Abstract
Suggestion, a powerful factor in everyday social interaction, is most effective during hypnosis. Subjective evaluations and brain‐imaging findings converge to propose that hypnotic suggestion strongly modulates sensory processing. To reveal the brain regions that mediate such a modulation, we analyzed data from a functional‐magnetic‐resonance‐imaging study on hypnotic‐suggestion‐induced pain on 14 suggestible subjects. Activation strengths in the right dorsolateral prefrontal cortex (DLPFC) during initiation of suggestion for pain correlated positively with the subjective intensity of the subsequent suggestion‐induced pain, as well as with the strengths of the maximum pain‐related activation in the in the secondary somatosensory (SII) cortex. Furthermore, activation of the insula and the anterior cingulate cortex predicted the pain‐related SII activation. The right DLPFC, as an area important for executive functions, likely contributes to functional modulation in the modality‐specific target areas of given suggestions. Hum Brain Mapp 2009. © 2009 Wiley‐Liss, Inc.
Keywords: suggestion, dorsolateral prefrontal cortex, hypnosis, brain, pain, functional magnetic resonance imaging
INTRODUCTION
Suggestion is widely involved in everyday human interaction. Effectiveness of suggestion is at strongest during hypnosis that is characterized by relaxation, focused attention and feeling of confidence [Barber, 2000; Kallio and Revonsuo, 2003]. Hypnosis therefore seems a useful tool in the study of suggestion‐related brain function [Raz and Shapiro, 2002]. Hypnosis is typically induced by suggestions for deepening relaxation and focused attention [Weitzenhoffer and Hilgard, 1962]. The resulting change in the subject's mental state is clinically effective in alleviating pain, especially when the pain is enhanced by increased arousal [Vickers and Zollman, 1999].
The clearest evidence of the effect of suggestion on brain activation comes, however, from studies that use specific suggestions in addition to the induction of hypnosis. For example, when subjects in a positron‐emission‐tomography (PET) study looked at a grey‐scale figure and either imagined or were suggested to see it in colors [Kosslyn et al., 2000], color‐processing brain areas were more strongly activated during hypnotic suggestion than during imagination alone, in agreement with the subjects' reports. After posthypnotic suggestion, modulations of brain activity in visual areas and in the anterior cingulate cortex were associated with enhanced performance in a Stroop task [Raz et al., 2005]. Along similar lines, fMRI demonstrated pain‐related brain areas to be more strongly activated during suggestion‐induced pain than during imagination of pain [Derbyshire et al., 2004]. Furthermore, change in activation and functional connectivity of the pain‐related midcingulate cortex correlated to modulation of pain by suggestion during hypnosis [Faymonville et al., 2000, 2003; Rainville et al., 1997].
These interesting findings arise the question how such a modulation is mediated in the brain. A PET study unravelled a wide‐spread frontal and parietal activation during pain‐modulating suggestions, advocating for the involvement of multiple cognitive functions during suggestion [Rainville et al., 1999]. Whether the output from some of these brain areas would result in the observed modulation of the pain‐related brain regions, remains, however elusive. As the impetus for the present study, we proposed that the relationship between the strength of brain activation during initiation of suggestion for pain and the intensity of the resulting pain—as well as the strength of the pain‐related brain activation—could be informative of such suggestion‐related modulation of brain activity.
We therefore analyzed data of a recent fMRI study [Raij et al., 2005] on 14 highly suggestible subjects. Raij et al. [ 2005] compared brain activation during suggestion‐induced pain with brain activation during laser‐induced pain. Instead of brain activation during the “suggestion‐induced pain,” i.e., the period from the subject's signal for maximum pain to the beginning of the suggestion for pain relief [Raij et al., 2005], we now focused on brain activation during the verbal initiation of suggestion for pain. This period is of interest, because it coincided with the subjects' reports of a gradual increase of pain. To avoid confusion between this early period of suggestion and the subsequent period of stable suggestion‐induced pain, we call the period of the initiation of suggestion for pain as “InitPain” and the subsequent period of suggestion‐induced pain as “SuggPain.” We specifically tested the hypothesis that activation during InitPain in the dorsolateral prefrontal cortex (DLPFC) would predict SuggPain‐related brain activation and subjective ratings of the pain. Such an effect can be expected by the role of the DLPFC in cognitive and perceptual control [Miller, 2000] and because the strength of the DLPFC activation during expectation of placebo analgesia is correlated with the amount of subsequent pain relief [Wager et al., 2004].
MATERIALS AND METHODS
This article utilizes fMRI data of our previous study on brain correlates of laser and suggestion‐induced pain [Raij et al., 2005]. Subjects, study procedure, and imaging are explained here to the extent needed to follow the present analysis that focuses on the period the hypnotic suggestion was verbally initiated [for further details, see Raij et al., 2005].
Subjects
The fMRI recordings were run on the 14 most suggestible subjects among 103 volunteers applying Stanford Hypnotic Susceptibility Scale: Form C [scores ≥ 8; Weitzenhoffer and Hilgard, 1962]. The subjects (ages 20–36years, mean 26 years; 11 females, 3 males; 13 right‐handed, one ambidextrous) gave a written informed consent before participation in the study approved by the local ethics committee.
Stimuli and Design
Before the subject entered the MRI scanner, researcher S.N. induced hypnosis for the whole imaging period by sequential relaxation of body parts and by suggesting deepening relaxation during number count from 1 to 10 (see Fig. 1). The subject was asked to signal with a small movement of the right foot when the pain had reached its maximum and when the pain was totally relieved. After a 30‐s hypnosis baseline, S.N. initiated the suggestion for pain: “Sensations in the back of your left hand start to become painful, more and more painful. Unpleasant experience of pain gets stronger and stronger, and when it reaches the limit you can tolerate it will not increase any further but will stay stable until I tell you that all pain disappears.” When necessary, the verbal suggestion was repeated until the subject signaled maximum pain. No new suggestions were given during the subsequent 30‐s period of suggestion‐induced pain. Thereafter, suggestion was given for pain relief: “The pain goes further and further away, and soon you do not feel any pain at all. The pain is relieved, and your hand feels totally normal.” Subject's signal for pain relief was followed by a 30‐s hypnosis baseline. During this period, no suggestion was given. After the hypnosis baseline, suggestion for pain was repeated. These procedures were repeated throughout the 12‐min scanning session. Immediately after the imaging session, the subject filled in a questionnaire about the experience of pain. These questions included the location and type of the pain as well as the temporal changes in pain intensity, and the subject filled visual analog scales (VAS) regarding intensity, unpleasantness, and the reality of the pain (latter on scale from imaginary pain to real pain). For the analysis, the end points of VAS ratings were anchored to 0 and 100.
Figure 1.
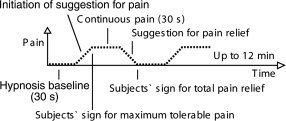
Study procedure.
Data Acquisition
Blood‐oxygenation‐level‐dependent (BOLD) signals were collected by Signa VH/i 3.0T MRI scanner (GE Healthcare, Chalmont St Giles, UK) with gradient‐echo (GRE) echo‐planar imaging (EPI) sequence (TR = 3 s, TE = 32 ms, flip angle = 90°, FOV = 20 cm, 96 × 96 matrix, slice thickness 3 mm without spacing, whole‐head coverage by 37 oblique axial slices). Structural images were collected by T1‐weighted 3D‐SPGR sequence (TR = 8.4 ms, TE = 1.8 ms, TI = 300 ms, flip angle 15°, NEX = 2).
Data Analysis
The imaging data were pre‐processed and analyzed with Statistical Parametric Mapping software (SPM2, http://www.fil.ion.ucl.ac.uk/spm). Functional images were realigned for head motion, and the image volumes were then spatially normalized [Friston et al., 1995a] into the average brain of the Montreal Neurological Institute (MNI), resulting in voxel size of 2 mm × 2 mm × 2 mm, and smoothed with a 8‐mm (full width at half maximum) Gaussian kernel.
In the analysis applying the general linear model [Friston et al., 1995b], separate box‐car functions were created to model brain activity related to individual alterations during InitPain, suggestion for pain relief, hypnosis baseline, as well as SuggPain and the subject's motor signaling. All these functions were convolved with a hemodynamic response function, and a first‐order autoregressive model was included to compensate for autocorrelation error [Bullmore et al., 1996]. Model function was then fit voxel by voxel to individual data, resulting in parameter estimates that were statistically compared to obtain individual contrast images for each condition of interest [Friston et al., 1995b]. The contrast images for “InitPain vs. hypnosis baseline” and for “InitPain vs. SuggPain” were then fed into group‐level one‐sample t tests. The “InitPain” refers here to the period from the beginning of the verbal suggestion for pain to the subject's signal for the maximum pain, and the “SuggPain” refers to the period from the subject's signal for the maximum pain to the beginning of the verbal suggestion for pain relief.
The individual pain ratings and the maximum pain‐related brain activations during SuggPain were tested for correlation with InitPain‐related activation strengths (i.e., parameter estimates from the contrast “InitPain vs. hypnosis baseline”). The pain‐related individual activation strengths were defined from the site where the group‐level activation had a global maximum in the contrast “SuggPain vs. hypnosis baseline.” This activation in the right (contralateral) secondary somatosensory (SII) cortex (x = 52, y = −30, z = 26) was reported in our earlier study on suggestion‐induced pain [Raij et al., 2005]. We extracted the individual mean parameter estimates from the voxels with P < 0.001 (n = 250; volume 2.0 cm3) in this SII cluster and entered these values to voxel‐wise correlation analysis with the parameter estimates from the contrast “InitPain vs. hypnosis baseline.”
Clusters of significant activation or correlation were identified by setting the height threshold at P < 0.05 (corrected for multiple comparisons at the cluster level according to random field theory).
In the DLPFC, we considered correlation of the InitPain‐related activation with pain ratings or pain‐related activation significant, when contiguous voxels (with P < 0.05 each for correlation, uncorrected) overlapped with the activation cluster related to InitPain. This overlap was visually searched for and is presented in Figure 3.
Figure 3.
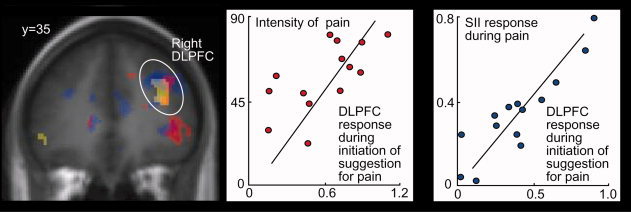
Left: Overlap of (i) activation during verbal initiation of suggestion for pain (InitPain; yellow), (ii) correlation across subjects between the activation strengths during InitPain and the subsequent intensity of suggestion‐induced pain (SuggPain; red), and (iii) correlation across subjects between the activation strengths during InitPain and activation strengths in the global maximum of SuggPain‐related activation in the contralateral second somatosensory (SII) cortex [blue; x = 52, y = −30, z = 26; Raij et al., 2005]. Middle: Correlation between activation strengths of the dorsolateral prefrontal cortex (DLPFC) during InitPain and the intensity of SuggPain (r = 0.66 at x = 40, y = 32, z = 26). Right: Correlation between activation strengths of the dorsolateral prefrontal cortex during InitPain and activation strengths in the global maximum of the SuggPain‐related activation in the contralateral second somatosensory cortex (r = 0.80 at x = 28, y = 32, z= 34).
To visualize overlap of the correlations in other brain regions, we created inclusive mask image for correlation (t > 1.78, P < 0.05, uncorrected) of activation in the contrast “InitPain vs. hypnosis baseline” with the subsequent pain intensity. We then used this mask image in above‐described voxel‐wise correlation analysis of the individual contrast images “InitPain vs. hypnosis baseline” with the SuggPain‐related SII activation.
We also tested whether the pain‐related activation in the right SII cortex would be stronger during SuggPain than during InitPain. In this one‐sample t test of individual contrast images for “SuggPain vs. InitPain,” we considered results statistically significant when contiguous voxels with P < 0.05 overlapped with the above‐described pain‐related SII cluster. Because several regions were activated in the contrast “InitPain vs. hypnosis baseline,” we applied statistical threshold of P < 0.005 in >20 contiguous voxels to test whether these regions would be activated also in the contrast “Init Pain vs. SuggPain.”
RESULTS
Behavioral Data
As reported previously [Raij et al., 2005], subjects signaled the maximum tolerable pain 29 ± 4 s (mean ± SEM; range 9−57 s) after the beginning of the suggestion. They reported the pain to increase gradually during InitPain and then stay stable until the suggestion for pain relief. On the 0–100 VAS, the reported intensity of pain was 57 ± 5, the unpleasantness of pain was 51 ± 6, and the reality of pain was 62 ± 5.
Imaging Data
The following brain areas were more active during InitPain than during hypnosis baseline: the bilateral temporal lobes, the right inferior frontal gyrus, the supplementary motor and anterior cingulate cortices, the premotor cortices, the right dorsolateral prefrontal cortex (DLPFC), the middle insula, and the cerebellum (P < 0.05, corrected for multiple comparisons; Fig. 2 and Table I). All these areas—except for the insula—were more active also during the InitPain than during SuggPain (P < 0.005 in >20 contiguous voxels, uncorrected).
Figure 2.
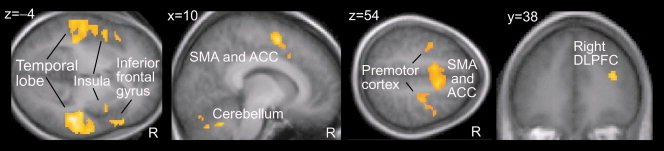
Activated brain areas during verbal initiation of suggestion for pain (referred as InitPain in the main text) overlaid on the average (normalized) structural MR image. SMA, supplementary motor cortex; ACC, anterior cingulate cortex.
Table I.
Brain regions that were more active during the initiation of suggestion for pain (referred as InitPain in the main text) than during hypnosis baseline
| Region | MNI | Peak z‐score | P cluster level corrected | Extent (cm3) | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Right temporal lobe | 50 | −22 | −6 | 5.4 | <0.001 | 18.9 |
| Left temporal lobe | −53 | −20 | −4 | 5.1 | <0.001 | 19.1 |
| Right inferior frontal gyrus | 56 | 22 | −8 | 5.2 | <0.001 | 8.1 |
| Right insula* | 38 | 8 | 11 | 4.3 | <0.001 | 3.3 |
| Left insula* | −38 | 18 | 5 | 5.4 | <0.001 | 5.8 |
| Right anterior cingulate cortex and supplementary motor cortex | 10 | 7 | 53 | 4.9 | <0.001 | 3.0 |
| Left anterior cingulate cortex and supplementary motor cortex | −10 | 2 | 50 | 4.4 | <0.001 | 2.1 |
| Cerebellar vermis | 10 | −58 | −24 | 4.6 | <0.001 | 3.7 |
| Right cerebellum | 26 | −73 | −20 | 3.9 | 0.002 | 1.3 |
| Left cerebellum | −44 | −64 | −27 | 4.4 | <0.001 | 3.2 |
| Right premotor cortex | 26 | −11 | 50 | 4.6 | <0.001 | 2.6 |
| Left premotor cortex | −36 | −2 | 44 | 4.0 | 0.011 | 2.4 |
| Right dorsolateral prefrontal cortex | 34 | 38 | 17 | 4.5 | 0.014 | 0.9 |
All suggestion‐related regions, except those two marked with an asterisk (*), were more active also compared with the following suggestion‐induced pain (referred as SuggPain in the main text; P < 0.005, uncorrected). Extent refers to the contiguous area of voxels with P < 0.001.
In contrast, the right SII cortex was more strongly activated during SuggPain than during InitPain (P = 0.002 for the maximum voxel at x = 48, y = −20, z = 26, P < 0.05 in 61 voxels, uncorrected; MNI coordinates reported throughout). Subjective intensity of pain explained 27% of the variance of the pain‐related activation at the second somatosensory cortex SII (r = 0.52, r 2 = 0.27).
Correlations in the Regions Activated During InitPain
In the right DLPFC, the individual activation strengths in the contrast “InitPain vs. hypnosis baseline” correlated with the subjective intensities of the subsequent pain (r = 0.66, P = 0.005 at x = 40, y = 32, z = 26; uncorrected P value for the maximum voxel). In an overlapping DLPFC region, these activation strengths correlated also with the SuggPain‐related activation strengths in the global maximum at the contralateral SII cortex (r = 0.89, P < 0.03, corrected, at x = 28, y = 32, z = 34; Fig. 3, Table II). In addition, the pain‐related SII‐activation strengths were predicted by activation strengths during InitPain (contrast “InitPain vs. hypnosis baseline”) in the bilateral medial thalamus, the premotor and motor cortices, the cerebellum, bilateral anterior cingulate cortex, bilateral insula, the right dorsolateral prefrontal cortex and the midbrain (r > 0.8, P < 0.05, corrected for multiple comparisons; Table II). Pain intensities were predicted by InitPain‐related activation strengths in these regions (P < 0.05; Fig. 4), except for the left insula, the premotor and the motor cortex.
Table II.
Brain regions in which activation strengths during initiation of suggestion for pain (InitPain) correlated with the maximum activation strengths (in the contralateral secondary somatosensory cortex) during subsequent suggestion‐induced pain (SuggPain)
| Region | MNI | Peak r | Peak z‐score | P cluster level corrected | Extent (cm3) | ||
|---|---|---|---|---|---|---|---|
| x | y | Z | |||||
| Right medial thalamus | 9 | −10 | 13 | 0.79 | 4.6 | <0.001 | 11.3 |
| Left medial thalamus | −6 | −12 | 12 | 0.82 | 4.6 | <0.001 | |
| Premotor and motor cortices | 10 | −28 | 72 | 0.91 | 4.6 | <0.001 | 5.7 |
| −12 | −10 | 64 | 0.91 | ||||
| 10 | −6 | 64 | 0.88 | ||||
| Cerebellum | −4 | −40 | −12 | 0.91 | 4.5 | <0.001 | 6.9 |
| −4 | −34 | −24 | 0.87 | ||||
| Bilateral anterior cingulate cortex | −6 | 2 | 40 | 0.90 | 4.4 | <0.001 | 6.9 |
| −10 | 12 | 30 | 0.87 | ||||
| 6 | 4 | 44 | 0.85 | ||||
| Right insula | 34 | 6 | 8 | 0.90 | 4.4 | 0.017 | 1.6 |
| Left insula* | −38 | 8 | 2 | 0.90 | 4.3 | 0.001 | 2.3 |
| Right dorsolateral–anterior prefrontal cortex* | 26 | 44 | 12 | 0.89 | 4.3 | 0.032 | 1.0 |
| Midbrain | 6 | −20 | −20 | 0.90 | 4.3 | 0.016 | 1.2 |
Activation strengths in all these regions, except those two marked with an asterisk (*), correlated with the maximum pain‐related activation also during SuggPain. Extent refers to the contiguous area of voxels with P < 0.001.
Figure 4.
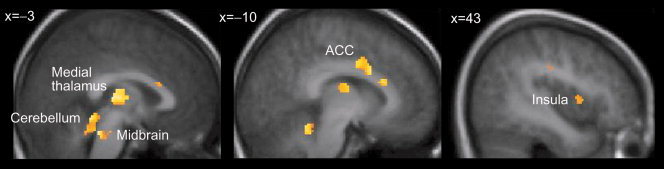
Other brain regions whose activation strengths during verbal initiation of suggestion for pain (InitPain) correlated both with the pain intensity and the maximum of the pain‐related activation in the contralateral second somatosensory (SII) cortex during the suggestion‐induced pain (SuggPain). Correlations are overlaid on the average (normalized) structural MR image. ACC, anterior cingulate cortex.
In addition to InitPain‐related activations, also SuggPain‐related activation strengths of the bilateral medial thalamus, the premotor and motor cortices, the cerebellum, the bilateral anterior cingulate cortex, and the midbrain correlated with the SuggPain‐related SII activation strengths (P < 0.05, corrected for multiple comparisons). The right DLPFC and the left insula showed a similar trend. The midbrain correlation peaked in the region of reticular formation and of the periaqueductal grey.
Correlations in Other Brain Regions
The InitPain‐related brain‐activation strengths showed a trend towards correlation with the reality ratings of the following experience of pain in the right orbitofrontal cortex (x = 26, y = 28, z = −16), the left parietal operculum (x = −36, y = −34, z = 22), and the bilateral medial prefrontal cortex (x = 16, y = 42, z = 18 and x = −8, y = 42, z = 14; P < 0.005 for the peak voxels, uncorrected).
DISCUSSION
Our most compelling finding was the positive correlation between the response strengths in the right DLPFC during InitPain and the pain‐related brain activation strengths and subjective intensity of SuggPain.
Correlation Between InitPain‐Related DLPFC‐Activation Strengths and SuggPain
To the best of our knowledge, similar correlation between suggestion‐related DLPFC‐activation strength and the following experience has not been reported before. The role of the prefrontal cortex in cognitive and perceptive control is, however, well known [Miller, 2000], and the right DLPFC‐activation strength has been shown to predict the effectiveness of placebo analgesia [Wager et al., 2004]. In contrast to SII cortex that is related to the sensory dimension of the pain, the DLPFC activation was weaker during the SuggPain than during InitPain. These findings suggest that the DLPFC correlation is not simply due to the involvement of the DLPFC in the processing of pain intensity.
Other Correlations Between InitPain and SuggPain
In addition to the DLPFC, both pain‐related subjective intensity and SII activation were predicted by InitPain‐related activation strengths in the bilateral medial thalamus, the cerebellum, the bilateral anterior cingulate cortex, the right insula, and the midbrain regions of the reticular formation, and the periaqueductal grey. In most of these regions, both InitPain‐ and SuggPain‐related activation strengths correlated with SuggPain‐related SII activation strengths.
The anterior cingulate cortex and the insula comprise the cortical regions of the medial pain system that processes the emotional‐motivational dimensions of pain. These regions have been shown to be active during anticipation of pain, social exclusion, and empathy for pain, without concurrent activation of the lateral (sensory) pain system [Eisenberger et al., 2003; Ploghaus et al., 1999; Singer et al., 2004]. In our previous study on suggestion‐induced pain, these regions were activated—although less strongly than during physically induced pain—in addition to the medial system [Raij et al., 2005]. Altogether, our findings suggest that, at least under expectation of physical pain, the medial pain‐system may contribute to the emergence and maintenance of the sensory experience of pain. This interpretation agrees with the proposal that the posterior ACC modulates activation in the pain‐related brain circuitries during pain‐modulating suggestions [Faymonville et al., 2003].
Further cues for the mechanisms of suggestion‐related modulation of the pain‐related brain activation may arise from the correlation between activation of the thalamus and the superior pons with the subsequent pain. These sites of activation agree with brain areas involved in descending pain control [Willis and Westlund, 1997].
Correlations Between InitPain‐Related Activation and the Subjective Reality of SuggPain
Correlation analysis suggested that orbitofrontal and medial prefrontal activation strengths during InitPain predict subjective reality of the subsequent pain. These correlations did not, however, overlap with the activation during InitPain. Because we focused on DLPFC function, these correlations were treated with conservative statistics that they did not survive. Both the orbitofrontal and the medial prefrontal cortex are, however, well suited correlates of the experience of the reality of pain. The medial prefrontal cortex is involved in processing of emotional and attentional information and has been linked to subjective reality of suggested experiences [Raij et al., 2005; Szechtman et al., 1998]. The orbitofrontal cortex, on the other hand, has been related to fear of the consequences of pain [Ochsner et al., 2006], and such fear likely contributes to the experience of pain reality.
Activation During InitPain vs. Activation During SuggPain
The brain activation during InitPain comprised a wide‐spread neuronal network that has been associated with pain‐modulating suggestion [Rainville et al., 1999]. Most regions of this network were activated more strongly during InitPain than during SuggPain. Interpretation of these findings is, however, difficult due to multiple cognitive functions involved in the speech comprehension and hypnotic suggestion; for an extensive proposal of such functions, see Rainville et al. [ 1999]. As the DLPFC can contribute to the functional states of distant brain regions by direct and indirect signaling [Miller, 2000], we suggest the DLPFC to be involved in activation of the target areas of a given suggestion.
CONCLUSIONS
Our findings extend the previous literature by showing that strengths of the DLPFC activation during InitPain correlate with the SuggPain‐related brain activations and with the intensity of the pain. These results speak for an active involvement of the right DLPFC in the modulation of the brain's pain circuitry during InitPain. In addition to the DLPFC, activation of the medial pain system and the midbrain regions related to descending pain control predicted SuggPain. Further studies are needed to address the exact mechanisms of such modulations and the extent to which these mechanisms overlap with those of the placebo effect as well as with those of suggestions without hypnosis in everyday communication.
Acknowledgements
The authors thank S. Aulanko, N. Forss, R. Joensuu, V. Jousmäki, M. Kattelus, S. Malinen, H. Renvall, M. Schürmann, and A. Tarkiainen for their expert help and advice.
REFERENCES
- Barber TX ( 2000): A deeper understanding of hypnosis: Its secrets, its nature, its essence. Am J Clin Hypn 42: 208–272. [DOI] [PubMed] [Google Scholar]
- Bullmore E,Brammer M,Williams SC,Rabe‐Hesketh S,Janot N,David A,Mellers J,Howard R,Sham P ( 1996): Statistical methods of estimation and inference for functional MR image analysis. Magn Reson Med 35: 261–277. [DOI] [PubMed] [Google Scholar]
- Derbyshire SW,Whalley MG,Stenger VA,Oakley DA ( 2004): Cerebral activation during hypnotically induced and imagined pain. Neuroimage 23: 392–401. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI,Lieberman MD,Williams KD ( 2003): Does rejection hurt? An FMRI study of social exclusion. Science 302: 290–292. [DOI] [PubMed] [Google Scholar]
- Faymonville ME,Laureys S,Degueldre C,DelFiore G,Luxen A,Franck G,Lamy M,Maquet P ( 2000): Neural mechanisms of antinociceptive effects of hypnosis. Anesthesiology 92: 1257–1267. [DOI] [PubMed] [Google Scholar]
- Faymonville ME,Roediger L,Del Fiore G,Delgueldre C,Phillips C,Lamy M,Luxen A,Maquet P,Laureys S ( 2003): Increased cerebral functional connectivity underlying the antinociceptive effects of hypnosis. Brain Res Cogn Brain Res 17: 255–262. [DOI] [PubMed] [Google Scholar]
- Friston KJ,Ashburner J,Frith CD,Poline J‐P,Heather JD,Frackowiak RSJ ( 1995a): Spatial registration and normalization of images. Hum Brain Mapp 2: 165–189. [Google Scholar]
- Friston KJ,Holmes AP,Worsley KJ,Poline J‐P,Frith CD,Frackowiak RSJ ( 1995b): Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp 2: 189–210. [Google Scholar]
- Kallio S,Revonsuo A ( 2003): Hypnotic phenomena and altered states of consciousness: A multilevel framework of description and explanation. Contemp Hypn 20: 111–164. [Google Scholar]
- Kosslyn SM,Thompson WL,Costantini‐Ferrando MF,Alpert NM,Spiegel D ( 2000): Hypnotic visual illusion alters color processing in the brain. Am J Psychiatry 157: 1279–1284. [DOI] [PubMed] [Google Scholar]
- Miller EK ( 2000): The prefrontal cortex and cognitive control. Nat Rev Neurosci 1: 59–65. [DOI] [PubMed] [Google Scholar]
- Ochsner KN,Ludlow DH,Knierim K,Hanelin J,Ramachandran T,Glover GC,Mackey SC ( 2006): Neural correlates of individual differences in pain‐related fear and anxiety. Pain 120: 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploghaus A,Tracey I,Gati JS,Clare S,Menon RS,Matthews PM,Rawlins JN ( 1999): Dissociating pain from its anticipation in the human brain. Science 284: 1979–1981. [DOI] [PubMed] [Google Scholar]
- Raij TT,Numminen J,Närvänen S,Hiltunen J,Hari R ( 2005): Brain correlates of subjective reality of physically and psychologically induced pain. Proc Natl Acad Sci USA 102: 2147–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainville P,Duncan GH,Price DD,Carrier B,Bushnell MC ( 1997): Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science 277: 968–971. [DOI] [PubMed] [Google Scholar]
- Rainville P,Hofbauer RK,Paus T,Duncan GH,Bushnell MC,Price DD ( 1999): Cerebral mechanisms of hypnotic induction and suggestion. J Cogn Neurosci 11: 110–125. [DOI] [PubMed] [Google Scholar]
- Raz A,Shapiro T ( 2002): Hypnosis and neuroscience: A cross talk between clinical and cognitive research. Arch Gen Psychiatry 59: 85–90. [DOI] [PubMed] [Google Scholar]
- Raz A,Fan J,Posner MI ( 2005): Hypnotic suggestion reduces conflict in the human brain. Proc Natl Acad Sci USA 102: 9978–9983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T,Seymour B,O'Doherty J,Kaube H,Dolan RJ,Frith CD ( 2004): Empathy for pain involves the affective but not sensory components of pain. Science 303: 1157–1162. [DOI] [PubMed] [Google Scholar]
- Szechtman H,Woody E,Bowers KS,Nahmias C ( 1998): Where the imaginal appears real: A positron emission tomography study of auditory hallucinations. Proc Natl Acad Sci USA 95: 1956–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers A,Zollman C ( 1999): ABC of complementary medicine. Hypnosis and relaxation therapies. BMJ 319: 1346–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD,Rilling JK,Smith EE,Sokolik A,Casey KL,Davidson RJ,Kosslyn SM,Rose RM,Cohen JD ( 2004): Placebo‐induced changes in fMRI in the anticipation and experience of pain. Science 303: 1162–1167. [DOI] [PubMed] [Google Scholar]
- Weitzenhoffer AM,Hilgard ER ( 1962): Stanford Hypnotic Susceptibility Scale: Form C. Palo Alto: Consulting Psychologists Press. [Google Scholar]
- Willis WD,Westlund KN ( 1997): Neuroanatomy of the pain system and the pathways that modulate pain. J Clin Neuropsysiol 14: 2–31. [DOI] [PMC free article] [PubMed] [Google Scholar]


