Abstract
Experimental panic induction with cholecystokinin tetrapeptide (CCK‐4) is considered as a suitable model to investigate the pathophysiology of panic attacks. While only a few studies investigated the brain activation patterns following CCK‐4, no data are available on the putative involvement of the amygdala in the CCK‐4 elicited anxiety response. We studied the functional correlates of CCK‐4‐induced anxiety in healthy volunteers by means of functional magnetic resonance imaging (fMRI) and region of interest (ROI) analysis of the amygdala. Sixteen healthy volunteers underwent challenge with CCK‐4 compared with placebo in a single‐blind design. Functional brain activation patterns were determined for the CCK‐4‐challenge, the placebo response and anticipatory anxiety (AA). CCK‐4‐induced anxiety was accompanied by a strong and robust activation (random effects analysis, P < 0.00001, uncorrected for multiple testing) in the ventral anterior cingulate cortex (ACC), middle and superior frontal gyrus, precuneus, middle and superior temporal gyrus, occipital lobe, sublobar areas, cerebellum, and brainstem. In contrast, random effects group analysis for placebo and AA using the same level of significance generated no significant results. Using a more liberal level of significance, activations could be observed in some brain regions such as the dorsal part of the ACC during AA (random effects analysis, P < 0.005). Overall functional responses did not differ between panickers and nonpanickers. Only 5 of 11 subjects showed strong amygdala activation. However, ROI analysis pointed towards higher scores in fear items in these subjects. In conclusion, while overall brain activation patterns are not related to the subjective anxiety response to CCK‐4, amygdala activation may be involved in the subjective perception of CCK‐4‐induced fear. Hum Brain Mapp, 2009. © 2007 Wiley‐Liss, Inc.
Keywords: fMRI, CCK‐4, anxiety, amygdala, ACC
INTRODUCTION
In the past two decades panic induction with cholecystokinin‐tetrapeptide (CCK‐4) as an experimental model of human anxiety has become a focus of intensive research. CCK‐4 is a synthetic analog of the endogenous neuropeptide cholecystokinin (CCK) [Rehfeld and Neilson, 1995; van Megen et al., 1996], which has been found in different brain regions [Beinfeld et al., 1981]. Particularly high concentrations of CCK have been detected in regions, which have been implicated in the mediation of panic attacks such as the cerebral cortex, amygdala, hippocampus, and brainstem nuclei. Moreover, there is increasing evidence that CCK is acting there as a neurotransmitter [Rehfeld, 1985]. Two different CCK‐receptor subtypes have meanwhile been identified, a peripheral CCK‐1 (CCK‐A) and a central CCK‐2 (CCK‐B) receptor [Moran et al., 1986]. The latter one exhibits high affinity for CCK‐4 and the synthetic pentapeptide pentagastrin. It has been suggested that alterations in endogenous CCK‐metabolism [Akiyoshi et al., 1997; Brambilla et al., 1993; Lydiard et al., 1992] and CCK‐B receptor gene variants [Kennedy et al., 1999] may contribute to the pathophysiology of panic disorder (PD).
CCK‐4 and pentagastrin dose dependently induce panic attacks in patients with PD and healthy controls. The CCK‐4‐induced panic symptomatology closely resembles spontaneously occurring panic attacks in patients with PD [Bradwejn et al., 1990, 1991]. CCK‐4‐induced panic attacks are reproducible [Bradwejn et al., 1992] and accompanied by a marked somatic reaction [Bradwejn et al., 1998; Koszycki et al., 1998]. In contrast to other provocation tests [Aronson et al., 1989; Rapee et al., 1991; Spinhoven et al., 1993] cognitive factors do not crucially affect the CCK‐4‐induced panic response [Aluoja et al., 1997; Koszycki et al., 1993, 1996a; van Megen et al., 1994]. Thus, CCK‐4 has been postulated to fulfil the criteria for an ideal and valid panicogenic agent [Bradwejn and Koszycki, 1994b, 2001]. Moreover, several studies demonstrated that successful treatment with antipanic drugs such as imipramine [Bradwejn and Koszycki, 1994a], fluvoxamine [van Megen et al., 1997], citalopram [Shlik et al., 1997], or benzodiazepines [de Montigny, 1989; Zwanzger et al., 2003] attenuate CCK‐4‐induced panic attacks. Therefore, the CCK‐4 challenge paradigm does not only constitute a model to study the pathophysiology of PD but can serve as a useful tool to evaluate the antipanic potential of novel anxiolytic compounds in phase I proof‐of‐concept trials [Eser et al., 2007].
However, only few studies addressed the functional neuroanatomy of CCK‐4‐induced anxiety. Preliminary positron emission tomography (PET) studies in healthy volunteers suggested that CCK‐4‐induced panic attacks are associated with cerebral blood flow (CBF) increase in the anterior cingulate cortex (ACC), the claustrum‐insular‐amygdala region and the cerebellar vermis [Benkelfat et al., 1995; Javanmard et al., 1999]. So far, only one study investigated the brain activity pattern related to CCK‐4‐induced panic using a functional magnetic resonance imaging (fMRI) approach [Schunck et al., 2006]. This study suggested that fMRI might be suitable to study the functional neuroanatomy of CCK‐4‐induced anxiety [Schunck et al., 2006]. However, cerebral activity in the amygdala region was not investigated in this study, although this region has been hypothesized to represent the center of a “fear network” in the brain, which mediates panic attacks [Gorman et al., 2000]. Therefore, we investigated the functional neuroanatomy of CCK‐4‐induced panic in healthy volunteers by means of fMRI and questioned whether experimentally induced panic is related to the activation of the amygdala.
MATERIALS AND METHODS
Subjects
Seventeen subjects were recruited through advertisement and participated in the study after giving their written informed consent. To avoid putative confounding effects of the menstrual cycle phase on the response to CCK‐4 [Le Melledo et al., 1995, 1999, 2001], only healthy males were studied. Subjects were free of any personal or family history of psychiatric illness. Somatic diseases were ruled out by means of physical examination, electrocardiogram, electroencephalogram, and routine laboratory testing including haematological screening, blood chemistry with glucose, total protein, total bilirubin, liver enzymes, electrolytes, creatinine, urea, uric acid, cholesterol, triglycerides, semiquantitative urinalysis, and thyroid hormones. Any intake of drugs was ruled out by urine toxicology screening for at least 4 weeks prior to baseline screening. Finally, 16 subjects with a mean age of 25.6 ± 4.2 years underwent the CCK‐4 challenge. One subject dropped out immediately before the fMRI procedure, because he experienced symptoms of claustrophobia when he entered the scanner. The Ethics Committee for Human Experiments at the Ludwig‐Maximilian University, Munich, Germany, approved the protocol.
Procedure
The study consisted of one MRI session lasting 40 min. During this session two runs lasting 15 min each were performed. Upon arrival subjects were first given an overview of the sequence of events. Subjects were instructed that they would receive two injections during the whole session. They were told that they would receive once CCK‐4 and once placebo in a random order during the first and the second run. Subjects were blind to the exact time points of injections. However, they were ensured that the injections would not be administered during the first 3 min of each run. After this resting period it was announced by interphone that the injection could be delivered within the next 12 min of the run. Actually, all subjects received both injections exactly 5 min after the beginning of each run. The 2 min of recording prior to CCK‐4 injection were used to examine effects of anticipatory anxiety (AA).
To evaluate putative differences in cerebral activity between AA and the panicogenic effects of CCK‐4 the sequence of injections was only single‐blinded. The first run was designed to generate placebo effects and therefore all subjects received 2 ml saline as a bolus injection (placebo condition). Ten minutes apart from the first run the second run started, which was designed to generate CCK‐4 effects (CCK‐4 condition). It was hypothesized that subjects which had first experienced the placebo injection during the first run would expect the CCK‐4 injection after the end of the resting period of the second run and therefore would develop higher AA. AA effects were investigated during the 2 min of recording after the resting period prior to CCK‐4 injection.
CCK‐4 Administration
For injections an intravenous catheter was inserted into a forearm vein and was connected to a syringe outside the scanner from where CCK‐4 or placebo injections were performed. During the whole challenge period, 0.9% isotonic sodium chloride solution was infused (∼50 ml/h) to avoid occlusion of the cannula and to keep subjects blinded with regard to the exact time point of CCK‐4 injection. Fifty microgram CCK‐4 (dissolved in 2 ml 0.9% saline, Clinalfa, Läufelfingen, Switzerland) were administered as a bolus injection. Saline was used as placebo.
Imaging
Imaging was performed in a 1.5‐T Siemens Magnetom Avanto scanner with echoplanar capability. For functional BOLD imaging forty‐two slices with a T2*‐weighted EPI sequence (TR: 4 s; TE: 40 ms; FOV: 192/192 mm; matrix: 64 × 64; interleaved slice acquisition; slice thickness: 3 mm; interslice gap: 0.75 mm; resulting pixel size: 3 × 3 mm2) were acquired in the same position as the anatomical images. Each functional time series consisted of 226 volumes. Two functional time series were acquired (CCK‐4 condition and placebo condition). The first four functional image frames of each time series were discarded to allow for signal equilibration (T1 saturation effects), giving a total of 222 frames per run used in analysis. For each subject a three‐dimensional MPRAGE data set (T1‐weighted) was acquired. The subject's head was immobilized during the whole experiment to minimize involuntary head movements.
Image Processing and Statistical Analysis
The complete postprocessing of the data including motion correction, statistics, and transfer of the data into Talairach space was done with the BrainVoyager QX software package (version 1.7, Rainer Goebel, Maastricht, Netherlands). Motion correction was done using Trilinear Interpolation on a reduced (12.5%) data set. Data smoothing was performed as temporal data smoothing and linear trend removal. Spatial data smoothing has been performed using an 8 mm FWHM Gaussian filter. Functional data were transformed into Talairach space, aligned with the three‐dimensional anatomical volumes from the same session and interpolated to a resolution of 1 mm3.
For placebo and CCK‐4 time series a corresponding design matrix was modeled and convoluted with a haemodynamic response function which contained the idealized response functions assuming a period of CCK‐4 or placebo effects in the minute after the beginning of injection (predictor 1, placebo/CCK‐4 period, corresponding to 15 images). This assumption was based on the fact that panic symptoms occur immediately following CCK‐4 injection, which are paralleled by cardiovascular effects with a maximum increase in heart rate and systolic blood pressure in the first minute after CCK‐4 administration [Eser et al., 2007]. Furthermore, a period of effects of AA in the 2 min before injection (predictor 2, AA period, corresponding to 30 images) was assumed. Baseline activity was defined as the first 3 min of each run (baseline 1 period, corresponding to 42 images) when subjects felt safe from the application of CCK‐4 or placebo to rule out effects of AA during this period and the last 5 min of each run (baseline 2 period, corresponding to 75 images) following an interval of 4 min after the period of CCK‐4 or placebo effects (predictor 3, restoration period, corresponding to 60 images) to be sure that any effects of CCK‐4 or placebo had restored during this period.
For further statistical analysis, multiple regression analysis was performed on the three‐dimensional functional volume time courses (one for every subject with 222 time points each) using the general linear model and the three aforementioned predictors “placebo/CCK‐4,” “AA period” and “restoration period.”
Statistical maps were computed for analysis of individuals and thresholded at P < 0.05 (uncorrected for multiple testing). To obtain information about activations which are consistent for the entire group, two random effects statistical group analyses thresholded at P < 0.00001 (uncorrected for multiple testing) and at P < 0.005 (uncorrected for multiple testing) were performed.
Region of Interest Analysis
Analysis of extent of activation in both amygdalae was based on the number of activated voxels in regions of interest as described previously [Heinz et al., 2005] placed as a cube with an edge length of 12 mm in the center of both amygdalae (x, y, z = ±24, −4, −12). Statistical weighted mass was computed by adding the t values of each single voxel in the defined region of interest for CCK‐4 and placebo effects, respectively.
Panic Symptom Assessment
Panic symptoms were evaluated with a DSM‐III‐R derived Panic Symptom Scale (PSS). This scale was established by Bradwejn et al. [1991] to assess CCK‐4‐induced panic symptoms in PD patients [Bradwejn and Koszycki, 1994a; Bradwejn et al., 1994] and healthy controls [Bradwejn et al., 1998; Koszycki et al., 1998]. Each of the 18 PSS items can be rated from 0 (absent) to 4 (extremely severe). In addition to the sum intensity (PSS sum of symptoms) score, also the number of positively rated PSS symptoms (PSS number of symptoms) was recorded. Subjects were asked to report PSS symptoms before each scan (baseline) and immediately thereafter. At the latter time point subjects were instructed to rate the intensity of their strongest feeling after the CCK‐4 and placebo injection.
Keeping in line with previous investigations using the PSS [Flint et al., 1998; Koszycki et al., 1998; Zwanzger et al., 2003] subjects were classified as panickers if they fulfilled all of the following criteria after CCK‐4 administration: a sudden onset of panic symptoms, an endorsement of at least four PSS symptoms and a subjective experience of anxiety/fear/apprehension of at least moderate intensity (a score of 2 or higher in the respective PSS item), the remaining subjects were considered as nonpanickers.
Heart rate was continuously monitored using a specialized MRI safety monitor (Patientenmonitor, model number 3150MRI, Invivo Research Inc., Orlando, FL). Values recorded 1 min before placebo or CCK‐4 injection and 1, 2, 3, 4, and 5 min thereafter entered further analysis.
Statistical Analysis
Behavioral and heart rate measurements are expressed as mean ± SEM. For analysis of clinical scales and heart rate measurements of the whole group Student's t test was employed. Analysis of variance (ANOVA) was performed for intergroup comparison of panic rating scores between panickers and nonpanickers with panic status as between subject factor. For statistical analysis of cardiovascular data at baseline and 1, 2, 3, 4, and 5 min following CCK‐4 challenge a multivariate analysis of variance (MANOVA) with time as within subject factor and panic as between subject factor was performed. For statistical comparisons between the number of activated voxels during the CCK‐4 and the placebo condition a paired t‐test was conducted. α = 0.05 was set as the nominal level of significance.
RESULTS
Primary Analysis Population
In contrast to placebo CCK‐4 elicited a marked and statistically significant increase in panic rating scores (PSS sum score: T = −3.01, P < 0.001, PSS number of symptoms: T = −13.7, P < 0.001) over preCCK‐4 baseline values. This panic response to CCK‐4 was accompanied by a significant increase in heart rate with a maximum 1 min after CCK‐4 administration. Although the comparison of panic symptom severity before and after placebo reached also statistical significance, PSS sum scores and PSS numbers of symptoms were markedly lower after placebo administration compared to postCCK‐4 injection scores. PSS scores for the whole group are presented in Table I.
Table I.
Behavioral responses to placebo and cholecystokinin‐tetrapeptide (CCK‐4) in the whole study population
| Placebo scan | CCK‐4 scan | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | After placebo | Baseline | After CCK‐4 | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| PSS sum of symptoms | 3.25 | 0.86 | 5.88 | 1.26 | 2.00 | 0.84 | 26.25 | 2.42 |
| PSS number of symptoms | 2.63 | 0.63 | 4.38 | 0.85 | 1.88 | 0.76 | 12.06 | 0.74 |
Sum score of symptoms in the PSS and number of symptoms reported in the PSS, at baseline, after placebo administration and after CCK‐4 challenge. Values are given as mean ± SD.
fMRI
Using random effects analysis and on a strict level of significance (P < 0.00001, uncorrected for multiple testing) large responses to CCK‐4 were detected in the ventral ACC, middle and superior frontal gyrus, precuneus, middle and superior temporal gyrus, occipital lobe, sublobar areas, cerebellum, and brainstem. For details on activated brain regions see Table II, mean activation maps of the functional neuroanatomical response to CCK‐4 for the whole group are shown in Figure 1.III
Table II.
Random effects analysis: Regions activated by injection of CCK‐4
| Location | Center of gravity (Talairach coordinatesa) | Cluster size | Average t score | Maximum t score | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Frontal lobe | ||||||
| Middle FG left | −32 | −5 | 40 | 264 | 7.51 | 10.10 |
| Anterior Cingulate right | 3 | 32 | −4 | 1,091 | 7.38 | 10.16 |
| Precentral Gyrus left | −43 | 19 | 36 | 190 | 7.13 | 8.76 |
| Middle FG right | 47 | 22 | 40 | 78 | 6.83 | 7.50 |
| Superior FG right | 45 | 40 | 32 | 43 | 6.79 | 7.25 |
| Superior FG right | 7 | 21 | 59 | 15 | 6.64 | 6.87 |
| Brainstem | ||||||
| Pons left | −23 | −28 | −29 | 1,117 | 7.23 | 11.55 |
| Midbrain left | −8 | −25 | −6 | 824 | 7.06 | 8.69 |
| Midbrain right | 7 | −37 | −12 | 541 | 6.91 | 8.34 |
| Pons left | −4 | −14 | −20 | 182 | 6.84 | 7.54 |
| Cerebellum | ||||||
| PL right, Uvula | 28 | −80 | −23 | 645 | 7.14 | 9.53 |
| PL left, Declive | −16 | −76 | −14 | 762 | 7.09 | 8.54 |
| PL left, Cerebellar Tonsil | −23 | −57 | −33 | 1,973 | 7.07 | 8.25 |
| PL left, Declive | −15 | −78 | −19 | 748 | 6.99 | 8.31 |
| PL right, Uvula | 2 | −63 | −29 | 2,876 | 6.85 | 7.84 |
| PL right, Cerebellar Tonsil | 26 | −47 | −32 | 905 | 6.85 | 7.74 |
| Anterior Lobe right, Culmen | 19 | −28 | −22 | 348 | 6.74 | 7.54 |
| Parietal Lobe | ||||||
| Precuneus right | 16 | −68 | 44 | 838 | 7.09 | 9.14 |
| Precuneus left | −4 | −70 | 35 | 2,610 | 7.04 | 9.02 |
| Precuneus right | 19 | −67 | 32 | 1,544 | 7.03 | 9.14 |
| Inferior Parietal Lobule left | −50 | −39 | 28 | 163 | 6.81 | 7.74 |
| Supramarginal Gyrus left | −36 | −49 | 32 | 44 | 6.75 | 7.50 |
| Temporal Lobe | ||||||
| Superior TG right | 55 | −25 | 7 | 766 | 7.06 | 8.76 |
| Middle TG left | −48 | −47 | 1 | 747 | 6.97 | 9.30 |
| Superior TG right | 49 | −50 | 10 | 290 | 6.95 | 7.78 |
| PHG left | −42 | −40 | −1 | 77 | 6.94 | 7.97 |
| Superior TG left | −58 | −33 | 14 | 347 | 6.84 | 7.83 |
| PHG right | 29 | −25 | −12 | 14 | 6.58 | 7.06 |
| Occipital lobe | ||||||
| Middle Occipital Gyrus left | −24 | −86 | −1 | 345 | 6.95 | 8.20 |
| Lingual Gyrus right | 4 | −86 | −12 | 406 | 6.87 | 7.53 |
| Cuneus right | 10 | −80 | 28 | 506 | 6.79 | 7.82 |
| Sublobar | ||||||
| Putamen left | −20 | −2 | 7 | 107 | 6.93 | 8.38 |
| Claustrum right | 24 | 4 | 19 | 71 | 6.74 | 7.27 |
| Insula left | −47 | −6 | 5 | 64 | 6.67 | 7.21 |
The statistical threshold used to define the clusters was P = 0.00001 (uncorrected for multiple testing).
According to Talaraich and Tournoux [1988]. FG, frontal gyrus; PL, posterior lobe; GT, temporal gyrus; PHG, parahippocampal gyrus.
Figure 1.
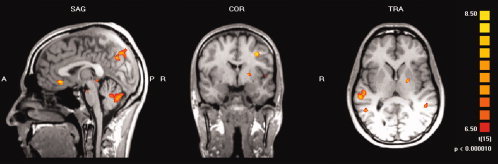
Group analysis (n = 16) of the BOLD signal related to the effects of CCK‐4 injection (random effects analysis, P < 0.00001, uncorrected for multiple testing). Activation is seen in the ventral anterior cingulate cortex, left middle frontal gyrus, left precuneus, left middle temporal gyrus, right superior temporal gyrus, left insula, left putamen, cerebellum and brainstem.
Table III.
Behavioral responses to placebo and cholecystokinin‐tetrapeptide (CCK‐4) in panickers and nonpanickers. Sum score of symptoms of the PSS, number of symptoms reported in the PSS, sum of fear items of the PSS and sum of somatic panic items of the PSS before and after CCK‐4
| PSS sum of symptoms | PSS number of symptoms | PSS fear items | PSS somatic items | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | After CCK‐4 | Baseline | After CCK‐4 | Baseline | After CCK‐4 | Baseline | After CCK‐4 | |
| Panickers (n = 9) | 2.11 ± 1.2 | 30.56 ± 3.1 | 2.0 ± 1.1 | 12.56 ± 0.9 | 0.11 ± 0.11 | 3.22 ± 0.8 | 1.89 ± 1.8 | 24.8 ± 2.7 |
| Nonpanickers (n = 7) | 1.86 ± 1.2 | 20.71 ± 2.9 | 1.71 ± 1.1 | 11.43 ± 1.3 | 0.00 ± 0.00 | 0.57 ± 0.2 | 1.71 ± 1.1 | 19.6 ± 3.3 |
Values are given as mean ± SD.
In contrast, the random effects group analysis for effects of placebo and of AA (P < 0.00001, uncorrected for multiple testing, same level of significance as used for the description of the effects of CCK‐4) generated no significant results.
To show eventual effects of the injection of placebo we used a more liberal level of significance (random effects analysis, P < 0.005, uncorrected for multiple testing). We found three clusters of activated voxels with considerably lower maximum t levels than found in the CCK‐4 condition. The centers of gravity of these clusters were located in the left hippocampus and the right middle frontal gyrus. Details on activated brain regions are presented in Table IV.
Table IV.
Random effects analysis: Regions activated by injection of placebo
| Location | Center of gravity (Talairach coordinatesa) | Cluster size | Average t score | Maximum t score | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Frontal lobe | ||||||
| Middle FG right | 24 | 1 | 48 | 476 | 3.92 | 5.89 |
| Middle FG right | 35 | 6 | 41 | 30 | 3.56 | 4.19 |
| Temporal lobe | ||||||
| Hippocampus left | −26 | −35 | 3 | 80 | 3.44 | 3.72 |
The statistical threshold used to define the clusters was P < 0.005 (uncorrected for multiple testing).
According to Talaraich and Tournoux [1988].
Using the more liberal level of significance (random effects analysis, P < 0.005, uncorrected for multiple testing) for analysis of the AA condition some activation was found in the ACC, which, however, in contrast to the response to CCK‐4, was located in the dorsal part of the ACC. Additionally, we found activations for the AA condition in the right cingulate gyrus, right and left middle frontal gyrus, right inferior frontal gyrus, left superior temporal gyrus, left middle temporal gyrus, left supramarginal gyrus and left angular gyrus. For details of activated brain regions see Table V. Mean activation maps for the AA condition are presented in Figure 2.
Table V.
Random effects analysis: Regions activated during anticipatory anxiety condition
| Location | Center of gravity (Talairach coordinatesa) | Cluster size | Average t score | Maximum t score | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Frontal lobe | ||||||
| Anterior Cingulate | 0 | 30 | 30 | 1,555 | 3.67 | 4.90 |
| Cingulate gyrus right | 3 | −23 | 33 | 615 | 4.05 | 5.91 |
| Precentral gyrus right | 18 | −24 | 71 | 36 | 3.66 | 4.44 |
| Middle FG right | 38 | 4 | 47 | 49 | 3.49 | 3.83 |
| Middle FG left | −44 | 34 | 17 | 89 | 3.53 | 4.26 |
| Inferior FG right | 48 | 28 | 12 | 53 | 3.45 | 3.83 |
| Inferior FG right | 28 | 14 | −17 | 46 | 3.49 | 3.85 |
| Temporal lobe | ||||||
| Supramarginal gyrus left | −54 | −53 | 20 | 637 | 3.73 | 4.89 |
| Middle TG left | −63 | −44 | 0 | 1,490 | 4.19 | 6.62 |
| Superior TG left | −51 | −18 | −2 | 15 | 3.48 | 3.91 |
| Parietal lobe | ||||||
| Angular gyrus left | −44 | −63 | 36 | 115 | 3.61 | 4.55 |
The statistical threshold used to define the clusters was P < 0.005 (uncorrected for multiple testing).
According to Talaraich and Tournoux [1988].
Figure 2.
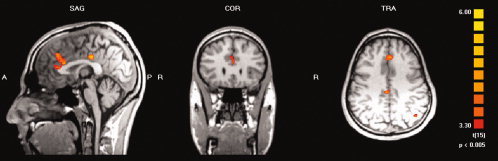
Group analysis (n = 16) of the BOLD signal related to effects of anticipatory anxiety (random effects analysis, P < 0.005, uncorrected for multiple testing). Activation is seen in the dorsal anterior cingulate cortex, posterior cingulate cortex and left parietal lobe.
Region of Interest Analysis of Amygdala Activity
Compared with the placebo condition we found significantly higher numbers of activated voxels in the regions of interest in both amygdalae in the CCK‐4 condition (see Fig. 3; left amygdala: T = 3.2, P = 0.007; right amygdala: T = 2.7, P = 0.015).
Figure 3.
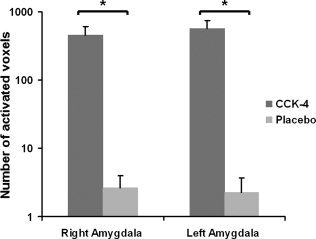
Comparison between placebo and CCK‐4 condition of the number of activated voxels in the regions of interest in the right and left amygdala. Values are given as mean ± SEM. The asterisks indicate statistical significance.
With regard to the extent of amygdala activation, we divided the subjects into two groups: a group of five subjects with high amygdala activation (a total of activated voxels in left and right amygdala more than 1,000 voxels, mean 2,784.6 ± 810.0 voxels) showed considerably higher ratings for PSS fear items (fear of dying, fear of going crazy, fear of loosing control) in comparison to the group of eleven subjects with low amygdala activation (average: 212.8 ± 239.7 voxels) (see Fig. 4). While both groups differed significantly with regard to the extent of amygdala activation (T = 7.0, P = 0.002), the observed difference in perception of fear symptoms failed to reach statistical significance, probably due to the small sample size. An example for the time course of activity in the right amygdala region of interest of a single subject with high amygdala activation is shown in Figure 5.
Figure 4.
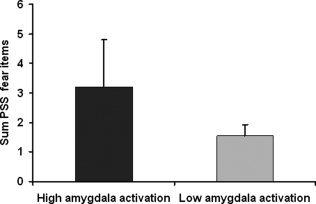
Sum of PSS fear items (fear of dying, fear of going crazy, fear of loosing control) in subjects with high amygdala activation (n = 5) compared to subjects with low amygdala activation (n = 11). Values are given as mean ± SEM.
Figure 5.
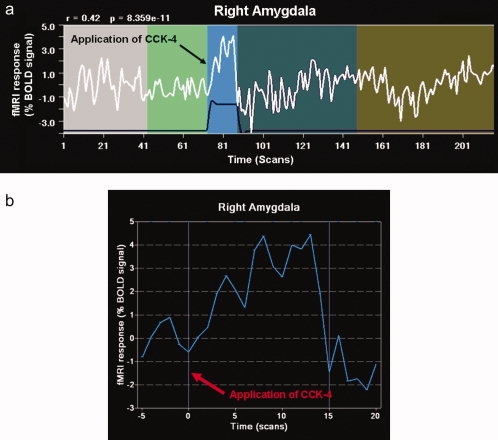
(a) Time course of activity in a right amygdala region of interest of a single subject with high amygdala activation during the whole fMRI run following CCK‐4 administration. (b) Time course of activity in a right amygdala region of interest of a single subject with high amygdala activation during the first minute after CCK‐4 injection.
Comparison of Subjects According to their Subjective Anxiety Response
PSS scores for panickers and nonpanickers are presented in Table II. According to the PSS derived panic criterion, 9 out of 16 subjects (56.3%) were classified as panickers after CCK‐4 injection. In contrast, no subject experienced a panic attack during the placebo condition. Panickers and nonpanickers did not differ in panic rating scores before and after placebo challenge or prior to CCK‐4 injection. As to be expected, panickers reported a significantly higher PSS sum score (F 1,14 = 5.21; P = 0.04) after CCK‐4 injection compared with nonpanickers. However, the number of reported PSS symptoms did not significantly differ between panickers and nonpanickers (F 1,14 = 0.56; P = 0.47). With regard to panic symptomatology panickers compared with nonpanickers reported a significantly higher sum intensity of PSS fear items (fear of going crazy, fear of loosing control and fear of dying) after CCK‐4 (F 1,14 = 8.2; P = 0.013). In contrast, panickers and nonpanickers displayed similar subjective somatic PSS responses to CCK‐4 (F 1,14 = 1.6; P = 0.23). This lack of difference in peripheral PSS items between panickers and nonpanickers was paralleled by the observation that MANCOVA revealed a significant time effect for heart rate acceleration after CCK‐4 (F2.6,30.8 = 47.01, P < 0.001) but no time x panic interaction. Time course of heart rate acceleration in panickers and nonpanickers is shown in Figure 6.
Figure 6.
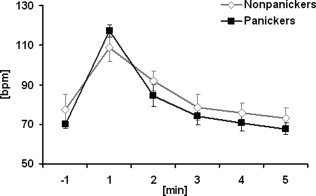
Effect of CCK‐4 on heart rate in panickers (n = 8, squares) and nonpanickers (data from 2 subjects are missing, n = 6, triangles). Scores are given as mean ± SEM. Time is expressed as minutes relative to CCK‐4 injection. Repeated measured ANOVA indicated a significant time effect for heart rate (F 2.6,30.8 = 47.01, P < 0.001) however, no significant time × panic status interaction.
In contrast to the differences in panic symptom severity between panickers and nonpanickers we found no significant difference in the BOLD response to CCK‐4 injection between these two groups.
DISCUSSION
We investigated the functional neuroanatomy of CCK‐4‐induced panic attacks in healthy volunteers by means of fMRI in order to clarify whether experimentally induced anxiety by CCK‐4 is reflected by distinct brain activation patterns in neuronal fear circuits. Because the amygdala plays a key role for the neuroanatomy of panic [Gorman et al., 2000], we questioned to what extent amygdala activation contributes to CCK‐4‐induced panic attacks.
In line with previous reports [Eser et al., 2007], CCK‐4 elicited a strong subjective panic response together with marked cardiovascular reactions in the majority of subjects. These CCK‐4‐induced panic symptoms were associated with enhanced cerebral activity in various brain regions such as the ventral ACC, middle and superior frontal gyrus, precuneus, middle and superior temporal gyrus, occipital lobe, sublobar areas, cerebellum and brainstem. This activation pattern is in line with previous studies using the CCK‐4 challenge paradigm, which detected CBF increases in the ACC, the claustrum‐insular region and the cerebellar vermis using PET [Benkelfat et al., 1995; Javanmard et al., 1999] and with enhanced cerebral activity in the ACC, insular region and cerebellum observed with fMRI [Schunck et al., 2006]. Moreover, functional imaging studies reported increases in CBF in the claustrum‐insular region and cerebellum also during lactate induced panic attacks in patients with PD [Reiman et al., 1989].
In addition to the effects of CCK‐4, we studied the brain activation patterns during AA. During the AA period a certain activation was observed in the dorsal ACC, right middle frontal gyrus, left medial frontal gyrus, and the temporal lobes. Thus, the activation pattern observed during the AA period partly overlapped with that observed after CCK‐4 injection. However, these effects of AA were much less pronounced when compared with those of CCK‐4 and disappeared when a comparable level of significance was adopted. Therefore, we conducted additional analysis on a more liberal level of significance. Interestingly, the activation in the ACC was located in the dorsal part during AA, whereas CCK‐4‐induced panic symptoms were accompanied by a functional response in the ventral part of the ACC. Although previous functional imaging studies with CCK‐4 and pentagastrin observed also a functional response in the ACC during AA in healthy controls [Benkelfat et al., 1995; Javanmard et al., 1999; Schunck et al., 2006] and in PD patients [Boshuisen et al., 2002], these studies did not distinguish between the ventral and dorsal part of the ACC. Nevertheless, neuroanatomically, the ACC consists of the ventral‐rostral subdivison and the dorsal subdivision [Devinsky et al., 1995], which are distinguishable also from a neurofunctional point of view. The dorsal subdivision (Brodman areas 24b′‐c′, 32′) has been implicated in the modulation of attention or executive functions [Bush et al., 2000] and cognitive information processing [Devinsky et al., 1995]. In contrast, the rostral‐ventral subdivision, which is mainly activated after CCK‐4 challenge, is involved in the regulation of autonomic and endocrine functions [Devinsky et al., 1995] but also in conditioned emotional learning [Bush et al., 2000] and attribution of emotional context [Devinsky et al., 1995]. Therefore, the observed differences in activation in the ACC between the AA and CCK‐4 condition might reflect a differential functional neuroanatomy between cognitive anticipation and real autonomic and affective responses to CCK‐4 in our subjects.
According to the PSS derived panic criterion, which relies on the severity of subjective feelings of anxiety and a quorum of other panic symptoms, CCK‐4 elicited a panic attack in 56.3% (9 out of 16) of our subjects, which is comparable to the panic rate observed in previous CCK‐4 challenge studies involving healthy volunteers [Bradwejn et al., 1998; Koszycki et al., 1993, 1996b, 1998]. Panickers and nonpanickers differed neither in baseline panic scores nor in the CCK‐4‐induced increase in heart rate, thereby confirming previous reports, which showed that neither baseline anxiety levels nor the cardiovascular response to CCK‐4 is related to the severity of subjective panic response [Eser et al., 2007]. As to be expected, panickers scored significantly higher on the PSS after CCK‐4 injection than nonpanickers. This difference was only attributable to significantly higher levels of subjective anxiety, whereas severity of somatic symptoms was uniform between the two groups. It is intriguing that in spite of the significant difference in CCK‐4‐induced anxiety severity cerebral activation patterns did not differ between panickers and nonpanickers. This lack of difference in functional neuroantomical responses between panickers and nonpanickers is in line with previous studies reporting that CCK‐4‐induced overall CBF changes or fMRI activation patterns [Benkelfat et al., 1995; Schunck et al., 2006] are not more pronounced or extended in subjects, who panicked after CCK‐4 challenge.
Although the amygdala plays a key role for the neuroanatomy of panic [Gorman et al., 2000], so far, previous studies investigating the functional neuroanatomy of CCK‐4‐induced panic did not investigate the amygdala region during the experimental challenge procedure. We questioned to what extent amygdala activation contributes to CCK‐4‐induced panic attacks. Therefore, we performed a region of interest analysis for this special brain area. In contrast to placebo, CCK‐4‐induced a significant activation in both amygdalae. However, opposite to the observed overall brain activation patterns after CCK‐4, the activation pattern in the amygdala region was not uniform in all subjects. Pronounced amygdala activation was only found in 5 of 11 subjects. We therefore distinguished subjects with high amygdala activation from those with low activation. Subjects with high amygdala activation rated considerably higher PSS fear items, but did not report a higher overall panic symptom severity. Although difference in fear severity did not reach statistical significance, probably due to the small sample size, our results indicate that in contrast to overall panic symptom severity the perception of CCK‐4‐induced fear symptom severity might be related to the extent of amygdala activation.
A neuroanatomical model of PD and experimentally induced panic attacks has been proposed, which suggests that the administration of a panicogenic substance causes a nonspecific activation, while only susceptible subjects with an abnormally sensitive neuroanatomical fear network respond with a panic attack [Gorman et al., 2000; Windmann, 1998]. Taking into account that the pattern of autonomic and neuroendocrine response is not uniform during panic attacks and that different acting agents are equally capable to induce panic it has been suggested that the abnormally sensitive fear network does not only include the amygdala and its projections to the brainstem and hypothalamus but also the prefrontal cortex, the insula, and the thalamus [Gorman et al., 2000; Windmann, 1998]. Therefore, experimental panicogens do not interact only with a specific brainstem area but activate the entire fear network. In addition, it may be suggested that intraindividual differences in the sensitivity of the endogenous fear network may account for intraindividual differences in panic sensitivity to experimental panic induction in healthy controls.
So far, amygdala activation has not been studied during experimental panic provocation in PD patients. However, enhanced amygdala activity detected in these patients during rest [Sakai et al., 2005] points towards enhanced amygdala sensitivity and suggests a pathophysiological preattentive cognitive bias towards threat stimuli in PD.
In addition, the importance of the amygdala as the center of the endogenous fear network, that plays a crucial role for fear perception, has been demonstrated in several neuroimaging studies. In contrast to neutral, happy, anger or disgust stimuli a specific activation of the amygdala has been found in healthy subjects after presentation of fearful faces [Breiter et al., 1996; Morris et al., 1996, 1998, 2002; Whalen et al., 2001], even if these faces were not perceived consciously [Whalen et al., 1998].
In patients suffering from different anxiety disorders, experimental order‐specific anxiety provocation, such as exposure to imagery scripts in PTSD [Rauch and Shin, 1997], to ideographic tailored stimuli designed to provoke the patients symptoms in OCD [Breiter et al., 1996], or to neutral face expression in social phobia [Birbaumer et al., 1998] was found to be accompanied by a significant amygdala activation. Furthermore, activation of the amygdala and the periamygdaloid cortex has been demonstrated during conditioned fear acquisition [LaBar et al., 1998] which rapidly habituates [Buchel et al., 1998], suggesting an important role of the amygdala as a rapid information processing pathway for novel, behavioral relevant signals [Buchel et al., 1998].
Our observation of considerably higher levels of fear points toward the major role of the amygdala in the early perception of CCK‐4‐induced threat in single subjects with high amygdala activation. However, these subjects did not report higher overall levels of CCK‐4‐induced panic symptom severity. In contrast to the role of the amygdala in the early perception of fear the impact in the subsequent subjective process of feeling anxiety is less clear. During self‐generated fear, induced by recalled stimulus images, no amygdala activation has been detected [Damasio et al., 2000]. This lack of amygdala activation has been attributed to the fact that the amygdala is more likely activated during the induction of emotion but not during the subsequent feeling of emotion [Buchel et al., 1998; Damasio et al., 2000; Davidson and Irwin, 1999] where the recruitment of additional limbic or paralimbic areas may be relevant. Within the prefrontal cortex (PFC) which is supposed to play a crucial role for affective memory working, the right inferior and right medial orbital PFC have been found to be activated during experimental provocation of anxiety [Rauch and Shin, 1997]. Furthermore, within the PFC the ACC is the region where attention and emotional functions are integrated and which plays a major role in the modulation of emotional responses [Damasio et al., 2000]. In addition, it has been suggested that depending on the degree of conscious awareness of fear [Williams et al., 2006] other brain areas may be further involved in the subjective experience of anxiety. Enhanced amygdala activity has been found in both, during subliminal and supraliminal fearful face perception. However, time series analysis revealed right amygdala response and right ventral ACC activity during subliminal fear perception, while supraliminal fear perception was associated with greater activity of the dorsal left amygdala, the dorsal ACC and with greater medial prefrontal activity [Williams et al., 2006]. Therefore, the amygdala may be relevant for the perception of fear, however, reciprocal connections between the amygdala and the sensory thalamus, the prefrontal cortex, the ACC and insula are suggested to be important for the neurocognitive processing of anxiety [Gorman et al., 2000] and, therefore, the observed enhanced brain activity after CCK‐4 in ACC, middle and superior frontal gyrus and other areas may be relevant for the subjective feeling of CCK‐4‐induced anxiety.
As a limitation of our study it should be considered that we studied only healthy men. Therefore we cannot exclude certain gender‐related effects on CCK‐4‐induced brain activity. However, because previous studies suggested that the reactivity to CCK‐4 in women may vary during the menstrual cycle [Le Melledo et al., 1995, 1999] the study was restricted to healthy males in order to avoid putative confounding effects of gender on the sensitivity to CCK‐4. Furthermore, an increased rate of subjects who panicked after CCK‐4 might have enhanced the observed functional effects. However, as an additional objective was to answer the question whether panickers versus nonpanickers differ in their functional response to CCK‐4, we were not able to examine exclusively highly CCK‐4 sensitive subjects. Therefore, further studies should not only address the impact of gender on CCK‐4‐induced brain activity but may also enhance the intensity of functional effects by recruitment of highly CCK‐4 sensitive subjects.
In conclusion, while overall brain activation patterns are not related to the subjective anxiety response to CCK‐4, amygdala activation may be involved in the subjective perception of CCK‐4‐induced anxiety. Further studies should assess the effects of anxiolytic compounds in the combined CCK‐4 challenge/fMRI paradigm to gain further insights into the involvement of the underlying fear network in the pathophysiology of panic attacks and the functional neuroanatomy of anxiolytic treatment.
REFERENCES
- Akiyoshi J,Isogawa K,Tsutsumi T,Kasturagi S,Kohno K,Furuta M,Yamamoto Y,Yamada K,Fujii I ( 1997): Cholecystokinin tetrapeptide‐induced calcium mobilization in T cells of patients with panic disorder, major depression, or schizophrenia. Biol Psychiatry 42: 151–154. [DOI] [PubMed] [Google Scholar]
- Aluoja A,Shlik J,Vasar V,Kingisepp PH,Jagomagi K,Vasar E,Bradwejn J ( 1997): Emotional and cognitive factors connected with response to cholecystokinin tetrapeptide in healthy volunteers. Psychiatry Res 66: 59–67. [DOI] [PubMed] [Google Scholar]
- Aronson TA,Whitaker‐Azmitia P,Caraseti I ( 1989): Differential reactivity to lactate infusions: the relative role of biological, psychological, and conditioning variables. Biol Psychiatry 25: 469–481. [DOI] [PubMed] [Google Scholar]
- Beinfeld MC,Meyer DK,Eskay RL,Jensen RT,Brownstein MJ ( 1981): The distribution of cholecystokinin immunoreactivity in the central nervous system of the rat as determined by radioimmunoassay. Brain Res 212: 51–57. [DOI] [PubMed] [Google Scholar]
- Benkelfat C,Bradwejn J,Meyer E,Ellenbogen M,Milot S,Gjedde A,Evans A ( 1995): Functional neuroanatomy of CCK4‐induced anxiety in normal healthy volunteers. Am J Psychiatry 152: 1180–1184. [DOI] [PubMed] [Google Scholar]
- Birbaumer N,Grodd W,Diedrich O,Klose U,Erb M,Lotze M,Schneider F,Weiss U,Flor H ( 1998): fMRI reveals amygdala activation to human faces in social phobics. Neuroreport 9: 1223–1226. [DOI] [PubMed] [Google Scholar]
- Boshuisen ML,Ter Horst GJ,Paans AM,Reinders AA,den Boer JA ( 2002): rCBF differences between panic disorder patients and control subjects during anticipatory anxiety and rest. Biol Psychiatry 52: 126–135. [DOI] [PubMed] [Google Scholar]
- Bradwejn J,Koszycki D ( 1994a): Imipramine antagonism of the panicogenic effects of cholecystokinin tetrapeptide in panic disorder patients. Am J Psychiatry 151: 261–263. [DOI] [PubMed] [Google Scholar]
- Bradwejn J,Koszycki D ( 1994b): The cholecystokinin hypothesis of anxiety and panic disorder. Ann NY Acad Sci 713: 273–282. [DOI] [PubMed] [Google Scholar]
- Bradwejn J,Koszycki D ( 2001): Cholecystokinin and panic disorder: Past and future clinical research strategies. Scand J Clin. Lab Invest 61( Suppl): 19–27. [PubMed] [Google Scholar]
- Bradwejn J,Koszycki D,Meterissian G ( 1990): Cholecystokinin‐tetrapeptide induces panic attacks in patients with panic disorder. Can J Psychiatry 35: 83–85. [DOI] [PubMed] [Google Scholar]
- Bradwejn J,Koszycki D,Shriqui C ( 1991): Enhanced sensitivity to cholecystokinin tetrapeptide in panic disorder. Clinical and behavioral findings. Arch Gen Psychiatry 48: 603–610. [DOI] [PubMed] [Google Scholar]
- Bradwejn J,Koszycki D,Payeur R,Bourin M,Borthwick H ( 1992): Replication of action of cholecystokinin tetrapeptide in panic disorder: Clinical and behavioral findings. Am J Psychiatry 149: 962–964. [DOI] [PubMed] [Google Scholar]
- Bradwejn J,Koszycki D,Couetoux du TA,van Megen H,den Boer J,Westenberg H ( 1994): The panicogenic effects of cholecystokinin‐tetrapeptide are antagonized by L‐365,260, a central cholecystokinin receptor antagonist, in patients with panic disorder. Arch Gen Psychiatry 51: 486–493. [DOI] [PubMed] [Google Scholar]
- Bradwejn J,LeGrand JM,Koszycki D,Bates JH,Bourin M ( 1998): Effects of cholecystokinin tetrapeptide on respiratory function in healthy volunteers. Am J Psychiatry 155: 280–282. [DOI] [PubMed] [Google Scholar]
- Brambilla F,Bellodi L,Perna G,Garberi A,Panerai A,Sacerdote P ( 1993): Lymphocyte cholecystokinin concentrations in panic disorder. Am J Psychiatry 150: 1111–1113. [DOI] [PubMed] [Google Scholar]
- Breiter HC,Etcoff NL,Whalen PJ,Kennedy WA,Rauch SL,Buckner RL,Strauss MM,Hyman SE,Rosen BR ( 1996): Response and habituation of the human amygdala during visual processing of facial expression. Neuron 17: 875–887. [DOI] [PubMed] [Google Scholar]
- Buchel C,Morris J,Dolan RJ,Friston KJ ( 1998): Brain systems mediating aversive conditioning: An event‐related fMRI study. Neuron 20: 947–957. [DOI] [PubMed] [Google Scholar]
- Bush G,Luu P,Posner MI ( 2000): Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4: 215–222. [DOI] [PubMed] [Google Scholar]
- Damasio AR,Grabowski TJ,Bechara A,Damasio H,Ponto LL,Parvizi J,Hichwa RD ( 2000): Subcortical and cortical brain activity during the feeling of self‐generated emotions. Nat Neurosci 3: 1049–1056. [DOI] [PubMed] [Google Scholar]
- Davidson RJ,Irwin W ( 1999): The functional neuroanatomy of emotion and affective style. Trends Cogn Sci 3: 11–21. [DOI] [PubMed] [Google Scholar]
- de Montigny C ( 1989): Cholecystokinin tetrapeptide induces panic‐like attacks in healthy volunteers. Preliminary findings. Arch Gen Psychiatry 46: 511–517. [DOI] [PubMed] [Google Scholar]
- Devinsky O,Morrell MJ,Vogt BA ( 1995): Contributions of anterior cingulate cortex to behaviour. Brain 118: 279–306. [DOI] [PubMed] [Google Scholar]
- Eser D,Schüle C,Baghai T,Floesser A,Krebs‐Brown A,Enunwa M,de la MS,Engel R,Kucher K,Rupprecht R ( 2007): Evaluation of the CCK‐4 model as a challenge paradigm in a population of healthy volunteers within a proof‐of‐concept study. Psychopharmacology 192: 479–487. [DOI] [PubMed] [Google Scholar]
- Flint AJ,Koszycki D,Vaccarino FJ,Cadieux A,Boulenger JP,Bradwejn J ( 1998): Effect of aging on cholecystokinin‐induced panic. Am J Psychiatry 155: 283–285. [DOI] [PubMed] [Google Scholar]
- Gorman JM,Kent JM,Sullivan GM,Coplan JD ( 2000): Neuroanatomical hypothesis of panic disorder, revised. Am J Psychiatry 157: 493–505. [DOI] [PubMed] [Google Scholar]
- Heinz A,Braus DF,Smolka MN,Wrase J,Puls I,Hermann D,Klein S,Grusser SM,Flor H,Schumann G,Mann K,Buchel C ( 2005): Amygdala‐prefrontal coupling depends on a genetic variation of the serotonin transporter. Nat Neurosci 8: 20–21. [DOI] [PubMed] [Google Scholar]
- Javanmard M,Shlik J,Kennedy SH,Vaccarino FJ,Houle S,Bradwejn J ( 1999): Neuroanatomic correlates of CCK‐4‐induced panic attacks in healthy humans: A comparison of two time points. Biol Psychiatry 45: 872–882. [DOI] [PubMed] [Google Scholar]
- Kennedy JL,Bradwejn J,Koszycki D,King N,Crowe R,Vincent J,Fourie O ( 1999): Investigation of cholecystokinin system genes in panic disorder. Mol Psychiatry 4: 284–285. [DOI] [PubMed] [Google Scholar]
- Koszycki D,Cox BJ,Bradwejn J ( 1993): Anxiety sensitivity and response to cholecystokinin tetrapeptide in healthy volunteers. Am J Psychiatry 150: 1881–1883. [DOI] [PubMed] [Google Scholar]
- Koszycki D,Zacharko RM,Bradwejn J ( 1996a): Influence of personality on behavioral response to cholecystokinin‐tetrapeptide in patients with panic disorder. Psychiatry Res 62: 131–138. [DOI] [PubMed] [Google Scholar]
- Koszycki D,Zacharko RM,Le Melledo JM,Young SN,Bradwejn J ( 1996b): Effect of acute tryptophan depletion on behavioral, cardiovascular, and hormonal sensitivity to cholecystokinin‐tetrapeptide challenge in healthy volunteers. Biol Psychiatry 40: 648–655. [DOI] [PubMed] [Google Scholar]
- Koszycki D,Zacharko RM,Le Melledo JM,Bradwejn J ( 1998): Behavioral, cardiovascular, and neuroendocrine profiles following CCK‐4 challenge in healthy volunteers: A comparison of panickers and nonpanickers. Depress Anxiety 8: 1–7. [PubMed] [Google Scholar]
- LaBar KS,Gatenby JC,Gore JC,LeDoux JE,Phelps EA ( 1998): Human amygdala activation during conditioned fear acquisition and extinction: A mixed‐trial fMRI study. Neuron 20: 937–945. [DOI] [PubMed] [Google Scholar]
- Le Melledo JM,Bradwejn J,Koszycki D,Bichet D ( 1995): Premenstrual dysphoric disorder and response to cholecystokinin‐tetrapeptide. Arch Gen Psychiatry 52: 605–606. [DOI] [PubMed] [Google Scholar]
- Le Melledo JM,Merani S,Koszycki D,Bellavance F,Palmour R,Gutkowska J,Steinberg S,Bichet DG,Bradwejn J ( 1999): Sensitivity to CCK‐4 in women with and without premenstrual dysphoric disorder (PMDD): During their follicular and luteal phases. Neuropsychopharmacology 20: 81–91. [DOI] [PubMed] [Google Scholar]
- Le Melledo JM,Arthur H,Dalton J,Woo C,Lipton N,Bellavance F,Koszycki D,Boulenger JP,Bradwejn J ( 2001): The influence of Type A behavior pattern on the response to the panicogenic agent CCK‐4. J Psychosom Res 51: 513–520. [DOI] [PubMed] [Google Scholar]
- Lydiard RB,Ballenger JC,Laraia MT,Fossey MD,Beinfeld MC ( 1992): CSF cholecystokinin concentrations in patients with panic disorder and in normal comparison subjects. Am J Psychiatry 149: 691–693. [DOI] [PubMed] [Google Scholar]
- Moran TH,Robinson PH,Goldrich MS,McHugh PR ( 1986): Two brain cholecystokinin receptors: Implications for behavioral actions. Brain Res 362: 175–179. [DOI] [PubMed] [Google Scholar]
- Morris JS,Frith CD,Perrett DI,Rowland D,Young AW,Calder AJ,Dolan RJ ( 1996): A differential neural response in the human amygdala to fearful and happy facial expressions. Nature 383: 812–815. [DOI] [PubMed] [Google Scholar]
- Morris JS,Friston KJ,Buchel C,Frith CD,Young AW,Calder AJ,Dolan RJ ( 1998): A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain 121: 47–57. [DOI] [PubMed] [Google Scholar]
- Morris JS,deBonis M,Dolan RJ ( 2002): Human amygdala responses to fearful eyes. Neuroimage 17: 214–222. [DOI] [PubMed] [Google Scholar]
- Rauch SL,Shin LM ( 1997): Functional neuroimaging studies in posttraumatic stress disorder. Ann NY Acad Sci 821: 83–98. [DOI] [PubMed] [Google Scholar]
- Rapee RM,Telfer LA,Barlow DH ( 1991): The role of safety cues in mediating the response to inhalations of CO2 in agoraphobics. Behav Res Ther 29: 353–355. [DOI] [PubMed] [Google Scholar]
- Rehfeld JF ( 1985): Neuronal cholecystokinin: One or multiple transmitters? J Neurochem 44: 1–10. [DOI] [PubMed] [Google Scholar]
- Rehfeld JF,Neilson FC ( 1995): Molecular form and regional distribution of cholecystokinin in the central and peripheral nervous system In: Bradwejn J,Vasar E, editors. Cholecystokinin and Anxiety: From Neuron to Behaviour. Austin: Springer‐Verlag‐RG; pp 33–56. [Google Scholar]
- Reiman EM,Raichle ME,Robins E,Mintun MA,Fusselman MJ,Fox PT,Price JL,Hackman KA ( 1989): Neuroanatomical correlates of a lactate‐induced anxiety attack. Arch Gen Psychiatry 46: 493–500. [DOI] [PubMed] [Google Scholar]
- Sakai Y,Kumano H,Nishikawa M,Sakano Y,Kaiya H,Imabayashi E,Ohnishi T,Matsuda H,Yasuda A,Sato A,Diksic M,Kuboki T ( 2005): Cerebral glucose metabolism associated with a fear network in panic disorder. Neuroreport 16: 927–931. [DOI] [PubMed] [Google Scholar]
- Schunck T,Erb G,Mathis A,Gilles C,Namer IJ,Hode Y,Demaziere A,Luthringer R,Macher JP ( 2006): Functional magnetic resonance imaging characterization of CCK‐4‐induced panic attack and subsequent anticipatory anxiety. Neuroimage 31: 1197–1208. [DOI] [PubMed] [Google Scholar]
- Shlik J,Aluoja A,Vasar V,Vasar E,Podar T,Bradwejn J ( 1997): Effects of citalopram treatment on behavioural, cardiovascular and neuroendocrine response to cholecystokinin tetrapeptide challenge in patients with panic disorder. J Psychiatry Neurosci 22: 332–340. [PMC free article] [PubMed] [Google Scholar]
- Spinhoven P,Onstein EJ,Sterk PJ,Haen‐Versteijnen D ( 1993): Discordance between symptom and physiological criteria for the hyperventilation syndrome. J Psychosom Res 37: 281–289. [DOI] [PubMed] [Google Scholar]
- Talaraich J,Tournoux P ( 1988): Co‐planar stereotaxic atlas of the human brain. Stuttgart: Thieme. [Google Scholar]
- van Megen HJ,Westenberg HG,den Boer JA,Haigh JR,Traub M ( 1994): Pentagastrin induced panic attacks: Enhanced sensitivity in panic disorder patients. Psychopharmacology (Berl) 114: 449–455. [DOI] [PubMed] [Google Scholar]
- van Megen HJ,Westenberg HG,den Boer JA,Kahn RS ( 1996): Cholecystokinin in anxiety. Eur Neuropsychopharmacol 6: 263–280. [DOI] [PubMed] [Google Scholar]
- van Megen HJ,Westenberg HG,den Boer JA,Slaap B,Scheepmakers A ( 1997): Effect of the selective serotonin reuptake inhibitor fluvoxamine on CCK‐4 induced panic attacks. Psychopharmacology (Berl) 129: 357–364. [DOI] [PubMed] [Google Scholar]
- Whalen PJ,Rauch SL,Etcoff NL,McInerney SC,Lee MB,Jenike MA ( 1998): Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci 18: 411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ,Shin LM,McInerney SC,Fischer H,Wright CI,Rauch SL ( 2001): A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion 1: 70–83. [DOI] [PubMed] [Google Scholar]
- Williams LM,Liddell BJ,Kemp AH,Bryant RA,Meares RA,Peduto AS,Gordon E ( 2006): Amygdala‐prefrontal dissociation of subliminal and supraliminal fear. Hum Brain Mapp 27: 652–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windmann S ( 1998): Panic disorder from a monistic perspective: Integrating neurobiological and psychological approaches. J Anxiety Disord 12: 485–507. [DOI] [PubMed] [Google Scholar]
- Zwanzger P,Eser D,Aicher S,Schule C,Baghai TC,Padberg F,Ella R,Moller HJ,Rupprecht R ( 2003): Effects of alprazolam on cholecystokinin‐tetrapeptide‐induced panic and hypothalamic‐pituitary‐adrenal‐axis activity: a placebo‐controlled study. Neuropsychopharmacology 28: 979–984. [DOI] [PubMed] [Google Scholar]


