Abstract
Using spinal cord functional magnetic resonance imaging (fMRI), 12 right‐handed healthy subjects were scanned during a tactile stimulation of the palm of the right hand. The task‐related mean signal change was computed for all activated voxels within the cervical cord, and separately, in the four cord quadrants (right and left anterior, right and left posterior) from C5 to C8. The frequency of fMRI activity at each cord level was obtained by assigning a score of 25% at each active quadrant and by averaging the percentage of active quadrants at each level of all subjects. The difference in the occurrence of fMRI activity (a) in right versus left, and anterior versus posterior cord, and (b) among the different cord levels, was evaluated using a random effect logistic regression model, with the frequency of fMRI activity as the dependent variable and the subject as the grouping factor. The task‐related mean signal change of all activated voxels of the cord was 3.2% (SD = 0.8%). During the tactile stimulation, subjects showed a higher occurrence of fMRI cord activity in the right than in the left cervical cord (odds ratio = 2.25, 95% confidence interval = 1.31–3.87, P = 0.003). A significant heterogeneity in frequency of fMRI activity between cord levels was also observed (P < 0.001), with the highest frequencies of fMRI activity detected at C6 and C7. Spinal cord fMRI enables to obtain reliable physiological information on the activity of human spinal circuits associated to tactile stimulation. This holds significant promise for a better planning and conduct of studies of people with diseased spinal cords. Hum Brain Mapp 2009. © 2007 Wiley‐Liss, Inc.
Keywords: functional magnetic resonance imaging, spinal cord functional magnetic resonance imaging, tactile stimulation, spinal cord, spinal cord interneurons
INTRODUCTION
Functional magnetic resonance imaging (fMRI) of the spinal cord is limited by severe technical challenges, which include the small size of the target tissue, the different magnetic susceptibilities of the bone, cartilage and cord, and the presence of motion, such as those linked to cardiac pulsation [Stroman, 2005]. Despite this, seminal studies have shown that spinal cord fMRI is feasible and enables to obtain reliable results both in healthy subjects [Govers et al., 2007; Komisaruk et al., 2002; Kornelsen and Stroman, 2004; Li et al., 2005; Madi et al., 2001; Maieron et al., 2007; Moffitt et al., 2005; Ng et al., 2006; Stracke et al., 2005; Stroman and Ryner, 2001; Stroman et al., 2001, 2002a, 2003a, 2005a; Yoshizawa et al., 1996] and in patients with spinal cord injury [Kornelsen and Stroman, 2007; Stroman et al., 2002b, 2004].
Several spinal cord fMRI studies have examined the regional distribution of cord activity during movements of the upper [Govers et al., 2007; Madi et al., 2001; Maieron et al., 2007; Ng et al., 2006; Yoshizawa et al., 1996] and lower limbs [Kornelsen and Stroman, 2004] as well as following thermal and painful stimulation of different dermatomes [Moffitt et al., 2005; Stroman et al., 2002a, b, 2004, 2005a] in healthy subjects, but only a few studies have investigated cord recruitment following a tactile stimulation [Stracke et al., 2005; Stroman et al., 2002a]. The advantages of tactile stimuli for spinal cord fMRI experiments is at least twofold. First, the tactile stimulation holds promise for investigating cord function in cases where active motor paradigms may not be feasible (e.g., in patients with paretic or plegic extremities). Second, tactile stimuli, unlike electrical stimuli, do not cause electromagnetic interference. Therefore, tactile stimulation might prove to be a useful paradigm to interrogate the nature and function of the human sensorimotor neural system in the context of spinal cord fMRI experiments.
Against this background, we used spinal cord fMRI based on proton density (PD)‐weighted spin‐echo (SE) fMRI in a 1.5 Tesla clinical MR system to assess cervical cord recruitment in healthy subjects, during the performance of a tactile stimulation of the palm of the right hand. We also investigated the topographical (both transversally and rostrocaudally) distribution of the tactile‐associated cervical cord activity to gain addition in vivo insight into human spinal cord physiology.
METHODS
Patients
We studied 12 right‐handed healthy subjects (seven women and five men; mean age = 37.7 years, range = 21–56 years), with no previous history of neurological dysfunction and a normal neurological exam. Handedness was established according to the 10‐item version of the Edinburg Handedness Inventory (EHI) scale [Oldfield, 1971]. The mean laterality quotient at the EHI was 0.96 (range =0.90–1.00). To be included in the study, subjects had to have no previous clinical episode attributable to the involvement of the cervical cord, such as acute myelitis, myelopathy, trauma, or other spinal diseases potentially affecting the cord, no other major medical conditions, no history of alcohol or drug abuse, and not being treated with any psychoactive drug. Local Ethical Committee approval and written informed consent from all individuals were obtained before study initiation.
MRI Acquisition
MRI data were obtained at 1.5 T in a Siemens Magnetom Avanto (Erlangen, Germany). Using a phased‐array spine receiver coil and a posterior neck coil, cervical cord fMRI data were acquired with a multishot turbo SE (TSE) sequence (TR = 2850 msec; TE = 11 msec; flip angle = 120°; field of view [FOV] = 100 × 100 mm2; matrix size = 256 × 244, turbo factor = 25, phase encoding direction: A‐P). Nine contiguous axial slices with a thickness of 7 mm, aligned with the vertebral body centers or with the intervertebral disks [Stroman and Ryner, 2001], covering cord segments from C5 to C8, were acquired. The in‐plane spatial resolution was 0.39 × 0.39 mm2. Spatial saturation pulses anterior and posterior to the cord were used to avoid aliasing and to reduce motion artifacts from breathing and swallowing. Flow compensation in the slice direction was applied to reduce artifacts from cerebrospinal fluid (CSF) flow. The time for acquiring one functional volume was 13 sec.
In the same scanning session, a dual‐echo TSE sequence was also obtained from the cervical cord (TR = 2000 msec; TE = 30/145 msec; flip angle = 150°; echo train length [ETL] = 23; FOV = 300 × 300 mm2; matrix size = 320 × 320; seven sagittal contiguous slices with a thickness of 4 mm) and the brain (TR = 3460 msec; TE = 27/109 msec; flip angle = 150°; ETL = 5; FOV = 250 × 250 mm2; matrix size = 512 × 512; 44 contiguous axial slices with thickness of 3 mm). For imaging the brain, the slices were positioned to run parallel to a line that joins the most inferoanterior and inferoposterior parts of the corpus callosum.
fMRI Experimental Design
Using a block design (ABAB), where four periods of rest were alternated with four periods of activity (each period of rest and activity consisting of five measurements), the subjects were scanned while performing a tactile stimulation of the palm of the right hand. To prevent any movement of the shoulder, arm, elbow or forearm, subjects' arms were restrained with straps. The forearm was in a supine position with respect to the scanner table. Head motion was minimized using foam padding and ear blocks. The stimulus, paced by a metronome at 1 Hz frequency, was administered to the subjects by an observer inside the scanner room, who pushed and pulled an homemade device which repeatedly tapped the center of the palm of the subjects' right hand with the back side (∼1 × 1 cm2) of a wooden spoon. This device had a locking mechanism with springs for controlling the amplitude of the wooden spoon displacement and the pressure exerted from the operator (the contact force of the device was about 2 N to the skin surface of the palm of the hand). Subjects were instructed not to aid the movements in any way. Subjects were trained outside the scanner room and were instructed to relax as much as possible and to keep their eyes closed during the whole duration of the experiment.
fMRI Analysis
All image postprocessing was performed on an independent computer workstation (Sun Microsystem, Mountain View, CA) by an experienced observer blinded to subjects' identity. FMRI data were analyzed using a custom‐made software written in MatLab (The MathWorks, Natick, MA) [Stroman et al., 2006]. To reduce the effects of subject's motion, images were first registered with respect to the first scan by means of rigid‐body translation and rotation. To avoid the effects of changes in the tone or position of the surrounding muscles [Stroman et al., 2001, 2002b], the registration process was applied after extraction of the cord tissue from the original images, using a semiautomated contouring technique [Rovaris et al., 1997]. From the rigid‐body registration, six motion parameters (three translations and three rotations) were generated for each subject's scan. Subjects with a maximum translation greater of 2.0 mm or with a maximum rotation greater of 0.04 radian in the x, y plane were excluded from the subsequent statistical analysis [Li et al., 2005]. A band‐pass filter was applied to the fMRI time‐courses, to remove low‐frequency baseline drift and high‐frequency noise from breathing and respiration. No spatial smoothing was applied to the realigned scans to preserve the in‐plane data resolution and compensate for the small size of the cord. Transformed fMRI data were then analyzed using a General Linear Model (GLM) approach [Friston et al., 1994]. This consisted of a basis set composed of a box‐car modelling the stimulus paradigm, a linear ramp to account for baseline trends, and a constant function to account for baseline intensity [Stroman et al., 2006]. Statistical t‐maps were generated for all subjects (P < 0.05, uncorrected). The presence of voxels of activity in the four quadrants of the cord (right and left anterior, and right and left posterior) at different levels was assessed by visual inspection on the activation maps from all subjects. The mean signal intensity changes induced by the PD increase during the task were computed for all activated voxels within the cervical cord, and separately, for each of the cervical cord sections studied.
Structural MRI Analysis
The presence of any disk protrusion or bony spondylosis, or both, was noted and graded [Thorpe et al., 1993]. Brain and cervical cord dual‐echo scans were inspected to detect any structural abnormalities.
Statistical Analysis
The frequency of fMRI activity at each cord level was obtained by assigning a score of 25% at each active quadrant and by averaging the percentage of active quadrants at each level of all subjects. The difference in the occurrence of fMRI activity (a) in right versus left and anterior versus posterior cord and (b) among the different cord levels, was evaluated using a random effect logistic regression model, with the frequency of fMRI activity as the dependent variable and the subject as the grouping factor.
RESULTS
No hyperintensities or any other abnormalities were seen on the conventional brain and cervical cord MR images obtained from all subjects. A mild (i.e., thecal indentation not reaching the cord) degree of spondylosis or disk protrusion was found in two subjects. During fMRI acquisition, no bulk body movement was noted. One subject was excluded from the analysis for the presence of motion artifacts above the a priori defined thresholds.
Activity of the cervical cord was detected in all subjects. Figure 1 shows an illustrative example of activation maps obtained from two subjects. The average signal change of all activated voxels within the cervical cord was 3.2% (SD = 0.8%). The distribution of the average signal changes in the different cord quadrants was as follows: 3.4% (SD = 0.7%) in the right anterior, 3.4% (SD = 1.0%) in the left anterior, 3.4% (SD = 0.7%) in the right posterior, 3.2% (SD = 0.8%) in the left posterior. Table I reports the average signal change for each of the cervical cord sections studied from C5 to C8.
Figure 1.
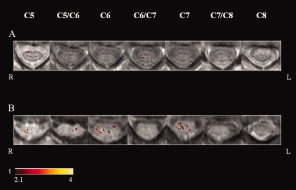
Illustrative activation maps (color coded for t values) of cervical cord superimposed on axial proton TSEs images from C5 to C8, from two healthy subjects (A and B) during a tactile stimulation of the palm of their right hands. Right (R) and left (L).
Table I.
Cervical Cord Average Signal Changes (SD) and Number of Subjects Having Activated Voxels During the Performance of a Tactile Stimulation of the Palm of the Subjects' Right Hand in the Right Anterior and Posterior, and left Anterior and Posterior Quadrants of the Cervical Cord at Different Cord Levels in Healthy Subjects
| Spinal level | C5 | C5/C6 | C6 | C6/C7 | C7 | C7/C8 | C8 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Side | R | R | R | R | R | R | R | |||||||
| Region | A | P | A | P | A | P | A | P | A | P | A | P | A | P |
| Average signal change [%] (SD) | 3.2 (0.5) | 3.5 (0.6) | 3.3 (0.6) | 3.0 (0.5) | 3.3 (0.7) | 3.4 (0.9) | 3.2 (0.8) | 3.3 (—) | 3.6 (0.7) | 3.5 (0.8) | 3.8 (1.8) | 2.8 (0.8) | 4.6 (—) | 3.6 (0.9) |
| Number of subjects | 5 | 5 | 3 | 4 | 8 | 8 | 2 | 1 | 4 | 8 | 2 | 2 | 1 | 3 |
| Side | L | L | L | L | L | L | L | |||||||
| Region | A | P | A | P | A | P | A | P | A | P | A | P | A | P |
| Average signal change [%] (SD) | 3.2 (1.7) | 2.9 (1.0) | 3.2 (1.0) | 3.5 (1.0) | 4.0 (1.2) | 3.1 (1.1) | 3.5 (1.4) | 3.1 (0.6) | 3.3 (0.2) | 3.3 (0.7) | — | 3.5 (0.3) | — | — |
| Number of subjects | 2 | 5 | 5 | 2 | 3 | 3 | 4 | 2 | 3 | 3 | — | 2 | — | — |
Abbreviations: SD, standard deviation; R, right; A, anterior; P, posterior; L, left.
During the tactile stimulation, healthy subjects showed a higher occurrence of fMRI cord activity in the right than in the left cervical cord (P = 0.003, odds ratio = 2.25, 95% confidence interval = 1.31–3.87). No significant difference in the frequency of activity in the anterior versus posterior portions of the cord was detected (P = 0.41). A significant heterogeneity in the frequency of fMRI activity between cord levels was observed (P < 0.001). The mean frequencies of fMRI activity were: 35% at C5, 29% at C5/C6, 46% at C6, 19% at C6/C7, 38% at C7, 13% at C7/C8, and 8% at C8.
DISCUSSION
In this study, we used a multishot TSE sequence to define the spinal cord regions activated by a tactile stimulation of the dominant, right upper limb in a group of healthy individuals. The choice of such a sequence, instead of the “classical” echo planar imaging (EPI) ones which are sensitive to the so‐called “blood oxygenation level dependent” effect (BOLD) [Ogawa et al., 1990, 1993], was based on the fact that previous studies [Stroman et al., 2002c, 2003b, 2005b] postulated the existence of an additional source of contrast in spinal cord fMRI, which is likely to be related to a PD change. This effect, called “signal enhancement by extravascular protons” (SEEP) [Stroman et al., 2002c, 2003b, 2005b], is thought to arise from a local change in fluid balance, which in turn may result from changes in perfusion pressure, production of extracellular fluid, neurons and glial cells swelling, and maintenance of ion and neurotransmitter concentrations at sites of neuronal activity [Fujita et al., 1997; Ohta et al., 1996; Stroman and Andrew, 2007; Stroman et al., 2002c, 2003b, 2005b]. Previous studies showed that both the BOLD and the SEEP effects give a contribution to the signal change observed during spinal fMRI activity, with different relative proportions depending on field strength and TE value used [Stroman, 2005; Stroman et al., 2002c]. Although a BOLD effect is likely to have contributed to the signal changes we observed, spinal cord fMRI scans based on SE imaging using a low TE, which is the case of the present study, are likely to be more sensitive to SEEP than to BOLD effect [Stroman et al., 2002c]. In addition, the use of a non‐EPI, fast, multishot sequence minimizes the distortions typically associated to EPI sequences [Stroman, 2005], thus allowing to obtain images of higher quality than EPI and to limit the consequences of a reduced signal contribution of Fourier lines acquired with late echoes, which may affect single‐shot TSE scans [Constable and Gore, 1992]. Finally, images with a high in‐plane resolution (about 0.4 mm voxel size) and reduced partial volume effects were obtained, which is desirable because of the small size of the cervical cord. To further reduce image artifacts, additional strategies were applied, including: (a) the use of flow‐compensation gradients and spatial saturation pulses (to eliminate all confounding signal arising from areas outside the cord and to reduce the impact of breathing artifacts); (b) the restraint of subjects' head and arm (although repeated tactile stimulation of the palm of the hand did not include a component of task‐related motion); and (c) the inclusion of a rigid‐body registration of the images in the fMRI analysis.
The main result of this study is the demonstration that fMRI can reliably detect activity in regions of the human cervical cord following a tactile stimulation of the palm of the hand [Brodal, 1981; Kandel et al., 1991; Williams and Warwick, 1980]. We indeed showed an average signal changes of around 3.2% (range = 2.8–4.6%), which is consistent with previous findings [Stroman and Ryner, 2001]. Similar findings have been previously reported by spinal cord fMRI studies performed in healthy subjects [Maieron et al., 2007; Stracke et al., 2005; Stroman and Ryner, 2001; Yoshizawa et al., 1996], which showed that both movement [Maieron et al., 2007; Yoshizawa et al., 1996] and tactile stimulation [Stracke et al., 2005; Stroman and Ryner, 2001] of the hand have the potential to elicit cord activity bilaterally, albeit largely in the cord side ipsilateral to the moving/stimulated hand. According to the expected anatomical distribution of the tactile‐associated cord recruitment [Brodal, 1981; Kandel et al., 1991; Williams and Warwick, 1980], fMRI activity was predominantly detected on the side of the body being stimulated.
In line with the results of a previous spinal cord fMRI study [Stracke et al., 2005], which showed that a tactile stimulation of the right hand elicits cord activity not only dorsally, but also in the ventral cord, we found that cord recruitment spanned throughout the entire cord cross‐sectionally. To explain this widespread distribution of cord recruitment following a tactile stimulation the function of spinal cord interneuronal systems should be considered [Brodal, 1981; Kandel et al., 1991; Williams and Warwick, 1980]. The tactile sensation travels from the skin, along the axons, passes the neuronal cell bodies in the dorsal root ganglia, and enters the dorsal columns of the spinal cord. Somatosensory axons from the upper limbs are more lateral than those from the lower extremities, and run up the dorsal columns in the cuneate tract. At the level of the medulla oblongata, these axons synapse with the secondary neurons in the cuneate nucleus. However, fibers mediating tactile input from the periphery also synapse extensively with spinal interneurons before reaching the medulla oblongata. Two prominent classes of neurons in the spinal cord contribute to the somatosensory system: dorsal association interneurons and relay neurons [Gross et al., 2002]. Association neurons, that comprise the substantia gelatinosa, predominantly project along the ipsilateral pathways [Brown, 1981; Helms and Johnson, 2003; Szentagothai, 1964], and form a closed system that integrates sensory information within the spinal cord. Relay interneurons, many of which are commissural, are located in the marginal zone (lamina I), the deep dorsal horn, and the ventral spinal cord [Brown, 1981]. They relay somatosensory information to the brain and project to the contralateral side of the cord by way of the spinocerebellar, spinotectal, and spinothalamic tracts [Brown, 1981]. As the consequence, the input directing to and departing from these spinal interneurons might account for the observed extensive cord recruitment that we found following tactile stimulation.
Another important finding was that fMRI activity was distributed across several cervical cord segments (from C5 to C8), but the higher frequency of activity was found to correspond to the stimulated dermatome. The cord levels with the highest frequency of fMRI activity were C6 and C7 (mean frequencies of 46 and 38%, respectively), while cord levels with the lowest frequency of fMRI activity were C7/C8 and C8 (mean frequencies of 13 and 8%, respectively). Our results are in agreement with those obtained by Stroman et al. [2002a], who showed that a thermal stimulation alternatively applied to different dermatomes (the forearm [C5], the thumb side of the hand [C6], and the little finger side of the hand [C8]) elicits cord activity, which is predominantly located in the side of the body ipsilateral to the stimulus and at the spinal cord level corresponding with the stimulated dermatome. Intriguingly, we also found a moderate fMRI activity at the level of the upper cervical cord segments (C5 and C5/C6, mean frequencies of 35 and 29%, respectively). These latter activations might reflect the recruitment of premotor interneurons of the spinal cord [Pierrot‐Deseilligny, 2002]. Several studies have suggested the presence of a rostral propriospinal system which should transmit descending excitatory and inhibitory inputs onto motoneurons for target‐reaching movements [Pierrot‐Deseilligny, 2002]. These studies demonstrated that the propriospinal system also receives peripheral inputs from the moving limb allowing the cortical command to be updated at the premotoneuronal level [Pierrot‐Deseilligny, 2002]. Moreover, these propriospinal neurons also receive inhibitory inputs by cutaneous afferents from the skin field, which meets the target at the end of a movement [Nielsen and Pierrot‐Deseilligny, 1991].
Clearly, our study is not without limitations. These include the relatively small number of subjects studied and the still suboptimal spatial resolution of the adopted technique with respect to the complex anatomy of the structure studied. In addition, considering the variability of fMRI findings, our results need to be replicated. Despite we applied several strategies to reduce possible biases on our results, including extraction of cord tissue, the application of a band‐pass filter and the inclusion in the GLM of a linear ramp and a constant function, we can not completely exclude that part of our results are due to false‐positive activations, which are a rather common finding in spinal fMRI [Stroman, 2005, 2006] due to the aliased physiological noise related to both respiration and cardiac cycle contained in the spinal fMRI time courses [Stroman, 2006]. Nevertheless, the present study adds to the growing field of spinal cord fMRI by providing, for the first time, a quantitative regional mapping of cervical cord activity elicited by tactile stimulus in healthy subjects. Further studies are now warranted to investigate the dependence of the cord response from the frequency and amplitude of the tactile stimulation. Our findings may also have important clinical implications, since we have shown that it is possible to obtain reliable physiological information on the activity of spinal circuits in humans using a tactile stimulus at a routine clinical scanner. This is not a trivial issue given the fact that the spinal cord is an eloquent area of the central nervous system (CNS). As a consequence, assessing tactile‐associated recruitments might prove to be a rewarding strategy to interrogate damage/dysfunction of this CNS structure in many neurological/neurosurgical conditions (even in disabled patients where other approaches are most likely not to be informative) and to monitor cord plasticity following neurorehabilitation.
Acknowledgements
This study was partially supported by a grant from Fondazione Italiana Sclerosi Multipla (FISM 2005/R/12).
REFERENCES
- Brodal A ( 1981): Neurological Anatomy in Relation to Clinical Medicine. New York: Oxford University Press. [Google Scholar]
- Brown AG ( 1981): Organization of the Spinal Cord. New York: Springer Verlag. [Google Scholar]
- Constable RT,Gore JC ( 1992): The loss of small objects in variable TE imaging: Implications for FSE, RARE and EPI. Magn Reson Med 28: 9–24. [DOI] [PubMed] [Google Scholar]
- Friston KJ,Jezzard P,Turner R ( 1994): Analysis of functional MRI time series. Hum Brain Mapp 1: 153–171. [Google Scholar]
- Fujita H,Meyer E,Reutens DC,Kuwabara H,Evans AC,Gjedde A ( 1997): Cerebral [15O] water clearance in humans determined by positron emission tomography. II. Vascular responses to vibrotactile stimulation. J Cereb Blood Flow Metab 17: 73–79. [DOI] [PubMed] [Google Scholar]
- Govers N,Beghin J,Van Goethem JW,Michiels J,van den Hauwe L,Vandervliet E,Parizel PM ( 2007): Functional MRI of the cervical spinal cord on 1.5 T with fingertapping: To what extent is it feasible? Neuroradiology 49: 73–81. [DOI] [PubMed] [Google Scholar]
- Gross MK,Dottori M,Goulding M ( 2002): Lbx1 specifies somatosensory association interneurons in the dorsal spinal cord. Neuron 34: 535–549. [DOI] [PubMed] [Google Scholar]
- Helms AW,Johnson JE ( 2003): Specification of dorsal spinal cord interneurons. Curr Opin Neurobiol 13: 42–49. [DOI] [PubMed] [Google Scholar]
- Kandel E,Schwartz JH,Jessell TM ( 1991): Principles of Neural Science. New York: Elsevier Science. [Google Scholar]
- Komisaruk BR,Mosier KM,Liu WC,Criminale C,Zaborszky L,Whipple B,Kalnin A ( 2002): Functional localization of brainstem and cervical spinal cord nuclei in humans with fMRI. AJNR Am J Neuroradiol 23: 609–617. [PMC free article] [PubMed] [Google Scholar]
- Kornelsen J,Stroman PW ( 2004): fMRI of the lumbar spinal cord during a lower limb motor task. Magn Reson Med 52: 411–414. [DOI] [PubMed] [Google Scholar]
- Kornelsen J,Stroman PW ( 2007): Detection of the neuronal activity occurring caudal to the site of spinal cord injury that is elicited during lower limb movement tasks. Spinal Cord 45: 485–490. [DOI] [PubMed] [Google Scholar]
- Li G,Ng MC,Wong KK,Luk KD,Yang ES ( 2005): Spinal effects of acupuncture stimulation assessed by proton density‐weighted functional magnetic resonance imaging at 0.2 T. Magn Reson Imaging 23: 995–999. [DOI] [PubMed] [Google Scholar]
- Madi S,Flanders AE,Vinitski S,Herbison GJ,Nissanov J ( 2001): Functional MR imaging of the human cervical spinal cord. AJNR Am J Neuroradiol 22: 1768–1774. [PMC free article] [PubMed] [Google Scholar]
- Maieron M,Iannetti GD,Bodurka J,Tracey I,Bandettini PA,Porro CA ( 2007): Functional responses in the human spinal cord during willed motor actions: evidence for side‐ and rate‐dependent activity. J Neurosci 27: 4182–4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt MA,Dale BM,Duerk JL,Grill WM ( 2005): Functional magnetic resonance imaging of the human lumbar spinal cord. J Magn Reson Imaging 21: 527–535. [DOI] [PubMed] [Google Scholar]
- Ng MC,Wong KK,Li G,Lai S, Yang, ES ,Hu Y,Luk KD ( 2006): Proton‐density‐weighted spinal fMRI with sensorimotor stimulation at 0.2 T. NeuroImage 29: 995–999. [DOI] [PubMed] [Google Scholar]
- Nielsen J,Pierrot‐Deseilligny E ( 1991): Pattern of cutaneous inhibition of the propriospinal‐like excitation to human upper limb motoneurones. J Physiol 434: 169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S,Lee TM,Kay AR,Tank DW ( 1990): Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA 87: 9868–9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S,Menon RS,Tank DW,Kim SG,Merkle H,Ellermann JM,Ugurbil K ( 1993): Functional brain mapping by blood oxygenation level‐dependent contrast magnetic resonance imaging. A comparison of signal characteristics with a biophysical model. Biophys J 64: 803–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta S,Meyer E,Fujita H,Reutens DC,Evans A,Gjedde A ( 1996): Cerebral [15O]water clearance in humans determined by PET. I. Theory and normal values. J Cereb Blood Flow Metab 16: 765–780. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Pierrot‐Deseilligny E ( 2002): Propriospinal transmission of part of the corticospinal excitation in humans. Muscle Nerve 26: 155–172. [DOI] [PubMed] [Google Scholar]
- Rovaris M,Filippi M,Calori G,Rodegher M,Campi A,Colombo B,Comi G ( 1997): Intra‐observer reproducibility in measuring new putative markers of demyelination and axonal loss in multiple sclerosis: A comparison with conventional T2‐weighted images. J Neurol 18: 895–901. [DOI] [PubMed] [Google Scholar]
- Stracke CP,Pettersson LG,Schoth F,Moller‐Hartmann W,Krings T ( 2005): Interneuronal systems of the cervical spinal cord assessed with BOLD imaging at 1.5 T. Neuroradiology 47: 127–133. [DOI] [PubMed] [Google Scholar]
- Stroman PW ( 2005): Magnetic resonance imaging of neuronal function in the spinal cord: Spinal fMRI. Clin Med Res 3: 146–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroman PW ( 2006): Discrimination of errors from neuronal activity in functional MRI of the human spinal cord by means of General Linear Model analysis. Magn Reson Med 56: 452–456. [DOI] [PubMed] [Google Scholar]
- Stroman PW,Andrew RD ( 2007): Functional magnetic resonance imaging of cortical tissue slices by means of signal enhancement by extravascular water protons (SEEP) contrast. Proc Int Soc Mag Reson Med 15: 1966. [Google Scholar]
- Stroman PW,Ryner LN ( 2001): Functional MRI of motor and sensory activation in the human spinal cord. Magn Reson Imaging 19: 27–32. [DOI] [PubMed] [Google Scholar]
- Stroman PW,Krause V,Malisza KL,Frankenstein UN,Tomanek B ( 2001): Characterization of contrast changes in functional MRI of the human spinal cord at 1.5 T. Magn Reson Imaging 19: 833–838. [DOI] [PubMed] [Google Scholar]
- Stroman PW,Krause V,Malisza KL,Frankenstein UN,Tomanek B ( 2002a): Functional magnetic resonance imaging of the human cervical spinal cord with stimulation of different sensory dermatomes. Magn Reson Imaging 20: 1–6. [DOI] [PubMed] [Google Scholar]
- Stroman PW,Tomanek B,Krause V,Frankenstein UN,Malisza KL ( 2002b): Mapping of neuronal function in the healthy and injured human spinal cord with spinal fMRI. NeuroImage 17: 1854–1860. [DOI] [PubMed] [Google Scholar]
- Stroman PW,Krause V,Malisza KL,Frankenstein UN,Tomanek B ( 2002c): Extravascular proton‐density changes as a non‐BOLD component of contrast in fMRI of the human spinal cord. Magn Reson Med 48: 122–127. [DOI] [PubMed] [Google Scholar]
- Stroman PW,Malisza KL,Onu M ( 2003a): Functional magnetic resonance imaging at 0.2 Tesla. NeuroImage 20: 1210–1214. [DOI] [PubMed] [Google Scholar]
- Stroman PW,Tomanek B,Krause V,Frankenstein UN,Malisza KL ( 2003b): Functional magnetic resonance imaging of the human brain based on signal enhancement by extravascular protons (SEEP fMRI). Magn Reson Med 49: 433–439. [DOI] [PubMed] [Google Scholar]
- Stroman PW,Kornelsen J,Bergman A,Krause V,Ethans K,Malisza KL,Tomanek B ( 2004): Noninvasive assessment of the injured human spinal cord by means of functional magnetic resonance imaging. Spinal Cord 42: 59–66. [DOI] [PubMed] [Google Scholar]
- Stroman PW,Kornelsen J,Lawrence J ( 2005a): An improved method for spinal functional MRI with large volume coverage of the spinal cord. J Magn Reson Imaging 21: 520–526. [DOI] [PubMed] [Google Scholar]
- Stroman PW,Kornelsen J,Lawrence J,Malisza KL ( 2005b): Functional magnetic resonance imaging based on SEEP contrast: Response function and anatomical specificity. Magn Reson Imaging 23: 843–850. [DOI] [PubMed] [Google Scholar]
- Szentagothai J ( 1964): Neuronal and synaptic arrangement in the substantia gelatinosa Rolandi. J Comp Neurol 122: 219–239. [DOI] [PubMed] [Google Scholar]
- Thorpe JW,Kidd D,Kendall BE,Tofts PS,Barker GJ,Thompson AJ,MacManus DG,McDonald WI,Miller DH ( 1993): Spinal cord MRI using multi‐array coils and fast spin echo. I. Technical aspects and findings in healthy adults. Neurology 43: 2625–2631. [DOI] [PubMed] [Google Scholar]
- Williams PL,Warwick R. 1980. Gray's Anatomy, 36th ed Edinburgh: Chirchill Livingstone. [Google Scholar]
- Yoshizawa T,Nose T,Moore GJ,Sillerud LO ( 1996): Functional magnetic resonance imaging of motor activation in the human cervical spinal cord. NeuroImage 4: 174–182. [DOI] [PubMed] [Google Scholar]


