Abstract
Activity within the default‐mode network (DMN) is thought to be related to self‐referential processing, such as thinking about one's preferences or personality traits. Although the DMN is generally considered to function as a network, evidence is starting to accumulate that suggests that areas of the DMN are each specialized for different subfunctions of self‐referential processing. Here, we address the issue of functional specialization by investigating changes in coupling between areas of the DMN during self‐referential processing. To this aim, brain activity was assessed during a task in which subjects had to indicate whether a trait adjective described their own personality (self‐referential, Self condition), that of another person (other‐referential, Other condition), or whether the trait was socially desirable (nonreferential, Control condition). To exclude confounding effects of cardiorespiratory processes on activity and functional coupling, we corrected the fMRI signal for these effects. Activity within areas of the DMN was found to be modulated by self‐referential processing. More specifically, during the Self condition compared to the Other and Control condition, activity within the dorsal medial prefrontal cortex, ventral medial prefrontal cortex, and posterior cingulate cortex was increased. Moreover, coupling between areas of the DMN was reduced during the Self condition compared to the Other and Control condition, while coupling between regions of the DMN and regions outside the network was increased. As such, these results provide an indication for functional specialization within the DMN and support the notion that each area of the DMN is involved in different subfunctions of self‐referential processing. Hum Brain Mapp, 2010. © 2010 Wiley‐Liss, Inc.
Keywords: fMRI, default‐mode network, self‐referential, social cognition, functional connectivity, respiration, heart rate
INTRODUCTION
During rest, a network of brain areas shows increased activation compared to when people are performing a complex task [Shulman et al., 1997]. This network is called the default‐mode network (DMN) and includes the dorsal and ventral medial prefrontal cortex [dMPFC and vMPFC; Brodmann areas (BAs) 10, 9, 32, and 24], the posterior cingulate cortex/retrosplenial cortex (PCC, BA 23/31, and RSC, BA 29/30), and the lateral posterior cortex (LP, BA 39/40) [Buckner et al., 2008; Fox et al., 2005; Greicius et al., 2003; Gusnard and Raichle, 2001; Raichle et al., 2001]. Studies in humans using diffuse tensor imaging [Greicius et al., 2009; van den Heuvel et al., 2008] as well as studies in macaques [Kobayashi and Amaral, 2003, 2007; Ongur and Price, 2000] reported dense anatomical connections between the areas of the DMN. Furthermore, several functional connectivity studies showed strong positive correlations within the DMN both during resting‐state [Damoiseaux et al., 2006; Fox et al., 2005; Greicius et al., 2003; Lowe et al., 1998; van Buuren et al., 2009] and during cognitive tasks [Greicius et al., 2003; Kjaer et al., 2002], indicating that activity within the network is highly correlated.
Several authors propose self‐referential, introspective processing to underlie activity within the DMN [Amodio and Frith, 2006; Fox et al., 2005; Gusnard and Raichle, 2001; Mason et al., 2007; Uddin et al., 2008]. For example, Mason et al. [ 2007] reported positive correlations between DMN activity and so‐called mind‐wandering and negative correlations between DMN activity and task‐demand. A number of studies investigated DMN activity during tasks that invoked self‐referential processing such as responding to statements describing one's own personality, attitudes, or preferences. These studies show that activity within the midline areas of the DMN, the dMPFC, vMPFC, and PCC, is increased during self‐referential conditions as compared to a control task [Craik et al., 1999; Fossati et al., 2003; Gusnard et al., 2001; Johnson et al., 2002, 2006; Kelley et al., 2002; Macrae et al., 2004; Mitchell et al., 2006; Ochsner et al., 2004, 2005; Schmitz et al., 2004; Schneider et al., 2008; Zysset et al., 2002]. Activity within the LP is also found to be increased during self‐referential conditions [Craik et al., 1999; Fossati et al., 2003; Johnson et al., 2006; Mitchell et al., 2006; Ochsner et al., 2004; Zysset et al., 2002], although this finding is less consistent across studies.
Taken together, these findings suggest that the DMN functions as a network. However, evidence is now starting to accumulate that suggest some form of functional specialization within the DMN [Buckner et al., 2008; Northoff and Bermpohl, 2004; Northoff et al., 2006]. That is, regions of the DMN are thought to be involved in different subfunctions of self‐referential processing, such as monitoring, evaluating, and integrating of self‐referential stimuli [Northoff and Bermpohl, 2004; Northoff et al., 2006]. In a meta‐analysis of 27 PET and fMRI studies on self‐referential tasks, Northoff and co‐workers investigated functional specialization within the cortical midline structures. Using cluster analyses, they distinguished between three different subregions within the DMN, being the vMPFC/posterior ACC, the dMPFC, and the PCC, suggesting that the DMN consists of separate components each involved in different subfunctions of self‐referential processing [Northoff et al., 2006]. Furthermore, Schmitz and Johnson [ 2006] investigated functional specialization within the MPFC. They reported an increase in connectivity during self‐referential processing, dissociating a dorsal and a ventral MPFC network, which they interpreted as support for the notion that these regions are engaged in different subfunctions of self‐referential processing.
Surprisingly, changes in connectivity between areas of the DMN during self‐referential processing have not yet been reported. Such changes are to be expected if these areas are engaged in different subfunctions during self‐referential processing. The failure to report changes within the DMN could be caused by influences of cardiorespiratory (CR) processes on the connectivity measures. That is, CR processes such as heart rate (HR) and respiration affect the BOLD signal independently of neuronal activity [Glover et al., 2000; Wise et al., 2004] and are found to contaminate measures of activity within as well as connectivity between brain areas [Birn et al., 2006; Shmueli et al., 2007; van Buuren et al., 2009]. Changes in connectivity due to changes in task conditions and cognitive processes could therefore be obscured by these CR effects.
Here, we address the issue of functional specialization within the DMN by investigating changes in coupling between areas of the DMN during self‐referential processing. To this aim, brain activity, heart beat, and respiration are measured in 19 healthy control subjects during a self‐referential task. In this task, subjects are asked to indicate whether a trait adjective describes their own personality (self‐referential, Self condition), that of another person (other‐referential, Other condition), or whether the trait is socially desirable (nonreferential, Control condition). To exclude confounding effects of CR processes on connectivity and activity in the brain, the fMRI signal is corrected for CR effects [van Buuren et al., 2009].
METHODS
Participants
Nineteen right‐handed (mean ± SD Edinburgh Handedness Inventory [Oldfield, 1971] quotient, 0.82 ± 0.15) healthy subjects (eight males; mean ± SD years, 21.5 ± 1.9) were included in this study. None of the participants had a history of psychiatric or neurological disorders, substance abuse, or medical disorders or had any contraindications for MRI. Participants were recruited from the University of Utrecht and received monetary compensation for participation. All gave written informed consent. The ethics committee of the University Medical Center of Utrecht approved this study.
Task
In the self‐referential task [Craik et al., 1999; Kelley et al., 2002], subjects were instructed to make judgments about trait adjectives. Depending on the condition, the subjects were asked to indicate whether a trait adjective described their own personality (Self condition), the Dutch prime‐minister's personality (Other condition), or whether the trait was social desirable (nonreferential, Control condition) by pressing the left “yes” or the right “no” button (see Fig. 1). The words were extracted from a list with trait adjectives validated for likableness [Anderson, 1968] and translated into Dutch. Half of the adjectives were of positive valence and half were of negative valence. The task consisted of five blocks of eight trials (28 s per block) per condition alternated with rest periods of 30 s. During each trial, one trait adjective was presented for 3.5 s or until the subject responded. After each response, a fixation cross was presented for the remaining trial duration. Each task block started with an instruction trial of 2 s indicating the onset of a task condition.
Figure 1.
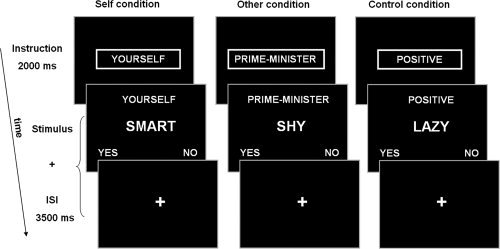
Self‐reflection task. The stimulus duration and interstimulus interval (ISI) duration were dependent on the reaction time; the fixation cross appeared as soon as the subject responded for the remaining trial time.
Cardiorespiratory Processes
Measurements
Four electrocardiogram electrodes were affixed to the subjects' chest and a respiration band was placed at the level of the abdomen. In this way, we obtained two signals; a heartbeat signal with a trigger marking times at which an R‐peak was detected and a respiratory signal measuring the expansion of the respiration band. From the heartbeat data, three signals were derived: the phase of the heartbeat (i.e. cardiac cycle), the HR, and heart rate variability (HRV). From the respiration data, the respiration cycle (i.e. respiration phase) was derived and respiration volume per time (RVT) was calculated by multiplying respiration amplitude with respiration frequency (see van Buuren et al. [ 2009] for a detailed description of the calculation of the CR signals). Heartbeat phase, HR, HRV, respiration phase, and RVT were used to correct the fMRI time series data for confounding effects of CR processes.
Correction
The correction for the CR processes was performed using custom Matlab software (Aztec, http://www.ni-utrecht.nl/downloads/aztec). This method is described in detail in our previous study [van Buuren et al., 2009]. In short, the BOLD signal was corrected for the effects of the cardiac and respiratory cycle using RETROICOR [Glover et al., 2000]. Subsequently, the remaining BOLD signal was corrected for the effects of HR, HRV, and RVT using a multiple regression approach. To account for the variable delay between fluctuations in the BOLD signal and fluctuations in these CR processes, the time courses of these variables were shifted with multiple lags. The optimal lags for HR, HRV, and RVT were determined by calculating, for every voxel separately, the correlation between the CR time course and the BOLD signal at a range of lags. The optimal lag was then selected as the lag having the strongest absolute correlation and the lagged signal was included in the multiple regression.
The effect of the CR correction on the BOLD signal was investigated by calculating how much of the variance in BOLD signal could be explained by the CR processes. The proportion of explained variance (EV) was calculated as EV (%) = 100 × [1 − (σpost/σpre)], where σpost was the variance after correction and σpre variance before correction.
Functional Magnetic Resonance Imaging
Measurements
All imaging was performed on a Philips 3.0T Achieva whole‐body MRI scanner (Philips Medical Systems, Best, The Netherlands). Functional images were obtained using a 2D‐EPI‐SENSE sequence with the following parameters: voxel size 4 mm isotropic; TR = 1,600 ms; TE = 23 ms; flip angle = 72.5°; matrix 52 × 30 × 64; field of view 208 × 120 × 256; 30‐slice volume; SENSE‐factor R = 2.4 (anterior–posterior). A total of 395 functional images were acquired during the self‐reflection task.
After the acquisition of the functional images, an 3D Fast Field Echo (FFE) T1‐weighted structural image of the whole brain was made (scan parameters: voxel size 1 mm isotropic, TR = 25 ms; TE = 2.4 ms; flip angle = 30°; field of view 256 × 150 × 204, 150 slices).
Image preprocessing
Image preprocessing and analyses were carried out with SPM5 (http://www.fil.ion.ucl.ac.uk/spm/). After realignment, the structural scan was coregistered to the mean functional scan. Next, using unified segmentation, the structural scan was segmented and normalization parameters were estimated. Subsequently, all scans were registered to a MNI T1‐standard brain using these normalization parameters and a 3D Gaussian filter (8‐mm full width at half maximum) was applied to all functional images.
Whole brain analyses
The preprocessed functional images were submitted to a general linear model regression analysis after correcting for CR effects. The design matrix contained factors modeling the onsets and durations of the Self, Other, and Control conditions as well as the instructions that were presented during the task. These factors were convolved with a canonical hemodynamic response function [Friston et al., 1995]. To correct for head motion, the six realignment parameters were included in the design matrix as regressors of no interest. A high‐pass filter was applied to the data with a cut‐off frequency of 0.0055 Hz to correct for drifts in the signal. Subsequently, for each subject, first level contrast images were created for each condition compared to baseline and compared to all other conditions.
Next, group activation maps were calculated for each contrast using a random effects analysis approach. All group activation maps were tested for significance at a cluster‐defining threshold of P < 0.001 with a P < 0.05 familywise error‐corrected (FWE‐corrected) cluster size of 18 voxels.
To provide more insight in the underlying pattern of activity, exploratory regions of interest (ROI) analyses were conducted [Poldrack, 2007] using a 4‐mm‐radius sphere around the peaks of activation within the midline areas of the DMN, dMPFC, vMPFC, and PCC, in the contrast [Self > Control]. These ROI analyses were not used for inference and are depicted in Supporting Information Figure 1.
Psychophysiological interaction analyses
To investigate changes in coupling within the DMN during self‐referential processing, psychophysiological interaction (PPI) analyses [Friston et al., 1997] were conducted using SPM5. A PPI shows changes in functional coupling between a seed region and other regions in relation to a psychological factor (i.e. the various task conditions). For each seed region, two PPI analyses were conducted to investigate changes in functional coupling during, respectively, the Self versus Control condition and Self versus Other condition (i.e. psychological factor). A 4‐mm‐radius sphere around the peaks of activation within the midline areas of the DMN, dMPFC, vMPFC, and PCC, in the contrast (Self > Control) were taken as seed regions for the PPI analyses as follows. For each subject, the first eigenvariate of the BOLD signal within each seed region (i.e. volume of interest) was calculated and adjusted for average activation during the task (i.e. F‐contrast showing effects of task) and head motion. The interaction between activity within the seed regions and each psychological factor (i.e. PPI regressor) was then calculated and activity positively related to each interaction as well as negatively related activity was investigated. Subsequently, these individual contrast images of the PPI analyses were entered in second level analyses to test for groupwise effects. To test in which areas activity was significantly explained (positively or negatively) by the PPI regressor (i.e. interaction between the presence of self‐referential processing and activity in one of the seed regions), t‐tests were performed. Significance of the group t‐maps was assessed at a cluster‐defining threshold of P < 0.001 and a P < 0.05 FWE‐corrected cluster size of 23 voxels.
RESULTS
Behavioral Results
During the Control condition, more than 90% of the responses matched those of the validated ratings on likableness (mean ± SD, 92.8 ± 3.1%), indicating that the subjects paid attention to the task. Reaction times differed significantly between the task conditions [F(2,36) = 17.35, P < 0.0005]. Subsequent paired‐sample t‐tests showed that the subjects responded faster during the Control condition (mean ± SD, 1299 ± 182 ms) compared to the Self condition [mean ± SD, 1403 ± 162 ms; t(18) = −4.62, P < 0.0005] and the Other condition [mean ± SD, 1434 ± 188 ms; t(18) = −5.06, P < 0.0005]. Reaction times during the Self and Other condition did not differ (P = 0.19).
Effects of CR Correction
To investigate the impact of the correction for CR processes on the BOLD signal, the variance explained by the CR processes was calculated. On average, 22.2% (±SD 3.8%) of the variance in the whole brain could be explained by the CR correction, indicating that the CR correction removed a considerable proportion of the variance within the BOLD signal. This is consistent with results from our previous study [van Buuren et al., 2009].
Whole Brain Analyses
Results of the whole brain analyses are presented in Figure 2 and Table I.
Figure 2.
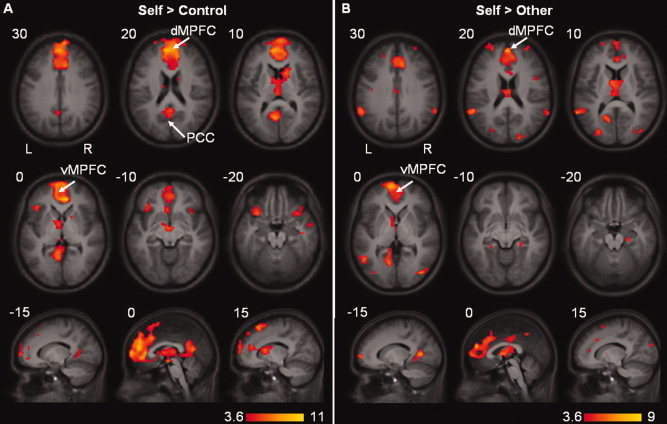
Whole brain activation overlaid on the mean anatomical image. (A) Significant activation during the Self condition compared to the Control condition. (B) Significant activation during the Self condition compared to the Other condition. Cluster‐defining threshold of P < 0.001 with a P < 0.05 FWE‐corrected cluster size of 18 voxels. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Table I.
Whole‐brain activation levels
| Brain region | BA | MNI coordinates | Voxels | Z score | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Self > Control | ||||||
| Dorsal medial prefrontal cortex | 10 | −4 | 48 | 16 | 1625 | 6.06 |
| Retrosplenial cortex | 30 | −8 | −60 | 8 | 228 | 5.15 |
| Inferior frontal gyrus | 47 | −32 | 16 | −24 | 119 | 4.64 |
| Inferior temporal pole | 20 | 44 | −16 | −20 | 127 | 4.42 |
| Other > Control | ||||||
| Middle temporal gyrus | 21 | −60 | −12 | −16 | 58 | 5.73 |
| Posterior cingulate cortex | 23 | 0 | −56 | 16 | 108 | 5.27 |
| Middle temporal pole | 38 | 48 | 12 | −28 | 121 | 5.09 |
| Dorsal medial prefrontal cortex | 9 | −4 | 52 | 32 | 244 | 4.88 |
| Inferior temporal gyrus | 21 | −44 | 8 | −36 | 79 | 4.67 |
| Orbital frontal cortex | 11 | 0 | 48 | −16 | 42 | 4.01 |
| Self > Other | ||||||
| Retrosplenial cortex | 30 | −8 | −64 | 4 | 133 | 5.52 |
| Dorsal medial prefrontal cortex | 9 | 0 | 52 | 20 | 516 | 5.04 |
| Middle temporal lobe | 21 | −64 | −52 | 8 | 148 | 5.03 |
| Caudate | −8 | 0 | 8 | 248 | 4.68 | |
| Middle occipital lobe | 19 | 40 | −80 | 0 | 83 | 4.64 |
| Hippocampus | 24 | −24 | −16 | 39 | 4.54 | |
| Superior frontal cortex | 9 | −20 | 40 | 40 | 37 | 4.32 |
| Precuneus | 7 | −4 | −48 | 44 | 22 | 4.12 |
| Middle occipital lobe | 19 | −32 | −84 | 4 | 55 | 4.10 |
| Inferior parietal lobe | 40 | 64 | −48 | 28 | 40 | 4.07 |
| Insula | 13 | 44 | 8 | 16 | 21 | 4.03 |
| Middle cingulate | 24 | −36 | −16 | 36 | 20 | 3.95 |
| Middle frontal cortex | 10 | 32 | 52 | 24 | 31 | 3.77 |
| Supplementary motor area | 6 | 12 | 12 | 64 | 41 | 3.73 |
BA = Brodmann area.
Clusters showing significant activation during the Self condition compared to the Control condition (upper part), during the Other condition compared to the Control condition (middle part), or during the Self condition compared to the Other condition (lower part).
MNI coordinates represent the location of the peak voxels.
Cluster‐defining threshold of P < 0.001 with a P < 0.05 FWE‐corrected cluster size of 18 voxels.
The dMPFC, extending into the vMPFC, and the retrosplenial cortex (RSC) were more active during the Self condition compared to the Control condition. When comparing the Self condition to the Other condition, the dMPFC, extending into the vMPFC, and the RSC as well as the inferior parietal gyrus (i.e. lateral posterior cortex, LP) showed increased activity (see Table I). These findings of increased activity within areas of the DMN even after correcting for CR effects indicate that these areas are engaged in some form of self‐referential processing. In addition, when comparing the Other condition to the Control condition, increased activity was found within a more superior region of the dMPFC and the PCC.
Psychophysiological Interaction Analyses
Psychophysiological interaction (PPI) analyses were performed to investigate changes in functional coupling during self‐referential processing. Peaks of activation within the three midline areas of the DMN in the contrast (Self > Control) were taken as seed regions: a seed region located in the dMPFC (x,y,z = −4, 48, 16), the vMPFC (x,y,z = 0, 64, 4), and the PCC (x,y,z = −4, −52, 28). Two PPI analyses were performed for each seed region, showing changes in functional coupling between each seed region and other areas during the Self versus Control condition and the Self versus Other condition, respectively. Results of the PPI analyses are presented in Figure 3 and Tables II and III.
Figure 3.
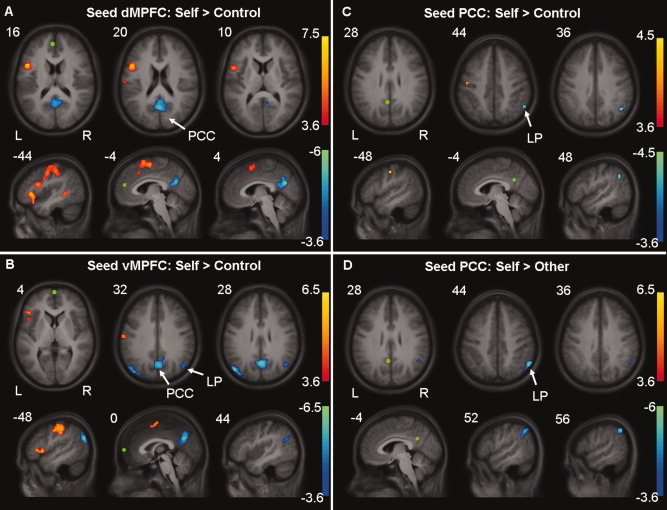
Areas showing significant psychophysiological interactions overlaid on the mean anatomical image. Areas showing decreased (blue) or increased (red) coupling with (A) the dMPFC, (B) the vMPFC, (C) the PCC during the Self condition compared to the Control condition, and with (D) the PCC during the Self condition compared to the Other condition. Seed regions are depicted in green. Cluster‐defining threshold of P < 0.001 with a P < 0.05 FWE‐corrected cluster size of 23 voxels. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Table II.
PPI results Self versus Control
| Brain region | BA | MNI coordinates | Voxels | Z score | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| dMPFC: Positive | ||||||
| Inferior frontal gyrus | 44 | −48 | 12 | 16 | 682 | 5.01 |
| Middle temporal gyrus | 21 | −48 | −44 | −4 | 28 | 4.31 |
| Anterior cingulate cortex | 32 | 12 | 24 | 36 | 38 | 3.93 |
| Precuneus | 7 | ‐24 | −64 | 40 | 47 | 3.87 |
| dMPFC: Negative | ||||||
| Posterior cingulate cortex | 23 | 4 | −64 | 24 | 137 | 4.52 |
| vMPFC: Positive | ||||||
| Postcentral gyrus | 3 | −56 | −12 | 44 | 216 | 4.43 |
| Inferior frontal gyrus | 45 | −40 | 24 | 0 | 41 | 4.20 |
| Supplementary motor area | 6 | −4 | −8 | 60 | 46 | 3.88 |
| vMPFC: Negative | ||||||
| Angular gyrus | 39 | −48 | −76 | 28 | 72 | 4.58 |
| Posterior cingulate cortex | 31 | 4 | −64 | 28 | 168 | 4.40 |
| Angular gyrus | 39 | 44 | −64 | 28 | 29 | 3.63 |
| PCC: Positive | ||||||
| Precentral gyrus | 4 | −48 | −12 | 44 | 32 | 3.58 |
| PCC: Negative | ||||||
| Angular gyrus | 39 | 48 | −56 | 36 | 32 | 3.50 |
BA = Brodmann area.
Clusters showing positive or negative PPI with the dMPFC, vMPFC, and PCC during the Self condition compared to the Control condition.
MNI coordinates represent the location of the peak voxels.
Cluster‐defining threshold of P < 0.001 with a P < 0.05 FWE‐corrected cluster size of 23 voxels.
Table III.
PPI results Self versus Other
| Brain region | BA | MNI coordinates | Voxels | Z score | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| dMPFC: Positive | ||||||
| Insula | 13 | −40 | 0 | 16 | 188 | 4.77 |
| Superior temporal gyrus | 38 | −40 | 12 | −32 | 55 | 4.63 |
| Postcentral gyrus | 3 | −48 | −24 | 56 | 195 | 4.52 |
| Supplementary motor area | 6 | −4 | 12 | 56 | 172 | 4.39 |
| Superior frontal gyrus | 8 | −8 | 44 | 40 | 25 | 4.13 |
| dMPFC: Negative | ||||||
| ns | ||||||
| vMPFC: Positive | ||||||
| Inferior frontal gyrus | 45 | −44 | 28 | −4 | 335 | 5.20 |
| Supplementary motor area | 6 | −8 | 36 | 60 | 355 | 4.89 |
| Postcentral gyrus | 3 | −40 | −16 | 60 | 67 | 3.82 |
| Hippocampus | −28 | −20 | −12 | 33 | 3.76 | |
| vMPFC: Negative | ||||||
| ns | ||||||
| PCC: Positive | ||||||
| ns | ||||||
| PCC: Negative | ||||||
| Inferior parietal lobe | 40 | 56 | −56 | 44 | 39 | 4.31 |
BA = Brodmann area; ns = nonsignificant.
Clusters showing positive or negative PPI with the dMPFC, vMPFC, and PCC during the Self condition compared to the Other condition.
MNI coordinates represent the location of the peak voxels.
Cluster‐defining threshold of P < 0.001 with a P < 0.05 FWE‐corrected cluster size of 23 voxels.
Self versus Control condition—dMPFC
There was an increase in coupling between activity within the inferior frontal gyrus (IFG) extending into the supplementary motor area (SMA), middle temporal gyrus, precuneus and dorsal anterior cingulate cortex (dorsal ACC), and activity in the dMPFC during the Self condition compared to the Control condition. Results of the negative interaction contrast showed that there was a decrease in coupling between activity within the PCC and activity within the dMPFC during the Self condition compared to the Control condition.
Self versus Control condition—vMPFC
An increase in coupling was found between the vMPFC and postcentral gyrus, SMA, and IFG during the Self condition compared to the Control condition. A decrease in coupling with activity within the vMPFC was found within the PCC, left and right angular gyrus (i.e. lateral posterior cortices, LP) during the Self condition compared to the Control condition.
Self versus Control condition—PCC
There was an increase in coupling between activity within the PCC and activity within the precentral gyrus during the Self condition compared to the Control condition. Coupling between activity within the PCC and activity within the right angular gyrus (i.e. LP) was found to be decreased when comparing the Self to the Control condition.
Self versus Other condition—dMPFC
An increase in coupling between activity within the dMPFC and insula, extending into the IFG, the superior temporal gyrus, postcentral gyrus, SMA, and superior frontal gyrus was found during the Self condition compared to the Other condition (Table III). Results of the negative interaction contrast did not reach significance, indicating that functional coupling between the dMPFC and other areas of the DMN did not decrease during the Self condition as compared to the Other condition.
Self versus Other condition—vMPFC
When comparing the Self condition to the Other condition, an increase in coupling was found between activity within the vMPFC and activity within the IFG, extending into the insula, the superior frontal gyrus, postcentral gyrus as well as the left hippocampus. No decreases in coupling with activity within the vMPFC were observed.
Self versus Other condition—PCC
Results of the positive interaction contrast did not reach significance; however, a decrease in coupling between activity within the PCC and right inferior parietal lobule was found during the Self condition compared to the Other condition.
DISCUSSION
In this study, we addressed functional specialization in the DMN by investigating functional coupling during self‐referential processing. We used an extensive correction method to remove confounding effects of CR processes, namely heartbeat and respiration. Consistent with previous reports, activation within the midline areas of the DMN was increased during self‐referential processing [Craik et al., 1999; D'Argembeau et al., 2005; Fossati et al., 2003; Johnson et al., 2002; Kelley et al., 2002; Kjaer et al., 2002; Macrae et al., 2004; Mitchell et al., 2006; Moran et al., 2006; Schmitz et al., 2004; Zysset et al., 2002]. More specifically, activity within the dorsal medial prefrontal cortex (dMPFC), ventral MPFC (vMPFC), and posterior cingulate cortex/retrosplenial cortex (PCC/RSC) was increased when judging personality traits with respect to oneself (i.e. self‐referential processing) as compared to making judgements about another person (i.e other‐referential) or the social desirability of the traits (i.e. nonreferential). In addition, we found increased activation within the lateral posterior cortices (LP) when making judgments about personality traits with respect to oneself compared to making judgments with respect to another person. Furthermore, when engaged in self‐referential processing, functional coupling between areas of the DMN was decreased, while coupling with areas outside the network was increased, providing an indication for functional specialization within the DMN.
Activity within the midline areas of the DMN was increased not only during self‐referential processing but also when subjects were involved in making judgments about personality traits with respect to another person. This is in line with previous studies, which suggest that when making judgments about another person, people are engaged in some form of self‐referential processing [Amodio and Frith, 2006; D'Argembeau et al., 2007; Johnson et al., 2006; Northoff et al., 2006; Schmitz et al., 2004; Vogeley and Fink, 2003]. That is, when judging another person's personality or feelings, people tend to reflect on their own feelings, personality, and experiences. However, the extent of self‐referential processing is thought to be greater when thinking of one's own personality as compared to another person's personality. Indeed, activity was increased within the DMN during self‐referential processing when compared to other‐referential processing. It is important to note that these increases in activity represent relative increases. That is, although the dMPFC and vMPFC show increased activation during self‐referential processing, these areas as well as the PCC show less deactivation instead of increased activation during other‐referential processing compared to nonreferential processing.
In addition to these activation changes, we found that during self‐referential processing functional coupling between the dMPFC and PCC, between the vMPFC and PCC and LP as well as between the PCC and right LP was decreased when compared to nonreferential control processing. When comparing self‐referential processing to other‐referential processing, a decrease in coupling was observed between the PCC and right LP. In contrast, functional coupling between the vMPFC, dMPFC and PCC and areas outside the DMN, including the inferior frontal gyrus, superior frontal gyrus, insula, middle temporal gyrus, somatosensory cortex and hippocampus, was increased during self‐referential processing. This increased coupling with regions outside the DMN suggests that the areas of the DMN are also part of other networks.
To our knowledge, this is the first study to report a decrease in functional coupling within the DMN during self‐referential processing. A previous study by Schmitz and Johnson [ 2006] did report an increase in coupling between activity in areas of the DMN and activity in areas outside the network during self‐referential processing, but did not find changes in coupling between areas of the DMN. The differences between the results of the current study and the study of Schmitz and Johnson could be due to confounding effects of CR processes, but may also be caused by differences in seed regions, which were located closer to the midline of the brain in the current study. Also, the task of the current study differed from the task used by Schmitz and Johnson in that an other‐referential condition as well as periods of rest were included in addition to the self‐referential and control condition.
Although the results from the current study provide support for the notion that areas of the DMN are engaged in different subfunctions of self‐referential processing, these findings do not necessarily imply that self‐referential processing solely drives activity within the DMN during resting‐state. In addition, the task used in this study did not allow us to specify the possible subfunctions of self‐referential processing. However, results of previous studies do provide some indication of the nature of the subfunctions. That is, the ventral part of the MPFC is thought to play a role predominantly in identifying stimuli as self‐relevant by integrating cognitive and emotional processing [Gusnard et al., 2001; Northoff and Bermpohl, 2004; Northoff et al., 2006; Schmitz and Johnson, 2007], whereas the dorsal part of the MPFC has been associated with introspective self‐referential processing such as the appraisal and evaluation of the self‐relevant stimuli [Gallagher and Frith, 2003; Gusnard et al., 2001; Lane et al., 1997]. The PCC and the LP are, given their dense connections with the hippocampal formation [Kobayashi and Amaral, 2003, 2007], thought to be involved in integrating new self‐referential stimuli in a context of autobiographic memories and past self‐referential stimuli. To further investigate functional specialization within the DMN, it would be useful to investigate changes in functional coupling during a more elaborate task that encompasses the various components of self‐referential processing such as identifying, monitoring, evaluating, and integrating of self‐referential stimuli.
Future studies investigating functional specialization may also benefit from the use of different effective connectivity techniques such as dynamic causal modelling [Friston et al., 2003] or structural equation modelling [McIntosh and Gonzalez‐Lima, 1994]. In the current study, psychophysiological interaction (PPI) analyses were used to investigate changes in functional coupling. By using PPI, we were only able to model contributions from a single area at a time (i.e. modulation by the dMPFC, vMPFC, and PCC), as a PPI only gives an indication in which area activity covariates with one other region (i.e. the seed region) as a function of the psychological variable. In contrast, dynamic causal modelling and structural equation modelling allow inference of causal interactions between multiple areas.
The current study confirms that, even after correcting for confounding effects of CR processing, activity within areas of the DMN is related to self‐referential processing. More specifically, we found increased activity within the dMPFC, vMPFC, PCC as well as the LP during self‐referential processing. In addition, functional coupling between these regions was reduced during such processing, while coupling with areas outside the DMN was increased, suggesting that subregions of the DMN are also part of different networks. As such, these results provide an indication for functional specialization within the DMN, suggesting that each area of the DMN is involved in different subfunctions of self‐referential processing.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information Figure 1
REFERENCES
- Amodio DM, Frith CD ( 2006): Meeting of minds: The medial frontal cortex and social cognition. Nat Rev Neurosci 7: 268–277. [DOI] [PubMed] [Google Scholar]
- Anderson NH ( 1968): Likableness ratings of 555 personality‐trait words. J Pers Soc Psychol 9: 272–279. [DOI] [PubMed] [Google Scholar]
- Birn RM, Diamond JB, Smith MA, Bandettini PA ( 2006): Separating respiratory‐variation‐related fluctuations from neuronal‐activity‐related fluctuations in fMRI. Neuroimage 31: 1536. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews‐Hanna JR, Schacter DL ( 2008): The brain's default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124: 1–38. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Moroz TM, Moscovitch M, Stuss DT, Winocur G, Tulving E, Kapur S ( 1999): In search of the self: A positron emission tomography study. Psychol Sci 10: 26–34. [Google Scholar]
- Damoiseaux JS, Rombouts SARB, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF ( 2006): Consistent resting‐state networks across healthy subjects. Proc Natl Acad Sci USA 103: 13848–13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Argembeau A, Collette F, Van der Linden M, Laureys S, Del Fiore G, Degueldre C, Luxen A, Salmon E ( 2005): Self‐referential reflective activity and its relationship with rest: A PET study. Neuroimage 25: 616. [DOI] [PubMed] [Google Scholar]
- D'Argembeau A, Ruby P, Collette F, Degueldre C, Balteau E, Luxen A, Maquet P, Salmon E ( 2007): Distinct regions of the medial prefrontal cortex are associated with self‐referential processing and perspective taking. J Cogn Neurosci 19: 935–944. [DOI] [PubMed] [Google Scholar]
- Fossati P, Hevenor SJ, Graham SJ, Grady C, Keightley ML, Craik F, Mayberg H ( 2003): In search of the emotional self: An fMRI study using positive and negative emotional words. Am J Psychiatry 160: 1938–1945. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME ( 2005): From the cover: The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102: 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Turner R, Frackowiak RS ( 1995): Characterizing evoked hemodynamics with fMRI. Neuroimage 2: 157–165. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ ( 1997): Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 6: 218–229. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W ( 2003): Dynamic causal modelling. Neuroimage 19: 1273–1302. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Frith CD ( 2003): Functional imaging of 'theory of mind'. Trends Cogn Sci 7: 77–83. [DOI] [PubMed] [Google Scholar]
- Glover GH, Li TQ, Ress D ( 2000): Image‐based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med 44: 162–167. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V ( 2003): Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 100: 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF ( 2009): Resting‐state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex 19: 72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME ( 2001): Searching for a baseline: Functional imaging and the resting human brain. Nat Rev Neurosci 2: 685. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME ( 2001): Medial prefrontal cortex and self‐referential mental activity: Relation to a default mode of brain function. Proc Natl Acad Sci USA 98: 4259–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP ( 2002): Neural correlates of self‐reflection. Brain 125: 1808–1814. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Raye CL, Mitchell KJ, Touryan SR, Greene EJ, Nolen‐Hoeksema S ( 2006): Dissociating medial frontal and posterior cingulate activity during self‐reflection. Scan 1: 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF ( 2002): Finding the self? An event‐related fMRI study. J Cogn Neurosci 14: 785–794. [DOI] [PubMed] [Google Scholar]
- Kjaer TW, Nowak M, Lou HC ( 2002): Reflective self‐awareness and conscious states: PET evidence for a common midline parietofrontal core. Neuroimage 17: 1080–1086. [PubMed] [Google Scholar]
- Kobayashi Y, Amaral DG ( 2003): Macaque monkey retrosplenial cortex: II. Cortical afferents. J Comp Neurol 466: 48–79. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Amaral DG ( 2007): Macaque monkey retrosplenial cortex: III. Cortical efferents. J Comp Neurol 502: 810–833. [DOI] [PubMed] [Google Scholar]
- Lane RD, Fink GR, Chau PM, Dolan RJ ( 1997): Neural activation during selective attention to subjective emotional responses. Neuroreport 8: 3969–3972. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, Sorenson JA ( 1998): Functional connectivity in single and multislice echoplanar imaging using resting‐state fluctuations. Neuroimage 7: 119–132. [DOI] [PubMed] [Google Scholar]
- Macrae CN, Moran JM, Heatherton TF, Banfield JF, Kelley WM ( 2004): Medial prefrontal activity predicts memory for self. Cereb Cortex 14: 647–654. [DOI] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN ( 2007): Wandering minds: The default network and stimulus‐independent thought. Science 315: 393–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AR, Gonzalez‐Lima F ( 1994): Structural equation modelling and its application to network analysis in functional brain imaging. Hum Brain Mapp 2: 2–22. [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR ( 2006): Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron 50: 655. [DOI] [PubMed] [Google Scholar]
- Moran JM, Macrae CN, Heatherton TF, Wyland CL, Kelley WM ( 2006): Neuroanatomical evidence for distinct cognitive and affective components of self. J Cogn Neurosci 18: 1586–1594. [DOI] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F ( 2004): Cortical midline structures and the self. Trends Cogn Sci 8: 102. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J ( 2006): Self‐referential processing in our brain—A meta‐analysis of imaging studies on the self. Neuroimage 31: 440. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, Hanelin J, Ramachandran T, Glover G, Mackey SC ( 2004): Reflecting upon feelings: An fMRI study of neural systems supporting the attribution of emotion to self and other. J Cogn Neurosci 16: 1746–1772. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Beer JS, Robertson ER, Cooper JC, Gabrieli JD, Kihsltrom JF, D'Esposito M ( 2005): The neural correlates of direct and reflected self‐knowledge. Neuroimage 28: 797–814. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Ongur D, Price JL ( 2000): The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex 10: 206–219. [DOI] [PubMed] [Google Scholar]
- Poldrack RA ( 2007): Region of interest analysis for fMRI. Soc Cogn Affect Neurosci 2: 67–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL ( 2001): Inaugural article: A default mode of brain function. Proc Natl Acad Sci USA 98: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz TW, Johnson SC ( 2006): Self‐appraisal decisions evoke dissociated dorsal–ventral aMPFC networks. Neuroimage 30: 1050–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz TW, Johnson SC ( 2007): Relevance to self: A brief review and framework of neural systems underlying appraisal. Neurosci Biobehav Rev 31: 585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz TW, Kawahara‐Baccus TN, Johnson SC ( 2004): Metacognitive evaluation, self‐relevance, and the right prefrontal cortex. Neuroimage 22: 941. [DOI] [PubMed] [Google Scholar]
- Schneider F, Bermpohl F, Heinzel A, Rotte M, Walter M, Tempelmann C, Wiebking C, Dobrowolny H, Heinze HJ, Northoff G ( 2008): The resting brain and our self: self‐relatedness modulates resting state neural activity in cortical midline structures. Neuroscience 157: 120–131. [DOI] [PubMed] [Google Scholar]
- Shmueli K, van Gelderen P, de Zwart JA, Horovitz SG, Fukunaga M, Jansma JM, Duyn JH ( 2007): Low‐frequency fluctuations in the cardiac rate as a source of variance in the resting‐state fMRI BOLD signal. Neuroimage 38: 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE ( 1997): Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J Cogn Neurosci 9: 648–663. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Kelly AM, Biswal BB, Margulies DS, Shehzad Z, Shaw D, Ghaffari M, Rotrosen J, Adler LA, Castellanos FX, Milham MP ( 2008): Network homogeneity reveals decreased integrity of default‐mode network in ADHD. J Neurosci Methods 169: 249–254. [DOI] [PubMed] [Google Scholar]
- van Buuren M, Gladwin TE, Zandbelt BB, van den Heuvel M., Ramsey NF, Kahn RS, Vink M ( 2009): Cardiorespiratory effects on default‐mode network activity as measured with fMRI. Hum Brain Mapp 30: 3031–3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel M, Mandl R, Luigjes J, Hulshoff PH ( 2008): Microstructural organization of the cingulum tract and the level of default mode functional connectivity. J Neurosci 28: 10844–10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogeley K, Fink GR ( 2003): Neural correlates of the first‐person‐perspective. Trends Cogn Sci 7: 38–42. [DOI] [PubMed] [Google Scholar]
- Wise RG, Ide K, Poulin MJ, Tracey I ( 2004): Resting fluctuations in arterial carbon dioxide induce significant low frequency variations in BOLD signal. Neuroimage 21: 1652–1664. [DOI] [PubMed] [Google Scholar]
- Zysset S, Huber O, Ferstl E, von Cramon DY ( 2002): The anterior frontomedian cortex and evaluative judgment: An fMRI study. Neuroimage 15: 983. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information Figure 1


