Abstract
The pattern of degenerative changes in the brain white matter (WM) in aging, mild cognitive impairment (MCI), and Alzheimer's disease (AD) has been under debate. Methods of image analysis are an important factor affecting the outcomes of various studies. Here we used diffusion tensor imaging (DTI) to obtain fractional anisotropy (FA) measures of the WM in healthy young (n = 8), healthy elderly (n = 22), MCI (n = 8), and AD patients (n = 16). We then applied “tract‐based spatial statistics” (TBSS) to study the effects of aging, MCI, and AD on WM integrity. Our results show that changes in WM integrity (that is, decreases in FA) are different between healthy aging and AD: in healthy older subjects compared with healthy young subjects decreased FA was primarily observed in frontal, parietal, and subcortical areas whereas in AD, compared with healthy older subjects, decreased FA was only observed in the left anterior temporal lobe. This different pattern of decreased anatomical connectivity in normal aging and AD suggests that AD is not merely accelerated aging. Hum Brain Mapp, 2009. © 2008 Wiley‐Liss, Inc.
Keywords: aging, Alzheimer's disease, DTI, white matter, connectivity
INTRODUCTION
Normal aging is associated with a global decline in cognitive functions. Attention, information processing, and working memory are particularly compromised in the elderly [Craik and Salthouse, 2000; Salthouse and Ferrer‐Caja, 2003]. In Alzheimer's disease (AD), an even more prominent decline in cognitive function is observed. Memory function is primarily compromised along with impairment in at least one other cognitive domain such as language or visuospatial abilities [Lindeboom and Weinstein, 2004; Pasquier, 1999]. In addition to cognitive impairment, aging and AD are both also related to changes in brain function and anatomy.
In this study we investigated the anatomical connectivity of the brain in aging, mild cognitive impairment (MCI), and AD. It has been suggested that classic approaches towards the anatomy‐cognitive function relation (based on correlations) are not sufficient in understanding a dynamic system like the brain [Stephan, 2004]. As an alternative to study brain function and anatomy, studies of connectivity of neural subsystems using several imaging techniques such as EEG, MEG, and MRI, have attracted considerable interest in recent years (for a review see [Uhlhaas and Singer, 2006]). Although it seems that functional connectivity is shaped at least in some part by anatomical connectivity, the nature of the relationship between these two remains uncertain [Kotter and Sommer, 2000; Sporns et al., 2004; Stephan, 2004]. While knowledge of functional connectivity changes in the brain in aging and dementia has improved in recent years (e.g. [Greicius et al., 2004; Stam et al., 2006]), less is currently known about the underlying anatomical connectivity. The main cause is lack of techniques and technical limitations of existing techniques to measure anatomical brain connectivity in vivo. One approach to study anatomical connectivity is to correlate regional cortical thickness measures using anatomical MRI [He et al., 2007]. Another more frequently applied technique is diffusion tensor imaging (DTI). DTI is based on measurement of characteristics of water diffusion, i.e. fractional anisotropy (FA), apparent diffusion coefficient (ADC) and diffusion direction (principle eigenvector) in the brain. The fact that water molecules will more readily diffuse along the major axis of the fiber bundle than perpendicular to it [Moseley et al., 1990] provides the basis for in vivo visualization of white matter (WM) tracts [Le Bihan, 2003]. One of the most important factors affecting the FA value is the integrity of axons and their myelin sheaths. Degeneration of neural tracts can therefore be detected by measurement of FA value [Gupta et al., 2006].
Previous studies into aging and WM tract integrity generally showed compatible results: reduced FA in healthy elderly subjects most prominently in frontal WM areas and the anterior corpus callosum [Head et al., 2004; Pfefferbaum et al., 2005; Salat et al., 2005; Sullivan and Pfefferbaum, 2006; Sullivan et al., 2006]. However, some other studies showed decreased FA in parietal and temporal WM [Lehmbeck et al., 2006] or lower global FA across the brain [Madden et al., 2004]. For MCI and AD the results of previous studies are more variable than those seen in aging. Most studies reported greater decrease of FA in frontal areas, however, the location of additional FA changes varied, e.g. additional changes were observed in parietal [Medina et al., 2006], temporal [Naggara et al., 2006; Takahashi et al., 2002] or both parietal and temporal lobes [Bozzali et al., 2002; Huang and Auchus, 2007; Xie et al., 2006]. There may be several explanations for these ambiguous results, such as differences in sample composition (mild vs. moderate AD patients, amnestic MCI vs. multi‐domain MCI) and the methods used for analyzing the data. Several previous studies have applied a voxelwise comparison of whole‐brain anisotropy using voxel‐based morphometry (VBM), e.g. [Naggara et al., 2006; Xie et al., 2006]. This technique was originally developed for finding GM density changes in T1‐weighted MRI images [Ashburner and Friston, 2000] and has potential pitfalls mainly related to registration and smoothing [Bookstein, 2001]. Other methods like ROI analysis and tractography [Behrens et al., 2003; Conturo et al., 1999], although useful for specific regions or tracts, are affected by the accuracy of the manually defined region of interests (ROI). For a more extensive discussion on the limitations of these approaches, see Smith et al. [ 2006]. Because of the atrophy commonly observed in AD patients, misalignment of the data is a common problem when studying these patients. A VBM analysis therefore may not be the most appropriate approach for analyzing these data. Tract‐based spatial statistics (TBSS, [Smith et al., 2006]) is a recently developed fully automated whole brain analysis technique that uses voxelwise statistics on FA data but simultaneously minimizes the effects of misalignment [Smith et al., 2006]. TBSS provides more consistent results across subjects and sessions than after VBM‐preprocessing or manual placement of ROIs [Smith et al., 2006]. In this study we used TBSS to investigate the effect of normal aging, MCI, and AD, on anatomical connectivity (i.e. WM tract integrity) and additionally explored the association between WM tract integrity and cognitive function.
MATERIALS AND METHODS
Subjects
Diffusion tensor images were obtained from four groups of right‐handed participants: 8 young healthy subjects (age 22.8 ± 2.5, range 19–26; mini mental status examination (MMSE) 29.5 ± 0.5; 4 female), 22 old healthy subjects without memory complaints (age 70.7 ± 6.0, range 60–81 years; MMSE 28.7 ± 1.4; 13 female), 8 patients with MCI (age 73.9 ± 4.9 years, range 65–81 years; MMSE 25.9 ± 2.6; 5 female), and 16 patients with mild AD (age 69.5 ± 6.9 years, range 59–79 years; MMSE 22.9 ± 3.2; 8 female). Patients were recruited at the Alzheimer Center of the VU University Medical Center, Amsterdam, the Netherlands. MCI patients were diagnosed using criteria for amnestic MCI [Petersen et al., 2001a], and clinical dementia rating (CDR) scale scores of 0.5 [Morris, 1993]. Diagnostic criteria of AD were that of NINCDS‐ADRDA [McKhann et al., 1984], with MMSE scores >18 and CDR < 2. These values correspond to what is known as mild AD. Healthy subjects were recruited by two means: (1) asking family members of patients and (2) advertisements posted in the medical center, the medical faculty of the university and activity centers for the elderly in the community. The Ethical Review Board of the VU University Medical Center Amsterdam approved the study. All subjects provided informed consent; patients under supervision of a lawful caregiver if necessary. Participants were excluded if they had any significant medical, neurological (except for the diseases under study here in the patient groups), or psychiatric illness; a history of brain damage; or if they were taking medication known to influence cerebral function (except for AD medication in the AD group). T2‐weighted fluid attenuation inversion recovery (FLAIR) scans of each subject were reviewed by a neuroradiologist to assess the presence of vascular lesions. Subjects with WM abnormalities outside the normal range were excluded from participation in the study. Evenly distributed across all groups (except for the young healthy subjects) some, probably age‐related, WM abnormalities were observed (29 subjects with Fazekas‐score range 1–3 (1.21 ± 0.41) [Fazekas et al., 1987]; and three subjects with 1–3 lacunes).
Neuropsychological Assessment
All participants underwent an MMSE, a geriatric depression scale (GDS) and the Dutch version of the new adult reading test (NLV), as an indicator of (premorbid) IQ, followed by an extensive neuropsychological test battery including tests measuring attention/concentration, processing speed, episodic memory, executive functioning and praxis (see Table I for details). Scores on neuropsychological tests are compared between groups using t tests for the aging data (younger versus older healthy subjects) and for the patient data (AD, MCI, and older healthy subjects). The significance threshold was set at P < 0.0005 after correction for multiple comparisons.
Table I.
Patient characteristics and neuropsychological profile
| YHS M ± SD | OHS M ± SD | MCI M ± SD | AD M ± SD | YHS vs. OHS t | AD vs. OHS t | MCI vs. OHS t | MCI vs. AD t | |
|---|---|---|---|---|---|---|---|---|
| Gender (male/female) | 4/4 | 9/13 | 3/5 | 8/8 | 0.16 | 0.54 | 0.16 | 0.56 |
| Age | 22.8 ± 2.7 | 70.7 ± 6.0 | 73.9 ± 4.9 | 69.5 ± 6.7 | 21.80* | 0.60 | 1.33 | 1.63 |
| MMSE | 29.6 ± 05 | 28.7 ± 1.4 | 25.9 ± 2.6 | 22.9 ± 3.2 | 1.77 | 6.78* | 2.91 | 2.26 |
| GDS | 0.13 ± 0.4 | 0.6 ± 1.0 | 1.1 ± 1.2 | 1.2 ± 1.2 | 1.34 | 1.51 | 1.07 | 0.10 |
| NLV | 100 ± 14.5 | 110.7 ± 18.7 | 111 ± 17.3 | 102.5 ± 19.7 | 1.45 | 1.29 | 0.04 | 1.03 |
| Attention, concentration, and speed | ||||||||
| Digit span | ||||||||
| Forward span | 6.3 ± 1.0 | 6.2 ± 1.0 | 6.0 ± 0.6 | 5.0 ± 1.4 | 0.05 | 3.07 | 0.52 | 1.65 |
| Backward span | 5.5 ± 0.8 | 5.1 ± 0.9 | 5.3 ± 1.5 | 3.9 ± 1.0 | 1.03 | 4.07* | 0.41 | 2.64 |
| WAIS Symbol substitution/encoding | 81.4 ± 13.6 | 58.7 ± 15.3 | 48.9 ± 6.3 | 36.8 ± 21.4 | 3.69 | 3.65 | 2.50 | 2.03 |
| Trail making test A | 27.9 ± 9.2 | 43.6 ± 15.4 | 45.8 ± 12.2 | 72.2 ± 29.0 | 2.68 | 3.50 | 0.36 | 3.06 |
| Stroop | ||||||||
| Word card | 39.6 ± 6.1 | 46.2 ± 6.4 | 54.3 ± 9.3 | 54.7 ± 13.6 | 2.51 | 2.32 | 2.66 | 0.08 |
| Color card | 54.1 ± 7.1 | 60.8 ± 11.0 | 74.8 ± 23.1 | 84.7 ± 26.0 | 1.54 | 3.46 | 2.26 | 0.91 |
| Color‐word card | 78 ± 17.4 | 116.7 ± 37.3 | 144.5 ± 37.1 | 180.3 ± 72.8 | 2.80 | 2.94 | 1.74 | 1.29 |
| Episodic memory | ||||||||
| 15 word test | ||||||||
| Total immediate recall | 55 ± 6.3 | 42.9 ± 10.7 | 21.6 ± 6.1 | 21.9 ± 5.9 | 3.00 | 7.75* | 5.31* | 0.10 |
| Delayed recall | 11.8 ± 2.3 | 8.4 ± 3.1 | 0.9 ± 1.1 | 1.6 ± 1.7 | 2.76 | 8.57* | 9.70* | 1.12 |
| Visual association test | ||||||||
| A | 12 ± 0.0 | 11.5 ± 1.1 | 7.1 ± 4.1 | 5.1 ±3.8 | 2.13 | 6.47* | 2.97 | 1.18 |
| B | 11.5 ± 0.8 | 10.6 ± 1.5 | 5.1 ± 4.4 | 3.1 ± 3.6 | 3.19 | 4.85* | 2.40 | 1.22 |
| WAIS Symbol substitution/memory | ||||||||
| Cued reproduction | 15 ± 3.9 | 9.4 ± 4.0 | 1.9 ± 2.6 | 2.5 ± 2.8 | 3.35 | 5.37* | 4.85* | 0.47 |
| Free reproduction | 8.1 ± 1.0 | 6.4 ± 1.6 | 4.0 ± 2.1 | 3.1 ± 1.5 | 2.88 | 6.19* | 3.38 | 1.17 |
| Memory impairment screen plus | ||||||||
| Direct reproduction | 12 ± 0.0 | 11.0 ± 1.6 | 10.9 ± 1.1 | 8.8 ± 2.5 | 2.98 | 3.40 | 0.62 | 2.73 |
| Delayed reproduction | 11.8 ± 0.5 | 10.4 ± 1.7 | 3.1 ± 2.2 | 2.9 ± 2.7 | 3.36 | 9.67* | 10.91* | 0.25 |
| Executive function | ||||||||
| WISC maze | ||||||||
| Total time | 118.3 ± 26.5 | 217.5 ± 87.8 | 278 ± 188.8 | 299 ± 147.1 | 4.74* | 2.12 | 1.08 | 0.28 |
| Mistakes | 0.6 ± 0.5 | 2.5 ± 1.6 | 4.0 ± 4.3 | 4.6 ± 4.1 | 3.10 | 1.91 | 1.14 | 0.30 |
| Trail making test B | 57.5 ± 18.7 | 101.1 ± 44.2 | 128.6 ± 38.0 | 251.4 ± 134.2 | 3.78 | 4.26 | 2.27 | 3.21 |
| Fluency | ||||||||
| Animals 2 min | 43.3 ± 5.4 | 35.2 ± 7.9 | 26.9 ± 8.8 | 18.3 ± 6.7 | 2.65 | 6.82* | 2.49 | 2.63 |
| Insects 1 min | 13.8 ± 2.7 | 9.0 ± 3.1 | 5.5 ± 1.8 | 4.7 ± 2.4 | 3.87 | 4.50* | 2.97 | 0.86 |
| Praxis | ||||||||
| Rey complex figure | ||||||||
| Copy | 34.9 ± 1.1 | 32.7 ± 3.0 | 31.6 ± 8.3 | 25.9 ± 8.6 | 1.57 | 2.76 | 0.53 | 1.51 |
| Organization | 4.6 ± 1.8 | 3.3 ± 2.0 | 2.9 ± 2.2 | 1.9 ± 1.9 | 2.61 | 2.00 | 0.53 | 1.05 |
YHS, younger healthy subjects; OHS, older healthy subjects; MCI, mild cognitive impairment; AD, Alzheimer's disease; MMSE, mini mental status examination; GDS, geriatric depression scale; NLV, the Dutch version of the New Adult Reading test; WAIS, Wechsler adult intelligence scale; WISC, Wechsler intelligence scale for children; TMT, trail making test.
Significant at P = 0.0005.
Imaging Methods
Magnetic resonance imaging (MRI) examinations were conducted on a 1.5T Sonata system (Siemens, Erlangen, Germany), including a T1‐weighted 3D gradient sequence (TR = 2,700 ms; TE = 3.97 ms; flip angle = 8°, 160 coronal slices; voxel size: 1 × 1.5 × 1 mm3). DTI was measured using an echo planar imaging (EPI) sequence [Reese et al., 2003] with the following specifications: TR = 8500 ms, TE = 86 ms, voxel size: 2‐mm isotropic, 59 consecutive slices, acquisition matrix 128 mm × 128 mm (FOV = 256 mm), 6/8 partial Fourier, 60 diffusion directions with b‐value = 700 s/mm2, and 10 images with no diffusion weighting. The bandwidth was 1860 Hz/pixel. Head motion was minimized by the use of tightly padded clamps attached to the head coil.
Data Analysis
Statistical analysis of the FA data was carried out using TBSS [Smith et al., 2006] part of FSL [Smith et al., 2004]. First, raw DTI images were corrected for motion and eddy current effects. Subsequently, FA images were created by fitting the diffusion tensor to the raw diffusion data and then brain‐extracted [Smith, 2002]. The next analysis steps were performed separately for the aging data (younger and older healthy subjects) and the patient data (older healthy subjects, MCI, and AD). All subjects' FA data were aligned into a common space using non‐linear registration as applied in the ‘image registration toolkit’ (IRTK) [Rueckert et al., 1999]. For each dataset (aging and patient) a study‐specific target image was selected by aligning every FA image to every other one and then identifying the most representative one. Next, the mean FA image was created and thinned to create a mean FA skeleton, which represents the centers of all tracts common to the groups. Each subject's aligned FA data was then projected onto this skeleton and the resulting data was fed into cross‐subject statistics. Differences in global FA integrity were calculated by comparing mean FA values within the skeleton mask (thresholded at a mean FA value of 0.25) between groups using nonparametric two‐sample Mann‐Whitney U‐tests. Voxelwise statistics were performed using a permutation‐based inference tool for nonparametric statistical thresholding (“randomize,” part of FSL). Further specific details of the analyses are given in [Smith et al., 2006]. All voxelwise group comparisons were performed using simple two‐sample t tests. The group's mean FA skeleton was used as a mask (thresholded at a mean FA value of 0.25) and the number of permutations was set to 5000. The significance threshold for between‐group differences was set at P < 0.05 (corrected for multiple comparisons across voxels), using the threshold‐free cluster‐enhancement option in the “randomize” permutation‐testing tool in FSL [Smith and Nichols, 2007].
In an additional exploratory analysis the presence of correlations between patient characteristics and neuropsychological measures, and mean FA was assessed. The mean FA value per subject was extracted out of the WM areas showing significant between‐group differences in the contrast younger versus older healthy subjects and AD versus older healthy subjects. The patient characteristics and neuropsychological measures included in the correlation analyses were those measures that differed significantly between groups. Correlation analyses were performed using Spearman's rho one‐tailed, corrected for multiple comparisons.
RESULTS
Neuropsychological Profile of Each Group
In Table I the patient characteristics and neuropsychological profile of each group, and comparisons between groups are presented. The older healthy subjects showed worse performance than younger subjects on the Wechsler intelligence scale for children (WISC) maze, total time; a neuropsychological test measuring executive functioning. AD patients performed worse than older healthy subjects on tests across all cognitive domains measured with this test‐battery except for praxis. Specifically, worse performance was demonstrated on the Digit span, backward span; all episodic memory tests (15 word test, Visual association test (VAT), Wechsler adult intelligence scale (WAIS) substitution/memory, Memory impairment screen (MIS) plus); and the Fluency task. Impaired performance in MCI patients compared to older healthy subjects was only observed in tests measuring episodic memory function, i.e. in the 15 word test, WAIS substitution/memory cued reproduction, and MIS plus delayed reproduction. No significant differences in cognitive function were found between MCI and AD patients.
Changes in Fractional Anisotropy Across the Groups
Figure 1 depicts mean global FA values per group. A significant decrease in global FA integrity (i.e. average FA across all skeleton voxels) was shown in older subjects compared with younger (OHS < YHS) (P = 0.008, one‐tailed) and for AD versus MCI (P < 0.047, one‐tailed). In AD versus older healthy subjects a trend towards a reduced global FA was shown (P < 0.065, one‐tailed), this is probably less significant than the AD versus MCI difference because of the high spread in mean FA across the healthy older group. No difference in global FA values was observed when comparing MCI patients with the older healthy subjects. The spatial distribution of significant differences between the groups in FA (across all voxels within the skeleton mask) is shown in Figure 2. Compared with the younger subjects, the older healthy subjects showed significantly lower FA values in the frontal, parietal and temporal lobes, corpus callosum (particularly the genu and body) and the internal capsule (P < 0.05, corrected). These changes in FA were mainly bilateral, although after visual inspection appeared more pronounced in the left hemisphere. No significant differences were observed in the opposite contrast. AD patients showed decreased FA, compared with older healthy subjects in the anterior part of the left temporal lobe, probably in the uncinate fasciculus (P < 0.05 corrected). No significant differences were observed in the opposite contrast. In MCI no significant differences were observed with neither AD nor older healthy subjects (P < 0.05 corrected).
Figure 1.
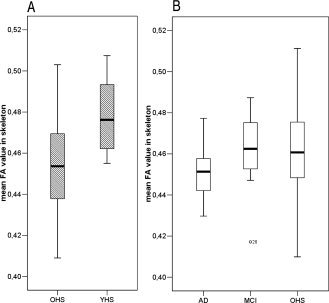
Box plots depicting mean global FA values per group. The effect of aging and disease was computed separately, with a slightly (sub‐study specific) different mean FA‐skeleton. Therefore (A) aging and (B) disease are depicted separately in this figure. A significant decrease in global FA integrity (i.e. average FA across all skeleton voxels) is shown in figure (A) for normal aging (older healthy subjects (OHS) < younger healthy subjects (YHS); P = 0.008, one‐tailed) and in figure (B) for disease in AD < MCI (P < 0.047, one‐tailed). For AD < OHS a trend towards reduced global FA is shown (P < 0.065, one‐tailed); this is less significant than the AD < MCI difference because of the high spread in mean FA across the older healthy subjects. MCI and healthy older subjects do not differ in global FA.
Figure 2.
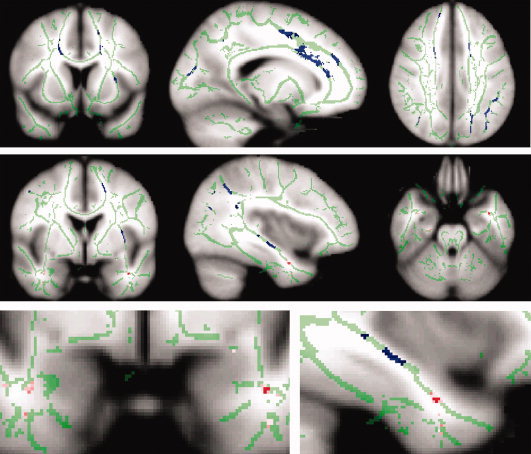
Spatial maps of the results of between group voxel‐wise statistics. Differences in WM tract integrity are displayed in blue for older compared with younger healthy subjects and in red for AD compared with older healthy subjects. Images (coronal, sagittal, and axial view) are t‐statistics, P < 0.05 corrected. For visualization purposes the differences between AD and healthy older subjects is also displayed in pink at a more liberal threshold (P < 0.001, uncorrected). In green the “mean_FA_skeleton” is shown, only FA values projected on the skeleton were compared. These images are overlaid on the MNI152 standard brain. The left hemisphere of the brain corresponds to the right side of the image.
Correlation Between FA and Neuropsychology
Within the healthy older subjects a significant correlation was found between age and mean FA (ρ = −0.66, P = 0.0004; see Fig. 3). This shows that the effect of aging on WM tract integrity is not only visible between groups that differ largely in age but also between subjects within the same group (age range 60–81 years). No significant correlation was observed between performance on the WISC maze, total time and mean FA of the older healthy subjects. Of the 11 neuropsychological measures that were significantly different between AD and healthy older subjects (see Table I), evidently all showed a correlation with mean FA across the older healthy and AD groups together but no significant correlations were found for the groups separately.
Figure 3.
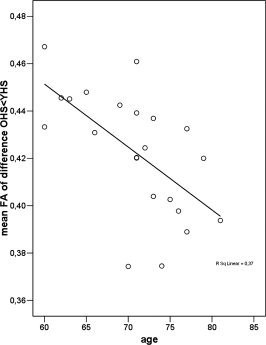
Correlations between FA integrity and age. Scatter plot of the correlation between mean FA within the WM areas affected by aging and age within the older healthy controls. A significant inverse correlation (ρ = −0.66, P = 0.0004) was observed, i.e. older age was related to lower mean FA.
DISCUSSION
Our study confirmed white matter degradation in the frontal lobe as an effect of aging consistent with previous studies [Head et al., 2004; Pfefferbaum et al., 2005; Salat et al., 2005; Sullivan and Pfefferbaum, 2006; Sullivan et al., 2006]. Other regions of decreased FA with aging in this study (the parietal and temporal lobes and interhemispheric tracts through the corpus callosum particularly in the genu and body) have also been observed in previous studies [Kochunov et al., 2007; Lehmbeck et al., 2006; Pfefferbaum et al., 2000]. These tracts through the corpus callosum are essentially related to the prefrontal cortical connections [Zarei et al., 2006]. A decrease in cognitive performance was observed in older compared to younger subjects on the WISC maze (total time). This test measures executive functioning, which is known to be dependent on the integrity of the frontal lobe, subcortical structures and their connections with the rest of the brain [Gazzaniga et al., 1998; Graham et al., 2004].
Our results of the patient study are consistent with our understanding of pathology in AD that is focused on the degeneration of the medial temporal lobe. In AD a decrease in FA compared with healthy older subjects was shown in the left anterior temporal lobe. This difference in FA appeared to be located in the uncinate fasciculus that connects the hippocampus with prefrontal cortex. At a more liberal threshold (as is displayed in pink in Fig. 2) FA reduction was shown in both the left and right anterior temporal lobe. The symmetrical pattern of FA changes in AD suggests that this is not a chance finding. We believe that in AD WM integrity is decreased bilaterally in the temporal lobe, however here we can only reliably report the effect observed in the left hemisphere. No significant difference in FA was observed between MCI and the other groups. It appears that FA values of MCI patients lie somewhere in between those of older healthy subjects and AD, as they do not differ compared to both other groups but the other groups do exhibit between‐group differences. This is in line with the concept of MCI as a transitional stage between normal aging and AD [Petersen et al., 2001b]. A lack of statistical power, possibly related to the small sample size, could have caused the absence of between‐group effects with the MCI group and the other groups.
In the healthy older group a high spread in global mean FA was observed. We do not know what this variance is due to; perhaps it is related to the larger number of subjects included in the healthy older group compared to the MCI and AD groups. However, we do think this high spread could have caused the lack of statistical significance in the comparison of global mean FA between AD and healthy older subjects. As expected, AD patients performed worse than healthy older subjects across all cognitive domains tested with our neuropsychological test battery except for praxis. Episodic memory function differentiated MCI from older healthy subjects and no significant differences were observed between MCI and AD.
Our results showed a distinction between the spatial pattern of FA changes observed in normal aging and in AD; the effect of old versus young is primarily located in frontal, parietal and subcortical areas whereas the effect of AD versus healthy older subjects is located in the temporal lobe.
The observed effects in AD are small compared with previous findings in AD [Bozzali et al., 2002; Huang and Auchus, 2007; Naggara et al., 2006; Takahashi et al., 2002; Xie et al., 2006]. Previous studies might show spurious results related to misalignment and spatial smoothing. However, the observed effects are also relatively small compared with the effects currently observed in healthy aging. Therefore, another possibility is that pathology in these AD patients reduced FA so strongly that potential areas of interest were mistakenly excluded from analysis (e.g. due to the thresholding of the mean FA values on the skeleton). However, this is unlikely as such large reductions in FA have not been identified previously in AD.
Patterns of functional interactions within the brain are likely affected by changes in the anatomical integrity of the WM network. That this is probably the case has been shown for instance by Kotter and Sommer [ 2000]. In the current study changes in cognitive function and WM integrity have been observed in healthy aging and AD. A correlation between these two was found across the groups (combining younger and older healthy subjects; and older healthy subjects and AD) but not for each group separately. Possible explanations could be the lack of statistical power per group and the small amount of variation within groups.
It should be noted that one possible limitation of this study is the relatively small number of subjects in the young healthy and MCI groups. This may have limited the conclusions of the statistical comparisons involving these two groups. Another limitation is the large difference in age between the two healthy subject groups. This makes it impossible to investigate when age‐related decreases in FA occur. Does this happen gradually or more abruptly after a certain age? For future studies on the influence of aging and MCI on white matter integrity we suggest to increase the number of subjects. Furthermore, for the study of aging specifically we would suggest to include more subject groups across different ages or include age as a continuous factor.
In this study, we provided a global map of white matter changes in healthy elderly, MCI and AD patients by measuring FA changes across the brain. However, decreased FA values only provided us with an indication of compromised connectivity within a certain WM tract. To measure changes within fiber tracts of interest, we would have to perform DTI tractography to visualize and quantify the tracts. Preferably this then has to be related to the actual functioning of the tracts. In a previous study we have shown functional connectivity within several “resting state networks” in the brain [Damoiseaux et al., 2006]. Relating functional connectivity as observed in these “resting state networks” with anatomical connectivity could provide us with more insight in the association between functional and anatomical connectivity.
Acknowledgements
We thank Christian F. Beckmann for discussion and comments.
REFERENCES
- Ashburner J,Friston KJ ( 2000): Voxel‐based morphometry—The methods. Neuroimage 11: 805–821. [DOI] [PubMed] [Google Scholar]
- Behrens TE,Johansen‐Berg H,Woolrich MW,Smith SM,Wheeler‐Kingshott CA,Boulby PA,Barker GJ,Sillery EL,Sheehan K,Ciccarelli O,Thompson AJ,Brady JM,Matthews PM ( 2003): Non‐invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci 6: 750–757. [DOI] [PubMed] [Google Scholar]
- Bookstein FL ( 2001): Voxel‐based morphometry should not be used with imperfectly registered images. Neuroimage 14: 1454–1462. [DOI] [PubMed] [Google Scholar]
- Bozzali M,Falini A,Franceschi M,Cercignani M,Zuffi M,Scotti G,Comi G,Filippi M ( 2002): White matter damage in Alzheimer's disease assessed in vivo using diffusion tensor magnetic resonance imaging. J Neurol Neurosurg Psychiatry 72: 742–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conturo TE,Lori NF,Cull TS,Akbudak E,Snyder AZ,Shimony JS,McKinstry RC,Burton H,Raichle ME ( 1999): Tracking neuronal fiber pathways in the living human brain. Proc Natl Acad Sci USA 96: 10422–10427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik FIM,Salthouse TA ( 2000): Handbook of Aging and Cognition II. Mahwah,NJ: Erlbaum. [Google Scholar]
- Damoiseaux JS,Rombouts SA,Barkhof F,Scheltens P,Stam CJ,Smith SM,Beckmann CF ( 2006): Consistent resting‐state networks across healthy subjects. Proc Natl Acad Sci USA 103: 13848–13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazekas F,Chawluk JB,Alavi A,Hurtig HI,Zimmerman RA ( 1987): MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol 149: 351–356. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS,Ivry RB,Mangun GR ( 1998): Executive functions and frontal lobes In: Gazzaniga MS,Ivry RB, Mangun GR, editors. Cognitive Neuroscience: The Biology of the Mind. New York: W. W. Norton & Company; pp 423–464. [Google Scholar]
- Graham NL,Emery T,Hodges JR ( 2004): Distinctive cognitive profiles in Alzheimer's disease and subcortical vascular dementia. J Neurol Neurosurg Psychiatry 75: 61–71. [PMC free article] [PubMed] [Google Scholar]
- Greicius MD,Srivastava G,Reiss AL,Menon V ( 2004): Default‐mode network activity distinguishes Alzheimer's disease from healthy aging: Evidence from functional MRI. Proc Natl Acad Sci USA 101: 4637–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RK,Saksena S,Hasan KM,Agarwal A,Haris M,Pandey CM,Narayana PA ( 2006): Focal Wallerian degeneration of the corpus callosum in large middle cerebral artery stroke: Serial diffusion tensor imaging. J Magn Reson Imaging 24: 549–555. [DOI] [PubMed] [Google Scholar]
- He Y,Chen ZJ,Evans AC ( 2007): Small‐world anatomical networks in the human brain revealed by cortical thickness from MRI. Cereb Cortex 17: 2407–2419. [DOI] [PubMed] [Google Scholar]
- Head D,Buckner RL,Shimony JS,Williams LE,Akbudak E,Conturo TE,McAvoy M,Morris JC,Snyder AZ ( 2004): Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type: Evidence from diffusion tensor imaging. Cereb Cortex 14: 410–423. [DOI] [PubMed] [Google Scholar]
- Huang J,Auchus AP ( 2007): Diffusion tensor imaging of normal appearing white matter and its correlation with cognitive functioning in mild cognitive impairment and Alzheimer's disease. Ann N Y Acad Sci 1097: 259–264. [DOI] [PubMed] [Google Scholar]
- Kochunov P,Thompson PM,Lancaster JL,Bartzokis G,Smith S,Coyle T,Royall DR,Laird A,Fox PT ( 2007): Relationship between white matter fractional anisotropy and other indices of cerebral health in normal aging: Tract‐based spatial statistics study of aging. NeuroImage 35: 478–487. [DOI] [PubMed] [Google Scholar]
- Kotter R,Sommer FT ( 2000): Global relationship between anatomical connectivity and activity propagation in the cerebral cortex. Philos Trans R Soc Lond B Biol Sci 355: 127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan D ( 2003): Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci 4: 469–480. [DOI] [PubMed] [Google Scholar]
- Lehmbeck JT,Brassen S,Weber‐Fahr W,Braus DF ( 2006): Combining voxel‐based morphometry and diffusion tensor imaging to detect age‐related brain changes. Neuroreport 17: 467–470. [DOI] [PubMed] [Google Scholar]
- Lindeboom J,Weinstein H ( 2004): Neuropsychology of cognitive ageing, minimal cognitive impairment, Alzheimer's disease, and vascular cognitive impairment. Eur J Pharmacol 490: 83–86. [DOI] [PubMed] [Google Scholar]
- Madden DJ,Whiting WL,Huettel SA,White LE,MacFall JR,Provenzale JM ( 2004): Diffusion tensor imaging of adult age differences in cerebral white matter: Relation to response time. Neuroimage 21: 1174–1181. [DOI] [PubMed] [Google Scholar]
- McKhann G,Drachman D,Folstein M,Katzman R,Price D,Stadlan EM ( 1984): Clinical diagnosis of Alzheimer's disease: Report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 34: 939–944. [DOI] [PubMed] [Google Scholar]
- Medina D,Toledo‐Morrell L,Urresta F,Gabrieli JD,Moseley M,Fleischman D,Bennett DA,Leurgans S,Turner DA,Stebbins GT ( 2006): White matter changes in mild cognitive impairment and AD: A diffusion tensor imaging study. Neurobiol Aging 27: 663–672. [DOI] [PubMed] [Google Scholar]
- Morris JC ( 1993): The clinical dementia rating (CDR): Current version and scoring rules. Neurology 43: 2412–2414. [DOI] [PubMed] [Google Scholar]
- Moseley ME,Cohen Y,Kucharczyk J,Mintorovitch J,Asgari HS,Wendland MF,Tsuruda J,Norman D ( 1990): Diffusion‐weighted MR imaging of anisotropic water diffusion in cat central nervous system. Radiology 176: 439–445. [DOI] [PubMed] [Google Scholar]
- Naggara O,Oppenheim C,Rieu D,Raoux N,Rodrigo S,Dalla BG,Meder JF ( 2006): Diffusion tensor imaging in early Alzheimer's disease. Psychiatry Res 146: 243–249. [DOI] [PubMed] [Google Scholar]
- Pasquier F ( 1999): Early diagnosis of dementia: Neuropsychology. J Neurol 246: 6–15. [DOI] [PubMed] [Google Scholar]
- Petersen RC,Doody R,Kurz A,Mohs RC,Morris JC,Rabins PV,Ritchie K,Rossor M,Thal L,Winblad B ( 2001a): Current concepts in mild cognitive impairment. Arch Neurol 58: 1985–1992. [DOI] [PubMed] [Google Scholar]
- Petersen RC,Stevens JC,Ganguli M,Tangalos EG,Cummings JL,DeKosky ST ( 2001b): Practice parameter: Early detection of dementia: Mild cognitive impairment (an evidence‐based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 56: 1133–1142. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A,Sullivan EV,Hedehus M,Lim KO,Adalsteinsson E,Moseley M ( 2000): Age‐related decline in brain white matter anisotropy measured with spatially corrected echo‐planar diffusion tensor imaging. Magn Reson Med 44: 259–268. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A,Adalsteinsson E,Sullivan EV ( 2005): Frontal circuitry degradation marks healthy adult aging: Evidence from diffusion tensor imaging. Neuroimage 26: 891–899. [DOI] [PubMed] [Google Scholar]
- Reese TG,Heid O,Weisskoff RM,Wedeen VJ ( 2003): Reduction of eddy‐current‐induced distortion in diffusion MRI using a twice‐refocused spin echo. Magn Reson Med 49: 177–182. [DOI] [PubMed] [Google Scholar]
- Rueckert D,Sonoda LI,Hayes C,Hill DL,Leach MO,Hawkes DJ ( 1999): Nonrigid registration using free‐form deformations: Application to breast MR images. IEEE Trans Med Imaging 18: 712–721. [DOI] [PubMed] [Google Scholar]
- Salat DH,Tuch DS,Greve DN,van der Kouwe AJ,Hevelone ND,Zaleta AK,Rosen BR,Fischl B,Corkin S,Rosas HD,Dale AM ( 2005): Age‐related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging 26: 1215–1227. [DOI] [PubMed] [Google Scholar]
- Salthouse TA,Ferrer‐Caja E ( 2003): What needs to be explained to account for age‐related effects on multiple cognitive variables? Psychol Aging 18: 91–110. [DOI] [PubMed] [Google Scholar]
- Smith SM ( 2002): Fast robust automated brain extraction. Hum Brain Mapp 17: 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM,Nichols TE ( 2007): Threshold‐Free Cluster‐Enhancement: Addressing the problem of threshold dependence in cluster inference. HBM, poster 363 W‐AM. Abstract. [DOI] [PubMed]
- Smith SM,Jenkinson M,Woolrich MW,Beckmann CF,Behrens TE,Johansen‐Berg H,Bannister PR,De Luca M,Drobnjak I,Flitney DE,Niazy RK,Saunders J,Vickers J,Zhang Y,De Stefano N,Brady JM,Matthews PM ( 2004): Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23 ( Suppl 1): S208–S219. [DOI] [PubMed] [Google Scholar]
- Smith SM,Jenkinson M,Johansen‐Berg H,Rueckert D,Nichols TE,Mackay CE,Watkins KE,Ciccarelli O,Cader MZ,Matthews PM,Behrens TE ( 2006): Tract‐based spatial statistics: Voxelwise analysis of multi‐subject diffusion data. Neuroimage 31: 1487–1505. [DOI] [PubMed] [Google Scholar]
- Sporns O,Chialvo DR,Kaiser M,Hilgetag CC ( 2004): Organization, development and function of complex brain networks. Trends Cogn Sci 8: 418–425. [DOI] [PubMed] [Google Scholar]
- Stam CJ,Jones BF,Manshanden I,van Cappellen van Walsum AM,Montez T,Verbunt JP,de Munck JC,van Dijk BW,Berendse HW,Scheltens P ( 2006): Magnetoencephalographic evaluation of resting‐state functional connectivity in Alzheimer's disease. Neuroimage 32: 1335–1344. [DOI] [PubMed] [Google Scholar]
- Stephan KE ( 2004): On the role of general system theory for functional neuroimaging. J Anat 205: 443–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV,Pfefferbaum A ( 2006): Diffusion tensor imaging and aging. Neurosci Biobehav Rev 30: 749–761. [DOI] [PubMed] [Google Scholar]
- Sullivan EV,Adalsteinsson E,Pfefferbaum A ( 2006): Selective age‐related degradation of anterior callosal fiber bundles quantified in vivo with fiber tracking. Cereb Cortex 16: 1030–1039. [DOI] [PubMed] [Google Scholar]
- Takahashi S,Yonezawa H,Takahashi J,Kudo M,Inoue T,Tohgi H ( 2002): Selective reduction of diffusion anisotropy in white matter of Alzheimer disease brains measured by 3.0 Tesla magnetic resonance imaging. Neurosci Lett 332: 45–48. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ,Singer W ( 2006): Neural synchrony in brain disorders: Relevance for cognitive dysfunctions and pathophysiology. Neuron 52: 155–168. [DOI] [PubMed] [Google Scholar]
- Xie S,Xiao JX,Gong GL,Zang YF,Wang YH,Wu HK,Jiang XX ( 2006): Voxel‐based detection of white matter abnormalities in mild Alzheimer disease. Neurology 66: 1845–1849. [DOI] [PubMed] [Google Scholar]
- Zarei M,Johansen‐Berg H,Smith S,Ciccarelli O,Thompson AJ,Matthews PM ( 2006): Functional anatomy of interhemispheric cortical connections in the human brain. J Anat 209: 311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]


