the self. Trends Cogn Sci 8: 102–107. [DOI] [PubMed] [Google Scholar]
- Palmen SJ, van Engeland H, Hof PR, Schmitz C ( 2004): Neuropathological findings in autism. Brain 127: 2572–2583. [DOI] [PubMed] [Google Scholar]
- Parvizi J, Van Hoesen GV, Buckwalter J, Damasio A ( 2006): Neural connections of the posteromedial cortex in the macaque. Proc Natl Acad Sci USA 103: 1563–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran VS ( 2004): A Brief Tour of Human Consciousness. New York: PI Press; p 96. [Google Scholar]
- Rodriguez E, George N, Lachaux JP, Martinerie J, Renault B, Varela FJ ( 1999): Perception's shadow: Long‐distance synchronization of human brain activity. Nature 397: 430–433. [DOI] [PubMed] [Google Scholar]
- Schiff ND, Giacino JT, Kalmar K, Victor JD, Baker K, Gerber M, Fritz B, Eisenberg B, Bionchi T, O'Connor J, Kobylarz EJ, Farris S, Machado A, McCagg C, Plum F, Fins JJ, Rezai AR ( 2007): Behavioral improvements with thalamic stimulation after severe traumatic brain injury. Nature 448: 600–603. [DOI] [PubMed] [Google Scholar]
- Sekihara K, Nagaraja SS, Poeppel D, Marantz A, Miyashita Y ( 2002): Application of an MEG eigenspace beamformer to reconstructing spatio‐temporal activities of neural sources. Hum Brain Mapp 15: 199–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Edelman GM ( 2000): Schizophrenia and the mechanism of conscious integration. Brain Res Brain Res Rev 31: 391–400. [DOI] [PubMed] [Google Scholar]
- Tulving E ( 1985): Memory and consciousness. Can Psychol 26: 1–12. [Google Scholar]
- van Veen B, van Drongelen W, Yuchtman M, Suzuki A ( 1997): Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans Biomed Eng 44: 867–880. [DOI] [PubMed] [Google Scholar]
- Varela F, Lachaux JP, Rodriguez E, Martinerie J ( 2001): The brainweb: Phase synchronization and large‐scale integration. Nat Rev Neurosci 2: 229–239. [DOI] [PubMed] [Google Scholar]
- Vogeley K, May M, Ritzl A, Falkai P, Zilles K, Fink GR ( 2004): Neural correlates of first‐person perspective as one constituent of self‐consciousness. J Cogn Neurosci 16: 817–827. [DOI] [PubMed] [Google Scholar]


