Abstract
Our aim in this study was to explore the neural substrates of executive function in frontal and nonfrontal white matter using diffusion tensor imaging (DTI). We studied the relationship between executive dysfunction and DTI measurements on 13 subjects with amnesic mild cognitive impairment (aMCI), 11 subjects with early Alzheimer's disease (AD), and 16 control subjects. All participants underwent an examination of their intelligence, memory, and executive function and were subjected to DTI. Both aMCI and early AD subjects showed executive function impairment with differential performance in frontal‐related behaviors. Both aMCI and early AD subjects showed increased mean diffusivity in the genu of the corpus callosum and left frontal periventricular white matter (PVWM), whereas subjects with early AD showed an additional decrease in the fractional anisotropy of bilateral frontal PVWM and in the genu of the corpus callosum. The frontal PVWM was associated with performance on the Verbal Fluency Test, the Wisconsin Card Sorting Test (WCST), and Part B of the Trail Making Test. The parietal PVWM was associated with perseverative errors on the WCST and Part A of the Trail Making Test. In summary, executive function was impaired in subjects with aMCI and early AD and was associated with frontal and parietal PVWM changes. These changes may be due to early AD degeneration of the lateral cholinergic projections or to early change of the superior longitudinal fasciculus. Hum Brain Mapp, 2009. © 2009 Wiley‐Liss, Inc.
Keywords: diffusion tensor imaging, Alzheimer's disease, mild cognitive impairment, executive function, periventricular white matter
INTRODUCTION
Alzheimer's disease (AD) is the most common type of dementia in elderly people. AD was previously considered to be a kind of cortical dementia associated with a loss of large pyramidal neurons primarily in the associative cortexes [Pearson et al.,1985]. More recently, however, the important role of white matter pathology in AD pathogenesis (such as myelin breakdown and oligodendrocytic loss) has been increasingly recognized [Brun and Englund,1986; Hirono et al.,2000; Sjöbeck and Englund,2003]. Studies showing that oligodendrocyte damage is an initial step in the disease suggest myelin‐breakdown as an important component of the AD process [Bartzokis et al.,2003]. White matter pathology in AD may be indicative of anterograde Wallerian degeneration, white matter rarefaction with axonal damage and gliosis, reactive astrocytosis, or myelin breakdown [Englund et al.,2004]. Unique spatial distributions of white matter changes may reflect a particular pathogenesis. Wallerian degeneration may involve lesions close to cortical areas having the greatest pathological burden. In contrast, diffusely confluent rarefaction is frequently vascular or ischemic in origin, usually at periventricular areas or near the hemispheric semiovale [Frazekas et al.,1993].
Because MCI is a heterogeneous condition, subjects with this impairment are classified into four clinical subtypes: amnesic MCI‐single domain, amnesic MCI‐multiple domains, nonamnesic MCI‐single domain, and nonamnesic MCI‐multiple domains [Busse et al.,2006]. The prognosis of each subtype varies. In a 4‐year follow‐up study in Pittsburgh, 70% of the subjects with amnesic MCI (aMCI) progressed to dementia (all of them to AD) and none returned to normal [Lopez et al.,2007]. In another study with a 6‐year observation period, 60% of the subjects with aMCI‐multiple domains developed dementia at follow‐up, and 100% of the participants with dementia had AD [Busse et al.,2006]. Therefore, in the present study, we included only subjects with aMCI (single or multiple‐domain subtypes), as they have a higher probability of developing AD at follow‐up and would be regarded as being in a very early phase of AD.
Magnetic resonance imaging (MRI) was used in the study of AD through hippocampal volumetry and quantification of increased white matter hyper‐intensities (WMHI). WMHI have been widely studied using conventional structure MRI techniques such as T2‐weighted and fluid‐attenuated inversion recovery (FLAIR) images. By these methods, WMHI have been reported to be associated with a decrement of executive functions in normal aging [O'sullivan et al.,2004; Marshall et al.,2006]. Conflicting findings have arisen, however, particularly in the earliest clinical stage of AD [Bartzokis et al.,2003; de Groot et al.,2000]. Although T2‐weighted changes are sensitive in the detection of subcortical vascular disease, they provide little information about the severity of the underlying pathological changes [Awad et al.,1986], and the correlation between T2‐lesion load and cognitive dysfunction was relatively modest [Sabri et al.,1999]. Diffusion tensor imaging (DTI) may allow for the detection of white matter changes in normal appearing white matter (NAWM). DTI provides increased sensitivity in the detection of alterations in the microstructure of white matter in vivo. Such alterations are indicative of diseases that cause axonal damage and demyelination.
DTI measures the directionality of the molecular diffusion of water, representing the structural integrity of white matter [Medina et al.,2006]. Mean diffusivity (MD) is a measurement of the mean motion of bulk water in all directions, and fractional anisotropy (FA) is a measurement of the direction consistency [Rose et al.,2006].
Neuropsychological investigations of early AD and aMCI were characterized by subtle deficits in multiple domains such as attention, learning and memory, executive function, processing speed, and language function [Twamley et al.,2006]. Among these, executive function was referred to as a supervisory, or high‐level cognitive process because of the combination and organization of complex behaviors and thoughts involved [Alvarez and Emory,2006; Stuss et al.,2000]. Subjects with frontal lobe insult, however, might not demonstrate significant executive dysfunction; and subjects with nonfrontal lesions might perform poorly in the executive function tests [Ahola et al.,1996; Anderson et al.,1991]. Therefore, we used DTI to explore the association between executive dysfunction and white matter changes in the frontal or nonfrontal structures in subjects with aMCI and early AD. We also examined whether there were any other differences in performance in the various aspects of executive function tested in these subjects.
SUBJECTS AND METHODS
Subjects
Thirteen subjects with aMCI, 11 subjects with early AD, and 16 control subjects participated in the study. All participants were right‐handed Taiwan Chinese. All three groups were evaluated with the same battery of tests, including the Wechsler Adult Intelligence Scale‐III (WAIS‐III), the Taiwan version of the Wechsler Memory Scale‐Third Edition (WMS‐III) [Hua et al.,2005], the Clinical Dementia Rating (CDR), and executive function tests. We also performed the WAIS‐III test on all participants to obtain a quotient for general intelligence (IQ) and evaluated them with CDR to determine the global severity of dementia.
Subjects with amnesic MCI and early AD were selected from the memory clinic at the National Taiwan University Hospital. Following tests at the memory clinic, they received a comprehensive clinical checkup including a review of their history, physical and neurological examinations, and laboratory tests. Subjects with early AD fulfilled the NINCDS‐ADRDA criteria for probable AD with CDR scores of 0.5 or 1.0 [McKhann et al.,1984]. The early AD subjects were reviewed using the Hachinski ischemic scale and those with a Hachinski ischemic score greater than four were excluded [Moroney et al.,1997].
Subjects with deficits in any recall subtest of the WMS‐III, a CDR score of 0.5, and consistently normal ADL/IADL, were diagnosed as aMCI in keeping with the Mayo clinic criteria [Petersen et al.,2001].
The control subjects were selected from healthy volunteers in an MCI project. In this project, the volunteers were given a medical check‐list for major systemic disease, operation, and hospitalization. Those having uncontrolled medical conditions such as heart failure, recent myocardial infarction (in the past 6 months), malignancy (in the past 2 years), or poorly controlled diabetes (Hb A1C > 8.5) were excluded. They also received physical and neurological examinations and were scored on a short form Geriatric Depression Scale (GDS‐S); those who had a GDS‐S score greater than nine were excluded. Control subjects had normal cognitive function, confirmed by the neuropsychological test battery. All subjects or their primary caregivers gave their informed consent. The study was approved by the ethics committee of the university hospital.
Executive Function Tests
Executive function was examined with (1) a Verbal Fluency (VF) task; (2) the Wisconsin Card Sorting Test (WCST), Modified version; (3) the Trail Making Test (TMT), part A and B; and (4) the Frontal Behavioral Inventory (FBI).
In the VF task, subjects were instructed to name as many vegetables, fish, and fruits as possible in 1 min for each category [Hua et al.,1997]. We used the WCST with 52 cards and calculated the number of completed categories and perseverative errors as indices of executive function [Nelson,1976]. Both written TMT‐A and TMT‐B [Reitan,1958] were applied as a measure of attention, speed, and mental flexibility. The scoring was expressed in terms of the time in seconds required for completion of each of the two parts of the test and truncated at 150 s for Part A and at 300 s for Part B. The FBI is a 24‐item caregiver‐based behavioral questionnaire. It was originally designed for the diagnosis and quantification of frontotemporal dementia symptoms, but is also helpful in the differential diagnosis of dementia [Kertesz,1997; Milan et al.,2007]. There were four possible response options, resulting in a total score ranging from 0 to 72: (0), mild and occasional (1), moderate (2), or serious and most of the time (3). Behavioral problems were further divided into two categories, each with a 12‐item Negative Behavioral Score (0–36) and Disinhibition Score (0–36).
MRI Acquisition
Images were acquired with a 1.5‐T GE Excite MRI system. We performed a routine whole brain MRI scan for every patient, including an axial T2‐weighted image (TR/TE, 5,600/90, slice thickness 5 mm), an axial FLAIR (TR/TI/TE 9,000/2250/85, slice thickness: 5 mm), and a coronal 3D T1WI using inversion prepared spoiled gradient echo (IR‐SPGR) sequence (TR/TE/flip angle 10.5/2.2/15°, slice thickness: 1.5 mm). The T2‐weighted and FLAIR images were used to identify lacunar infarct and diffusely confluent WMHI. We excluded those subjects with the image possibility of subcortical ischemic vascular dementia as WMHI of extending caps or irregular halos (>10 mm), diffusely confluent hyperintensities (>25 mm), and deep gray matter lacunes [Román et al.,2002].
A 3D IR‐SPGR Helped to Identify the Proper Position for Examining the ROI
For DTI acquisition, we used single shot spin echo EPI acquisition with TR/TE = 6000 ms/90 ms, 25 uniformly distributed gradient directions, a b‐value of 1,000 s/mm2, and a NEX of two. In total, 24 contiguous axial slices were acquired with 5‐mm slice thickness, a 128 × 128 matrix, and a 24 cm × 24 cm field of view; the slices were positioned to run parallel to the anterior commissural and posterior commissural plane. The total acquisition time was 5 min and 40 s.
DTI Measurement
Postprocessing of the DTI data was done on a GE workstation (FuncTool, Advantage Workstation 4.2, GE, Medical System, Milwaukee, WI). This system uses automatic correction of the EPI distortion by scaling, deskewing, and translating to align each image with the reference image (b = 0) and minimize the mismatch between diffusion and reference images. The MD and FA maps were computed and displayed along with a b = 0 reference map. We measured the MD and FA at different locations of white matter using ROI‐based analysis. The neuroradiologist (YFC) responsible for the placement of ROIs was blind to the clinical diagnosis of all the participants. We placed 12 ovoid ROIs in the white matter regions on two slices, which included the temporal subcortical white matter (SCWM) adjacent to the temporal horns, the genu and splenium of the corpus callosum, the anterior SCWM, the anterior periventricular white matter (PVWM), and the posterior SCWM and the posterior PVWM (see Fig. 1). The nonmidline regions were measured on both sides. The size of the ROIs was 8–12 voxels, measuring 1.878 mm × 1.875 mm × 5 mm each. For further details regarding ROI placement, see our previous work (Chen et al.,2008).
Figure 1.
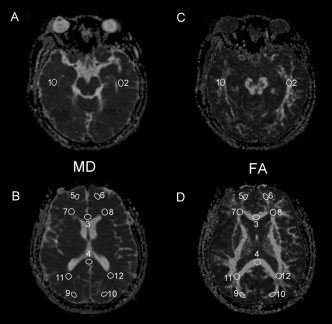
Selection of ROI in MD and FA maps. Twelve ROIs in MD (A and B) and FA (C and D) maps were selected. Brain regions are indicated as: 1 and 2 = Right and left temporal subcortical white matter (SCWM), 3 and 4 = genu and splenium of corpus callosum, 5 and 6 = right and left anterior medial SCWM, 9 and 10 = right and left posterior SCWM, 7 and 8 = right and left anterior periventricular white matter (PVWM), and 11 and 12 = right and left posterior PVWM.
Quantitative WMHI Measurement
The FLAIR images were used for automatic measurement of WMHI. We developed a user‐friendly MATLAB‐based program (ROITOOL) for the quantitative analysis of WMHI. The WMHI had higher signal intensity than the surrounding area, resulting in a signal intensity histogram with a peak of high intensities. The operator then set the intensity threshold to obtain the area of WMHI. The volume was calculated using outlined area multiplied by slice thickness. This analysis was performed separately in the frontal, temporal, and parieto‐occipital lobes of either hemisphere (Chen et al.,2006).
Statistics
Demographic and clinical data with nonparametric characteristics were compared using a Chi‐square test, and parametric data were examined using analysis of variance (ANOVA). Multivariate ANOVA (MANOVA) was used to compare between group DTI, WMHI measures, and cognitive function tests with pairwise post hoc. Pearson correlation was used to assess the association between executive function and the DTI measurements. To correlate executive function with multiple ROIs, a stepwise linear regression was performed to clarify the contribution of each related ROI. All statistical analyses were performed with SPSS version 11.0 (SPSS, Chicago, IL, 2003).
RESULTS
Demographic, Clinical Data, and Memory Functions
There was no significant difference between groups for gender (Pearson Chi = 1.665, P = 0.435), age (F = 2.622, P = 0.086), and years of education (F = 2.213, P = 0.124).
There was a significant group effect for full IQ (F = 8.321, P < 0.01) and PIQ (F = 11.394, P < 0.001). Post hoc analyses revealed that early AD subjects had lower full IQ (P < 0.01) and PIQ (P < 0.001) than the control subjects. Early AD subjects also had lower PIQ than the aMCI subjects (P < 0.05; Table I).
Table I.
Demographic profile and general intelligence
| Group | CS (n = 16) | MCI (n = 13) | AD (n = 11) |
|---|---|---|---|
| Gender (women/men) | 7/9 | 5/8 | 7/4 |
| Age (years) | 69.0 (8.4) | 73.2 (9.3) | 76.7 (8.5) |
| Education (years) | 10.5 (3.8) | 11.4 (4.3) | 7.8 (4.9) |
| Full IQ | 114.4 (11.8) | 101.3 (19.0) | 86.2 (20.7)* |
| PIQ | 112.9 (10.3) | 100.9 (17.8)** | 83.2 (17.7)* |
| VIQ | 114.1 (12.2) | 102.1 (18.4) | 96.8 (32.0) |
Mean (standard deviation); CS, control subject; MCI, mild cognitive impairment; AD, Alzheimer's disease; *P < 0.01, analysis of variance with post hoc between AD and CS; **P < 0.05 analysis of variance with post hoc between MCI and AD.
Both early AD and aMCI subjects had lower memory scores than the control subjects in immediate recall, delayed recall and recognition (all P < 0.01; Table II).
Table II.
Performance of cognitive function tests
| Tests | CS | MCI | AD |
|---|---|---|---|
| VF | 39.8 (6.8) | 22.8 (6.6)** | 19.8 (4.5)** |
| WCST‐c | 5.8 (1.1) | 2.7 (2.3)** | 1.3 (0.5)** |
| WCST‐p | 2.3 (1.6) | 20.2 (15.6)** | 19.2 (14.4)* |
| TMT‐A (s) | 49.3 (11.6) | 89.0 (43.1)* | 110.2 (36.7)** |
| TMT‐B (s) | 129.9 (46.0) | 250.7 (85.6)** | 278.5 (33.4)** |
| FBI‐n | 0.0 (0.0) | 5.2 (3.3)**† | 10.5 (5.1)** |
| FBI‐d | 0.0 (0.0) | 1.2 (1.2)†† | 7.2 (5.0)** |
| I‐recall | 57.8 (12.7) | 26.2 (15.8)** | 23.7 (12.6)** |
| D‐recall | 35.8 (8.5) | 11.5 (10.4)** | 4.2 (6.5)** |
| Recognition | 25.8 (2.9) | 17.2 (2.5)** | 11.2 (8.2)** |
Mean (standard deviation); VF, Verbal Fluency Test; WCST, Wisconsin Card Sorting Test; c, number of completed categories; p, number of perseverative errors; TMT, trail making, A, part A; B, part B; FBI, frontal behavioral inventory; n, negative behavioral score; d, disinhibition score; I‐recall, immediate recall of logical memory; D‐recall, delayed recall of logical memory of Wechsler Memory Scare 3rd Version; *P < 0.05; **P < 0.01 multiple variates analysis of variance with post hoc between AD and CS; †P < 0.05, ††P < 0.01 with post hoc between MCI and AD.
Executive Functions
The group effect was significant for all executive function tests, including total scores on the VF task (F = 26.28, P < 0.001), numbers of completed categories (F = 23.02, P < 0.001), perseverative errors on the WCST (F = 8.43, P < 0.01), TMT‐A (F = 9.92, P < 0.01), TMT‐B (F = 17.80, P < 0.001), negative behavioral scores (F = 26.12, P < 0.001), and disinhibition scores of FBI (F = 16.92, P < 0.001; Table II). Post hoc analyses showed that both aMCI and early AD subjects had significantly lower VF than the control subjects (both P < 0.001). Both aMCI and early AD subjects had significantly lower numbers of completed categories in the WCST than the control subjects (both P < 0.01); both aMCI (P < 0.01) and early AD subjects (P < 0.05) committed more perseverative errors than the control subjects. Similarly, both subjects with aMCI (P < 0.05 for TMT‐A, P < 0.01 for TMT‐B) and early AD (P < 0.01 for TMT‐A, P < 0.001 for TMT‐B) required significantly more time to complete the trail‐making tasks than the control subjects.
For frontal‐related behavioral symptoms, subjects with early AD exhibited significantly more negative behaviors and more disinhibition than the control subjects (both P < 0.001). The aMCI subjects, however, reported only more negative behaviors (P < 0.01), but not more disinhibition than the controls. FBI was the only frontal‐related function where there was a significant difference in the post hoc analysis between aMCI and early AD subjects for both negative behaviors (P < 0.05) and disinhibition (P < 0.01).
DTI Measurement (Tables III and IV)
Table III.
Mean diffusivity of region‐of‐interest in CS, MCI and AD
| MD (10−9 m2/s) | CS (n = 16) | MCI (n = 13) | AD (n = 11) | MCI/AD (n = 24) |
|---|---|---|---|---|
| PVWM | ||||
| Anterior right | 0.87 (0.09) | 0.92 (0.16) | 0.93 (0.14) | 0.93 (0.15) |
| Anterior left | 0.79 (0.09) | 0.90 (0.18)* | 0.90 (0.16)* | 0.90 (0.16)* |
| Posterior right | 0.87 (0.09) | 0.95 (0.17) | 0.92 (0.18) | 0.93 (0.17) |
| Posterior left | 0.89 (0.13) | 0.95 (0.22) | 0.92 (0.12) | 0.94 (0.18) |
| SCWM | ||||
| Anterior right | 0.76 (0.03) | 0.76 (0.06) | 0.80 (0.14) | 0.78 (0.10) |
| Anterior left | 0.69 (0.06) | 0.72 (0.07) | 0.72 (0.10) | 0.72 (0.08) |
| Posterior right | 0.81 (0.05) | 0.78 (0.08) | 0.78 (0.07) | 0.78 (0.05) |
| Posterior left | 0.81 (0.06) | 0.78 (0.08) | 0.80 (0.06) | 0.79 (0.07) |
| Temporal right | 0.85 (0.07) | 0.89 (0.08) | 0.87 (0.06) | 0.88 (0.07) |
| Temporal left | 0.85 (0.05) | 0.86 (0.63) | 0.84 (0.08) | 0.85 (0.07) |
| Corpus callosum | ||||
| Genu | 0.81 (0.34) | 1.00 (0.25)* | 1.01 (0.22)* | 1.01 (0.23)* |
| Splenium | 0.87 (0.06) | 0.91 (0.11) | 0.89 (0.09) | 0.91 (0.10) |
MD, mean diffusivity (standard deviation); PVWM, periventricular white matter; SCWM, subcortical white matter; CS, control subjects; MCI, mild cognitive impairment; AD, Alzheimer's disease; *P < 0.05 analysis of variance with post hoc between AD and/or MCI and CS.
Table IV.
Fraction anisotropy of region‐of‐interest in CS, MCI and AD
| FA | CS (n = 16) | MCI (n = 13) | AD (n = 11) | MCI/AD (n = 24) |
|---|---|---|---|---|
| PVWM | ||||
| Anterior right | 0.38 (0.08) | 0.34 (0.10) | 0.30 (0.05)* | 0.32 (0.08)* |
| Anterior left | 0.39 (0.05) | 0.34 (0.10) | 0.30 (0.05)** | 0.32 (0.09)** |
| Posterior right | 0.47 (0.08) | 0.45 (0.10) | 0.44 (0.09) | 0.45 (0.09) |
| Posterior left | 0.47 (0.09) | 0.44 (0.11) | 0.43 (0.08) | 0.44 (0.10) |
| SCWM | ||||
| Anterior right | 0.45 (0.05) | 0.44 (0.04) | 0.42 (0.06) | 0.43 (0.05) |
| Anterior left | 0.45 (0.05) | 0.47 (0.05) | 0.47 (0.06) | 0.47 (0.05) |
| Posterior right | 0.43 (0.06) | 0.44 (0.05) | 0.42 (0.06) | 0.43 (0.05) |
| Posterior left | 0.44 (0.04) | 0.46 (0.06) | 0.41 (0.05)† | 0.44 (0.06) |
| Temporal right | 0.46 (0.06) | 0.43 (0.07) | 0.44 (0.04) | 0.44 (0.06) |
| Temporal left | 0.49 (0.06) | 0.45 (0.07) | 0.48 (0.05) | 0.47 (0.06) |
| Corpus callosum | ||||
| Genu | 0.78 (0.07) | 0.74 (0.09) | 0.70 (0.09)* | 0.72 (0.09)* |
| Splenium | 0.75 (0.06) | 0.75 (0.05) | 0.75 (0.05) | 0.75 (0.05) |
FA, fraction anisotropy (standard deviation); PVWM, periventricular white matter; SCWM, subcortical white matter; CS, control subjects; MCI, mild cognitive impairment; AD, Alzheimer's disease; *P < 0.05; **P < 0.01, analysis of variance with post hoc between AD and/or MCI and CS; †P < 0.05, analysis of variance with post hoc between AD and MCI.
The DTI measurements showed a significant group effect with increased MD in the genu of the corpus callosum (F = 3.35, P < 0.05) and decreased FA in the genu of the corpus callosum and in the left anterior PVWM (F = 4.96, P < 0.05). Post hoc analyses revealed that subjects with both aMCI and early AD had increased MD in the genu of the corpus callosum and in the left anterior PVWM (both P < 0.05). Only early AD subjects, however, had decreased FA in the genu of the corpus callosum (P < 0.05) and in bilateral anterior PVWM (right P < 0.05 and left P < 0.01). The left posterior SCWM was the only area that differentiated aMCI from early AD (P < 0.05).
Quantitative WMHI Measurements
Total WMHI measurements of the three groups were highest in the subjects with early AD (5.83 ± 7.34 cm3), followed by the subjects with aMCI (4.54 ± 5.72 cm3) and the controls (1.51 ± 1.71 cm3). However, there was no significant group effect in total WMHI, right temporal, left temporal, right frontal, right parietal, and left temporal regions (all P > 0.05). Group effect existed only in the left frontal WMHI; post hoc analysis revealed that the difference was between the controls (0.44 ± 0.59 cm3) and the early AD subjects (1.92 ± 2.41 cm3; P < 0.05) in agreement with our DTI finding.
Correlation Analyses
To decrease type I error from multiple correlations, we used P < 0.01 for the level of significance in the correlation analyses of DTI, WMHI measurements, and cognitive performance.
In subjects with aMCI, both MD of the anterior PVWM (r = 0.750 for the right and r = 0.800 for the left, both P < 0.01) and MD of the posterior PVWM (r = 0.852 for the right and r = 0.899 for the left, both P < 0.001) were positively correlated with perseverative errors on the WCST. FA of the left anterior PVWM was positively associated with completed categories on the WCST (r = 0.759, P < 0.01) and FA of the left posterior PVWM was negatively associated with perseverative errors on the WCST (r = −0.753, P < 0.01). In subjects with early AD, MD of the left temporal SCWM was negatively associated with the total scores of VF (r = −0.840, P < 0.01).
In this study, we investigated both subjects with aMCI and mild AD. Although the performance of cognitive tasks was worse in AD subjects than in aMCI subjects in all domains, the only significant difference between the two groups was in FBI scores representing frontal‐related behavioral problems. The only significant difference in DTI measurements between the groups was in FA of the left posterior SCWM. Therefore, it is reasonable to regard the transition from aMCI to early AD as a continuum, at least for subjects in this study. In light of this assessment, we combined results from aMCI and early AD in the correlation analysis to increase the statistical power from a greater number of subjects.
In this combined population, MD of the right anterior PVWM (r = −0.533), FA of genu of the corpus callosum (r = 0.483), and FA of the posterior PVWM (r = 0.457 for the right and r = 0.555 for the left) were significantly associated with VF (all P < 0.01). Both MD (r = −0.488 for the right and r = −0.492 for the left, both P < 0.01) and FA (r = 0.568 for the right and r = 0.591 for the left, both P < 0.001) of the anterior PVWM, and right anterior SCWM (r = 0.447, P < 0.01) were significantly associated categories that were completed on the WCST. In the same vein, MD of both anterior (r = 0.568 for the right and r = 0.622 for the left, both P < 0.001) and posterior PVWM (r = 0.545 for the right, P < 0.01 and r = 0.580 for the left, both P < 0.001); and FA of both anterior (r = −0.455 for the right and r = −0.467 for the left, both P < 0.01) and posterior PVWM (r = −0.520 for the left, P < 0.001) were significantly associated with perseverative errors on the WCST. MD of the left anterior PVWM was positively associated with both the time needed to complete TMT‐A (r = 0.536, P < 0.01) and TMT‐B (r = 0.438, P < 0.01). In addition, MD of the right posterior PVWM (r = 0.436, P < 0.01) and FA of the left posterior PVWM (r = −0.518, P < 0.01) were significantly associated with TMT‐A; FA of the anterior PVWM were negatively associated with TMT‐B (r = −0.423, P < 0.01 for the right and r = −0.611, P < 0.001 for the left). FA of the left anterior PVWM was negatively associated with negative behavioral scores of the FBI (r = −0.446, P < 0.01).
Total WMHI or WMHI measured in all areas were not significantly associated with cognitive performances (all P > 0.01). The results of correlation analyses were summarized in Table V with symbols.
Table V.
Areas of significant association between diffusion tensor, white matter hyper‐intensities measurements and executive dysfunctions
| VF | WCST‐c | WCST‐p | TMT‐A | TMT‐B | FBI‐n | FBI‐d | |
|---|---|---|---|---|---|---|---|
| Diffusion tensor measurements in periventricular white matter | |||||||
| Frontal R/L | */− | *†/* | *†/*† | −/* | */* | −/* | −/− |
| Parietal R/L | */* | −/− | *†/*† | */* | −/− | −/− | −/− |
| Diffusion tensor measurements in subcortical white matter | |||||||
| Frontal R/L | −/− | */− | −/− | −/− | −/− | −/− | −/− |
| Parietal R/L | −/− | −/− | −/− | −/− | −/− | −/− | −/− |
| Temporal R/L | −/‡ | −/− | −/− | −/− | −/− | −/− | −/− |
| Diffusion tensor measurements in corpus callosum | |||||||
| Genu | −/* | −/− | −/− | −/− | −/− | −/− | −/− |
| Splenium | −/− | −/− | −/− | −/− | −/− | −/− | −/− |
| White matter hyper‐intensities | |||||||
| Frontal R/L | −/− | −/− | −/− | −/− | −/− | −/− | −/− |
| Parietal R/L | −/− | −/− | −/− | −/− | −/− | −/− | −/− |
| Temporal R/L | −/− | −/− | −/− | −/− | −/− | −/− | −/− |
For all subjects; †For MCI; ‡For early AD, P < 0.01, Pearson correlation analysis; VF, verbal fluency test; WCST, Wisconsin card sorting test; c, number of completed categories; p, number of perseverative errors; TMT, trail making; A, part A; B, part B; FBI, frontal behavioral inventory; n, negative behavioral score; d, disinhibition score.
To clarify the contributions of different ROIs, we performed stepwise linear regression of the seven ROIs (merging measurements from both hemispheres, independent variables) on the executive function (dependent variables) that were correlated to more than one ROI of the DTI measure. Perseverative errors on the WCST were positively associated with MD in both anterior and posterior PVWM and were negatively associated with FA of the posterior PVWM and posterior SCWM. Completed categories on the WCST were positively associated with FA in the anterior PVWM and anterior SCWM.
Regression analyses showed that the MD of the posterior PVWM predicted 45.8% (F = 14.38, P < 0.01) of the perseverative errors on the WCST and that the MD of the anterior SCWM predicted an additional 17.8% (F = 7.82, P < 0.05) of these errors (Fig. 2A–C). Similarly, FA of the posterior PVWM predicted 42.9% (F = 12.8, P < 0.01) of the perseverative errors on the WCST (Fig. 2D). FA of the anterior PVWM predicted 32.9% (F = 8.33, P < 0.05) of the completed categories on the WCST (see Fig. 3).
Figure 2.
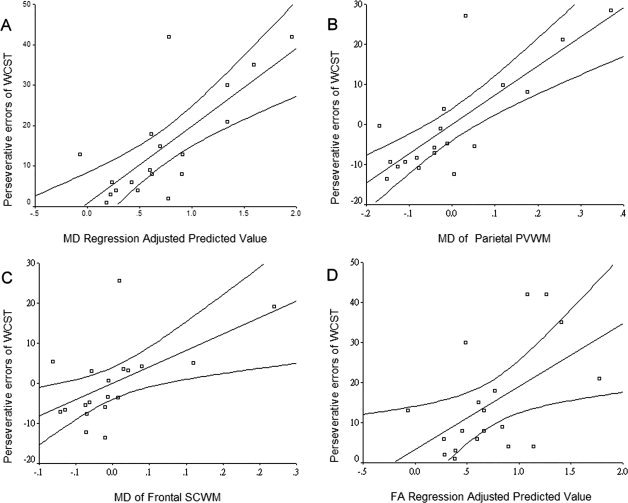
A: Perseverative errors on Wisconsin Card Sorting Test (WCST) regression‐adjusted predicted values of mean diffusivity (MD, R square = 0.5645); (B) partial regression plot of MD at parietal periventricular white matter (PVWM); (C) partial regression plot of MD at frontal subcortical white matter (SCWM); (D) regression‐adjusted predicted values of fraction anisotropy (FA, R square = 0.2839) of the parietal PVWM; the plots were obtained from both subjects with amnesic mild cognitive impairment and early Alzheimer's disease.
Figure 3.
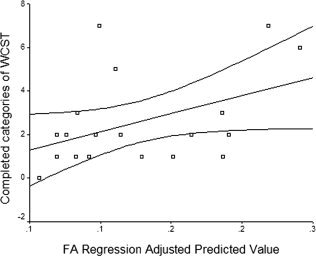
Completed categories on Wisconsin Card Sorting Test (WCST) regression‐adjusted predicted values of fractional anisotropy (FA, R square = 0.1922) for frontal periventricular white matter. The plot was obtained from both subjects with amnesic mild cognitive impairment and early Alzheimer's disease.
DISCUSSION
AD subjects may exhibit changes in DTI measurements in both the anterior and posterior cerebral white matter. This finding was discovered in our previous study on AD subjects with different severity [Chen et al.,2008] and is compatible with other reports [Chua et al.,2008; Medina et al.,2006; Naggara et al.,2006]. In this study, aMCI and early AD could be differentiated by FA at the left posterior (posterior parietal) SCWM, which may imply a consequence of a parietal associate cortex lesion, possibly through Wallerian degeneration.
We found executive dysfunctions in aMCI and early AD compatible with previous reports [Baudic et al.,2006; Twamley et al.,2006]. The semantic fluency task is useful in the detection of preclinical and early AD [Auriacombe et al.,2006]. Subjects with both aMCI and early AD displayed significantly impaired performances of the VF task. VF performance was associated with white matter changes in the frontal and parietal PVWM as well as the corpus callosum. The association of left frontal changes with semantic fluency deficits has been confirmed by a previous functional MRI study [Gillard et al.,2003].
Performance on the WCST represents flexibility in shifting mental sets [Miayake et al.,2000]. Subjects with both aMCI and early AD showed significantly impaired performance on the WCST. The WCST appears sensitive to frontal lobe damage, but cannot be used alone to predict a focal frontal lesion [Demakis,2003]. Rather, it requires a distributed neural network [Stuss et al.,2000]. Both subjects with aMCI and early AD had significantly lower numbers of completed categories and more perseverative errors on the WCST than the control subjects. Category completion is related to conceptual processing and formation of the stimulus attributes, that is, color, form, or number. It depends heavily on dorsolateral prefrontal regions [MacPherson et al.,2002]. In this study, we found that the number of completed categories on the WCST was related to frontal PVWM and anterior (frontal) SCWM. Perseverative errors were related to frontal and posterior (parietal) PVWM. Previous neuroimaging studies have revealed that shifting‐related tasks were completed through cooperation between the dorsolateral prefrontal cortex and the posterior parietal cortex [Buchsbaum et al.,2005; Smith et al.,2004]. Regression analyses in this study further supported the importance of this cooperation, in that both FA and MD of the parietal PVWM predicted more than 40% of the perseverative errors of WCST while the MD of frontal SCWM predicted an additional 17.8% (Fig. 2A–D). Another study differentiated that “stuck‐in‐set” perseverative errors were related to frontal dysfunction while “recurrent” perseverative errors were associated with parietal lesions [Nagahama et al.,2005]. In short, we found that frontal and parietal periventricular DTI measurements were associated with executive dysfunctions.
The TMT is a measure of attention, speed, and mental flexibility. TMT‐B, which is sensitive to cognitive flexibility, was also impaired in our subjects with aMCI and early AD [Kortte et al.,2002; Collette et al.,2005]. The performance of TMT‐B was related to frontal PVWM, which is critical for mental set shifting. On the other hand, TMT‐A is heavily dependent on attention, which has been considered to have an important role in the posterior parietal cortex [Nachev and Husain,2006]. We found that TMT‐A was related to the parietal PVWM, in addition to its frontal involvement.
In this study, frontal‐related behaviors differentiated subjects with aMCI from early AD, which is compatible with and comparable to previous studies [Milan et al.,2007]. We found that negative behaviors were significantly associated with DTI measurement of the frontal PVWM.
We noticed that significant correlations within an individual group occurred mainly in the group of aMCI. This is probably from a “floor” effect of AD patients on some executive tasks. For example, we truncated the time needed to complete trail‐making A and B at 150 and 300 s. Low‐education AD subjects might fail the WCST and perform poorly in verbal fluency.
Questions remain, however, about why the PVWM plays such an important role in executive function. The cholinergic neurons in the basal forebrain have widespread projections to the cortex, and the degeneration of basal forebrain cholinergic neurons is an early AD process. White matter of the frontal and parietal PVWM may impact executive dysfunction by affecting the critical lateral cholinergic fibers that pass through [Behl et al.,2007]. Furthermore, early pathological change of the periventricular part of the superior longitudinal fasciculus, which joins the prefrontal and parietal region, is another possible account for executive dysfunction. Superior longitudinal fasciculus may be compromised in different clinical conditions such as MCI and AD [Damoiseaux et al.,2009], traumatic brain injury [Kraus et al.,2007], and schizophrenia [Karigodt et al.,2008].
One limitation of this study is rooted in the DTI measurement itself. Artifacts in ROI‐based analyses are related to the shape and size of the ROIs used. In this study, we used an experienced rater to place the ROIs (YFC), and the criteria for selecting ROIs sustained a good reliability. The intraobserver and interobserver reliability were computed as coefficients of variation (CV) centered to mean for precision of measurement and intraclass correlation (ICC) for degree of agreement in every ROI. Results showed a high precision of intraobserver (CV, FA: 4.3% ± 1.6% and MD: 4.0% ± 2.3%) and interobserver measurement (CV, MD: 3.8% ± 1.4% and FA: 8.0% ± 3.4%), and high intraobserver (ICC, FA: 0.98 and MD 0.97) and interobserver agreement (ICC, MD: 0.97 and FA: 0.92; Chen et al.,2008).
There is a second limitation of this study that may constrain the generalization of our interpretations. The majority of the ROIs in this study were placed on NAWM. In cases with minor punctuate WMHI, often of nonischemic origin, an ROI was situated on NAWM in the proximity of the WMHI. Although in cases with WMHI around the anterior and posterior horns of lateral ventricles, an ROI was either entirely within the abnormal appearing white matter or across the NAWM and WMHI. Axonal membrane and myelin were the major barriers to water diffusivity. In the WMHI or leukoaraiosis where axonal damage and myelin‐breakdown occurred, the MD increased and FA decreased. If an ROI was situated entirely within a WMHI lesion or across areas of NAWM and WMHI, the variations of DTI measurements would be increased. We excluded those subjects with lacunar infracts or subjects with diffusely confluent WMHI which were most often of ischemic origin.
In this study, we obtained significant correlation between executive dysfunction and brain white matter change through DTI but not through WMHI measurement. Although we investigated subjects with very limited WMHI, our study demonstrated that DTI outrivaled WMHI as an indicator of cognitive dysfunction. T2‐weighted images are sensitive in the detection of subcortical lesions but provide little information about the severity of the pathological changes [Awad et al.,1986].
CONCLUSION
In this study, we demonstrated executive dysfunction in subjects with aMCI and early AD. Differential performance of executive tasks was also observed in our participants. The most important finding was that white matter change in frontal and nonfrontal (parietal) PVWM accounted for the observed executive dysfunction. This may be due to the early AD degenerative process on the lateral cholinergic bundle or superior longitudinal fasciculus involvement.
REFERENCES
- Ahola K, Vilkki J, Servo A ( 1996): Frontal tests do not detect frontal infarctions after ruptured intracranial aneurysm. Brain Cogn 31: 1– 16. [DOI] [PubMed] [Google Scholar]
- Alvarez JA, Emory E ( 2006): Executive function and the frontal lobes: A meta‐analytic review. Neuropsychol Rev 16: 17– 42. [DOI] [PubMed] [Google Scholar]
- Anderson SW, Damasio H, Jones RD, Tranel D ( 1991): Wisconsin Card Sorting Test performance as a measure of frontal lobe damage. J Clin Exp Neuropsychol 13: 909– 922. [DOI] [PubMed] [Google Scholar]
- Auriacombe S, Lechevallier N, Amieva H, Harston S, Raoux N, Dartigues JF ( 2006): A longitudinal study of quantitative and qualitative features of category verbal fluency in incident Alzheimer's disease subjects: Results from the PAQUID study. Dement Geriatr Cogn Disord 21: 260– 266. [DOI] [PubMed] [Google Scholar]
- Awad IA, Johnson PC, Spetzler RF, Hodak JA ( 1986): Incidental subcortical lesions identified on magnetic resonance imaging in the elderly. II. Post‐mortem pathological correlations Stroke 17: 1090– 1097. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Cummings JL, Sultzer D, Henderson VW, Nuechterlein KH, Mintz J ( 2003): White matter structure integrity in healthy aging adults and subjects with Alzheimer's disease. Arch Neurol 60: 393– 398. [DOI] [PubMed] [Google Scholar]
- Baudic S, Barba GD, Thibaudet MC, Smagghe A, Remy P, Traykov L ( 2006): Executive function deficits in early Alzheimer's disease and their relations with episodic memory. Arch Clin Neuropsychol 21: 15– 21. [DOI] [PubMed] [Google Scholar]
- Behl P, Bocti C, Swartz RH, Gao FQ, Sahlas DJ, Lanctot KL, Streiner DL, Black SE ( 2007): Strategic subcortical hyperintensities in cholinergic pathways and executive function decline in treated Alzheimer patients. Arch Neurol 64: 266– 272. [DOI] [PubMed] [Google Scholar]
- Brun A, Englund E ( 1986): A white matter disorder in dementia of the Alzheimer type: A pathoanatomical study. Ann Neurol 19: 253– 262. [DOI] [PubMed] [Google Scholar]
- Buchsbaum BR, Greer S, Chang WL, Berman KF ( 2005): Meta‐analysis of neuroimaging studies of the Wisconsin Card‐Sorting Task and component processes. Hum Brain Mapp 25: 35– 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse A, Hensel A, Gühne U, Angermeyer MC, Riedel‐Heller S ( 2006): Mild cognitive impairment—Long‐term course of four clinical subtypes. Neurology 67: 2176– 2185. [DOI] [PubMed] [Google Scholar]
- Chen YF, Wang H, Chu Y, Huang YC, Su MY ( 2006): Regional quantification of white matter hyperintensity in normal aging, mild cognitive impairment, and Alzheimer's disease. Dementia Geriatr Cogn Disord 22: 177– 184. [DOI] [PubMed] [Google Scholar]
- Chen TF, Lin CC, Chen YF, Liu HM, Hua MS, Huang YC, Chiu MJ ( 2008): Diffusion tensor changes in subjects with amnesic mild cognitive impairment and various dementias. Psychiatry Res Neuroimaging; DOI: 10.1016/j.psychresns. 2008.09.002. [DOI] [PubMed] [Google Scholar]
- Chua TC, Wen W, Slavin MJ, Sachdev PS ( 2008): Diffusion tensor imaging in mild cognitive impairment and Alzheimer's disease: A review. Curr Opin Neurol 21: l83– 192. [DOI] [PubMed] [Google Scholar]
- Collette F, Van der Linden M, Laureys S, Delfiore G, Degueldre C, Luxen A, Salmon E ( 2005): Exploring the unity and diversity of the neural substrate of executive functioning. Human Brain Mapping 25: 409– 423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Smith SM, Witter MP, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, Zarei M, Rombouts SA ( 2009): White matter tract integrity in aging and Alzheimer's disease. Hum Brain Mapp 30: 1051– 1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot JC, de Leeuw FE, Oudkerk M, van Gijn J, Hofman A, Jolles J, Breteler MM ( 2000): Cerebral white matter lesions and cognitive function: The Rotterdam scan study. Ann Neurol 47: 145– 151. [DOI] [PubMed] [Google Scholar]
- Demakis GJ ( 2003): A meta‐analysis review of the sensitivity of the Wisconsin Card Sorting Test to frontal and lateralized frontal brain damage. Neuropsychology 17: 255– 264. [DOI] [PubMed] [Google Scholar]
- Englund E, Sjöbeck M, Brockstedt S, Lätt J, Larsson EM ( 2004): Diffusion tensor MRI post mortem demonstrated cerebral white matter pathology. J Neurol 251: 350– 352. [DOI] [PubMed] [Google Scholar]
- Frazekas F, Kleinert R, Offenbacher H, Schmidt R, Kleinert G, Payer F, Radner H, Lechner H ( 1993): Pathological correlates of incidental MRI white matter signal hyperintensities. Neurology 43: 1683– 1689. [DOI] [PubMed] [Google Scholar]
- Gillard MD, Sachs BC, Whitnah JR, Ahmad Z, Balsamo LM, Petrella JR, Braniecki SH, McKinney CM, Hunter K, Xu B, Grandin CB ( 2003): Development aspects of language processing: fMRI of verbal fluency in children and adults. Hum Brain Mapp 18: 176– 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirono N, Kitagaki H, Kazui H, Hashimoto M, Mori E ( 2000): Impact of white matter changes on clinical manifestation of Alzheimer's disease: A quantitative study. Stroke 31: 2182– 2188. [DOI] [PubMed] [Google Scholar]
- Hua MS, Chang SH, Chen ST ( 1997): Factor structure and age effects with an aphasia test battery in normal Taiwanese adults. Neuropsychology 11: 156– 162. [DOI] [PubMed] [Google Scholar]
- Hua MS, Chang BS, Lin KN, Yang JM, Lu SR, Chen SY ( 2005): Wechsler Memory Scale, 3rd ed (Chinese) [Manual]. Taipei: Chinese Behavioral Science Corp. [Google Scholar]
- Karigodt KH, van Erp TG, Poldrack RA, Bearden CE, Nuechterlein KH, Cannon TD ( 2008): Diffusion tensor imaging of the superior longitudinal fasciculus and working memory in recent‐onset schizophrenia. Biol Psychiatry 63: 512– 518. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Davidson W, Fox H ( 1997): Frontal behavioral inventory: Diagnostic criteria for frontal lobe dementia. Can J Neurol Sci 24: 29– 36. [DOI] [PubMed] [Google Scholar]
- Kortte KB, Horner MD, Windham WK ( 2002): The trail making test, Part 2: Cognitive flexibility or ability to maintain set? Appl Neuropsychol 9: 106– 109. [DOI] [PubMed] [Google Scholar]
- Kraus MF, Susmaras T, Caughlin BP, Walker C, Sweeney JA, Little DM ( 2007): White matter integrity and cognition in chronic traumatic brain injury: A diffusion tensor imaging study. Brain 130: 2508– 2519. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Kuller LH, Becker JT, Dulber C, Sweet RA, Gach HM, DeKosky ST ( 2007): Incidence of dementia in mild cognitive impairment in the cardiovascular health study cognition study. Arch Neurol 64: 416– 420. [DOI] [PubMed] [Google Scholar]
- MacPherson SE, Philips LH, Della Salla S ( 2002): Age, executive function, and social decision making: A dorsolateral prefrontal theory of cognitive aging. Psychol Aging 17: 598– 609. [PubMed] [Google Scholar]
- Marshall GA, Hendrickson R, Kaufer DI, Ivanco LS, Bohnnen NI ( 2006): Cognitive correlates of brain MRI subcortical signal hyperintensities in non‐demented elderly. Int J Geriatr Psychiatry 21: 32– 35. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman D, Price D, Stadlan EM ( 1984): Clinical diagnosis of Alzheimer's type: Report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology 34: 939– 944. [DOI] [PubMed] [Google Scholar]
- Medina D, DeToledo‐Morrell L, Urresta F, Gabrieli JD, Moseley M, Fleischman D, Bennett DA, Leurgans S, Turner DA, Stebbins GT ( 2006): White matter changes in mild cognitive impairment and AD: A diffusion tensor imaging study. Neurobiol Aging 27: 663– 672. [DOI] [PubMed] [Google Scholar]
- Miayake A, Friedman NP, Emerson M, Witzki AH, Howerter A ( 2000): The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognit Psychol 41: 49– 100. [DOI] [PubMed] [Google Scholar]
- Milan G, Lamenza MG, Iavarone A, Galeone F, Lorè E, de Falco C, Sorrentino P, Postiglione A ( 2007): Frontal behavioral inventory in the differential diagnosis of dementia. Acta Neurol Scand 117: 260– 265. [DOI] [PubMed] [Google Scholar]
- Moroney J, Bagiella E, Desmond D, Hachinski V, Molsa P, Gustafson L, Brun A, Fischer P, Erkinjuntti T, Rosen W, Paik M, Tatemichi T ( 1997): Meta‐analysis of the Hachinski Ischemic Score in pathologically verified dementias. Neurology 49: 1096– 1105. [DOI] [PubMed] [Google Scholar]
- Nachev P, Husain M ( 2006): Disorders of visual attention and the posterior parietal cortex. Cortex 42: 766– 773. [DOI] [PubMed] [Google Scholar]
- Nagahama Y, Okina T, Suzuki N, Nabatame H, Matsuda M ( 2005): The cerebral correlates of different types of perseveration in the Wiscon Card Sorting Test. J Neurol Neurosur Psychiatry 76: 169– 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naggara O, Oppenheim C, Rieu D, Raoux N, Rodrigo S, Dalla Barba G, Meder JF ( 2006): Diffusion tensor imaging in early Alzheimer's disease. Psychiatry Res 146: 243– 249. [DOI] [PubMed] [Google Scholar]
- Nelson HE ( 1976): A modified card sorting test sensitive to frontal lobe defects. Cortex 12: 313– 324. [DOI] [PubMed] [Google Scholar]
- O'sullivan M, Morris RG, Huckstep B, Jones DK, Williams SC, Markus HS ( 2004): Diffusion tensor MRI correlates with executive dysfunction in subjects with ischaemic leukoaraiosis. J Neurol Neurosurg Psychiatry 75: 441– 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson RC, Esiri MM, Hiorns RW, Wilcock GK, Powell TP ( 1985): Anatomical correlates of the distribution of the pathological changes in the neocortex in Alzheimer disease. Proc Natl Acad Sci USA 82: 4531– 4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B ( 2001): Current concepts in mild cognitive impairment. Arch Neurol 58: 1985– 1992. [DOI] [PubMed] [Google Scholar]
- Reitan RM ( 1958): Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills 8: 271– 276. [Google Scholar]
- Román GC, Erkinjuntti T, Wallin A, Pantoni L, Chui HC ( 2002): Subcortical ischaemic vascular dementia. Lancet Neurol 1: 426– 436. [DOI] [PubMed] [Google Scholar]
- Rose SE, McMahon KL, Janke AL, O'Dowd B, de Zubicaray G, Strudwick MW, Chalk JB ( 2006): Diffusion indices on magnetic resonance imaging and neuropsychological performance in amnesic mild cognitive impairment. J Neurol Neurosurg Psychiatry 77: 1122– 1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabri O, Ringelstein EB, Hellwig D, Schneider R, Schreckenberger M, Kaiser HJ, Mull M, Buell U ( 1999): Neuropsychological impairment correlates with hypoperfusion and hypometabolism but not with severity of white matter lesions on MRI in patients with cerebral microangiopathy. Stroke 30: 556– 566. [DOI] [PubMed] [Google Scholar]
- Sjöbeck M, Englund E ( 2003): Glial levels determine severity of white matter disease in Alzheimer's disease: A neuropathological study of glial changes. Neuropathol Appl Neurobiol 29: 159– 169. [DOI] [PubMed] [Google Scholar]
- Smith AB, Taylor E, Brammer M, Rubia K ( 2004): Neural correlates of switching set as measured in fast, event‐related functional magnetic resonance imaging. Hum Brain Mapp 21: 247– 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT, Levine B, Alexander MP, Hong J, Palumbo C, Hamer L, Murphy KJ, Izukawa D ( 2000): Wisconsin Card Sorting Test performance in subjects with focal frontal and posterior brain damage: Effects of lesion location and test structure on separable cognitive processes. Neuropsychologia 38: 388– 402. [DOI] [PubMed] [Google Scholar]
- Twamley EW, Ropacki SA, Bondi MW ( 2006): Neuropsychological and neuroimaging changes in preclinical Alzheimer's disease. J Int Neuropsychol Soc 12: 707– 735. [DOI] [PMC free article] [PubMed] [Google Scholar]


