Abstract
Developmental dyslexia has been assumed to arise from general auditory deficits, compromising rapid temporal integration both of linguistic and nonlinguistic acoustic stimuli. Because the effort of auditory temporal processing of speech and nonspeech test materials may depend on presentation rate, fMRI measurements were performed in dyslexics and controls during passive listening to series of syllable and click sounds, using a parametric approach. Controls showed a decrease of hemodynamic brain activation within the right and an increase within the left anterior insula as a function of the presentation rate both of click as well as syllable trains. By contrast, dyslexics exhibited this profile of hemodynamic responses under the nonspeech condition only. As concerns syllables, activation in dyslexics did not depend on presentation rate. Moreover, a subtraction analysis of hemodynamic main effects across conditions and groups revealed decreased activation both of the left and right anterior insula in dyslexics compared to controls during application both of click and syllables. These results indicate, in line with preceding studies, that the insula of both hemispheres is involved in auditory temporal processing of nonlinguistic auditory stimuli and demonstrate, furthermore, that these operations of intrasylvian cortex also extend to the linguistic domain. In addition, our data suggest that the anterior insula represents an important neural correlate of deficient temporal processing of speech and nonspeech sounds in dyslexia. Hum Brain Mapp 2009. © 2008 Wiley‐Liss, Inc.
Keywords: reading, developmental dyslexia, temporal processing deficit, auditory processing, syllables, click sounds, fMRI, insula
INTRODUCTION
Developmental dyslexia is considered a specific disorder of the acquisition of reading skills, which does not reflect general cognitive impairments, sensory deficits, and/or inadequate schooling [American Psychiatric Association,1994; Shaywitz,1998]. Often this syndrome is accompanied by poor spelling abilities [Shaywitz and Shaywitz,2005]. Longitudinal studies indicate dyslexia to represent a persistent condition rather than a “transient developmental lag” [Shaywitz and Shaywitz,2005; Svensson and Jacobson,2006]. This definition is a purely descriptive characterization because even after more than 100 years of research, no consensus about the etiological basis of dyslexia has been achieved so far [see Démonet et al.,2004, for a review]. Most studies published during the last four decades focus on deficits in phonological processing [e.g., Siegel,1998; Snowling,1998]. However, it is still unsettled whether these problems are confined to the linguistic domain [Snowling,2000; Stanovich,1988] or whether they must be considered a secondary symptom, due to basic nonlinguistic deficits bound to either the visual [Becker et al.,2005; DiLollo et al.,1983; Talcott et al.,2000], the auditory [Ben‐Artzi et al.,2005; Cohen‐Mimran and Sapir,2007], or both domains [see Farmer and Klein,1995, for review]. Tallal [1980], for instance, suggests a general sensory deficit in rapid temporal processing to be the core deficit in dyslexia. According to this approach, the basic temporal processing impairment leads to an inability to integrate sensory information entering in rapid succession to the central nervous system and causes a cascade of effects that disrupt the normal development of the phonological system and subsequently the acquisition of reading skills [Tallal et al.,1993].
Functional imaging studies have begun to provide new insights into the neural basis of temporal auditory processing and, therefore, may help to resolve the debate on the biological basis of dyslexia. A number of studies demonstrated that rapid auditory temporal features of speech and nonspeech stimuli are preferentially processed by the left hemisphere, whereas contralateral regions appear to be specifically involved in the analysis of slower fluctuations of the acoustic signal [Fiez et al.,1995; Zatorre et al.,2002]. In accordance with these observations, Ivry and Robertson [1998] assumed the left and right hemisphere to operate as a high‐ or low‐pass filter, respectively, acting on auditory input (“double filtering by frequency theory”). According to this model, the left hemisphere seems to predominantly process consonantal sounds characterized by segments extending across a few tens of milliseconds, whereas the right counterpart mediates suprasegmental information such as speech intonation contours as well as musical melodies acting upon longer time frames [Ivry and Robertson,1998].
In a previous fMRI study [Ackermann et al.,2001], we found evidence in support of the “double filtering by frequency theory” of cerebral laterality effects: Healthy adults had been asked to passively listen to click trains, varying in frequency from 2 to 6 Hz. Based on this design, the course of neural activation could be mapped depending on the presentation rate, and thus, to delineate temporal processing. Distinct rate‐response relationships were found at the level of the right cerebellar hemisphere, the left thalamus, the left and right anterior insula, the left inferior frontal gyrus, and the tectum. Most noteworthy, the anterior insula showed a profile resembling the operation of a high‐pass (left hemisphere) or low‐pass (right hemisphere) filter across the series of stimulus frequencies applied—a pattern in line with the model by Ivry and Robertson [1998].
This fMRI study used the passive listening paradigm of our preceding investigation to further elucidate the neural correlates of temporal auditory processing in dyslexic adults. To determine whether the linguistic content of the test materials has a significant impact on hemodynamic brain activation, the syllable/pa/ was added as a second stimulus category. Both events—click trains and syllable repetitions—were presented at six different frequencies each, ranging from 1 to 9 Hz, to delineate the response profiles associated with the temporal processing of linguistic and nonlinguistic stimulus materials in dyslexics and controls. The question was whether dyslexics and controls show different rate–response profiles of hemodynamic brain activation and whether, in case such discrepancies emerge, they are exclusively bound to the speech condition.
MATERIALS AND METHODS
Subjects
This study included seven participants with a diagnosis of developmental dyslexia (two females; mean age: 18.0 years, SD = 1.8 years) and seven healthy subjects (two females; mean age 23.7 years, SD = 4.3 years), serving as controls. German was the native language of all participants, and all of them were right‐handed as determined by means of the Edinburgh Handedness Inventory [Oldfield,1971; lateralization index >80%]. None of the participants reported a history of neurological diseases or psychiatric disorders. Informed consent had been obtained in line with the Institutional Review Board of the University of Ulm.
In all dyslexic participants, the respective diagnosis had been established in primary school. They had a documented history of both reading and spelling difficulties across their entire school career and reported persistent difficulties up to the date of investigation. Indeed, the dyslexics were as a group a few years younger than their controls (t(12) = 3.43, P < 0.01, two‐tailed). However, age‐related differences in brain activation between these two groups of young adults must not be expected.
To validate the previously given diagnosis, all participants, dyslexics and controls, were tested again up to 4 weeks prior to the fMRI experiment. Inclusion in this study required an average or above average nonverbal intelligence as measured by the Culture Fair Intelligence Test [German version, Weiß,1997]. Furthermore, a standardized test of reading and spelling skills was given to all subjects. The evaluation of reading abilities (reading time and reading errors for real words and pseudowords) relied upon a German reading test for adults [Schulte‐Körne,2001]. The mean error score for real words (maximum = 48) amounted to 4.1 (SD = 2.3) in the dyslexics and to 0.1(SD = 0.4) in the control group, yielding a significant group difference (t(12) = −4.60, P < 0.001, one‐tailed). The mean reading time for real words was 61.3 s (SD = 14.4) in the dyslexic group and 33.1 s (SD = 5.8) in the control group. Real word reading was significantly slower in dyslexics than in controls (t(12) = −4.76, P < 0.001, one‐tailed). The mean error score for pseudowords (maximum = 48) amounted to 13.6 (SD = 5.6) in dyslexics and 0.86 (SD = 0.9) in controls, also with a significant difference between groups (t(12) = −5.97, P < 0.001, one‐tailed). Finally, groups differed in reading time for pseudowords (t(12) = −4.58, P < 0.001, one‐tailed), with a mean reading time of 113.9 s (SD = 27.6) in dyslexics and 66.9 s (SD = 12.1) in the control group. Spelling was measured by means of a standardized German spelling test for adults [Kersting and Althoff,2004] demonstrating very poor spelling skills for the dyslexic group (percentage rank <15). The control group showed an average or above average spelling skills (percentage rank >66). Of a total of 60 words, the dyslexic group produced incorrect spellings for 37.6 words (SD = 7.7), whereas the control group produced only 4.0 words (SD = 2.9) incorrectly. Dyslexics made significantly more spelling errors than controls (t(12) = −10.77, P < 0.001, one‐tailed).
Stimuli and Procedure
Isochronous trains of click sounds and syllables served as the acoustic stimulus materials. In line with a preceding study [Ackermann et al.,2001], clicks were generated by manual editing of a recorded natural sound (stroke of a pen against the desk). To improve the audibility within the MR scanner environment, a double‐spike (spaced 2 ms apart) was superimposed on the initial excitation phase (about 3 ms) of broadband noise, followed by a dampened signal (duration ∼10 ms, spectral energy distribution centered at 3 kHz). In difference to the previous experiment [Ackermann et al.,2001; 2.0–6.0 Hz], we used a modified frequency band from 1.0 to 9.0 Hz. As in Ackermann et al. [2001], click stimuli were presented at six different frequency rates. In this study, the following stimulation frequencies were used: 1.0, 2.5, 3.0, 5.0, 7.0, and 9.0 Hz. The duration of each stimulus train amounted to 6 s. The speech condition included the syllable/pa/, produced with a rather flat intonation by a female speaker. Syllable trains were generated by means of a signal editing software (Computer Speech Lab 4300, Kay Elemetrics, USA). The same train duration time and frequency rates as in the click condition were used. Both conditions, the clicks as well as the syllables, were repeated 15 times. Altogether, the experiment consisted of 180 stimulus trains per subject (2 stimulus conditions × 6 frequency rates × 15 repetitions) presented in a counterbalanced order. Stimuli were presented via headphones simultaneously to both ears and were well discernible at a comfortable loudness level against the background of scanner noise. Subjects were instructed to passively listen to the acoustic stimuli and to strictly refrain from any motor or cognitive responses such as finger lifting and silent or overt counting. Single stimulus trains started in randomized order either simultaneously with the onset of a measurement period or at a delay of 1 or 2 s to decouple the hemodynamic responses to target stimuli from scanner noise.
fMRI Data Acquisition
Subjects lay supine in a 3.0 T head scanner (Siemens Allegra), their heads being secured by foam rubber to minimize the movement artifacts. Using an echo‐planar imaging sequence (64 × 64 matrix, field of view = 192 × 192 mm2, TE = 35 ms, TR = 3 s, flip angle = 90°), 36 parallel axial slices (thickness = 3 mm, gap = 0.75 mm) were obtained across the entire brain volumes (5 runs × 205 images, resulting, altogether, in 1025 image volumes), including five initial dummy scans for the equilibration of T1 saturation effects. For the sake of anatomical localization of hemodynamic activation effects, fMRI maps were superimposed on a T1‐weighted 3D sequence, averaged across all subjects (MPRAGE; 208 sagittal slices, thickness = 1.0 mm, 256 × 256 matrix, field of view = 256 × 256 mm2, TE = 4.38 ms, TR = 5.5 ms).
fMRI Data Analysis
After data preprocessing, parametric analysis including the calculation of main effects and rate‐to‐response functions was performed to determine the influence of stimulation rate on the magnitude of the BOLD signal. All brain areas identified as activated areas on the basis of this procedure served as the volumes of interest for the subsequent analysis. Subsequently, a subtraction analysis across groups and conditions was performed. The height threshold of this study at voxel level was set at P < 0.001 (T > 3.10) with an extension threshold of k ≥ 67 voxels.
Data preprocessing
fMRI data were transformed to an ANALYZE‐compatible format and analyzed using SPM5. Anatomical T1‐weighted images were realigned to the standard T1 template. Coregistration of the functional images then relied on the same transformation matrix. Subsequently, spatial normalization and correction of MRI images into a standard space as defined by an ideal template were performed. Finally, the normalized data sets were smoothed with an isotropic Gaussian kernel (10 mm).
Main effects of hemodynamic activation and rate‐to‐response functions
The resulting contrast images during passive listening to click and syllable trains from each participant provided the data base for all subsequent steps of statistical analysis. A parametric approach using polynomial basis algorithms up to the second order allowed for the determination of the relationship between stimulation rate and regional BOLD signal. This procedure models three different rate‐to‐response functions: (a) categorical on‐off responses between the acoustic stimulation and the silent baseline conditions irrespective of presentation rate (main effect; Fig. 1), (b) linear BOLD signal changes along with increasing acoustic stimulation (first order term), and (c) nonlinear (quadratic) relationships between stimulus frequency and hemodynamic activation. At the activation maximum within each volume of interest, group means and the respective standard deviation of these measures were determined and displayed in a bar graph (Figs. 3 and 4).
Figure 1.
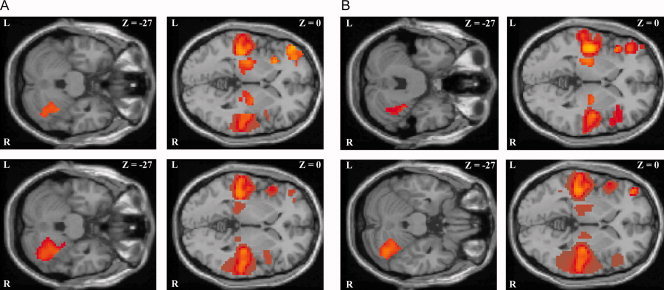
(A) Healthy control subjects: main effect of the parametric analysis irrespective of acoustical stimulation rate during presentation of clicks (upper row) and syllables (lower row) displayed on transverse sections of the anatomical reference images (SPM5 template). L, left; R, right; z, distance to the intercommisural plane. (B) Dyslexic subjects: main effect of the parametric analysis irrespective of acoustical stimulation rate during presentation of clicks (upper row) and syllables (lower row) displayed on transverse sections of the anatomical reference images (SPM5 template). L, left; R, right; z, distance to the intercommisural plane. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Figure 3.
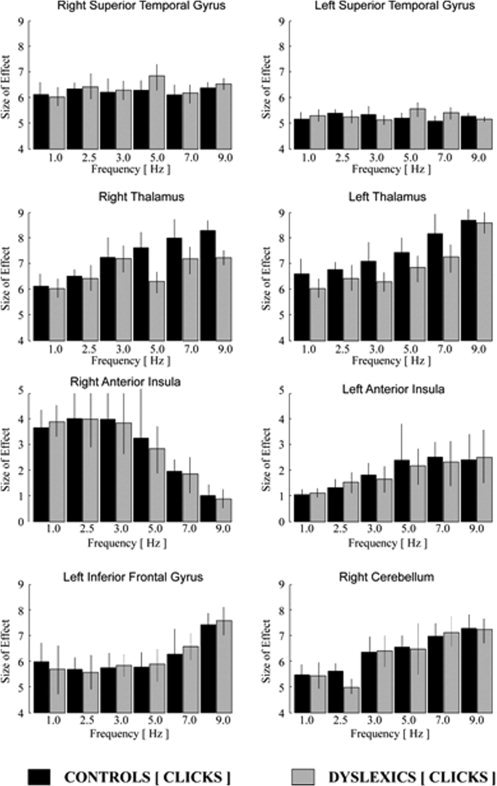
Analysis of frequency‐dependent activation for click stimuli: Linear and nonlinear changes of signal intensity for different frequencies of acoustical stimulation displayed by the size of effect and the variance of signal intensity (calculated in arbitrary units by SPM5; gray colored in dyslexics, black colored in healthy control subjects). Note: scaling of the hemodynamic effects of the anterior insula differs from that of all other regions.
Figure 4.
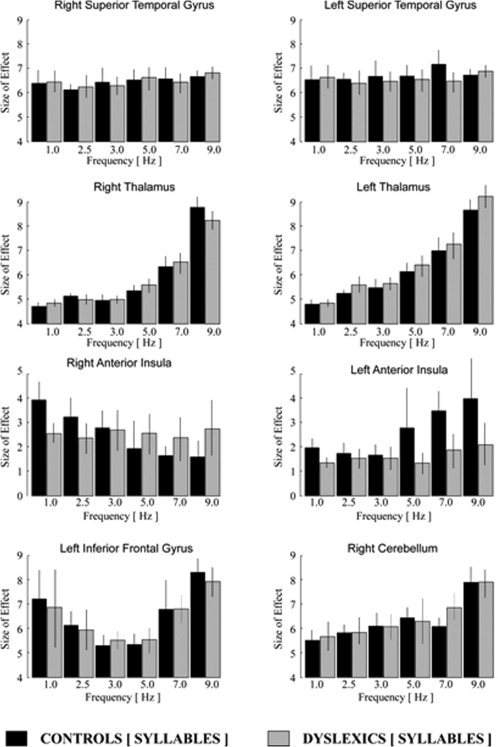
Analysis of frequency‐dependent activation for syllable stimuli: Linear and nonlinear changes of signal intensity for different frequencies of acoustical stimulation displayed by the size of effect and the variance of signal intensity (calculated in arbitrary units by SPM5; gray colored in dyslexics, black colored in healthy control subjects). Note: scaling of the hemodynamic effects of the anterior insula differs from that of all other regions.
Subtraction analysis
On the one hand, a subtraction approach was performed across acoustical stimulation with clicks in healthy versus dyslexic subjects and in dyslexic versus healthy subjects. On the other hand, a subtraction analysis was performed across acoustical stimulation with syllables in healthy versus dyslexic subjects and in dyslexic versus healthy subjects (see Fig. 2).
Figure 2.
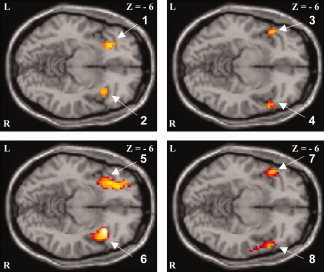
Subtraction analysis of the respective main effects during: (a) acoustical stimulation with clicks in healthy control subjects versus dyslexics (upper row, left illustration) and vice versa (upper row, right illustration); (b) acoustical stimulation with syllables in healthy control subjects versus dyslexics (lower row, left illustration) and vice versa (lower row, right illustration). L, left; R, right; z, distance to the intercommisural plane. Activated brain region, T values [SPM‐coordinates]: 1. Left anterior insula, 3.89 [−30 24 −6], 2. Right anterior insula, 3.46 [39 21 −6], 3. Left rolandic operculum (frontal part), 4.05 [−42 15 −6], 4. Right rolandic operculum (frontal part), 3.73 [51 24 −6], 5. Left anterior insula, 4.71 [−33 12 −6], 6. Right anterior insula, 4.37 [36 18 −6], 7. Left rolandic operculum (frontal part), 3.92 [−42 18 −6], and 8. Right rolandic operculum (frontal part), 4.52 [48 24 −6]. Brain regions were determined using the SPM anatomy toolbox (http://www.fz-juelich.de/inb/inb-3//spm_anatomy_toolbox) and the AAL (anatomical automatic labeling) toolbox (http://www.cyceron.fr/freeware). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
RESULTS
On the basis of the computation of the parametric analysis, we defined the superior temporal gyrus, the thalamus, and the anterior insula of both hemispheres as well as the left inferior frontal gyrus and the right superior cerebellum as the regions of interest for all subsequent statistical data analyses (Fig. 1 and Tab. I).
Table I.
Parametric analysis of rate‐dependent effects during acoustic stimulation with click sounds and syllables in healthy controls and dyslexic subjects
| Clicks/syllables | Main effect (all frequencies) | First‐order term (positive linear effect) | First‐order term (negative linear effect) | Second‐order term (nonlinear effect) | |
|---|---|---|---|---|---|
| A. Healthy controls | |||||
| Inferior frontal gyrus (BA 44/45) | Left | 6.75/6.18 [−57 33 3]/[−57 36 0] | n.s./n.s. | n.s./n.s. | 4.93/4.54 [−54 36 0]/[−57 33 3] |
| Anterior insula | Left | 3.49/3.86 [−30 24 0]/[−42 18 −3] | 4.17/3.91 [−33 21 0]/[−36 24 −3] | n.s./n.s. | n.s./n.s. |
| Right | 4.37/4.80 [39 24 0]/[39 21 3] | n.s./n.s. | 4.78/4.93 [39 24 0]/[39 31 −3] | n.s./n.s. | |
| Thalamus | Left | 4.12/3.87 [−18 −18 0]/[−15 −18 3] | 3.93/4.21 [−18 −15 0]/[−15 −15 3] | n.s./n.s. | n.s./n.s. |
| Right | 4.84/3.51 [15 −21 3]/[15 −18 0] | 4.23/4.45 [15 −18 0]/[21 −18 3] | n.s./n.s. | n.s./n.s. | |
| Superior temporal gyrus (BA 40/41) | Left | 5.89/7.47 [−51 −15 6]/[−48 −12 0] | n.s./n.s. | n.s./n.s. | n.s./n.s. |
| Right | 8.93/9.04 [51 −15 3]/[48 −18 6] | n.s./n.s. | n.s./n.s. | n.s./n.s. | |
| Cerebellum | Right | 4.73/6.54 [27 −57 −27]/[30 −54 −27] | 3.34/4.21 [27 −54 −27]/[33 −57 −27] | n.s./n.s. | n.s./n.s. |
| B. Dyslexic subjects | |||||
| Inferior frontal gyrus (BA 44/45) | Left | 6.34/5.76 [−57 30 0]/[−54 33 6] | n.s./n.s. | n.s./n.s. | 4.363/4.72 [−51 39 3]/[−54 36 0] |
| Anterior insula | Left | 3.72/3.51 [−33 21 −3]/[−39 15 −3] | 4.83/n.s. [−42 15 0]/n.s. | n.s./n.s. | n.s./n.s. |
| Right | 5.37/4.69 [33 21 −3]/[36 24 3] | n.s./n.s. | 3.91/n.s. [45 12 0]/n.s. | n.s./n.s. | |
| Thalamus | Left | 4.29/5.77 [−15 −15 0]/[−15 −18 0] | 4.78/5.01 [−21 −18 3]/[−15 −21 3] | n.s./n.s. | n.s./n.s. |
| Right | 3.21/3.47 [15 −18 6]/[21 −15 3] | 3.95/4.81 [15 −18 0]/[18 −18 0] | n.s./n.s. | n.s./n.s. | |
| Superior temporal gyrus (BA 40/41) | Left | 6.34/7.33 [−48 −12 3]/[−48 −9 3] | n.s./n.s. | n.s./n.s. | n.s./n.s. |
| Right | 6.38/8.24 [51 −12 0]/[45 −15 3] | n.s./n.s. | n.s./n.s. | n.s./n.s. | |
| Cerebellum | Right | 5.45/6.13 [33 −54 −30]/[27 −51 −27] | 4.92/5.24 [30 −51 −27]/[30 −54 −30] | n.s./n.s. | n.s./n.s. |
The first number refers to clicks/the second number to syllables. T values represent activation maximum within each region. SPM‐coordinates are printed in square brackets. Brain regions were determined using the SPM Anatomy Toolbox (http://www.fz-juelich.de/inb/inb-3//spm_anatomy_toolbox) and the AAL (anatomical automatic labeling) toolbox (http://www.cyceron.fr/freeware). BA, Brodmann area; n.s., not significant.
Using the parametric signal analysis, healthy controls demonstrated a linearly increasing hemodynamic response during both conditions (click and syllable trains) in parallel with an increasing stimulation rate at the right cerebellum, the thalamus of both sides, and the left insular cortex (Figs. 3 and 4), whereas the hemodynamic BOLD activity decreased at the level of the right anterior insula. Additionally, at the level of the left inferior frontal gyrus, a significant nonlinear rate‐to‐response function could be detected for both conditions in healthy controls. Consistent with previous studies of our group, no linear or nonlinear increasing or decreasing hemodynamic activation effects at the superior temporal gyrus of both hemispheres in parallel with an increasing acoustical stimulation frequency were detected [Ackermann et al.,2001].
The hemodynamic activation in dyslexic subjects did not differ significantly from the activation patterns in healthy control subjects during acoustical presentation of click sounds. However, in contrast to acoustical presentation of clicks, the presentation of syllables demonstrated a significant discrepancy between healthy controls and dyslexics at the level of the anterior insula of both hemispheres (Figs. 3 and 4). Although healthy controls showed a decreasing activity at the right and an increasing hemodynamic activation at the left anterior insula with increasing acoustical presentation rate of click and syllable trains, the dyslexic group demonstrated a similar activation pattern only during the click condition, but not during the syllable presentation (Figs. 3 and 4). Remarkably, increasing syllable presentation rate, neither elicited a linear nor a nonlinear increasing or decreasing BOLD signal change along with an increasing acoustical stimulation frequency at the anterior insula in dyslexics (see Fig. 4).
Finally, the subtraction analysis of the main effects across conditions and groups revealed a significant activation at the level of the anterior insula of both hemispheres during acoustical stimulation of clicks and syllables in healthy controls. Strikingly, significant activation in dyslexics was located more lateral at the level of the rolandic operculum (frontal part) of both hemispheres (see Fig. 2). The distance between the activation maxima within the respective activation clusters found for dyslexics and controls is differing about 3 voxels. Also, there is a tendency toward a lateralization effect to the left anterior insula during the syllable condition compared with the click condition in healthy controls but not in dyslexics.
DISCUSSION
Using fMRI, this study aimed to investigate the neural correlates of auditory temporal processing of linguistic and nonlinguistic test materials in developmental dyslexia. A previous fMRI study of our group [Ackermann et al.,2001] had introduced a parametric passive listening paradigm, applying click trains at six different frequency rates (2–6 Hz) as a means to test rate‐dependent auditory processing capabilities in healthy adults. This experiment extended this design in two directions. First, six presentation rates—ranging from 1 to 9 Hz—were considered for analysis. Second, syllable trains served as an additional stimulus category to compare the temporal auditory processing of speech and nonspeech test materials in dyslexics.
The two stimulus conditions elicited in both subject groups bilateral hemodynamic main effects at the level of the superior temporal gyrus, thalamus, and anterior insula. Furthermore, significant responses emerged within the left inferior frontal gyrus and the right cerebellar hemisphere. The right cerebellum showed a linear relationship between hemodynamic activation and stimulation rate in both groups and under both the speech and nonspeech conditions. Such a rate‐dependent response is in line with other neuroimaging studies demonstrating the cerebellum to be involved in the representation of temporal aspects of auditory‐perceptual information [Ackermann et al.,2001; Mathiak et al.,2002,2004]. In both groups, furthermore, the left inferior frontal gyrus showed significant hemodynamic activation in response to speech and nonspeech stimuli. Again, these findings are consistent with results of earlier fMRI studies [Burton and Small,2006; Joanisse and Gati,2003]. In dyslexics as well as controls, nonlinear rate‐dependent BOLD signal changes could be observed at the level of left inferior frontal gyrus, both in response to clicks and syllables. These rate‐dependent effects of hemodynamic activation support the notion that left inferior frontal gyrus and right cerebellum interact during auditory temporal processing [Mathiak et al.,2004].
At the level of right and left thalamus, the hemodynamic response to click and syllable trains increased in parallel with stimulation rate both in dyslexics and controls. Our preceding study [Ackermann et al.,2001], by contrast, had reported the rate‐dependent activation pattern of the thalamus to be restricted to the left hemisphere. In line with the latter data, a recent fMRI experiment [Tervaniemi et al.,2006] found that only the thalamic nuclei of the left hemisphere respond to length variation of speech stimuli. Conceivably, the observed differences in thalamic rate‐dependent activation patterns between our previous [Ackermann et al.,2001] and this study are due to methodological differences such as the extension of the frequency range, the additional introduction of stimuli with linguistic content, and the usage of a higher magnetic field intensity.
Rate had no significant impact on the hemodynamic responses of the left and right superior temporal gyrus in both groups and under both stimulus conditions, arguing against a critical contribution of these structures to the processing of rate information. This assumption is in accordance with other fMRI studies using nonspeech stimuli in healthy adults as test materials [Ackermann et al.,2001; Jamison et al.,2006]. Nevertheless, other studies support the notion that the superior temporal gyrus has been found to be involved in the analysis of speech and nonspeech sounds [Liebenthal et al.,2005].
At the level of the anterior insula, a profile resembling the operation of a left‐sided high‐pass filter—in terms of an increasing hemodynamic response in parallel with increasing presentation rate—and a right‐sided low‐pass filter—in terms of a decreasing hemodynamic response in parallel with increasing presentation rate—emerged in both groups during application of click trains. It is well established that both insular cortices participate in several key auditory processes, including different aspects of auditory temporal processing [see Bamiou et al.,2003, for review), and that the anterior part of the insula is especially relevant for the encoding of auditory information [see Köttter,2008, for review]. The rate‐to‐response functions of the anterior insular cortex—as observed in this and in previous studies [Ackermann et al.,2001]—support the notion that the “double filtering by frequency” mechanisms of both hemispheres [Ivry and Robertson,1998] critically depend on this brain area. Given a fundamental role of the anterior insula in auditory temporal processing, it is noteworthy that group differences emerged particularly within this region. Controls showed under both stimulus conditions higher activation maxima within the anterior insular cortex of either hemisphere. Dyslexics, by contrast, exhibited larger hemodynamic responses of the left and right frontotemporal operculum. Using rhyming and verbal short‐term memory tasks, requiring active phonological processing, a variety of neuroimaging studies reported diminished activation of insular areas in dyslexia [Corina et al.,2001; Paulesu et al.,1996]. That these differential responses also emerged during passive listening conditions and also in association with nonspeech stimuli can be interpreted as evidence for a basic nonlinguistic deficit in temporal auditory processing in dyslexics.
Paulesu et al. [1996] have proposed that insular dysfunctions in dyslexia might give rise to a disconnection between anterior and posterior speech areas, resulting in a defective phonological processing system. Recent functional neuroanatomy studies were able to demonstrate bidirectional connections between the anterior insula, on the one hand, and with the cingular, parietal, and prefrontal cortex, on the other [Kötter,2008]. The insula, thus, might serve as an anatomical bridge between various components of the cerebral language network. Although the results of this fMRI study offer an additional evidence for insular dysfunctions in dyslexia, a preceding investigation of our group [Steinbrink et al.,2008] documented disrupted connections within the cerebral network underlying reading skills in these patients. The previous study [Steinbrink et al.,2008] had evaluated white matter neuroanatomy in dyslexics, using diffusion tensor imaging (DTI) in subjects of a similar age when compared with the participants of this investigation. DTI revealed decreased fractional anisotropy (possibly indicative of reduced axonal myelination) in dyslexics in bilateral frontotemporal and left temporoparietal white matter regions connecting brain areas involved in language‐ or reading‐related processes [see Steinbrink et al.,2008, for more details]. These DTI findings of reduced white matter anisotropy in dyslexia—in combination with this fMRI findings of decreased insular activation in dyslexia—support the disconnection hypothesis by Paulesu et al. [1996]. The aforementioned activation of the frontotemporal opercula (frontal part of the rolandic operculum) in dyslexics may be interpreted as a compensatory process, as previous brain imaging studies have shown that healthy adults do not activate the frontal opercular cortices in passive listening to speech and nonspeech stimuli [Fiez et al.,1995].
In addition to decreased activation, material‐specific differences in hemodynamic activation of the anterior insula could be found in dyslexics. By contrast to click trains, the syllable condition did not elicit rate‐dependent BOLD effects in the dyslexics while controls exhibited the same rate‐to‐response profiles under both stimulus conditions. In consideration of differential hemispheric specialization of rapid and slow auditory processing [Fiez et al.,1995; Zatorre et al.,2002], dyslexics' aberrant rate‐to‐response profiles during application of syllables might reflect a functional deficit in the specialization of the left hemisphere for rapid temporal processing of speech.
The findings of this fMRI study provide evidence for a insular contribution to auditory temporal processing deficits in dyslexia. The question arises, however, why the differences in hemodynamic activation were restricted to the speech condition. Syllables and clicks not only differ in linguistic content but also in spectrotemporal complexity. Indeed, a number of fMRI studies on auditory temporal processing in dyslexic adults and children found deficits in the left‐hemispheric specialization for rapid auditory processing for both speech [Ruff et al.,2002] and nonspeech stimuli [Gaab et al.,2007; Temple et al.,2000]. The nonspeech stimuli used in these studies were much more complex than ours, because they were designed to mimic the spectrotemporal characteristics of syllables [Temple et al.,2000]. Thus, differences in the auditory, i.e., spectrotemporal complexity of the stimulus materials might explain differences between our results and those of other fMRI studies on auditory temporal processing. This suggestion is in line with previous studies, using the mismatch negativity (MMN) component of the auditory event‐related potentials as a tool for the investigation of central auditory processing in dyslexia (Bishop,2007). Some of these studies found deviant MMN deflections in dyslexics for nonspeech, but not for speech stimuli [Corbera et al.,2006], others for speech but not for nonspeech stimuli [Schulte‐Körne et al.,1998], yet others for both kinds of stimuli [Lachmann et al.,2005]. These observations point at a critical impact of task demands and design upon auditory temporal processing in developmental dyslexia [Baldeweg et al.,1999; see Bishop,2007, for critical review].
Because this study did not rely on a sparse sampling technique, it might be assumed that background scanner noise influenced the results of the experiment in a systematic way. Indeed, several investigations were able to document that dyslexics show impaired perception of speech [Bradlow et al.,2003; Brady et al.,1983] and nonspeech stimuli embedded in noise [Chait et al.,2007]. Deficient noise exclusion even may represent an etiological factor of developmental dyslexia [Sperling et al.,2005]. As we were aware of this eventual confounding effect, participants were offered the opportunity to adjust the volume of stimuli presentation to an individual comfortable loudness level. Still, we cannot exclude the possibility that the group differences we observed do partly stem from differential impacts of scanner noise on neural activations. For three reasons, we think however, that it is unlikely that group differences can be explained by scanner noise alone. In most brain regions, first, both groups showed the same global activation levels and rate‐dependent activation profiles. These observations argue against a differential impact of scanner noise upon hemodynamic responses in dyslexics and controls. Second, group differences in rate‐dependent activation patterns in the anterior insula were restricted to the speech condition, and are thus unlikely to result from scanner noise. Third, dyslexics' insular activation patterns differed in the speech and nonspeech condition, indicating that they are not produced by scanner noise. As a consequence, the observed group differences can be expected not to reflect a specific influence of scanner noise on auditory processing in dyslexia.
CONCLUSIONS
Taken together, this study yielded the following central findings
-
1
In healthy adults, the anterior insula exhibited a rate‐dependent pattern of hemodynamic activation resembling a high‐pass filter in the left hemisphere and a low‐pass filter in the right hemisphere during passive listening to clicks and syllables applied at various rates. This profile of insular activation corroborates the suggestion of differential hemispheric specialization for temporal auditory processing and indicates an important contribution of intrasylvian cortex to the encoding of temporal aspects both of nonlinguistic as well as linguistic material.
-
2
Dyslexics showed decreased activation of the left and right anterior insula in passive listening to click and syllable stimuli. Given the importance of the insula for temporal auditory processing, this finding suggests a basic nonlinguistic temporal auditory processing deficit in dyslexia.
-
3
The click condition elicited almost equivalent rate‐dependent activation of the anterior insula both in dyslexics and controls. During application of syllable trains, by contrast, only the control but not the dyslexic group showed a similar impact of stimulus frequency upon hemodynamic responses of intrasylvian cortex. As syllables and clicks not only differ with respect to linguistic content but also in signal complexity, it remains unclear whether these results should be interpreted as evidence for a speech‐specific temporal auditory processing deficit in dyslexia or whether these data simply reflect differential sensitivity of the two groups to acoustic stimulus characteristics. Such ambiguities represent a general dilemma of studies addressing the question of general auditory versus specific linguistic deficits of temporal processing in dyslexia.
To conclude, this fMRI study provides evidence that the anterior insula of both hemispheres (1) engages in the temporal encoding of linguistic and nonlinguistic auditory stimuli, (2) contributes to temporal auditory processing deficits in dyslexia, and (3) appears to be involved in the phonological problems of dyslexic subjects.
Acknowledgements
Many thanks to Ingo Hertrich for preparation of the acoustical stimuli, Margarete Linner for assistance with participant recruitment and care, Gerwin Müller, Baerbel Herrnberger, and Katrin Vogt for technical assistance and helpful comments. Many thanks are also to anonymous reviewers for helpful comments on an earlier draft of this article.
REFERENCES
- Ackermann H,Riecker A,Mathiak K,Erb M,Grodd W,Wildgruber D ( 2001): Rate‐dependent activation of a prefrontal‐insular‐cerebellar network during passive listening to trains of click stimuli: An fMRI study. Neuroreport 12: 4087–4092. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association ( 1994): Diagnostic and Statistical Manual on Mental Disorders, 4th ed. (DSM‐IV). Washington, DC: American Psychiatric Press. [Google Scholar]
- Baldeweg T,Richardson A,Watkins S,Foale C,Gruzelier J ( 1999): Impaired auditory frequency discrimination in dyslexia detected with mismatch evoked potentials. Ann Neurol 45: 495–503. [DOI] [PubMed] [Google Scholar]
- Bamiou D‐E,Musiek FE,Luxon LM ( 2003): The insula (Island of Reil) and its role in auditory processing. Literature review. Brain Res Rev 42: 143–154. [DOI] [PubMed] [Google Scholar]
- Becker C,Elliott MA,Lachmann T ( 2005): Evidence for impaired visuoperceptual organisation in developmental dyslexics and its relation to temporal processes. Cogn Neuropsychol 22: 499–522. [DOI] [PubMed] [Google Scholar]
- Ben‐Artzi E,Fostick L,Babkoff H ( 2005): Deficits in temporal‐order judgments in dyslexia: Evidence from diotic stimuli differing spectrally and from dichotic stimuli differing only by perceived location. Neuropsychologia 43: 714–723. [DOI] [PubMed] [Google Scholar]
- Bishop DVM ( 2007): Using mismatch negativity to study central auditory processing in developmental language and literacy impairments: Where are we, and where should we be going? Psychol Bull 133: 651–672. [DOI] [PubMed] [Google Scholar]
- Bradlow AR,Kraus N,Hayes E ( 2003): Speaking clearly for children with learning disabilities: Sentence perception in noise. J Speech Lang Hear Res 46: 80–97. [DOI] [PubMed] [Google Scholar]
- Brady S,Shankweiler D,Mann V ( 1983): Speech perception and memory coding in relation to reading ability. J Exp Child Psychol 35: 345–367. [DOI] [PubMed] [Google Scholar]
- Burton MW,Small SL ( 2006): Functional neuroanatomy of segmenting speech and nonspeech. Cortex 42: 644–651. [DOI] [PubMed] [Google Scholar]
- Chait M,Eden G,Poeppel D,Simon JZ,Hill DF,Flowers DL ( 2007): Delayed detection of tonal targets in background noise in dyslexia. Brain Lang 102: 80–90. [DOI] [PubMed] [Google Scholar]
- Cohen‐Mimran R,Sapir S ( 2007): Auditory temporal processing deficits in children with reading disabilities. Dyslexia 13: 175–192. [DOI] [PubMed] [Google Scholar]
- Corbera S,Escera C,Artigas J ( 2006): Impaired duration mismatch negativity in developmental dyslexia. Neuroreport 17: 1051–1055. [DOI] [PubMed] [Google Scholar]
- Corina DP,Richards TL,Serafini S,Richards AL,Steury K,Abbott RD,Echelard DR,Maravilla KR,Berninger VW( 2001): fMRI auditory language differences between dyslexic and able reading children. Neuroreport 12: 1195–1201. [DOI] [PubMed] [Google Scholar]
- Démonet J‐F,Taylor MJ,Chaix Y ( 2004): Developmental dyslexia. Lancet 363: 1451–1460. [DOI] [PubMed] [Google Scholar]
- DiLollo V,Hanson D,McIntyre JS ( 1983): Initial stages of visual information processing in dyslexia. J Exp Psychol Hum Percept Perform 9: 923–935. [DOI] [PubMed] [Google Scholar]
- Farmer ME,Klein RM ( 1995): The evidence for a temporal processing deficit linked to dyslexia: A review. Psychon Bull Rev 2: 460–493. [DOI] [PubMed] [Google Scholar]
- Fiez JA,Raichle ME,Miezin FM,Petersen SE,Tallal P,Katz WF ( 1995): PET studies of auditory and phonological processing: Effects of stimulus characteristics and task demands. J Cogn Neurosci 7: 357–375. [DOI] [PubMed] [Google Scholar]
- Gaab N,Gabrieli JDE,Deutsch GK,Tallal P,Temple E ( 2007): Neural correlates of rapid auditory processing are disrupted in children with developmental dyslexia and ameliorated with training: An fMRI study. Restor Neurol Neurosci 25: 295–310. [PubMed] [Google Scholar]
- Ivry RB,Robertson LC ( 1998): The Two Sides of Perception. Cambridge, MA: MIT Press. [Google Scholar]
- Jamison HL,Watkins KE,Bishop DVM,Matthews PM ( 2006): Hemispheric specialization for processing auditory nonspeech stimuli. Cereb Cortex 16: 1266–1275. [DOI] [PubMed] [Google Scholar]
- Joanisse MF,Gati JS ( 2003): Overlapping neural regions for processing rapid temporal cues in speech and nonspeech signals. Neuroimage 19: 64–79. [DOI] [PubMed] [Google Scholar]
- Kersting M,Althoff K ( 2004): Rechtschreibungstest (RT). Göttingen: Hogrefe. [Google Scholar]
- Kötter R ( 2008): Funktionelle neuroanatomie der inselrinde (functional neuroanatomy of the insular cortex). Nervenheilkunde 27: 425–429. [Google Scholar]
- Lachmann T,Berti S,Kujala T,Schröger E ( 2005): Diagnostic subgroups of developmental dyslexia have different deficits in neural processing of tones and phonemes. Int J Psychophysiol 56: 105–120. [DOI] [PubMed] [Google Scholar]
- Liebenthal E,Binder JR,Spitzer SM,Possing ET,Medler DA ( 2005): Neural substrates of phonemic perception. Cereb Cortex 15: 1621–1631. [DOI] [PubMed] [Google Scholar]
- Mathiak K,Hertrich I,Grodd W,Ackermann H ( 2002): Cerebellum and speech perception: A functional magnetic resonance imaging study. J Cogn Neurosci 14: 902–912. [DOI] [PubMed] [Google Scholar]
- Mathiak K,Hertrich I,Grodd W,Ackermann H ( 2004): Discrimination of temporal information at the cerebellum: Functional magnetic resonance imaging of nonverbal auditory memory. Neuroimage 21: 154–162. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Paulesu E,Frith U,Snowling M,Gallagher A,Morton J,Frackowiack RSJ,Frith CD ( 1996): Is developmental dyslexia a disconnection syndrome?—Evidence from PET scanning. Brain 119: 143–157. [DOI] [PubMed] [Google Scholar]
- Ruff S,Cardebat D,Marie N,Démonet JF ( 2002): Enhanced response of the left frontal cortex to slowed down speech in dyslexia: An fMRI study. Neuroreport 13: 1285–1289. [DOI] [PubMed] [Google Scholar]
- Schulte‐Körne G ( 2001): Lese‐Rechtschreibstörung und Sprachwahrnehmung. Münster: Waxmann. [Google Scholar]
- Schulte‐Körne G,Deimel W,Bartling J,Remschmidt H ( 1998): Auditory processing and dyslexia: Evidence for a speech specific processing deficit. Neuroreport 9: 337–340. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE ( 1998): Dyslexia. N Engl J Med 338: 307–312. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE,Shaywitz BA ( 2005): Dyslexia (specific reading disability). Biol Psychiatry 57: 1301–1309. [DOI] [PubMed] [Google Scholar]
- Siegel LS ( 1998): Phonological processing deficits and reading disabilities In Metsala JL,Ehri LC, editors. Word Recognition in Beginning Literacy. Mahwah, NJ: Erlbaum; pp 141–160. [Google Scholar]
- Snowling M ( 1998): Dyslexia as a phonological deficit: Evidence and implications. Child Psychol Psychiatry Rev 3: 4–11. [Google Scholar]
- Snowling M ( 2000): Dyslexia. Oxford: Blackwell. [Google Scholar]
- Sperling AJ,Lu Z‐L,Manis FR,Seidenberg MS ( 2005): Deficits in perceptual noise exclusion in developmental dyslexia. Nat Neurosci 8: 862–863. [DOI] [PubMed] [Google Scholar]
- Stanovich KE ( 1988): Explaining the differences between the dyslexic and the garden‐variety poor reader: The phonological‐core‐variable‐difference‐model. J Learn Disabil 21: 590–604. [DOI] [PubMed] [Google Scholar]
- Steinbrink C,Vogt K,Kastrup A,Müller H‐P,Juengling FD,Kassubek J,Riecker A ( 2008): The contribution of white and gray matter differences to developmental dyslexia: Insights from DTI and VBM at 3.0 Tesla. Neuropsychologia 46: 3170–3178. [DOI] [PubMed] [Google Scholar]
- Svensson I,Jacobson C ( 2006): How persistent are phonological difficulties? A longitudinal study of reading retarded children. Dyslexia 12: 3–20. [DOI] [PubMed] [Google Scholar]
- Talcott JB,Hansen PC,Assoku EL,Stein JF ( 2000): Visual motion sensitivity in dyslexia: Evidence for temporal and energy integration deficits. Neuropsychologia 38: 935–943. [DOI] [PubMed] [Google Scholar]
- Tallal P ( 1980): Auditory temporal perception, phonics, and reading disabilities in children. Brain Lang 9: 182–198. [DOI] [PubMed] [Google Scholar]
- Tallal P,Miller S,Fitch RH ( 1993): Neurobiological basis of speech: A case for the preeminence of temporal processing. Ann N Y Acad Sci 682: 27–47. [DOI] [PubMed] [Google Scholar]
- Temple E,Poldrack RA,Protopapas A,Nagarajan S,Salz T,Tallal T ( 2000): Disruption of the neural response to rapid acoustic stimuli in dyslexia: Evidence from functional MRI. Proc Natl Acad Sci USA 97: 13907–13912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervaniemi M,Szameitat AJ,Kruck S,Schröger E,Alter K,De Baene W,Friederici A ( 2006): From air oscillations to music and speech: Functional magnetic resonance imaging evidence for fine‐tuned neural networks in audition. J Neurosci 26: 8647–8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiß RH ( 1997): Grundintelligenztest Skala 2 (CFT 20). Göttingen: Hogrefe. [Google Scholar]
- Zatorre RJ,Belin P,Penhune VB ( 2002): Structure and function of auditory cortex: Music and speech. Trends Cogn Sci 6: 37–46. [DOI] [PubMed] [Google Scholar]


