Abstract
A number of studies have investigated differences in neural correlates of abstract and concrete concepts with disagreement across results. A quantitative, coordinate‐based meta‐analysis combined data from 303 participants across 19 functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) studies to identify the differences in neural representation of abstract and concrete concepts. Studies that reported peak activations in standard space in contrast of abstract > concrete or concrete > abstract concepts at a whole brain level in healthy adults were included in this meta‐analysis. Multilevel kernel density analysis (MKDA) was performed to identify the proportion of activated contrasts weighted by sample size and analysis type (fixed or random effects). Meta‐analysis results indicated consistent and meaningful differences in neural representation for abstract and concrete concepts. Abstract concepts elicit greater activity in the inferior frontal gyrus and middle temporal gyrus compared to concrete concepts, while concrete concepts elicit greater activity in the posterior cingulate, precuneus, fusiform gyrus, and parahippocampal gyrus compared to abstract concepts. These results suggest greater engagement of the verbal system for processing of abstract concepts and greater engagement of the perceptual system for processing of concrete concepts, likely via mental imagery. Hum Brain Mapp, 2010. © 2010 Wiley‐Liss, Inc.
Keywords: meta analysis, language, concept representation, abstract words, concrete words
INTRODUCTION
How abstract and concrete concepts are represented in the brain is relevant to understanding language function in both healthy and clinical populations [Eviatar et al., 1990; Kuperberg et al., 2008; Mervis and John, 2008]. Behavioral differences in processing between abstract and concrete concepts have been well documented and referred to as the concreteness effect: concrete words are acquired earlier, and are remembered and recognized more rapidly than abstract words [Kroll and Merves, 1986; Schwanenflugel, 1991]. The concreteness effect is increased in patients with aphasia caused by left‐hemispheric damage [Goodglass et al., 1969] and dyslexia [Shallice and Warrington, 1975]. A reverse concreteness effect [Warrington, 1975], showing more severe impairment for understanding concrete than abstract concepts, has been found in patients with semantic dementia [Breedin et al., 1994; Reilly et al., 2006]. This double dissociation suggests a difference in neural representation of abstract and concrete concepts.
One possible explanation of the concreteness effect is the difference in imageability between abstract and concrete concepts. Concrete concepts have higher imageability than abstract concepts: for example, hammer is more easily visualized than freedom. The engagement of mental imagery in semantic concept processing has been argued to contribute to the difference in abstract and concrete concept representation [Marschark et al., 1987; Paivio, 1991]. Mental imagery is similar to a perceptual experience, but happens without the presence of external stimuli [Thomas, 2008]. Neuroimaging studies have indicated that mental imagery generates neural activity in sensory and motor systems of the same modality [Borst and Kosslyn, 2008; Farah, 1995; Jeannerod and Decety, 1995; Kosslyn et al., 1995], suggesting that common neural mechanisms underlie imagery and perception [Kosslyn et al., 2001].
Mental imagery has been associated with basic cognitive processes such as memory [Brewer and Pani, 1996; Paivio, 1969]. It has also been proposed to be involved with semantic representation through the engagement of neural activity for sensory‐motor processes. Perceptual symbol systems theory postulates that all concepts are grounded in action, perceptual and emotional experience; language comprehension can also be interpreted as the construction of perceptual and motor simulation [Barsalou, 1999, 2003; Barsalou et al., 2003]. Another suggestion is that mental imagery may rely on the mechanism of reenactment [Halpern and Zatorre, 1999; Jeannerod and Decety, 1995], which is viewed as underlying conceptualization and comprehension [Barsalou, 1999]. Thus, mental imagery may play a role in concept processing. A central question in the debate of whether processing mechanisms of abstract and concrete concepts differ is whether concepts are represented by words or by nonlanguage factors, such as perceptual and motor experiences.
Specific theories of abstract and concrete concepts representation have addressed the function of mental imagery. The dual‐coding hypothesis suggests a common verbal representation for both concrete and abstract concepts and an additional mental imagery process for concrete concepts [Paivio, 1991]. From a neural perspective, dual‐coding theory predicts that representation of concrete concepts is based more on neural substrates of the imagery processing system compared with abstract concepts. On the other hand, the concreteness effect can also be interpreted without incorporating mental imagery. The context availability theory argues that representational information and connections to semantic knowledge lead to richer context, resulting in more efficient processing of concrete concepts in comparison to abstract concepts [Schwanenflugel et al., 1988].
This theoretical debate has led to neuroimaging studies comparing representation of abstract and concrete concepts. To date, results of these studies have been inconsistent. Some studies have found greater activations for concrete compared to abstract concepts only [Mestres‐Missé et al., 2008; Wise et al., 2000]. Specifically, imagery‐based and perceptual regions were reported to be activated for concrete > abstract contrast in several studies [Binder et al., 2005; Fiebach and Friederici, 2004; Grossman et al., 2002]; however, the foci for mental imagery generation varied from predominantly right hemisphere [Paivio, 1991] to left hemisphere [D'Esposito et al., 1997], to bilateral areas [Binder et al., 2005]. Other studies only identified areas with greater activation for abstract compared to concrete concepts [Friederici et al., 2000; Grossman et al., 2002; Jessen et al., 2000; Kiehl et al., 1999; Noppeney and Price, 2004; Perani et al., 1999; Pexman et al., 2007]. At the same time, there were also studies that found regions activated for both concrete > abstract and abstract > concrete contrasts [Binder et al., 2005; Fiebach and Friederici, 2004; Fliessbach et al., 2006; Harris et al., 2006; Sabsevitz et al., 2005; Tettamanti et al., 2008; Wallentin et al., 2005; Whatmough et al., 2004].
Typically, a single neuroimaging experiment does not have enough power to reveal the neural substrates of a cognitive process, partly due to the limited sample size. Desmond and Glover [2002] have found that to detect a signal change of 0.5% with 80% power, approximately 25 participants are necessary, and many studies have far fewer participants [Thirion et al., 2007]. A number of studies examining the neural representation of abstract and concrete concepts have been conducted, and it is possible to examine the consistency among results using a quantitative approach. Two studies [Fiebach and Friederici, 2004; Pexman et al., 2007] reviewed the reported activity coordinates in relevant studies; Fiebach and Friederici [2004] also offered an integrated visualization of these peaks in one brain template. Binder et al. [2009] performed a meta‐analysis of functional neuroimaging studies on semantic processing, and identified representational differences in abstract and concrete concepts. The aim of this study is to clarify differences in neural representation of abstract and concrete concepts by integrating existing neuroimaging evidence through meta‐analysis. Multilevel kernel density analysis [Etkin and Wager, 2007] was applied to evaluate the activation consistency across published neuroimaging studies of abstract and concrete concept representation.
METHODS
Study Selection
Peer‐reviewed journals in PsycARTICLES, PsycCRITIQUES, PsycINFO, Web of Science, and Psychology & Behavioral Sciences Collection databases were searched for neuroimaging studies of abstract and concrete concepts. In addition, we searched the reference lists of identified studies to ensure inclusion of all relevant studies fitting our criteria. To compare abstract and concrete concepts directly, the criteria for study inclusion were (1) participants were healthy adults; (2) the selected studies reported the peak activations in Montreal Neurological Institute (MNI) or Talairach coordinates [Talairach and Tournoux, 1988] in either condition, i.e., brain regions where concrete concepts showed greater activations compared to abstract concepts (concrete > abstract) or the reverse (abstract > concrete); (3) contrasts were performed at a whole brain level (i.e., not at a region‐of‐interest level). These criteria resulted in a total of 303 participants across nineteen studies eligible for inclusion in the meta‐analysis (Table I).
Table I.
Studies included in the meta‐analysis
| Study | Imaging modality | Number of participants | Random or fixed effect | Materials | Input modality | Task |
|---|---|---|---|---|---|---|
| Mestres‐Missé et al. [2008] | 3T fMRI | 15 | Random | Sentence pairs | Visual | Recognition |
| Tettamanti et al. [2008] | 3T fMRI | 18 | Random | Sentences | Auditory | Passive listening |
| Pexman et al. [2007] | 3T fMRI | 20 | Random | Words | Visual | Semantic categorization (consumable or not) |
| Fliessbach et al. [2006] | 1.5T fMRI | 21 | Random | Words | Visual | Recognition |
| Harris et al. [2006] | 1.5T fMRI | 20 | Random | Words | Visual | Semantic judgment (positive or negative) |
| Binder et al. [2005] | 1.5T fMRI | 24 | Random | Words | Visual | Lexical decision |
| Sabsevitz et al. [2005] | 1.5T fMRI | 28 | Random | Word triads | Visual | Semantic similarity decision |
| Wallentin et al. [2005] | 1.5T fMRI | 18 | Random | Sentences | Visual and auditory | Sentence comprehension |
| Fiebach and Friederici [2004] | 3T fMRI | 12 | NA | Words | Visual | Lexical decision |
| Noppeney and Price [2004] | 2T fMRI | 15 | Random | Word triads | Visual | Semantic similarity decision |
| Whatmough et al. [2004] | PET | 15 | NA | Word pairs | Visual | Semantic similarity decision (read aloud if the pair is similar in meanings) |
| Grossman et al. [2002] | 4T fMRI | 16 | Fixed | Words | Visual | Semantic judgment (pleasant or not) |
| Friederici et al. [2000] | 3T fMRI | 14 | NA | Words | Visual | Semantic categorization (syntactic task: noun or function word; semantic task: concrete or abstract) |
| Jessen et al. [2000] | 1.5T fMRI | 14 | Fixed | Words | Visual | Memory encoding |
| Wise et al. [2000]a | PET | 18 | Fixed | Words | Auditory | Passive listening |
| Fixed | Word triads | Auditory | Semantic similarity decision sample | |||
| Fixed | Words | Visual/ auditory | Passive listening/viewing | |||
| Kiehl et al. [1999] | 1.5T fMRI | 6 | Fixed | Words | Visual | Lexical decision |
| Perani et al. [1999] | PET | 14 | Fixed | Words | Visual | Lexical decision |
| Mellet et al. [1998] | PET | 8 | Fixed | Words with definitions | Auditory | Mental image generation (concrete) and passive listening (abstract) |
| D'Esposito et al. [1997] | 1.5T fMRI | 7 | NA | Words | Auditory | Mental image generation (concrete) and passive listening (abstract) |
Relevant results taken from multistudy analysis conducted by Wise et al. [2000].
Multilevel Kernel Density Analysis
The multilevel kernel density analysis (MKDA) is a coordinate‐based meta‐analysis method where the statistical indicator is the probability of activation of a given voxel in the brain [Kober et al., 2008; Wager et al., 2007, 2009]. The general null hypothesis is that peak coordinates of activated regions are randomly distributed. If the number of nearby active peaks for a peak coordinate is greater than the number expected by chance, the null hypothesis is rejected. A number of meta‐analysis methods are available; the MKDA method was selected for its several advantages. First, MKDA emphasizes the multilevel hierarchy of the data: multiple peaks are nested in a contrast, and multiple contrasts are nested in a study. Second, MKDA allows weighting contrasts by study sample size and quality. Compared with other commonly used meta‐analysis methods in brain imaging [Turkeltaub et al., 2002], this method prevents the result from being dominated by any single study with a large number of reported activations. It has the ability to weight the included studies by the number of participants and the quality of analysis based on random or fixed effects designs, such that studies with fewer participants or fixed effects designs are given less weight while studies with a larger numbers of participant or random effects designs are given more weight. Finally, the MKDA test statistic offers a straightforward interpretation as the weighted proportion of activated contrasts in a kernel around each voxel [Kober et al., 2008].
For this meta‐analysis, relevant study variables were sample size, analysis type (fixed or random effects), and peak coordinates in the contrasts concrete > abstract and abstract > concrete. We retained significance criteria set by individual studies. For those studies using multiple tasks for one contrast, data from only one task were retained to avoid inclusion of data from the same participants more than once (however, we cannot guarantee against data from the same participants being reported in different studies). Analyses were performed in Matlab 2009a (Mathworks, Naticks, MA) based on the MKDA tool package created by Wager et al. [2009] (http://www.columbia.edu/cu/psychology/tor/). Peaks from each study were convolved with a spherical kernel of 10 mm radius (kernels of 5 mm and 15 mm were also investigated). The studies were weighted by the number of participants (N) and type of analysis (δ):
where c is the index factor for the number of comparison maps I [Kober et al., 2008]. Studies that used random effects analysis had an adjusted weight of 1.0 and studies that used fixed effects, or when analysis type was unknown, had an adjusted weight of 0.75 [Kober et al., 2008]. The test statistic P represents the proportion of studies that found significantly active voxels within the 10 mm radius of each voxel. The threshold for statistical significance was determined using a Monte Carlo simulation procedure with 5,000 iterations; increasing the number of iterations to greater than 5,000 did not change the results. The significance threshold was set at the proportion exceeding 95% of the Monte Carlo simulation maxima and controlled by familywise error (FWE) rate. In addition, we examined FWE‐corrected results based on cluster extent.
RESULTS
Meta‐analysis results indicated different neural representation patterns for abstract and concrete concepts (Fig. 1). Regions with significant proportions of stronger activation for abstract compared to concrete concepts were in the inferior frontal gyrus (IFG) and middle temporal gyrus (MTG) in the left hemisphere (Table II). Regions that showed stronger activation for concrete concepts were found in the left precuneus, parahippocampal gyrus, posterior cingulate, and fusiform gyrus (Table III). These results were robust to changes in kernel size. Additional activated foci corrected on cluster extent at the 10 mm kernel were located within these regions. Applying a 15 mm kernel resulted in additional regions for each of the contrasts. Abstract concepts elicited greater activation in the left precentral gyrus, whereas concrete concepts were more strongly activated in left superior occipital gyrus, angular gyrus, and culmen.
Figure 1.
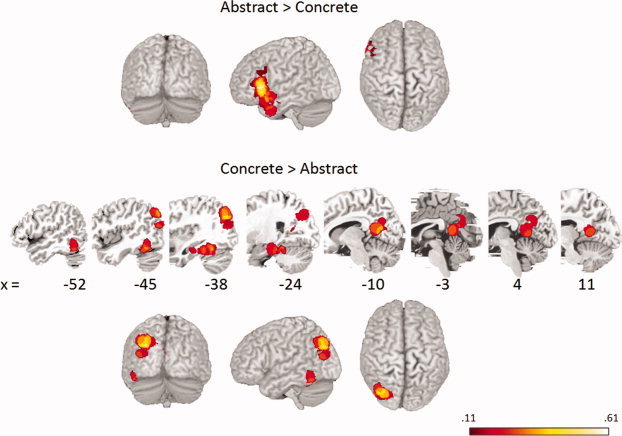
Regions surpassing the maximum activation proportion expected under the null hypothesis, within 10 mm of each voxel for abstract > concrete and concrete > abstract comparisons (P < 0.05 FWE corrected, cluster extent).
Table II.
Consistently activated foci across all studies for abstract greater than concrete concepts (P < 0.05, FWE corrected)
| Region | MNI | Number of voxels | ||
|---|---|---|---|---|
| x | y | z | ||
| Left | ||||
| Inferior frontal gyrus | –48 | 18 | –2 | 738 |
| Inferior frontal gyrus | –50 | 20 | 4 | 373 |
| Inferior frontal gyrus | –42 | 20 | –4 | 138 |
| Middle temporal gyrus | –52 | 10 | –18 | 68 |
| Superior temporal gyrus | –48 | 18 | –10 | 117 |
| Superior temporal gyrus | –48 | 10 | –8 | 42 |
| Middle temporal gyrus | –52 | 8 | –32 | 37 |
| Middle temporal gyrus | –58 | –42 | –4 | 7 |
Table III.
Consistently activated foci across all studies for concrete greater than abstract concepts (P < 0.05, FWE corrected)
| Region | MNI | Number of voxels | ||
|---|---|---|---|---|
| x | y | z | ||
| Left | ||||
| Precuneus | –34 | –76 | 34 | 147 |
| Posterior cingulate | –12 | –58 | 12 | 111 |
| Posterior cingulate | –14 | –56 | 12 | 45 |
| Posterior cingulate | –10 | –62 | 14 | 41 |
| Posterior cingulate | –12 | –56 | 6 | 25 |
| Fusiform gyrus | –40 | –52 | –22 | 16 |
| Parahippocampal gyrus | –32 | –32 | –20 | 8 |
| Parahippocampal gyrus | –28 | –34 | –20 | 1 |
DISCUSSION
This study used the multilevel kernel density method to conduct a meta‐analysis on 19 neuroimaging studies to investigate the neural representation of abstract and concrete concepts. Although the individual results of studies used in our analysis were varied, the meta‐analysis presented a consistent tendency for a difference in the representation of abstract and concrete concepts. Two contrasts were examined: abstract > concrete and concrete > abstract. Results clearly suggest a greater engagement of the verbal system for processing of abstract concepts, and a greater engagement of the perceptual system for processing of concrete concepts.
Abstract > Concrete
The comparison of abstract > concrete concepts showed significant consistent activation among studies in the left IFG and MTG (Fig. 1). The anterior inferior portion of the IFG as well as the prefrontal regions at large have been linked to verbally‐mediated semantic knowledge processing [Goldberg et al., 2007; Petersen et al., 1988]. Semantic task requirements have been shown to alter activity in the IFG and posterior cingulate [Zatorre et al., 1992]. This effect on semantic processing in the IFG has been successfully dissociated from the effect of task difficulty, and it has been argued that IFG may act as a specialized central executive area for semantic retrieval [Demb et al., 1995]. Similarly, Fliessbach et al. [2006] posited that the increased left IFG activation associated with abstract words reflects more strategic retrieval of semantic knowledge, consistent with both context availability and dual‐coding theories. The context availability theory would predict that the access to semantic knowledge for concrete words will be easier than for abstract words, so the effortful processing of abstract words is required for successful encoding. The dual‐coding theory would predict that semantic processing will be more important when dealing with abstract compared to concrete concepts due to the lack of imagery system involvement, which explains the increased activity of the left IFG elicited by abstract words.
The left IFG has also been implicated in phonological processing during working memory tasks [Fiebach and Friederici, 2004]. Lesions to the left IFG produce deficits in phonological and syntactic processes [Bookheimer, 2002; Caramazza et al., 1981]. Sabsevitz et al. [2005] proposed that activation in the more posterior parts of the frontal lobe by abstract concepts may represent phonological working memory processing, while the more anterior regions of the inferior frontal gyrus may play a role in the putative verbal semantic system. Binder et al. [2005] suggested that the stronger left IFG activation reflects the additional semantic processing for abstract words compared to concrete words during a lexical decision task, as abstract words are held in working memory in phonological form to a greater degree than concrete words. These inferences suggest that neural representational differences between abstract and concrete concepts might also be ascribed to phonological processing differences caused by different levels of semantic processing difficulties.
Left MTG has been shown to play a role in several aspects of language processing, including processing of abstract concepts [for a review, see Pexman et al., 2007]. Noppeney and Price [2004] attributed the difference in left MTG activation between abstract and concrete concepts to distinct retrieval mechanisms or strategies, rather than different neural representations or processing demands.
Taken together, the consistent activation across studies resulting from processing of abstract compared to concrete concepts in both left IFG and MTG is linked to activation elicited by a language‐based or verbal system. Representation of abstract concepts may be held in working memory to a greater degree and require more efforts of semantic retrieval.
Concrete > Abstract
Among studies included in the meta‐analysis, the comparison of concrete > abstract concepts showed significant consistent activation in the left precuneus, posterior cingulate, parahippocampal gyrus, fusiform gyrus, and culmen, with a trend of left temporal, occipital, and parietal regions (Fig. 1). These results imply greater engagement of mental image generation in concrete compared to abstract concept processing. The left parietal lobe is predominant in generating mental images, and activation in parietal and occipital lobes has been attributed to different mental imagery tasks [Kosslyn et al., 2001; Sack et al., 2005]. Specifically, the precuneus has been associated with memorizing verbally described scenes which requires mental image generation [Mellet et al., 2000]. The comparison of concrete concepts to abstract concepts also elicited activity in the left supramarginal gyrus and posterior cingulate. The posterior cingulate has been associated with mental imagery processes [Johnson et al., 2006; Kilts et al., 2004]. This region has also been linked with episodic and visuospatial memory function [Aggleton and Pearce, 2001; Epstein et al., 2007; Gainotti et al., 1998; Rudge and Warrington, 1991; Valenstein et al., 1987; Vincent et al., 2006], possibly because mental imagery plays a role in those processes. However, others have suggested that the bilateral posterior cingulate is semantically more engaged in abstract information processing [Pexman et al., 2007; Tettamanti et al., 2005]. This opposite effect may be due to the deactivation of this region in response to concrete concepts [Ghio and Tettamanti, in press].
The left fusiform gyrus and culmen have been implicated in the mental generation of visual features of objects [D'Esposito et al., 1997; Ganis et al., 2004; Mestres‐Missé et al., 2008]. The left fusiform and parahippocampal gyrus have been found to contribute to the processing of visual, imageable spatial property knowledge during explicit semantic tasks [Sabsevitz et al., 2005; Wallentin et al., 2005]. Although the increased activation of the left fusiform gyrus for concrete concepts might be confounded by task differences rather than the concreteness difference in some studies [D'Esposito et al., 1997; Mellet et al., 1998], this left fusiform activation effect was also found in the concrete > abstract comparison by using a precisely controlled semantic similarity task [Sabsevitz et al., 2005]. Sabsevitz et al. [2005] attributed the absence of fusiform activation in some previous studies to the shallow processing demand of the task, such as lexical decision. Moreover, the fusiform gyrus was found to be more active in the learning of new concrete words than abstract words [Mestres‐Missé et al., 2008]. According to Mestres‐Missé et al. [2008], this was in agreement with the dual‐coding theory, which predicts that the regions within the pathway of object properties processing would be activated because of the imagery system activation. Further, they suggested that contrary to the prediction of context availability theory, this left fusiform activation was less likely to be ascribed to a contextual difference between concrete and abstract concepts, because novel concrete and abstract words were both presented in sentences with a similar degree of supportive context. In addition, Mestres‐Missé et al. [2008] suggested that anterior ventral left fusiform activation might reflect the activation of several competing alternatives associated with the target concrete word in looking for the matching concept, which is in line with the hypothesis that concrete words have a better‐organized, categorical structure, thus allowing the sharing of features with semantically overlapping concepts [Crutch and Warrington, 2005].
Concrete concepts are more easily visualized than abstract concepts; they are connected with highly visual specific items, whereas abstract concepts may only be visualizable through the use of loosely‐connected symbols. Mental imagery has been linked with activation of perceptual systems [Kosslyn et al., 2001]. For example, generation of mental images results in activation similar to the perception of specific objects, such as faces [O'Craven and Kanwisher, 2000]. These findings suggest that mental imagery is a key component in processing of concrete compared to abstract concepts. Taken together, results from both contrasts suggest neural representation of abstract concepts relies more heavily on the verbal system, whereas concrete concept representation involves more mental imagery than abstract and relies more heavily on the perceptual system.
Potential Factors Influencing Results Discrepancies Among Studies
The meta‐analysis results revealed the consistent difference between the neural representation of abstract and concrete concepts across studies. However, not all studies used in meta‐analysis reported activations in regions identified by the meta‐analysis. For instance, the most consistently reported region, the left IFG in abstract > concrete comparison, was found only in 10 of the 19 studies. On the other hand, some other brain regions that were reported by several studies were not identified by the meta‐analysis results. Some of these regions were in close proximity to the consistently activated regions or were right hemisphere homologues, such as the inferior temporal gyrus [Mellet et al., 1998; Sabsevitz et al., 2005; Tettamanti et al., 2008], or the right MTG for abstract > concrete comparison [Mellet et al., 1998; Pexman et al., 2007; Wallentin et al., 2005]. Additional regions were not identified by the meta‐analysis, including the superior frontal gyrus for the abstract > concrete comparison [D'Esposito et al., 1997; Pexman et al., 2007; Sabsevitz et al., 2005; Wallentin et al., 2005], or the precentral gyrus for the concrete > abstract comparison [Mellet et al., 1998; Sabsevitz et al., 2005; Wallentin et al., 2005]. The effects of task and stimuli may have contributed to the discrepancies in results. We discuss each of them later.
Effects of task
One might argue that the type of task used to elicit semantic processing can affect neural activation. For example, the relation of the left IFG activation to working memory [Binder et al., 2005; Fiebach and Friederici, 2004; Sabsevitz et al., 2005] makes it reasonable to assume a moderation effect of task load on this region. To determine the effect of task on the representation of concepts, we conducted additional meta‐analyses examining the aforementioned contrasts according to task type. We divided tasks into superficial (passive listening and lexical decision on words and pseudowords) or deep processing (semantic categorization, semantic judgment, and semantic similarity) categories. Several studies using tasks, such as recognition, were not included in this additional analysis because they did not fit our selection criterion as either superficial or deep processing tasks. However, the numbers of studies in the two groups were too small (7 and 8, respectively, of 19 studies) to detect consistent effects of task across the included studies.
Effects of stimuli
The materials were similar across studies, so the form of the stimuli was unlikely to have an effect on our results. The stimuli used in individual studies were single words (real and pseudo), word groups, or sentences. Sixteen studies used words, while three used whole sentences as stimuli. Most studies presented the stimuli visually; also six studies presented auditory stimuli, some of which used both presentation modalities (Table I).
The effects of organizations of semantic categories in the brain have long been discussed [Bookheimer, 2002; Caramazza and Shelton, 1998; Hillis and Caramazza, 1991]. The semantic categories selected to represent abstract or concrete concepts varied among studies. Most studies had one general abstract and one general concrete concept category, whereas some others used more specific subcategories. For example, Noppeney and Price [2004] used one semantic category for abstract concepts and three (sound, visual, or hand motion) for concrete words; Harris et al. [2006] used one category for concrete and two (metaphysical or mental state) for abstract words. Regions identified by the current meta‐analysis, such as the fusiform gyrus, have been associated with object recognition and naming [Bookheimer et al., 1995]. The evidence raises the question of whether the representational differences between abstract and concrete concepts are content specific, in which case the change of specific word category would change the patterns of representational difference [Martin and Chao, 2001]. This question could be tested by including diverse subcategories in both abstract and concrete conditions.
Stimuli characteristics such as the word frequency, length, familiarity, and phonological or orthographic match between abstract and concrete words can also be critical. A lower average word frequency of abstract words might activate additional regions not associated with semantic differences. In some studies the stimuli were not balanced on these factors, possibly due to the difficulty in finding semantically suitable words. As a consequence, the differences in activation may be attributed to missing controls. One example is the debate concerning the role of the superior temporal gyrus. The left superior temporal gyrus was consistently activated across studies for abstract > concrete concepts, but not all studies reporting this region controlled for phonological factors. In fact, it recently has been argued that the superior temporal gyrus may be engaged in phonological processing [e.g., Bradley and Mark, 2008; Graves et al., 2008] rather than semantic comprehension [Demonet et al., 1992; Hillis et al., 2001; Wise et al., 1991].
Meta‐Analysis Methodology
Meta‐analysis combines data from multiple studies, resulting in a large total number of participants. The total number of participants included in the current analysis was 303, a number far beyond what is feasible within a typical neuroimaging study. MKDA was selected because it has several advantages suited to the investigation of representational differences for abstract and concrete concepts. It allowed weighting studies by the sample size and analysis type, and the results of MKDA provided an intuitive interpretation, representing the proportion of studies activating within the chosen radius of a voxel [Kober et al., 2008].
Despite its clear advantages, meta‐analysis has some inherent limitations. Because the meta‐analysis was based on spatial coordinates from neuroimaging data, it was limited to PET and fMRI studies, and excluded EEG/ERP studies despite the large body of literature in that field. In addition, coordinate‐based meta‐analysis methods such as MKDA incorporate information only from published coordinates. Thus, these methods do not account for different within‐study variability and cannot model random variation across studies [Salimi‐Khorshidi et al., 2009].
SUMMARY
We have identified meaningful and consistent differences in the neural representation of abstract and concrete concepts by using meta‐analysis to combine data from 303 participants across 19 published studies. Abstract concepts elicit greater activity in the inferior frontal gyrus and middle temporal gyrus compared to concrete concepts, while concrete concepts elicit greater activity in the posterior cingulate, precuneus, fusiform gyrus, and parahippocampal gyrus compared to abstract concepts. These results suggest greater engagement of the verbal system for processing of abstract concepts and greater engagement of the perceptual system for processing of concrete concepts, likely via mental imagery.
REFERENCES
- Aggleton JP, Pearce JM ( 2001): Neural systems underlying episodic memory: insights from animal research. Philos Trans R Soc Lond Ser B: Biol Sci 356: 1467–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsalou LW ( 1999): Perceptual symbol systems. Behav Brain Sci 22: 577–660. [DOI] [PubMed] [Google Scholar]
- Barsalou LW ( 2003): Abstraction in perceptual symbol systems. Philos Trans R Soc Lond: Biol Sci 358: 1177–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsalou LW, Simmons WK, Barbey AK, Wilson CD ( 2003): Grounding conceptual knowledge in modality‐specific systems. Trends Cogn Sci 7: 84–91. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL ( 2009): Where is the semantic system? A critical review and meta‐analysis of 120 functional neuroimaging studies. Cereb Cortex bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Westbury CF, McKiernan KA, Possing ET, Medler DA ( 2005): Distinct brain systems for processing concrete and abstract concepts. J Cogn Neurosci 17: 905–917. [DOI] [PubMed] [Google Scholar]
- Bookheimer S ( 2002): Functional MRI of language: New approaches to understanding the cortical organization of semantic processing. Ann Rev Neurosci 25: 151–188. [DOI] [PubMed] [Google Scholar]
- Bookheimer S, Zeffiro T, Blaxton T, Gaillard W, Theodore W ( 1995): Regional cerebral blood flow during object naming and word reading. Hum Brain Mapp 3: 93–106. [Google Scholar]
- Borst G, Kosslyn SM ( 2008): Visual mental imagery and visual perception: Structural equivalence revealed by scanning processes. Mem Cogn 36: 849–862. [DOI] [PubMed] [Google Scholar]
- Bradley RB, Mark DE ( 2008): The search for the phonological store: from loop to convolution. J Cogn Neurosci 20: 762–778. [DOI] [PubMed] [Google Scholar]
- Breedin SD, Saffran EM, Coslett HB ( 1994): Reversal of the concreteness effect in a patient with semantic dementia. Cogn Neuropsychol 11: 617–660. [Google Scholar]
- Brewer WF, Pani JR ( 1996): Reports of mental imagery in retrieval from long‐term memory. Consciousness Cogn 5: 265–287. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Berndt RS, Basili AG, Koller JJ ( 1981): Syntactic processing deficits in aphasia. Cortex 17: 333–348. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Shelton JR ( 1998): Domain‐specific knowledge systems in the brain: The animate‐inanimate distinction. J Cogn Neurosci 10: 1–34. [DOI] [PubMed] [Google Scholar]
- Crutch SJ, Warrington EK ( 2005): Abstract and concrete concepts have structurally different representational frameworks. Brain 128: 615–627. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Detre JA, Aguirre GK, Stallcup M, Alsop D, Tippet LJ, Farah MJ ( 1997): A functional MRI study of mental image generation. Neuropsychologia 35: 725–730. [DOI] [PubMed] [Google Scholar]
- Demb JB, Desmond JE, Wagner AD, Vaidya CJ, Glover GH, Gabrieli JD ( 1995): Semantic encoding and retrieval in the left inferior prefrontal cortex: A functional MRI study of task difficulty and process specificity. J Neurosci 15: 5870–5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demonet J‐F, Chollet F, Ramsay S, Cardebat D, Nespoulous J‐L, Wise R, Rascol A, Frackowiak R ( 1992): Anatomy of phonologic and semantic processing in normal subjects. Brain 115: 1753–1768. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Glover GH ( 2002): Estimating sample size in functional MRI (fMRI) neuroimaging studies: Statistical power analyses. J Neurosci Methods 118: 115–128. [DOI] [PubMed] [Google Scholar]
- Epstein RA, Higgins JS, Jablonski K, Feiler AM ( 2007): Visual scene processing in familiar and unfamiliar environments. J Neurophysiol 97: 3670–3683. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD ( 2007): Functional neuroimaging of anxiety: A meta‐analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 164: 1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eviatar Z, Menn L, Zaidel E ( 1990): Concreteness: Nouns, verbs, and hemispheres. Cortex 26: 611–624. [DOI] [PubMed] [Google Scholar]
- Farah MJ ( 1995): The neural bases of mental imagery In: Gazzaniga MS, editor. The Cognitive Neurosciences. Cambridge, MA: The MIT Press; pp 963–975. [Google Scholar]
- Fiebach CJ, Friederici AD ( 2004): Processing concrete words: fMRI evidence against a specific right‐hemisphere involvement. Neuropsychologia 42: 62–70. [DOI] [PubMed] [Google Scholar]
- Fliessbach K, Weis S, Klaver P, Elger CE, Weber B ( 2006): The effect of word concreteness on recognition memory. Neuroimage 32: 1413–1421. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Opitz B, von Cramon DY ( 2000): Segregating semantic and syntactic aspects of processing in the human brain: An fMRI investigation of different word types. Cereb Cortex 10: 698–705. [DOI] [PubMed] [Google Scholar]
- Gainotti G, Almonti S, Betta AMD, Silveri MC ( 1998): Retrograde amnesia in a patient with retrosplenial tumour. Neurocase: The Neural Basis of Cognition 4: 519–526. [Google Scholar]
- Ganis G, Thompson WL, Kosslyn SM ( 2004): Brain areas underlying visual mental imagery and visual perception: An fMRI study. Cogn Brain Res 20: 226–241. [DOI] [PubMed] [Google Scholar]
- Ghio M, Tettamanti M: Semantic domain‐specific functional integration for action‐related vs. abstract concepts. Brain Lang (in press). [DOI] [PubMed] [Google Scholar]
- Goldberg RF, Perfetti CA, Fiez JA, Schneider W ( 2007): Selective retrieval of abstract semantic knowledge in left prefrontal cortex. J Neurosci 27: 3790–3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodglass H, Hyde MR, Blumstein S ( 1969): Frequency, picturability and availability of nouns in aphasia. Cortex 5: 104–119. [DOI] [PubMed] [Google Scholar]
- Graves WW, Grabowski TJ, Mehta S, Gupta P ( 2008): The left posterior superior temporal gyrus participates specifically in accessing lexical phonology. J Cogn Neurosci 20: 1698–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, Koenig P, DeVita C, Glosser G, Alsop D, Detre J ( 2002): The neural basis for category‐specific knowledge: An fMRI study. Neuroimage 15: 936–948. [DOI] [PubMed] [Google Scholar]
- Halpern AR, Zatorre RJ ( 1999): When that tune runs through your head: A PET investigation of auditory imagery for familiar melodies. Cereb Cortex 9: 697–704. [DOI] [PubMed] [Google Scholar]
- Harris GJ, Chabris CF, Clark J, Urban T, Aharon I, Steele S, McGrath L, Condouris K, Tager‐Flusberg H ( 2006): Brain activation during semantic processing in autism spectrum disorders via functional magnetic resonance imaging. Brain Cogn 61: 54–68. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Caramazza A ( 1991): Category‐specific naming and comprehension impairment: A double dissociation. Brain 114: 2081–2094. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Wityk RJ, Tuffiash E, Beauchamp NJ, Jacobs MA, Barker PB, Selnes OA ( 2001): Hypoperfusion of Wernicke's area predicts severity of semantic deficit in acute stroke. Ann Neurol 50: 561–566. [DOI] [PubMed] [Google Scholar]
- Jeannerod M, Decety J ( 1995): Mental motor imagery: A window into the representational stages of action. Curr Opin Neurobiol 5: 727–732. [DOI] [PubMed] [Google Scholar]
- Jessen F, Heun R, Erb M, Granath D, Klose U, Papassotiropoulos A ( 2000): The concreteness effect: Evidence for dual‐coding and context availability. Brain Lang 74: 103–112. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Raye CL, Mitchell KJ, Touryan SR, Greene EJ, Nolen‐Hoeksema S ( 2006): Dissociating medial frontal and posterior cingulate activity during self‐reflection. Social Cogn Affective Neurosci 1: 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl KA, Liddle PF, Smith AM, Mendrek A, Forster BB, Hare RD ( 1999): Neural pathways involved in the processing of concrete and abstract words. Hum Brain Mapp 7: 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilts CD, Gross RE, Ely TD, Drexler KPG ( 2004): The neural correlates of cue‐induced craving in cocaine‐dependent women. Am J Psychiatry 161: 233–241. [DOI] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss‐Moreau E, Lindquist K, Wager TD ( 2008): Functional grouping and cortical–subcortical interactions in emotion: A meta‐analysis of neuroimaging studies. Neuroimage 42: 998–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosslyn SM, Ganis G, Thompson WL ( 2001): Neural foundations of imagery. Nat Rev Neurosci 2: 635–642. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Thompson WL, Klm IJ, Alpert NM ( 1995): Topographical representations of mental images in primary visual cortex. Nature 378: 496–498. [DOI] [PubMed] [Google Scholar]
- Kroll JF, Merves JS ( 1986): Lexical access for concrete and abstract words. J Exp Psychol: Learn Mem Cogn 12: 92–107. [Google Scholar]
- Kuperberg GR, West WC, Lakshmanan BM, Goff D ( 2008): Functional magnetic resonance imaging reveals neuroanatomical dissociations during semantic integration in schizophrenia. Biol Psychiatry 64: 407–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschark M, Richman CL, Yuille JC, Hunt RR ( 1987): The role of imagery in memory: On shared and distinctive information. Psychol Bull 102: 28–41. [PubMed] [Google Scholar]
- Martin A, Chao LL ( 2001): Semantic memory and the brain: structure and processes. Curr Opin Neurobiol 11: 194–201. [DOI] [PubMed] [Google Scholar]
- Mellet E, Tzourio‐Mazoyer N, Bricogne S, Mazoyer B, Kosslyn SM, Denis M ( 2000): Functional anatomy of high‐resolution visual mental imagery. J Cogn Neurosci 12: 98–109. [DOI] [PubMed] [Google Scholar]
- Mellet E, Tzourio N, Denis M, Mazoyer B ( 1998): Cortical anatomy of mental imagery of concrete nouns based on their dictionary definition. NeuroReport 9: 803–808. [DOI] [PubMed] [Google Scholar]
- Mervis CB, John AE ( 2008): Vocabulary abilities of children with Williams syndrome: Strengths, weaknesses, and relation to visuospatial construction ability. J Speech Lang Hear Res 51: 967–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestres‐Missé A, Münte TF, Rodriguez‐Fornells A ( 2008): Functional neuroanatomy of contextual acquisition of concrete and abstract words. J Cogn Neurosci 20: 2153–2166. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Price CJ ( 2004): Retrieval of abstract semantics. Neuroimage 22: 164–170. [DOI] [PubMed] [Google Scholar]
- O'Craven KM, Kanwisher N ( 2000): Mental imagery of faces and places activates corresponding stiimulus‐specific brain regions. J Cogn Neurosci 12: 1013–1023. [DOI] [PubMed] [Google Scholar]
- Paivio A ( 1969): Mental imagery in associative learning and memory. Psychol Rev 76: 241–263. [Google Scholar]
- Paivio A ( 1991): Dual coding theory: Retrospect and current status. Can J Psychol 45: 255–287. [Google Scholar]
- Perani D, Cappa SF, Schnur T, Tettamanti M, Collina S, Rosa MM ( 1999): The neural correlates of verb and noun processing: An PET study. Brain 122: 2337–2344. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME ( 1988): Positron emission tomographic studies of the cortical anatomy of single‐word processing. Nature 331: 585–589. [DOI] [PubMed] [Google Scholar]
- Pexman PM, Hargreaves IS, Edwards JD, Henry LC, Goodyear BG ( 2007): Neural correlates of concreteness in semantic categorization. J Cogn Neurosci 19: 1407–1419. [DOI] [PubMed] [Google Scholar]
- Reilly J, Grossman M, McCawley G ( 2006): Concreteness effects in lexical processing of semantic dementia. Brain Lang 99: 147–148. [Google Scholar]
- Rudge P, Warrington EK ( 1991): Selective impairment of memory and visual perception in splenial tumours. Brain 114: 349–360. [DOI] [PubMed] [Google Scholar]
- Sabsevitz DS, Medler DA, Seidenberg M, Binder JR ( 2005): Modulation of the semantic system by word imageability. Neuroimage 27: 188–200. [DOI] [PubMed] [Google Scholar]
- Sack AT, Camprodon JA, Pascual‐Leone A, Goebe R ( 2005): The dynamics of interhemispheric compensatory processes in mental imagery. Science 308: 702–704. [DOI] [PubMed] [Google Scholar]
- Salimi‐Khorshidi G, Smith SM, Keltner JR, Wager TD, Nichols TE ( 2009): Meta‐analysis of neuroimaging data: A comparison of image‐based and coordinate‐based pooling of studies. Neuroimage 45: 810–823. [DOI] [PubMed] [Google Scholar]
- Schwanenflugel PJ ( 1991): Why are abstract concepts hard to understand? In: Schwanenflugel PJ, editor. The Psychology of Word Meanings. Hillsdale, NJ: Erlbaum; pp 223–250. [Google Scholar]
- Schwanenflugel PJ, Harnishfeger KK, Stowe RW ( 1988): Context availability and lexical decisions for abstract and concrete words. J Mem Lang 27: 499–520. [Google Scholar]
- Shallice T, Warrington EK ( 1975): Word recognition in a phonemic dyslexic patient. Q J Exp Psychol 27: 187–199. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P ( 1988): Co‐planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical. [Google Scholar]
- Tettamanti M, Buccino G, Saccuman MC, Gallese V, Danna M, Scifo P, Fazio F, Rizzolatti G, Cappa SF, Perani D ( 2005): Listening to action‐related sentences activates fronto‐parietal motor circuits. J Cogn Neurosci 17: 273–281. [DOI] [PubMed] [Google Scholar]
- Tettamanti M, Manenti R, Rosa PAD, Falini A, Perani D, Cappa SF, Moro A ( 2008): Negation in the brain: Modulating action representations. Neuroimage 43: 358–367. [DOI] [PubMed] [Google Scholar]
- Thirion B, Pinel P, Mériaux S, Roche A, Dehaene S, Poline J‐B ( 2007): Analysis of a large fMRI cohort: Statistical and methodological issues for group analyses. Neuroimage 35: 105–120. [DOI] [PubMed] [Google Scholar]
- Thomas NJT ( 2008): Mental imagery In: Zalta EN, editor. The Stanford Encyclopedia of Philosophy. http://plato.stanford.edu/archives/win2008/entries/mental-imagery/. [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA ( 2002): Meta‐analysis of the functional neuroanatomy of single‐word reading: Method and validation. Neuroimage 16: 765–780. [DOI] [PubMed] [Google Scholar]
- Valenstein E, Bowers D, Verfaellie M, Heilman KM, Day A, Watson RT ( 1987): Retrosplenial amnesia. Brain 110: 1631–1646. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, Buckner RL ( 2006): Coherent spontaneous activity identifies a hippocampal‐parietal memory network. J Neurophysiol 96: 3517–3531. [DOI] [PubMed] [Google Scholar]
- Wager TD, Lindquist M, Kaplan L ( 2007): Meta‐analysis of functional neuroimaging data: Current and future directions. SCAN 2: 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Lindquist MA, Nichols TE, Kober H, van Snellenberg JX ( 2009): Evaluating the consistency and specificity of neuroimaging data using meta‐analysis. Neuroimage 45: S210–S221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallentin M, Østergaard S, Lund TE, Østergaard L, Roepstorff A ( 2005): Concrete spatial language: See what I mean? Brain Lang 92: 221–233. [DOI] [PubMed] [Google Scholar]
- Warrington EK ( 1975): The selective impairment of semantic memory. Q J Exp Psychol 27: 635–657. [DOI] [PubMed] [Google Scholar]
- Whatmough C, Verret L, Fung D, Chertkow H ( 2004): Common and contrasting areas of activation for abstract and concrete concepts: an HO PET study. J Cogn Neurosci 16: 1211–1226. [DOI] [PubMed] [Google Scholar]
- Wise R, Chollet F, Hadar URI, Friston K, Hoffner E, Frackowiak R ( 1991): Distribution of cortical neural networks involved in word comprehension and word retrieval. Brain 114: 1803–1817. [DOI] [PubMed] [Google Scholar]
- Wise RJS, Howard D, Mummery CJ, Fletcher P, Leff A, Büchel C ( 2000): Noun imageability and the temporal lobes. Neuropsychologia 38: 985–994. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Evans AC, Meyer E, Gjedde A ( 1992): Lateralization of phonetic and pitch discrimination in speech processing. Science 256: 846–849. [DOI] [PubMed] [Google Scholar]


