Abstract
Children and adolescents born before 33 weeks of gestation, that is very preterm, may experience problems with the inhibitory control of behaviour and the allocation of attention. Previous functional magnetic resonance imaging (fMRI) studies have found preterm‐born adolescents to display altered brain activation in tasks measuring inhibitory control. However, adolescence is a period during which dynamic changes are occurring in the brain, and it is not yet known whether these functional alterations will persist into adulthood, or instead reflect developmental delay. This study used an event‐related fMRI Go/No‐Go motor response inhibition paradigm, which included an oddball task measuring attention allocation to infrequent stimuli, to compare blood‐oxygen‐level‐dependent (BOLD) signal between 26 preterm‐born adults and 21 controls. Group differences in brain activation were observed in inhibition and attention networks during both conditions. During motor response inhibition, preterm‐born participants compared to controls showed increased BOLD signal in medial and right lateral posterior brain regions, including middle temporal/occipital gyrus, posterior cingulate gyrus and precuneus. During oddball trials, preterm‐born young adults displayed attenuated brain activation in a fronto‐parietal‐cerebellar network which is involved in mediating attention allocation. This pattern of reduced brain activation in task‐relevant regions of attention allocation, and increased activation in posterior brain regions during inhibitory control, suggests adult alteration of inhibition and attention processing following very preterm birth, which may reflect a developmental delay. Hum Brain Mapp, 2009. © 2008 Wiley‐Liss, Inc.
Keywords: preterm, Go/No‐go, selective attention, response inhibition, fMRI
INTRODUCTION
There is a wealth of literature on cognitive and behavioural impairments following very preterm birth. For instance, preterm‐born children tend to show problems with both shifting and focussing attention, which may predict later behavioural problems [Andersen and Doyle, 2004; Lawson and Ruff, 2004]. In addition, increased psychiatric symptomatology such as externalising behaviours and incidence of attention deficit and hyperactivity disorder (ADHD) have been described in preterm‐born and very low birth weight (e.g., <1,500 g: VLBW) cohorts [Bhutta et al., 2002; Botting et al., 1997; Breslau and Chilcoat, 2000; Foulder‐Hughes and Cooke, 2003; Lawson and Ruff, 2004]. Increased parental ratings of impulsivity have also been recorded [Eriksson and Pehrsson, 2003].
Cognitive and behavioural impairments have partly been explained by structural brain alterations in very preterm populations [Abernethy et al., 2004; Allin et al., 2001; Nosarti et al., 2004, 2008]. In adolescence, an association has been observed between caudate volume and hyperactivity scores in individuals who were born very preterm [Nosarti et al., 2005]. Because of its periventricular location, the caudate nucleus is at elevated risk of damage in preterm neonates, as a result of cerebral haemorrhage and perinatal asphyxia. In addition, preterm adolescents (mean gestational age <30 weeks) when compared with controls have been reported to have smaller caudate volumes [Abernethy et al., 2004; Nosarti et al., 2008]. Decreased caudate volume has also been observed in preterm children with low IQ scores (<80) [Abernethy et al. 2004]. Together these data suggest that cognitive and behavioural functions reliant on fronto‐striatal pathways may be impaired in very preterm samples. Similarly, alterations of fronto‐striatal networks have been described in ADHD in terms of structural brain development [Castellanos et al., 2002; Krain and Castellanos, 2006] and functional neuronal substrates during tasks involving inhibitory control [Durston et al., 2003; Rubia et al., 1999].
Inhibitory control is one of the latest cognitive functions to develop, maturing well into adolescence [Tamm et al., 2002; Wildenberg and Molen, 2004]. The ability to monitor and inhibit responses is required in many psychological domains, such as the regulation of emotion [Lewis and Stieben, 2004], the protection of attentional focus from interference, as well as the withdrawal of motor responses [Rubia, 2002]. Motor response inhibition is mediated by lateral prefrontal areas such as the dorsolateral and inferior frontal cortex, anterior cingulate and the caudate nucleus [Bunge et al., 2002; Horn et al., 2003; Rubia et al., 2001, 2003, 2006, 2007a].
In line with the behavioural findings of late development of inhibitory control are functional imaging findings of late progressive maturation of the fronto‐striatal activation in networks [Booth et al., 2003; Bunge et al., 2002; Rosso et al., 2004; Rubia et al., 2000, 2006, 2007a]. In particular, activation in networks in the prefrontal and caudate regions have been shown to increase in a linear fashion between childhood and mid‐adulthood during inhibitory task performance [Rubia et al., 2000a, 2006]. Recent data using diffusion tensor imaging also found that the maturation of fronto‐striatal white matter fibre tracts (from age 7 to 31) related to enhanced performance on a Go/No‐go inhibition tasks [Liston et al., 2006]. In addition, research suggests that younger age groups rely more on posterior brain regions as compared with the task‐relevant fronto‐striatal networks recruited by older participant groups [Booth et al., 2003; Bunge et al., 2002; Rubia et al., 2006, 2007a]. Bearing this in mind, it is of interest to investigate whether such late maturing functions and their underlying fronto‐striatal neural networks are impaired in preterm‐born adults who may show signs of functional and structural developmental delay [Nosarti et al., 2006, 2008].
The extent to which preterm‐born adults show response inhibition impairments is not yet clear. Work by our group exploring executive function suggests that preterm‐born adults may still display difficulties on tasks with inhibitory components, such as the Hayling sentence completion test which includes a verbal inhibition component [Nosarti et al., 2007]. Another study using functional magnetic resonance imaging (fMRI) and the same Go/No‐go task employed in this study, found that preterm‐born adolescent boys (mean age 16 years) displayed differential brain activation when compared with a sample of non‐preterm‐born adolescent boys, despite showing comparable cognitive performance [Nosarti et al., 2006]. Preterm‐born adolescents in contrast to controls showed decreased blood‐oxygenated‐level‐dependent (BOLD) signal in caudate nucleus, left inferior frontal and anterior cingulate gyri. They also showed increased brain activation mainly in temporal regions, which was interpreted as being possibly compensatory. In addition, a recent large‐scale study by our group found an increase in delayed responses in preterm‐born adults on a neuropsychological task that requires interference inhibition [Nosarti et al., 2007]; i.e., the Incompatibility subscale of the Test of Attentional Performance.
Other fMRI studies have revealed differences in brain activation between full‐term and preterm‐born children and adolescents, even in the presence of intact cognitive performance [Ment and Constable, 2007; Petersen et al., 2002]. This may implicate differential developmental time courses, processes of neural plasticity, i.e. the process whereby compensatory neural events facilitate the re‐organisation of existing brain tissue [Nosarti et al., 2003], and/or increased neurocognitive compensation with age. fMRI is particularly well‐placed to detect such process, which may not be evident at a neuropsychological level [Rubia, 2002; Rushe et al., 2001].
The aim of this study was to use event‐related fMRI to explore the neuronal substrates of response inhibition and attention allocation in young adults who were born very preterm (<33 weeks of gestation) and a group of control participants of similar age and gender. The rationale for undertaking this study was threefold: first to test whether alterations in brain activation during motor response inhibition would be observed in preterm‐born adults. To date, current knowledge of the functional neuronal consequences of preterm birth is largely confined to child and adolescent samples (see Ment and Constable, 2007 for a review). Second, to investigate whether preterm‐born adults would show differences in attention networks during a simple Oddball task measuring perceptive selective attention allocation. Third, to include female participants, as the majority of fMRI studies has investigated preterm‐born boys, despite evidence that the gender may influence the mechanisms of brain development [DeBellis et al., 2001; Gur et al., 1999; Reiss et al., 2004; Thompson et al., 2007], as well as the relationship between brain structure and function [Nosarti et al., 2004, 2005].
We hypothesised that preterm‐young adults would continue to show BOLD signal alterations, when compared with healthy controls, during inhibition and attention processing, possibly reflecting developmental delay, and/or neurocognitive compensation. This may manifest as attenuation in task‐specific neural networks underpinning motor inhibition and attention allocation, e.g., fronto‐striatal and fronto‐parieto‐temporal, respectively, or increased brain activation in regions that underpin less specialised attention processes.
METHODS
Participants
In 1983–1984, 252 infants born at less than 33 weeks gestation were admitted within 5 days of birth to the Neonatal Unit at University College London Hospital (UCLH), survived and were discharged. Of this cohort, all individuals born at 28 or less weeks of gestation were enrolled for long‐term follow‐up, as well as a random sample of those born from 29 to 33 weeks of gestation. This selection was necessitated by an expansion in capacity of UCLH in 1983, which prevented inclusion of the entire consecutive series due to limited research resources. One hundred forty‐seven (40% of the entire sample) adolescents were selected for the study (78 born at <28 weeks gestation and 69 born at 29–33 weeks gestation). Of the 107 individuals assessed in adolescence, 55 were born at 28 or less weeks of gestation and 52 were born from 29 to 33 weeks of gestation.
For the present fMRI study, 26 randomly selected right‐handed preterm‐born individuals with no history of cerebral palsy, grade 3/4 intraventricular hemorrhage or periventricular leucomalacia (12 born at <28 weeks gestation and 14 born at 29–33 weeks gestation; 16 males; mean age 20.1; age range 18.9–21.2 years) and 21 controls (9 males; mean age 20.1, range 17.8–23.4 years) were recruited (see Table I). This sample was comparable with a larger group of very preterm individuals, of a similar age and selected from the same cohort as in this study, [Allin et al., in press] in terms of proportion of individuals who were born at <28 weeks (χ = 0.28, P > 0.05), and estimated Wechsler Abbreviated Scale of Intelligence (WASI) [Wechsler, 1999] Full Scale IQ (t (90) = 1.16, P > 0.05).
Table I.
Demographic details and behavioural data for preterm‐born participants and controls
| Variable | Controlsa | Preterm‐born | ||||
|---|---|---|---|---|---|---|
| Mean (SD) | Males (n = 9) | Females (n = 12) | Total (n = 21) | Males (n = 16) | Females (n = 10) | Total (n = 26) |
| Age (years) | 19.88 ± 1.39 | 20.31 ± 1.96 | 20.13 ± 1.7 | 20.20 ± 0.65 | 19.98 ± 0.65 | 20.11 ± 0.65 |
| Gestation (weeks) | — | — | — | 28.25 ± 1.95 | 29.60 ± 1.71 | 28.70 ± 1.95 |
| Birth weight | 1378.81 ± 101.49 | 1269.70 ± 90.08 | 1336.84 ± 361.97 | |||
| Go error rate: omissionsb , c | 2.22 ± 3.70 | 0.46 ± 0.93 | 1.25 ± 2.7 | 0.93 ± 2.67 | 1.33 ± 2.96 | 1.09 ± 2.73 |
| No‐go error rate: commissions | 1.67 ± 1.12 | 0.82 ± 1.47 | 1.20 ± 1.4 | 1.50 ± 1.46 | 1.90 ± 1.85 | 1.65 ± 1.60 |
| Reaction time: Go stimuli (s)b | 0.41 ± 0.06 | 0.46 ± 0.07 | 0.44 ± 0.07 | 0.41 ± 0.06 | 0.45 ± 0.04 | 0.43 ± 0.06 |
| Reaction time: Oddball stimuli (s) | 0.44 ± 0.08 | 0.49 ± 0.07 | 0.47 ± 0.08 | 0.43 ± 0.06 | 0.49 ± 0.05 | 0.45 ± 0.06 |
There was one instance of missing data for behavioural measures.
Includes Oddball trials.
Three outliers were excluded.
Twenty‐one controls were recruited from advertisements in the local press and universities selected according to age, handedness and gender. Inclusion criteria were full‐term birth (37–42 completed weeks of gestation) and English as a first language; exclusion criteria included birth complications (e.g., low birth weight defined as <2,500 g, endotracheal mechanical ventilation), prolonged gestation (greater than 42 weeks), history of psychiatric illness, severe hearing and motor impairment.
Mean WASI Full Scale IQ was 107.7 (±4.3) for controls (n = 16) and 103.7 (±2.3) for preterm‐born participants, and did not differ between groups (t (39) = 0.90, P > 0.05). Verbal IQ was 94.5 (±9.5) for controls and 94.9 (±13.2) for participants born very preterm (t (39) = −0.9, P > 0.05), with performance IQ being 101.1 (±8.8) for controls; 99.2 (±11.8) for preterm‐born participants (t (39) = 0.42, P > 0.05). The groups did not differ in ratings of parental social economic class [HMSO, 1991; χ = 4.02, P > 0.05).
Ethical approval for the study was obtained from the local ethical committee. Written informed consent for the assessment, including MRI, was obtained from all participants and a parent of the only participant (control) who had not yet reached age 18. All participants received travel expenses, refreshments and a nominal remuneration for participation in the study.
fMRI Paradigm: Go/No‐Go Task
In the Go/No‐go task, a motor response has to be selectively inhibited or executed depending on whether a ‘go’ or a ‘no‐go’ signal is displayed on the screen. It requires motor response inhibition and selective attention. A rapid, randomised, mixed trial event‐related fMRI was used in combination with a Go/NoGo activation paradigm including Oddball trials to control for the attentional oddball effect of low‐frequency no‐go appearance (for details of tasks please see Nosarti et al., 2006; Rubia et al., 2005a, 2006]. Inter‐stimulus intervals (ISI) were randomly jittered between 1.6 and 2.0 and presentation was randomised to optimise statistical efficiency [Dale, 1999; Dale and Buckner, 1997].
The basic task is a choice reaction time task. Arrows of 500‐ms duration appear on the middle of the screen and point to either the left or right side. After the 500 ms stimulus duration, there is a blank screen of 1.1–1.5 s, so that each inter‐trial‐interval amounts on average to 1.8 s. The participant is instructed to press the left or right response button as fast as possible, depending on the direction of the arrow. Infrequently (in 12% of trials), arrows pointing to the top (no‐go signals) appear in the middle of the screen with 500 ms duration. Participants have to inhibit their motor response to these arrows. In another 12% of trials (Oddball trials), slightly slanted arrows pointing left or right appear, and participants have to press a response button as fast as they can to either the left or right response button, corresponding to the direction of where the arrows point, just as to the go signals. These low‐frequency oddball stimuli control for the low‐frequency attentional oddball effect and difference in arrow tilt of the no‐go trials. Twenty‐four no‐go stimuli and 24 oddball stimuli were pseudorandomly interspersed with 160 go stimuli. The event‐related analysis investigates the neural correlates of three different functions: (1) No‐go > Oddball trials; pure motor response inhibition, controlling for selective attention to the rare No‐go trials when compared with frequent go trials by subtracting the equally rare oddball trials. (2) No‐go > Go trials; motor response inhibition, without controlling for the effects of attention allocation to infrequent stimuli. (3) Oddball > Go trials; measuring selective attention in the rare Oddball trials when compared with the frequent go trials. Task duration was 6.15 min.
The task was explained to the participants and each was trained once in each condition prior to MRI scanning. In the scanner, the task instructions were repeated. The paradigm was written in visual basic programming and projected from a PC onto a mirror within the MRI scanner during the scan and response data were recorded onto a PC at the same time.
Image Acquisition
Gradient‐echo echo‐planar MR images were acquired from participants using a 1.5 Tesla Neuro‐optimised GE MR Signa System (GE Medical Systems, Milwaukee, WI) at the Maudsley Hospital, London. A quadrature head coil was used for radio frequency (RF) pulse transmission and reception. In each of the 12 7‐mm thick near axial slices with a 0.7‐mm gap, 208 T2‐weighted MR images depicting BOLD contrast were acquired parallel to the intercommissural (AC‐PC) plane (time to repeat: TR 1,800 ms, time to echo‐TE 40 ms, flip angle 90°). A high‐resolution inversion recovery EPI dataset with 3‐mm thick slices and an in‐plane resolution of 1.5 mm was also acquired to facilitate mapping of the functional data into Talairach space.
Functional MRI Analyses
Individual analysis
The fMRI data were analysed with the software developed at the Institute of Psychiatry (XBAM V3.4), using a nonparametric approach to minimise assumptions. The data were first realigned [Bullmore et al., 1999] to minimise motion‐related artefacts and smoothed using a Gaussian filter (FWHM 7.2 mm). Time series analysis was then carried out by first convolving each experimental condition with gamma variate functions, modelling delays of 4 and 8 s, respectively (to allow variability within this range). The weighted sum of these two convolutions that gave the best fit (least‐squares) to the time series at each voxel was then computed, and a goodness of fit statistic computed at each voxel consisting of the ratio of the sum of squares of deviations from the mean intensity value due to the model (fitted time series) divided by the sum of squares due to the residuals (original time series minus model time series). This statistic is called the SSQ‐ratio. The appropriate null distribution for assessing significance of any given SSQ‐ratio was then computed using the wavelet‐based data re‐sampling method described in detail elsewhere [Bullmore et al., 2001] and applying the model‐fitting process to the re‐sampled data. This process was repeated 20 times at each voxel and the data combined over all voxels to give the overall null distribution of SSQ‐ratio. The same permutation strategy was applied at each voxel to preserve spatial correlational structure in the data. Voxels activated at any desired level of type I error can then be determined by obtaining the appropriate critical value of the SSQ‐ratio from the null distribution. Only activation related to successful trials were modeled, to circumnavigate effects due to differential cognitive performance.
Group mapping
The observed and randomised SSQ‐ratio maps were transformed into standard space by a two‐stage process involving first a rigid body transformation of the fMRI data into a high‐resolution inversion recovery image of the same subject followed by an affine transformation onto a Talairach template [Tailairach and Tournoux, 1988]. A generic brain activation map (GBAM) was then produced for each experimental condition by calculating the median observed SSQ‐ratio over all subjects at each voxel (median values were used to minimise outlier effects) at each intracerebral voxel in standard space [Brammer et al., 1997], and testing these median SSQ‐ratio values against the null distribution of median SSQ‐ratios computed from the identically transformed wavelet re‐sampled data [Brammer et al., 1997]. To increase sensitivity and reduce the multiple comparison problem encountered in fMRI, hypothesis testing was carried out at the cluster level using the method developed by Bullmore et al. [ 1999] initially for structural image analysis, and subsequently shown to give excellent cluster‐wise type I error control in both structural and functional fMRI analysis. For each condition, <1 false positive activated cluster per map was expected at a P‐value of <0.05 at the voxel level, and P < 0.005 at the cluster‐level.
ANOVA for group comparisons
Following transformation of the statistics maps (SSQ ratio) for each individual into standard space, it is possible to perform a randomisation‐based test for voxel or cluster‐wise differences. First, the difference between the median SSQ ratio values in each group was calculated at each voxel. The median ratio was then recalculated a large number of times at each voxel following random permutation of group membership. The latter operation yields the distribution of median differences under the null hypothesis of no effect of group membership. Voxel‐wise maps of significant group differences at any desired level of type I error can then be obtained using the appropriate threshold from the null distribution. Provided that identical permutations are carried out at each voxel (to preserve spatial correlations) this method can then be extended to yield cluster‐level differences [Bullmore et al., 1999]. For the group comparison, <1 false positive activated cluster per map was expected at a voxel‐wise P‐value of <0.05, and cluster‐wise P‐value of <0.005.
In fMRI analysis, most commonly used assessments of the significance of the fit of the resulting model use normal theory and the validity of the normality assumption is rarely tested. The method of analysis described earlier (XBAM) makes no such assumptions. Instead, it uses median statistics to control outlier effects and permutation rather than normal theory‐based inference. Furthermore, the most common test statistic is computed by standardising for individual difference in residual noise before embarking on second level, multi‐subject, testing using robust permutation‐based methods. This allows a mixed effects approach to analysis—an approach that has recently been recommended following a detailed analysis of the validity and impact of normal theory based inference in fMRI in large number of subjects [Thirion et al., 2007].
RESULTS
Demographic and Behavioural Data
There were no age (t (45) = 0.05, P > 0.05) or gender (χ(1) = 1.63, P > 0.05) differences between groups. However, given the possible gender differences in behavioural performance, multiple group × gender analyses of variance were conducted to test for group differences on behavioural variables. The P values were adjusted for multiple testing using the False Discovery Rate [Benjamini and Hochberg, 1995] ‐ see Table I for descriptive statistics.
There were no group differences in error rate for No‐go trials (commissions: F(1,42) = 1.02, P > 0.05, ηp 2 = 0.024), nor on reaction time for Go stimuli (F(1,42) = 0.02, P > 0.05; ηp 2 = 0.001). Women were, however, slower to respond to the Go trials (F(1,42) = 5.83, P = 0.05, ηp 2 = 0.122), although no interaction between group × gender was observed (F(1,42) = 0.18, P > 0.05, ηp 2 = 0.004). Further analyses suggest the observed gender effect was driven by reaction times on the Oddball trials (F(1,42) = 8.37, P = 0.025; ηp 2 = 0.174), but again no group × gender interaction was observed (F(1,42) = 1.01, P > 0.05; ηp 2 = 0.002).
After exclusion of three outliers (±2 SD from mean) from the preterm‐born adult group, no between group differences were observed for error rate for Go stimuli (omissions: F(1,39) = 0.062, P > 0.05, ηp 2 = 0.002), nor gender differences (F(1,39) = 0.67, P > 0.05, ηp 2 = 0.017) or gender × group interaction (F(1,39) = 1.7, P > 0.05, ηp 2 = 0.042). These patterns of findings mirrored those from a whole group analysis, i.e., including outliers.
Functional MRI Data
Generic brain activation
During the motor inhibition trials controlling for the attentional oddball effect (No‐go > Oddball trials after ‘go’ trials), control participants showed brain activation in the superior temporal gyrus. In this contrast, the preterm‐born group displayed brain activation in the cuneus, posterior cingulate gyrus and post‐central gyrus (see Table II).
Table II.
Within‐group activation foci for controls and preterm‐born participants for each contrast
| Talairach co‐ordinates | Cluster P value | No. of Voxels | |||
|---|---|---|---|---|---|
| x | y | z | |||
| Control participants | |||||
| No‐Go > Oddball | |||||
| R superior temporal gyrus (BA 41) | 51 | −33 | 9 | 0.0020 | 17 |
| No‐Go > Go | |||||
| R cerebellum/anterior lobe/culmen | 40 | −48 | −29 | 0.0002 | 385 |
| R superior temporal gyrus (BA 41) | 47 | −37 | 15 | 0.0010 | 185 |
| Oddball > Go | |||||
| R subgyral parietal lobe (BA 40) | 36 | −41 | 37 | 0.0002 | 461 |
| R inferior frontal gyrus (BA 9) | 43 | 11 | 26 | 0.0002 | 202 |
| L middle occipital gyrus (BA 18) | −40 | −78 | −7 | 0.0020 | 123 |
| Preterm‐born participants | |||||
| No‐Go > Oddball | |||||
| Cuneus (BA 18) | 0 | −74 | 20 | 0.0002 | 138 |
| L posterior cingulate gyrus (BA 24) | −4 | −22 | 37 | 0.0020 | 30 |
| R postcentral gyrus (BA 3) | 40 | −26 | 48 | 0.0030 | 23 |
| No‐Go > Go | |||||
| R precuneus (BA 31) | 7 | −70 | 26 | 0.0003 | 503 |
| Oddball > Go | |||||
| L middle frontal gyrus (BA 46) | −47 | 22 | 26 | 0.0002 | 284 |
| R middle occipital gyrus | 36 | −70 | 9 | 0.0030 | 124 |
During the No‐go > Go trials which tap motor inhibition without controlling for the attentional oddball effect, control participants showed brain activation in cerebellum and superior temporal gyrus. In the preterm‐born group, there was increased BOLD signal in precuneus.
Control participants showed increased BOLD response in the Oddball > Go trials in inferior frontal gyrus, subgyral parietal lobe and middle occipital gyrus. The preterm‐born group displayed increased BOLD signal in middle frontal gyrus and middle occipital gyrus.
Group comparison
For the contrast of No‐go > Oddball trials, preterm‐born individuals showed increased brain activation in right hemispheric posterior regions in the middle temporal gyrus, middle cingulate gyrus, posterior cingulate and precuneus during No‐go trials when contrasted with Oddball trials (see Table III; Fig. 1A).
Table III.
Between‐group foci for preterm‐born and controls for each contrast
| Talairach co‐ordinates | Cluster P value | No. of voxels | |||
|---|---|---|---|---|---|
| x | y | z | |||
| Preterm‐born > controls | |||||
| No‐Go > Oddball | |||||
| R middle temporal/occipital gyrus (BA 39/19) | 40 | −67 | 26 | 0.001 | 53 |
| R middle cingulate gyrus (BA 31) | 4 | −44 | 42 | 0.002 | 48 |
| R posterior cingulate (BA 30) | 4 | −67 | −13 | 0.009 | 31 |
| R precuneus (BA 31) | 7 | −70 | 26 | 0.002 | 28 |
| Controls > preterm‐born | |||||
| No‐Go > Go | |||||
| L cerebellum/posterior | −18 | −41 | −40 | 0.007 | 86 |
| R cerebellum/culmen | 40 | −48 | −29 | 0.009 | 44 |
| Oddball > Go | |||||
| L cerebellum–posterior/uvula | −18 | −74 | −24 | 0.002 | 119 |
| R inferior frontal gyrus (BA 44) | 51 | 19 | 26 | 0.003 | 117 |
| R precentral gyrus (BA 4/6) | 25 | −19 | 53 | 0.005 | 84 |
| R supramarginal gyrus (BA 40) | 36 | −41 | 31 | 0.008 | 38 |
Figure 1.
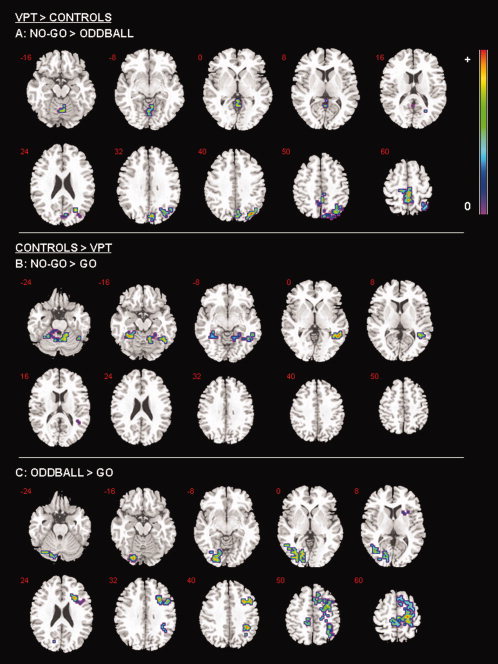
Between‐group foci of brain activation for each contrast. The left side of the brain is shown on the left side of the images.
For the contrast of No‐Go > Go trials, the preterm‐born group showed reduced BOLD signal in the left posterior cerebellum and the right supramarginal gyrus (see Table III; Fig. 1B).
For the contrast of Oddball > Go preterm‐born participants displayed attenuated brain activation in the left cerebellum, right inferior frontal gyrus, right premotor cortex and the right supramarginal gyrus (see Table III; Fig. 1C).
Gender
To explore the interaction between group (preterm‐born and control) and gender, SSQs representing the magnitude of brain activation (see description in Methods section) at the peak co‐ordinate for each cluster in which preterm‐born individuals displayed differential brain activation in comparison to controls, were entered into a Group × Gender analysis of variance. No significant interactions between group and gender survived adjustment for multiple testing, for clusters in the No‐go > Oddball which were differentially activated between preterm‐born participants and controls.
There were no significant Group × Gender interactions in the No‐go > Go contrast.
For the Oddball > Go contrast, in addition to the expected main effect for group, a trend towards a Group × Gender interaction was observed, for activation in the right supramarginal gyrus which was decreased in preterm‐born females (F(1,43) = 6.7 df, P = 0.05), who showed decreased BOLD signal when compared with male and female controls, with a trend towards being statistically different from preterm males (P = 0.07).
DISCUSSION
Preterm‐born adults displayed differential brain activation, in comparison to controls, when presented with both motor response inhibition and oddball attention allocation trials. Increased brain activation was observed in the preterm‐born group in right hemispheric middle occipital/temporal gyrus, precuneus and posterior cingulate gyrus during No‐go trials after subtraction of attentional Oddball trials. During No‐go contrasted with Go trials, preterm‐born participants showed reduced brain activation in the left posterior cerebellum and a lateral region of the right cerebellum/culmen. During the attentional Oddball condition, preterm‐born individuals displayed attenuated activation in the right inferior prefrontal and parietal cortices and left cerebellum.
Motor Response Inhibition
The increased right hemispheric brain activation observed in the preterm‐born group during motor inhibition controlling for attention allocation to low‐frequency stimuli (No‐go > Oddball) was localised in middle temporal/occipital cortex, posterior cingulate and precuneus. The middle temporal/occipital cortex is thought to respond to the volitional control of attention [Newman et al., 2007], or effortful, internally mediated orientation to visual spatial stimuli [Mayer et al., 2004]. The posterior cingulate is also involved in the dynamic reallocation of spatial attention [Mesulam et al., 2001; Small et al., 2003], and is part of a ‘generic midline attentional network’ [Rubia et al., 2006] alongside anterior regions in the cingulate gyrus and medial parietal lobe. Our data suggest these regions were recruited to a greater extent in the preterm‐born participants to successfully inhibit the motor response.
Similarly, in a different younger sample of preterm‐born adolescent boys aged 14–18 years, we previously observed increased brain activation during motor inhibition in right middle temporal and posterior cingulate regions. However, in the earlier study such increases were alongside decreases in brain activation in the prefrontal cortex and caudate nucleus [Nosarti et al., 2006]. No decreases in brain activation during motor response inhibition were observed in this study. In the absence of longitudinal data, it is difficult to place these results in the context of the earlier dataset. However, it is interesting to note that grey matter decreases have been observed in preterm‐born adolescents in both the caudate and regions of the prefrontal cortex [Nosarti et al., 2008]. One possibility is that structural differences could have accounted for decreases in brain activation in these regions in adolescents, but may no longer be apparent in adulthood. Longitudinal evidence of delays in cortical maturation has started to emerge in other conditions such as ADHD [Shaw et al., 2007]. More specifically, caudate abnormalities have been found to normalise with age in ADHD [Castellanos et al., 2002]. Future studies could explore this hypothesis using longitudinal studies to ascertain whether differences in brain structure in preterm cohorts remain into adulthood.
Although both children and adults have been found to have increased brain activation in medial posterior regions such as the precuneus during response inhibition tasks, in children such activation has been found to be directly related to the success of inhibition [Bunge et al., 2002], while older subjects rely more on fronto‐striatal networks [Rubia et al., 2006, 2007a]. Booth and colleagues [ 2003] found additional recruitment of the bilateral posterior cingulate in children as compared with adults during a response inhibition task, and there is evidence that younger people rely more heavily on these posterior brain regions. For instance, the precuneus and posterior cingulate gyrus, as well as temporal and occipital brain areas, show negative correlations with age, between adolescent and adulthood, during several tasks of motor response inhibition including the Go/no‐go task used here [Rubia et al., 2006, 2007a], and also in tasks of selective attention [Marsh et al., 2007]. More diffuse brain activation that is less concentrated on task‐specific regions distinguishes children from adults, and it is thought that these less task‐specific posterior brain regions may play a compensatory role in task completion when task‐specific regions are yet to fully mature [Durston and Casey, 2006]. This suggests the pattern of increased posterior activation in the preterm‐born participants in this study resembles that of younger participants and may indicate a less mature pattern of brain activation in preterm‐born adults during motor response inhibition.
During inhibition trials that were not controlled for the attentional oddball effect, preterm‐born participants showed decreased activation in a medial region of the left cerebellum and a more lateral region of the right cerebellum (culmen). The cerebellum is an important part of fronto‐striato‐cerebellar networks where brain activation correlates linearly with age between childhood and adulthood [Rubia et al., 2007a] and has been found to be activated during Go/No‐go tasks [Bunge et al., 2002; Garavan et al., 2003; Nosarti et al., 2006; Rubia et al., 2006]. In addition, activation in the cerebellum has been shown to correlate with inhibitory capacity [Rubia et al., 2007a]. In this study, a reduction in cerebellar activation is likely to reflect a less mature pattern of brain activation in preterm‐born participants, which may be compounded by regional structural differences as decreased cerebellar volume has also been observed in preterm‐born adolescents [Allin et al., 2001; Nosarti et al., 2008].
Preterm‐born young adults' behavioural performance on the motor response inhibition trials in this study did not differ statistically from controls. This is in line with findings of a younger group of preterm‐born males [Nosarti et al., 2006], as well as of individuals with other pathologies such as ADHD [Rubia et al., 1999, 2005, 2007b; Smith et al., 2006]. The findings of differential activation patterns and yet seemingly unimpaired performance may be indicative of neurocognitive compensation. These data are also consistent with previous observations that brain activation may be better placed to reveal group differences than behavioural performance, whereby compensatory processes may be more difficult to identify [Nosarti et al., 2006; Rubia et al., 1999, 2005a, b, 2007b; Smith et al., 2006].
Alternatively, the absence of performance differences between groups may be due to a lack of statistical power. It is possible that the absence of group × gender interaction resulted from a type 2 error, as the sample size of this and many fMRI studies, is relatively small for neuropsychological analyses, and the statistical power to detect subtle differences in inhibition and attention may be insufficient. In fact, a larger sample of preterm‐born adults displayed performance deficits on a two tasks with an inhibitory component, i.e., the Incompatibility subscale of the Test of Attentional Performance and the Hayling sentence completion test [Nosarti et al., 2007]. In addition, although not statistically significant, the gender ratio of the two groups did differ, which may have impacted upon the findings. However, the effect sizes in this study were generally weak, making it difficult to ascertain whether these data represent a lack of power or lack of an effect. Either way, to observe brain activation similar to those displayed in children, alongside intact or relatively minor behavioural differences, i.e., small effect sizes, may suggest neurocognitive compensation similar to that displayed by children whose brains are yet to fully mature.
Attention Allocation
Pronounced group differences in brain activation were observed during the Oddball trials (Oddball > Go), which measure the allocation of attention to infrequent stimuli. During these trials, the preterm‐born group displayed reduced activation in the right inferior frontal gyrus, right precentral gyrus, right supramarginal gyrus and cerebellum. Brain activation in each of these regions has previously been found during tasks of attention allocation [Clark et al., 2000; Kiehl et al., 2005; Rubia et al., 2007b].
Preterm‐born adults displayed attenuated activation in the right precentral gyrus during the Oddball trials, in line with results obtained in a similar sample of preterm‐born adolescent males [Nosarti et al., 2006]. The precentral gyrus is thought to be involved in target detection [Kiehl et al., 2005]. Preterm‐born adults also showed a decrease in BOLD signal in supramarginal gyrus during the trials which measure attention allocation (Oddball). Activation in the parietal lobes has been observed during tasks of inhibition, although it is not considered part of an inhibitory network and may be related to attentional functions necessary to manage the switch from a ‘go’ to a ‘no‐go’ response [Bunge et al., 2002; Garavan et al., 2003; Rubia et al., 2006]. The supramarginal gyrus, in part, underpins the detection of novel salient stimuli [Downar et al., 2002; Kiehl et al., 2005]. Although the Oddball trials in this study were not novel, they were salient by virtue of their infrequency. A recent study have found a similar pattern of hypo‐activation in parietal regions, including BA40, in adolescents with ADHD during Oddball trials [Rubia et al., 2007b; Tamm et al., 2006].
The inferior frontal gyrus (IFG) is thought to be involved in evaluating stimuli per se [Downar et al., 2002]. This may explain why this brain area is consistently activated during both No‐go and Oddball trials, which often require the evaluation of stimuli, in order to inhibit and execute responses [Madden et al., 2004; Rubia et al., 2006]. More specifically, the right IFG has been found to be involved in target detection [Madden et al., 2004], and to display increased activation in trials requiring the detection of salient, novel stimuli when contrasted with familiar stimuli [Downar et al., 2002]. In this study, preterm‐born participants showed attenuated activation in the right IFG during Oddball > Go suggesting functional brain activation differences in selective attention, and in particular, the detection of infrequent stimuli. Developmental studies of selective attention found increased task‐related prefrontal activation to occur in adults, when contrasted with adolescents and children [Adleman et al., 2002; Rubia et al., 2006], with functional age‐related linear increases also displayed in this region [Adleman et al., 2002; Tamm et al., 2002; Rubia et al., 2006]. These data suggest the observed under‐activation of the right inferior prefrontal cortex in this study may be indicative of a developmental delay in preterm‐born adults. It is of interest that attenuated activation in this region has been found in adolescents with ADHD during inhibitory and attention tasks [Rubia et al., 2005b; Smith et al., 2006].
Alongside hypo‐activation in fronto‐parietal networks during trials involving allocation of attention to infrequent stimuli, attenuated activation was also observed in the left lateral cerebellum. The cerebellum has been implicated in visual‐spatial attention [Townsend et al., 1999], with some studies suggesting a role in response reassignment [Bischoff‐Grethe et al., 2002]. Recent lesion data also point to a more specific role in overt shifts of attention characterised by saccades to a target as opposed to covert attentional shifts [Golla et al., 2005]. Functional abnormalities in the cerebellum during motor inhibition and attention allocation are in line with previous observations of structural and functional differences in the cerebellum in preterm‐born adolescent samples [Allin et al., 2001; Nosarti et al., 2006, 2008].
Neural Development
Taken together, these data suggest that preterm‐born adults show reduced activation when compared with controls in response to rare, infrequent stimuli including attenuated activation in task‐specific fronto‐parietal‐cerebellar circuits. The findings of reduced focal activation in task‐specific fronto‐parieto‐cerebellar circuits where functional neuronal development has been shown to continue into adulthood [Adleman et al., 2002; Rubia et al., 2006, 2007a], suggest that the neural correlates of attention allocation may remain underdeveloped in preterm‐born adults. In the motor inhibition trials, preterm‐born adults show more diffuse activation in right hemispheric posterior temporal, occipital and cingulate brain regions, similar to activation patterns observed in children and adolescents and where functional activation is known to decrease linearly with age between childhood and adulthood [Casey et al., 1997, 2000; Rubia et al., 2006, 2007a].
The pattern of increased activation in one condition, and decreased activation in the other, may at first glance appear contradictory. However, these data may suggest that preterm‐born adults when compared with controls display brain activation patterns that are reflective of less mature functional brain development. Our current understanding of functional neuronal maturation suggests it is reflected by more efficient and focal neural recruitment, such as an increase in the magnitude of activation in regions which are critical for task completion, and attenuation of activation in regions which are not essential for the task [Durston and Casey, 2006; Tamm et al., 2002]. Younger when compared with older participants show reduced activation in task‐specific brain regions, but more diffuse and extensive activation in posterior and less task‐relevant brain areas. This pattern of reduced activation in task‐specific regions of attention allocation, and increased activation of less task‐relevant regions during inhibitory trials, is similar to that observed in children, and thought to represent neurocognitive compensation in brains that are yet to fully develop. This suggests that preterm‐born adults are showing signs of functional neuronal developmental delay. In addition, to find regional hyper‐ and hypo‐activation suggests the observed results are not simply an artefact of either increased or decreased global brain activation in preterm‐born individuals [Nosarti et al., 2006].
The differential brain activation observed between preterm‐born participants and controls can be interpreted in the context of documented brain alterations following preterm birth [Abernethy et al., 2004; Inder et al., 1999; Nosarti et al., 2002, 2004, 2008; Thompson et al., 2007]. Disconnection or damage to white matter tracts following preterm birth [Huppi et al., 2001] could directly or indirectly affect the development of grey matter and the neuronal substrates underlying high‐order cognitive functions [Inder et al., 1999; Olsen et al., 1997]. A recent large‐scale study suggests that structural variations in brain structure in preterm‐born adolescents accounted for 29% of the variance in neuro‐developmental outcome, while group membership did not reach statistical significance [Nosarti et al., 2008]. Another smaller scale study also found task‐specific functional neural plasticity in preterm‐born adults with corpus callosum damage in contrast to preterm‐born individuals without damage [Santhouse et al., 2002]. It is of note that the current sample was ‘low risk’ in terms of neonatal characteristics, i.e., no cerebral palsy, grade 3/4 intraventricular hemorrhage, or periventricular leucomalacia. This may mean our results are specific to those preterm‐born adults likely to have the most favourable neurodevelopmental outcome.
These findings also suggest that functional neuronal development in preterm‐born populations may be to some extent mediated by gender. The decreased BOLD signal in the right supramarginal gyrus during the Oddball > Go were mainly driven by preterm‐born women. As discussed earlier, this region is thought to underpin the detection of salient stimuli [Downar et al., 2002], and is under‐activated in clinical cohorts such as ADHD [Smith et al., 2006; Tamm et al., 2006]. It is of interest that overall women showed slower reaction times on the Oddball trials, although this effect was not specific to preterm‐born female participants. Previous studies suggest women show attenuated brain activation in a range of networks during response inhibition tasks [Li et al., 2006]. However, one study found no male/female differences in a spatial attention task [Bell et al., 2006], and increased activation in females have also been observed in the inferior parietal lobe and right middle frontal gyrus (BA6) during error processing; i.e., failed inhibition attempts [Hester et al., 2004]. Structural brain differences between males and females have been observed in children and adolescents [DeBellis et al., 2001] as well as adults [Gur et al., 1999]. Structural gender differences in selective brain areas have also been reported in preterm infants [Thompson et al., 2007], in line with data suggesting that the developmental trajectories of preterm‐born men and women may differ [Ingemarsson, 2003; Reiss et al., 2004].
CONCLUSION
Preterm‐born adults show decreased BOLD signal in task‐relevant brain regions during attention allocation and increased signal in task‐irrelevant posterior brain regions during motor response inhibition. This suggests continuing functional and neural plasticity following very preterm birth, which is testament to the resilience of the human brain. This observed pattern of brain activation in preterm‐born adults resembles that of younger cohorts, and so is suggestive of a maturational delay. Future studies should attempt to adjust for task difficulty and allow less room for cognitive or neural compensation Rubia et al., 2007a].
REFERENCES
- Abernethy LJ,Cooke RW,Foulder‐Hughes( 2004): Caudate and hippocampal volumes, intelligence, and motor impairment in 7‐year‐old children who were born preterm. Childhood Pediatric Res 55: 884–893. [DOI] [PubMed] [Google Scholar]
- Adleman N,Menon V,Blasey C,White C,Warsofsky I,Glover G,Reiss A ( 2002): A developmental fMRI study of the stroop colour‐word task. Neuroimage 16: 61–75. [DOI] [PubMed] [Google Scholar]
- Allin M,Matsumoto H,Santhouse A,Nosarti C,AlAsady MH,Stewart A,Rifkin L,Murray RM ( 2001): Cognitive and motor function and the size of the cerebellum in adolescents born very pre‐term. Brain 124: 60–66. [DOI] [PubMed] [Google Scholar]
- Allin M,Walshe M,Fern A,Nosarti C,Rushe TM,Cuddy M,Wyatt J,Rifkin L,Murray RM( 2008): Cognitive maturation in preterm and term born adolescents. J Neurol Neurosurg Psychiatry 79: 381–386. [DOI] [PubMed] [Google Scholar]
- Anderson P,Doyle L ( 2004): Executive functioning in school‐aged children who were born very preterm or with extremely low birth weight in the 1990s. Pediatrics 114: 50–57. [DOI] [PubMed] [Google Scholar]
- Bell E,Willson M,Wilman A,Dave S,Silverstone P ( 2006): Males and females differ in brain activation tasks during cognitive tasks. Neuroimage 30: 529–538. [DOI] [PubMed] [Google Scholar]
- Benjamini Y,Hochberg Y ( 1995): Controlling the false discovery rate—A practical and powerful approach to multiple testing. J R Stat Soc B Met 57: 289–300. [Google Scholar]
- Bhutta A,Cleves M,Casey P,Cradock M,Anand K ( 2002): Cognitive and behavioural outcomes of school‐aged children who were born very preterm: A meta‐analysis. JAMA 288: 728–737. [DOI] [PubMed] [Google Scholar]
- Bischoff‐Grethe A,Ivry R,Grafton S ( 2002): Cerebellar involvement in response reassignment rather than attention. J Neurosci 22: 546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth J,Burman D,Meyer J,Lei Z,Trommer B,Davenport N,Li W,Parrish T,Gitelman D,Mesulam M ( 2003): Neural development of selective attention and response inhibition. Neuroimage 20: 737–751. [DOI] [PubMed] [Google Scholar]
- Botting N,Powls A,Cooke R,Marlow N ( 1997): Attention deficit hyperactivity disorders and other psychiatric outcomes in very low birthweight children at 12 years. J Chil Psychol Psychiatry 38: 931–941. [DOI] [PubMed] [Google Scholar]
- Brammer M,Bullmore E,Simmons A,Williams S,Grasby P,Howards R,Woodruff PW,Rabe‐Hesketh S ( 1997): Generic brain activation mapping in functional magnetic resonance imaging: A non‐parametric approach. Magn Reson Imaging 5: 763–770. [DOI] [PubMed] [Google Scholar]
- Breslau N,Chilcoat H ( 2000): Psychiatric sequelae of low birth weight at 11 years of age. Biol Psychiatry 47: 1005–1011. [DOI] [PubMed] [Google Scholar]
- Bullmore E,Long C,Suckling J,Fadili J,Calvert G,Zelaya F. ( 2001): Colored noise and computational inference in neurophysiological (fMRI) time series analysis: Resampling methods in time and wavelet domains. Hum Brain Mapp 12: 61–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore ET,Suckling J,Overmeyer S,Rabe‐Hesketh S,Taylor E,Brammer M ( 1999): Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural Mr images of the brain. IEEE Trans Med Imaging 18: 32–42. [DOI] [PubMed] [Google Scholar]
- Bunge S,Dudukovic N,Thomason M,Vaidya C,Gabrieli J ( 2002): Immature frontal lobe contributions to cognitive control in children: Evidence from fMRI. Neuron 33: 301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmody D,Bendersky M,DeMarco J,Hiatt M,Dunn S,Hegyi T,Lewis M ( 2006): Early risk, attention and brain activation in adolescents born preterm. Child Dev 77: 384–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B,Trainor R,Oredni J,Schubert A,Nystrom J,Giedd J,Castellanos J,Haxby J,Noll C,Cohen J,Forman S,Dahl R,Rapoport J ( 1997): A developmental functional MRI study of prefrontal activation during performance of a Go‐Nogo task. J Cogn Neurosci 9: 835–847. [DOI] [PubMed] [Google Scholar]
- Casey B,Giedd J,Thomas K ( 2000): Structural and functional brain development and its relation to cognitive development. Biol Psychol 54: 241–257. [DOI] [PubMed] [Google Scholar]
- Castellanos FX,Lee P,Sharp MS,Jeffries N,Greenstein D,Clasen L,Blumenthal M,James R,Ebens L,Walter J,Zijdenbos A,Evans C,Giedd J,Rapoport J ( 2002): Developmental trajectories of brain volume abnormalities in children and adolescents with attention‐deficit/hyperactivity disorder. JAMA 288: 1740–1748. [DOI] [PubMed] [Google Scholar]
- Clark V,Fannon S,Lai S,Benson R,Bauer L ( 2000): Responses to rare visual target and distractor stimuli using event‐related fMRI. J Neurophysiol 83: 3133–3139. [DOI] [PubMed] [Google Scholar]
- Dale A ( 1999): Optimal experimental design for event‐related fMRI. Hum Brain Mapp 8: 109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale A,Buckner R ( 1997): Selective averaging of rapidly presented individual trials using fMRI. Hum Brain Mapp 5: 329–340. [DOI] [PubMed] [Google Scholar]
- DeBellis M,Keshavan M,Beers S,Hall J,Frustaci K,Masalehdan A,Noll J,Boring A ( 2001): Sex differences in brain maturation during childhood and adolescence. Cereb Cortex 11: 552–557. [DOI] [PubMed] [Google Scholar]
- Downar J,Crawley A,Mikulis D,Davis K ( 2002): A cortical network sensitive to stimulus salience in a neutral behavioural context across multiple sensory modalities. J Neurophysiol 87: 615–620. [DOI] [PubMed] [Google Scholar]
- Durston S,Casey B ( 2006): What have we learned about cognitive development from neuroimaging? Neuropsychologia 44: 2149–2157. [DOI] [PubMed] [Google Scholar]
- Durston S,Tottenham N,Thomas K,Davidson M,Eigsti I,Yang Y,Ulug A,Casey B ( 2003): Differential patterns of striatal activation in young children with and without ADHD. Biol Psychiatry 53: 871–878. [DOI] [PubMed] [Google Scholar]
- Eriksson B,Pehrsson G ( 2003): Relationships between the family's way of functioning and children's temperament as rated by parents of pre‐term children. J Child Health Care 7: 89–100. [DOI] [PubMed] [Google Scholar]
- Foulder‐Hughes L,Cooke R ( 2003): Motor, cognitive, and behavioural disorders in children born very preterm. Dev Med Child Neurol 45: 97–103. [PubMed] [Google Scholar]
- Garavan H,Ross T,Kaufman J,Stein E ( 2003): A midline dissociation between error‐processing and response conflict monitoring. Neuroimage 20: 1132–1139. [DOI] [PubMed] [Google Scholar]
- Golla H,Thier P,Haarmeier T ( 2005): Disturbed overt but normal covert shifts of attention in adult cerebellar patients. Brain 128: 1525–1535. [DOI] [PubMed] [Google Scholar]
- Gur R,Turetsky B,Matsui M,Yan M,Bilker W,Hughett P, et al. ( 1999): Sex differences in brain grey and white matter in healthy young adults: Correlations with cognitive performance. J Neurosci 19: 4065–4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R,Passbender C,Garavan H ( 2004): Individual differences in error processing: A review and reanalysis of three event‐related FMRI studies using the Go/Nogo task. Cereb Cortex 14: 986–994. [DOI] [PubMed] [Google Scholar]
- HMSO ( 1991): Office of Population Censuses and Surveys, Standard Occupational Classification. London: HMSO. [Google Scholar]
- Horn N,Dolan M,Elliott R,Deakin J,Woodruff P ( 2003): Response inhibition and impulsivity: An fMRI study. Neuropsychologia 41: 1959–1966. [DOI] [PubMed] [Google Scholar]
- Huppi P,Murphy B,Maier S,Zientra G,Inder T,Barnes P, et al. ( 2001): Microstructural brain development after perinatal cerebral white matter injury assessed by diffusion tensor magnetic resonance imaging. Pediatrics 107: 455–460. [DOI] [PubMed] [Google Scholar]
- Inder T,Huppi P,Warfield S,Kikinis R,Zientara GP,Barnes PD,Jolesz F,Volpe JJ ( 1999): Periventricular white matter injury in the premature infant is followed by reduced cerebral cortical gray matter volume at term. Ann Neurol 46: 755–760. [DOI] [PubMed] [Google Scholar]
- Ingemarsson I ( 2003): Gender aspects of preterm birth. BJOG 20: 34–38. [DOI] [PubMed] [Google Scholar]
- Kiehl K,Stevens M,Laurens K,Pearlson G,Calhun V,Liddle P ( 2005): An adaptive reflexive processing model of neurocognitive function: Supporting evidence from a large‐scale (N = 100) fMRI study of an auditory Oddball task. Neuroimage 25: 899–915. [DOI] [PubMed] [Google Scholar]
- Krain A,Castellanos FX ( 2006): Brain development and ADHD. Clin Psychol Rev 26: 433–444. [DOI] [PubMed] [Google Scholar]
- Lawson K,Ruff H ( 2004): Early focused attention predicts outcome for children born prematurely. Dev Behav Pediatr 25: 399–406. [DOI] [PubMed] [Google Scholar]
- Lewis M,Stieben J ( 2004): Emotion regulation in the brain: Conceptual issues and directions for developmental research. Child Dev 75: 371–376. [DOI] [PubMed] [Google Scholar]
- Li C,Huang C,Constable R,Sinha R ( 2006): Gender differences in the neural correlates of response inhibition during a stop signal task. Neuroimage 32: 918–929. [DOI] [PubMed] [Google Scholar]
- Liston C,Watts R,Tottenham N,Davidson S,Niogi S,Ulug A,Casey B ( 2006): Fronto‐striatal microstructure modulates efficient recruitment of cognitive control. Cereb Cortex 16: 553–560. [DOI] [PubMed] [Google Scholar]
- Madden D,Whiting W,Provenzale J,Huettel S ( 2004): Age‐related changes in neural activity during visual target detection measured by fMRI. Cereb Cortex 14: 143–155. [DOI] [PubMed] [Google Scholar]
- Marsh R,Zhu H,Wang Z,Skudlarski P,Peterson B ( 2007): A developmental fMRI study of self‐regulatory control in Tourette's syndrome. Am J Psychiatry 164: 955–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A,Dorflinger J,Rao S,Seidenberg M ( 2004): Neural networks underlying endogenous and exogenous visual‐spatial orienting. Neuroimage 23: 534–541. [DOI] [PubMed] [Google Scholar]
- Ment L,Constable RT ( 2007): Injury and recovery in the developing brain: Evidence from functional MRI studies of prematurely born children. Nat Clin Pract Neurol 3: 558–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M,Noble A,Yun‐Hee K,Parrish T,Gittleman D ( 2001): Heterogeneity of cingulate contributions to spatial attention. Neuroimage 13: 1065–1072. [DOI] [PubMed] [Google Scholar]
- Newman S,Keller T,Just M ( 2007): Volitional control of attention and brain activation in dual task performance. Hum Brain Mapp 28: 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosarti C,Al Asady M,Frangou S,Stewart A,Rifkin L,Murray RM( 2002): Adolescents who were born very preterm have decreased brain volumes. Brain 125: 1616–1623. [DOI] [PubMed] [Google Scholar]
- Nosarti C,Rifkin L,Murray R ( 2003): Long‐term consequences of preterm birth In: Cicchetti D,Walker E, editors. Neurodevelopmental Mechanisms in Psychopathology. Cambridge: Cambridge University Press. [Google Scholar]
- Nosarti C,Rushe T,Woodruff P,Stewart A,Rifkin L,Murray R ( 2004): Corpus callosum size and very preterm birth: Relationship to neuropsychological outcome. Brain 127: 2080–2089. [DOI] [PubMed] [Google Scholar]
- Nosarti C,Allin M,Frangou S,Rifkin L,Murray R ( 2005): Decreased caudate volume is associated with hyperactivity in adolescents born very preterm. Biol Psychiatry 13: 339. [DOI] [PubMed] [Google Scholar]
- Nosarti C,Rubia K,Smith A,Frearson S,Williams S,Rifkin L,Murray R ( 2006): Altered functional neuroanatomy of response inhibition in adolescent boys who were born very preterm. Dev Med Child Neurol 48: 265–271. [DOI] [PubMed] [Google Scholar]
- Nosarti C,Giouroukou E,Micali N,Rifkin L,Morris R,Murray R ( 2007): Impaired executive functioning in young adults born very preterm. J Int Neuropsychol Soc 18: 1–11. [DOI] [PubMed] [Google Scholar]
- Nosarti C,Giouroukou E,Healy E,Rifkin L,Walshe M,Chitnis X,Williams S,Murray R ( 2008): Abnormalities of grey and white matter distribution in adolescents who were born very preterm predict neurodevelopmental outcome. Brain 131: 205–217. [DOI] [PubMed] [Google Scholar]
- Olsen P,Paakko E,Vainionpaa L,Pyhtinen J,Jarvelin M ( 1997): Magnetic resonance Imaging of periventricular leukomalacia and its clinical correlation in children. Ann Neurol 41: 754–761. [DOI] [PubMed] [Google Scholar]
- Petersen B,Vohr B,Kane M,Whalen D,Schneider K,Katz K,Zhang H,Duncan C,Makuch R,Gore C,Ment L ( 2002): A functional magnetic resonance imaging study of language processing and its cognitive correlates in prematurely born children. Pediatrics 110: 1153–1162. [DOI] [PubMed] [Google Scholar]
- Reiss AL,Kesler SR,Vohr B,Duncan CC,Katz KH,Pajot S,Schneider K,Makuch R,Ment L ( 2004): Sex differences in cerebral volumes of 8‐year olds born preterm. J Pediatr Psychol 145: 242–249. [DOI] [PubMed] [Google Scholar]
- Rosso I,Young A,Femia L,Yurgelun‐Todd D ( 2004): Cognitive and emotional components of frontal lobe functioning in childhood and adolescence. Ann NY Acad Sci 1021: 355–362. [DOI] [PubMed] [Google Scholar]
- Rubia K ( 2002): The dynamic approach to neurodevelopmental psychiatric disorders: Use of fMRI combined with neuropsychology to elucidate the dynamics of psychiatric disorders, exemplified in ADHD and Schizophrenia. Behav Brain Res 130: 47–56. [DOI] [PubMed] [Google Scholar]
- Rubia K,Overmeyer S,Taylor E,Brammer M,Williams S,Simmons A,Bullmore E ( 1999): Hypofrontality in attention deficit hyperactivity disorder during higher order motor control: A study with functional MRI. Am J Psychiatry 156: 891–896. [DOI] [PubMed] [Google Scholar]
- Rubia K,Overmyer S,Taylor E,Brammer M,Williams S,Simmons A,Bullmore E ( 2000): Functional frontalisation with age: Mapping neurodevelopmental trajectories with fMRI. Neurosci Biobehav Rev 24: 13–19. [DOI] [PubMed] [Google Scholar]
- Rubia K,Russell T,Overmeyer S,Brammer M,Bullmore E,Sharma T,Simmons A,Williams S,Giampietro V,Andrew C,Taylor E ( 2001): Mapping motor inhibition: Conjunctive brain activations across different versions of Go/Nogo and stop tasks. Neuroimage 13: 250–261. [DOI] [PubMed] [Google Scholar]
- Rubia K,Smith A,Brammer M,Taylor E ( 2003): Right inferior prefrontal cortex meditates response inhibition while mesial prefrontal cortex is responsible for error detection. Neuroimage 20: 351–358. [DOI] [PubMed] [Google Scholar]
- Rubia K,Lee F,Cleare A,Tunstall N,Fu C,Brammer M,McGuire P ( 2005a) Tryptophan depletion reduces right inferior prefrontal activation during response inhibition in fast, event‐related fMRI. Psychopharmacology (Berl) 179: 791–801. [DOI] [PubMed] [Google Scholar]
- Rubia K,Smith A,Brammer M,Toone B,Taylor E ( 2005b) Abnormal brain activation during inhibition and error detection in medication naive adolescents with ADHD. Am J Psychiatry 162: 1067–1075. [DOI] [PubMed] [Google Scholar]
- Rubia K,Smith A,Woolley J,Nosarti C,Heyman I,Brammer M,Taylor E ( 2006): Progressive increase of fronto‐striatal brain activation from childhood to adulthood during event related tasks of cognitive control. Hum Brain Mapp 27: 973–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K,Smith A,Taylor E,Brammer M ( 2007a) Linear age‐correlated functional development of right inferior fronto‐striato‐cerebellar networks during response inhibition and anterior cingulate during error‐related processes. Hum Brain Mapp 28: 1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K,Smith A,Brammer M,Taylor E ( 2007b) Temporal lobe dysfunction in medication‐naïve boys with attention‐deficit/hyperactivity disorder during attention allocation and its relation to response variability. Biol Psychiatry 62: 999–1006. [DOI] [PubMed] [Google Scholar]
- Rushe T,Rifkin L,Stewart A,Townsend J,Roth S,Wyatt J,Murray R ( 2001): Neuropsycholgical outcome at adolescence of very preterm birth and its relation to brain structure. Dev Med Child Neurol 43: 226–233. [DOI] [PubMed] [Google Scholar]
- Santhouse A,Ffytche D,Howard R,Williams S,Stewart A,Rooney M,Wyatt J,Rifkin L,Murray R ( 2002): The functional significance of perinatal corpus callosum damage: An fMRI study in young adults. Brain 125: 1782–1792. [DOI] [PubMed] [Google Scholar]
- Shaw P,Eckstrand K,Sharp W,Blumenthal J,Lerch J,Greenstein D,Clasen L,Evans A,Giedd J,Rapoport J ( 2007): Attention‐deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci USA 104: 19649–19654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small D,Gitelman D,Gregory M,Nobre A,Parrish T,Mesalum M ( 2003): The posterior cingulate and medial prefrontal cortex meditate the anticipatory allocation of spatial attention. Neuroimage 18: 633–641. [DOI] [PubMed] [Google Scholar]
- Smith A,Taylor E,Brammer M,Toone B,Rubia K ( 2006): Task‐specific hypoactivation in prefrontal and temporoparietal brain regions during motor inhibition and task switching in medication‐naive children and adolescents with attention deficit hyperactivity disorder. Am J Psychiatry 163: 1044–1051. [DOI] [PubMed] [Google Scholar]
- Tailairach J,Tournoux P ( 1998): Co‐Planar Stereotaxic Atlas of the Human Brain. Stuttgart: Thieme Verlag. [Google Scholar]
- Tamm L,Menon V,Reiss A ( 2002): Maturation of brain function associated with response inhibition. J Am Acad Child Adolesc Psychiatry 41: 1231–1238. [DOI] [PubMed] [Google Scholar]
- Tamm L,Menon V,Reiss A ( 2006): Parietal attentional system abberrations during target detection in adolescents with attention hyperactivity disorder: Event‐related fMRI evidence. Am J Psychiatry 163: 1033–1043. [DOI] [PubMed] [Google Scholar]
- Thirion B,Pinel P,Meriaux S,Roche A,Dehaene S,Poline J ( 2007): Analysis of a large fMRI cohort: Statistical and methodological issues for group analysis. Neuroimage 35: 105–120. [DOI] [PubMed] [Google Scholar]
- Thompson D,Warfield S,Carlin J,Pavlovic M,Wang H,Bear M,Kean M,Doyle L,Egan G,Inder T ( 2007): Perinatal risk factors altering regional brain structure in the preterm infant. Brain 130: 667–677. [DOI] [PubMed] [Google Scholar]
- Townsend J,Courchesne E,Covington E,Westerfield M,Harris N,Lyden P,Lowry T,Press G ( 1999): Spatial attention deficits in patients with acquired or developmental cerebellar abnormality. J Neurosci 19: 5632–5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D ( 1999): Abbreviated Scale of Intelligence. New York: The Psychological Corporation. [Google Scholar]
- Wildenberg W,Molen M ( 2004): Developmental trends in simple and selective inhibition of compatible and incompatible responses. J Exp Child Psychol 87: 201–220. [DOI] [PubMed] [Google Scholar]


