Abstract
Navigated transcranial magnetic stimulation (TMS) can be used to stimulate functional cortical areas at precise anatomical location to induce measurable responses. The stimulation has commonly been focused on anatomically predefined motor areas: TMS of that area elicits a measurable muscle response, the motor evoked potential. In clinical pathologies, however, the well‐known homunculus somatotopy theory may not be straightforward, and the representation area of the muscle is not fixed. Traditionally, the anatomical locations of TMS stimulations have not been reported at the group level in standard space. This study describes a methodology for group‐level analysis by investigating the normal representation areas of thenar and anterior tibial muscle in the primary motor cortex. The optimal representation area for these muscles was mapped in 59 healthy right‐handed subjects using navigated TMS. The coordinates of the optimal stimulation sites were then normalized into standard space to determine the representation areas of these muscles at the group‐level in healthy subjects. Furthermore, 95% confidence interval ellipsoids were fitted into the optimal stimulation site clusters to define the variation between subjects in optimal stimulation sites. The variation was found to be highest in the anteroposterior direction along the superior margin of the precentral gyrus. These results provide important normative information for clinical studies assessing changes in the functional cortical areas because of plasticity of the brain. Furthermore, it is proposed that the presented methodology to study TMS locations at the group level on standard space will be a suitable tool for research purposes in population studies. Hum Brain Mapp, 2010. © 2010 Wiley‐Liss, Inc.
Keywords: navigated brain stimulation, transcranial magnetic stimulation, group‐level analysis, brain, motor cortex, hand knob, excitability, thenar muscle, anterior tibial muscle
INTRODUCTION
The traditional concept of homunculus somatotopy of the peripheral musculature [Penfield and Rasmussen,1950] has been studied extensively [Barinaga,1995; Beisteiner et al.,2004; Kapreli et al.,2007]. However, the exact point‐to‐point representation of homunculus somatotopy has been shown to be much more complex because there are large variations between individuals in the motor‐sensory cortex [Branco et al.,2003; Farrell et al.,2007]. Furthermore, functional representation areas of different muscles have been reported to change in relation to certain diseases and conditions such as joint immobilization [Liepert et al.,1995; Zanette et al.,1997], chronic pain [Flor,2002,2003; Flor et al.,1997] or stroke [Byrnes et al.,2001; Cramer and Crafton,2006]. On the other hand, brain plasticity may also be affected by therapy [Levy et al.,2001] or learning and experience [Büchel et al.,1998; Kilgard et al.,2001; Münte et al.,2002; Singer,1995].
At present, several different brain mapping methods such as functional magnetic resonance imaging (fMRI), magnetoencephalography (MEG), and transcranial magnetic stimulation (TMS) can be used in clinical studies assessing functional representation areas. TMS is a non‐invasive method that selectively stimulates cortical areas using a magnetic pulse [Barker et al.,1985]. There are several methods to position the TMS coil over the head and target the TMS to certain areas on the cortex. It has been shown that positioning the TMS coil based on individual fMRI results yields a stronger behavioral effect size than when positioning of the coil is based either solely on individual anatomy (MR images), group‐averaged fMRI results or 10–20 EEG positioning [Herwig et al.,2003; Sack et al.,2009; Sparing et al.,2008]. Furthermore, systems have been developed to assist in TMS coil placement and stimulation based on MR images, while the individual is inside the MR scanner [Bohning et al.,2003; Denslow et al.,2005]. Positioning the TMS coil and performing the whole stimulation session inside the MR scanner enables accurate coil location in relation to individual anatomy, but it limits the moving of the coil and stimulating over several locations due to lack of space. In addition, the measurement of the TMS induced response is technically demanding. Therefore, it is not always possible to perform an fMRI study before TMS, and it is especially difficult during the procedure.
When the stimulation system includes an MRI‐based navigation method, the targeting of the magnetic pulse to a specific cortical area can be performed visually based on individual cortex anatomy [Herwig et al.,2001; Julkunen et al.,2009]. This provides a very specific focusing of the TMS pulse to a specific area on the cortex and enables precise repetitive stimulation to the chosen site. When the TMS induced electromyography (EMG) response is measured simultaneously, the optimal site on the motor cortex can be determined based on both anatomical and functional information. Furthermore, some MRI‐based navigation systems keep a record of the stimulation sites in MR coordinates for subsequent analysis and this information can be utilized to compare representative areas found with different brain mapping modalities, e.g. TMS and fMRI. It has been shown that these two methods correspond well with each other [Boroojerdi et al.,1999; Herwig et al.,2002; Krings et al.,2001a,b; Lotze et al.,2003]. In our study, the coil was positioned using both the information of individual anatomy (MR images) and the induced function (EMG responses measured from the target muscle). We believe that this procedure provides the best possible functional and anatomical localization of the TMS at the same time maximizing subject comfort and enabling stimulation over a large area.
TMS as a method to study brain function has lately gained more and more popularity. Traditionally, the locations of the TMS stimulations have not been reported on standard space at the group level, presumably because this requires specialized equipment that can record the stimulation sites in the MR coordinates. Earlier, Denslow et al. [2005] reported stimulation locations normalized to standard space. However, their objective was to examine the differences between function‐guided and image‐guided TMS using fMRI, not to study variations in the locations of the optimal stimulation sites. There are no large‐scale normalized materials for the representation areas of hand and leg muscles determined with TMS. One can hypothesize that baseline information on the normal variation in the optimal representation of muscles would improve detection of abnormal excitation sites. The aim of this study was to devise a methodology to report TMS locations in standard space and to determine baseline of representation areas on the motor cortex for thenar and anterior tibial muscles in a healthy population using navigated TMS. Furthermore, we utilized the methodology to investigate whether age and gender had any effect on the location of the optimal stimulation sites and to investigate the correlation of the location of the optimal stimulation site and anatomically defined hand‐knob at the group level. We postulate that the normative data which we collected may in future be used to provide baseline information for clinical studies of brain plasticity.
METHODS
Subjects
This study examined 59 healthy right‐handed volunteers (31 women and 28 men) recruited from hospital and university staff, student body, and the local community. The same subjects participated in our recent study, where we reported normal clinical neurophysiological values for most common TMS parameters determined using navigation [Säisänen et al.,2008b]. None of the subjects had any central nervous system (CNS) diseases or psychiatric disorders and none of them were taking medications with known CNS effects, or had any commonly accepted contraindications for MRI or TMS. The subjects gave their written informed consent prior to participating. The study was approved by the local ethics committee.
Age range of the subjects was 22–79 years, evenly distributed (appr. 10 subjects per decade). Handedness was determined according to the Waterloo Handedness Questionnaire‐revised and reduced form with 20 items.
Measurement Setups
Magnetic resonance imaging (MRI) was performed with a 1.5T Siemens Magnetom Avanto (Erlangen, Germany) scanner using a standard 8‐channel head coil and a T1‐weighted sequence (TR 1980 ms, TE 3.93 ms, matrix 256 × 256, 176 sagittal slices, slice thickness 1.0 mm, in‐slice resolution of 1.0 mm × 1.0 mm). The MR images were used in the rendering of a 3D image of each subject's head for the navigation (Fig. 1).
Figure 1.
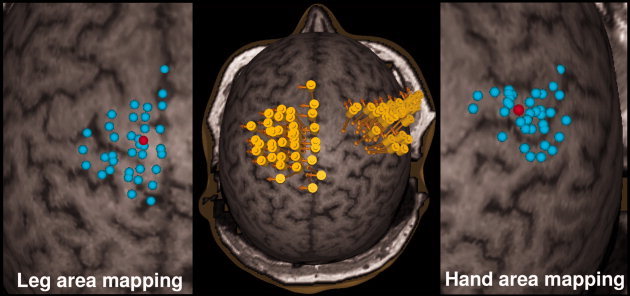
The primary motor cortex and the surrounding areas were thoroughly mapped for thenar (hand) and anterior tibial (leg) muscle representation areas. The middle image shows the cortical stimulation areas with individual stimuli for leg and hand areas. The yellow cylinders represent the location of the coil during stimulation. The direction of the induced electrical current is illustrated with orange arrows. Stimulation foci are shown in the close‐ups for both areas (leg on the left and hand on the right). Red dots represent the optimal stimulation sites inducing the highest MEP response.
The TMS setup consisted of eXimia navigation system combined with a magnetic stimulator and two figure‐of‐eight TMS coils with mean wing radius of 50 mm (eXimia NBS System and TMS stimulator, Nexstim Helsinki Finland). Both biphasic pulse type and monophasic pulse type were used. The navigation system combines individual MR images with online infrared‐tracking of the subject's head and the TMS coil in use. With the information of the coil geometry and orientation relative to the subject's head, the eXimia navigation software estimates the magnetic stimulation induced electric field on the visualized reconstructed cortex surface [Ruohonen and Ilmoniemi,1999]. The stimulation site in eXimia navigation software is defined as the maximum of the induced electric field on the chosen surface. Thus, the location of the maximum electric field on the surface is based on mathematical modeling of the electrical conductivity of the subject's head and not only a projection of a virtual rod from the TMS coil.
During stimulation, EMG was recorded and monitored continuously on‐line (ME 6000, Mega Electronics Ltd., Kuopio, Finland). EMG was measured using pregelled disposable Ag/AgCl electrodes. The active electrode was attached to the skin overlying the thenar (abductor pollicis brevis) and the reference electrode on the first metacarpophalangeal joint when examining the hand motor area. On the lower limb, the active electrode was placed on the anterior tibial muscle with the reference on the tibial bone approximately 10 cm distally. The EMG signals were filtered to the 8–500 Hz band, amplified, displayed and stored for off‐line analysis. The TMS system delivered triggering pulses that synchronized the TMS and EMG systems.
Localization of the Optimal Stimulation Sites
The induced electric field is greatest near the TMS coil and predominantly causes excitation at bends of the pyramidal axons [Ruohonen and Ilmoniemi,1999]. Therefore, in this study, we chose the depth to be as near the TMS coil as possible (strong electrical field) with as much axonal bends as possible (large probability to cause activation). The depth was chosen for each subject similarly based on their individual anatomy to be the outermost surface where gray and white matter are distinguishable. We wanted to focus on variation on anteroposterior and lateral‐medial directions in the optimal location, as they are the directions that can be measured when using TMS and that affect the placement of the TMS coil. The depth was therefore fixed as well as possible based on individual anatomy. The optimal cortical stimulation site for studied muscles was defined as the location of the maximum of the electric field on the visualized surface where TMS evoked the largest EMG‐response in the relaxed target muscle. To ensure the relaxation of the muscle, the background EMG activity was visually monitored during mapping, and stimulations were given only when the examined limb was relaxed [Säisänen et al.,2008a].
The localization of the optimal stimulation site was separately performed with both bi‐ and mono‐phasic pulse forms for the thenar muscle, and with the biphasic pulse form for the anterior tibial muscle, thus resulting in six mapped stimulation locations for each subject. The optimal stimulation site using the monophasic pulse form for the anterior tibial muscle was not defined, because in most of the subjects, the maximum stimulator output of the TMS system was insufficient to evoke EMG‐responses with the monophasic pulse form [Säisänen et al.,2008b]. To locate the optimal stimulation site for the thenar muscle, the hand muscle area around the anatomically defined cortical “hand knob” [Yousry et al.,1997] was extensively mapped. With the biphasic pulse form, the starting stimulation intensity was 45 or 50% of the stimulator maximum output depending on the age of the subject. The mapping intensity was adjusted individually (average: 52%, range: 35–75% of the stimulator maximum output) so that MEPs of around 200–300 μV were elicited somewhere around the hand knob. With the monophasic pulse form the required mapping intensity was higher; average: 74%, range: 55–99%. The mapping was continued in each direction from the hand knob as long as MEPs were elicited. During stimulation, the coil was kept tangential to the surface of the head and the direction of the induced current perpendicular to the anatomically defined central sulcus (Fig. 1). For the anterior tibial muscle, the mapping was begun at the midline at the level of the central sulcus, then continued both anteriorly and posteriorly, and a few centimetres laterally (Fig. 1). The average mapping intensity was 76% of the stimulator maximum output (range: 50–99%). On average, 40 ± 15 stimuli were required to locate the optimal stimulus site and coil orientation. The stimulation order of the hemispheres was randomized in each subject.
Data Analysis
The navigation software was used to assess the location of the maximum of the TMS induced electric field in the cortex and it expresses this in MR coordinates. These coordinates were used to define the optimal stimulation site anatomically in the MR images. Subsequently, as a way to enable the group comparison of these sites, the individual MR images were spatially normalized to standard space as defined by the Montreal Neurological Institute ICBM‐152‐template using the SPM5‐software (The Wellcome Department of Imaging Neuroscience, Institute of Neurology, University College London, UK) running on Matlab 7.4 (The Mathworks, Natick, USA). The images were first manually aligned to the AC‐PC‐line in SPM5 to provide the best possible starting estimates for the normalization procedure. The normalized images were resampled into (2 × 2 × 2) mm3 voxel size. The location of a single voxel in the standard space can be determined using the deformation field calculated from each subject's inverse normalization parameterization. It is essential to use the inverse parameterization since the normal parameterization which is saved in the normalization step defines the warps from standard space to individual space. This procedure was used to normalize the MR‐coordinates of the optimal stimulation into the standard space. Normalization of the MR‐coordinates enables comparison of the optimal stimulation sites at the group‐level. To determine the normal variation of the optimal stimulation sites, ellipsoids of the 95% confidence interval were fitted to the clusters of the individual sites by estimating the lengths and directions of the ellipsoid main axis based on chi‐square distribution using Matlab.
Anatomical Measurements
The location of the hand‐knob in standard space was defined for each subject to check the anatomical consistency of the normalized images. The location of the hand‐knob was defined using three points of the inverted Ω‐shaped hand‐knob [Yousry et al.1997]: medial, central, and lateral corner (Fig. 2). The coordinates of these points were defined on both hemispheres at the depth of the individual optimal TMS‐location if the shape of the hand‐knob was visible at that level or at the nearest depth where it was visible.
Figure 2.
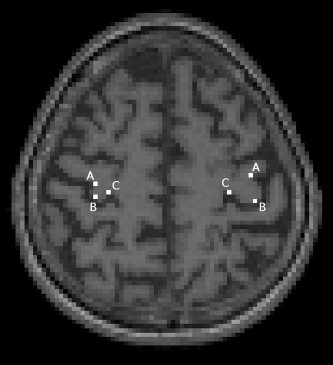
The anatomical measurements of the hand‐knob in the normalized space. Three points were defined in each hand‐knob: lateral (A), central (B), and medial (C) corner of the inverse Ω‐shape.
Statistical Analysis
All statistical analyses were performed using the normalized location coordinates. For each optimal stimulation site, the centre of mass of the individual normalized coordinates was calculated. The Euclidian distance and the distance in each Cartesian direction between the individual site and the centre of mass were calculated for each subject. The statistical analyses were performed with SPSS 14.0 (SPSS Inc., Chicago, IL). The correlation of the variance in the optimal stimulation site between age, head circumference, and hand‐knob coordinates was tested with Pearson correlation. Independent samples t‐test was used to test if there was a gender difference in optimal stimulation sites. Differences between hand‐knob coordinates and optimal stimulation site coordinates in both lateral‐medial and anteroposterior direction were tested with paired samples t‐test.
RESULTS
As expected, the optimal stimulation site for the thenar muscle was usually found at the hand knob or in the near vicinity and for anterior tibial muscle the optimal location was near to the longitudinal fissure on M1. The centres of mass of optimal stimulation sites in MNI‐coordinates are illustrated in Figure 3. There were no statistically significant differences in any direction in the locations of the optimal stimulation sites or anatomically defined hand‐knob between genders.
Figure 3.
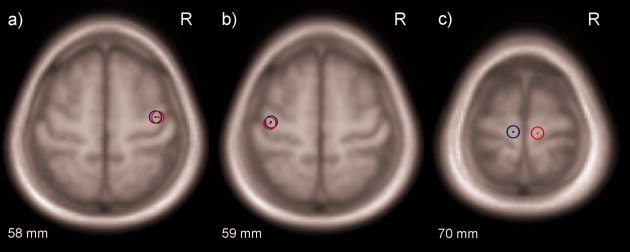
The centres of masses of the normalized optimal stimulation sites for (a) left thenar muscle (biphasic pulse form in red and monophasic in blue), b) right thenar muscle (biphasic pulse form in red and monophasic in blue), and c) right (blue) and left (red) anterior tibial muscle.
The coordinates of the centres of mass of the normalized optimal stimulation sites and the basis vectors of 95% confidence interval ellipsoids main axis, as well as the volumes of the ellipsoids are presented in Table I for both coils and both muscles. The normalized optimal stimulation sites with 95% confidence ellipsoids are illustrated in Figure 4. The observed variance in the optimal stimulation site did not correlate with age or head circumference.
Table I.
95% Confidence interval ellipsoids
| Centre of mass | Main axis' basis vectors (mm) | V (ml) | |
|---|---|---|---|
| Biphasic pulse form, thenar muscle | |||
| Right hemisphere | 40, −12, 57 | 4.0i + 0.4j + 4.5k | 5.9 |
| −3.0i – 13.0j + 4.0k | |||
| 12.2i – 6.0j – 10.2k | |||
| Left hemisphere | −37, −16, 59 | −3.5i – 0.1j + 4.2k | 5.4 |
| 11.1i + 4.0j + 9.3k | |||
| −3.4i + 15.0j – 2.4k | |||
| Biphasic pulse form, tibial muscle | |||
| Right hemisphere | 10, −24, 70 | 1.9i + 0.1j + 7.0k | 9.1 |
| 14.8i + 1.7j – 4.0k | |||
| −2.2i + 19.3j + 0.3k | |||
| Left hemisphere | −8, −23, 70 | −1.4i – 0.6j + 7.7k | 7.1 |
| 11.0i – 0.2j + 2.0k | |||
| 0.1i + 19.1j + 1.6k | |||
| Monophasic pulse form, thenar muscle | |||
| Right hemisphere | 38, −12, 58 | −3.9i – 0.6j – 4.2k | 5.0 |
| 6.5i + 8.6j – 7.2k | |||
| 8.6i – 11.8j – 6.2k | |||
| Left hemisphere | −36, −15, 59 | −2.8j + 0.1j + 4.3k | 5.2 |
| 9.4i – 2.5j + 6.1k | |||
| 4.1i + 20.6j + 2.1k | |||
The table presents the centres of mass of optimal stimulation sites in MNI‐coordinates, the basis vectors of the 95% confidence interval ellipsoids' main axis and the corresponding ellipsoids' volumes (V) in milliliters for all subjects according to the pulse form and studied muscle.
Figure 4.
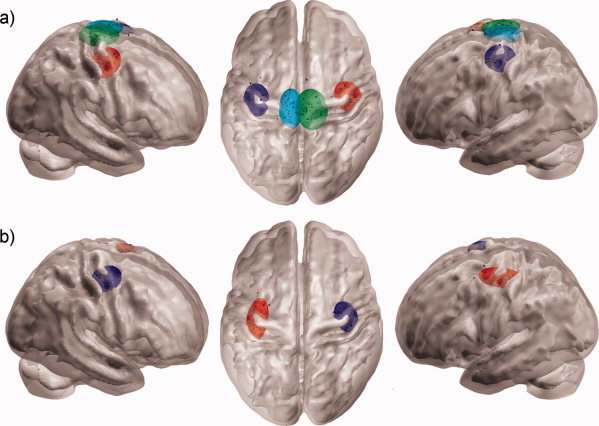
The normalized optimal stimulation sites (black dots) and their corresponding 95% confidence interval ellipsoids using (a) biphasic pulse form for left and right thenar muscle (red and dark blue ellipsoids, respectively) and left and right anterior tibial muscle (green and light blue ellipsoids, respectively), and (b) monophasic pulse form for left and right thenar muscle (dark blue and red ellipsoids, respectively). The outer surface of the rendered brain represents the gray matter/CSF boundary and the inner surface represents the gray matter/white matter boundary.
There were no statistically significant differences between lateral or central hand‐knob coordinates and optimal stimulation site coordinates for the thenar muscle using either of the coils in the lateral‐medial direction. However, with the medial hand‐knob coordinate, there was a significant difference (P < 0.001) so that the optimal stimulation sites were more laterally located. In anteroposterior direction, the optimal stimulation sites for thenar muscle were located more anteriorly than the hand‐knob. This difference was significant (P < 0.001) with all three hand‐knob coordinates.
There were no significant correlations between the hand‐knob coordinates and the optimal stimulation sites in the anteroposterior direction using either of the pulse forms, or in lateral direction when using biphasic pulse form. However, on the left hemisphere, the optimal stimulation site for the monophasic pulse form did correlate with all the hand‐knob points in lateral‐medial direction (P < 0.05, R = 0.29–0.40).
DISCUSSION
In this study, the normal optimal TMS sites were defined for thenar and anterior tibial muscles in the healthy population. Somatotopy of the thenar and anterior tibial representation areas in primary motor cortex followed the order reported with several imaging methods [Beisteiner et al.,2004; Hlustík et al.,2001; Van Gelderen et al.,2005; van Westen et al.,2004].
The individual optimal stimulation sites were normalized into standard space to enable group comparison. The spatial variation in the location of the optimal stimulation sites proved to be relatively high especially in the anterior‐posterior direction. Our finding of spatial variation between subjects is in line with previous findings of individual variability in motor cortex somatotopy. In particular, in studies using direct electrical stimulation extensive individual variation has been identified and the concept of homunculus somatotopy of the motor‐sensory cortex has been criticized [Branco et al.,2003; Farrell et al.,2007]. The largest variation in our study was found in anteroposterior direction. This is in agreement with an earlier test–retest study where the variation for the representation area of APB was larger in the anteroposterior direction than in the lateral direction [Malcolm et al.,2006]. Although these researchers studied variation within subjects, the observed intra‐individual variation in the anteroposterior direction may also have an effect on our inter‐individual results. The variation in optimal stimulation sites was further examined by fitting 95% confidence interval ellipsoids in clusters of optimal stimulation sites. The volumes of the ellipsoids represent the variation between the sites, i.e. the larger the volume, the greater the variation. The variations in optimal stimulation sites for the anterior tibial muscles were larger than for thenar muscle. This is logical since the representation area of leg muscles is known to be located on the medial surface of each hemisphere where it is more difficult to target the magnetic pulse as accurately as on the hand area. The monophasic pulse type has been shown to be more accurate in motor cortex excitation [Alexander and Maciunas,1999] which might explain the slightly smaller variation in the locations of the optimal stimulation site for thenar muscle using the monophasic pulse type than biphasic pulse type. The difference in variation between these two pulse forms, however, was not large in our study population, highlighting the larger variation between‐subjects than within‐subjects.
The optimal stimulation site for the thenar muscle was usually found anterior to the central sulcus and laterally from the medial corner of the anatomically defined hand‐knob. However, as there was no correlation between the hand‐knob location and the optimal stimulation site, the stimulation site in the TMS studies should not be defined purely based on individual anatomy. This is in line with previous studies in which different coil positioning approaches has been compared [Denslow et al.,2005; Sack et al.,2009; Sparing et al.,2008]. In these studies, positioning the coil based on individual fMRI results was found to be the most reliable approach. If individual fMRI results are not available, accurate and thorough mapping to identify the optimal site needs to be performed.
Navigated TMS enables the mapping of the cortex accurately only bidimensionally, i.e. in the anteroposterior and lateral directions. The third dimension, the depth, cannot be defined unambiguously due to the nature of the TMS technique. In this study, the depth has been systematically defined similarly in each subject as the depth where the gray and white matter first can be distinguished on the reconstructed surface of the 3D‐rendered MRI head. Therefore, the differences in the depth direction are not within the scope of this study. To study deeper structures of the brain in 3D and also more complex movement‐related functions, other methods, such as fMRI, need to be used. However, the better spatial resolution of navigated TMS on the cortex supports its use for mapping changes in the sensorimotor cortical areas. It has been proposed that combining the fMRI or PET with MEG and navigated TMS might be the optimal way to study brain plasticity [Rossini and Dal Forno,2004].
In general, navigated TMS is considered as very specific in mapping functional areas of the brain, especially the peripheral muscles [Ruohonen and Ilmoniemi,2002]. The accuracy of the aiming tool in the eXimia navigation software gives a precision of ±2 mm and ±2 degrees in rotation when consecutive stimuli are given to the same location. The specificity of individual optimal stimulation sites, however, may vary even in healthy subjects, e.g. due to varying level of alertness during the measurement session [Salih et al.,2005], fluctuating relaxation level in the target muscle [Begum et al.,2005], or errors caused by the measurement system. Commonly the reproducibility of the mapped location of the representation area of muscles is good from session‐to‐session using the eXimia navigation system [Danner et al.,2008; Julkunen et al.,2009]. The issues mentioned earlier may have led to some inaccuracy in defining the optimal stimulation sites in some of our subjects. However, because of the large number of subjects in this study, any systematic effect of the individual inaccuracies has been minimized. Another possible source of inaccuracy is the normalization of the individual MR images and stimulation sites into standard space. Although normalization is an essential step in comparing results between subjects, it may contribute some additional variation, depending on the success of the procedure. In fMRI studies; however, spatial normalization is regarded as a standard procedure when studying populations [Ashburner and Friston,1999] and the normalization method used in this study is one of the most commonly used techniques in fMRI studies. The precision of anatomical normalization of this method has been reported to have a standard deviation of about 1 mm in the medial temporal lobe [Salmond et al.,2002]. To further study the accuracy of the normalization, the hand‐knob location was defined in normalized MR images for each subject. The average standard deviation in the hand‐knob coordinates (medial, central, and lateral points, x‐ and y‐coordinates) was 4.4 mm (range: 3.6–4.9 mm) in our study population. This variation is not solely attributable to the normalization procedure; there is also variation in the shape of the hand‐knob thus affecting the chosen coordinates. Therefore, the possible variation caused by the normalization is acceptable and the accuracy was considered as adequate.
In summary, the normal functional area of healthy brain motor cortex was mapped and placed into standard space. MRI‐navigated TMS combined with image‐normalization procedure was found to be a suitable method with which to study group differences in localizing brain motor function. Furthermore, the methodology presented in this study could be used to study possible differences in the location of functional representation areas of muscles or other functionally active cortical areas caused by disease, learning, or experience. It is predicted that navigated TMS will prove to be a valuable method in the study of brain plasticity with an outcome comparable to fMRI.
Acknowledgements
The authors wish to thank all the subjects who volunteered for this study.
References
- Alexander E,Maciunas RJ ( 1999): Advanced Neurosurgical Navigation. New York: Thieme; 605 p. [Google Scholar]
- Ashburner J,Friston KJ ( 1999): Nonlinear spatial normalization using basis functions. Hum Brain Mapp 7: 254–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barinaga M ( 1995): Remapping the motor cortex. Science 268: 1696–1698. [DOI] [PubMed] [Google Scholar]
- Barker AT,Jalinous R,Freeston IL ( 1985): Non‐invasive magnetic stimulation of human motor cortex. Lancet 1: 1106–1107. [DOI] [PubMed] [Google Scholar]
- Begum T,Mima T,Oga T,Hara H,Satow T,Ikeda A,Nagamine T,Fukuyama H,Shibasaki H ( 2005): Cortical mechanisms of unilateral voluntary motor inhibition in humans. Neurosci Res 53: 428–435. [DOI] [PubMed] [Google Scholar]
- Beisteiner R,Gartus A,Erdler M,Mayer D,Lanzenberger R,Deecke L ( 2004): Magnetoencephalography indicates finger motor somatotopy. Eur J Neurosci 19: 465–472. [DOI] [PubMed] [Google Scholar]
- Bohning DE,Denslow S,Bohning PA,Walker JA,George MS ( 2003): A TMS coil positioning/holding system for MR image‐guided TMS interleaved with fMRI. Clin Neurophysiol 114: 2210–2219. [DOI] [PubMed] [Google Scholar]
- Boroojerdi B,Foltys H,Krings T,Spetzger U,Thron A,Topper R ( 1999): Localization of the motor hand area using transcranial magnetic stimulation and functional magnetic resonance imaging. Clin Neurophysiol 110: 699–704. [DOI] [PubMed] [Google Scholar]
- Branco DM,Coelho TM,Branco BM,Schmidt L,Calcagnotto ME,Portuguez M,Neto EP,Paglioli E,Palmini A,Lima JV,Da Costa JC ( 2003): Functional variability of the human cortical motor map: electrical stimulation findings in perirolandic epilepsy surgery. J Clin Neurophysiol 20: 17–25. [DOI] [PubMed] [Google Scholar]
- Büchel C,Price C,Frackowiak RS,Friston K ( 1998): Different activation patterns in the visual cortex of late and congenitally blind subjects. Brain 121: 409–419. [DOI] [PubMed] [Google Scholar]
- Byrnes ML,Thickbroom GW,Phillips BA,Mastaglia FL ( 2001): Long‐term changes in motor cortical organisation after recovery from subcortical stroke. Brain Res 889: 278–287. [DOI] [PubMed] [Google Scholar]
- Cramer SC,Crafton KR ( 2006): Somatotopy and movement representation sites following cortical stroke. Exp Brain Res 168: 25–32. [DOI] [PubMed] [Google Scholar]
- Danner N,Julkunen P,Könönen M,Säisänen L,Nurkkala J,Karhu J ( 2008): Navigated transcranial magnetic stimulation and comuted electric field strength reduce stimulator‐dependent differences in the motor threshold. J Neurosci Methods 174: 116–122. [DOI] [PubMed] [Google Scholar]
- Denslow S,Bohning DE,Bohning PA,Lomarev MP,George MS ( 2005): An increased precision comparison of TMS‐induced motor cortex BOLD fMRI response for image‐guided versus function‐guided coil placement. Cog Behav Neurol 18: 119–126. [DOI] [PubMed] [Google Scholar]
- Farrell DF,Burbank N,Lettich E,Ojemann GA ( 2007): Individual variation in human motor‐sensory (rolandic) cortex. J Clin Neurophysiol 24: 286–293. [DOI] [PubMed] [Google Scholar]
- Flor H,Braun C,Elbert T,Birbaumer N ( 1997): Extensive reorganization of primary somatosensory cortex in chronic back pain patients. Neurosci Lett 224: 5–8. [DOI] [PubMed] [Google Scholar]
- Flor H ( 2002): The modification of cortical reorganization and chronic pain by sensory feedback. Appl Psychophysiol Biofeedback 27: 215–227. [DOI] [PubMed] [Google Scholar]
- Flor H ( 2003): Cortical reorganisation and chronic pain: implications for rehabilitation. J Rehabil Med 41( Suppl): 66–72. [DOI] [PubMed] [Google Scholar]
- Herwig U,Schonfeldt‐Lecuona C,Wunderlich AP,von Tiesenhausen C,Thielscher A,Walter H,Spitzer M ( 2001): The navigation of transcranial magnetic stimulation. Psychiatry Res: Neuroimaging 108: 123–131. [DOI] [PubMed] [Google Scholar]
- Herwig U,Schonfeldt‐Lecuona C,Wunderlich AP,von Tiesenhausen C,Thielscher A,Walter H,Spitzer M ( 2002): Spatial congruence of neuronavigated transcranial magnetic stimulation and functional neuroimaging. Clin Neurophysiol 113: 462–468. [DOI] [PubMed] [Google Scholar]
- Herwig U,Satrapi P,Schonfeldt‐Lecuona C ( 2003): Using the international 10‐20 EEG system for positioning of transcranial magnetic stimulation. Brain Topogr 16: 95–99. [DOI] [PubMed] [Google Scholar]
- Hlustík P,Solodkin A,Gullapalli RP,Noll DC,Small SL ( 2001): Somatotopy in human primary motor and somatosensory hand representations revisited. Cereb Cortex 11: 312–321. [DOI] [PubMed] [Google Scholar]
- Julkunen P,Säisänen L,Danner N,Niskanen E,Hukkanen T,Mervaala E,Könönen M ( 2009): Comparison of navigated and non‐navigated transcranial magnetic stimulation for motor cortex mapping, motor threshold and motor evoked potentials. Neuroimage 44: 790–795. [DOI] [PubMed] [Google Scholar]
- Kapreli E,Athanasopoulos S,Papathanasiou M,Van Hecke P,Keleki D,Peeters R,Strimpakos N,Sunaert S ( 2007): Lower limb sensorimotor network: issues of somatotopy and overlap. Cortex 43: 219–232. [DOI] [PubMed] [Google Scholar]
- Kilgard MP,Pandya PK,Vazquez J,Gehi A,Schreiner CE,Merzenich MM ( 2001): Sensory input directs spatial and temporal plasticity in primary auditory cortex. J Neurophysiol 86: 326–338. [DOI] [PubMed] [Google Scholar]
- Krings T,Chiappa KH,Foltys H,Reinges MH,Cosgrove GR,Thron A ( 2001a): Introducing navigated transcranial magnetic stimulation as a refined brain mapping methodology. Neurosurg Rev 24: 171–179. [DOI] [PubMed] [Google Scholar]
- Krings T,Foltys H,Reinges MH,Kemeny S,Rohde V,Spetzger U,Gilsbach JM,Thron A ( 2001b): Navigated transcranial magnetic stimulation for presurgical planning—Correlation with functional MRI. Minim Invasive Neurosurg 44: 234–239. [DOI] [PubMed] [Google Scholar]
- Levy CE,Nichols DS,Schmalbrock PM,Keller P,Chakeres DW ( 2001): Functional MRI evidence of cortical reorganization in upper‐limb stroke hemiplegia treated with constraint‐induced movement therapy. Am J Phys Med Rehabil 80: 4–12. [DOI] [PubMed] [Google Scholar]
- Liepert J,Tegenthoff M,Malin JP ( 1995): Changes of cortical motor area size during immobilization. Electroencephalogr Clin Neurophysiol 97: 382–386. [DOI] [PubMed] [Google Scholar]
- Lotze M,Kaethner RJ,Erb M,Cohen LG,Grodd W,Topka H ( 2003): Comparison of representational maps using functional magnetic resonance imaging and transcranial magnetic stimulation. Clin Neurophysiol 114: 306–312. [DOI] [PubMed] [Google Scholar]
- Malcolm MP,Triggs WJ,Light KE,Shechtman O,Khandekar G,Gonzalez Rothi LJ ( 2006): Reliability of motor cortex transcranial magnetic stimulation in four muscle representations. Clin Neurophysiol 117: 1037–1046. [DOI] [PubMed] [Google Scholar]
- Münte TF,Altenmüller E,Jäncke L ( 2002): The musician's brain as a model of neuroplasticity. Nat Rev Neurosci 3: 473–478. [DOI] [PubMed] [Google Scholar]
- Penfield W, Rasmussen T, editors ( 1950): The Cerebral Cortex of Man: A Clinical Study of Localization of Function. New York: Macmillan. [Google Scholar]
- Rossini PM,Dal Forno G ( 2004): Integrated technology for evaluation of brain function and neural plasticity. Phys Med Rehabil Clin N Am 15: 263–306. [DOI] [PubMed] [Google Scholar]
- Ruohonen J,Ilmoniemi RJ ( 1999): Modeling of the stimulating field generation in TMS. Electroencephalogr Clin Neurophysiol Suppl 51: 30–40. [PubMed] [Google Scholar]
- Ruohonen J,Ilmoniemi RJ ( 2002): Physical principles for transcranial magnetic stimulation In: Pascual‐Leone A, Davey NJ, Rothwell JC, Wassermann EM, Puri BK, editors. Handbook of TMS. New York: Oxford University Press; pp 18‐19–29. [Google Scholar]
- Sack AT,Kadosh RC,Schuhmann T,Moerel M,Walsch V,Goebel R ( 2009): Optimizing functional accuracy of TMS in cognitive studies: a comparison of methods. J Cognitive Neurosci 21: 207–221. [DOI] [PubMed] [Google Scholar]
- Säisänen L,Pirinen E,Teitti S,Könönen M,Julkunen P,Määttä S,Karhu J ( 2008a): Factors influencing cortical silent period: Optimized stimulus location, intensity and muscle contraction. J Neurosci Methods 169: 231–238. [DOI] [PubMed] [Google Scholar]
- Säisänen L,Julkunen P,Niskanen E,Danner N,Hukkanen T,Lohioja T,Nurkkala J,Mervaala E,Karhu J,Könönen M ( 2008b): Motor potentials evoked by navigated transcranial magnetic stimulation in healthy subjects. J Clin Neurophysiol 25: 367–372. [DOI] [PubMed] [Google Scholar]
- Salmond CH,Ashburner J,Vargha‐Khadem F,Connelly A,Gadian DG,Friston KJ ( 2002): The precision of anatomical normalization in the medial temporal lobe using spatial basis functions. Neuroimage 17: 507–512. [DOI] [PubMed] [Google Scholar]
- Salih F,Khatami R,Steinheimer S,Hummel O,Kühn A,Grosse P ( 2005): Inhibitory and excitatory intracortical circuits across the human sleep‐wake cycle using paired‐pulse transcranial magnetic stimulation. J Physiol 565: 695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W ( 1995): Development and plasticity of cortical processing architectures. Science 270: 758–764. [DOI] [PubMed] [Google Scholar]
- Sparing R,Buelte D,Meister IG,Paus T,Fink GR ( 2008): Transcranial magnetic stimulation and the challenge of coil placement: a comparison of conventional and stereotaxic neuronavigational strategies. Hum Brain Mapp 29: 82–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gelderen P,Wu CWH,De Zwart JA,Cohen L,Hallett M,Duyn JH ( 2005): Resolution and reproducibility of BOLD and perfusion functional MRI at 3.0 Tesla. Magn Reson Med 54: 569–576. [DOI] [PubMed] [Google Scholar]
- van Westen D,Fransson P,Olsrud J,Rosén B,Lundborg G,Larsson E ( 2004): Fingersomatotopy in area 3b: An fMRI‐study. BMC Neurosci 5: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousry TA,Schmid UD,Alkadhi H,Schmidt D,Peraud A,Buettner A,Winkler P ( 1997): Localization of the motor hand area to a knob on the precentral gyrus. Brain 120: 141–157. [DOI] [PubMed] [Google Scholar]
- Zanette G,Tinazzi M,Bonato C,di Summa A,Manganotti P,Polo A,Fiaschi A ( 1997): Reversible changes of motor cortical outputs following immobilization of the upper limb. Electroencephalogr Clin Neurophysiol 105: 269–279. [DOI] [PubMed] [Google Scholar]


