Abstract
Our sense of self is strongly colored by emotions although at the same time we are well able to distinguish affect and self. Using functional magnetic resonance imaging, we here tested for the differential effects of self‐relatedness and emotion dimensions (valence, intensity) on parametric modulation of neural activity during perception of emotional stimuli. We observed opposite parametric modulation of self‐relatedness and emotion dimensions in the dorsomedial prefrontal cortex and the ventral striatum/nucleus accumbens, whereas neural activity in subcortical regions (tectum, right amygdala, hypothalamus) was modulated by self‐relatedness and emotion dimensions in the same direction. In sum, our results demonstrate that self‐relatedness is closely linked to emotion dimensions of valence and intensity in many lower subcortical brain regions involved in basic emotional systems and, at the same time, distinct from them in higher cortical regions that mediate cognitive processes necessary for becoming aware of one's self, for example self‐consciousness. Hum Brain Mapp, 2009. © 2007 Wiley‐Liss, Inc.
Keywords: functional imaging, affect, prefrontal cortex, self
INTRODUCTION
Our humans' sense and subjective experience of the self is most often strongly colored by emotions; for instance, we experience ourselves as positive, for example showing for instance pride, or negative, for example feeling for instance guilty. At the same time, we are however well able to distinguish self and emotion. The question of the nature of the self has been one of the most salient problems throughout the history of philosophy and more recently psychological and neural correlates of the self and self‐relatedness have also been addressed in psychology and neuroscience [Amodio and Frith, 2006; Beer, 2007; Damasio, 1999, 2003; D'Argembeau et al., 2005; Frith and Frith, 2006; Kalisch et al., 2006; Keenan et al., 2000; Kelley et al., 2002; Lambie and Marcel, 2002; LeDoux, 2002; Moran et al., 2006; Northoff and Bermpohl, 2004; Northoff et al., 2006; Ochsner et al., 2004, 2005; Phan et al., 2004; Schore, 2003]. Along with others, we consider the self as a separate neurobiological system that serves to relate internal states and external stimuli to the organism, for example self‐related processing, by valuing and attributing meaning to them thereby establishing a sense of belongingness [Northoff and Bermpohl, 2004; Panksepp, 1998, 2003, 2005]. If this is true, self‐related processing should be closely related to emotional processing in lower regions of the brain where emotions are predominantly processed, whereas at the same time, the biological system underlying the self should also be capable of being dissociated from other biological systems like emotions; this might be realized by for instance the way they differentially modulate neural activity in certain higher brain regions when compared with emotions. However, recent neuroimaging studies show involvement of various brain regions in both self and emotions; these include cortical and subcortical regions like the medial orbitofrontal cortex (MOFC), the ventromedial prefrontal cortex, dorsomedial prefrontal cortex (DMPFC), the ventrolateral prefrontal cortex (VLPFC), the amygdala, ventral striatum including the nucleus accumbens (VS/NACC), and other subcortical regions like the tectum and the hypothalamus [Beer, 2007; Gillihan and Farah, 2005; Gusnard et al., 2001; Kelley et al., 2002; Moran et al., 2006; Northoff et al., 2006; Ochsner and Feldmann Barrett, 2002; Ochsner and Gross, 2005; Ochsner et al., 2004; Panksepp, 2005; Phan et al., 2002, 2004; Satpute and Lieberman, 2006; Turk et al., 2002, 2003; Vogeley et al., 2004]. Based on a recent meta‐analysis [Northoff et al., 2006], these regions have been subsumed under the concept of the subcortical–cortical midline system, which we assume to be the core system mediating self‐relatedness. Though other regions like the insula, the somatosensory cortex, and the temporal pole have also been associated with self‐relatedness, we therefore focused on this subcortical–cortical midline system as the presumed core neural system of our self.
Using functional magnetic resonance imaging (fMRI), we tested for parametric modulation of self‐relatedness in neuroanatomically overlapping regions that have been implicated in emotional and self‐related neural processing. Unlike in previous studies on the self, we here relied on a parametric approach (degree of correlation between brain and psychological states), which allowed us to test for similar or differential effects of self‐relatedness and emotion dimensions on regional BOLD signal changes. We directly mapped the degree of self‐relatedness of emotional stimuli to the degree of regional signal changes during perception of the very same stimuli. Because recent studies demonstrated considerable impact of cognitive components like judgment, discrimination, and explicit appraisal on neural activity in medial cortical regions during emotional perception [Grimm et al., 2006; Ochsner and Feldmann Barrett, 2002; Ochsner and Gross, 2005; Ochsner et al., 2004; Taylor et al., 2003], we, unlike others [Moran et al., 2006; Phan et al., 2004], explicitly sought to avoid such cognitive task biases in our paradigm. Instead, subjects had to merely perceive emotional stimuli in fMRI and had to make only an arbitrary mouse click to assure vigilance, whereas they evaluated the very same stimuli in a postscanning session with regard to their degree of self‐relatedness and affective dimensions (valence and intensity) on a visual‐analog scale ranging from 1 (low) to 9 (high). Individual subjects' self‐relatedness and affective scores of each picture were then included as regressors in the analyses of neural activity during emotional perception. This allowed us to test for positive and negative parametric modulation using partial correlation analysis as well as for direct comparison of parametric modulation by self‐relatedness with one by emotional valence and intensity.
MATERIAL AND METHODS
Subjects
We investigated 15 female and male subjects (7 females, 8 males; age: 24.4 ± 2.72, mean ± SD, min: 21, max: 31). All were right‐handed as assessed by the Edinburgh Inventory for Handedness [Oldfield, 1971]. After detailed explanation of the study design and potential risks all subjects gave written informed consent. The study was approved by the institutional review board of the Otto‐von‐Guericke University of Magdeburg.
Experimental Stimuli and Design
Photographs from the International Affective Picture System (IAPS) were shown to the subjects for a duration of 5 s. Picture sets were counterbalanced across subjects as well as within each subject according to the two categories, high‐ and low‐positive emotional valence, and medium‐positive emotional valence. For exact matching procedures see previous work of our group [Grimm et al., 2006]. The paradigm consisted of eight runs with different emotional (i.e. high‐ and low‐positive emotional valence) and neutral (i.e. medium‐positive emotional valence) pictures; each picture was presented only once. In the respective runs, pictures were presented in a randomized order and subjects were instructed to view the pictures passively. An arbitrary button press was requested to be made as quick as possible, which allowed to assure a constant level of attention during picture viewing. Reaction times from picture onset to button press were measured. At the time of scanning, subjects were not aware of any postscanning ratings. To exclude the possible effects of anticipation or preceding attention (see Grimm et al. [2006] for details), half of the pictures were preceded by an expectancy period with a duration of 4 s, in which the type (high/low positive valence and medium‐positive valence) of the following picture was indicated by a white arrow on a dark background pointing to different directions. An upward pointing arrow was followed by an emotional picture (i.e. high‐ and low‐positive emotional valence), and a downward pointing a neutral (i.e. medium‐positive emotional valence) picture. The other half of the pictures were presented directly after a fixation cross, which was presented after every picture for 8.5 s and served as a baseline condition and allowed the subjects to recover from the emotional stimulation. The nonpictorial stimuli (arrows, fixation cross) were of equal size, color, and luminance and were centered on a black background. A total of 256 trials were presented in the eight runs. The different types of IAPS pictures and expectancy tasks were pseudorandomized within and across the eight runs. Prior to the experimental session, subjects were familiarized with the paradigm by completing a test run. During fMRI, pictures were projected automatically via a computer and a forward projection system on a screen placed at the end of the subject's gurney. Subjects lay supine in the scanner and viewed the screen through a mirror positioned on the head coil. Subjects were asked to keep their eyes open and fixate the middle of the screen in front of them. They were asked not to move the fingers, head, or body during picture presentation and viewing with the exception of the button press for the response, for which we measured reaction times.
Behavioral Ratings
Subjective ratings of self‐relatedness and emotional dimensions (valence, intensity) were made on a visual‐analog scale ranging from 0 to 9. These three ratings (self‐relatedness, emotional valence, and emotional intensity) were made in all subjects immediately afterward in a postscanning session to avoid cognitive influences during scanning, which can confound neural activity during emotional perception [Grimm et al., 2006; Taylor et al., 2003].
Self‐relatedness was assessed using the question “How much do I personally associate with or relate to this picture?” (translated from German) and ranged from “low personal association” (1) to “high personal association” (9). Subjects were instructed to re‐experience the picture with regard to themselves. Rather than evaluating the picture with regard to the distinction between self and nonself, they were instructed to rate the personal association and meaning based on the strength of their subjective or personal experience of themselves while viewing the pictures. Prior to beginning the task, participants were also explicitly asked what they understood by “personal association” which was then explained in the way the concept was to be used here, that is, as affective‐experiential [Northoff et al., 2006]; for example, how much their own person is experientially and emotionally involved. This was done to exclude other potential meanings of “personal association” like, for example imagination of oneself in the picture, recollection of associated autobiographical memories, introspection or observation of themselves while viewing the picture, or evaluation of self‐relevance. We are aware that this focus on implicit, subjective, and phenomenal aspects and thus on what is called “phenomenal experience” might be rather difficult to specify and validate through independent measures, but we believe it is a critical perspective for understanding brain–mind interrelations during experiential states. What was most important here was to establish the experimental conditions that minimized predominantly cognitive‐evaluative tendencies as, for example, might be required by recollection of autobiographical memories, imagination, etc. Because even our postscanning evaluation with the question “How much do I personally associate with or relate to this picture?” requires some at least implicit cognitive‐evaluative elements, we did not attempt to monitor that psychological dimension during the scanning session itself.
Subjective ratings of emotion dimensions included emotional valence and intensity. Emotional valence was assessed using the question “How unpleasant/pleasant is that picture?” and ranged on a continuum from “less positive” to “very positive” on a 1–9 point Likert scale. Emotional intensity was assessed using the question “How intense is this picture?” and ranged on a continuum from “low” (1) to “high” (9). Because we did not include a measure of “dominance” (or “surgency”) in our postscanning ratings, we relied on the given standard IAPS ratings for this dimension (4.91 ± 1.26). We considered this as justified because our subjects' ratings for emotional valence (4.72 ± 2.21) and intensity (5.19 ± 2.54) did not differ significantly from standard IAPS norms (intensity: 5.10 ± 2.32, valence: 4.81 ± 1.74), and the concept of “dominance” is the most difficult for subjects to understand.
To validate ratings of self‐relatedness and their relation to emotion dimensions, we tested the very same pictures and ratings (self‐relatedness, emotional valence, emotional intensity) in another group of subjects (n = 13), which we will here refer to as “the behavioral control group”.
Based on the individual subjects' rating, we distinguished between high (7–9 on the visual analog scale),medium (4–6 on the visual analogue scale), and low (1–3 on the visual analog scale) categories in our analysis of self‐relatedness. We then analyzed the ratings of emotional valence and intensity in orientation on the same three categories of self‐relatedness, so that these measures could be interrelated. In sum, we performed ANOVA and post hoc t‐tests to directly compare ratings of emotion dimensions between the three different categories of self‐relatedness. Finally, we performed Pearson Product Moment Correlation analysis of self‐relatedness with emotion dimensions.
FMRI Acquisition
Data acquisition was conducted on a 1.5‐T General Electric Signa scanner using a standard headcoil. Imaging procedures included collection of (a) structural high‐resolution images (RF‐spoiled GRASS sequence 60 slices sagittal, 2.8 mm thickness), (b) T1‐weighted anatomic images coplanar with the functional images (23 slices, aligned to the plane connecting the anterior and posterior commissure axis covering the whole head in oblique axial orientation), (c) inversion recovery T1‐weighted echo planar images coplanar with the functional images, and (d) echo planar functional images sensitive to BOLD contrast (257 sequential acquisitions, 23 slices with 3.125 mm in‐plane resolution, 5 mm thickness, 1 mm gap; T2*‐weighted gradient echo sequence: TR 2s, TE 40 ms). The first seven images were discarded due to T1 saturation effects. Subjects were positioned in the scanner and the head was immobilized with foam pieces and a velcro strip around the forehead. By a mounted mirror on the headcoil a screen was visible, on which the stimuli were projected using an LCD projector.
Image Analysis
Image processing and statistical analyses were carried out using MATLAB 6.5.1 (Mathworks, Natick, MA) and SPM2 (Statistical parametric mapping software, SPM; Wellcome Department of Imaging Neuroscience, London, UK; http://www.fil.ion.ucl.ac.uk) and included realignment, normalization and smoothing. We considered emotional (i.e. high‐ and low‐positive emotional valence) and neutral (i.e. medium‐positive emotional valence) pictures as well as, based upon postscanning ratings, high‐ and low‐self‐related pictures as regressors (expectancy conditions were included as regressor of no interest). We conducted two different types of analyses, categorical and parametric, for self‐relatedness, valence, and intensity. First, categorical analysis was performed by calculating statistical parametric maps that were generated for emotional (i.e. high‐ and low‐positive emotional valence) versus neutral (i.e. medium‐positive emotional valence) pictures and then for high self‐related pictures versus low self‐related pictures for each subjects by using a general linear model. Signal percent changes (%) were calculated for all four conditions, emotional (i.e. high‐ and low‐positive emotional valence) and neutral (i.e. medium‐positive emotional valence) pictures and high‐ and low‐self‐related pictures. Random effect analysis was used to determine each subject's voxel‐wise activation during emotional (when compared with neutral) pictures and high self‐related (when compared with low self‐related) pictures. For the entire group of 15 subjects, significant clusters of activation were determined by using the threshold P < 0.001, (uncorrected), k > 10. Second, parametric analysis was performed by using partial correlation analysis that was conducted along the recommendations by SPM (http://www.mrc-cbu.cam.ac.uk/). Individual subject's rating values of self‐relatedness, valence, and intensity for each picture presented in fMRI were entered as regressors in the design matrix. First, the positive and negative correlation for each dimension (self‐relatedness, valence, intensity) was calculated by including the respective two others as covariates. This was followed in a second step by direct comparison of self‐relatedness with valence and intensity, respectively. The level of statistical significance was again set to P < 0.001, (uncorrected), k > 10. Correlation curves were extracted from original SPM data. Time courses using Marsbar program were calculated for high, medium, and low levels of self‐relatedness.
RESULTS
Behavioral Data
Reaction times as a function of self‐relatedness
Reaction time (means ± SD) in the scanner, as calculated from an arbitrary button click to ensure vigilance, for all pictures was 824 ± 295 ms. ANOVA (df (2) = 44, F = 0.008, P > 0.05) revealed no significant difference in reaction times between high (780 ± 257 ms), medium (830 ± 277 ms), and low (835 ± 312) self‐related pictures. Reaction times (means ± SD) were also calculated for high (825 ± 291 ms), medium (833 ± 289 ms) and low (814 ± 305 ms) emotional intensity as well as for high, that is, more positive (814 ± 274), medium, that is, less positive (821 ± 299 ms) and low, that is, not at all positive (838 ± 305 ms) valenced pictures, which both revealed no significant effects ANOVA (emotional intensity: df (2) = 44, F = 0.222, P > 0.05; emotional valence: df (2) = 44, F = 0.139, P > 0.05). This suggests that no robust differences in vigilance were evident as a function of degree to which the pictures were deemed self‐related during the course of the study.
Self‐relatedness
Subjective evaluation of self‐relatedness of stimuli revealed the following results in the subjects who underwent imaging. Mean average ratings (means ± SD) of self‐relatedness were for all pictures 3.71 ± 2.37 and distinguished also between pictures with high (7.48 ± 0.63), medium (5.08 ± 0.77), and low (1.79 ± 0.80) self‐relatedness (see Supplementary Fig. 1a). We investigated self‐relatedness combined for those pictures with lowest and highest positive valence values and obtained a self‐relatedness value (means ± SD) of 4.79 ± 1.56 whereas the self‐relatedness value (means ± SD) for pictures with medium positive valence (which are considered to be medium positive and thus neutral) was 2.03 ± 0.82. Self‐relatedness was significantly higher (T = 4.10, df = 43, P < 0.005) in pictures with high/low positive emotional valence (4.79 ± 1.56) when compared with those with medium‐positive valence (2.03 ± 0.82). Finally, we investigated a behavioral control group with the same behavioral measures, yielding very similar results (see Supplementary Fig. 1a), which indicates a certain degree of intersubject reliability. Postscanning ratings for emotion dimensions (valence, intensity) were also obtained and are described together with intercorrelations between those measures in the Supplementary material.
FMRI Data
Emotional perception and self‐relatedness
Comparison between emotional (i.e. high‐ and low‐positive valenced pictures) and neutral (i.e. medium‐positive valenced pictures) picture perception yielded signal intensities in the DMPFC, the tectum/midbrain, the dorsomedial thalamus, right amygdala, right lateral premotor cortex, and other regions (see Fig. 1A and Supplementary Table I). Based on our postscanning ratings, we then compared all pictures rated as high self‐related (scores between 7 and 9 on our visual analog scale) with those rated as low self‐related (scores between 1 and 3 on our visual analog scale). High self‐related pictures showed higher signal intensities in the DMPFC (with effects localized dorsally from those revealed by the contrast emotional > neutral pictures), MOFC, tectum/midbrain, right amygdala, hypothalamus, VS/NACC, and some other regions when compared with low self‐related pictures (see Fig. 1B and Supplementary Table I for all regions). To reveal how emotional perception and self‐relatedness were related in the brain, we then exclusively masked the contrast High self‐related pictures > Low self‐related pictures with the one Emotional (i.e. high‐ and low‐positive valenced) pictures > Neutral (i.e. medium‐positive valenced) pictures. This yielded signal intensities in the MOFC, DMPFC, VS/NACC, right amygdale, and tectum/midbrain (see Fig. 1C and Supplementary Table I). Finally, to search for regions commonly recruited in both high degrees of self‐relatedness and emotional (i.e. high‐ and low‐positive valenced pictures) pictures, we also performed conjunction analysis (High self‐related pictures > Low self‐related pictures) versus (Emotional pictures, i.e. high‐ and low‐positive valenced pictures) > Neutral pictures (i.e. medium‐positive valenced pictures) [Friston et al., 2005]. This yielded significant signal changes in the bilateral parietal cortex whereas no signal changes were observed in the above‐mentioned subcortical and cortical midline regions (even when the threshold was lowered to P < 0.01) (see Supplementary Table I).
Figure 1.
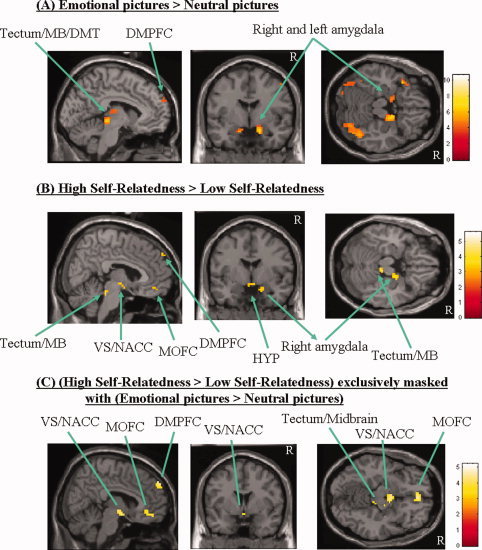
Signal intensities during emotional perception and self‐relatedness. (A) The signal intensities during emotional perception when compared (categorical comparison of conditions) with perception of neutral pictures. The sagittal view depicts the left hemisphere; the threshold of significance is set to P < 0.001 (uncorr), k > 10. See Supplementary Table I for exact coordinates. (B) The figure shows the signal intensities when one compares (categorical comparison of conditions) all (emotional and neutral) pictures rated as high self‐related with those rated as low self‐related. The sagittal view depicts the right hemisphere; the threshold of significance is set to P < 0.001 (uncorr), k > 10. See Supplementary Table I for exact coordinates. (C) The signal intensities when one exclusively masks the contrasts High self‐related pictures > Low self‐related pictures with the one Emotional pictures > Neutral pictures. The sagittal view depicts the right hemisphere; the threshold of significance is set to P < 0.001 (uncorr), k > 10 (the one for the mask was set to P < 0.05 (uncorr), k > 10). See Supplementary Table I for exact coordinates. Abbreviations: DMPFC, dorsomedial prefrontal cortex; MB, midbrain; MOFC, medial orbitofrontal cortex; HYP, hypothalamus; VS/NACC, ventral striatum/nucleus accumbens; DMT, dorsomedial thalamus; R, right; DMT, dorsomedial thalamus. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Parametric modulation of self‐relatedness
We first investigated the modulation of neural activity by self‐relatedness using partial correlation analysis (P < 0.001, uncorrected, k > 10) for which we entered self‐relatedness scores for each picture from individual subjects as regressors (and emotional valence and intensity as covariates). Significantly positive correlation with self‐relatedness was obtained in the DMPFC, VS/NACC, right amygdala, tectum, hypothalamus, dorsomedial thalamus, and the bilateral posterior parietal cortex (see Fig. 2A and Supplementary Table II). This indicates that higher signal intensities in these regions were accompanied by higher degrees of self‐relatedness, whereas significantly negative correlation with self‐relatedness was observed in the right VLPFC, right lateral premotor cortex, and various other regions (see Fig. 2B and Supplementary Table II). This indicates that higher signal intensities in these regions were accompanied by lower degrees of self‐relatedness.
Figure 2.
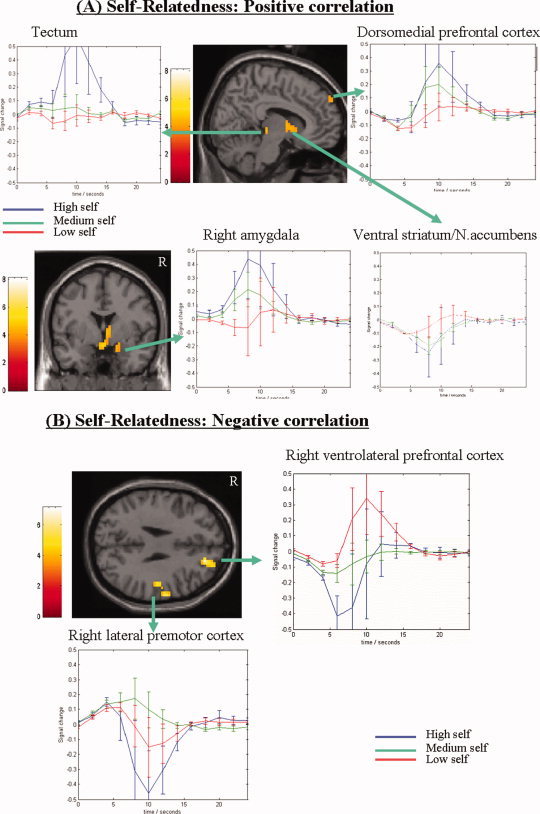
Signal intensities in positively (A) and negatively (B) correlating parametric maps of self‐relatedness. The image represents all regional signal intensities that correlate either positively (A) or negatively (B) with the degree of self‐relatedness (1–9 on visual analog scale) (see Supplementary Table II for exact coordinates). Subject‐specific partial correlation analysis of self‐relatedness was done at P < 0.001 uncorrected with extent threshold k = 10 voxels. The sagittal images depict the right hemisphere. The curves (x‐axis represent time and y‐axis signal percent change) demonstrate the BOLD‐signals for high (6–9 on visual analog scale; blue curve), medium (4–6 on visual analog scale, green curve), and low (1–3 on visual analog scale, red curve) self‐relatedness within each region. Abbreviations: R, right; DMPFC, dorsomedial prefrontal cortex; VLPFC, ventrolateral prefrontal cortex; VS/NACC, ventral striatum/nucleus accumbens. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Parametric modulation of emotion dimensions
To investigate the neural relation between self‐relatedness and emotion dimensions, we investigated correlation of neural activity with subjective ratings of emotional valence and intensity in those regions that were parametrically modulated by self‐relatedness. Our results suggest that emotional valence and intensity correlate in a different, that is, opposite direction than self‐relatedness in the DMPFC and the VS/NACC (see Fig. 3A,B and Supplementary Fig. 2a), whereas our data indicate that emotional valence and/or intensity are modulated in the same way as self‐relatedness in the tectum, the right amygdala, and the right VLPFC (see Fig. 3C–E and Supplementary Fig. 2b). Finally, we also correlated ratings for dominance, as given by IAPS, because we did not evaluate them in our subjects, with fMRI results. We here did not obtain any significant correlating voxels in the right VLPFC, DMPFC, VS/NACC, tectum, and right amygdale, which makes it rather unlikely that modulation of self‐relatedness in these regions was confounded by dominance. Because we here specifically focused on self‐relatedness as distinct and overlapping with emotion, we did not report those regions that were specifically associated with emotional dimensions and differed between them.
Figure 3.
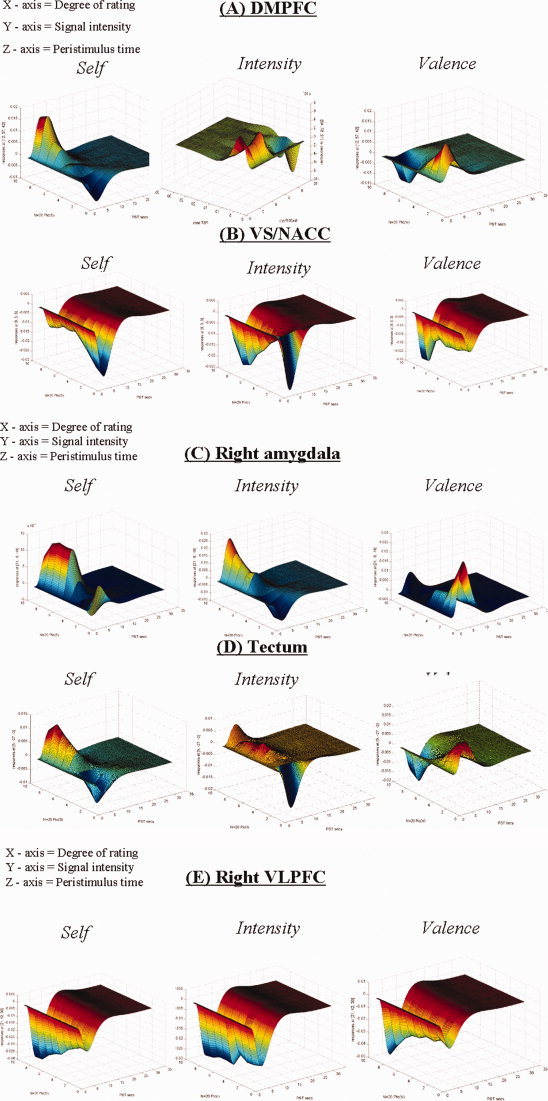
Three‐dimensional representation of parametric correlation of self‐relatedness and emotion dimensions with regional signal changes. The figure shows the three‐dimensional visualization of parametric correlation of self‐relatedness, emotional valence and emotional intensity with signal changes in different regions (A–E). The x‐axis represents the subjective evaluation scores as obtained on the visual‐analog scale ranging from 1 to 9; the y‐axis represents the percent signal changes; and the z‐axis represents the time in seconds. Abbreviations: DMPFC, dorsomedial prefrontal cortex; VLPFC, ventrolateral prefrontal cortex; VS/NACC, ventral striatum/nucleus accumbens. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Comparison of parametric modulation between self‐relatedness and emotion dimensions
To investigate overlapping or dissociable regions between self‐relatedness and emotion dimensions (valence, intensity, dominance), we contrasted effects of self‐relatedness with those of emotional valence and intensity by directly comparing (P < 0.001, uncorrected, k > 10) the respective partial correlation analyses. Self‐relatedness correlated significantly stronger than emotional intensity in the DMPFC, VS/NACC, and the right VLPFC as well as in the posterior cingulate cortex (bordering to the retrosplenium) and some other regions (see Fig. 4A, Supplementary Table II). Self‐relatedness correlated significantly stronger than emotional valence (i.e. high‐, medium‐, and low‐positive valence) in the DMPFC, VS/NACC, right VLPFC, right amygdala, and tectum as well as in the dorsomedial thalamus, and the bilateral parietal cortex (see Fig. 4B, Supplementary Table II).
Figure 4.
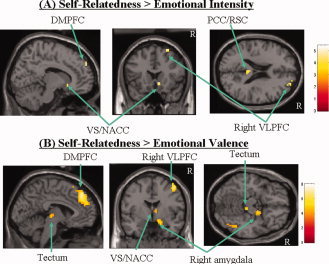
Comparison between parametric correlation maps of self‐relatedness and those of emotion dimensions. The figure shows the comparison of regions that parametrically correlate significantly stronger with the degree of self‐relatedness when compared with the ones for emotion dimensions like emotional intensity (A) and valence (B) (see Supplementary Table II for exact coordinates). Statistical comparisons (P < 0.001 uncorrected with extent threshold k = 10 voxels) were done on the basis of contrasts that relied on partial correlation analyses for all three regressors (self, valence, and intensity). The sagittal images depict the right hemisphere. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
We observed inversely related parametric modulation of neural activity in the DMPFC and the VS/NACC by the degree of self‐relatedness when compared with emotion dimensions like intensity, valence, and dominance. This distinguished the DMPFC and the VS/NACC from other regions like the right amygdala, the right VLPFC, and the tectum that were modulated by both self‐relatedness and emotion dimensions in the same direction. Self‐relatedness thus does not seem to be related directly to affective experience in the DMPFC and the VS/NACC whereas it is clearly related to affect in VLPFC and several lower brain regions that have been implicated in emotional processing [Panksepp, 1998]. In general, our observation of different brain regions that differentially mediate self‐relatedness and emotion dimensions may contribute to a better understanding why our self, though it is strongly and emotionally colored, must be distinguished from emotions.
The DMPFC showed positive correlation with self‐relatedness and negative correlation with emotional intensity and valence. Involvement of the DMPFC in processing self‐relatedness is in accordance with the previous studies showing involvement of the DMPFC in cognitive discrimination and explicit appraisal of self‐relatedness [Gusnard et al., 2001; Johnson et al., 2002; Kelley et al., 2002; Macrae et al., 2004; Moran et al., 2006; Ochsner and Gross, 2005; Phan et al., 2004; Zysset et al., 2002]. Though recent studies [Moran et al., 2006; Phan et al., 2004] demonstrated a similar parametric modulation in the DMPFC by self‐relatedness, these studies presupposed a cognitive component because subjects had to evaluate the stimuli's degree of self‐relatedness during scanning. Our paradigm avoided such cognitive paradigm because subjects had to merely perceive but not judge emotional pictures. Thus, our study avoids the task‐related effects of self‐relatedness and, therefore, complements these findings by demonstrating parametric modulation of DMPFC neural activity by self‐relatedness during mere perception of emotional pictures. The more signal intensities in the DMPFC, the stronger subjects rated the degree of self‐relatedness while rating the same stimuli as less intense and of lower positive emotional valence. Self‐relatedness and emotional dimensions thus seem to be oppositely related to the degree of neural activity in the DMPFC. These findings suggest a differential role of the DMPFC in self‐related and affective processing of positive emotional valence. Based on the earlier findings (see above) and a recent review [Ochsner and Gross, 2005], the DMPFC is assumed to be involved in explicitly discriminating and appraising self‐related stimuli and consequently becoming aware of its degree of self‐relatedness resulting in what may be called self‐consciousness (see also Northoff and Bermpohl [2004] and Northoff et al. [2006]). Previous studies suggest that higher self‐relatedness of a stimulus requires more explicit discrimination and appraisal of self‐relatedness and may therefore lead to higher self‐consciousness which may, in turn, induce higher activity in the DMPFC [Gusnard et al., 2001; Kelley et al., 2002; Mitchell et al., 2005; Ochsner et al., 2004; Phan et al., 2004; Schmitz et al., 2004]. However, this assumption is not consistent with the results of Moran et al. [2006]. They found an inverted U‐shape curve in reaction time as self‐relatedness increased indicating less explicit discrimination in high self‐relatedness. This difference may be related to the different paradigms applied by Moran et al. [2006] and the present study. With regard to Moran et al. [2006] it seems conceivable that stimuli, which are unambiguously self‐relevant or not self‐relevant, are faster to judge than those stimuli, which are partly self‐relevant or partly not self‐relevant as in our study, which may explain the difference in reaction times. Thus, it may be speculated that, although the stimuli were evaluated as less self‐related, more explicit discrimination processing was needed to decide on the self‐relatedness of the stimulus in our study. Therefore, it may be argued that in our study the processing of high self‐related stimuli (without accompanying explicit cognitive decision tasks) may involve more explicit discrimination processing than lower self‐relatedness stimuli. Furthermore, because in our study the subjects were instructed to passively view the pictures, any explicit discrimination processing is not related to an explicit cognitive decision task. However, the exact relationship of self‐related processing and explicit discrimination processing is still unclear so that more studies are needed to further elucidate this point.
Concerning the central role of the DMPFC in self‐relatedness, our findings are in accordance with those by Moran et al. [2006]. We included emotional valence (and emotional intensity) as covariate in correlation analysis with self‐relatedness and yielded the DMPFC; this means that parametric modulation of self‐relatedness in this region cannot be traced back to emotional valence (and intensity). This confirms the findings made by Moran et al. [2006]. The same kind of analysis though in a reverse way was done for emotional valence with self‐relatedness as covariate, which again yielded parametric modulation in the DMPFC though in an opposite direction, for example negative. This second analysis was not done by Moran et al. [2006] because they controlled for emotional valence in a merely categorical way in the design itself, which precluded investigation of parametric modulation of emotional valence with self‐relatedness as covariate. Differences between our and Moran's results may thus, at least in part, be due to methodological differences. Taken together, our findings indicate that the DMPFC parametrically mediates both self‐relatedness and emotional valence (and intensity) though in opposite, that is, positive and negative, ways. We interpret this finding in a two‐fold way. (i) The DMPFC may be crucially involved in mediating self‐relatedness thus confirming Moran's findings. (ii) As being crucially involved in self‐relatedness, the DMPFC needs to distinguish self‐relatedness from emotion dimensions (valence, intensity) for which our data suggest the mechanisms of opposite direction in parametric modulation. The latter finding may be considered to differ from Morans' results who only observed self‐relatedness, but not emotional valence to be associated with the DMPFC. In addition to the above‐mentioned methodological difference, this discrepancy could also in part be due to the inclusion of a cognitive‐evaluative component in the fMRI task in Morans' study, which may have down‐modulated possible emotional processing in the DMPFC (see Panksepp [1998]). Our study, in contrast, did not include such cognitive‐evaluative component in fMRI, which indeed has been shown to down‐modulate neural activity in the DMPFC (and other regions) when compared with mere emotional perception [Grimm et al., 2006; Taylor et al., 2003]. Functionally, our findings suggest that the role of the DMPFC in self‐related processing cannot solely consist in the explicit appraisal and evaluation of self‐relatedness. Interestingly, we observed opposite relationship between self‐elatedness and emotional intensity. In contrast to self‐relatedness, we observed that higher degrees of intensity were rather associated with relatively low neural activity in the DMPFC. The observation of opposite modulation of emotional intensity and self‐relatedness is even more remarkable when one considers the fact that, on the behavioral level, both parameters correlated positively with each other. Psychologically, such neural dissociation between self and emotion may contribute to the lay persons' ability to dissociate self and emotion and the cognitive neuroscientists' functional distinction between self‐relatedness and emotion dimensions.
As in the DMPFC, neural activity of the VS/NACC showed positive parametric modulation by self‐relatedness as distinguished from both emotional intensity and valence. The higher the degree of self‐relatedness of a stimulus, the higher the activity induced in the VS/NACC. This is not only in accordance with other recent studies [Lieberman et al., 2004; Phan et al., 2004] but extends them by showing opposite parametric modulation (i.e. positive and negative correlation) of self‐relatedness and emotion dimensions by neural activity in the VS/NACC. Interestingly, the degree of neural activity in the VS/NACC has also been associated with the process of valuing the potential pleasure and/or incentive value of reward stimuli for the organism [Calder et al., 2004; Knutson and Cooper, 2005; Schultz et al., 2003]. Association of the VS/NACC with both self‐relatedness and reward raises the question whether the process of valuing may be an integral aspect of self‐related processing. Valuing establishes the relevance of stimuli for organisms, which first and foremost makes judgment of the stimulus' degree of self‐relatedness possible at all; henceforth the stimulus' reward value must be considered a crucial part of self‐relatedness (see above and Northoff et al. [2006]). If this is true, one would expect comparable involvement of the VS/NACC in both self‐relatedness and reward value. However, no studies directly comparing self‐relatedness and reward value have been reported yet.
In contrast to the DMPFC and the VS/NACC, we observed parametric modulation of neural activity in the right amygdala by both self‐relatedness and emotional intensity in the same direction. This is in accordance with previous findings by Phan et al. [2004], who also showed involvement of this region during judgment of this emotional dimension, as well as with previous studies associating emotional intensity with the amygdala [Anderson et al., 2003; Kim et al., 2003, 2004]. Our results complement these findings by showing a negative correlation with valence and thus an inversed pattern compared with emotional intensity and self‐relatedness in the right amygdala. This reflects not only the general observation that the amygdala is associated with negative emotions [Phan et al., 2002; Wager et al., 2003] but also sheds some light on the relationship between self and emotions. The opposite correlation of self‐relatedness and emotional valence with neural activity in the right amygdala suggests that this region may be involved in modulating the degree of less positive emotional valence (and emotional intensity), that is, attributed to the self: The more the neural activity in the right amygdala, the higher the degrees of self‐relatedness and the less‐positive emotional valence (and emotional intensity) are attributed to the respective stimulus. We therefore would suggest that the right amygdala may be crucially involved in attributing and assigning less‐positive emotional valence and emotional intensity to the self‐related stimuli.
Similar to the right amygdala, other subcortical midline regions like the tectum, the hypothalamus, and the dorsomedial thalamus were also parametrically modulated by both self‐relatedness and emotional intensity in the same direction. The more neural activity was observed in these regions during picture viewing, the more self‐relatedness and the higher emotional intensity was attributed to these pictures. The involvement of subcortical midline regions in self‐relatedness complements the recent neuroimaging studies, which showed more arousal in subcortical regions than the cortical ones [Damasio et al., 2000], a finding that is congruent with abundant animal work [Panksepp, 1998, 2005]. At present, we interpret our results to be most consistent with the view that subcortical regions of the brain are more critically involved in the actual generation of affective experience than neocortical ones, which regulate emotions. As demonstrated in both animal and human studies, these subcortical regions are involved in integrative processing of interoceptive bodily functions and have therefore been associated with what has been called “bodily self” or “proto‐self” [Craig, 2002; Damasio, 1999; Panksepp, 2005].
Another region that showed similar parametric modulation of self and emotion though in a negative direction was the right VLPFC. This region has been associated with explicit discrimination and appraisal of self‐relatedness, especially with self‐awareness of one's own face [Keenan et al., 2000; Platek et al., 2004; Turk et al., 2002, 2003]. Our results complement these findings in two respects. First, we observed parametric modulation of neural activity in this region by the degree of self‐relatedness. Second, we observed analogous parametric modulation of self‐relatedness and emotion dimensions. Hence, it is worth considering that this region may be important in recognizing and becoming aware of self‐related stimuli by means of their emotional valence and intensity.
Methodologically, a central issue is how to conceptualize self‐relatedness in a standard manner. Self‐relatedness may be understood differently by different people. We tried to adjust for such interpretive difference by giving explicit instructions (see methods) on how the concept was to be applied. Moreover, we have now investigated self‐relatedness of IAPS pictures in this manner in several groups of subjects and have found no significant differences between groups in our behavioral measure of self‐relatedness. Though this does not validate our self‐relatedness ratings procedures, it does indicate that people may be conceptualizing the measure in the same way. What needs to be done in the future is to establish other equally reliable rating instruments for self‐relatedness, and assess how they relate to each other. Another issue is that self‐relatedness may involve different psychological processes like explicit appraisal, representation, constitution, and monitoring of self‐relatedness, which may have not been fully controlled for during both scanning and post hoc tests. Future imaging studies of self‐relatedness should not only seek to go beyond mere attempts to discriminate between levels of self‐relatedness of stimuli but also to evaluate the extent to which certain stimuli have other kinds of personal meaning, including job‐related issue, culture‐related ones, and perhaps matters of personal beliefs and philosophies. There are many distinct ways in which individuals may feel connected to stimuli in the world in self‐referential ways. The current approach investigating self‐relatedness in the context of dimensional emotional evaluations might be considered only a first step into the psychological complexities that emerge from our many distinct relationships with diverse aspects of the world.
Unlike other studies, we intentionally did not include a strong cognitive task component in our paradigm; we focused on emotional perception rather than on discrimination and explicit appraisal of self‐relatedness requiring self‐consciousness or self‐awareness. This may have helped reveal the presently observed linkage of self‐relatedness to neural activity in the subcortical midline regions which otherwise, perhaps in the presence of strong cognitive load, may have been masked. Indeed, we suspect that cognitively induced inhibition of affective processes may have been responsible for the neural dissociation, for example the differential modulation, between self‐relatedness and emotion in the DMPFC and the VS/NACC. If this is true, one may assume that our findings support the recent assumption [Beer, 2007; Moran et al., 2006] of the distinction between affective and cognitive aspects of self‐relatedness by assigning them not only different brain regions but also different ways of modulation. Thus, our study confirms (at least partially) and specifies the findings by Moran et al. [2006]. Unlike Moran et al. [2006] we did not include an explicitly cognitive component, for example a judgment, in our paradigm. Similar to their analysis, we controlled for emotional valence and intensity in our parametric analysis of self‐relatedness by including them as covariates and, like them, yielded the DMPFC as crucial region. Our design also allowed us to make the reverse analysis for emotional valence and intensity, respectively each time including self‐relatedness and the respective other emotion dimension as covariate. As such we could independently map parametric modulation of all three dimensions, self‐relatedness, emotional valence, and emotional intensity onto regional signal changes. This allowed us to investigate and compare their relationship, that is the direction of correlation being either positive or negative, and to reveal similar or different patterns of neural modulation between self‐relatedness and emotion dimensions. Other methodological differences between our study and the one by Moran et al. [2006] consist in the kind of rating dimensions of self‐relatedness (Moran: from 1–4 with categorization into 1/2 and 3/4; we: 1–9 applied in a parametric way without categorization) and the kind of emotion dimensions values (intensity, valence) used for fMRI analyses (Moran relied on the standard norms provided by IAPS whereas we used our subjects' subjective ratings of emotion dimensions for fMRI analysis). Despite these methodological differences, both studies yielded more or less similar results for the parametric modulation of self‐relatedness in the DMPFC.
One may also discuss the need for two different types of analyses, the categorical and the parametric one. We want to point out that the two main types of analyses (categorical and parametric) conducted here must be considered complementary for two reasons. First, results from the first approach, the categorical, can be supported by a second type of analysis, the parametric one, over the same data. This yielded indeed confirmation with regard to the regions being involved in self‐relatedness as was the case for the DMPFC, the right amygdala, the tectum, the ventral striatum, the right dorsomedial thalamus, and the bilateral parietal cortex that showed significant changes in both categorical (Supplementary Table I) and parametric (Supplementary Table II) analyses. Second, the parametric approach provides additional information by showing the direction of signal changes in relation to the continuum of self‐relatedness or emotional valence which in turn can contribute to differentiate both dimensions. For instance, it shows that the DMPFC is activated in both comparisons, high self versus low self picture and emotional versus neutral picture, in the categorical analyses, whereas the parametric analyses allow to further distinguish between both self and emotional valence with regard to the direction of signal change, the self showing positive correlation and emotional valence being modulated in a negative direction; for instance two other regions, the right amygdala and the tectum, that were also activated in both contrasts (self, emotion) in the categorical analyses (see Supplementary Table I), showed similar direction of signal changes in the parametric analyses. The main purpose of the parametric analyses was thus to compare and potentially differentiate the kind of signal changes that were induced by both self and emotion within those regions that were recruited by self‐relatedness, whereas it was not the purpose to reveal those regions in either analysis that were exclusively related with emotional valence itself independent of those regions recruited by self‐relatedness.
Finally, another relevant issue is that in this work we only evaluated linear modulation of brain activity. However, modulation of neural activity may also be in a nonlinear, for example, exponential, U‐shape, or independent ways [Lewis et al., 2006]. We suspect that modulation of self‐relatedness especially in the MOFC might occur nonlinearly and therefore was not covered by linear modulation as applied in our study. Such nonlinear modulation in MOFC is in accordance with previous work [Elliott et al., 2003], and would also be consistent with our observation that we observed involvement of the MOFC in self‐relatedness in the categorical analysis (e.g. in the contrast high vs. low self), whereas it did not appear in parametric correlation analysis. It is also possible that the psychological representation of self‐relatedness could benefit from nonlinear modulation to enable more clear‐cut distinctions between self‐ and nonself‐related stimuli; in this case, one would consequently expect exponential or U‐shape‐like curves signifying the relationship between regional signal changes and the degree of self‐relatedness. However, further studies directly comparing different modes of neural modulation in relation to self‐relatedness would be necessary to evaluate this proposition. It is also possible that commonly used measures of valence and arousal may not cover all the relevant emotional measures. For instance, in this work there was no attempt to monitor the intensity of the many specific emotions that exist in mammalian brains [Panksepp, 1998]. Once that is done, there may be different patterns for different emotions. For instance, it is possible that within the DMPFC and the VS/NACC, which is closely associated with mesolimbic dopamine innervation, self‐relatedness is associated with generalized affective feelings monitored by dimensional tools, but it may not respond in a similar way to different degrees of positive emotional valence. From that perspective, a general principle that may eventually emerge from this kind of work may be that different emotional processes are associated with the experience of self‐relatedness in different regions of the brain.
In summary, we demonstrate differential direction of parametric modulation of neural activity in the DMPFC and the VS/NACC by self‐relatedness and emotion dimensions. Although emotion dimensions (i.e. valence, intensity) were also associated with neural activity in these same brain regions, they were modulated in just the opposite direction. Such differential parametric modulation of self‐relatedness and emotion distinguished the DMPFC and the VS/NACC from other regions like the right amygdala, the right VLPFC and the tectum that were modulated by both self‐relatedness and emotion dimensions in the same direction. This suggests that in many lower predominantly subcortical regions of the brain, a sense of self seems to be more closely related to affective processes than in higher rather cortical brain regions. Our findings may thus contribute to better understand why we subjectively experience our self strongly emotional while, at the same time, at a higher‐order cognitive and more conceptual level we can easily distinguish our self from its emotions as distinct and separate function.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Figure 1a
Figure 1b
Figure 1c
Figure 2a
Figure 2b
Supporting Table 1
Supporting Table 2
Supporting Material results
REFERENCES
- Amodio DM,Frith CD ( 2006): Meeting of minds: The medial frontal cortex and social cognition. Nat Rev Neurosci 7: 268–277. [DOI] [PubMed] [Google Scholar]
- Anderson AK,Christoff K,Stappen I,Panitz D,Ghahremani DG,Glover G,Gabrieli JD,Sobel N ( 2003): Dissociated neural representations of intensity and valence in human olfaction. Nat Neurosci 6: 196–202. [DOI] [PubMed] [Google Scholar]
- Beer JS ( 2007): The default self: Feeling good or being right? Trends Cogn Sci 11: 187–189. [DOI] [PubMed] [Google Scholar]
- Calder AJ,Keane J,Lawrence AD,Manes F ( 2004): Impaired recognition of anger following damage to the ventral striatum. Brain 127: 1958–1969. [DOI] [PubMed] [Google Scholar]
- Craig AD ( 2002): How do you feel? Interoception: The sense of the physiological condition of the body. Nat Rev Neurosci 3: 655–666. [DOI] [PubMed] [Google Scholar]
- D'Argembeau A,Collette F,Van der LM,Laureys S,Del FG,Degueldre C,Luxen A,Salmon E ( 2005): Self‐referential reflective activity and its relationship with rest: A PET study. Neuroimage 25: 616–624. [DOI] [PubMed] [Google Scholar]
- Damasio A ( 1999): The Feeling of What Happens: Body and Emotion in the Making of Consciousness. New York: Harcourt Brace. [Google Scholar]
- Damasio A ( 2003): Mental self: The person within. Nature 423: 227. [DOI] [PubMed] [Google Scholar]
- Damasio AR,Grabowski TJ,Bechara A,Damasio H,Ponto LL,Parvizi J,Hichwa RD ( 2000): Subcortical and cortical brain activity during the feeling of self‐generated emotions. Nat Neurosci 3: 1049–1056. [DOI] [PubMed] [Google Scholar]
- Elliott R,Newman JL,Longe OA,Deakin JF ( 2003): Differential response patterns in the striatum and orbitofrontal cortex to financial reward in humans: A parametric functional magnetic resonance imaging study. J Neurosci 23: 303–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ,Penny W,David O ( 2005): Modeling brain responses. Int Rev Neurobiol 66: 89–124. [DOI] [PubMed] [Google Scholar]
- Frith CD,Frith U ( 2006): The neural basis of mentalizing. Neuron 50: 531–534. [DOI] [PubMed] [Google Scholar]
- Gillihan SJ,Farah MJ ( 2005): Is self special? A critical review of evidence from experimental psychology and cognitive neuroscience. Psychol Bull 131: 76–97. [DOI] [PubMed] [Google Scholar]
- Grimm S,Schmidt CF,Bermpohl F,Heinzel A,Dahlem Y,Wyss M,Hell D,Boesiger P,Boeker H,Northoff G ( 2006): Segregated neural representation of distinct emotion dimensions in the prefrontal cortex‐an fMRI study. Neuroimage 30: 325–340. [DOI] [PubMed] [Google Scholar]
- Gusnard DA,Akbudak E,Shulman GL,Raichle ME ( 2001): Medial prefrontal cortex and self‐referential mental activity: Relation to a default mode of brain function. Proc Natl Acad Sci USA 98: 4259–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC,Baxter LC,Wilder LS,Pipe JG,Heiserman JE,Prigatano GP ( 2002): Neural correlates of self‐reflection. Brain 125: 1808–1814. [DOI] [PubMed] [Google Scholar]
- Kalisch R,Wiech K,Herrmann K,Dolan RJ ( 2006): Neural correlates of self‐distraction from anxiety and a process model of cognitive emotion regulation. J Cogn Neurosci 18: 1266–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan JP,Wheeler MA,Gallup GG Jr,Pascual‐Leone A ( 2000): Self‐recognition and the right prefrontal cortex. Trends Cogn Sci 4: 338–344. [DOI] [PubMed] [Google Scholar]
- Kelley WM,Macrae CN,Wyland CL,Caglar S,Inati S,Heatherton TF ( 2002): Finding the self? An event‐related fMRI study. J Cogn Neurosci 14: 785–794. [DOI] [PubMed] [Google Scholar]
- Kim H,Somerville LH,Johnstone T,Alexander AL,Whalen PJ ( 2003): Inverse amygdala and medial prefrontal cortex responses to surprised faces. Neuroreport 14: 2317–2322. [DOI] [PubMed] [Google Scholar]
- Kim H,Somerville LH,Johnstone T,Polis S,Alexander AL,Shin LM,Whalen PJ ( 2004): Contextual modulation of amygdala responsivity to surprised faces. J Cogn Neurosci 16: 1730–1745. [DOI] [PubMed] [Google Scholar]
- Knutson B,Cooper JC ( 2005): Functional magnetic resonance imaging of reward prediction. Curr Opin Neurol 18: 411–417. [DOI] [PubMed] [Google Scholar]
- Lambie JA,Marcel AJ ( 2002): Consciousness and the varieties of emotion experience: A theoretical framework. Psychol Rev 109: 219–259. [DOI] [PubMed] [Google Scholar]
- LeDoux J ( 2002): The Synaptic Self: How Our Brain Become Who we Are? New York: Viking. [Google Scholar]
- Lewis PA,Critchley HD,Rotshtein P,Dolan RJ ( 2006): Neural correlates of processing valence and arousal in affective words. Cereb Cortex 17: 742–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD,Jarcho JM,Satpute AB ( 2004): Evidence‐based and intuition‐based self‐knowledge: An FMRI study. J Pers Soc Psychol 87: 421–435. [DOI] [PubMed] [Google Scholar]
- Macrae CN,Moran JM,Heatherton TF,Banfield JF,Kelley WM ( 2004): Medial prefrontal activity predicts memory for self. Cereb Cortex 14: 647–654. [DOI] [PubMed] [Google Scholar]
- Mitchell JP,Banaji MR,Macrae CN ( 2005): The link between social cognition and self‐referential thought in the medial prefrontal cortex. J Cogn Neurosci 17: 1306–1315. [DOI] [PubMed] [Google Scholar]
- Moran JM,Macrae CN,Heatherton TF,Wyland CL,Kelley WM ( 2006): Neuroanatomical evidence for distinct cognitive and affective components of self. J Cogn Neurosci 18: 1586–1594. [DOI] [PubMed] [Google Scholar]
- Northoff G,Bermpohl F ( 2004): Cortical midline structures and the self. Trends Cogn Sci 8: 102–107. [DOI] [PubMed] [Google Scholar]
- Northoff G,Heinzel A,de GM,Bermpohl F,Dobrowolny H,Panksepp J ( 2006): Self‐referential processing in our brain—A meta‐analysis of imaging studies on the self. Neuroimage 31: 440–457. [DOI] [PubMed] [Google Scholar]
- Ochsner KN,Feldmann Barrett L ( 2002): A multiprocess perspective on the neuroscience of emotion In: Mayne TJ,Bonanno GA, editors. Emotions: Currrent Issues and Future Directions. New York: Guilford; pp 38–81. [Google Scholar]
- Ochsner KN,Gross JJ ( 2005): The cognitive control of emotion. Trends Cogn Sci 9: 242–249. [DOI] [PubMed] [Google Scholar]
- Ochsner KN,Knierim K,Ludlow DH,Hanelin J,Ramachandran T,Glover G,Mackey SC ( 2004): Reflecting upon feelings: An fMRI study of neural systems supporting the attribution of emotion to self and other. J Cogn Neurosci 16: 1746–1772. [DOI] [PubMed] [Google Scholar]
- Ochsner KN,Beer JS,Robertson ER,Cooper JC,Gabrieli JD,Kihsltrom JF,D'Esposito M ( 2005): The neural correlates of direct and reflected self‐knowledge. Neuroimage 28: 797–814. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Panksepp J ( 1998): Affective Neuroscience: The Foundations of Human and Animal Emotions. New York: Oxford University Press. [Google Scholar]
- Panksepp J ( 2003): At the interface of the affective, behavioral, and cognitive neurosciences: Decoding the emotional feelings of the brain. Brain Cogn 52: 4–14. [DOI] [PubMed] [Google Scholar]
- Panksepp J ( 2005): Affective consciousness: Core emotional feelings in animals and humans. Conscious Cogn 14: 30–80. [DOI] [PubMed] [Google Scholar]
- Phan KL,Wager T,Taylor SF,Liberzon I ( 2002): Functional neuroanatomy of emotion: A meta‐analysis of emotion activation studies in PET and fMRI. Neuroimage 16: 331–348. [DOI] [PubMed] [Google Scholar]
- Phan KL,Taylor SF,Welsh RC,Ho SH,Britton JC,Liberzon I ( 2004): Neural correlates of individual ratings of emotional salience: A trial‐related fMRI study. Neuroimage 21: 768–780. [DOI] [PubMed] [Google Scholar]
- Platek SM,Keenan JP,Gallup GG Jr,Mohamed FB ( 2004): Where am I? The neurological correlates of self and other. Brain Res Cogn Brain Res 19: 114–122. [DOI] [PubMed] [Google Scholar]
- Satpute AB,Lieberman MD ( 2006): Integrating automatic and controlled processes into neurocognitive models of social cognition. Brain Res 1079: 86–97. [DOI] [PubMed] [Google Scholar]
- Schmitz TW,Kawahara‐Baccus TN,Johnson SC ( 2004): Metacognitive evaluation, self‐relevance, and the right prefrontal cortex. Neuroimage 22: 941–947. [DOI] [PubMed] [Google Scholar]
- Schore AN ( 2003): Affect Regulation and the Repair of the Self. New York: Norton. [Google Scholar]
- Schultz W,Tremblay L,Hollerman JR ( 2003): Changes in behavior‐related neuronal activity in the striatum during learning. Trends Neurosci 26: 321–328. [DOI] [PubMed] [Google Scholar]
- Taylor SF,Phan KL,Decker LR,Liberzon I ( 2003): Subjective rating of emotionally salient stimuli modulates neural activity. Neuroimage 18: 650–659. [DOI] [PubMed] [Google Scholar]
- Turk DJ,Heatherton TF,Kelley WM,Funnell MG,Gazzaniga MS,Macrae CN ( 2002): Mike or me? Self‐recognition in a split‐brain patient. Nat Neurosci 5: 841–842. [DOI] [PubMed] [Google Scholar]
- Turk DJ,Heatherton TF,Macrae CN,Kelley WM,Gazzaniga MS ( 2003): Out of contact, out of mind: The distributed nature of the self. Ann N Y Acad Sci 1001: 65–78. [DOI] [PubMed] [Google Scholar]
- Vogeley K,May M,Ritzl A,Falkai P,Zilles K,Fink GR ( 2004): Neural correlates of first‐person perspective as one constituent of human self‐consciousness. J Cogn Neurosci 16: 817–827. [DOI] [PubMed] [Google Scholar]
- Wager TD,Phan KL,Liberzon I,Taylor SF ( 2003): Valence, gender, and lateralization of functional brain anatomy in emotion: A meta‐analysis of findings from neuroimaging. Neuroimage 19: 513–531. [DOI] [PubMed] [Google Scholar]
- Zysset S,Huber O,Ferstl E,von Cramon DY ( 2002): The anterior frontomedian cortex and evaluative judgment: An fMRI study. Neuroimage 15: 983–991. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Figure 1a
Figure 1b
Figure 1c
Figure 2a
Figure 2b
Supporting Table 1
Supporting Table 2
Supporting Material results


