Abstract
Magnetoencephalographic, electromyographic (EMG), work, and reaction time (RT) were recorded from nine subjects during visually triggered intermittent isometric contractions of the middle finger under two conditions: unloaded and loaded (30% of maximal voluntary contraction). The effect of muscle fatigue was studied over three consecutive periods under both conditions. In the loaded condition, the motor evoked field triggered by the EMG onset decreased with fatigue, whereas movement‐evoked fields (MEFs) increased (P < 0.01). Fatigue was demonstrated in the loaded condition, since (i) RT increased due to an increase in the electromechanical delay (P < 0.002); (ii) work decreased from Periods 1 to 3 (P < 0.005), while (iii) the myoelectric RMS amplitude of both flexor digitorum superficialis and extensor muscles increased (P < 0.003) and (iv) during Period 3, the spectral deflection of the EMG median frequency of the FDS muscle decreased (P < 0.001). In the unloaded condition and at the beginning of the loaded condition, a parallel network including M1‐S1, posterior SII‐insular, and posterior cingulate cortices accounted for the MEF activities. However, under the effect of fatigue, medial insular and posterior cingulate cortices drove this network. Moreover, changes in the location of insular and M1‐S1 activations were significantly correlated with muscle fatigue (increase of RMS‐EMG; P < 0.03 and P < 0.01, respectively). These results demonstrate that a plastic network controls the strength of the motor command as fatigue occurs: sensory information, pain, and exhaustion act through activation of the medial insular and posterior cingulate cortices to decrease the motor command in order to preserve muscle efficiency and integrity. Hum Brain Mapp, 2009. © 2008 Wiley‐Liss, Inc.
Keywords: isometric contraction, reaction time, muscle fatigue, event‐related magnetic fields, MEG, brain plasticity, M1‐S1, SII‐insula, medial insula, posterior cingulate cortex
INTRODUCTION
Fatigue is generally defined as a reduction in the neuromuscular system's capacity to generate force or to perform work [Asmussen, 1979; Bigland‐Ritchie, 1981]. Two types of fatigue can be identified: peripheral (muscular) and central (cortical) [Asmussen, 1979; Bigland‐Ritchie, 1981, 1986; Gandevia, 1998; Maton, 1991]. Peripheral fatigue implies that the ability of muscles to produce force is reduced, whereas central fatigue refers to the inability to fully execute voluntary muscle activation.
Peripheral and central fatigue have been studied by recording reaction time (RT) [Pääsuke et al., 1999; Yeung et al., 1999; Zhou et al., 1996], force, and electromyographic (EMG) activities during maximal voluntary contractions [Bigland‐Ritchie, 1981; Gandevia, 2001; Nordlund et al., 2004], submaximal continuous contractions [Fuglevand et al., 1993; Garland et al., 1994; Löscher et al., 1996; Maton and Gamet, 1989], and intermittent isometric contractions [Bigland‐Ritchie et al., 1986]. During isometric contractions, muscles undergo a continuous reduction in force‐producing capability counterbalanced by a gradual recruitment of new unfatigued motor units until endurance time elapses [Bigland‐Ritchie et al., 1986; Maton, 1981]. It is well known that changes in EMG signal indirectly indicate an increase in voluntary effort during a prolonged submaximal motor task [Fuglevand et al., 1993; Hagberg, 1981; Löscher et al., 1996].
Enhancement of voluntary effort during submaximal isometric contraction has been shown by data recorded from the brain [Dettmers et al., 1996; Liu et al., 2002, 2003]. More specifically, Liu et al. [2002] suggested that an early central adjustment strengthens the descending motor command in order to compensate for force loss and subsequent inhibition by sensory feedback as fatigue became more severe. Neurophysiological factors substantiate such a claim. When fatigue sets in, the cortical cell firing changes [Belhaj‐Saïf et al., 1996] as well as the location of the sensorimotor cortex activation [Wannier et al., 1991]. Indeed, in monkeys, S1 neurons exhibit different properties from M1 neurons during isometric contractions since the discharge rate of S1 neurons varies with the force produced. This information is used as feedback to regulate fine‐graded force prehension. Moreover, the nociceptive activity during maintained contractions is likely to have synaptic connections to a large pool of motoneurons and, as such, probably helps to synchronize the firing rate of the motor unit [Ciubotariu et al., 2004] and/or to decrease motor unit activity [Sohn et al., 2000]. Since muscle afferents provide significant information to cortical cells that are functionally linked to their target motor fibers, changes in motor cortex activity have been defined as a central sign of fatigue [Gandevia, 2001; Maton, 1991]. In this framework, central fatigue mainly depends on the subject's willpower and determination [Gandevia, 2001; Jouanin et al., 1993; Nordlund et al., 2004].
Numerous authors have studied the role played by cerebral structures during fatigue induced by maximal voluntary contractions. The main result was that central fatigue is expressed by a reduction in neural activation (for review, see Gandevia, 2001]. Furthermore, during fatiguing sustained isometric contractions, positron emission tomography (PET), and functional magnetic resonance imaging (fMRI) identified the activation of the primary sensory‐motor cortices, supplementary motor areas, parietal area, bilateral secondary somatosensory (SII) area and the insula, anterior cingulate, basal ganglia (thalamus, globus pallidus, putamen); [Dettmers et al., 1996], and cerebellum [Liu et al., 2003]. Moreover, pain has been reported to activate different parts of the insula and cingulate region [Brooks et al., 2005; Niddam et al., 2002; Peyron et al., 2000, 2002].
However, these studies were unable to reveal the precise timing of the spatiotemporal cortical activations because of the poor time resolution of PET and fMRI methods. Therefore, in keeping with such findings, we used magnetoencephalography (MEG) to study the timing of cortical networks involved during muscle fatigue to show evidence of activation related to central fatigue, and activation dedicated to the preservation of muscle efficiency and integrity. Indeed, MEG and also EEG allow for very precise studies of the timing and localization of cortical activations occurring after finger stimulation [Baumgartner et al., 1991; Ishibashi et al., 2000; Meunier et al., 2001; Pizzela et al., 1999; Suk et al., 1991; Yang et al., 1993], finger movements with different inertial loads [Kristeva et al., 1990], hand movements whether spontaneous or triggered by responses to visual stimuli [Endo et al., 1996, 1999; Kristeva et al., 1991; Kristeva‐Feige et al., 1994; Weinberg et al., 1990], or by peripheral nerve stimuli [Forss and Jousmäki, 1998; Hari and Forss, 1999]. In these studies, MEG has clearly contributed to mapping activity sources at their peak latencies with high spatiotemporal resolution. Different neuromagnetic field components accompanying a voluntary movement have been identified during RT: the readiness field, the motor field (MF), the movement‐evoked field [MEF; Weinberg et al., 1990; Kristeva et al., 1991]. More recently, several MEFs (MEF I and II, 40–150 ms following EMG onset activity) have been proposed to reflect cortical sensory feedback from the periphery. By using dipole‐modeling, Cheyne et al. [2006] brought new evidence that MEFI arises from locations in the postcentral gyrus consistent with activation of area 3b in the posterior wall of the central sulcus that receives afferent input from cutaneous and joint receptors (3a), whereas MEF II components are likely reflecting a secondary activation of the motor precentral gyrus. Moreover, MEF III has also been described at latencies greater than 100 ms with rather complex topography, but its generators have not yet been identified [Cheyne et al., 1997, 2006; Kristeva‐Feige et al., 1994].
Further, MEG studies have demonstrated that nonfatiguing sustained isometric contractions, [Conway et al., 1995] or peripheral nerve stimulation during low‐level isometric contractions [Forss and Jousmäki, 1998] facilitate the activation of sensorimotor cortices in response to sensory afferent feedback. These studies substantiate the fact that during fatiguing isometric contractions, sensory and proprioceptive feedback contributes to adjusting motor cortex activity, which in turn controls the spinal motoneuronal pool and thus optimizes motor execution [Gross et al., 2000; Salenius et al., 1997]. Classically, these afferences are studied during voluntary movements by triggering the MEFs on EMG onset or RT [RT as the sum of premotor time (PMT) and electromechanical delay (EMD); Yeung et al., 1999; Zhou et al., 1996].
Therefore, we assume that as muscles fatigue sets in, changes in the neuromagnetic field prior to the isometric contraction (i.e. during the RT) should reflect the sensorimotor cortex adaptation to sensory, proprioceptive and nociceptive feedback. These inputs should increase during fatigue and as a result could decrease or even prohibit the motor command itself.
METHOD
Subjects and Experimental Conditions
A group of high‐performance male athletes (N = 9), field hockey players from the “Bataillon de Joinville”—French elite sports battalion, all right‐handed, well motivated to participate in this experiment (age: 24.0 ± 2.0 years, weight: 74.3 ± 3.9 kg, height: 175.1 ± 3.3 cm) were studied. They were chosen because of their high level of arm muscle endurance, which allowed us to record a higher number of experimental trials than with sedentary people. None had a history of trauma or neuromuscular disease. The Ethics Committee of the University of Paris V (Paris‐Cochin) approved this study, and subjects gave their informed consent.
The experimental design was influenced by the characteristics of the MEG signal, which requires signal averaging to extract the evoked magnetic field. Subjects were asked to perform intermittent isometric contractions as quickly as possible, after an 80‐ms LED signal trigger (RT task). Intermittent isometric contractions were carried out for 3 s (between two consecutive LED signals) during a maximum of 200 successive trials, with an interstimulus interval of 8 s. Two experimental conditions were recorded: 1st unloaded and 2nd with weightlifting.
For each subject, before the beginning of the experiment, resting heart rate was recorded for 1 min. Subsequently, subjects executed during 3 s maximal voluntary isometric contractions (MVC) of the flexor digitorum superficialis (FDS) muscle of the right middle finger. Three MVCs were completed at intervals of 90 s and recorded by a strain gauge (average value of 3.52 ± 0.62 kg across subjects).
The unloaded condition was then recorded (force level < 5% MVC) over 200 trials. After a 20‐min rest period, the loaded condition in which subjects were asked to lift a weight corresponding to 30% of the higher value of their three MVC was recorded (see Table I). The weight, made of amagnetic material, was set in front of the subject to provide a visual feedback of finger force and position. The task was performed either 200 times or until endurance time elapsed.
Table I.
Subject performance under the loadedcondition [endurance time (ET)] and their reportsat the end of the experiment
| Subject | 30% MVC (kg) | Trials | ET (s) | Report |
|---|---|---|---|---|
| S1 | 0.9 | 133 | 1,064 | Exhaustion |
| S2 | 0.96 | 158 | 1,264 | Exhaustion |
| S3 | 0.96 | 196 | 1,568 | Exhaustion |
| S4 | 1.1 | 200 | 1,600 | Pain |
| S5 | 0.81 | 177 | 1,416 | Pain |
| S6 | 1.05 | 100 | 800 | Pain |
| S7 | 1.1 | 143 | 1,144 | Exhaustion |
| S8 | 1.4 | 134 | 1,072 | Pain, exhaustion |
| S9 | 1.1 | 200 | 1,600 | Exhaustion |
When subjects reported a more intense middle finger than forearm pain this was classified as pain (in the sense of cutaneous pain), whereas when the forearm pain was the highest this was classified as exhaustion (in the sense of muscular pain); only one subject reported the same level for both.
Procedure
MEG data was collected with a whole‐head 151‐channel magnetometer (VMS/CTF, Canada) in a magnetically shielded room (Vacuumschmelze, Germany). Data were digitized at 625 Hz with an online 0–100 Hz band‐pass. Anatomical magnetic resonance imaging (MRI) was also recorded for each subject (characteristics of the slices were 0.9 × 0.9 in the axial plane and acquired every 1.3 mm).
Each subject was seated in the armchair of the MEG system, with his semipronated forearm Velcro‐taped to a nonmagnetic custom‐designed ergometer solidly attached to the armchair. The apparatus for fixating the arm and the hand, except for the middle finger, was constructed in keeping with the finger ergograph after Mosso [Asmussen, 1979; Di Guilio et al., 2006].
To prevent inactive fingers (2nd, 4th, and 5th) from moving, the proximal and distal interphalangeal joints of the right hand and the metacarpus were taped to a plastic splint. With this device, only the metacarpophalangeal joint of the middle finger could move. It was taped to a mobile plastic sheet so that it could only move 1.5 cm, and subjects kept their thumb pronated (Fig. 1). A dacron hydraulic line transmitted the pressure variations due to finger displacement (force rise, maintained contraction, and force release) out of the shielded room to a pressure sensor. To reduce the effects of remote co‐contractions on the MEG signal, subjects were attached to the armchair with Velcro bandages crossed over shoulders and chest.
Figure 1.
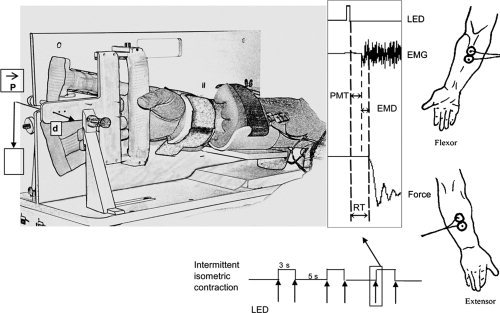
From left to right: (a) nonmagnetic ergometer: P = load, d = displacement; (b) arrows: LED triggering, RT (reaction time) is the sum of the premotor time (PMT) and electromechanical delay (EMD); (c) schematic positions of the EMG electrodes.
Before each experimental condition started, MEG sensors had to be relocated according to the subject's individual anatomy: exact head site and orientation were measured by collecting magnetic signals from three positioning coils fixed on the nasion and both tragi. The activities of the FDS‐profundus and the extensor digitorum (ED) muscles were recorded using EMG. EMG activity was recorded twice: (1) for the EMG analysis proper, at a sampling rate of 1,000 Hz by using a Biopac‐sytems (CEROM, France) with a 10–300 Hz bandpass. In this case EMG analysis was triggered by the LED onset. (2) EMGs were also recorded by the MEG system with a 0–100 Hz bandpass and a 625‐Hz sampling rate. In order to determine the EMG onset, a trial‐by‐trial visual analysis of the EMG activity was done by two independent scorers for each subject and condition. These measurements were used for triggering the MF and MEFs evoked fields.
EKG was recorded via a bipolar derivation placed under the two‐midclavicular lines. At the end of the experiment, subjects were asked to rate their own perceived exhaustion: pain, inability to sustain the effort, muscular fatigue, and associated pain (Table I).
Biomechanical and Electrophysiological Data
Heart rate (EKG), muscle activity (EMG), RT, work (W), MF, and MEFs brain activities were recorded in unloaded and loaded conditions. Experimental conditions were divided into three periods: (1) 0–33%; (2) 33–66%, and (3) 66–100%. As a result, the loaded condition periods' durations were related to each subject's endurance time.
During both conditions (unloaded and loaded), EMG onset activity was defined as the beginning of the raw FDS‐EMG after LED triggering.
RT was defined as the sum of PMT and EMD. PMT corresponds to the interval between LED onset and the beginning of EMG activity; EMD is the interval between the beginning of EMG activity and the finger movement onset.
W was computed using W (J) = ∫Ftp, where F is the force, t = 3 s (work time), and p = 3/8 [i.e. work time, 3 s, divided by the total time, 8 s; Kahn and Monod, 1989].
In the maintained phase of the intermittent isometric contraction, root mean square (RMS) and median frequency of EMG activity were computed on a recording window of 500 ms (to avoid instability due to the movement onset) to 2.5 s after EMG onset. RMS was expressed as a percentage of the maximum RMS value obtained during MVC.
Prior to processing, after a careful visual inspection of MEG data, artifacts (blinking, ocular movement, task‐unrelated EMG bursts, or abnormal slow drifts of the MEG signal) were removed. Trials where the EKG signal was of high amplitude and superimposed onto the MEG signal were also discarded. The average evoked magnetic field was then computed for each subject, for every sensor, under both conditions and for each period under analysis. Averages were triggered by the onset of EMG activity. The averaging time window went from −400 (retro averaging) to 400 ms; the baseline was computed between −400 and −200 ms. The grand average across subjects was computed and normalized by the number of trials for each subject (see Table I).
Dipole Fit Modeling and Measurements
For each subject, MEG‐evoked fields (computed on the 151 sensors) for the different conditions and different periods were imported into the dipole‐fit analyzer (CTF). MF recorded between –50 and 50 ms and MEFs between 50 and 250 ms were estimated with the standard dipole model. Each subject's MRI was also imported into the “MRI viewer” (Dipole‐Fit, CTF) for dipole modeling. Thus, each subject's cortical anatomy was used to estimate the best fitting sphere for dipole localization procedure. For each subject, two scorers agreed on the choice in space and time of the best dipole maps around the peak or peaks of the evoked MF and MEFs. If a single dipole was able to account for 90% of these visually chosen data, then it was retained. Otherwise, one or even two supplementary localization procedures were conducted in order to add one or two dipoles, which in turn were able to explain at least 90% of the variance. During the loaded condition or during the last part of the unloaded condition, the single dipole solution only explained about 60% of the MEFs data. Thus, two or three successive dipole model‐fitting procedures were used in those cases. Finally, x, y, z coordinates in the CTF system were transformed into x, y, z coordinates of the Talairach and Tournoux [1988] atlas. These data were submitted to statistical analysis and also averaged across subjects.
Statistical Analysis
Two‐way repeated measures of analysis of variances (ANOVAs) were used to analyze differences for all dependent variables (RT, PMT, EMD, W, RMS‐EMG, median frequency, heart rate, MF, and MEFs) in both conditions (loaded and unloaded) for the three periods (P1: 0–33%, P2: 33–66%, P3: 66–100% of the endurance time). When necessary, a Student‐Newman‐Keul test was used to correct pairwise multiple comparisons (Sigmastat, SPSS Science).
A Student‐Fischer t‐test for paired data was used to assess dipole parameter x,y,z coordinate and strength variations. When data were missing, the Mann‐Whitney Rank Sum Test was used. In order to have the same number of data points for all the subjects, regression analyses were done for 18 values extracted from P1, P2, and P3 where the P2 values were assigned to P1 or P3 as a function of the missing values of each subject.
RESULTS
Biomechanical Data
Subjects performed 200 trials in the unloaded condition, and only 160.1 ± 35.5 trials in the loaded condition (30% of the MVC). For the latter, the endurance time amounted to 21 min 20 s ± 4 min 49 s. Five subjects experienced exhaustion (i.e. inability to maintain the workload), three other subjects experienced painful sensations, and one subject felt both (Table I).
Work (W), RT, RMS‐EMG, spectral mean frequency (median frequency), and heart rate (HR) changes provided evidence of muscle fatigue in the loaded condition (Fig. 2).
Figure 2.
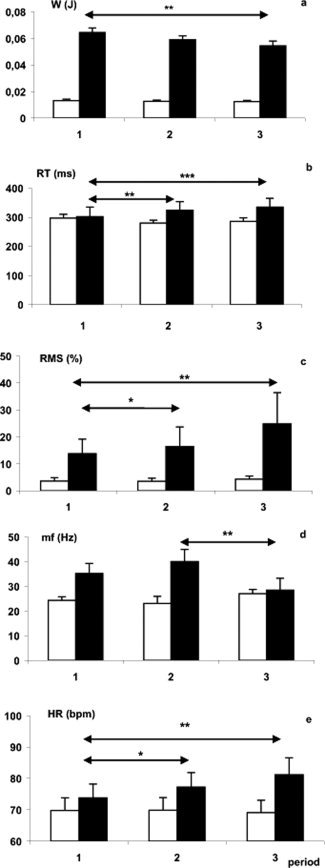
(a) Pattern of static work (W (J)); (b) reaction time (RT (ms)); (c) RMS (%) of the flexor digitorum superficialis (FDS) EMG expressed as a percentage of the RMS‐EMG of the maximal voluntary force of the FDS; (d) spectral deflection of median FDS‐EMG frequency (mf (Hz)); (e) heart rate (HR (bpm). Black bars: loaded; white bars: unloaded (*P < 005, **P < 001).
For W, the ANOVA showed a significant increase of W with the load (F 8,1 = 27.2; P < 0.002) and a decrease with the period (F 8,2 = 19.6; P < 0.001); a load × period interaction (F 8,2 = 5.23; P < 0.02) due to a decrease of W along with the periods was observed only in the loaded condition (see Fig. 2a). Since the weight to be held remained constant throughout the loaded condition, this decrease in work may only be accounted for by a reduction in the displacement of the middle finger. That is exactly what we observed when comparing displacement values between Periods 1 and 3 (1.38 ± 0.13 versus 0.92 ± 0.07 cm; P < 0.05).
RT was 86 ± 17 ms higher under the loaded condition (F 8,1 = 19.87; P < 0.002). No effect of the period on RT was observed for the unloaded condition; however, for the loaded condition, there was a significant difference in RT between Periods 1 and 2 and Periods 1 and 3 (all P < 0.05), (Fig. 2b). Moreover, a load × period interaction was significant (F 8,2 = 5.47; P < 0.02) because of the absence of any period effect under the unloaded condition. Across the three periods, a significant decrease of the PMT (21 ± 5 ms) was observed (F 8,1 = 8.9; P < 0.02) in the loaded condition when comparing with unloaded condition. On the contrary, the EMD increased by 53.4 ± 4.5 ms in the loaded condition (F 8,1 = 26.94; P < 0.002).
RMS‐EMG (FDS) increased of 27.4 ± 4.6% with load (F 8,1 = 17.55; P < 0.003) with a significant period effect (F 8,2 = 5.2; P < 0.02) and a load × period interaction (F 8,2 = 8.06; P < 0.004) due to the absence of any period effect under the unloaded condition (Fig. 2c). Similar results were also observed for the ED muscle.
The median frequency of FDS muscle increased of 35.4 ± 4 Hz in the loaded condition (F 7,1 = 13.4; P < 0.001), and there was also a period effect (F 7,2 = 31.75; P < 0.001) with a load × period interaction (F 7,2 = 11.84; P < 0.001) which revealed a significant decrease in median frequency under the loaded condition for Period 3 (28.4 Hz) when compared with Period 1 (35.2 Hz) and 2 (40 Hz), (Fig. 2d). Conversely, median frequency of the ED muscle increased during Period 3 (P < 0.02).
The RMS‐EMG of the FDS muscle increased with the EMD (F 16,1 = 5.604, R = 0.52; P < 0.03). These results confirm that additional motor units must be recruited to perform the task when fatigue develops [for a review see Bigland‐Ritchie, 1981]. Moreover, since the RMS‐EMG of the FDS and ED muscles covaried (F 17,1 = 5.34, R = 0.5; P < 0.003), this suggests that both muscles cooperate to execute the task.
Heart rate increased with load (F 8,1 = 11.66; P < 0.01) regularly during the three periods. A significant load × period interaction (F 8,2 = 7.36; P < 0.005) revealed that no heart rate variations existed in the unloaded condition (Fig. 2e). Accordingly, when fatigue increased (i) heart rate and EMD increased (F 16, 1 = 7.116, R = 0.567, P < 0.02) and (ii) the work decreased whereas the RMS‐EMG of the FDS muscle increased (F 15, 1 = 7.628, R = −0.568, P < 0.014).
MEG Event‐Related Fields
On Figure 3 (map), grand‐averaged data of the magnetic field obtained from the controlateral hemisphere including medial sensors are clearly consistent with the description of the MF peaking around the EMG onset. It displays an anterior localization and orientation, suggesting a current flow away from the anterior bank of the central sulcus, consistent with an activation of the primary sensorimotor cortex. This MF is followed by a second evoked field peaking around 100–150 ms with a posterior localization and orientation, suggesting a current flow away from the posterior bank of the central sulcus consistent with a postcentral gyrus activation which identifies the MEFs.
Figure 3.
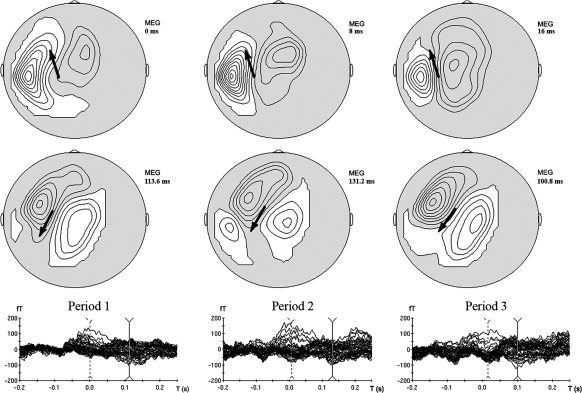
Upper row: Isocontour maps of the MF at the EMG onset (grand averaged data). Lower row: Isocontour maps of MEF at the mean peak latency (grand averaged data). Arrows represent an estimation of the main “equivalent dipole.”
Subject by subject measurements of peak latencies and amplitude (Fig. 4) were done for MF on the following sensors: middle left temporal 24, 33, 34; left central 21, 41; and zenith central 01. For MEFs the sensors were left frontal 45; left temporal 12, 13, 23; left parietal 31; zenith parieto‐occipital 2; right parietal 31, 32.
Figure 4.
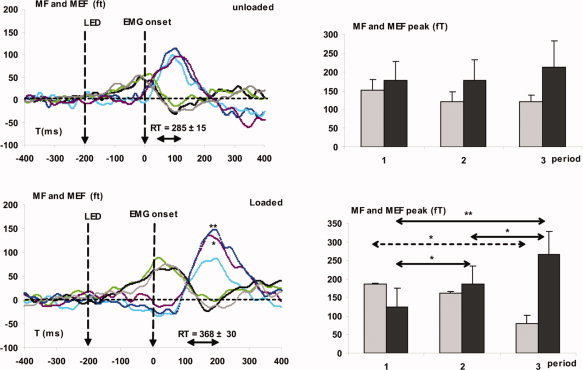
On the left: grand average across subjects of the motor field (MF) and movement‐evoked field (MEFs) during the unloaded (up) and loaded conditions (down) for the three periods. Respectively for MF and MEF: Period 1, light green and blue lines; Period 2, gray and purple lines; Period 3, black and dark blue lines. On the right: mean amplitude of the peak across subjects. Gray bars: MF, Black bars: MEFs (*P < 005, **P < 001).
These measurements revealed the following:
-
1
MF and MEFs peak latencies did not vary between unloaded and loaded conditions.
-
2
In the loaded condition, MF peak amplitude decreased (F 8, 2 = 3.7; P < 0.05) across periods (P < 0.02). Moreover, during the 3rd period, the MF peak amplitude decreased with endurance time (F 8, 1 = 9.91, R = −0.766, P < 0.02), which supports the assumption of a decrease of the motor command with fatigue.
-
3
Contrariwise, under the loaded condition, MEFs peak amplitude increased across periods (P < 0.001) which support an increase of the sensory feedback with fatigue.
Current Dipole Localization and Variations
For each subject under the unloaded and loaded conditions, the MF was well fitted by one single current dipole generally located in the vicinity of M1‐S1 (see Fig. 5 and Table II) but not the MEFs which were fitted by two or even three dipoles. In general, these dipoles were located in M1‐S1 and SII‐insula (see Fig. 6 and Table II), but posterior cingulate activations were observed in five of the subjects who experienced muscle fatigue with exhaustion (see Table I).
Figure 5.
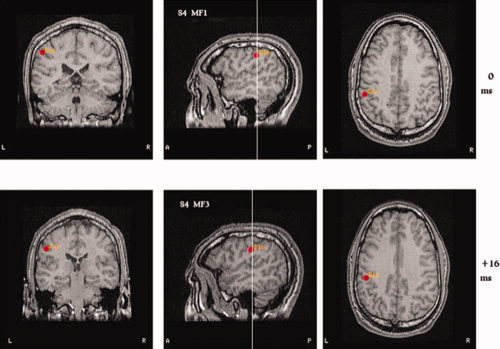
Dipole localization for the MF during the loaded condition for Subject 4. Upper MRI: The motor field during the Period 1 is labeled MF1; lower MRI: The motor field of the Period 3 is labeled MF3. The location of the MF dipole was found in the central sulcus whatever the load.
Table II.
Average values and standard errors across subjects of all the dipole locations found in this study during P1 and P3 periods, under the unloaded and loaded conditions
| Period | Latency (ms) | Strength (nAm) | Declination (deg) | Azimuth (deg) | x (mm) | y (mm) | z (mm) | n | Localization |
|---|---|---|---|---|---|---|---|---|---|
| MF | |||||||||
| Unloaded | |||||||||
| P1 | 17.2 ± 6.8 | 27.9 ± 5.5 | 72.5 ± 11.3 | 221.9 ± 42.3 | −33.9 ± 2.5 | −14.5 ± 2.1 | 44.1 ± 1.1 | 6 | Central sulcus M1 |
| P3 | 9.2 ± 7.3 | 21.9 ± 6.3 | 71.8 ± 6.3 | 154.5 ± 50.4 | −36.1 ± 3.4 | −18 ± 6.4 | 41.4 ± 5.5 | 6 | Central sulcus M1 |
| Loaded | |||||||||
| P1 | 4.3 ± 5.0 | 17.7 ± 2.1 | 98.5 ± 9.7 | 179.9 ± 40.0 | −32.5 ± 2.8 | −12.1 ± 2.4 | 45.2 ± 2.5 | 8 | Central sulcus M1 |
| P3 | 9.7 ± 5.0 | 31.6 ± 11.3 | 103.9 ± 8.3 | 139.1 ± 34.0 | −37.5 ± 1.8 | −13.1 ± 2.5 | 44.9 ± 2.2 | 6 | Central sulcus M1 |
| MEF | |||||||||
| Unloaded | |||||||||
| P1 | 138.3 ± 10.5 | 33.7 ± 3.3 | 93.3 ± 10.2 | 260.6 ± 31.5 | −32.4 ± 1.9 | −16.7 ± 2.9 | 46.6 ± 1.6 | 7 | Central sulcus M1 |
| P3 | 162.6 ± 20.4 | 39.5 ± 7.3 | 84.6 ± 5.0 | 189.8 ± 19.5 | −33.5 ± 2.2 | −17.0 ± 2.5 | 45.1 ± 1.9 | 6 | Central sulcus M1 |
| Loaded | |||||||||
| P1 | 144.7 ± 10.1 | 48.7 ± 12.7 | 91.7 ± 7.7 | 207.4 ± 35.1 | −34.4 ± 1.5 | −17.7 ± 1.2 | 47.7 ± 1.2 | 7 | Central sulcus M1 |
| P3 | 180.5 ± 17.0* | 28.9 ± 3.8 | 74.2 ± 3.9 | 218.3 ± 23.6 | −38.1 ± 1.1 | −30 ± 1.3** | 47.3 ± 1 | 7 | Postcentral gyrus S1 |
| Unloaded | |||||||||
| P1 | 162.8 ± 22.0 | 41.9 ± 8.6 | 76.7 ± 21.1 | 279.2 ± 27.8 | −35.3 ± 2.9 | −28.7 ± 1.7 | 15.0 ± 2.8 | 7 | SII‐posterior insula |
| P3 | 147.7 ± 11.4 | 64.0 ± 26.1 | 125.0 ± 11.6 | 279.0 ± 47.7 | −37.1 ± 2.7 | −18.8 ± 6.3 | 18.2 ± 3.0 | 6 | SII‐posterior insula |
| Loaded | |||||||||
| P1 | 153.8 ± 19.1 | 46.9 ± 9 | 74.6 ± 16.8 | 248.8 ± 39.2 | −34.3 ± 1.7 | −26.3 ± 2.9 | 14.9 ± 4 | 9 | SII‐posterior insula |
| P3 | 144.4 ± 14.3$ | 37.3 ± 7.2 | 93.0 ± 13.0 | 255.6 ± 36.5 | −36.3 ± 1.2 | −8,4 ± 4.8** | 17.8 ± 3.2 | 8 | Medial insula |
| Unloaded | |||||||||
| P3 | 150.0 ± 28.7 | 49.7 ± 12 | 98.0 ± 12.5 | 187.8 ± 13.6 | −6.1 ± 2.1 | −30 ± 7 | 40.6 ± 3.5 | 4 | Posterior cingulate |
| Loaded | |||||||||
| P1 | 121.0 ± 18.1 | 91.2 ± 20.2 | 88.6 ± 4.1 | 231.7 ± 32.3 | −10.3 ± 1.9 | −32.6 ± 7.7 | 25.7 ± 5.4 | 5 | Posterior cingulate |
| P3 | 136.0 ± 19.8$$ | 93.3 ± 17 | 89.0 ± 3.5 | 200.7 ± 42.2 | −7.8 ± 3 | −26.1 ± 8.6 | 34 ± 5.9 | 6 | Posterior cingulate |
Significant variations levels are noted as follows: (1) *P < 0.05 and **P < 0.01 for the P3 versus P1 latency and y coordinate comparisons; (2) $ P < 0.05 and $$ P < 0.01 for latencies comparisons during the Period 3 of the medial‐insular or the posterior cingulate area versus the postcentral gyrus (S1).
Figure 6.
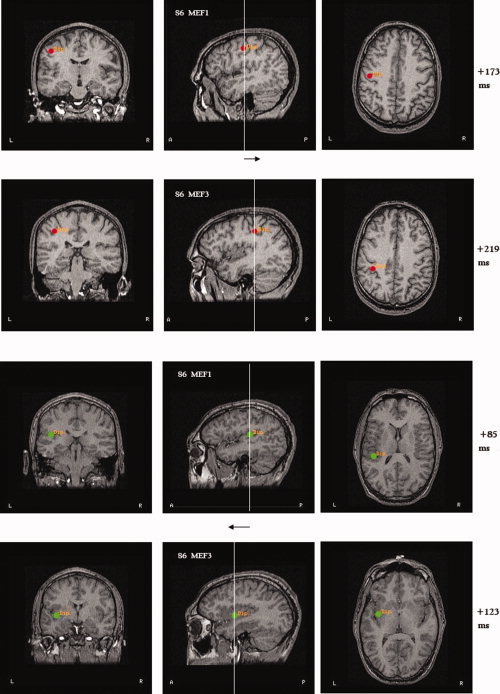
Examples of dipole shifts for the Subject 6 during the MEF. The two upper rows show the shift of the dipole location from the precentral to the postcentral gyrus. The two lower rows show the dipole shift from the posterior SII‐insula (in Period 1, labeled here MEF1) to the medial insula (in Period 3: MEF3). Note that for this subject SII‐insula and medial insula activations precede M1 and S1 activations by about 100 ms.
During MEFs when comparing Periods 1–3, two significant shifts in dipole locations were observed in M1‐S1 and SII‐insula with fatigue:
-
1
Dipole location shifted an average of −12.3 ± 1.3 mm, from an anteroinferior position in the central sulcus (around 145 ms) to a postcentral gyrus position (toward 180 ms): the more posterior the location, the higher the latency (F 12, 1 = 5.224, R = −0.567; P < 0.05, see Table II). Moreover, this location shift correlated with signs of muscle fatigue across subjects: increase of RMS‐EMG of both FDS and ED muscles (F 13, 1 = 17.08, R = −0.766, P < 0.001 and F 13, 1 = 6.311, R = −0.587, P < 0.03, respectively) and increase of the EMD (F 12, 1 = 4.926, R = −0.556, P < 0.05). It should also be underlined that during Period 3, the higher the median frequency shift of the FDS‐EMG muscle, the higher the dipole location shift was toward S1 (F 8, 1 = 23.47, R = 0.908, P < 0.005). To sum up, this shift in dipole activation from an M1 to a S1 maximum is clearly related to sensory afferences from the fatiguing muscle.
-
2
Dipole location shifted anteriorly 17.9 ± 3.9 mm from the posterior SII‐insula to the medial insula (P < 0.01; see Table II, y coordinate). When the RMS‐EMG activities of both FDS and ED muscles increased, the shift toward the medial insula position increased (F 15, 1 = 6.03, R = 0.549, P < 0.03; F 16, 1 = 6.15, R = 0.539, P < 0.03; respectively). It is noteworthy that during the 3rd Period, SII‐insular activations were the shortest, i.e. about 35 ms before those of S1 (Table II; P < 0.03). These results provide evidence that SII‐posterior‐insular and medial insular cortices play a primary role in the integration of sensory and nociceptive information.
DISCUSSION
In this study, fatigue is clearly demonstrated in the loaded condition by biomechanical, behavioral, cardiovascular, and neurophysiological data. As expected, during the loaded condition, the linear increase in RT stems mainly from an increase in the EMD [Pääsuke et al., 1999; Yeung et al., 1999; Zhou et al., 1996]. Moreover, as also described by Onishi et al. [2006], during RT task, our MEG results show that MF and MEF latencies are not different between the loaded and unloaded conditions, a finding which supports that the sensorimotor cortices are activated at the same time, regardless of load and EMD. In other words, it is the EMD and the RT, which increase with fatigue.
In the loaded condition, work decreased linearly from Periods 1 to 3, while the heart rate and the myoelectric RMS amplitude of both FDS and ED muscles increased. In addition, during Period 3, the spectral deflection of the EMG median frequency of FDS muscle shifted to a lower frequency. These results are well‐recognized signs of fatigue [Gerdle and Karlsson, 1994; Hagberg, 1981; Kleine et al., 2001; Löscher et al., 1996; Mannion and Dolan, 1996]. Further, it is noteworthy that, in the loaded condition, RMS‐EMGs of the FDS and ED muscles increase even though the work decreases, a finding which substantiates the view that subjects produced a voluntary effort to execute the task until the endurance time elapsed [Gandevia, 2001; Jouanin et al., 1993]. To sum up, with fatigue the reduced motor drive very likely leads to a decrease in motor units firing rate or recruitment while the motoneurons discharge decreased. Moreover the increase in RMS at the end of the task that is lower than the RMS of the maximal voluntary contraction supports a decrease in motor drive with central fatigue [Löscher et al., 1996].
Neuroimaging studies [Dai et al., 2001; Dettmers et al., 1995] have reported a proportional relationship between cortical signals and exerted force, indicating that brain signals are positively correlated to voluntary effort, as a higher level of effort is required for exerting greater muscle force. However, during muscle fatigue, a number of studies have also reported “suboptimal” central drive, indicating that the maximal central drive may decline, may not be able to reach the maximal level [Gandevia, 1998, 2001; Taylor et al., 2000] or plateaued [Liu et al., 2003]. Moreover, it has also been shown that motor cortical excitability assessed by transcranial magnetic stimulation was lower at the end of fatiguing contractions, suggesting that cortical output neurons may have been affected by inputs from inhibitory sources [Brasil‐Neto et al., 1994; Gandevia, 2001]. Our MEG data contribute to clarify when and why different intensities of the central drive may be observed. Indeed, we found that MF decreases whereas MEFs increase during the task (Table II). Thus, if the time window of measured activations does not allow separating MF from MEFs activities (which is the case with PET and fMRI studies), activations can remain stable, and increase or decrease with no clear relationships with the fatigue induced by the task since MF and MEFs activations are pooled.
In our results, MEFs are not only explained by M1‐S1 activations [Cheyne et al., 2006; Kristeva‐Feige et al., 1994] but also by activation of SII‐insula, medial insula, and posterior cingulate. Indeed, sensory afferent feedbacks in S1 and SII‐insula and medial insula were demonstrated in humans during cutaneous pain [Bingel et al., 2004; Garcia‐Larea et al., 2003; Howland et al., 1995; Kakigi et al., 2004; Oshiro et al., 1998; Qiu et al., 2004; Tran et al., 2002; Youell et al., 2004], muscle pain [Ferretti et al., 2003; Schreckenberger et al., 2005; Svensson et al., 1997], and with mechanical [Gelnar et al., 1999; Ringler et al., 2003] or electrical stimuli [Kakigi et al., 1995]. Moreover, MEFs are known to reflect afferent input to the sensorimotor area from moving muscles (muscle spindles or Golgi tendon organs) and cutaneous input [Cheyne et al., 1997; Hari and Forss, 1999; Weinberg et al., 1990]. More precisely, muscles spindle responses are activated 50–100 ms after EMG onset during isometric contractions [Vallbo, 1971]. These latencies are in the same latency range as MEFI [Cheyne et al., 2006], which supports an increase in MEFs amplitude due to these afferences.
In our study the dipole shift from M1 to S1 during the loaded condition does covary with myoelectric signs of fatigue. It should be underlined that dipole location is at the barycenter of a complex piece of cortical surface of which the extension may approximate 1 or 2 cm2. Thus, this shift should not be interpreted as a deactivation of M1 but as a balance of activity between M1 and S1, which is centered in M1 at the beginning of the loaded condition and in S1 at the end. This further substantiates the view that muscle fatigue induces a sensory feedback in S1 (especially tactile and proprioceptive afferent input to area 3b and 3a), which in turn contributes to a decrease of motor cortex activity (in the hand region of the precentral gyrus areas 6 and 4). This interpretation is corroborated by the results of Balzamo et al. [2004] and Masakado et al. [2004], who described direct nociceptive afferences (cutaneomuscular) from the sensorimotor area (S1) to the primary motor cortex.
Indeed, we also found a shift of activation from posterior SII‐insular to medial insular cortex: the higher this shift, the higher the increase of the RMS‐EMG. This clearly supports a relationship between medial‐insular activation and fatigue accompanied by pain and exhaustion. As also supported by our results, the electrogenesis of the MEF components should depend on sensory and nociceptive afferences projecting directly in S1 and in the caudal part of SII‐retroinsular cortex [Iannetti et al., 2005; Inui et al., 2003; Mima et al., 1998, Peyron et al., 2000, 2002; Qiu et al., 2004; Tran et al., 2002]. Mechanoreceptor inputs appear to be dominant in S1 [Ringler et al., 2003], and the insula also receives inputs from several areas associated with pain processing such as SI, SII, and the anterior cingulate cortex [Niddam et al., 2002; Svensson et al., 1997]. Ferretti et al. [2003] described two SII representations: a medial one for muscle nociception and a posterior one for skin nociception. Given that, in our loaded condition, the displacement of the middle finger reduces with fatigue, skin nociceptive receptors should be less activated than muscle receptors, which could explain the shift to the medial‐insula. Actually, Henderson et al. [2007] have demonstrated that posterior insula activation is observed in response to cutaneous pain, whereas muscle pain induces more anterior activation. This is also supported by our study since posterior or more medial‐anterior insula activations are, respectively, observed in relation to cutaneous pain during Period 1 and mainly in relation to muscular pain during Period 3. Moreover, painful and nonpainful somaesthesic representations in human insula overlap [Craig et al., 1996; Ostrowsky et al., 2002]. Neurons in the anterior insular cortex project into various limbic structures; hence, it may be argued that this region endorses a critical role inasmuch as it correlates ongoing pain with prior pain experiences and with both the motivational and affective components of pain [Brooks and Tracey, 2007; Svensson et al., 1997). In addition, attention‐related influence is known to enhance SII responses [Mima et al., 1998], because maintaining an isometric contraction requires sustained attention to the site of stimulation [Vrána et al., 2005].
Co‐contractions of other digits, hand, arm, and shoulder muscles, which occur in our study with fatigue, were most likely involved in the changes in S1 and medial‐insular activities. Two studies support this assumption. Lin et al. [2000] have shown that SII responses were differently affected by isometric contractions of various body parts: SII responses were greater during the isometric contraction of the thenar muscles, and also enhanced by the contraction of the deltoid muscle. Furthermore, Forss and Jousmäki [1998] measured the somatosensory evoked field during a submaximal isometric contraction of thenar muscles. They argued that isometric contractions facilitate the activation of SII cortices to tactile stimuli, possibly by decreasing inhibition from S1 cortex. Modulation of SII‐insula activity, therefore, seems to depend on the topographical proximity of contracting muscles with the stimulated body part. Indeed, our results are in accordance with Ruben et al. [2001] who described a somatotopic representation in SII within the controlateral parietal operculum. They found finger representations within the controlateral operculum, roughly halfway between the lip of the lateral sulcus and its fundus, whereas the representation site of the hallux was found more medially to this position at the fundus of the lateral sulcus near the posterior pole of the insula. Since at the end of the loaded task (P3) subjects also griped the handrail with the hallux, this can explain a part of the observed variability in dipole locations across subjects.
In our study, the six subjects who depicted activations of the posterior cingulate cortex also experienced exhaustion (or pain and exhaustion; see Table I). The posterior part of the cingulate cortex has been shown to be a skeletomotor region [Vogt et al., 2003] also activated during pain‐motor interactions [Vràna et al., 2005]. Moreover, Vogt et al. suggested that the posterior cingulate cortex could coordinate skeletomotor reflex responses. Our results support this assumption for muscular nociceptive input. Indeed, it is only during the third part of the fatiguing loaded task, but not during the unloaded condition, that posterior cingulate and medial insula activations do significantly precede S1 activation (40 ms).
To our knowledge, this MEG study provides for the first time evidence that, during MEFs, the nodes of a spatiotemporal network differently cooperate as a function of muscular fatigue. The main nodes of this network are primary motor cortex (M1), somatosensory cortex (S1), SII‐posterior insular area, medial insula, and posterior cingulate cortices. The timing of activation of these nodes and the relative balance of its activities do vary with fatigue. Indeed, during unloaded or nonfatiguing periods, activations of the nodes are massively parallel. On the contrary, during fatiguing and exhausting parts of the task, activations appear to be under the control of both medial insula and posterior cingulate cortices whose latencies of activation are shorter than those of the sensorimotor cortex. This supports that these regions are critically involved in the control of movement execution by increasing the response time (EMD) and/or inducing skeletomotor reflexes in order to preserve muscle efficiency and integrity.
Finally, these results were obtained for a homogenous group of high‐performance male athletes, with a high level of endurance, who may demonstrate abilities somehow different from those that would have been observed for a more sedentary control group. Nevertheless, we think that they represent a good picture of the neural basis of central fatigue that could be challenged in future by further studies.
Acknowledgements
The authors thank Drs. S. Lebozec, B. Maton, P. Satabin, and C.Y. Guézennec for helpful advices on previous versions of this article; Drs. F. Ash and J.‐R. Soss for very careful readings of the manuscript and English corrections.
Contributor Information
Jean‐Claude Jouanin, Email: jcjouanin@imassa.fr.
Bernard Renault, Email: bernard.renault@chups.jussieu.fr.
REFERENCES
- Asmussen E ( 1979): Muscle fatigue. Med Sci Sports Exerc 11: 313–321. [PubMed] [Google Scholar]
- Balzamo E,Marquis P,Chauvel P,Regis J ( 2004): Short‐latency components of evoked potentials to median nerve stimulation recorded by intracerebral electrodes in the human pre‐ and postcentral areas. Clin Neurophysiol 115: 1616–1623. [DOI] [PubMed] [Google Scholar]
- Baumgartner C,Doppelbauer L,Deecke L,Barth DS,Zeithlofer J,Lindinger G,Sutherling W ( 1991): Neuromagnetic investigation of somatotopy of human hand somatosensory cortex. Exp Brain Res 87: 641–648. [DOI] [PubMed] [Google Scholar]
- Belhaj‐Saïf A,Fourment A,Maton B ( 1996): Adaptation of the precentral cortical command to elbow muscle fatigue. Exp Brain Res 111: 405–416. [DOI] [PubMed] [Google Scholar]
- Bigland‐Ritchie B ( 1981): EMG/force relations and fatigue in human voluntary contractions. Exerc Sports Sci Rev 9: 75–117. [PubMed] [Google Scholar]
- Bigland‐Ritchie B,Furbush F,Woods JJ ( 1986): Fatigue of intermittent submaximal voluntary contractions: Central and peripheral factors. J Appl Physiol 61: 421–429. [DOI] [PubMed] [Google Scholar]
- Bingel U,Lorenz J,Glauche V,Knab R,Glascher J,Weiller C,Buchel C ( 2004): Somatotopic organization of human somatosensory cortices for pain: A single trial fMRI study. Neuroimage 23: 224–232. [DOI] [PubMed] [Google Scholar]
- Brasil‐Neto JP,Cohen LG,Hallet M ( 1994). Central fatigue as revealed by postexercise decrement of motor evoked potentials. Muscle Nerve 17: 713–719. [DOI] [PubMed] [Google Scholar]
- Brooks JCW,Tracey I ( 2007). The insula: A multidimensional integration site of pain. Pain 128: 1–2. [DOI] [PubMed] [Google Scholar]
- Brooks JCW,Zambreanu L,Godinez A,Craig AD,Tracey I ( 2005): Somatotopic organization of the human insula to painful heat studied with high‐resolution functional imaging. Neuroimage 27: 201–209. [DOI] [PubMed] [Google Scholar]
- Cheyne D,Endo H,Takeda T,Weinberg H ( 1997): Sensory feedback contributes to early mouvement‐evoked fields during voluntary finger movements in humans. Brain Res 771: 196–202. [DOI] [PubMed] [Google Scholar]
- Cheyne D,Bakhtazad L,Gaetz W ( 2006). Spatiotemporal mapping of cortical activity accompanying voluntary movements using an event‐related beamforming approach. Hum Brain Mapp 27: 213–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciubotariu A,Arendt‐Nielsen L,Graven‐Nielsen T ( 2004): The influence of muscle pain and fatigue on the activity of synergistic muscles of the leg. Eur J Appl Physiol 91: 604–614. [DOI] [PubMed] [Google Scholar]
- Conway BA,Halliday DM,Farmer SF,Shanani U,Mass P,Weir AI,Rosenberg JR ( 1995): Synchronization between motor cortex and spinal motoneural pool during the performance of a maintained motor task in man. J Physiol 489: 917–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD,Reiman EM,Evans A,Bushnell MC ( 1996): Functional imaging of an illusion of pain. Nature 384: 258–260. [DOI] [PubMed] [Google Scholar]
- Dai TH,Liu JZ,Sahgal V,Brown RW,Yue GH ( 2001). Relationship between muscle output and functional MRI‐measured brain activation. Exp Brain Res 140: 290–300. [DOI] [PubMed] [Google Scholar]
- Dettmers C,Fink GR,Leman RN,Stephan KM,Passingham RE,Silbersweigh D,Holmes A,Ridding MC,Brooks DJ,Frackowiak RS ( 1995). Relation between cerebral activity and force in the motor areas of the human brain. J Neurophysiol 74: 802–815. [DOI] [PubMed] [Google Scholar]
- Dettmers C,Lemon RN,Stephan KM,Fink GR,Fractowiak RS ( 1996): Cerebral activation during the exertion of sustained static force in man. Neuroreport 7: 2103–2110. [DOI] [PubMed] [Google Scholar]
- Di Guilio C,Daniele F,Tipton C ( 2006): Angelo Mosso and muscular fatigue: 116 years after the first congress of physiologists: IUPS commemoration. Adv Physiol Edu 30: 51–57. [DOI] [PubMed] [Google Scholar]
- Endo H,Kizuka T,Masuda T,Takeda T ( 1999): Automatic activation in the human primary motor cortex synchronized with movement preparation. Cogn Brain Res 3: 229–239. [DOI] [PubMed] [Google Scholar]
- Endo H,Takeda T,Kizuka T,Kikuchi Y,Masuda T,Kumagai T ( 1996): Estimation of movement‐related brain activities with visual stimuli. Visualization of information processing in the human brain: Electroencephal Clin Neurophysiol, Suppl. 47: 273–281. [PubMed] [Google Scholar]
- Ferretti A,Babiloni C,Del Gratta C,Caulo M,Tartao A,Bonomo L,Rossini PM,Romani GL ( 2003): Functional topography of the secondary somatosensory cortex for nonpainful and painful stimuli: An fMRI study. Neuroimage 20: 1625–1638. [DOI] [PubMed] [Google Scholar]
- Forss N,Jousmaki V ( 1998): Sensorimotor integration in human primary and secondary somatosensory cortices. Brain Res 781: 259–267. [DOI] [PubMed] [Google Scholar]
- Fuglevand AJ,Zackowski KM,Juey KA,Enoka RM ( 1993): Impairment of neuromuscular propagation during human fatiguing contractions at submaximal forces. J Physiol 460: 549–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC ( 1998): Neural control in human muscle fatigue: Changes in muscle afferents, moto neurons and moto cortical drive. Acta Physiol Scand 162: 275–283. [DOI] [PubMed] [Google Scholar]
- Gandevia SC ( 2001): Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81: 1725–1789. [DOI] [PubMed] [Google Scholar]
- Garcia‐Larrea L,Frot M,Valeriani M ( 2003): Brain generators of laser‐evoked potentials: From dipoles to functional significance. Clin Neurophysiol 33: 279–292. [DOI] [PubMed] [Google Scholar]
- Garland SJ,Enoka RM,Serrano LP,Robinson GA ( 1994): Behaviour of motor units in human biceps brachii during a submaximal fatiguing contraction. J Appl Physiol 76: 2411–2419. [DOI] [PubMed] [Google Scholar]
- Gelnar PA,Krauss BR,Sheehe PR,Szeverenyl NM,Apkarian V ( 1999): A comparative fMRI study of cortical representations for thermal painful, vibrotactile, and motor performance tasks. Neuroimage 10: 460–482. [DOI] [PubMed] [Google Scholar]
- Gerdle B,Karlssson S ( 1994): The mean frequency of the knee extensors is torque dependent both in the unfatigued and the fatigued states. Clin Physiol 14: 419–432. [DOI] [PubMed] [Google Scholar]
- Gross J,Tass PA,Salenius S,Hari R,Freund HJ,Schnitzler A ( 2000): Cortico‐muscular synchronization during isometric muscle contraction in humans as revealed by magnetoencephalography. J Physiol 527: 623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg M ( 1981): Muscular endurance and surface electromyogram in isometric and dynamic exercise. J Appl Physiol 51: 1–7. [DOI] [PubMed] [Google Scholar]
- Hari R,Forss N ( 1999): Magnetoencephalography in the study of human somatosensory cortical processing. Phil Trans R Soc Lond 354: 1145–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson LA,Gandevia SC,Macefield VG ( 2007). Somatotopic organizationb of the processing of muscle and cutaneous pain in the left and right insula cortex: A single‐trial fMRI study. Pain 128: 20–30. [DOI] [PubMed] [Google Scholar]
- Howland EW,Wakai RT,Mjaanes BA,Balog JP,Cleeland CS ( 1995): Whole head mapping of magnetic fields following painful electric finger shock. Cogn Brain Res 2: 165–172. [DOI] [PubMed] [Google Scholar]
- Iannetti GD,Zambreanu L,Cruccu G,Tracey I ( 2005): Operculoinsular cortex encodes pain intensity at the earliest stages of cortical processing as indicated by amplitude of laser‐evoked potentials in humans. Neuroscience 131: 199–208. [DOI] [PubMed] [Google Scholar]
- Inui K,Tran DT,Qiu Y,Wang X,Hoshiyama M,Kakigi R ( 2003): A comparative magnetoencephalographic study of cortical activations evoked by noxious and innocuous somatosensory stimulations. Neuroscience 120: 235–248. [DOI] [PubMed] [Google Scholar]
- Ishibashi H,Tobimatsu S,Shigeto H,Morioka T,Yamamoto T,Fukui M ( 2000): Differential interaction of somatosensory inputs in the human primary sensory cortex: A magnetoencephalographic study. Clin Neurophysiol 111: 1095–1102. [DOI] [PubMed] [Google Scholar]
- Jouanin JC,Kahn JF,Grucza R,Monod H ( 1993): Changes in the heart rate and electromyogram beyond the limit time of an isotonic isometric contraction. Eur J Appl Physiol 67: 208–212. [DOI] [PubMed] [Google Scholar]
- Kahn JF,Monod H ( 1989): Fatigue induced by static work. Ergonomics 32: 839–846. [DOI] [PubMed] [Google Scholar]
- Kakigi R,Koyama S,Hoshiyama M,Wanatabe S,Shimojo M,Kitamura Y ( 1995): Gating of somatosensory evoked responses during active finger movements: Magnetoencephalographic studies. J Neurol Sci 128: 195–204. [DOI] [PubMed] [Google Scholar]
- Kakigi R,Inui K,Tran DT,Qiu Y,Wang X,Watanabe S,Hoshiyama M ( 2004): Human brain processing and central mechanisms of pain as observed by electro‐ and magneto‐encephalography. J Chin Med Assoc 67: 377–386. [PubMed] [Google Scholar]
- Kleine BU,Stegeman DF,Mund D,Anders C ( 2001): Influence of motoneurons firing synchronization on SEMG characteristics in dependence of electrode position. J Appl Physiol 91: 1588–1599. [DOI] [PubMed] [Google Scholar]
- Kristeva R,Cheyne D,Lang W,Lindinger G,Deecke L ( 1990): Movement‐related potentials accompanying unilateral and bilateral finger movements with different inertial loads. Electroencephal Clin Neurophysiol 75: 410–418. [DOI] [PubMed] [Google Scholar]
- Kristeva R,Cheyne D,Deecke L ( 1991): Neuromagnetic fields accompanying unilateral and bilateral voluntary movements: Topography and analysis of cortical sources. Electroenceph Clin Neurophysiol 81: 284–298. [DOI] [PubMed] [Google Scholar]
- Kristeva‐Feige R,Walter H,Lutkenhoner B,Hampson S,Ross B,Knorr Y,Steinmetz H,Cheyne D ( 1994): A neuromagnetic study of the functional organization of the sensorimotor cortex. Eur J Neurosci 6: 632–639. [DOI] [PubMed] [Google Scholar]
- Lin YY,Simoes C,Forss N,Hari R ( 2000): Differential effects of muscle contraction form various body parts on neuromagnetic somatosensory responses. Neuroimage 11: 334–340. [DOI] [PubMed] [Google Scholar]
- Liu JZ,Dai TH,Sahghal V,Brown RW,Yue GH ( 2002): Nonlinear cortical modulation of muscle fatigue: A functional MRI study. Brain Res 957: 320–329. [DOI] [PubMed] [Google Scholar]
- Liu JZ,Shan ZY,Zhang LD,Sahgal V,Brown RW,Yue GH ( 2003): Human brain activation during sustained and intermittent submaximal fatigue muscle contractions: An fMRI study. J Neurophysiol 90: 300–312. [DOI] [PubMed] [Google Scholar]
- Löscher WN,Cresswell AG,Thorstensson A ( 1996): Central fatigue during a long‐lasting submaximal contraction of the triceps surae. Exp Brain Res 108: 305–314. [DOI] [PubMed] [Google Scholar]
- Mannion AF,Dolan P ( 1996): Relationship between myoelectric and mechanical manifestations of fatigue in the quadriceps femoris muscle group. Eur J Appl Physiol 74: 411–419. [DOI] [PubMed] [Google Scholar]
- Maton B ( 1981): Human motor unit activity during the onset of muscle fatigue in submaximal isometric isotonic contraction. Eur J Appl Physiol 46: 271–281. [DOI] [PubMed] [Google Scholar]
- Maton B ( 1991): Central nervous changes in fatigue induced by local work In: Atlan G,Beliveau L,Bouissou P,Masson, editors. Muscle Fatigue: Biochemical and Physiological Aspects, Mason, Paris. pp 207–221. [Google Scholar]
- Maton B,Gamet D ( 1989): The fatigability of two agonistic muscles in human isometric voluntary submaximal contraction: An EMG study. II. Motor unit firing rate and recruitment. Eur J Appl Physiol 58: 369–374. [DOI] [PubMed] [Google Scholar]
- Masakado Y,Ushiba J,Tomita Y,Chino N ( 2004): Cortical neuromagnetic activity associated with cutaneomuscular reflex. Electromyog Clin Neurophysiol 44: 83–87. [PubMed] [Google Scholar]
- Meunier S,Garnero L,Ducorps A,Mazières L,Lehéricy S,Tézenas du Montcel S,Renault B,Vidailhet M ( 2001): Human brain mapping in dystonia reveals both endophenotypic traits and adaptive reorganization. Ann Neurol 50: 521–527. [DOI] [PubMed] [Google Scholar]
- Mima T,Nagamine T,Nakamura K,Shibasaki H ( 1998): Attention modulates both primary and second somatosensory cortical activities in humans: A magnetoencephalography study. J Neurophysiol 80: 2215–2221. [DOI] [PubMed] [Google Scholar]
- Niddam DM,Yeh TC,Wu YT,Lee PL,Ho LT,Arendt‐Nielsen L,Chen AC,Hsieh JC ( 2002): Event‐related functional MRI study on central representation of acute muscle pain induced by electrical stimulation. Neuroimage 17: 1437–1450. [DOI] [PubMed] [Google Scholar]
- Nordlund MN,Thortensson A,Cresswell AG ( 2004): Central and peripheral contributions to fatigue in relation to level of activation during repeated maximal voluntary isometric plantar flexions. J Appl Physiol 96: 218–225. [DOI] [PubMed] [Google Scholar]
- Onishi H,Soma T,Kameyama S,Oishi M,Fuijmoto A,Oyama M,Furusawa A,Kurokawa Y ( 2006). Cortical neuromagnetic activation accompanying two types of voluntary finger extension. Brain Res 1123: 112–118. [DOI] [PubMed] [Google Scholar]
- Oshiro Y,Fuijita N,Tanaka H,Hirabuki N,Nakamura H,Yoshiya I ( 1998): Functional mapping of pain‐related activation with echo‐planar MRI: Significance of the SII‐insular region. Neuroreport 9: 2285–2289. [DOI] [PubMed] [Google Scholar]
- Ostrowsky K,Magnin M,Ryvlin P,Isnard J,Guenot M,Maugière F ( 2002): Representation of pain and somatic sensation in the human insula: A study of responses to direct electrical cortical stimulation. Cerebral Cortex 12: 376–385. [DOI] [PubMed] [Google Scholar]
- Paasüke M,Ereline J,Gapeyeva H ( 1999): Neuromuscular fatigue during repeated exhaustive submaximal static contractions of knee extensor muscles in endurance‐trained, power‐trained and untrained men. Acta Physiol Scand 166: 319–326. [DOI] [PubMed] [Google Scholar]
- Peyron R,Laurent B,Garcia‐Larrea L ( 2000): Functional imaging of brain responses to pain. A review and meta‐analysis. Neurophysiol Clin 30: 263–288. [DOI] [PubMed] [Google Scholar]
- Peyron R,Frot M,Schneider F,Garcia‐Larrea L,Mertens P,Barral FG,Sindou M,Laurent B,Maugière F ( 2002): Role of operculoinsular cortices in human pain processing: Converging evidence from PET, fMRI, dipole modelling, and intracerebral recordings of evoked potentials. Neuroimage 17: 1336–1446. [DOI] [PubMed] [Google Scholar]
- Pizzela V,Tecchio F,Romani JL,Rossini PM ( 1999): Functional localization of the sensory hand area with respect to the motor central gyrus knob. Neuroreport 10: 3809–3814. [DOI] [PubMed] [Google Scholar]
- Qiu Y,Inui K,Wang X,Nguyen BT,Tran TD,Kakigi R ( 2004): Effects of distraction on magnetoencephalographic responses ascending through C‐fibers in humans. Clin Neurophysiol 115: 636–646. [DOI] [PubMed] [Google Scholar]
- Ringler R,Greiner L,Kohlloeffel L,Handwerker HO,Forster C ( 2003): BOLD effects in different areas of the cerebral cortex during painful mechanical stimulation. Pain 105: 445–453. [DOI] [PubMed] [Google Scholar]
- Ruben J,Schwiemann J,Deuchert M,Meyer M,Krause T,Curio G,Villringer K,Kurth R,Villringer A ( 2001): Somatotopic organization of human secondary somatosensory cortex. Cereb Cortex 11: 463–473. [DOI] [PubMed] [Google Scholar]
- Salenius S,Portin K,Salmelin R,Hari R ( 1997): Cortical control of human motoneuron firing during isometric contraction. J Neurophysiol 77: 3401–3405. [DOI] [PubMed] [Google Scholar]
- Schreckenberger M,Siessmeier T,Viertmann A,Landvogt C,Buchnolz HG,Rolke R,Treede RD,Bartenstein P,Birklein F ( 2005): The unpleasantness of tonic pain is encoded by the insular cortex. Neurology 64: 1175–1183. [DOI] [PubMed] [Google Scholar]
- Sohn MK,Garven‐Nielsen L,Arendt‐Nielsen l,Svensson P ( 2000): Inhibition of the motor unit firing during experimental muscle pain in humans. Muscle Nerve 23: 1219–1226. [DOI] [PubMed] [Google Scholar]
- Suk J,Ribary U,Cappell J,Yamamoto T,Llinas R ( 1991): Anatomical localization revealed by MEG recordings of the human somatosensory system. Electroencephalog Clin Neurophysiol 78: 185–196. [DOI] [PubMed] [Google Scholar]
- Svensson P,Minoshima S,Beydoun A,Morrow TJ,Casey KL ( 1997): Cerebral processing of acute skin and muscle pain in humans. J Neurophysiol 78: 450–460. [DOI] [PubMed] [Google Scholar]
- Talairach J,Tournoux P ( 1988): Coplanar Stereotaxic Atlas of the Human Brain. Stuttgart: Thieme Medical Publisher; p 122. [Google Scholar]
- Taylor JL,Allen GM,Butler JE,Gandevia SC ( 2000). Supraspinal fatigue during intermittent maximal voluntary contractions of the human elbow flexors. J Appl Physiol 89: 305–313. [DOI] [PubMed] [Google Scholar]
- Tran TD,Inui K,Hoshiyama M,Lam K,Qiu Y,Kakigi R ( 2002): Cerebral activation by the signals ascending through unmyelinated C‐fibers in humans: An magnetoencephalographic study. Neuroscience 113: 375–386. [DOI] [PubMed] [Google Scholar]
- Vallbo AB ( 1971): Muscle spindle response at the onset of isometric voluntary contractions in man. Time difference between fusimotor and skeletal motor effects. J Physiol 218: 405–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA,Berger GR,Derbyshire SW ( 2003): Structural and functional dichotomy of human midcingulate cortex. Eur J Neurosci 18: 3134–3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrána J,Poláček H,Stančák A ( 2005): Somatosensory‐evoked potentials are influenced differently by isometric muscle contraction of stimulated and non‐stimulated hand in humans. Neurosci Lett 386: 170–175. [DOI] [PubMed] [Google Scholar]
- Wannier TM,Maier MA,Hepp‐Reymond MC ( 1991): Contrasting properties of monkey somatosensory and motor cortex neurons activated during the control of force in precision grip. J Neurophysiol 65: 572–589. [DOI] [PubMed] [Google Scholar]
- Weinberg H,Cheyne D,Crisp D ( 1990): Electroencephalographic and magnetoencephalographic studies of motor function. Adv Neurol 54: 193–205. [PubMed] [Google Scholar]
- Yang TT,Gallen CC,Schwartz BJ,Bloom FE ( 1993): Noninvasive somatosensory homunculus mapping in humans by using a large‐array biomagnetometer. Proc Nat Acad Sci USA 90: 3098–3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung SS,Au AL,Chow CC ( 1999): Effects of fatigue on the temporal neuromuscular control of vastus medialis muscle in humans. Eur J Appl Physiol 80: 379–385. [DOI] [PubMed] [Google Scholar]
- Youell PD,Wise RG,Bentley DE,Dickinson MR,King TA,Tracey I,Jones AK ( 2004): Lateralization of nociceptive processing in the human brain: A functional magnetic resonance imaging study. Neuroimage 23: 1068–1077. [DOI] [PubMed] [Google Scholar]
- Zhou S,McKenna MJ,Lawson DL,Morrison WE,Fairweather I ( 1996): Effects of fatigue and sprint training on electromechanical delay of knee extensor muscles. Eur J Appl Physiol 72: 410–416. [DOI] [PubMed] [Google Scholar]


