Abstract
Our study investigates the dependence of response monitoring and error detection on genetic influences modulating the serotonergic system. This was done using the event‐related potentials (ERPs) after error (Ne/ERN) and correct trials (Nc/CRN). To induce a sufficient amount of errors, a standard flanker task was used. The subjects (N = 94) were genotyped for the functional 5‐HT1A C(−1019)G polymorphism. The results show that the 5‐HT1A C(−1019)G polymorphism specifically modulates error detection. Neurophysiological modulations on error detection were paralleled by a similar modulation of response slowing after an error, reflecting the behavioral adaptation. The 5‐HT1A −1019 CC genotype group showed a larger Ne and stronger posterror slowing than the CG and GG genotype groups. More general processes of performance monitoring, as reflected in the Nc/CRN, were not affected. The finding that error‐specific processes, but not general response monitoring processes, are modulated by the 5‐HT1A C(−1019)G polymorphism is underlined by a wavelet analysis. In summary, the results suggest a specific effect of the 5‐HT1A C(−1019)G polymorphism on error monitoring, as reflected in the Ne, and suggest a neurobiological dissociation between processes of error monitoring and general response monitoring at the level of the serotonin 1A receptor system. Hum Brain Mapp, 2010. © 2009 Wiley‐Liss, Inc.
Keywords: executive function, error processing, 5‐HT1A polymorphism, event‐related potential, wavelet analysis
INTRODUCTION
Humans constantly evaluate their own actions, which is important for goal‐directed behavior. Achieving goals in changing environments require a set of flexible cognitive functions (Matsumoto and Tanaka, 2004). One of these functions is the ability to monitor the outcome of ones actions, that is, to evaluate if an error has occurred. In neurophysiological studies, these processes are assumed to be reflected by the “error negativity (Ne)” (Falkenstein et al., 1991) or “error‐related negativity (ERN)” (Gehring et al., 1993). A prominent recent theory of the Ne proposes that the basal ganglia dopaminergic (DA) system and the anterior cingulate cortex (ACC) interact producing the Ne. If an event is worse than expected (i.e., an error), the DA system sends an error signal to the ACC, which in turn elicits the Ne (Holroyd and Coles, 2002; Vidal et al., 2000). In accordance with this theory, the Ne is reduced in Parkinson's disease (e.g. Willemssen et al., 2008, 2009) and Huntington's disease (Beste et al., 2006, 2008, 2009), both revealing striatal DA dysfunction. Furthermore, studies on psychiatric conditions accompanied by changes in the DA system (e.g. schizophrenia) and pharmacological studies revealed a modulation of the Ne by dopamine (for review: Jocham and Ullsperger, 2009).
The DA system is crucially modulated by the serotonergic system within the prefrontal cortex (PFC) (for review: de Almeida et al., 2008). These interactions may form the neurobiological basis of the observed influence of the serotonergic system on error processing functions that has been shown in studies examining depression (e.g. Jocham and Ullsperger, 2008; Ruchsow et al., 2004, 2006). As an additional argument for serotonergic influences on the Ne, a modulation of the Ne by the serotonin transporter gene linked polymorphic region (5‐HTTLPR) has been shown (Fallgatter et al., 2004). Another serotonergic polymorphism, the functional serotonin 1 A receptor polymorphism (5‐HT1A C(−1019)G) (Huang et al., 2004) has recently attracted considerable interest in psychiatric research. The 5‐HT1A receptor polymorphism influences serotonergic neurotransmission (Albert and Lemonde, 2004). More precisely, the presence of a −1019 G allele is accompanied by a derepression of 5‐HT1A autoreceptor expression by disrupting an inhibitory transcription factor‐binding site. This leads to a reduced serotonergic neurotransmission (Lemonde et al., 2003). The −1019 G allele has been found to be associated with mood and anxiety disorders (Baune et al., 2008; Fakra et al., 2009; Freitag et al., 2006; Hettema et al., 2008; Rothe et al., 2004; Strobel et al., 2003; for review: Albert and Lemonde, 2004; Drago et al., 2008). Anxiety and error negativity are also intercorrelated. McDermott et al. (2009) have recently shown that response monitoring, as manifest in the error‐related negativity, moderates the association between behavioral inhibition in children and anxiety. Furthermore, the ERN may be considered as a marker for anxiety‐related traits (Hajcak and Simons, 2002; Olvet and Hajcak, 2008). So far, it is unclear if associations of the serotonin system and the error negativity with anxiety do share a common genetic basis. Therefore, we aimed to assess the relevance of the functional serotonin 1 A receptor polymorphism for error‐monitoring functions.
Moreover, it has recently been shown that the Ne reflects two distinct subprocesses; a “cognitive” and a “motor” subprocess (Yordanova et al., 2004). A subcomponent from the delta frequency band (1.5–3.5 Hz) was related to error‐specific monitoring at the cognitive level, and a second subcomponent from the theta frequency band (4–8 Hz) was associated with general response monitoring, irrespective if there was an error or not (Yordanova et al., 2004). Yordanova et al. (2004) state that such general response monitoring processes are reflected in the Nc/CRN, observed after a response on correct trials. It has been shown that especially the error‐specific “cognitive” subprocess is likely influenced by factors modulating DA activity (Beste et al., 2007). Based upon the interaction of the DA and serotonergic system (de Almeida et al., 2008), it may be hypothesized that modulations depending on the 5‐HT1A polymorphism may selectively affect these “cognitive” subprocesses and spare more general response monitoring subprocesses occurring irrespective of the outcome of a trials (i.e., error or correct), which are evident in correct as well as error trials.
Related to error‐processing functions, the Pe is supposed to reflect late conscious error recognition (for review: Overbeek et al., 2005) and has a parietal maximum (usually at Pz). Less is known about the neurobiological mechanisms underlying this component. As such, this study further tries to elucidate, where processes reflected by Pe are influence by serotonergic neural transmission, too.
In summary, the study examines the relevance of the functional serotonin 1 A receptor 5‐HT1A C(−1019)G) polymorphism for error‐monitoring functions and a potential neurobiological dissociation between error‐specific cognitive processes and general response monitoring processes applying neurophysiological techniques.
METHODS
Subjects
A sample of N = 94 genetically unrelated subjects of Caucasian descent was recruited by newspaper announcements. The mean and standard deviation (SD) are given. The mean age of the subjects was 25.3 years (6.2). The sample consisted of 29 males and 65 females. Calculating a Kruskal–Wallis test (H‐test), it is shown that the sexes were comparably distributed across the different 5‐HT1A C(−1019)G genotype groups (χ2 = 0.003; df = 1; P > 0.9). The mean BDI score was 3.59 (3.12). A univariate ANOVA shows that the BDI scores did not differ between the genotype groups [F(2,91) = 1.46; P > 0.2] [CC: 2.52 (2.46); CG: 3.80 (2.71); GG: 3.43 (3.27)]. As the 5‐HT1A C(−1019)G polymorphism is also associated with anxiety (e.g. Hettema et al., 2008), anxiety sensitivity (ASI) was also examined. The mean ASI score was 19.57 (11.38). A univariate ANOVA revealed a statistically significant difference between genotype groups (CC: 15.3 ± 10.7, CG: 22.5 ± 9.8, GG: 18.9 ± 12.9) originating from a higher ASI score in the CG compared to the CC genotype group [F(2,91) = 3.15; P = 0.045]. The other genotype groups did not differ from each other (all P > 0.4). Hardy–Weinberg equilibrium was examined using the program Finetti provided as an online source (http://ihg.gsf.de/cgi-bin/hw/hwa1.pl; Wienker TF and Strom TM). The distribution of 5‐HT1A C(−1019)G genotypes did not significantly differ from the expected numbers calculated on the basis of observed allele frequencies according to Hardy–Weinberg equilibrium (CC = 23, CG = 41, GG = 30; P = 0.298).
Volunteers were paid 8 euros per hour as compensation. The study was approved by decision of the ethics committee of the University of Münster. All subjects gave informed consent.
Genotyping
Genomic DNA was extracted from a 10 ml EDTA venous blood sample with the Qiagen FlexiGene DNA kit (Qiagen, Hilden, Germany). The 5‐HT1A C(−1019)G (rs6295) polymorphism was genotyped by means of a polymerase chain reaction (PCR)‐based restriction fragment length polymorphism assay. Primers were designed to amplify a 296‐bp DNA fragment containing the forward primer 122‐F 5′‐AGTTTTGTTCTTCATTTCGAGAT‐3′ and reverse mutagenic primer 122‐R 5′‐GAAGAAGACCGAGTGTGTCTAC‐3′. The mutagenic primer was constructed to introduce an artificial polymorphic restriction site. By using a Biometra T‐Gradient thermocycler (Whatman, Göttingen, Germany), standard PCR was carried out in a total volume of 20 μl containing 60 ng of genomic DNA, 1× PCR buffer, 8 pmol of each primer, 8 mM dNTPs, and 0.4 U of Taq polymerase (5′, Hamburg, Germany). After an initial step of denaturation at 94°C for 5 min, 35 cycles were carried out consisting of 94°C for 30 s, 54°C (annealing temperature) for 30 s, 72°C for 60 s, and a final extension step of 10 min at 72°C. Subsequent digestion overnight for 16 h at 65°C of an 8‐μl sample of the PCR product was accomplished with 3 U of TaiI (Fermentas, St. Leon‐Rot, Germany) in a total volume of 20 μl resulting in two patterns of fragments consisting of 203 + 57 + 36 bp for the G‐allele and 183 + 57 + 36 + 20 bp for the C‐allele. Digestion products were visualized by silver staining after separation on a 15% polyacrylamide gel in 1× TBE buffer (Tris–borate, EDTA) at 220 V for 3 h. Genotypes were determined independently by two investigators.
Experimental Paradigm
To measure error‐processing, we used a Flanker Task (Kopp et al., 1996), which reliably yields a high percentage of errors. In the Flanker task, vertically arranged visual stimuli were presented on a PC monitor. The target‐stimulus (white arrowhead or circle) was presented in the center of a black background with the arrowhead pointing to the right or left. These target‐stimuli were flanked by two vertically adjacent arrowheads, which pointed in the same (compatible) or opposite (incompatible) direction of the target stimulus. The flankers preceded the target by 100 ms to maximize premature responding to the flankers, which would result in errors in the incompatible and Nogo condition. The target was displayed for 300 ms. The response‐stimulus interval was 1,600 ms. Flankers and target were switched off simultaneously. Time pressure was administered by asking the subjects to respond within 550 ms, which additionally enhances the likelihood of errors. In trials with reaction times (RTs) exceeding this deadline a feedback stimulus (1,000 Hz, 60 dB SPL) was given 1,200 ms after the response; this stimulus had to be avoided by the subjects. Four blocks of 105 stimuli, each were presented in this task. Compatible (60%) and incompatible stimuli (20%) and Nogo‐stimuli (circle) (20%) were presented randomly. The Nogo‐trials were not included into the analysis. The subjects had to react depending on the direction of the central arrowhead and to refrain from responding to circles. There was a response cut‐off criterion, which was set at RT < 150 ms for the lower bound and 900 ms for the upper bound. Trials falling out of this interval were discarded from analysis.
Data Processing
During the task, the EEG was recorded from 28 Ag‐AgCl electrodes (Fpz, Fp1, Fp2, Fz, F3, F4, F7, F8, FCz, FC3, FC4, FC5, FC6, Cz, C3, C4, C7, C8, Pz, P3, P4, P7, P8, Oz, O1, O2, left mastoid—M1, right mastoid—M2) against a reference electrode located on Cz. Additionally, eye movements were monitored and recorded by means of two lateral and four vertical EOG electrodes. The sampling rate of all recordings was 500 samples/s, applying a filter bandwidth of 0.05–80 Hz to the EEG. Electrode impedances were kept below 5 kΩ. EEG was rereferenced off‐line to linked mastoids. Artifact rejection procedures were applied twice: automatically, with an amplitude threshold of ±80 μV, and visually by rejecting all trials contaminated by technical artifacts. Horizontal and vertical eye movements preserved in the accepted trials were corrected by means of a linear regression method for EOG correction (Gratton et al., 1983). Ne was clearly visible after response‐related averaging, indicating the presence of phase‐locked components in single sweeps. Therefore, analyses were performed for the averaged response‐triggered potentials to extract the time‐frequency components that were most stable and time‐locked to the response.
Time Domain Analysis
Epochs for time‐domain analysis of the response‐related (RRP) and event‐related potential (ERP) had a length of 4096 ms, which was chosen to keep comparability to the subsequent wavelet analyses. The Ne was identified as the most negative peak of RRPs at FCz within 100 ms after error responses. A similar, but smaller component was seen in the RRPs of correct trials, called Nc (Yordanova et al., 2004). A preresponse baseline was set at −800 till −600 ms before button press, which, according to the RTs, represents a time window from the prestimulus period and is free of activity related to stimulus or response processing (see Beste et al., 2007; Yordanova et al., 2004). To achieve a reference‐free evaluation, all data analyses were performed after calculation of current source density (CSD) of the signals (Nunez et al., 1997; Perrin et al., 1989). The CSD transform replaces the potential at each electrode with the CSD, thus eliminating the reference potential. The algorithm applies the spherical Laplace operator to the potential distribution on the surface of the head. Because the potential distribution is only known for the electrodes used, the procedure of spherical spline interpolation is used to calculate the continuous potential distribution. The exact mathematical procedure is explained in detail in Perrin et al. (1989). The CSD transformation leads to a spatial enhancement of the data, which is advantageous for a more reliable peak detection as well as quantification of wavelet transformation results. The amplitudes of the Ne and Nc were measured against the preceding positivity (peak‐to‐peak amplitudes) (e.g. Beste et al., 2008). The amplitude of the Pe was quantified at electrode Pz (Beste et al., 2008), relative to baseline.
Time‐Frequency Decomposition
To achieve a correct evaluation of low‐frequency components, the epochs used for time‐frequency analysis had a length of 4,096 ms (response in the middle of the epoch) to allow a reliable wavelet decomposition of even low frequencies. To represent RRPs in the time‐frequency domain, RRPs were analyzed by means of a continuous wavelet transform (Samar et al., 1999). Time‐frequency representations were calculated by Morlet's wavelets as described previously (e.g. Jensen et al., 2002; Tallon‐Baudry et al., 1997). The analytical presentation of Morlet's wavelet w(t,f) is:
where t is time, f is frequency, 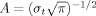 , σt is the wavelet duration, and i =
, σt is the wavelet duration, and i = 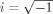 .
.
For time‐frequency plots, a ratio of f 0/σf = 5.5 was used, where f 0 is the central frequency and σf is the width of the Gaussian shape in the frequency domain. The choice of the ratio f 0/σf was oriented to the expected slower phase‐locked components present in the response‐related potentials, which had an effect on the shape of the Morlet's wavelet and decreased its decay (see e.g., Yordanova et al., 2004). The analysis was performed in the frequency range 0.5–20 Hz with a central frequency at 0.5‐Hz intervals. For different f 0, time and frequency resolutions can be calculated as 2σt and 2σf, respectively (Tallon‐Baudry et al., 1997). σt and σf are related by the equation σt = 1/(2πσf). For example, for f 0 = 1 Hz, 2σt = 1,770 ms, and 2σf = 0.36 Hz; for f 0 = 3 Hz, 2σt = 580 ms and 2σf = 1.09 Hz; for f 0 = 5 Hz, 2σt = 350 ms and 2σf = 1.82 Hz. To obtain the phase‐locked power, after time‐frequency decomposition of the averaged RRPs, amplitude values were squared and relevant time‐frequency (TF) components were extracted and analyzed. Their central frequencies were 1.5, 3, and 5 Hz, so that the corresponding TF components covered roughly the sub‐delta (0.5–2.5 Hz), delta (2.5–4 Hz), and theta (4–7 Hz) frequency bands. To focus specifically on Ne, magnitudes and latencies of the maximal phase‐locked power were measured within the time window 100–300 ms after response onset. This measurement was done at electrode revealing the strongest effect between correct and error responses in the time‐domain analysis. To normalize distributions, power values were log10‐transformed and then subjected to statistical analyses.
Statistical Analysis
Quantified data were analyzed using repeated measures ANOVAs. In general, the within factor was “correctness” (error, correct) and the between factor “group” (5‐HT1A CC, CG, GG genotype groups). The degrees of freedom were adjusted using the Greenhouse–Geisser correction when appropriate. In addition, separate univariate ANOVAs of the post hoc tests were calculated when necessary. For these analyses, Bonferroni corrections were applied. Tests of normal distribution using the Kolmogorov–Smirnov test revealed that each variable included to the ANOVAs was normal distributed (all z < 1.1; P > 0.2; one‐tailed). As a measure of variability, the standard error of the mean (SEM) together with the mean is given.
RESULTS
Behavioural Data
RTs on correct and error responses were analyzed using a repeated measures ANOVA with the within‐subject factor “correctness” (correct vs. error) and the between‐subject factor “group” (5‐HT1A CC, CG, GG genotype groups). The mean and SEM are given.
RTs were faster on error (325 ± 8) compared to correct trials (387 ± 9) [F(1,91) = 149.66; P < 0.001]. This effect was not different for genotype groups, as indicated by a nonsignificant interaction [F(2,91) = 1.68; P > 0.2]. The main effect “group” was also not significant [F(2,91) = 0.81; P > 0.4]. RTs of correct responses committed after an error (posterror‐RTs) are generally prolonged, which reflects the behavioral adaptation after an error (Rabbitt, 1966). To calculate this posterror slowing, we subjected the mean RT of correct responses in succession and those after an error (“sequence”) as within‐subject factor to a repeated measure ANOVA with “group” as between‐subject factor. RTs on correct response after an error were significantly longer (395 ± 8) [F(1,91) = 170.8; P < 0.001] than RTs on correct responses in succession (373 ± 10), indicating a slowing effect. This effect was different for genotype groups, as shown by the group by sequence interaction [F(2,91) = 25.9; P < 0.001]. Subsequently, we calculated a univariate ANOVA across the difference between RTs of correct responses after a correct response versus after an error. This difference varied across genotype groups [F(1,91) = 10.01; P < 0.001]. Bonferroni‐corrected post hoc tests revealed the difference was largest for the 5‐HT1A −1019 CC genotype group (33 ± 4), compared to GG (12 ± 3) and CG genotype groups (13 ± 4) (P < 0.001).
Error rates were analyzed using “trial type (compatible vs. incompatible)” as within‐subject factor and “group” as between‐subject factor. As expected, error rates were higher in the incompatible (19.4 ± 2.3), compared to the compatible condition (6.2 ± 1.2) [F(1,91) = 322.24; P < 0.001]. This effect did not differ between groups [interaction: F(2,91) = 0.70; P > 0.4] and also the mean error rate did not differ across groups [main effect group: F(2,91) = 0.2; P > 0.7].
As the ASI score differed between two of the examined functional genotype groups, we controlled for this possible confounder by examining the robustness of the above‐mentioned effects using the ASI score as covariate in ANCOVAs. These analyses showed that this score did not significantly modulate the pattern obtained in the above analyses (all F's < 1.2; P > 0.3). The results are further unbiased with respect to the BDI score as well as the unequal distribution of sexes did not differ the pattern of results (all F's < 0.6; P > 0.4).
NEUROPHYSIOLOGICAL DATA
Event‐Related Potentials
Response‐locked waveforms on error and correct trials are given in Figure 1.
Figure 1.
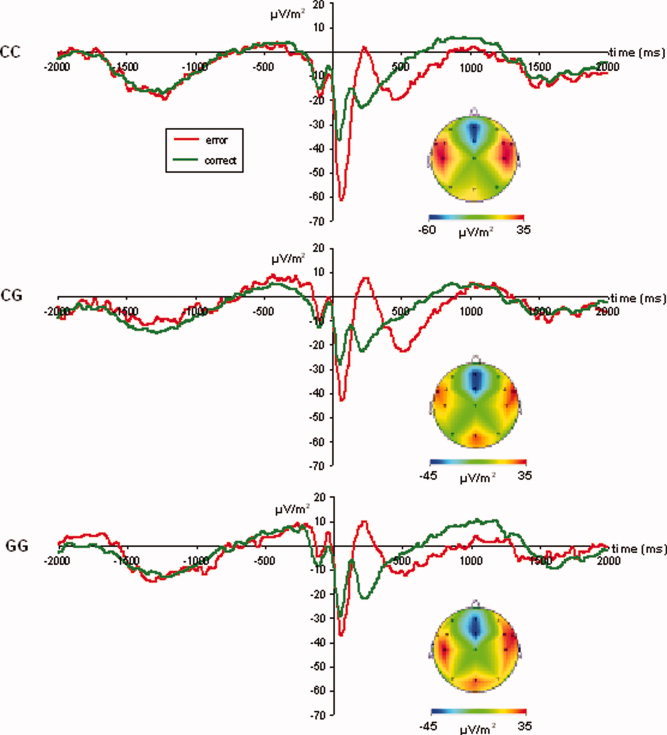
Response‐locked ERPs at electrode Fz. Green lines denote the ERP on correct trials, red lines denote the ERPs on error trials. The 5‐HT1A C(−1019)G genotype groups are plotted seperately. As can be seen, the Ne on was considerably larger in the CC genotype group than the other genotype groups. The Nc was comparably large in the different genotype groups. The CSD maps given for each genotype group denote a clear Ne‐topography, with negativities confined to electrodes Fz and FCz. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Response‐locked ERPs were analyzed using a repeated measures ANOVA with the within‐subject factors “electrode” and “error/correct” and the between‐subject factor “group.” Amplitudes were analyzed for electrodes Fz and FCz, as negativities on error trials were restricted to these electrodes (see map)1. The results showed that overall potentials (collapsed across error and correct trials) were stronger (i.e. more negative) at electrode Fz (−39.2 ± 0.7) than at electrode FCz (−34.8 ± 1.3) [F(1,91) = 11.42; P < 0.001]. Potentials were also more negative during error trials (−47.6 ± 1.2) than during correct trials (−26.3 ± 0.9) [F(1,91) = 240.75; P < 0.001].
Furthermore, the 5‐HT1A −1019 CC genotype group revealed the most negative potentials (−43.3 ± 1.8), differing from the CG (−34.0 ± 1.3) and GG genotype (−33.7 ± 1.5) groups (P < 0.001) [F(2,91) = 10.38; P < 0.001]. The latter groups did not differ from each other. Interestingly, there was a two‐way interaction “electrode × correctness × group” [F(2,91) = 9.47; P < 0.001]. Subsequent repeated measures ANOVAs for each electrode separately revealed that the interaction “correctness × group” was larger at electrode Fz [F(2,91) = 23.32; P < 0.001; η = 0.339] than at FCz [F(1,91) = 9.36; P < 0.001; η = 0.171].
Hence, only electrode Fz was analyzed further. The Ne differed between groups [F(2,91) = 40.79; P < 0.001], with the 5‐HT1A −1019 CC genotype group showing a larger Ne (−66.0 ± 2.1) than the other groups (CG: −42.5 ± 1.6; GG: −44.4 ± 1.9) (P < 0.001). The CG and GG genotype groups did not differ from each other (P > 0.4). The potential on correct trials (Nc) did not differ between groups [F(2,91) = 0.89; P > 0.4], showing that the observed effect is specific for error trials. As with the behavioral data, we examined a potential‐biasing effect of the ASI score for the ERPs in an ANCOVA, too. Also here, not statistically significant modulation of this factor was obtained (all F's < 0.9; P > 0.4). Similarly, the BDI score as well as the unequal distribution of sexes did not modify the pattern of results (all F's < 0.5; P > 0.5).
Concerning the Pe electrode, Pz was analyzed. For the Pe, the only main effect “error/correct” is obtained [F(1,91) = 325.2; P < 0.001]. It is shown that the Pe amplitude was larger at error trials (15.2 ± 3.3), compared to correct trials, where it turned negative (−5.2 ± 2.5). Neither the main effect group nor any interactions with this factor reached significance (all F's < 0.5; P > 0.5).
Time‐Frequency Decomposition
The time‐frequency plots of the Ne are given in Figure 2. As effects were maximal at electrode Fz (see above), only the phase‐locked wavelet power at this electrode was quantified and analyzed. For this analysis, the maximal log‐transformed power values (Beste et al., 2007) of sub‐delta, delta, and theta Ne components were analyzed.
Figure 2.
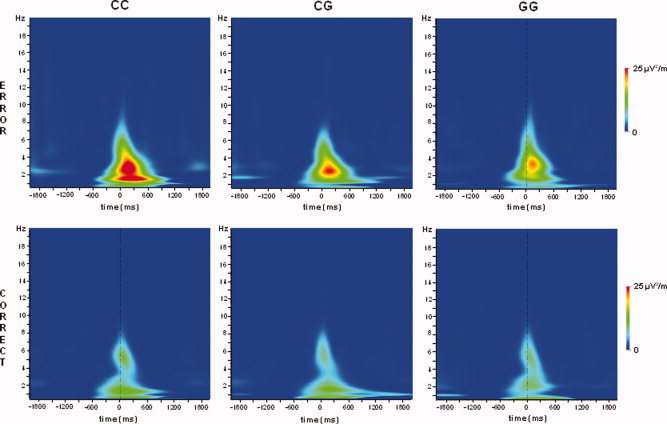
Time‐frequency plots on error (top row) and correct trials (bottom row), separated for the 5‐HT1A C(−1019)G genotype groups. The abscissa denotes the time scale in ms; time point 0 denotes the response. The ordinate denotes the frequency from 1 to 19 Hz. The power at each time point within the frequency range is color‐coded. As can be seen, the wavelet power is similar for correct responses. On error responses, the wavelet power is higher is increased in all genotype groups, relative to correct responses, but considerably more in the CC genotype group. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Phase‐locked power in the sub‐delta, delta, and theta frequency band were used as within‐subject factors, “group” was used as between‐subject factor in a repeated measures ANOVA. Interestingly, there was an interaction “error/correct × frequency × group” [F(4,182) = 7.80; P < 0.001; η = 0.146]. Subsequent repeated measures ANOVAs showed that an interaction “frequency × group” was evident for error trials [F(4,182) = 6.80; P < 0.001; η = 0.130], but not for correct trials [F(4,182) = 1.1; P > 0.2; η = 0.03]. For the error trials, subsequent univariate ANOVAs showed that the wavelet power differed between groups in the sub‐delta [F(2,91) = 29.66; P < 0.001; η = 0.395] and delta frequency band [F(2,91) = 30.90; P < 0.001; η = 0.404], but not in the theta frequency band [F(2,91) = 1.22; P > 0.2; η = 0.026]. For the sub‐delta, band power was highest for the CC genotype group (4.2 ± 0.03) and lower for the CG (3.9 ± 0.02) and GG allel groups (3.9 ± 0.02). The latter did not differ from each other (P > 0.9). For the delta frequency band, a similar pattern is shown. Here, power was highest for the CC genotype group (4.36 ± 0.02) (P < 0.001) and comparably low for the CG (4.12 ± 0.01) and GG genotype group (4.13 ± 0.02). In all, the FT analysis revealed a circumscribed higher power in error‐specific frequency bands (Yordanova et al., 2004). The error specificity of the sub‐delta and delta frequency band, opposed to the theta frequency band is underlined by the interaction “error/correct × frequency band” [F(2,182) = 50.65; P < 0.001]. This interactions show that differences between error and correct trials were only evident in the sub‐delta (error: 4.02 ± 0.02 correct: 3.68 ± 0.03; P < 0.001) and delta frequency band (error: 4.21 ± 0.02 correct: 3.40 ± 0.04; P < 0.001), but not in the theta frequency band (error: 3.85 ± 0.2 correct: 3.66 ± 0.2; P > 0.3).
As with the ERPs, the results reported by the above analysis of the TF‐data are also unbiased by the ASI score, as revealed by ANCOVAs showing no significant influence of this factor (all F's < 1.0; P > 0.3). As with the behavioral and time‐domain data, the results are further unbiased with respect to the possible influence of the BDI score and the factor sex (all F's < 0.7; P > 0.3).
DISCUSSION
In this current study, we examined the effects of the functional serotonin 1A receptor polymorphism (5‐HT1A C(−1019)G) on error processing, applying neurophysiological methods. We were particularly interested if this polymorphism modulates performance monitoring in general or if this polymorphism specifically modulates cognitive process related to error detection. Error specific and general response monitoring subprocesses were differentiated using a wavelet analysis (Yordanova et al., 2004).
We could show that the 5‐HT1A genotype groups differ with respect to their degree in posterror slowing, with the CC genotype showing the strongest slowing. Error rates were not different between the groups, suggesting that the results are unbiased by the frequency of errors or by a speed‐accuracy trade‐off. The posterror slowing effect is also reflected in the neurophysiological data. The Ne was strongest for the 5‐HT1A CC genotype. Both CG and GG genotype groups revealed a smaller Ne of equal size. Negativities occurring after correct responses (i.e. the Nc) were not influenced by the 5‐HT1A polymorphism. This is supported by the wavelet analysis, which also showed no differences in the phase‐locked power of the Nc between genotype groups. In contrast, the wavelet analysis across error‐trials revealed differences between the genotype groups, but these were restricted to the δ‐frequency band, supposed to specifically reflect cognitive subprocesses of error monitoring (Yordanova et al., 2004). Thus, the 5‐HT1A polymorphism seems to selectively modulate error‐specific subcomponents of performance monitoring, but not general response‐monitoring processes. The group with the strongest Ne also showed the strongest slowing, which nicely corroborates, with a complementary method, the findings of Debener et al. (2005) that the Ne plays a functional role in the adjustment of response strategies after an error. The results obtained are unbiased to possible influences of mood (BDI) and anxiety factors (ASI), as well as putative sex differences, as indicated by additional ANOVAs and ANCOVAs. It should be noted that the posterror slowing effect may be confounded by conflict adaptation. Yet, as trials types and error rates are equally distributed across groups, this may likely not affect group differences obtained. The Ne showed a quite frontal maximum (i.e. at Fz). In general, the Ne in easy choice reaction tasks, such as used here has a midline FCz maximum (e.g. Mathewson et al., 2005; Yordanova et al., 2004). Depending on the task, the Ne may be larger at Cz than at Fz (e.g. Boksem et al., 2006) or larger at Fz than at Cz (e.g. Hajcak et al., 2005; Ullsperger and von Cramon, 2001) as also found in this work. Precondition of a more frontal distribution may be flankerlike tasks (Ullsperger and von Cramon, 2001) and error correction (Fiehler et al., 2005), which also applies to our study.
The Pe did not differ between the genotype groups, suggesting that the serotonin 1 A receptor system less important for this function. Because of the parietal maximum, the Pe may be regarded to simply reflect an error‐related P3. For the P3, it has repeatedly been shown that this component does not depend on the serotonergic system (e.g. Oranje et al., 2008; Wienberg et al., 2009). The pattern observed for the Pe nicely fits to this.
The 5‐HT1A receptor polymorphism influences serotonergic neurotransmission (Albert and Lemonde, 2004). More precisely, the presence of a −1019 G allele is accompanied by a derepression of 5‐HT1A autoreceptor expression by disrupting an inhibitory transcription factor‐binding site. This leads to a reduced serotonergic neurotransmission (Lemonde et al., 2003). The −1019 G allele has been found to be associated with mood and anxiety disorders (Freitag et al., 2006; Rothe et al., 2004; Strobel et al., 2003; for review: Albert and Lemonde, 2004) and has been shown to modulate affective processing in these disorders (Dannlowski et al., 2007; Domschke et al., 2006).
In support of our findings, another line of evidence suggests that 5‐HT1A receptor agonists increase dopamine release in the PFC (for review: de Almeida et al., 2008). Therefore, the stronger Ne in homozygous carriers of the −1019 C allele may be explained by stronger stimulating effects on the DA system in carriers of the CC genotype compared to the CG and GG genotypes. Furthermore, our results are in line with Fallgatter et al. (2004) who observed an association of the less active 5‐HTTLPR s allele with a more pronounced Ne in 39 probands. The 5‐HTTLPR s allele increases serotonin availability in the synaptic cleft (Murphy and Lesch, 2008) as does the −1019 C allele eventually leading to an increased serotonergic neurotransmission (Lemonde et al., 2003). Hence, our results nicely corroborate the findings by Fallgatter et al. (2004) pointing to a genetically driven enhanced serotonergic transmission being associated with an increased Ne. Differential modulation of the DA system by serotonergic inputs may hence lead to modulations of error monitoring functions (Holroyd and Coles, 2002).
The results demonstrate a high sensitivity of the applied neurophysiological parameter in a healthy population and that serotonergic neural transmission selectively affects error‐specific subprocesses (delta and sub‐delta frequency band), but not general response monitoring functions (theta frequency band). The study by Yordanova et al. (2004) as well as the current data suggests that the critical point of the Ne/ERN is an increased in power of the δ‐frequency band. The theta component, evident in erroneous trials is not specific for the Ne, as it also appears during correct trials (Nc ERP‐component). Hence the theta component is not as closely linked to the Ne than the delta and sub‐delta component. The theta component may be more linked to the Nc, where we also found no differences. Thus, the results suggest a neurochemical dissociation between error‐specific and general response monitoring functions with respect to the importance of serotonergic neurotransmission. Although error‐specific monitoring processes seem to depend on the 5‐HT1A receptor system, general response‐monitoring functions seem to be independent of this receptor system. The Nc has been related to response monitoring (Falkenstein et al., 2000) or to conflict between the actual response and a response program (Bartholow et al., 2005), whereas others assume that the Nc is solely a smaller Ne (Vidal et al., 2000). As the Nc might be a smaller Ne (Vidal et al., 2000) and may hence reflect similar functions, the Nc may depend upon similar neurobiological mechanisms as the Ne, because processes that share functional principles are also mediated by similar neuronal substrates (Kosslyn and König, 1992). Because a neurobiological dissociation between these processes is observed, the Nc is unlikely reflecting only a smaller Ne, as then a similar modulation of both components would be more probable (Kosslyn and König, 1992). In line with the idea put forward by Yordanova et al. (2004), our results suggest that error monitoring adds error specific subprocesses to processes of general (motor) performance monitoring, which are likely related to different neurobiological mechanisms. Future studies may incorporate even larger sample sizes to assess such possible interactions on a genotype level and the differential impact of these on error processing and general response monitoring with sufficient power to detect even small effects.
In summary, the results suggest a differential impact of the 5‐HT1A C(−1019)G polymorphism on processes specific to error monitoring. More general processes of performance monitoring were not affected by this polymorphism. The results suggest that the 5‐HT1A −1019 G allele is associated with a decrease of neurophysiological processes underlying error monitoring processes and an attenuation of its behavioral consequences. Furthermore, our results provide evidence that the Ne is not simply an enlarged Nc, but error‐specific monitoring is rather accompanied by additional cognitive processes that most probably rely on serotonin 1A receptor‐mediated neural transmission.
Footnotes
The mean amplitude of the Ne at electrode Cz differed (−30.2 ± 2.6) from the mean Ne amplitudes electrodes FCz (−42.9 ± 1.8) and Fz (−48.9 ± 1.4) (P < 0.001).
REFERENCES
- Albert PR, Lemonde S ( 2004): 5‐HT1A receptors, gene repression, and depression: Guilt by association. Neuroscientist 10: 575–593. [DOI] [PubMed] [Google Scholar]
- Bartholow BD, Pearson MA, Dickter CL, Sher KJ, Fabiani M, Gratton G ( 2005): Strategic control and medial frontal negativity: Beyond errors and response conflict. Psychophysiology 42: 33–42. [DOI] [PubMed] [Google Scholar]
- Baune BT, Hohoff C, Roehrs T, Deckert J, Arolt V, Domschke K ( 2008): Serotonin receptor 1A‐1019C/G variant: Impact on antidepressant pharmacoresponse in melancholic depression? Neurosci Lett 436: 111–115. [DOI] [PubMed] [Google Scholar]
- Beste C, Saft C, Andrich J, Gold R, Falkenstein M ( 2006): Error processing in Huntington's disease. PloS One e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beste C, Saft C, Yordanova J, Andrich J, Gold R, Falkenstein M, Kolev V ( 2007): Functional compensation or pathology in cortico‐subcortical interactions in preclinical Huntington's disease. Neuropsychologia 45: 2922–2930. [DOI] [PubMed] [Google Scholar]
- Beste C, Saft C, Konrad C, Andrich J, Habbel A, Schepers I, Jansen A, Pfleiderer B, Falkenstein M ( 2008): Levels of error processing in Huntington's disease: A combined study using event‐related potentials and voxel‐based morphometry. Hum Brain Mapp 29: 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beste C, Willemssen R, Saft C, Falkenstein M ( 2009): Error processing in normal aging and in basal ganglia disorders. Neuroscience 159: 143–149. [DOI] [PubMed] [Google Scholar]
- Boksem MA, Meijman TF, Lorist MM ( 2006): Mental fatigue, motivation and action monitoring. Biol Psychol 72: 123–132. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Ohrmann P, Bauer J, Kugel H, Baune BT, Hohoff C, Kersting A, Arolt V, Heindel W, Deckert J, Suslow T ( 2007): Serotonergic genes modulate amygdala activity in major depression. Genes Brain Behav 6: 672–676. [DOI] [PubMed] [Google Scholar]
- de Almeida J, Palacois JM, Mengod G ( 2008): Distribution of 5‐HT and DA receptors in primate prefrontal cortex: Implications for pathophysiology and treatment. Prog Brain Res 172: 101–115. [DOI] [PubMed] [Google Scholar]
- Debener S, Ullsperger M, Siegel M, Fiehler K, von Cramon DY, Engel AK ( 2005): Trial‐by‐trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. J Neurosci 25: 11730–11737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domschke K, Braun M, Ohrmann P, Suslow T, Kugel H, Bauer J, Hohoff C, Kersting A, Engelien A, Arolt V, Heindel W, Deckert J ( 2006): Association of the functional‐1019C/G 5‐HT1A polymorphism with prefrontal cortex and amygdala activation measured with 3T fMRI in panic disorder. Int J Neuropsychopharmacol 9: 349–355. [DOI] [PubMed] [Google Scholar]
- Drago A, Ronchi DD, Serretti A ( 2008): 5‐HT1A gene variants and psychiatric disorders: A review of current literature and selection of SNPs for future studies. Int J Neuropsychopharmacol 11: 701–721. [DOI] [PubMed] [Google Scholar]
- Fakra E, Hyde LW, Gorka A, Fisher PM, Munoz KE, Kimak M, Halder I, Ferrell RE, Manuck SB, Hariri AR ( 2009): Effects of HT1A C(−1019)G on amygdale and trait anxiety. Arch Gen Psychiatry 66: 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L ( 1991): Effects of crossmodal divided attention on the ERP components. II. Error processing in choice reaction tasks. Electroencephalogr Clin Neurophysiol 78: 447–455. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Christ S, Hohnsbein J ( 2000): ERP components on reaction errors and their functional significance: A tutorial. Biol Psychol 51: 87–107. [DOI] [PubMed] [Google Scholar]
- Fallgatter AJ, Herrmann MJ, Römmler J, Ehlis AC, Wagener A, Heidrich A, Ortega G, Zeng Y, Lesch KP ( 2004): Allelic variation of serotonin transporter function modulates the brain electrical response for error processing. Neuropsychopharmacology 29: 1506–1511. [DOI] [PubMed] [Google Scholar]
- Fiehler K, Ullsperger M, von Cramon DY ( 2005): Electrophysiological correlated of error correction. Psychophysiology 42: 72–82. [DOI] [PubMed] [Google Scholar]
- Freitag CM, Domschke K, Rothe C, Lee YJ, Hohoff C, Gutknecht L, Sand P, Fimmers R, Lesch KP, Deckert J ( 2006): Interaction of serotonergic and noradrenergic gene variants in panic disorder. Psychiatry Genet 16: 59–65. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E ( 1993): A neural system for error detection and compensation. Psychol Sci 4: 385–390. [Google Scholar]
- Gratton G, Coles MG, Donchin E ( 1983): A new method for off‐line removal of ocular artefact. Electroencephalogr Clin Neurophysiol 55: 468–484. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Simons RF ( 2002): Error‐related brain activity in obsessive‐compulsive undergraduates. Psychiatry Res 110: 63–72. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Nieuwenhuis S, Ridderinkhof KR, Simons RF ( 2005): Error‐preceding brain activity: Robustness, temporal dynamics, and boundary conditions. Biol Psychol 70: 67–78. [DOI] [PubMed] [Google Scholar]
- Hettema JM, An SS, van den Oord EJ, Neale MC, Kendler KS, Chen X ( 2008): Association study between the serotonin 1A receptor (HTR1A) gene and neuroticism, major depression, and anxiety disorders. Am J Med Genet B Neuropsychiatr Genet 147: 661–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, Coles MGH ( 2002): The neural basis of human error processing: Reinforcement learning, dopamine, and the error‐related negativity. Psychol Rev 109: 679–709. [DOI] [PubMed] [Google Scholar]
- Huang YY, Battistuzzi C, Oquendo MA, Harkavy‐Friedman J, Greenhill L, Zalsman G, Brodsky B, Arango V, Brent DA, Mann JJ ( 2004): Human 5‐HT1A receptor C(−1019)G polymorphism and psychopathology. Int J Neuropsychopharmacol 7: 441–451. [DOI] [PubMed] [Google Scholar]
- Jensen O, Hari R, Kaila K ( 2002): Visually evoked gamma responses in the human brain are enhanced during voluntary hyperventilation. Neuroimage 15: 575–586. [DOI] [PubMed] [Google Scholar]
- Jocham G, Ullsperger M ( 2009): Neuropharmacology of performance monitoring. Neurosci Biobehav Rev 33: 48–60. [DOI] [PubMed] [Google Scholar]
- Kopp B, Rist F, Mattler U ( 1996): N200 in the flanker task as a neurobehavioral tool for investigating executive control. Psychophysiology 33: 282–294. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Koening O ( 1992): Wet mind: The New Cognitive Neuroscience. New York: Free Press. [Google Scholar]
- Lemonde S, Turecki G, Bakish D, Du L, Hrdina PD, Bown CD, Sequeira A, Kushwaha N, Morris SJ, Basak A, Ou XM, Albert PR ( 2003): Impaired repression at a 5‐hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. J Neurosci 23: 8788–8799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathewson KJ, Dyvwan J, Segalowitz SJ ( 2005): Brain bases of error‐related ERPs as influenced by age and task. Biol Psychol 70: 88–104. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Tanaka K ( 2004): The role of the medial prefrontal cortex in achieving goals. Curr Opin Neurobiol 14: 178–185. [DOI] [PubMed] [Google Scholar]
- Murphy DL, Lesch KP ( 2008): Targeting the murine serotonin transporter: Insights into human neurobiology. Nat Rev Neurosci 9: 85–96. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Srinivasan R, Westdorp AF, Wijesinghe RS, Tucker DM, Silberstein RB, Cadusch PJ ( 1997): EEG coherency. I. Statistics, reference electrode, volume conduction, Laplacians, cortical imaging, and interpretation at multiple scales. Electroencephalogr Clin Neurophysiol 103: 499–515. [DOI] [PubMed] [Google Scholar]
- Olvet M, Hajcak G ( 2008): The error‐related negativity (ERN) and psychopathology: Toward an endophenotype. Clin Psychol Rev 28: 1343–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oranje B, Jensen K, Wienberg M, Glenthoj BY ( 2008): Divergent effects of increased serotonergic activity on psychophysiological parameters of human attention. Int J Neuropsychopharmacol 11: 453–463. [DOI] [PubMed] [Google Scholar]
- Overbeeck TM, Nieuwenhuis S, Ridderinkhof KR ( 2005): Dissociable components of error processing: On the functional significance of the Pe vis‐à‐vis the ERN/Ne. J Psychophysiol 19: 319–329. [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF ( 1989): Spherical splines for scalp potential and current densitiy mapping. Eletroencephalogr Clin Neurophysiol 72: 184–187. [DOI] [PubMed] [Google Scholar]
- Rabbitt PM ( 1966): Error and error correction in choice‐response tasks. Nature 212: 438. [DOI] [PubMed] [Google Scholar]
- Rothe C, Gutknecht L, Freitag C, Tauber R, Mössner R, Francke P, Fritze J, Wagner G, Peikert G, Wenda B, Sand P, Jacob C, Rietschel M, Nöthen MM, Garritsen H, Fimmers R, Deckert J, Lesch KP ( 2004): Association of a functional 1019C>G 5‐HT1A receptor polymorphism with panic disorder with agoraphobia. Int J Neuropsychopharmacol 7: 189–192. [DOI] [PubMed] [Google Scholar]
- Ruchsow M, Herrnberger B, Wiesend C, Grön G, Spitzer M, Kiefer M ( 2004): The effect of errorneous responses on response monitoring in patients with major depressive disorder: A study with event‐related potentials. Psychophysiology 41: 833–840. [DOI] [PubMed] [Google Scholar]
- Ruchsow M, Herrnberger B, Beschoner P, Grön G, Spitzer M, Kiefer M ( 2006): Error processing in major depressive disorder: Evidence from event‐related potentials. J Psychiatr Res 40: 37–46. [DOI] [PubMed] [Google Scholar]
- Samar VJ, Bopardikar A, Rao R, Swartz K ( 1999): Wavelet analysis of neuroelectric waveforms: A conceptual tutorial. Brain Lang 66: 7–60. [DOI] [PubMed] [Google Scholar]
- Strobel A, Gutknecht L, Rothe C, Reif A, Mössner R, Zeng Y, Brocke B, Lesch KP ( 2003): Allelic variation in 5‐HT1A receptor expression is associated with anxiety‐ and depression‐related personality traits. J Neural Transm 110: 1445–1453. [DOI] [PubMed] [Google Scholar]
- Tallon‐Baudry C, Bertrand O, Delpuech C, Permier J ( 1997): Oscillatory γ‐band (30‐70 Hz) activity induced by a visual search task in humans. J Neurosci 17: 722–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY ( 2001): Subprocesses of performance monitoring: A dissociation of error processing and response competition revealed by event‐ related fMRI and ERPs. Neuroimage 14: 1387–1401. [DOI] [PubMed] [Google Scholar]
- Vidal F, Hasbroucq T, Grapperon J, Bonnet M ( 2000): Is the ‘error negativity’ specific to errors? Biol Psychol 51: 109–128. [DOI] [PubMed] [Google Scholar]
- Wienberg M, Glenthoi B, Jensen K, Oranje B ( 2009): A single dose of escitalopram increases mismatch negativity without affecting processing negativity or P300 amplitude in healthy volunteers. J Psychopharmacol [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Willemssen R, Müller T, Schwarz M, Hohnsbein J, Falkenstein M ( 2008): Error processing in patients with Parkinson's disease: The influence of medication state. J Neural Transm 115: 461–468. [DOI] [PubMed] [Google Scholar]
- Willemssen R, Müller T, Schwarz M, Falkenstein M, Beste C ( 2009): Response monitoring in de novo patients with Parkinson's disease. PLoS One e4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yordanova J, Falkenstein M, Hohnsbein J, Kolev V ( 2004): Parallel systems of error processing in the brain. Neuroimage 22: 590–602. [DOI] [PubMed] [Google Scholar]


