Abstract
The extrastriate body area (EBA) is one among the multiple, functionally specialized regions of the human visual cortex exhibiting modulation by body‐related stimuli. Here we investigate whether activation patterns differ for the perception of one's own body and the bodies of others. We used functional magnetic resonance imaging to identify body‐related brain areas and to see how these areas differentiate between images of one's own body and those of others in the absence of facial or motion cues. Whole brain explorative group‐level analysis identified body‐related blood oxygen level dependent (BOLD) signal enhancement in five regions of the right and in one region of the left hemisphere (right: in the extrastriate visual and parietal cortex and in the precentral gyrus, left: in the extrastriate visual cortex). General linear model group‐level random effects analysis of the self–other contrast revealed self‐related responses in the extrastriate and parietal regions in the right hemisphere but also in the right middle frontal gyrus. These results suggest the existence of a cortical network for the extraction of body‐related information and another cortical network for the extraction of self‐related body information. The two networks partially overlap in the right superior and inferior parietal cortices, but are clearly segregated in the extrastriate visual cortex and in the middle frontal gyrus. In addition, we report that the classical EBA is only involved in the analysis of body‐related information but not in the assignment of body identity. The latter appears to be accomplished by a network in right hemisphere comprising the fusiform body area, regions of the superior parietal lobe, the inferior parietal cortex, and the middle frontal gyrus. Hum Brain Mapp, 2009. © 2008 Wiley‐Liss, Inc.
Keywords: objects recognition, self, EBA, extrastriate cortex, body perception
INTRODUCTION
The human visual cortex is organized in multiple, functionally specialized regions, and some of them exhibit category‐specific modulation of their activity [Downing et al., 2006]. Thus, faces activate preferentially the occipital and fusiform face area (OFA and FFA) [Kanwisher et al., 1997; Puce et al., 1996; Tsao et al., 2006], places and scenes the parahippocampal place area [Epstein and Kanwisher, 1998], and images of the human body and of body parts the extrastriate body area (EBA) of the lateral occipitotemporal cortex [Downing et al., 2001]. In addition, the lateral occipital cortex (LOC), is activated by the shapes and structures of common objects showing no category specificity [Malach and Tootel, 1995].
The precise specialization of the aforementioned regions has been more deeply explored [Noppeney et al., 2005; Peelen and Downing, 2005]. For instance, Peelen and Downing [2005] claimed dual selectivity for faces and bodies in the previously specified FFA, but application of high‐resolution functional magnetic resonance imaging (fMRI) techniques revealed a segregation of face and body selective regions [Schwarzlose et al., 2005]. The EBA in the lateral occipitotemporal cortex is activated by images of human headless bodies and body parts [Downing et al., 2001 (fMRI); Thierry et al., 2006 (EEG)], but also by other body‐related stimuli such as point‐light biological motion, and the planning, imaging, and execution of limb movements [Astafiev et al., 2004; Grossmann and Blake, 2002; Peelen et al., 2006]. A causal involvement of the EBA in the visual processing of body‐related stimuli is suggested by the finding that inactivation of this region by repetitive transcranial magnetic stimulation causes an impairment in visual processing of body parts and an increase in reaction time for the discrimination of nonfacial body parts [Urgesi et al., 2004].
Faces and bodies constitute a particular category, because they allow for the distinction between self and others, raising the question whether this distinction is reflected in the activation of the respective processing areas. For faces, an extended network of areas has been identified, the activation of which differentiates between “self” and “others.” The network comprises the inferior occipital gyrus, the fusiform gyrus (FFG), the inferior parietal lobe (IPL), and the inferior frontal gyrus in the right hemisphere and the FFG, middle temporal cortex and the prefrontal cortex (PFC) of the left hemisphere [Keenan et al., 2000; Kircher et al., 2000, 2001; Nakamura et al., 2000; Platek et al., 2004; Sugiura et al., 2005]. Furthermore, there are gender‐related differences in face recognition tasks [Cellerino et al., 2004; Proverbio et al., 2006].
Differential activity distinguishing between anticipation, visualization, or observation of one's own movement versus that of others has been observed in the dorsal premotor cortex and in the lateral and medial PFC of the left hemisphere, in the paracingulate region and superior temporal cortex of the right hemisphere as well as bilaterally in the inferior and superior parietal cortices [Cunnington et al., 2006; Grezes et al., 2004; Jackson and Decety, 2004; Ramnani and Miall, 2004]. These fMRI results are in general agreement with clinical studies which indicated right hemisphere dominance in the recognition of oneself [Preilowski, 1977; Sperry et al., 1979]. On the basis of this evidence, Sugiura et al. [2006] proposed the existence of two distinct right‐hemispheric networks for self‐recognition: a posterior network that evaluates visuospatial cues and a frontal network encoding the concept of self.
The goal of the present study was to identify brain regions dedicated to human bodies and, in particular, to define brain regions specifically devoted to the perception of one's own body. We hypothesized that there are distinct networks for the analysis of bodies and the recognition of self for two reasons. First, networks involved in the encoding of self should comprise not only visual areas, since the concept of ownership requires polysensory integration [Saxe et al., 2006]. Second, Chan et al. [2004] had claimed that EBA does not differentiate between self and others. To further explore this claim we introduced different paradigms: we manipulated identity but not the viewpoint of body images, we opted for a uniform neutral upright body posture, and we contrasted self to unfamiliar others. Yet, another motivation for this study was our interest in eating disorders like anorexia nervosa and in the delusional misidentification syndrome (DMS) [Feinberg and Keenan, 2005], as these pathologies are related to disturbances in the perception of one's own body and the distinction between self and others, respectively.
Initially, we performed a ROI‐based analysis of body‐selective regions across subjects. Subsequently, in order to test which cortical areas respond preferentially to images of one's own body and whether body‐selective regions differentiate between self and other, we examined self–other contrasts using volume‐ and ROI‐based general linear model (GLM) fixed and random effect group‐level analysis. Because of the gender‐specific expression of eating disorders, we also performed a pilot examination of putative gender related differences in the networks processing body‐related information and supporting self–other distinctions, respectively.
METHODS
Two experiments were carried out. In the first experiment, the subjects were scanned in a 3‐T scanner while they were presented with photographs of their own bodies, of the bodies of other subjects, and of neutral objects. In the second experiment, the subjects' ability to recognize themselves was tested psychophysically outside an fMRI environment. In all images of bodies, the head was not shown in order to eliminate any face‐related cues.
Subjects
A total of 16 volunteers (average age = 29.2 years; 10 females) participated in the experiments. Twelve subjects (seven females) took part in Experiment I and four subjects (two females) in Experiment II. All subjects had normal or corrected‐to‐normal vision and a history free of neurological disorders. Participants in Experiment I were briefed about the fMRI procedure, and they completed a questionnaire about their physical conditions to ensure that there are no potential health risks and contraindications.
Stimuli
For the compilation of the stimulus material, subjects wore dark blue bikinis or swimming suites, and were photographed in neutral upright posture from four different angles (front, back, right, and left) against a nonreflective gray background. We opted for the presentation of different views in order to reduce habituation during the fMRI measurement. The pictures were taken with a digital camera Canon EOS‐30D (3.1 million effective pixels), and edited with PHOTO‐PAINT X3 graphic suite in order to obtain gray‐scale images of headless bodies. Images of neutral objects and their scrambled versions were edited in the same way. All images were put over a grid (400 × 400 pixels) to avoid differences in the stimulated portion of the visual field caused by the differences in the natural size of presented stimuli.
Task Paradigm
For the fMRI measurement, we used a block design with five stimulus conditions: images of self, of others of opposite gender, of others of same gender, of neutral objects, and of their scrambled versions. The blocks of others contained different views of one unfamiliar person of both sexes, there was no pairing of images for specific subjects, but the care was taken that the other was unfamiliar to subject. Each condition was repeated six times in one session, and each subject took part in two sessions. In each session, the first block was presented seven times and the first four scan volumes were skipped to avoid T1 saturation effects. In one block that lasted 16 s and comprised eight volumes, 16 pictures were presented, and in case of bodies, 4 × 4 different views in random order. One picture was shown for 400 ms followed by a 600 ms blank interval, and blocks were separated by 16 s.
Throughout a session, subjects were asked to fixate a fixation cross that was continuously presented in the center of the screen, but eye movements were not monitored. The images were randomized within and between blocks, with the blocks' order being counterbalanced within and between subjects using MATLAB (6.5). During one session that lasted 16.8 min, 504 functional volumes were recorded.
Stimuli were generated with custom‐made software based on the Microsoft DirectX library (Sounds and Images, Brain Innovation, Maastricht, The Netherlands). The stimuli were back‐projected onto a frosted screen with a liquid‐crystal‐display projector (VPL PX 20, Sony, Tokyo, Japan) and custom‐made lens. Subjects were viewing the screen through a mirror fixed onto the head coil. In order to maintain a constant level of vigilance, the subjects performed a “one back repetition task,” indicating the repetition of an image by pressing a button. The subjects were not familiarized with the stimulus material and were not instructed to discriminate between self and others.
Behavioral Experiment
In order to test whether the presentation time and the block design used in the scanner allowed the subjects to recognize themselves, we performed a second behavioral experiment in a different group of subjects. The paradigm was similar to the fMRI block design, and the stimulation was generated with Presentation (9.90) software (http://nbs.neuro-bs.com). Subjects were shown four blocks of images: their own body as seen by the camera, their own body as seen in the mirror, and two blocks of other peoples' bodies. The others' body images were of the same gender as the subjects. Presentation parameters were the same as in the fMRI experiment. Each condition was repeated four times in one session, and each subject took part in three sessions. The subjects' task was to indicate by pressing a button whether the body shown to them was their own. After the subjects had responded, the presentation sequence was interrupted and the question: “How sure are you?” appeared. The subjects judged their confidence between 50% (just guessing) and 100% (sure). Once the confidence judgment was given, the next body block started. The reaction times, correctness of given answers, and confidence levels were recorded.
fMRI measurement
Scanning was performed with a 3‐T Allegra or Trio scanner (Siemens, Brain Imaging Center, Frankfurt am Main, Germany), depending on the available scanning time. A gradient‐recalled echo‐planar‐imaging sequence was applied with the following parameters: 36 slices oriented approximately in parallel to the anterior commissure–posterior commissure plane; TR 2,000 ms; TE 30 ms; FA 90°; FOV 210 mm; in‐plane resolution 3.28 mm × 3.28 mm; slice thickness 2.4 mm and gap thickness 0.24 mm. In addition, a T1‐weighted anatomical scan was acquired with a magnetization‐prepared rapid‐acquisition gradient‐echo. In six subjects, acquired functional EPI scans were automatically corrected for geometric distortions by the reconstruction point spread function (PSF) algorithm. PSF is an algorithm that enables reliable and fully automated distortion correction of echo‐planar images at high field strengths [Zaitsev et al., 2004].
Data analysis
Data were analyzed with the BrainVoyager QX software package (BrainInnovation, Maastricht, The Netherlands). The first four volumes of each scan were discarded to avoid T1 saturation effects. Preprocessing of the functional data was performed using three‐dimensional motion correction, linear‐trend removal, temporal high‐pass filtering, and slice‐scan‐time correction. The functional data were coregistered with the structural images using 2D‐3D alignment for the creation of volume time course plots of the blood oxygen level dependent (BOLD) signal.
Statistical examination was based on the GLM employing a boxcar function for the stimulation and fixation conditions that was convolved with a γ distribution to account for the shape and delay of the hemodynamic response. We performed statistical analysis as follows:
-
a
Initially, we performed a single subject ROI analysis of the extrastriate body selective regions in the left and right hemisphere. EBA‐ROIs were defined in each individual by a whole brain GLM contrast for pictures of bodies versus pictures of objects (bodies > objects). ROIs in the inferior temporal cortex were selected as clusters of voxels (>10 neighbouring voxels) that exceeded the threshold by at least t > 2.4(df = 988; P < 0.01; uncorrected); however, the thresholds were adjusted for each subject individually (ranging between t > 2.4 and t > 12.6; see the Table I for exact values). Subsequently, we performed the statistical comparison of responses to own body (self) and other bodies (others, including both sexes) within the individually defined body‐selective ROIs. Finally, random effects analysis (RFX) of the average across all right and left body‐selective ROIs for the independent contrasts (bodies > objects and self > others) was performed.
-
b
Second, we applied a single subject explorative analysis, looking for regions in each individual subject preferring pictures of self over pictures of others (self > others).
-
c
Third, we applied a group‐level volume‐based analysis. This volume‐based analysis was performed for the contrast bodies > objects after individual Talairach transformation. Subsequently, the same volume‐based analysis was performed for the contrast self > others. Both contrasts were calculated using GLM fixed effects (FE) analysis at the threshold t(11,916) > 4; P < 0.05 [corrected for multiple comparison (MC)] for bodies > objects and t(11,916) > 3.5; P < 0.05 (corr. for MC) for self > others. Correction for MCs was performed using minimum cluster size and permutation testing‐based probability estimates of a false detection of a given clusters as proposed by Forman et al. [1995]. Initially, we applied a voxelwise threshold criterion of P < 0.005 and subsequently a spatial extent threshold of 130–208 contiguous functional voxels. This resulted in the whole brain corrected value of P < 0.05 determined by Monte Carlo simulations in BV QX [“Cluster threshold estimator plug‐in” (F. Esposito)].
-
d
Then we applied a group‐level volume‐based GLM analysis at t(11,916) > 3.5; P < 0.05 (corrected for MC using the same cluster thresholding procedure as described earlier) to define regions active for contrasts same > opposite gender and in an independent analysis for the contrast opposite > same gender.
-
e
We used the group‐level volume‐based analysis mentioned earlier (c) to define ROIs for three extrastriate visual areas that exhibited reliably a selective increase of responses for bodies as compared to objects. Similarly we defined cortical ROIs responding selectively to the images of self when compared to others. These functionally defined ROIs then entered a detailed ROI‐based random effects analysis, in which the contrasts self > others and bodies > objects were tested independently.
-
f
Finally, we reperformed a volume‐based group‐level analysis as mentioned earlier (d), and this time we performed the more conservative random effects (RFX) analysis at t(11) > 2.4; P < 0.05 again corrected for MCs using the aforementioned cluster size permutation testing. RFX analysis was performed for two volume‐based contrasts: (1) bodies > objects, and (2) self > other. The multisubject maps were obtained by z‐normalizing the time course of individual subjects and using a random effect procedure [Friston et al., 1999].
Table I.
ROI‐based single subject GLM (FE) analysis of the right and left hemisphere extrastriate body areas (EBAs) for the contrasts bodies > objects and self > others
| S. no. | EBA_RH | EBA_LH | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bodies > objects | Self > others | Bodies > objects | Self > others | |||||||||
| t | SE | P | t | SE | P | t | SE | P | t | SE | P | |
| 1 | >2.45 | 0.21 | <0.014 | >−0.62 | 0.21 | <0.53 | ||||||
| 2 | >7.58 | 0.22 | <0.000 | >0.61 | 0.22 | <0.54 | >8.11 | 0.20 | <0.000 | >1.56 | 0.20 | <0.12 |
| 3 | >3.0 | 0.26 | <0.002 | >−1.25 | 0.26 | <0.21 | ||||||
| 4 | >6.70 | 0.50 | <0.000 | >−3.39 | 0.26 | <0.007 | >6.16 | 0.20 | <0.000 | >−1.66 | 0.20 | <0.09 |
| 5 | >11.78 | 0.20 | <0.000 | >−2.52 | 0.20 | <0.01 | >8.11 | 0.22 | <0.000 | >−0.10 | 0.22 | <0.91 |
| 6 | >3.17 | 0.24 | <0.001 | >3.34 | 0.25 | <0.000 | >4.68 | 0.24 | <0.000 | >4.20 | 0.24 | <0.000 |
| 7 | >2.55 | 0.26 | <0.011 | >0.72 | 0.26 | <0.470 | >3.27 | 0.24 | <0.001 | >1.82 | 0.24 | <0.068 |
| 8 | >12.64 | 0.14 | <0.000 | >1.97 | 0.14 | <0.05 | >5.66 | 0.17 | <0.000 | >0.05 | 0.17 | <0.96 |
| 9 | >6.88 | 0.23 | <0.000 | >−1.09 | 0.23 | <0.27 | >7.62 | 0.22 | <0.000 | >−0.95 | 0.22 | <0.34 |
| 10 | >9.36 | 0.18 | <0.000 | >2.08 | 0.18 | <0.04 | >6.08 | 0.25 | <0.000 | >−0.01 | 0.25 | <0.98 |
| 11 | >8.21 | 0.40 | <0.000 | >0.43 | 0.40 | <0.66 | >7.04 | 0.26 | <0.000 | >−0.07 | 0.26 | <0.94 |
| 12 | >10.18 | 0.17 | <0.000 | >−0.19 | 0.17 | <0.85 | >8.78 | 0.21 | <0.000 | >−1.11 | 0.21 | <0.26 |
P values uncorrected.
RESULTS
Single Subject ROI Analysis
We found body selective regions in every subject (see Fig. 1). In 6 out of 12 subjects, we found two distinct body‐selective areas in the inferior temporal cortex of the right hemisphere. These distinct patches were grouped into a ventrally located fusiform body area (FBA) (Talairach coordinates across subjects 45(5); −63(3); −2(9)) and the occipitotemporal EBA (Talairach coordinates across subjects 48(5); −62(7); 3(5)). In the remaining six subjects, only the EBA of the right hemisphere showed significantly enhanced responses to bodies, and in 10 subjects, the EBA was activated significantly also in the left hemisphere (Talairach coordinates across subjects −45(4); −68(5); 3(5)). Additional to the extrastriate visual areas, nine subjects exhibited body selectivity in their parietal cortices (six subjects expressed selectiveness to the images of bodies in the right parietal cortex, while three subjects showed body selectiveness in the parietal cortices bilaterally).
Figure 1.
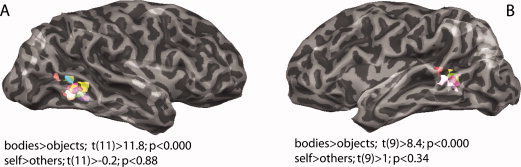
Body‐selective ROIs for GLM contrast: bodies > objects in the inferior temporal cortices shown on the transparent surfaces of the right (A) and left (B) hemisphere of one subject, with each participant's EBA cluster depicted with different color. The functional ROIs were selected by adjusting thresholds for each subject individually, ranging between t > 2.4 and t > 12.6 and with the lowest P value of P < 0.01 (uncorrected) (Table I shows exact values across subjects). Random effects analysis of the average across 12 right and 10 left hemisphere ROIs revealed significant BOLD signal modulation t(11) > 11.8, P < 0.00000 and t(9) > 8.4, P < 0.00001 (uncorrected) for the defining contrast bodies > objects. On the other hand, contrast of self > others within body‐selective ROIs did not show a significant effect.
For the subsequent single subject ROI analysis we only used the EBA, as it was activated most reliably across subjects as outlined in Table I. Detailed ROI‐based analysis of the ROIs defined on the single subject level for the contrast bodies > objects revealed significance in all 12 subjects for the right EBAs (P < 0.01, uncorrected) and in only 10 subjects for the left EBAs (P < 0.001, uncorrected). Further ROI analysis of the individually defined body‐selective ROIs for the contrast self > others showed that the right FBA in five subjects was responding selectively to images of self (P < 0.05, uncorrected) and the left EBA in one subject (P < 0.000, uncorrected). Consequently, after the random effects analysis of the average across all right and left EBA ROIs for the contrast self > others, we failed to find a significant modulation within these regions [t(11) > −0.2; SE = 0.11; P < 0.88 for the right and t(9) > 1.8; SE = 0.12; P < 0.343 (uncorrected) for the left hemisphere; Fig. 1].
Single Subject Explorative Analysis
Next we applied a single subject explorative analysis to detect regions that prefer images of the own body over images of others (self > others). This analysis revealed that nine subjects exhibited significant activation for the images of self in the extrastriate visual areas and the parietal cortex of the right hemisphere. Three of these subjects exhibited self‐related activity in the superior parietal cortex of both hemispheres and three subjects showed significant self‐related activation solely in the ventral and occipitotemporal extrastriate visual areas of the right hemisphere.
Group‐Level Volume‐Based Analysis
Group‐level volume‐based analysis (FE) was performed for three independent contrasts: bodies > objects, self > others, and same > opposite gender. The bodies > objects contrast (t(11,916) > 4.0; P < 0.05 (corrected for MC) identified three areas in the extrastriate visual cortex showing enhanced activation by body images: the FBA (Talairach coordinates: x = 45, y = −63, z = 0) and the EBA (Tal. x = 43, y = −65, z = 8) of the right hemisphere and the EBA (Tal. x = −46, y = −64, z = 1) of the left hemisphere. Additionally, there was a differential activation to the images of bodies in SPL (Tal. x = 19, y = −65, z = 50) and IPL (Tal. x = 33, y = −45, z = 50) as well as in the precentral gyrus of the right hemisphere (Tal. x = 42, y = 1, z = 33; Fig. 2A–I).
Figure 2.
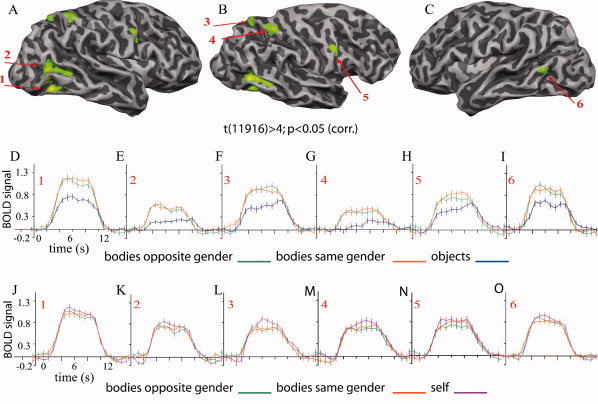
Cortical activation maps (A–C) and time courses (D–I) from group‐level volume‐based analysis of the entire brain for the contrast bodies (other bodies of the same gender and other bodies of the opposite gender) > objects shown on one individual surface at P < 0.05 (corrected for multiple comparisons). (A) Right hemisphere with extrastriate body selective regions (1 and 2), (B) lateral–dorsal view of the right hemisphere with parietal body selective areas (3 and 4), and the body‐selective region in the right precentral gyrus (5) and (C) extrastriate body selective region of the left hemisphere (5); event‐related time course graphs for the contrast bodies > objects in the ventral EBA of the right hemisphere (D); right occipitotemporal EBA (E); right superior parietal body‐selective region (F); right inferior parietal body‐selective region (G); body‐selective region in the right precentral sulcus (H) and left extrastriate body area (I). (J–O) Event‐related time course graphs show responses of all body‐selective regions to the contrast self > others. Error bars correspond to standard errors of the mean.
Further, GLM FEs group‐level explorative analysis for the contrast self > others (t(11,916) > 3.5; P < 0.05 corrected for MC) identified foci of enhanced responses to self in the extrastriate and parietal cortex as well as in the middle frontal gyrus of the right hemisphere. The augmentation of the BOLD signal by the images of self was observed in the extrastriate visual cortex (Tal. x = 48, y = −50, z = −12), in the superior parietal lobe (x = 24, y = −64, z = 39), in the inferior parietal sulcus (x = 34, y = −51, z = 47), and in the middle frontal gyrus (x = 43, y = 31, z = 20) of the right hemisphere. There was no significant modulation of the BOLD signal in the left hemisphere for this contrast (Fig. 3A–F).
Figure 3.
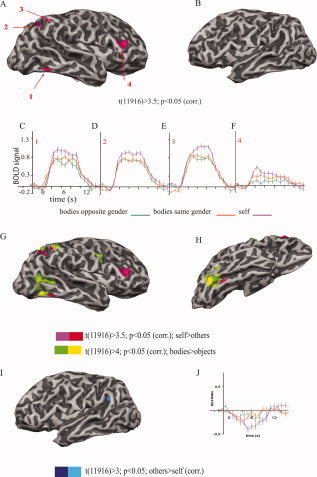
Significantly activated cortical areas (1–4) for the contrast self > others in the right (A) and in the left hemisphere (B), shown on the surface of one representative subject after GLM FEs group‐level analysis with P < 0.05 (corrected for multiple comparisons). The self‐related activity was detected in the extrastriate visual cortex (1), in the parietal cortex (2,3), and in the middle frontal gyrus (4) all in the right hemisphere. Note that no activation at the given contrast was detectable in the left hemisphere. Event‐related time course graphs (C–F) show an enhancement of the BOLD signal related to the images of self in the identified self‐selective areas (1–4). Error bars correspond to standard errors of the mean. The bodies (bodies > objects; yellow–green) and self (self > others; red–pink) selective regions are overlapping in the parietal cortex, but in the extrastriate visual cortex of the right hemisphere, self‐related activity is more directed toward the ventral bank (G: lateral view and H: ventral view). The opposite contrast others > self (dark–bright blue) revealed significant activity in the lateral sulcus of the left hemisphere (I,J).
Comparison of the body‐ and the self‐related maps revealed a correspondence in the superior parietal cortex, where the respective foci overlapped while in the extrastriate visual areas the self‐related activity extended more toward the ventral bank and the fusiform area than the body‐related activity (Fig. 3G,H). Further, conjunction analysis for the contrasts bodies > objects and self > other reveled an overlap of the related activity in the right superior and inferior parietal cortices, with t(11,916) > 3; P < 0.05 (corrected for MC) after GLM FE analysis.
GLM group‐level FE explorative analysis for the contrast others > self revealed significant BOLD signal modulation at t(11,916) > 3, P < 0.05 (corrected for MC) in the lateral sulcus of the left hemisphere (Fig. 3I,J).
In addition, GLM FE group‐level volume‐based explorative analysis for the contrast same > opposite gender at t(11,916) > 3.5 and P < 0.05 (corrected for MC) depicted the regions of the right frontal cortex as showing preference for the bodies of the same gender (especially pronounced in the inferior frontal gyrus; Tal. x = 41, y = 39, z = −2). No such modulation was observed in the left hemisphere (Fig. 4A,B). When the reverse contrast (opposite > same gender) was tested at the same P and t thresholds, no significant BOLD signal augmentation was observed in the right hemisphere. However, a preference for the body images of the opposite gender contrasted to the same gender was noticed in the postcentral gyrus (Tal. x = −47, y = −35, z = 40) of the left hemisphere (Fig. 4C,D).
Figure 4.
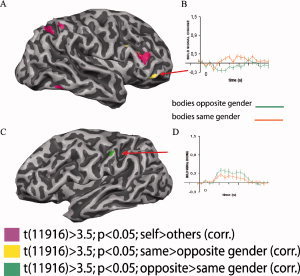
Significantly activated cortical areas for the contrasts same > opposite (yellow) and opposite > same gender (green) superimposed on previously described self‐selective network (pink) in the right (A) and in the left hemisphere (C), shown on the surface of one representative subject after GLM FEs group level analysis with P < 0.05 (corrected for multiple comparisons). The same > opposite gender contrast revealed significant enhancement of the BOLD signal in the right inferior frontal gyrus (A) while the opposite > same gender contrast has shown BOLD signal modulation in the left postcentral gyrus (C). Note that the selectiveness for the same or opposite gender was lateralized. Graphs B and D show event‐related time courses for the given conditions. Error bars correspond to standard errors of the mean.
Group‐Level Volume‐Based ROI Analysis
Detailed ROI‐based RFX analysis of the functionally defined body‐selective ROIs proved that all three regions exhibit strong selectivity for bodies when compared to objects (Table II) at t(11) > 4.4; SE = 0.19; P < 0.000 in EBA, t(11) > 5.9; SE = 0.15; P < 0.000 in FBA and t(11) > 3.4; SE = 0.18; P < 0.005 in EBA of the left hemisphere. However, none of these ROIs revealed selectivity to images of self and no specific gender effects were observed.
Table II.
Random effects group level GLM analysis of the right and left hemisphere extrastriate body areas (EBAs) for the contrast bodies > objectsand self > others
| Bodies > objects | Self > others | ||||||
|---|---|---|---|---|---|---|---|
| df | t | SE | P | t | SE | P | |
| EBA (RH) | 11 | >4.44 | 0.19 | <0.000 | >1.30 | 0.12 | <0.22 |
| FBA (RH) | 11 | >5.99 | 0.15 | <0.000 | >1.91 | 0.11 | <0.08 |
| EBA (LH) | 11 | >3.40 | 0.18 | <0.005 | >0.96 | 0.10 | <0.35 |
P values uncorrected.
Consequently, ROI‐based RFX analysis of the functionally defined self‐selective cortical areas (VEVC, ventral extrastriate visual cortex; IPS, inferior parietal sulcus; SPL, superior parietal lobe; MFG, middle prefrontal gyrus) revealed significance for the images of self ranging from t(11) > 2.8; SE = 0.13; P < 0.01 (uncorrected) in the IPS to t(11) > 4.43; SE = 0.14; P < 0.001(uncorrected) in the MFG (Table III). Furthermore, self‐selective ROIs were tested for the contrast bodies > objects, and we found that activity in the inferior pariatel sulcus was also modulated by the images of bodies (t(11); SE = 0.17; P < 0.045 (uncorrected)) while no such modulation was observed in the other self‐selective ROIs.
Table III.
Random effects group level GLM analysis of the “self”‐selective ROIs in the right hemisphere
| Self > others | Bodies > objects | ||||||
|---|---|---|---|---|---|---|---|
| df | t | SE | P | t | SE | P | |
| VEVC | 11 | >3.32 | 0.15 | <0.007 | >0.99 | 0.18 | <0.34 |
| IPS | 11 | >2.8 | 0.13 | <0.010 | >2.25 | 0.17 | <0.04 |
| SPL | 11 | >4.04 | 0.09 | <0.001 | >1.84 | 0.22 | <0.09 |
| MFG | 11 | >4.43 | 0.14 | <0.001 | >1.58 | 0.19 | <0.14 |
VEVC, ventral extrastriate visual cortex; IPS, inferior parietal sulcus; SPL, superior parietal lobe; MPG, middle prefrontal gyrus.
P values uncorrected.
Group‐Level Random Effects Analysis
After having identified putative areas of the network supporting perception of bodies and self, we performed a group‐level RFX analysis of the entire brain using a cluster threshold approach in which the initial voxelwise threshold criterion was P < 0.05 and a subsequent spatial extent threshold was 437–552 contiguous functional voxels. The newly computed more rigorous statistical maps confirmed all of our previous main findings. Importantly, we replicated the networks selective for bodies and self images, as identified with the GLM FE group‐level analysis (see Fig. 5). Thus, all described body‐selective ROIs (in extrastriate and parietal cortex of the right hemisphere) remained significant after RFX analysis. However, the small body selective area in left hemisphere did not reach significance level after RFX analysis. Unchanged was also the self selectivity of the ventral extrastriate visual cortex, the superior parietal cortex, and the middle frontal gyrus of the right hemisphere.
Figure 5.
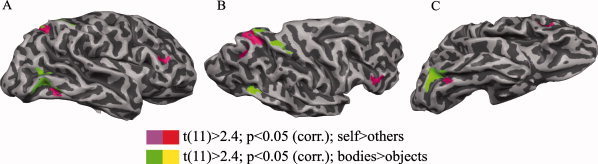
Body (green–yellow)‐ and self (pink–red)‐selective cortical networks in the right hemisphere after random effect group analysis of the whole brain, at P < 0.05 (corrected for multiple comparisons); (A) lateral; (B) lateral dorsal, and (C) lateral ventral view, shown on the surface of one representative subject.
Psychophysical experiments
In the debriefings after scanning experiment, subjects reported that the most difficult “one back task” was the identification of scrambled objects and the easiest task the identification of objects. However, we did not measure reaction time of the performance.
The results of the separate psychophysical control experiment indicated that subjects were able to distinguish between self and others when presented with the same stimulus material that was used in the fMRI experiment. In the first session, responses to self were correct in 92% (mean reaction time 5.99 ± 2.0 s) and in the second session in 100% (mean reaction time 4.64 ± 3.2 s) of the trials. In the third session, reaction times decreased further to 3.30 ± 1.9 s. The corresponding values for responses to others were 97% (4.35 ± 0.2 s), 96% (3.26 ± 1.0 s), and 100% (2.6 ± 0.9 s). Confidence of the correct classification increased from 92% to 96% for self and from 94% to 98% for other (see Fig. 6). There was no significant difference in the reaction time or correctness of responses to the images of self when the blocks with “mirror” and “camera” images are compared.
Figure 6.
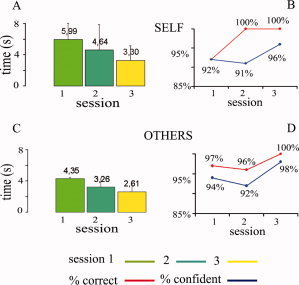
Reaction times for the body recognition task to the images of the subjects' own body (A) and bodies of others (C), with the corresponding percentage of correctness and level of confidence for the condition self (B) and others (D) obtained in three sessions. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
In agreement with previous studies [Downing et al., 2001; Schwarzlose et al., 2005], we identified EBAs of the ventral visual stream that respond preferentially to images of human bodies. In the right hemisphere, we often identified two body selective patches (EBA and FBA). In addition, we found three additional regions in the superior and inferior parietal cortex and in the precentral gyrus of the right hemisphere that also exhibited body‐related activation. This latter finding is in line with the notion that the parietal lobe plays an important role in the representation of body‐related information [Ehrsson et al., 2005; Pellijeff et al., 2006; Schimada et al., 2005], but is at variance with the report of Downing et al. [2006], who found no body‐selective regions outside the EBA and the right FFG.
We focused our study on the search for areas involved in extracting self‐related body information. In a recent study, Saxe et al. [2006] found differential BOLD responses in the EBA following presentation of body parts from either an allocentric or egocentric perspective. Similarly Chan et al. [2004] reported viewpoint dependent modulation of EBA responses but no self–other distinction. Our finding that EBA is not differentially activated in self–other distinctions is in line with the studies that had employed very different stimulation paradigms. This converging evidence contributes the notion that EBA is not involved in the distinction between self and others.
However, the results of the present study extend those of Saxe et al. [2006] and Chan et al. [2004] in that we observed self‐related responses in specific cortical areas outside extrastriate visual cortex that has not been identified previously. Chan et al. [2004] found the left superior parietal cortex as modulated by egocentric views of body parts, and Felician et al. [2004] suggested that this region is involved in the localization of body parts, but neither study implied that this region is involved in self–other distinction. Because the experimental paradigms of these studies differed from our approach, there is no conflict between results.
In the present experiment, body images were presented in several allocentric perspectives, and hence, ownership judgments could not be derived from spatial cues, suggesting that the activation patterns were associated with a view independent recognition of one's own body. We manipulated the identity of the shown images to check if the extrastriate body selective areas respond selectively to the own as compared to other bodies. Since self‐related activity was not found consistently in EBA, we have no reason to believe that EBA is involved in self detection and it might even not be involved in extracting body identity information.
However, we found other cortical areas in the right hemisphere that responded selectively to images of the own body. In ventral visual cortex, these areas were in close proximity to FBA positioned slightly more anterior. The localization of the self‐selective patch in the FBA is in agreement with the evidence that FFG responds to human attributes [Peelen and Downing, 2005; Peelen et al., 2006; Schwarzlose et al., 2005]. Since the FFA is involved in face identity discrimination [Loffler et al., 2005], its analogue for the discrimination of body identity might be the FBA rather than the EBA. However, more detailed mapping of EBA and FBA will be required to substantiate this hypothesis. The recent evidence that the OFA (occipitotemporal face area) and the EBA analyse faces and bodies, respectively, at the level of parts while the FFA and the FBA integrate face and body parts [Taylor et al., 2007], is compatible with the hypothesis that fusiform areas are involved in identity discrimination.
In parietal cortex, the self‐related areas were found to overlap with the body‐selective regions, which we defined in an independent analysis. The ventral, the parietal, and frontal regions were found predominantly in the right hemisphere, which agrees with the differential activation patterns evoked by one's own face contrasted to the faces of others that were also more pronounced in the right hemisphere [Platek et al., 2006; Uddin et al., 2005, 2006]. In agreement with classical neuropsychological studies on patients with right hemisphere lesions or hemispheric disconnection syndromes [Preilowski, 1977; Sperry et al., 1979], these data suggest a dominant role of the right hemisphere in the processing of signals related to self‐identification.
The activation maps for the contrast self–others comprised not only the body‐selective regions in the visual and parietal cortex but also a region in the middle frontal gyrus of the right hemisphere. This latter finding supports the proposal of Sugiura et al. [2006] that the right frontal cortex is part of a network dedicated to the representation of the concept of self, and it agrees with findings suggesting that the right frontal lobe is involved in the self‐attribution of stimuli such as faces and voices [Nakamura et al., 2001; Platek et al., 2004, 2006]. Likewise, we also show that the right frontal cortex is involved in the preferential processing of the images of the same gender when contrasted to the images of the opposite gender, while opposite gender preference was expressed in the left hemisphere. The self‐related frontal activation is also in agreement with the evidence that patients exhibiting a DMS often exhibit lesions in the right frontal lobe [for review see, Feinberg and Keenan, 2005].
The right inferior parietal regions identified in this study as distinguishing between self and other have also been shown to be activated in tasks involving the recognition of one's own face and comparisons between self and others [Platek et al., 2006]. This agrees with the notion that the parietal cortex participates in the network mediating awareness of ownership. Proprioceptive and visual signals relevant for the representation of the dynamic body scheme are processed and integrated in the right inferior parietal cortex [Jackson and Decety, 2004; Uher et al., 2005], in the right superior parietal lobe [Pellijeff et al., 2006; Shimada et al., 2005; Vogeley et al., 2001; Wolpert et al., 1998], and in the left superior parietal lobe [Felician et al., 2004]. Electrophysiological experiments in primates have demonstrated the involvement of superior parietal regions in monitoring limb positions [Graziano et al., 2000; Mountcastle et al., 1975; Sakata et al., 1973). And finally, there is evidence that direct stimulation of the parietal cortex can lead to a breakdown of the awareness of ownership, producing “out of body” experiences [Blanke et al., 2002].
The body‐ and self‐related activation within parietal regions might have been due to the mental rotation of the presented images, because parietal regions are activated by this operation [Halari et al., 2006; Hugdahl et al., 2006; Jordan et al., 2001; Just et al., 2001; Seurinck et al., 2004; Vingerhoets et al., 2002; Wraga et al., 2005]. However, we consider this possibility as unlikely, because the stimulation paradigm did not require mental rotation.
The enhanced responses to the views of the subjects' own bodies could also have been due to the increased arousal caused by the surprise to recognize one's own body rather than to selective processes of self‐recognition. Attention has been shown to enhance the activity of neurons in higher visual areas [for review see, Maunsell and Treue, 2006] and to increase the amplitude of BOLD responses [Wojciulik et al., 1998; Yi and Chun 2005; Yi et al., 2006]. Another possibility is that images of one's own body caused enhanced activations because of emotions associated with allocentic views of oneself. Humans use their facial expressions and body postures to convey their emotional state [Meeren et al., 2005] and data indicate that these emotional connotations influence neuronal responses in cortical areas responsive to faces and bodies [Hadjikhani and de Gelder, 2003]. Images of emotionally loaded body postures have been shown to activate the IPL even when presented in the blind hemifield of a patient with unilateral striate cortex lesion [de Gelder and Hadjikhani, 2006].
Although our data do not allow us to exclude attention‐ and emotion‐related effects as causes of the enhanced responses to the images of the subjects' own body, several arguments suggest that the enhancement is more specifically related to the activation of a network encoding identity and ownership. Subjects were unaware of the purpose of the study and were engaged in a one‐back matching task that kept the level of attention high but did not require them to distinguish between self and others. It is, thus, unlikely that the subjects attended preferentially to their own images or were surprised by them. As all photographs showed bodies in the same neutral upright posture, it is also unlikely that subjects associated particular emotions with these images while performing the one‐back discrimination task. Moreover, if attention or emotions would have been a major cause of the effects, we would have expected differential activations of networks involving further frontal cortices and/or limbic structures and we would have predicted more symmetric bilateral activation patterns. Yet another argument against an influence of attention and emotions on our data is the lack of identity‐related BOLD signal modulations in the classical EBAs of the left and right hemisphere.
As none of these patterns was observed, we favor the interpretation that the enhanced activation was specifically related to the, most likely, covert perception of the subject's own body. As discussed earlier, this interpretation agrees with the roles assigned to the respective areas in the analysis of body‐related visual and somatosensory information and the integrative functions which the right parietal cortex in particular has in the representation of the body scheme.
In conclusion, the present data indicate that cortical areas processing body‐related visual information form a specific and extended network adding to the notion that distributed processing is a general principle of cortical organization [Haxby et al., 2001]. In line with this notion, the data suggest further that the more abstract representation of ownership or self also involves a distributed network of cortical areas, and in this case mainly within the right hemisphere. The two networks partially overlap in the right superior and inferior parietal cortices but are clearly segregated in the extrastriate visual cortex and in the middle frontal gyrus. Our data as well as those of others [Chan et al., 2004; Saxe et al., 2006] indicate that the classical EBA is only involved in the analysis of body‐related information but not in the assignment of identity. The later appears to be accomplished by a network comprising the right FBA, positioned ventrally to the EBA, regions of the superior parietal lobe, the inferior parietal cortex, and the middle frontal gyrus.
Acknowledgements
We are grateful to D. Nikolic and L. Melloni for discussions on methods, to S. Weigelt for advice and comments on data analysis and to P. Janson for technical support.
REFERENCES
- Astafiev SV,Stanley CM,Shulman GL,Corbetta M ( 2004): Extrastriate body area in human occipital cortex responds to the performance of motor actions. Nat Neurosci 7: 542–548. [DOI] [PubMed] [Google Scholar]
- Blanke O,Ortigue S,Landis T,Seeck M ( 2002): Stimulating illusory own‐body perceptions. Nature 419: 269–270. [DOI] [PubMed] [Google Scholar]
- Cellerino A,Borghetti D,Sartucci F ( 2004): Sex differences in face gender recognition in humans. Brain Res Bull 63: 443–449. [DOI] [PubMed] [Google Scholar]
- Chan AW,Peelen MV,Downing PE ( 2004): The effect of viewpoint on body representation in the extrastriate body area. Neuroreport 25: 2407–2410. [DOI] [PubMed] [Google Scholar]
- Cunnington R,Windischberger C,Robinson S,Moser E ( 2006): The selection of intended actions and the observation of others' actions: A time‐resolved fMRI study. Neuroimage 15: 1294–1302. [DOI] [PubMed] [Google Scholar]
- Downing PE,Jiang Y,Shuman M,Kanwisher N ( 2001): A cortical area selective for visual processing of the human body. Science 293: 2470–2473. [DOI] [PubMed] [Google Scholar]
- Downing PE,Chan AWY,Peelen MV,Dodds CM,Kanwisher N ( 2006): Domain specificity in visual cortex. Cereb Cortex 16: 1453–1461. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH,Kito T,Sadato N,Passingham RE,Naito E ( 2005): Neural substrate of body size: Illusory feeling of shrinking of the waist. PLoS Biol 3: e412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein R,Kanwisher N ( 1998): A cortical reperesentation of the local visual environment. Nature 392: 598–601. [DOI] [PubMed] [Google Scholar]
- Feinberg TE,Keenan JP ( 2005): Where in the brain is the self? Conscious Cogn 14: 661–678. [DOI] [PubMed] [Google Scholar]
- Felician O,Romaiguere P,Anton JL,Nazarian B,Roth M,Poncet M,Roll JP ( 2004): The role of human left superior parietal lobule in body part localization. Ann Neurol 55: 749–751. [DOI] [PubMed] [Google Scholar]
- Forman SD,Cohen JD,Fitzgerald M,Eddy WF,Mintun MA,Noll DC ( 1995): Improved assessment of significant activation in functional magnetic‐resonance‐imaging (fMRI)—Use of a cluster‐size threshold. Magn Reson Med 33: 636–647. [DOI] [PubMed] [Google Scholar]
- Friston KJ,Holmes AP,Price CJ,Buchel C,Worsley KJ ( 1999): Multisubject fMRI studies and conjunction analyses. Neuroimage 10: 385–396. [DOI] [PubMed] [Google Scholar]
- de Gelder B,Hadjikhani N ( 2006): Non‐conscious recognition of emotional body language. Neuroreport 17: 583–586. [DOI] [PubMed] [Google Scholar]
- Graziano MS,Cooke DF,Taylor CS ( 2000): Coding the location of the arm by sight. Science 290: 1782–1786. [DOI] [PubMed] [Google Scholar]
- Grezes J,Frith CD,Passingham RE ( 2004): Inferring false beliefs from the actions of oneself and others: An fMRI study. Neuroimage 21: 744–750. [DOI] [PubMed] [Google Scholar]
- Grossmann ED,Blake R ( 2002): Brain areas active during visual perception of biological motion. Neuron 35: 1167–1175. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N,de Gelder B ( 2003): Seeing fearful body expressions activates the fusiform cortex and amygdala. Curr Biol 16: 2201–2205. [DOI] [PubMed] [Google Scholar]
- Halari R,Sharma T,Hines M,Andrew C,Simmons A,Kumari V ( 2006): Comparable fMRI activity with differential behavioural performance on mental rotation and overt verbal fluency tasks in healthy men and women. Exp Brain Res 169: 1–14. [DOI] [PubMed] [Google Scholar]
- Haxby JV,Gobbini MI,Furey ML,Ishai A,Schouten JL,Pietrini P ( 2001): Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science 293: 2425–2430. [DOI] [PubMed] [Google Scholar]
- Hugdahl K,Thomsen T,Ersland L ( 2006): Sex differences in visuo‐spatial processing: An fMRI study of mental rotation. Neuropsychologia 44: 1575–1583. [DOI] [PubMed] [Google Scholar]
- Jackson PL,Decety J ( 2004): Motor cognition: A new paradigm to study self–other interactions. Curr Opin Neurobiol 14: 259–263. [DOI] [PubMed] [Google Scholar]
- Jordan K,Heinze HJ,Lutz K,Kanowski M,Jancke L ( 2001): Cortical activations during the mental rotation of different visual objects. Neuroimage 13: 143–152. [DOI] [PubMed] [Google Scholar]
- Just MA,Carpenter PA,Maguire M,Diwadkar V,McMains S ( 2001): Mental rotation of objects retrieved from memory: A functional MRI study of spatial processing. J Exp Psychol Gen 130: 493–504. [DOI] [PubMed] [Google Scholar]
- Kanwisher N,McDermott J,Chun MM ( 1997): The fusiform face area: A module in human extrastriate cortex specialized for face perception. J Neurosci 17: 4302–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan JP,Ganis G,Freund S,Pascual‐Leone A ( 2000): Self‐face identification is increased with left hand responses. Laterality 5: 259–268. [DOI] [PubMed] [Google Scholar]
- Kircher TT,Senior C,Phillips ML,Benson PJ,Bullmore ET,Brammer M,Simmons A,Williams SC,Bartels M,David AS ( 2000): Towards a functional neuroanatomy of self processing: Effects of faces and words. Cogn Brain Res 10: 133–144. [DOI] [PubMed] [Google Scholar]
- Kircher TT,Senior C,Phillips ML,Rabe‐Hesketh S,Benson PJ,Bullmore ET,Brammer M,Simmons A,Bartels M,David AS ( 2001): Recognizing one's own face. Cognition 78: B1–B15. [DOI] [PubMed] [Google Scholar]
- Loffler G,Yourganov G,Wilkinson F,Wilson HR ( 2005): fMRI evidence for the neural representation of faces. Nat Neurosci 8: 1386–1390. [DOI] [PubMed] [Google Scholar]
- Malach R,Tootel RB ( 1995): Object‐related activity revealed by functional magnetic resonance imaging in human occipital cortex. Proc Natl Acad Sci USA 92: 8135–8139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunsell JH,Treue S ( 2006): Feature‐based attention in visual cortex. Trends Neurosci 29: 317–322. [DOI] [PubMed] [Google Scholar]
- Meeren HK,van Heijnsbergen CC,de Gelder B ( 2005): Rapid perceptual integration of facial expression and emotional body language. Proc Natl Acad Sci USA 8: 16518–16523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle VB,Lynch JC,Georgopoulos A,Sakata H,Acuna C ( 1975): Posterior parietal association cortex of the monkey: Command functions for operations within extrapersonal space. J Neurophysiol 38: 871–908. [DOI] [PubMed] [Google Scholar]
- Nakamura K,Kawashima R,Sato N,Nakamura A,Sugiura M,Kato T,Hatano K,Ito K,Fukuda H,Schormann T,Zilles K ( 2000): Functional delineation of the human occipito‐temporal areas related to face and scene processing. A PET study. Brain 123: 1903–1912. [DOI] [PubMed] [Google Scholar]
- Nakamura K,Kawashima R,Sugiura M,Kato T,Nakamura A,Hatano K,Naguma S,Kubota K,Fukuda H,Ito K,Kojima S ( 2001): Neural substrates for recognition of familiar voices: A PET study. Neuropsychologia 39: 1047–1054. [DOI] [PubMed] [Google Scholar]
- Noppeney U,Price CJ,Penny WD,Friston KJ ( 2005): Two distinct neural mechanisms for category‐selective responses. Cereb Cortex 16: 437–445. [DOI] [PubMed] [Google Scholar]
- Peelen MV,Downing PE ( 2005): Selectivity for the human body in the fusiform gyrus. J Neurophysiol 63: 603–605. [DOI] [PubMed] [Google Scholar]
- Peelen MV,Wiggett AJ,Downing PE ( 2006): Patterns of fMRI activity dissociate overlapping functional brain areas that respond to biological motion. Neuron 16: 815–822. [DOI] [PubMed] [Google Scholar]
- Pellijeff A,Bonilha L,Morgan PS,McKenzie K,Jackson SR ( 2006): Parietal updating of limb posture: An event‐related fMRI study. Neuropsychologia 44: 2685–2690. [DOI] [PubMed] [Google Scholar]
- Platek SM,Keenan JP,Gallup GG Jr,Mohamed FB ( 2004): Where am I? The neurological correlates of self and other. Brain Res 19: 114–122. [DOI] [PubMed] [Google Scholar]
- Platek SM,Loughead JW,Gur RC,Busch S,Ruparel K,Phend N,Panyavin IS,Langleben DD ( 2006): Neural substrates for functionally discriminating self‐face from personally familiar faces. Hum Brain Mapp 27: 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preilowski B ( 1977): Self‐recognition as a test of consciousness in left and right hemisphere of “split‐brain” patients. Act Nerv Super (Praha) 19 ( Suppl 2): 343–344. [PubMed] [Google Scholar]
- Proverbio AM,Brignone V,Matarazzo S,Del Zotto M,Zani A ( 2006): Gender differences in hemispheric asymmetry for face processing. BMC Neurosci 7: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puce A,Allison T,Asfgari M,Gore JC,Mccarthy G ( 1996): Differential sensitivity of human visual cortex to faces, letter strings, and textures: A functional magnetic resonanace imaging studies. J Neurosci 16: 5205–5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnani N,Miall RC ( 2004): A system in the human brain for predicting the actions of others. Nat Neurosci 7: 5–6. [DOI] [PubMed] [Google Scholar]
- Sakata H,Takaoka Y,Kawarasaki A,Shibutani H ( 1973): Somatosensory properties of neurons in the superior parietal cortex (area 5) of the rhesus monkey. Brain Res 21: 85–102. [DOI] [PubMed] [Google Scholar]
- Saxe R,Jamal N,Powell L ( 2006): My body or yours? The effect of visual perspective on cortical body representations. Cereb Cortex 16: 178–182. [DOI] [PubMed] [Google Scholar]
- Schwarzlose RF,Baker CI,Kanwisher N ( 2005): Separate face and body selectivity in the fusiform gyrus. J Neurosci 23: 11055–11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seurinck R,Vingerhoets G,de Lange FP,Achten E ( 2004): Does egocentric mental rotation elicit sex differences? Neuroimage 23: 1440–1449. [DOI] [PubMed] [Google Scholar]
- Shimada S,Hiraki K,Oda I ( 2005): The parietal role in the sense of self‐ownership with temporal discrepancy between visual and proprioceptive feedbacks. Neuroimage 15: 1225–1232. [DOI] [PubMed] [Google Scholar]
- Sperry RW,Zaidel E,Zaidel D ( 1979): Self recognition and social awareness in the deconnected minor hemisphere. Neuropsychologia 17: 153–166. [DOI] [PubMed] [Google Scholar]
- Sugiura M,Watanabe J,Maeda Y,Matsue Y,Fukuda H,Kawashima R ( 2005): Cortical mechanisms of visual self‐recognition. Neuroimage 24: 143–149. [DOI] [PubMed] [Google Scholar]
- Sugiura M,Sassa Y,Jeong H,Miura N,Akitsuki Y,Horie K,Sato S,Kawashima R ( 2006): Multiple brain networks for visual self‐recognition with different sensitivity for motion and body part. Neuroimage 32: 1905–1917. [DOI] [PubMed] [Google Scholar]
- Taylor JC,Wiggett AJ,Downing P ( 2007): Functional MRI analysis of body and body part representations in the extrastriate and fusiform body areas. J Neurophysiol 98: 1626–1633. [DOI] [PubMed] [Google Scholar]
- Thierry G,Pegna AJ,Dodds C,Roberts M,Basan S,Downing P ( 2006): An event‐related potential component sensitive to images of the human body. Neuroimage 32: 871–879. [DOI] [PubMed] [Google Scholar]
- Tsao DY,Freiwald WA,Tootell RB,Livingstone MS ( 2006): A cortical region consisting entirely of face‐selective cells. Science 3: 670–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ,Kaplan JT,Molnar‐Szakacs I,Zaidel E,Iacoboni M ( 2005): Self‐face recognition activates a frontoparietal mirror network in the right hemisphere: An event‐related fMRI study. Neuroimage 25: 926–935. [DOI] [PubMed] [Google Scholar]
- Uddin LQ,Molnar‐Szakacs I,Zaidel E,Iacoboni M ( 2006): rTMS to the right inferior parietal lobule disrupts self‐other discrimination. Soc Cogn Affect Neurosci 1: 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R,Murphy T,Friederich HC,Dalgleish T,Brammer MJ,Giampietro V,Phillips ML,Andrew CM,Ng VW,Williams SC,Campbell IC,Treasure J ( 2005): Functional neuroanatomy of body shape perception in healthy and eating‐disordered women. Biol Psychiatry 15: 990–997. [DOI] [PubMed] [Google Scholar]
- Urgesi C,Berlucchi G,Aglioti SM ( 2004): Magnetic stimulation of extrastriate body area impairs visual processing of nonfacial body parts. Curr Biol 14: 2130–2134. [DOI] [PubMed] [Google Scholar]
- Vingerhoets G,de Lange FP,Vandemaele P,Deblaere K,Achten E ( 2002): Motor imagery in mental rotation: An fMRI study. Neuroimage 17: 1623–1633. [DOI] [PubMed] [Google Scholar]
- Vogeley K,Bussfeld P,Newen A,Herrmann S,Happe F,Falkai P,Maier W,Shah NJ,Fink GR,Zilles K ( 2001): Mind reading: Neural mechanisms of theory of mind and self‐perspective. Neuroimage 14: 170–181. [DOI] [PubMed] [Google Scholar]
- Wojciulik E,Kanwisher N,Driver J ( 1998): Covert visual attention modulates face‐specific activity in the human fusiform gyrus: fMRI study. J Neurophysiol 79: 1574–1578. [DOI] [PubMed] [Google Scholar]
- Wolpert DM,Goodbody SJ,Husain M ( 1998): Maintaining internal representations: The role of the human superior parietal lobe. Nat Neurosci 1: 529–533. [DOI] [PubMed] [Google Scholar]
- Wraga M,Shephard JM,Church JA,Inati S,Kosslyn SM ( 2005): Imagined rotations of self versus objects: An fMRI study. Neuropsychologia 43: 1351–1361. [DOI] [PubMed] [Google Scholar]
- Yi DJ,Chun MM ( 2005): Attentional modulation of learning‐related repetition attenuation effects in human parahippocampal cortex. J Neurosci 6: 3593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi DJ,Kelley TA,Marois R,Chun MM ( 2006): Attentional modulation of repetition attenuation is anatomically dissociable for scenes and faces. Brain Res 29: 53–62. [DOI] [PubMed] [Google Scholar]
- Zaitsev M.Henning J,Speck O ( 2004): Point spread function mapping with parallel imaging techniques and high acceleration factors: Fast, robust, and flexible method for echo‐planar imaging distortion correction. Magn Reson Med 52: 1156–1166. [DOI] [PubMed] [Google Scholar]


