Abstract
Because humans seem to lack neuronal elements in the vomeronasal organ (VNO), many scientists believe that humans are unable to detect pheromones. This view is challenged by the observations that pheromone‐like compounds, 4,16‐androstadien‐3‐one (AND) and oestra‐1,3,5(10),16‐tetraen‐3‐ol (EST), activate the human hypothalamus. Whether these activations are mediated via VNO, venous blood or olfactory mucosa is presently unknown. To disentangle between the three alternatives, we conducted activation studies in 12 heterosexual males with chronic anosmia because of nasal polyps. Polyposis hampers signal transduction via the olfactory mucosa without interfering with the VNO or the pheromone transport via venous blood. Twelve healthy men served as controls. Subjects were investigated with 15O—H2O PET during smelling of odorless air (base line), AND, EST, vanillin, and acetone. Smelling of EST activated the anterior hypothalamus in controls, but not anosmics. Neither did the anosmics display cerebral activations with AND or vanillin. Clusters were detected only with the trigeminal odorant acetone, and only in the thalamus, brainstem, the anterior cingulate, and parts of the sensorimotor cortex. Direct comparisons with controls (controls–anosmics) showed clusters in the olfactory cortex (amygdala and piriform cortex) with AND, vanillin, and acetone, and in the anterior hypothalamus with EST. The observed absence of olfactory and presence of trigeminal activations in anosmics indicates that polyposis primarily affected signal processing via the olfactory mucosa. The anosmics inability to activate the hypothalamus with EST, therefore, suggests that in healthy men EST signals were primarily transmitted via the olfactory system. Hum Brain Mapp, 2009. © 2009 Wiley‐Liss, Inc.
Keywords: pheromones, olfaction, vomeronasal organ, anosmia, brain
INTRODUCTION
According to the original definition by Karlson and Luscher [ 1959], pheromones are “airborne chemical signals released by an individual into the environment and affecting the physiology and behavior of other members of the same species.” Both volatile and nonvolatile compounds can act as pheromones. These chemical signals provide information about gender and reproductive status, and mediate social and sexual behaviors, as well as neuroendocrine changes. The major sensory organ for the detection of pheromones in mammals is the vomeronasal organ (VNO). Because this organ is not functional in adult humans, the effect of pheromones on our behavior is highly questioned.
The VNO is located in a blind‐ended pouch within the septum of the nose, and is enclosed in a cartilaginous capsule that opens through a duct into the base of the nasal cavity [Meredith, 1991; Monti‐Bloch et al., 1998]. VNO is clearly distanced from the olfactory mucosa, which is located in the roof of the nasal cavity. Animal data show that axons from olfactory sensory neurons of the main olfactory epithelium in the nasal cavity project to the main olfactory bulb (MOB), whereas axons from neurons in the VNO project to the accessory olfactory bulb (AOB). The current view is that volatile odorants are detected by the olfactory epithelium, whereas pheromones are detected through the VNO [Keverne, 2007]. Behaviors affected by pheromone signaling such as sexual behavior, aggression, and pregnancy block in animals are typically attributed to VNO‐mediated processes [Keverne, 2007].
Although humans do have a VNO, there are several indications that it is not functional after birth [Trotier et al., 2000]. First, the human VNO epithelium resembles more strongly like the respiratory epithelium than the VNO neuroepithelium found in species with functional VNOs [Witt et al., 2002]. Second, the olfactory marker protein (OMP), which is a reliable marker for mature VNO neurons, is not expressed in human VNO [Dennis et al., 2004]. Third, genes coding for the TRCP2 ion channels necessary for pheromone signal transduction are pseudogenes in humans, as are most of the genes identified to code for receptor proteins in the mouse VNO, [Zufall et al., 2002]. Furthermore, the AOB is involved in humans, whereas in all other species with functional VNO, it is a relay structure for the processing of pheromone signals [Dennis et al., 2004]. As a consequence, pheromone signaling has long been questioned in humans. This view is, however, contradicted by growing arguments for an influence by pheromones on human physiology and behavior. One argument is that the well‐known synchronization of menstrual cycles among female roommates seems to be relayed by sweat, which contains pheromone‐like compounds [Stern and McClintock, 1998]. An other is that women smelling male sweat shift their luteinic hormone pulsatility to promote ovulation [Preti et al., 2003]. Furthermore, pheromone‐like compounds, 4,16‐androstadien‐3‐one (AND) oestra‐1,3,5(10),16‐tetraen‐3‐ol (EST), have several consecutive experiments shown to affect mood and arousal [Bensafi et al., 2004; Jacob et al., 2001; Jacob and McClintock, 2000; Lundstrom and Olsson, 2005]. Finally, PET and fMRI show that smelling of these compounds activates the specific reproduction‐related neuronal circuits of the human brain, in a sex and sexual orientation differentiated manner [Berglund et al., 2006; Savic, 2002; Savic et al., 2001, 2005; Sobel et al., 1999].
If humans do not posses a functional VNO, how is it possible that they can detect pheromone signals? As discussed in our previous studies, the observed cerebral activations could be mediated via VNO, the olfactory mucosa, or blood [Savic et al., 2001]. To disentangle these alternatives and investigate the impact of the olfactory mucosa, we carried out PET activation studies during smelling of EST and AND in 12 heterosexual males with chronic anosmia. Anosmia was based on nasal polyposis, which hampers signal transduction via the olfactory mucosa and should not affect the VNO. We postulated that if a VNO‐relayed pathway was functional, smelling of EST would yield a hypothalamic activation in anosmic men, just as it did in healthy heterosexual males [Berglund et al., 2006]. Absence of cerebral activation would, on the other hand, favor signal transduction via the olfactory mucosa.
To ensure that functional impairment was confined to the olfactory mucosa, activation experiments also included smelling of the pure olfactory odorant vanillin (VAN), and the trigeminal odorant acetone (ACE). Signals from VAN are mediated by the olfactory mucosa only, whereas those from ACE are transduced also by the respiratory epithelium [Cain and Murphy, 1980; Hummel and Welge‐Luessen, 2006; Savic et al., 2002].
METHODS
Subjects
Twelve right‐handed, nonsmoking, heterosexual men with chronic anosmia due to nasal polyps (age 21–42 years) were recruited for the study. Polyposis was diagnosed by a specialist in otorhinolaryngology and visualized with endoscopy, showing apical obstruction of the olfactory epithelium, but leaving the caudally located vomeronasal pits intact. Other than suffering from the polyposis subjects were in a good health, and reported no heredity for neuropsychiatric disorders or anosmia, as revealed by the detailed medical history.
The mean duration of anosmia was 4.7 years (range 1.5–10 years). The olfactory dysfunction was due to polyposis, and there was no history of hypo or anosmia secondary to trauma or upper respiratory infection, or heredity. Neither did any of the participants have congenital anosmia, nor a history of anosmia for specific odors. Olfactory thresholds were measured using n‐butyl alcohol test as described previously [Savic et al., 2000], and verified with phenyl ethyl alcohol test [Bensafi et al., 2007]. Only patients who did not detect any of the two odors even without any dilution were included in the study.
We also assessed trigeminal sensitivity based on the subject's ability to lateralize stimuli presented to either the left or the right nostril. For this purpose 99% eucalyptol was presented to either one nostril in a glass jar filled with 10ml with its spout placed in one nostril (Sigma Chemical, Deishofen, Germany), [Hummel et al., 2003]. Odorless air was presented in parallel, and via a separate jar with the spout to the other nostril. The instruction was to indicate the side of perceived irritation, odor, or burning/itching sensation, which was related to the side of eucalyptol presentation. A total of 10 stimuli were applied to blind‐folded subjects at an interstimulus interval of about 60 s. Stimulation to right and left nostril was counterbalanced and randomized. After each stimulus the subject was asked to identify the nostril where the odorant has been presented. The sum of correct identifications was used in the analysis.
The controls consisted of 12 right‐handed, nonsmoking, and heterosexual age and education matching men (age 21–36 years) without any upper respiratory problems of neuropsychiatric disorders or heredity. Data from 11 of these subjects were presented in one of our previous studies [Berglund et al., 2008]. The study was approved by the local ethics and safety radiation committees.
Odors
Pure (99%) vanillin (4‐hydroxy‐3‐methoxy‐benzaldehyde) and acetone (C3H6O, HPLC 99.9%) from Sigma Chemical (Deishofen, Germany) were used in liquid forms, without dilution. Like in our previous studies during the PET scans, AND and EST were presented in crystalline form and also odorous (200 mg, Steraloids, Newport, USA) [Savic et al., 2001].
Conditions and Experimental Procedure
PET scans were carried out during three separate conditions: birhinal, passive smelling of odorless air (AIR), acetone (ACE), vanillin (VAN), AND, and EST. Acetone and vanillin were used to investigate how anosmics process pure olfactory and mixed olfactory + trigeminal odorants [Savic et al., 2002]. These odorants were employed to ascertain that the inability to transduce signals from odorants in anosmics was restricted to the olfactory mucosa, and did not involve the respiratory nasal epithelium. AND is a steroid like EST, and was included to investigate whether a possible activation with EST in anosmic men was related to the specific compound rather than to its steroid structure.
The number of scans per subject was restricted to 14. All the subjects smelled odorless air, AND and EST during three scans; 50% of the subjects smelled VAN in three scans, and ACE in two; in the remaining subjects ACE was presented three times, and VAN twice. The order of conditions was balanced across the subjects. Thee scans were excluded from further analysis because of movement artifacts.
The odorants were contained in glass bottles with a cotton wand. When the condition consisted of smelling of air, the wand was soaked with distilled water. Each item was presented during a separate scan, at 10 mm distance from the nostrils. To minimize odor adaptation, the respective item was during the scan presented for 15 s, a total of four times. Each of these presentations was followed by a 5‐s interval of breathing the air in the scanner room, wherein a suction hood placed close to the scanner gantry, provided continuous suction and refreshment of the air. The first presentation started 5 s after the bolus injection of 15O—H2O. One stimulus session lasted 80 s, and each PET measurement for 60 s.
Independent of the condition, each presentation was indicated by touching the subject's right index finger. Subjects were informed that they would smell either odor or odorless air, without knowing the type or order of items. They were instructed to breathe passively (not sniff, even when no odor was perceived) and concentrate on perception of the presented item, without analyzing its features. During several preparatory psychophysical experiments, subjects were trained in the scanner with other odors to familiarize with an experimental procedure. Respiratory movements were recorded during each scan, using a strain gauge around the lower thorax (Comair AB, Stockholm). The respiratory frequency (breaths/min) and amplitude was recorded continuously, before and during each presentation of VAN, ACE, AND, EST, and AIR. The amplitude measured in millimeter is directly related to the degree of stretching of the strain gauge band, i.e., the circumferential increase of the lower thorax.
The Analysis of PET Data
PET experiments were in each subject preceded by a magnetic resonance imaging (MRI), (1.5 Tesla GE scanner; 3D SPGR; TE = 5 ms, TR = 21 ms; FOV = 256 mm). The PET scanner used was the CTI‐Siemens ECAT EXACT HR scanner, running in 3D mode. During the PET sessions each subject received bolus injections containing about 12 mCi of 15O—H2O. PET scans were carried out with the head fixed [Bergstrom et al., 1981], ears plugged, and eyes covered. The room temperature and air pressure in the PET room was standardized during all experiments (23°C, 997 hPa), and the contamination by odors avoided by a suction device connected to the scanner. Patients and controls were investigated over the overlapping time period, same time of the day, in identical conditions, and by same experimenters.
The relative regional cerebral blood flow (rCBF) was calculated during 60 s after the onset of acquisition, using a 10 mm Gaussian filter. The PET images were preprocessed including anatomical standardization and global normalization to 50 ml/100 g/min, using the SPM2 statistical package (Wellcome Foundation, London), [Frackowiak, 2004; Friston et al., 1999; Savic et al., 2000, 2001].
Significant activations were first evaluated in each separate group with one‐group random effect analysis (T‐threshold 0.001, corrected P‐value <0.05) for the following contrasts: AND–AIR and vv; EST–AIR and vv; VAN–AIR and vv; ACE–AIR and vv. Next, group differences were tested using a two‐group random effect analysis. Significant clusters were calculated at height threshold at T = 0.01, corrected P < 0.05 (SPM2, Wellcome Foundation, London), to avoid the risk that minor group differences remained undetected.
The between‐group evaluations relied on our previous findings showing that the AND‐ and EST‐related activations were confined to the hypothalamus and the olfactory brain. Considering that the SPM statistics is rather conservative, and to avoid type‐II error, we used a rectangular mask which covered only the horizontal sections of the brain between Z = +20 and Z = −20. For the contrasts involving ACE and VAN (ACE‐AIR, VAN‐AIR, and vv) the search space was the entire brain.
Volume of Interest Analysis
Volume of interest (VOI) analysis was used to evaluate whether anosmic men activated the anterior hypothalamus in a similar manner as controls. Because the anatomical boundaries of this region are difficult to determine with MRI, a so‐called functional VOI was employed. This VOI was generated in a previous study of heterosexual men who were investigated using a similar experimental procedure [Savic et al., 2001]. The VOI was constituted by the hypothalamus cluster, which was generated by the EST‐AIR contrast. It was defined from PET images that were reformatted to the same standard brain as those in this study, and was, therefore, possible to directly transfer to the individual PET images. The rCBF was in all subjects first normalized to the global cerebral mean of 50 ml/min/100 g, and the mean rCBF in this VOI extracted for all the scans in each subject [Savic et al., 2001]. Possible difference in the mean extracted rCBF for each condition was then compared between EST and AIR, AND and AIR, VAN and AIR, and ACE and AIR for each subject group using paired t‐tests. The number of degrees of freedom (df) was 11. The P‐level was 0.05, based on the hypothesis that only EST‐AIR would yield a significant activation. Possible differences between anosmics and controls for these activations were evaluated with a repeated measurement ANOVA using subject group as the between factor and the type of odorant as the within factor. When a significant interaction was detected the results were further explored with contrasts to determine which type of odorant determined the observed interaction. P values were considered significant when <0.05.
Analysis of the Psychophysical Data
Respiratory frequency and amplitude during the respective scan was related to the resting condition during 2 min immediately before each scan (defined as the baseline). For each scan, we calculated the product of respiratory frequency and respiratory amplitude and used this index to describe change from baseline in respiratory volume. Group was entered as in between variable and the four odorous compounds and air as within variable in the repeated measure ANOVA.
RESULTS
Controls had normal odor thresholds (5.0 × 10−5 ± 2.0 × 10−5 M). The correctly lateralized side of acetone presentation was 9.3 ± 0.4 in controls, and 8.7 ± 0.8 in anosmics (ns).
Activations
As reported previously, heterosexual male controls displayed clusters in the anterior hypothalamus and the left amygdala and piriform cortex when smelling EST (Table I) [Savic et al., 2001]. Anosmics, on the other hand, displayed no cerebral activations.
Table I.
Activations and deactivations in anosmics and controls
| Region | Controls | Anosmics | ||||
|---|---|---|---|---|---|---|
| Z level | Size, cm3 | Coordinates | Z level | Size, cm3 | Coordinates | |
| Activations in anosmics and controls | ||||||
| ACE‐AIR | ||||||
| R insular cortex (includes amygd + pirif cortex + caudate + anterior cingulated) | 6.5 | 9.0 | 38, −10, 6 | |||
| R caudate | ||||||
| L insular cortex (including the postcentral gyrus) | 5.2 | 5.0 | −30, −6, 0 | 3.0 | 1.4 | −50, 10, 30a |
| Ant cingulate | 3.5 | 5.6 | −22, 44, 8 | |||
| Hypothalamus and thalamusb | 3.5 | 2.8 | 16, −10, −20 | 4.2 | 2.2 | 2, −36, −6 |
| 4.2 | 5.0 | 8, −32, −20 | 12, 2, 16 | |||
| 5.1 | 2.4 | −2, −2, −6 | ||||
| Cerebellum | 4.3 | 0.8 | −48, −50, −28 | |||
| VAN‐AIR | ||||||
| R pirif + amygd | 5.1 | 1.6 | 20, −4, −20 | |||
| L pirif + amygd | 4.8 | 0.9 | −20, −4, −16 | |||
| L fusiform gyrus | 4.3 | 1.3 | −48, −42, −26 | |||
| AND‐AIR | ||||||
| R pirif + amygd | 4.5 | 0.9 | 36, −14, −6 | |||
| R lingular + fusiform gyri | 4.2 | 1.2 | 18, −60, −24 | |||
| EST‐AIR | ||||||
| Anterior hypothalamus | 4.6 | 1.2 | 6, −12, 2 | |||
| L pirif + amygd | 4.5 | 1.0 | −22, −6, 24 | |||
| Deactivations in anosmics and controls | ||||||
| AIR‐ACE | ||||||
| R superior temporal gyrus | 4.5 | 1.6 | 54, −12, −6 | 4.5 | 1.6 | 54, −12, −6 |
| R, L middle temporal gyrus | 4.4 | 1.2 | 38, −42, −14 | 4.5 | 3.2 | 42, −68, 8 |
| 3.3 | 3.2 | 38, −18, −20 | 38, −62, 12 | |||
| Parieto‐occipital cortex | 4.5 | 33 | 12, −68, 16 | 5.0 | 6.2 | −10, −82, 34 |
| 3.3 | 2.8 | −38, −36, 30 | ||||
| AIR‐AND | ||||||
| Medial prefrontal cortex | 3.7 | 2.5 | 4, 46, 20 | |||
| AIR‐EST | ||||||
| Anterior cingulate | 3.6 | 3.3 | 6, 58, 0 | |||
| AIR‐VAN | ||||||
| Medial prefrontal cortex | 4.5 | 5.6 | 38, 46, 8 | |||
| 4.8 | 3.6 | −14, 45, 2 | ||||
| Middle temporal gyrus | 4.4 | 1.2 | −40, −44, 6 | |||
| Cuneus, fusiform gyrus | 4.3 | 3.6 | 46, −74, −16 | |||
Values calculated using one group random effect analysis, with a height threshold at P = 0.001, and corrected P < 0.05. The Talairach's coordinates indicate the peak activation; the indicated regions describe the coverage of the respective cluster. pirif, piriform cortex; amygd, amygdale.
Includes only a portion of the granular insular cortex.
Includes the ventromedial nucleus and the pulvinar.
In controls, smelling of AND and VAN was associated with significant activations of the amygdala piriform, agranular insular cortex, and portions of the fusiform gyrus. Exposure to acetone resulted in additional clusters in the anterior cingulate, the brainstem (including the trigeminal nucleus), the thalamus (the ventromedial nucleus and the pulvinar), the sensorimotorcortex (corresponding to the topographical representation of face), and the cerebellum. Anosmics showed no activations with VAN or AND, and significant clusters appeared only for ACE–AIR. They were detected in the anterior cingulate, the thalamus (the ventromedial nucleus and the pulvinar), the brainstem (covering the trigeminal nucleus), and the sensorimoror cortex (the topographical representation of face). Notably, and at variance from controls, no amygdala, piriform, or insular clusters were detected (Table I). Neither was there any cerebellar activation.
Deactivations
Deactivations were defined as decreases in normalized rCBF when smelling odorants compared with air. ACE‐related deactivations were detected in controls as well as anosmics in the temporal and parieto‐occipital cortex. Controls displayed additional deactivations in the frontopolar and dorsolateral prefrontal cortex. During smelling of EST, AND, and VAN deactivations were detected only in controls; they were located in the frontopolar cortex and parts of the dorsolateral prefrontal cortex (Table I).
Group Comparisons
As expected, the contrast between controls and anosmics (controls–anosmics) showed a significant cluster in the anterior hypothalamus for EST–AIR, and also in the left amygdala (Table II, Fig. 1a). The corresponding group comparison for AND–AIR, VAN–AIR, and ACE–AIR showed clusters only in the amygdala, priform, and anterior insular cortex. Group comparisons with respect to VAN–AIR and ACE–AIR showed additional clusters in the anterior cingulate and the anterior thalamus, and also in the sensorimotor cortex for ACE‐AIR (Table II, Fig. 1b). When contrasting anosmics and controls (anosmmics–controls) significant clusters appeared only for ACE–AIR; they were located in the prefrontal cortex covering portions of the orbitofrontal cortex, and the parietal cortex; the latter region overlapped with the ACE‐related deactivation in controls (Tables I and II).
Table II.
Group differences
| Region | Controls–anosmics | Anosmics–controls | ||||
|---|---|---|---|---|---|---|
| Z level | Size, cm3 | Coordinates | Z level | Size, cm3 | Coordinates | |
| ACE‐AIR | ||||||
| R amygd + pirif cortex | 4.6 | 3.9 | 20, 4, −14 | |||
| L insular cortex + amygd | 3.6 | 0.7 | −36, −24, 10 | |||
| 3.4 | 1.2 | −12, 6, −10 | ||||
| Ant cingulate cortex | 3.9 | 1.3 | −4, 42, 0 | 4.5 | 1.7 | −18, 42, −4 |
| 12, 62, 14 | ||||||
| Subcallosum | 3.0 | 0.4 | 18, 20, −18 | |||
| Precentral + postcentral gyrus | 2.9 | 0.4 | −54, 0, 8 | 4.0 | 1.8 | −40, −6, 52 |
| Inferior frontal gyrus | 3.8 | 2.4 | −44, 20, 10 | |||
| Cuneus | 3.0 | 0.8 | −4, −84, 24 | |||
| VAN‐AIR | ||||||
| L amygd + pirif + agranular insular cortex + anterior thalamus | 3.9 | 5.3 | −44, 0, −26 | |||
| Cingular cortex | 3.8 | 1.7 | −6, 30, −8 | |||
| AND‐AIR | ||||||
| R amygd + pirif cortex | 4.6 | 3.9 | 20, 4, −14 | |||
| R lingular + fusiform gyri | ||||||
| EST‐AIR | ||||||
| Anterior hypothalamus | 4.0 | 0.5 | 2, −15, −4 | |||
| L amygd + pirif cortex | 4.5 | 1.0 | −22, −6, 24 | |||
Values calculated using two group random effect analysis, with a height threshold at P = 0.01, and corrected P < 0.05 for ACE‐AIR and VAN‐AIR, and uncorrected P < 0.05 for AND‐AIR and EST‐AIR. The Talairach's coordinates indicate the peak activation; the indicated regions describe the coverage of the respective cluster. R, right; L, left; amygd, amigdala; pirif, piriform cortex.
Figure 1.
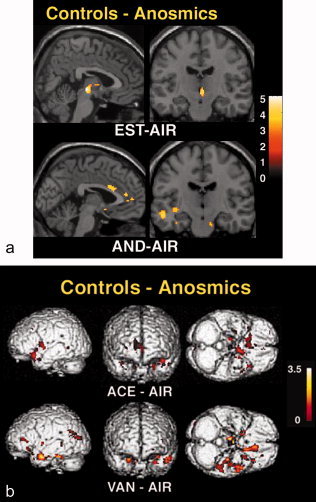
Group differences in activations with different odorants. Shown are the clusters calculated with two‐group random effect analysis, superimposed on standard brain MRI. The Sokoloff's color scale illustrates Z values reflecting the degree of difference in the respective activation. (a) Controls–anosmics with respect to EST–AIR (upper row) and AND–AIR (lower row). (b) Controls–anosmics with respect to ACE–AIR (upper row) and VAN–AIR (lower row). The dimensions are chosen to optimize illustrations of regions activated.
VOI Analysis
The post hoc VOI analysis confirmed that smelling EST elicited no activation in the anterior hypothalamus (corresponding to the hypothalamic VOI, P = 0.82, df = 11) in anosmics, but was clearly present in controls (P = 0.0056, df = 11), Figure 2. No hypothalamic activations were detected with VAN, ACE, or AND in anosmics (P = 0.92 for VAN–AIR; P = 0.54 for ACE–AIR; P = 0.38 for AND–AIR), or in controls (P = 0.44 for VAN–AIR; P = 0.53 for ACE–AIR; P = 0.44 for AND vs. AIR).
Figure 2.
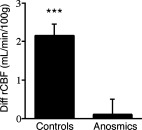
Volume of interest analysis of the rCBF in the anterior hypothalamus. There was a significant group × compound interaction (F = 16.3, P = 0.0002). The figure illustrates changes in rCBF in controls and anosmics for EST–AIR, which constituted the group difference. Mean and SD are given. ***P < 0.001.
VOI analysis showed a significant group difference (P = 0.0002, df = 3, power = 0.988, F = 16.3). It was constituted by EST–AIR, which showed significantly higher activation in controls (P < 0.0001).
Respiratory Responses
There was no significant group difference (F = 0.13; P = 0.7: df = 1) or group × compound interaction (F = 1.38; P = 0.2; df = 4) in the calculated respiratory index. See Figure S1 in supporting information.
DISCUSSION
In contrast to the male controls who displayed clear activations with EST in the anterior hypothalamus, no EST‐activations were detected in anosmic men. This finding was based on SPM analysis and confirmed with the VOI analysis. The methods applied have been reported in several of our previous studies yielding consistent and reproducible results [Berglund et al., 2006; Savic et al., 2001, 2002, 2005]. Therefore, they will not be further commented here.
Our choice to investigate anosmia on the basis of nasal polyposis was motivated by the assumption that polyps would affect only the olfactory mucosa. A minor affection of the surrounding epithelium cannot be excluded, but seems unlikely considering that all anosmic patients sensed acetone, and displayed clear cerebral activations with this compound. Furthermore, the VNO is located away from the nasal floor, which is the only region usually affected by polyps, and the endoscopic investigations did not suggest abnormality of the respiratory mucosa. In addition, none of our patients reported rhinitis, according to the available literature polyposis affects nasal mucosa primarily in conjunction with chronic nasorhinitis [Slater et al., 1996]. The age range was comparable in patients and controls and could not have contributed to the differences in odor sensitivity. Neither could anosmia have had other etiology than polyposis, considering that none of the patients had heredity for overall or selective anosmia, and that all patients reported a normal sense of smell before the congestion.
As expected, anosmics differed from controls by failing to activate regions attributed to olfactory processing in relation to VAN, AND, EST, as well as ACE. Thus, independently of the type of odorant or the type of chemical structure of the stimulus, no significant clusters were detected in the olfactory networks. To the contrary, the patients activated the neuronal circuits involved in trigeminal processing of odorants, [Boyle et al., 2007; Savic, 2002], confirming that they suffered from affection of the olfactory, but not respiratory epithelium. Activations with ACE were slightly less pronounced in anosmics compared to controls (Table II, Fig. 1b). This difference cannot be attributed to respiratory pattern, which did not differ significantly between the two groups. One possibility is that anosmics had reduced responsiveness to intranasal trigeminal stimuli because of loss of the olfactory enhancement of the trigeminal component of acetone [Cain and Murphy, 1980; Cashion et al., 2006; Gudziol et al., 2001; Hummel et al., 1996]. Another is that trigeminal processing was functionally reorganized in anosmics at a central level, as indicated by reports of reduced cerebral activation in anosmics with gaseous CO2, which is a nonodorous trigeminal stimulus [Iannilli et al., 2007]. The two mechanisms are not mutually exclusive and may coexist.
Theoretically, our male heterosexual controls could have processed signals from EST via VNO, via the olfactory mucosa or via venous blood (after absorption of EST into the nasal vasculature). Humoral transport seems improbable because it is much slower than the presently observed cerebral activations. For example, after nasal application of the bore pheromone androstenol, a maximum plasma concentration is observed between 40 and 50 min after application, whereas in our experiments the cerebral activation was detected already within 30 s [Stefanczyk‐Krzymowska et al., 2000]. Furthermore, if EST signals would be transduced to the hypothalamus by a humoral pathway the pattern of activation would be similar in controls and anosmics, which was not the case.
The present results also reject the hypothesis that signals from the pheromone component of EST are mediated by the VNO; rather, they favor the view that in healthy men signals from this component can be processed by the MOB via the olfactory epithelium. Interestingly, several lines of evidence suggest that the MOB may play a role in pheromone signal detection also in animals. The MOB is activated by pheromones in ferrets [Woodley and Baum, 2003] and in hamsters [O'Connell and Meredith, 1984]. Ablation of the VNO has no effect on the suckling behavior of rabbits [Hudson and Distel, 1986] or the mating behavior of male hamsters [Pfeiffer and Johnston, 1994]. Male mice with surgically removed VNO are still able to distinguish urine odors between males and estrous females. Likewise, removal of the VNO from female mice does not impair their ability to discriminate between males and females, whereas destruction of the olfactory epithelium with ZnSO4 reduces their sexual behavior [Keller et al., 2006]. The MOB is activated by pheromones also in ferrets and in hamsters [O'Connell and Meredith, 1984; Woodley and Baum, 2004].
A special family of pheromone sensing receptors, called “trace amine‐associated receptors” (TAARs), has recently been detected in the mouse olfactory epithelium. Interestingly, genes encoding these receptors are present also in humans [Liberles and Buck, 2006], which is in line with the previous report of Rodrigez et al. [ 2000] about a functional pheromone receptor gene expression in humanolfactory mucosa. Together these, yet anecdotal findings, provide further support for the hypothesis that humans, like several other mammals are capable of processing pheromone signals through the olfactory mucosa. Although the present data favor this possibility only indirectly and do not provide any definite evidence, they highly motivate further investigations in this controversial area.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Figure S1. The percent change in the respiratory index from baseline to presentation is illustrated for air and the four compounds. Mean and SEM are given. There was no significant group differences (F = 0.13; P = 0.7: Df = 1) or group × compound interaction (F = 1.38; P = 0.2; Df = 4) in the calculated respiratory index. Group was entered as in between variable and the four compounds and air as within variable in the repeated measure ANOVA. Air = Unscented air, Van = Vanillin, AND = Androstanediol, EST = Estratetraenol, Ace = Acetone.
Acknowledgements
We thank Dr. Balasz Gulyas for injecting the PET tracer during some of the experiments.
REFERENCES
- Bensafi M,Tsutsui T,Khan R,Levenson RW,Sobel N ( 2004): Sniffing a human sex‐steroid derived compound affects mood and autonomic arousal in a dose‐dependent manner. Psychoneuroendocrinology 29: 1290–1299. [DOI] [PubMed] [Google Scholar]
- Bensafi M,Frasnelli J,Reden J,Hummel T ( 2007): The neural representation of odor is modulated by the presence of a trigeminal stimulus during odor encoding. Clin Neurophysiol 118: 696–701. [DOI] [PubMed] [Google Scholar]
- Berglund H,Lindstrom P,Savic I ( 2006): Brain response to putative pheromones in lesbian women. Proc Natl Acad Sci USA 103: 8269–8274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund H,Lindstrom P,Dhejne‐Helmy C,Savic I ( 2008): Male‐to‐female transsexuals show sex‐atypical hypothalamus activation when smelling odorous steroids. Cereb Cortex 18: 1900–1908. [DOI] [PubMed] [Google Scholar]
- Bergstrom M,Boethius J,Eriksson L,Greitz T,Ribbe T,Widen L ( 1981): Head fixation device for reproducible position alignment in transmission CT and positron emission tomography. J Comput Assist Tomogr 5: 136–141. [DOI] [PubMed] [Google Scholar]
- Boyle JA,Frasnelli J,Gerber J,Heinke M,Hummel T ( 2007): Cross‐modal integration of intranasal stimuli: A functional magnetic resonance imaging study. Neuroscience 149: 223–231. [DOI] [PubMed] [Google Scholar]
- Cain WS,Murphy CL ( 1980): Interaction between chemoreceptive modalities of odour and irritation. Nature 284: 255–257. [DOI] [PubMed] [Google Scholar]
- Cashion L,Livermore A,Hummel T ( 2006): Odour suppression in binary mixtures. Biol Psychol 73: 288–297. [DOI] [PubMed] [Google Scholar]
- Dennis JC,Smith TD,Bhatnagar KP,Bonar CJ,Burrows AM,Morrison EE ( 2004): Expression of neuron‐specific markers by the vomeronasal neuroepithelium in six species of primates. Anat Record 281: 1190–1200. [DOI] [PubMed] [Google Scholar]
- Frackowiak RSJ ( 2004): Human Brain Function. Amsterdam: Elsevier Academic. [Google Scholar]
- Friston KJ,Holmes AP,Worsley KJ ( 1999): How many subjects constitute a study? Neuroimage 10: 1–5. [DOI] [PubMed] [Google Scholar]
- Gudziol H,Schubert M,Hummel T ( 2001): Decreased trigeminal sensitivity in anosmia. ORL J Otorhinolaryngol Relat Spec 63: 72–75. [DOI] [PubMed] [Google Scholar]
- Hudson R,Distel H ( 1986): Pheromonal release of suckling in rabbits does not depend on the vomeronasal organ. Physiol Behav 37: 123–128. [DOI] [PubMed] [Google Scholar]
- Hummel T,Welge‐Luessen A ( 2006): Assessment of olfactory function. Adv Otorhinolaryngol 63: 84–98. [DOI] [PubMed] [Google Scholar]
- Hummel T,Barz S,Lotsch J,Roscher S,Kettenmann B,Kobal G ( 1996): Loss of olfactory function leads to a decrease of trigeminal sensitivity. Chem Senses 21: 75–79. [DOI] [PubMed] [Google Scholar]
- Hummel T,Futschik T,Frasnelli J,Huttenbrink KB ( 2003): Effects of olfactory function, age, and gender on trigeminally mediated sensations: A study based on the lateralization of chemosensory stimuli. Toxicol Lett 140/141: 273–280. [DOI] [PubMed] [Google Scholar]
- Iannilli E,Gerber J,Frasnelli J,Hummel T ( 2007): Intranasal trigeminal function in subjects with and without an intact sense of smell. Brain Res 1139: 235–244. [DOI] [PubMed] [Google Scholar]
- Jacob S,McClintock MK ( 2000): Psychological state and mood effects of steroidal chemosignals in women and men. Horm Behav 37: 57–78. [DOI] [PubMed] [Google Scholar]
- Jacob S,Hayreh DJ,McClintock MK ( 2001): Context‐dependent effects of steroid chemosignals on human physiology and mood. Physiol Behav 74: 15–27. [DOI] [PubMed] [Google Scholar]
- Karlson P,Luscher M ( 1959): Pheromones': A new term for a class of biologically active substances. Nature 183: 55–56. [DOI] [PubMed] [Google Scholar]
- Keller M,Douhard Q,Baum MJ,Bakker J ( 2006): Destruction of the main olfactory epithelium reduces female sexual behavior and olfactory investigation in female mice. Chem Sens 31: 315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keverne EB ( 2007): Genomic imprinting and the evolution of sex differences in mammalian reproductive strategies. Adv Genet 59: 217–243. [DOI] [PubMed] [Google Scholar]
- Liberles SD,Buck LB ( 2006): A second class of chemosensory receptors in the olfactory epithelium. Nature 442: 645–650. [DOI] [PubMed] [Google Scholar]
- Lundstrom JN,Olsson MJ ( 2005): Subthreshold amounts of social odorant affect mood, but not behavior, in heterosexual women when tested by a male, but not a female, experimenter. Biol Psychol 70: 197–204. [DOI] [PubMed] [Google Scholar]
- Meredith M ( 1991): Sensory processing in the main and accessory olfactory systems: Comparisons and contrasts. J Steroid Biochem Mol Biol 39: 601–614. [DOI] [PubMed] [Google Scholar]
- Monti‐Bloch L,Jennings‐White C,Berliner DL ( 1998): The human vomeronasal system. A review. Ann NY Acad Sci 855: 373–389. [DOI] [PubMed] [Google Scholar]
- O'Connell RJ,Meredith M ( 1984): Effects of volatile and nonvolatile chemical signals on male sex behaviors mediated by the main and accessory olfactory systems. Behav Neurosci 98: 1083–1093. [DOI] [PubMed] [Google Scholar]
- Pfeiffer CA,Johnston RE ( 1994): Hormonal and behavioral responses of male hamsters to females and female odors: Roles of olfaction, the vomeronasal system, and sexual experience. Physiol Behav 55: 129–138. [DOI] [PubMed] [Google Scholar]
- Preti G,Wysocki CJ,Barnhart KT,Sondheimer SJ,Leyden JJ ( 2003): Male axillary extracts contain pheromones that affect pulsatile secretion of luteinizing hormone and mood in women recipients. Biol Reprod 68: 2107–2113. [DOI] [PubMed] [Google Scholar]
- Rodriguez I,Greer CA,Mok MY,Mombaerts P ( 2000): A putative pheromone receptor gene expressed in human olfactory mucosa. Nat Genet 26: 18–19. [DOI] [PubMed] [Google Scholar]
- Savic I ( 2002): Imaging of brain activation by odorants in humans. Curr Opin Neurobiol 12: 455–461. [DOI] [PubMed] [Google Scholar]
- Savic I,Gulyas B,Larsson M,Roland P ( 2000): Olfactory functions are mediated by parallel and hierarchical processing. Neuron 26: 735–745. [DOI] [PubMed] [Google Scholar]
- Savic I,Berglund H,Gulyas B,Roland P ( 2001): Smelling of odorous sex hormone‐like compounds causes sex‐differentiated hypothalamic activations in humans. Neuron 31: 661–668. [DOI] [PubMed] [Google Scholar]
- Savic I,Gulyas B,Berglund H ( 2002): Odorant differentiated pattern of cerebral activation: Comparison of acetone and vanillin. Hum Brain Mapp 17: 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savic I,Berglund H,Lindstrom P ( 2005): Brain response to putative pheromones in homosexual men. Proc Natl Acad Sci USA 102: 7356–7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater A,Smallman LA,Logan AC,Drake‐Lee AB ( 1996): Mucociliary function in patients with nasal polyps. Clin Otolaryngol Allied Sci 21: 343–347. [DOI] [PubMed] [Google Scholar]
- Sobel N,Prabhakaran V,Hartley CA,Desmond JE,Glover GH,Sullivan EV,Gabrieli JD ( 1999): Blind smell: Brain activation induced by an undetected air‐borne chemical. Brain 122 (Pt 2): 209–217. [DOI] [PubMed] [Google Scholar]
- Stefanczyk‐Krzymowska S,Krzymowski T,Grzegorzewski W,Ws B,Skipor J ( 2000): Humoral pathway for local transfer of the priming pheromone androstenol from the nasal cavity to the brain and hypophysis in anaesthetized gilts. Exp Physiol 85: 801–809. [DOI] [PubMed] [Google Scholar]
- Stern K,McClintock MK ( 1998): Regulation of ovulation by human pheromones. Nature 392: 177–179. [DOI] [PubMed] [Google Scholar]
- Trotier D,Eloit C,Wassef M,Talmain G,Bensimon JL,Doving KB,Ferrand J ( 2000): The vomeronasal cavity in adult humans. Chem Senses 25: 369–380. [DOI] [PubMed] [Google Scholar]
- Witt M,Georgiewa B,Knecht M,Hummel T ( 2002): On the chemosensory nature of the vomeronasal epithelium in adult humans. Histochem Cell Biol 117: 493–509. [DOI] [PubMed] [Google Scholar]
- Woodley SK,Baum MJ ( 2003): Effects of sex hormones and gender on attraction thresholds for volatile anal scent gland odors in ferrets. Horm Behav 44: 110–118. [DOI] [PubMed] [Google Scholar]
- Woodley SK,Baum MJ ( 2004): Differential activation of glomeruli in the ferret's main olfactory bulb by anal scent gland odours from males and females: An early step in mate identification. Eur J Neurosci 20: 1025–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zufall F,Kelliher KR,Leinders‐Zufall T ( 2002): Pheromone detection by mammalian vomeronasal neurons. Microsc Res Tech 58: 251–260. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Figure S1. The percent change in the respiratory index from baseline to presentation is illustrated for air and the four compounds. Mean and SEM are given. There was no significant group differences (F = 0.13; P = 0.7: Df = 1) or group × compound interaction (F = 1.38; P = 0.2; Df = 4) in the calculated respiratory index. Group was entered as in between variable and the four compounds and air as within variable in the repeated measure ANOVA. Air = Unscented air, Van = Vanillin, AND = Androstanediol, EST = Estratetraenol, Ace = Acetone.


