Abstract
Recent studies on spontaneous fluctuations in the functional MRI blood oxygen level‐dependent (BOLD) signal in awake healthy subjects showed the presence of coherent fluctuations among functionally defined neuroanatomical networks. However, the functional significance of these spontaneous BOLD fluctuations remains poorly understood. By means of 3 T functional MRI, we demonstrate absent cortico‐thalamic BOLD functional connectivity (i.e. between posterior cingulate/precuneal cortex and medial thalamus), but preserved cortico‐cortical connectivity within the default network in a case of vegetative state (VS) studied 2.5 years following cardio‐respiratory arrest, as documented by extensive behavioral and paraclinical assessments. In the VS patient, as in age‐matched controls, anticorrelations could also be observed between posterior cingulate/precuneus and a previously identified task‐positive cortical network. Both correlations and anticorrelations were significantly reduced in VS as compared to controls. A similar approach in a brain dead patient did not show any such long‐distance functional connectivity. We conclude that some slow coherent BOLD fluctuations previously identified in healthy awake human brain can be found in alive but unaware patients, and are thus unlikely to be uniquely due to ongoing modifications of conscious thoughts. Future studies are needed to give a full characterization of default network connectivity in the VS patients population. Hum Brain Mapp, 2009. © 2009 Wiley‐Liss, Inc.
Keywords: functional MRI, consciousness, resting state, vegetative state, default network
INTRODUCTION
During performance of a wide range of attention‐demanding cognitive tasks, two opposite types of responses are commonly observed. A given set of lateral frontal and parietal cortical regions (the so‐called ‘task‐positive network’) commonly exhibit activity increases (Fox et al., 2005). In contrast, a different set of regions, including posterior cingulate/precuneus (PCC), medial prefrontal cortex (MPF), temporoparietal junctions (TPJ), inferior temporal (IT) and parahippocampal (PH) cortices, routinely exhibit decreases in activity (Gusnard and Raichle, 2001). These latter areas were referred to as the ‘default network’ or ‘task‐negative network.’ While lateral frontoparietal regions have been related to attention to external world, the default network has been suggested to be involved in various aspects of self‐referential processing (Fox et al., 2005), inner or task‐unrelated thoughts (McKiernan et al., 2006), self‐reflective thinking in terms of planning for the future or simulation of behavior (Ingvar, 1979).
In recent years, brain activity fluctuations in the default resting state have received increasing interest. Even in the absence of sensory inputs, structured patterns of ongoing spontaneous activity can be observed in cortical and thalamic neurons (Kenet et al., 2003; Steriade et al., 1993; Tsodyks et al., 1999). Resting state functional connectivity studies using functional magnetic resonance imaging (fMRI) usually examine correlations in slow (0.1 Hz) spontaneous fluctuations in the blood oxygen level‐dependent (BOLD) signal (Cordes et al., 2001). The collective result of these studies is that regions similarly modulated by tasks or stimuli tend to exhibit correlated spontaneous fluctuations even in the absence of tasks or stimuli. In particular, several teams identified reproducible correlated BOLD fluctuations within the default network in resting healthy awake volunteers (Fox et al., 2005; Fransson, 2005; Greicius et al., 2003; Laufs et al., 2003). On the other hand, several independent studies (Fox et al., 2005; Fransson, 2005) recently showed that the dichotomy between lateral frontoparietal areas and the default network, observed in a wide range of cognitive tasks, is represented intrinsically in the resting human brain, in the absence of any overt task or behavior. However, the functional significance of these coherently distributed brain activity fluctuations remains largely unknown.
We hypothesized that system‐wide coherent BOLD fluctuations, if distinctively related to conscious thoughts, would not be present in well‐documented unaware patients. Vegetative state (VS) is defined by a total lack of awareness of self or of the environment, despite preserved arousal (Laureys et al., 2004). To test our hypothesis, we thus searched for the presence of BOLD functional connectivity among default network areas in a typical VS patient, using a validated correlational approach (Fox et al., 2005; Vincent et al., 2007). This study does not attempt to give a full account of the characteristics of resting state networks in VS patients at the population level. However, in our view, the presence of system‐wide coherent spontaneous BOLD oscillations in one single well‐documented VS patient would be sufficient to challenge the view that these fluctuations uniquely reflect the presence of conscious thoughts. To test for the specificity of our findings, we also present results of this approach obtained in a brain dead (BD) patient.
METHODS
Healthy Controls and Ethics
Six age‐matched healthy subjects [age (mean ± SD) 41 ± 11 years, range 26–54 years] were recruited from Liege University. None of the subjects had any neurological or psychiatric disease history. The study was approved by the Ethics Committee of the Faculty of Medicine of the University of Liège. Informed consent was obtained from all healthy subjects and from the people having legal responsibility for the patients.
VS Patient
A 48‐year‐old man was found comatose (score of 3 at the Glasgow Coma Scale) with cardio‐respiratory arrest following food airway obstruction and asphyxiation and was successfully resuscitated. Time elapsed between collapse and start of cardio‐respiratory resuscitation was estimated superior to 10 min. After resuscitation, the patient showed myoclonic status epilepticus. Following withdrawal of sedation, he recovered spontaneous breathing and eye opening, but failed to show any sign of volitional motor response. EEG showed a nonreactive low‐voltage delta activity with discontinuous generalized epileptiform activity. Somatosensory‐evoked potentials detected peripheral (brachial plexus) and lemniscal brainstem (P14), but no cortical (N20) responses. MRI showed a diffuse cortico‐subcortical atrophy and secondary hydrocephalus without focal lesion.
We examined the patient two and a half years after his cardio‐respiratory arrest. Repeated behavioral evaluations by experienced clinicians (MB, AV, CS, CB and SL) confirmed the diagnosis of VS as defined by currently accepted criteria (Bates, 2005; Giacino et al., 2002; The Multi‐Society Task Force on PVS, 1994). Employed videotaped, validated and standardized ‘consciousness scales’ included the Revised Coma Recovery Scale (CRS‐R) (Giacino et al., 2004), the Sensory Modality Assessment and Rehabilitation Technique (SMART) (Gill‐Thwaites and Munday, 2004), the Wessex Head Injury Matrix (WHIM) (Majerus and Van der Linden, 2000), the Western Neuro Sensory Stimulation Profile (WNSSP) (Ansell and Keenan, 1989) and the Glasgow–Liège Scale (GLS) (Born et al., 1985).
[18F]fluorodeoxyglucose‐PET, performed as described elsewhere (Laureys et al., 2000a), showed a massive and global decrease of cerebral metabolic rates for glucose. Statistical parametric mapping analysis (Laureys et al., 1999a), comparing FDG‐PET data from the patient to 40 healthy age‐matched controls, identified the most metabolically impaired areas in widespread frontoparietal polymodal associative regions (bilateral dorsolateral prefrontal, parieto‐ temporal and posterior cingulate, precuneal cortices)—as previously observed in VS (Laureys et al., 2004). Relatively preserved areas were identified in the brainstem (encompassing the pedunculopontine reticular formation), the hypothalamus and the basal forebrain—allowing for preserved arousal and autonomic functions (Laureys et al., 2004).
At the time of fMRI scanning, the patient showed spontaneous eye opening, preserved brainstem reflexes, oral reflexive movements and auditory and visual startle responses. He showed present grasp, palmomental and sucking reflexes; spastic quadriparesis with bilateral pyramidal signs and no spontaneous limb movements and flexion withdrawal to noxious stimuli. The patient failed to show any vocalization or sign of verbal or nonverbal intentional communication. Cognitive event‐related potentials to the patient's own as compared to other names, performed as described elsewhere (Perrin et al., 2006), repeatedly failed to elicit a P3 response. The patient failed to show volitional brain activity as tested on two occasions in an active fMRI paradigm [as described by Boly et al. (2007b) and Owen et al. (2006)]. No clinical recovery was observed at this day, 12 months after fMRI scanning.
Brain Dead Patient
A 50‐year‐old woman was admitted stuporous in the intensive care unit due to massive cranial hemorrhage and evolved to a comatose state with areactive mydriasis 2 days later. Computed tomography showed major cerebral hemorrhage accompanied by a severe vasospasm. Cerebral arteriography showed an aneurysm on the anterior communicant artery with signs of recent blooding. At the time of fMRI scanning, without any sedation, neither any evidence for electrolyte disturbance, endocrine disturbance or hypothermia, the patient showed a nonresponsive coma with confirmed diagnosis of brain death as defined by Wijdicks (1995). An apnoea test confirmed the diagnosis (Wijdicks, 2001). Electroencephalogram showed an isoelectric pattern, using the recording methodology described elsewhere (American Electroencephalographic Society, 1994). Magnetic resonance anatomical sequences showed a diffuse brain swelling consecutive to the vasospasm, with diffuse white matter damage and hemorrhagic sequela predominant in the medial frontal lobe. Cerebral MR angiography confirmed the absence of intracranial arterial flow.
Functional Imaging Acquisition
VS patient and controls functional MRI time series were acquired using a 3 T MR scanner (Allegra, Siemens, Germany). Three hundred and fifty‐five (355) to Three hundred and seventy‐five (375) multislice T2*‐weighted fMRI images were obtained with a gradient echo‐planar sequence using axial slice orientation (32 slices; voxel size = 3.4 × 3.4 × 3 mm3; matrix size = 64 × 64 × 32; repetition time = 2,460 ms; echo time = 40 ms; flip angle = 90°; field of view = 220 mm). BD patient fMRI data were acquired on a 1.5 T MR scanner (Siemens, Germany). Two hundred (200) multislice T2*‐weighted fMRI images were obtained with a gradient echo‐planar sequence using axial slice orientation (36 slices; voxel size = 3.75 × 3.75 × 3.6 mm3; matrix size = 64 × 64 × 36; repetition time = 3,000 ms; echo time = 30 ms; flip angle = 90°; field of view = 240 mm). Anatomical images were obtained by using a T1‐weigthed 3D MP‐RAGE sequence for each subject. A standard head coil was used in the data acquisition. Monitoring of vital parameters (electrocardiogram, blood pressure, O2 saturation, respiratory rate and PCO2 capnography) were performed in patients by a senior anesthesiologist throughout the experiment.
Data Analysis
Functional data were preprocessed and analyzed using Statistical Parametric Mapping (version SPM5; Wellcome Department of Imaging Neuroscience, London, UK; http://www.fil.ion.ucl.ac.uk/spm). The first three fMRI volumes were removed to allow for signal equilibration. Preprocessing steps included realignment and adjustment for movement‐related effects (Friston et al., 1995), spatial normalization into standard stereotactic MNI space (Friston et al., 1995) and spatial smoothing using a Gaussian kernel of 12 mm full width at half maximum (FWHM). Data were then temporally band‐passed filtered (0.009–0.08 Hz) using a Gaussian temporal filter (FSL software, version 3.2, Oxford Centre for Functional Magnetic Resonance Imaging of the Brain, Oxford, UK, http://www.fmrib.ox.ac.uk/fsl). The first eigenvariate of the time courses of voxels in a seed region of interest located in the PCC [6‐mm sphere centered on coordinates (x = −5 mm, y = −49 mm and z = 40 mm) from Fox et al. (2005)] was then extracted in each subject using SPM5. In each subject, similar time course extractions were performed for a white matter and a ventricle 6 mm radius voxel of interest. The two latter time courses, the global brain signal changes across time and their derivatives were then used as confound in the statistical model. The movement parameters and their derivatives were further added as regressors of no interest in the design matrix. Serial correlations were then estimated with a restricted maximum likelihood algorithm using an intrinsic autoregressive model during parameter estimation. The effects of interest were tested by linear contrasts, generating statistical parametric maps [SPM(T)] in each subject. Two contrast images were computed in each subject, respectively, identifying regions significantly correlated or anticorrelated to PCC after removal of the sources of spurious variance cited above. Results in patients were assessed at the single‐subject level. In healthy subjects, individual summary statistics images were entered in a second‐level analysis, corresponding to a random effects model in which subjects are considered random variables. A complementary random effect analysis compared correlations and anticorrelations to PCC in VS patient versus healthy controls. Patients statistical maps were masked inclusively with controls results (uncorrected P value < 0.01) for spatial comparison to controls' data. In controls, the random effect group analysis was thresholded at false discovery rate (FDR)‐corrected P value <0.05 at the whole‐brain level or (for anticorrelations) in a 10‐mm radius spherical small volume around a priori coordinates taken from previous studies (Fox et al., 2005; Fransson, 2005). In patients, results were thresholded at whole‐brain FDR‐corrected P value <0.05. All results were thresholded at a 10 voxels cluster extent.
RESULTS
Healthy controls showed significant positive correlations between PCC and cortical default network areas (i.e., MPF, TPJ, IT, PH) and with medial thalamus. Our VS patient showed residual long‐range cortico‐cortical functional connectivity with all cortical areas identified in controls but not with the thalamus (Table I, Fig. 1). The whole default network connectivity was found to be significantly reduced in VS as compared to controls. In both healthy controls and the VS patient, a number of areas previously reported as belonging to the ‘task‐positive network’ (i.e. lateral frontoparietal cortices, frontal eye field, inferior temporal/MT, supplementary motor area (SMA) and preSMA, premotor cortex, insula, inferior frontal cortex) showed significant anticorrelated BOLD activity compared to the PCC (Table II, Fig. 2). However, these anticorrelations were significantly reduced in the VS patient as compared to controls. Our BD patient did not show any long‐distance functional connectivity; correlations were only found locally, in the PCC voxel of interest (peak at MNI coordinates x = −6 mm, y = −46 mm, z = 42 mm, Fig. 1). No significant anticorrelations could be identified in the BD patient.
Table I.
Peak areas of significance for default network connectivity in healthy controls and in vegetative state patient
| Brain area | x | y | z | Z value | FDR‐corrected P |
|---|---|---|---|---|---|
| Healthy controls | |||||
| PCC/Precuneusa | 2 | −56 | 30 | 4.96 | 0.013 |
| MPF/SFSa | 2 | 62 | −2 | 5.01 | 0.013 |
| −4 | 50 | 34 | 3.94 | 0.015 | |
| Left TPJa | −48 | −66 | 48 | 5.42 | 0.006 |
| Right TPJa | 62 | −58 | 28 | 3.20 | 0.028 |
| Left PHa | −24 | −34 | −28 | 3.33 | 0.024 |
| Temporal cortexa | 54 | −10 | −30 | 4.70 | 0.013 |
| Medial thalamusa | −8 | −16 | 10 | 3.46 | 0.021 |
| Vegetative state patient | |||||
| PCC/Precuneus | −10 | −72 | 24 | Inf | <0.001 |
| MPFC/SFS | 8 | 48 | −20 | Inf | <0.001 |
| 4 | 52 | 28 | Inf | <0.001 | |
| Left TPJ | −52 | −58 | 42 | Inf | <0.001 |
| Right TPJ | 48 | −58 | 30 | Inf | <0.001 |
| Left PH | −16 | −34 | 0 | 6.26 | <0.001 |
| Temporal cortex | −62 | −38 | −2 | 7.02 | <0.001 |
Results thresholded at whole brain FDR‐corrected P value <0.05.
FDR, false discovery rate; PCC, posterior cingulate cortex; MPF, medial prefrontal cortex; SFS, superior frontal sulcus; TPJ, temporo‐parietal junction; PH, parahippocampal gyrus.
Areas less correlated to PCC in vegetative state patient compared to controls.
Figure 1.
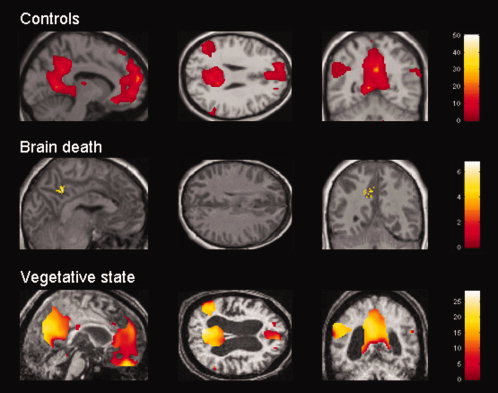
Positive correlations with posterior cingulate/precuneus BOLD activity in healthy controls, brain dead and vegetative state patients. Results thresholded for display at uncorrected P < 0.01 in random effect group analysis in healthy controls, and at FDR‐corrected P value <0.05 in patients. MNI coordinates of sections are as follows: x = −10 mm for controls, −4 mm for BD patient and 2 mm for VS patient, y = −56 mm, z = 26 mm. Note that there is no residual long‐range functional connectivity in the brain dead patient. In the VS patient, despite residual functional connectivity within the default network, the observed correlations were significantly reduced compared to controls. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Table II.
Peak cortical areas of significance for anticorrelations with posterior cingulate cortex/ precuneus—the so‐called ‘task‐positive network’—common to healthy controls and vegetative state patient
| Brain area | x | y | z | Z value | FDR‐corrected P |
|---|---|---|---|---|---|
| Healthy controls | |||||
| DLPFCa | −40 | 44 | 24 | 5.06 | 0.015 |
| 40 | 38 | −4 | 3.34 | 0.023* | |
| FEF/preCSa | −14 | −20 | 70 | 5.15 | 0.013 |
| Premotor cortexa | 20 | −2 | 66 | 4.55 | 0.047 |
| SMA/preSMAa | 8 | 10 | 52 | 3.75 | 0.012* |
| Inferior parietal cortexa | −62 | −36 | 28 | 3.40 | 0.013* |
| 44 | −38 | 52 | 4.00 | 0.011* | |
| Posterior parietal cortexa | −20 | −56 | 62 | 3.74 | 0.014* |
| Inferior temporal/MTa | 50 | −58 | −8 | 3.99 | 0.010* |
| Inferior occipitala | −34 | −84 | −4 | 3.79 | 0.038* |
| Insula/IFGa | −42 | 18 | 0 | 4.62 | 0.044 |
| 54 | 10 | 6 | 3.37 | 0.026* | |
| Vegetative state patient | |||||
| DLPFC | −40 | 50 | 20 | Inf | <0.001 |
| 36 | 58 | 6 | Inf | <0.001 | |
| FEF/preCS | −14 | −10 | 70 | Inf | <0.001 |
| Premotor cortex | 12 | −2 | 68 | Inf | <0.001 |
| SMA/preSMA | 6 | 4 | 50 | Inf | <0.001 |
| Inferior parietal cortex | 40 | −44 | 50 | 3.56 | 0.001 |
| Posterior parietal cortex | −22 | −50 | 62 | 2.71 | 0.009 |
| Inferior temporal/MT | 48 | −50 | −22 | Inf | <0.001 |
| Insula/IFG | −42 | 24 | −2 | Inf | <0.001 |
| 48 | 4 | −2 | Inf | <0.001 |
Results thresholded at P value <0.05 corrected for FDR at the whole brain level, or in a small volume around a priori coordinates (*).
FDR, false discovery rate; DLPFC, dorsolateral prefrontal cortex; FEF, frontal eye field; preCS, precentral sulcus; SMA, supplementary motor area; MT, visual motion area; IFG: inferior frontal gyrus.
Areas less anticorrelated to posterior cingulate cortex/precuneus in vegetative state patient as compared to controls.
Figure 2.
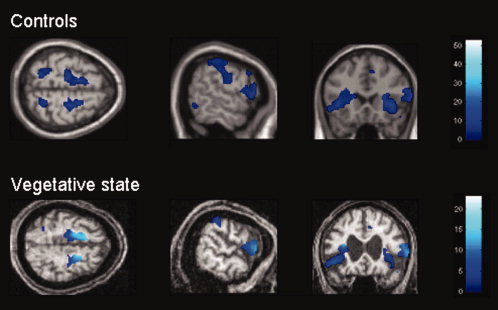
Anticorrelations with posterior cingulate/precuneus in healthy controls and in VS patient. For display purposes, results are thresholded at uncorrected P < 0.01 in healthy controls, and at FDR‐corrected P < 0.05 in VS patient. MNI coordinates of sections are z = 60 mm, x = 62 mm and y = 20 mm. Note that the observed residual connectivity was significantly reduced in VS patient compared to controls. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
Our VS patient fulfills all the behavioral criteria for VS, as documented by repeated and exhaustive behavioral assessments using a (videotaped) battery of internationally validated scales, properly aiming at detecting nonreflexive behaviors in noncommunicative patients (Laureys et al., 2005; Majerus et al., 2005). FDG‐PET showed a diffuse metabolic impairment in frontoparietal associative networks with preserved brainstem, hypothalamus and the basal forebrain as classically reported in vegetative patients (Laureys et al., 1999a, b, 2004). Repeated fMRI assessment failed to demonstrate signs of awareness employing mental imagery paradigms as described elsewhere (Boly et al., 2007b; Owen et al., 2006). The patient suffered from a well‐documented anoxic encephalopathy resulting in a VS enduring for 2.5 years at the time of fMRI. Given the etiology and the very long time interval, the chances of recovery of consciousness are in this case virtually inexistent, and the patient's condition can be regarded as permanent VS (The Multi‐Society Task Force on PVS, 1994).
Taken together, we consider this case as an extremely well‐documented VS and hence ideal to test our hypothesis that persistence of intrinsically functionally connected networks—if observed—are not necessarily reflecting a default brain state related to consciousness. Our fMRI data indeed showed not only significant remaining correlations between distant default network areas, but also anticorrelations between this default network and a set of regions formerly involved in the ‘task‐positive network’ (Fox et al., 2005; Fransson, 2005) in healthy volunteers. The reported anticorrelations with the default mode network were here calculated taking into account average brain signal as a regressor as reported elsewhere (Fox et al., 2005). It should however be stressed that this methodology is not yet generally accepted and could introduce artifactual anticorrelations. Taken as a whole, our findings reject the interpretation that spontaneous slow fluctuations in the fMRI BOLD uniquely reflect ongoing interoceptive state‐of‐minds (Fransson, 2005). On the contrary, dynamic intrinsically correlated brain fluctuations appear probably necessary, but not sufficient to support consciousness. In other words, though these fluctuations certainly modulate our perception of external and internal world, their presence or absence cannot be indicative of the presence of absence of awareness, i.e. spontaneous trains of thoughts.
Our findings are in line with recent data showing preserved default network connectivity in healthy humans during light sleep (Horovitz et al., 2007), during conscious sedation (Greicius et al., 2008), in the human infant brain (Fransson et al., 2007) and in nonhuman primates during general anesthesia (Vincent et al., 2007). The described preservation of functional connectivity in the absence of consciousness could be seen as reflecting preserved anatomical connections dissociated from higher cognitive functions. It could also be argued that the temporal dynamics of our ‘conscious stream of consciousness’ [classically considered around 500 ms (Libet, 2006)] are much faster than the slow fMRI BOLD oscillations occurring at around 10 s time period here observed. On the other hand, we show that both correlations and anticorrelations with PCC are reduced in the VS patient as compared to age‐matched controls. The observed reduced functional connectivity within the default network in VS is in line with the hypothesis that spontaneous fMRI signal changes may be partly related to ongoing interoceptive state‐of‐minds or conscious thoughts. It is possible that there exist several contributors to spontaneous signal fluctuations in the default mode, where some of these sources might reflect conscious thought processes, whereas others originate from unconscious processes. In the same line, several studies suggest that spontaneous signal fluctuations in the default‐mode network reorganize during the performance of a working memory task and are modulated in response to external work load (Esposito et al., 2006; Fransson, 2006). Moreover, the idea that spontaneous fMRI fluctuations is multilayered from a cognitive point of view has gained some further support by recent results that have shown that spontaneous baseline brain activity fluctuations seem also capable to influence human perception (Boly et al., 2007a) and behavior (Fox et al., 2007). Finally, the observed absence of cortico‐thalamic functional connectivity in our VS patient stresses the importance of cortico‐ thalamo‐cortical interaction in the emergence of consciousness (Dehaene and Changeux, 2005; Laureys et al., 2000b; Tononi, 2004; White and Alkire, 2003).
Our BD patient fullfills all the standard clinical criteria for brain death (Wijdicks, 2001), and this diagnostic was confirmed by paraclinical examinations. fMRI results did not show any long‐range significant functional connectivity. This could be expected, as there is no neuronal activity left in BD brains. It should be noted that in the BD patients we acquired less EPI volumes as compared to the VS and control subjects. This could be a bias toward our negative findings in BD. To address this issue, we scanned six supplementary healthy controls using the same acquisition parameters and number of scans than in the reported BD patient. In all controls, we could identify default network connectivity at the single subject level, reinforcing our interpretation that differences in acquisition parameters are not sufficient to explain the complete lack of functional connectivity here reported in BD. The absence of long‐ distance correlations in T2* signal in the BD patient also indicates that our results are unlikely to be due to respiratory artifacts, known to be source of spurious signal in default network areas as measured in healthy volunteers (Birn et al., 2006), as this patient was still breathing at the time of the fMRI data acquisition.
In conclusion, spontaneous system‐wide functional connectivity patterns previously identified in the healthy human brain are unlikely to be exclusively due to ongoing modifications of conscious thoughts, as they have here been identified in a well‐documented patient in a permanent VS. However, our VS patient showed absent cortico‐thalamic and significantly reduced cortico‐cortical functional connectivity patterns compared to our age‐matched controls. These results are in line with the hypothesis that the spontaneous signal fluctuations in the default mode have several contributors, some related to conscious thoughts and others originating from unconscious processing. It should be stressed that our study is a single‐case study, for which only one VS patient was included. Therefore, the validity of the current results on a population level of statistical inference is limited, and care should be exercised when interpreting the results in a general sense. A full account of the characteristics of resting state networks in VS patients would only be reached by studying a significantly larger cohort of patients. Future studies on patients in pathological comatose states, in sleep and in pharmacological coma, are needed to better understand the functional meaning of the human ‘default mode of brain function’ (Raichle et al., 2001).
Acknowledgements
MB, CP, SL and PM are, respectively, Research Fellow, Research Associate, Senior Research Associate and Research Director at FNRS. We thank Jacques Trantsieaux and Dimitri Haye for their assistance in acquiring the fMRI data.
REFERENCES
- American Electroencephalographic Society ( 1994): Guideline three: Minimum technical standards for EEG recording in suspected cerebral death. American Electroencephalographic Society. J Clin Neurophysiol 11: 10–13. [PubMed] [Google Scholar]
- Ansell BJ,Keenan JE ( 1989): The Western Neuro Sensory Stimulation Profile: A tool for assessing slow‐to‐recover head‐injured patients. Arch Phys Med Rehabil 70: 104–108. [PubMed] [Google Scholar]
- Bates D ( 2005): The vegetative state and the Royal College of Physicians guidance. Neuropsychol Rehabil 15(3/4): 175–183. [DOI] [PubMed] [Google Scholar]
- Birn RM,Diamond JB,Smith MA,Bandettini PA ( 2006): Separating respiratory‐variation‐related fluctuations from neuronal‐ activity‐related fluctuations in fMRI. Neuroimage 31: 1536–1548. [DOI] [PubMed] [Google Scholar]
- Boly M,Balteau E,Schnakers C,Degueldre C,Moonen G,Luxen A,Phillips C,Peigneux P,Maquet P,Laureys S( 2007a): Baseline brain activity fluctuations predict somatosensory perception in humans. Proc Natl Acad Sci USA 104: 12187–12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boly M,Coleman MR,Davis MH,Hampshire A,Bor D,Moonen G,Maquet PA,Pickard JD,Laureys S,Owen AM ( 2007b): When thoughts become action: An fMRI paradigm to study volitional brain activity in non‐communicative brain injured patients. Neuroimage 36: 979–992. [DOI] [PubMed] [Google Scholar]
- Born JD,Albert A,Hans P,Bonnal J ( 1985): Relative prognostic value of best motor response and brain stem reflexes in patients with severe head injury. Neurosurgery 16: 595–601. [DOI] [PubMed] [Google Scholar]
- Cordes D,Haughton VM,Arfanakis K,Carew JD,Turski PA,Moritz CH,Quigley MA,Meyerand ME ( 2001): Frequencies contributing to functional connectivity in the cerebral cortex in “resting‐state” data. AJNR Am J Neuroradiol 22: 1326–1333. [PMC free article] [PubMed] [Google Scholar]
- Dehaene S,Changeux JP ( 2005): Ongoing spontaneous activity controls access to consciousness: A neuronal model for inattentional blindness. PLoS Biol 3: e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito F,Bertolino A,Scarabino T,Latorre V,Blasi G,Popolizio T,Tedeschi G,Cirillo S,Goebel R,Di Salle F ( 2006): Independent component model of the default‐mode brain function: Assessing the impact of active thinking. Brain Res Bull 70(4–6): 263–269. [DOI] [PubMed] [Google Scholar]
- Fox MD,Snyder AZ,Vincent JL,Corbetta M,Van Essen DC, et al. ( 2005): The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102: 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD,Snyder AZ,Vincent JL,Raichle ME ( 2007): Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron 56: 171–184. [DOI] [PubMed] [Google Scholar]
- Fransson P ( 2005): Spontaneous low‐frequency BOLD signal fluctuations: An fMRI investigation of the resting‐state default mode of brain function hypothesis. Hum Brain Mapp 26: 15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P ( 2006): How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia 44: 2836–2845. [DOI] [PubMed] [Google Scholar]
- Fransson P,Skiold B,Horsch S,Nordell A,Blennow M,Lagercrantz H,Aden U ( 2007): Resting‐state networks in the infant brain. Proc Natl Acad Sci USA 104: 15531–15536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K,Ashburner J,Frith C,Poline JB,Heather J,Frackowiak RSJ ( 1995): Spatial realignement and normalization of images. Hum Brain Mapp 2: 165–189. [Google Scholar]
- Giacino JT,Ashwal S,Childs N,Cranford R,Jennett B,Katz DI,Kelly JP,Rosenberg JH,Whyte J,Zafonte RD,Zasler ND ( 2002): The minimally conscious state: Definition and diagnostic criteria. Neurology 58: 349–353. [DOI] [PubMed] [Google Scholar]
- Giacino JT,Kalmar K,Whyte J ( 2004): The JFK Coma Recovery Scale‐Revised: Measurement characteristics and diagnostic utility. Arch Phys Med Rehabil 85: 2020–2029. [DOI] [PubMed] [Google Scholar]
- Gill‐Thwaites H,Munday R ( 2004): The Sensory Modality Assessment and Rehabilitation Technique (SMART): A valid and reliable assessment for vegetative state and minimally conscious state patients. Brain Inj 18: 1255–1269. [DOI] [PubMed] [Google Scholar]
- Greicius MD,Krasnow B,Reiss AL,Menon V ( 2003): Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 100: 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD,Kiviniemi V,Tervonen O,Vainionpaa V,Alahuhta S,Reiss AL,Menon V ( 2008): Persistent default‐mode network connectivity during light sedation. Hum Brain Mapp 29: 839–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA,Raichle ME ( 2001): Searching for a baseline: Functional imaging and the resting human brain. Nat Rev Neurosci 2: 685–694. [DOI] [PubMed] [Google Scholar]
- Horovitz SG,Fukunaga M,de Zwart JA,van Gelderen P,Fulton SC,Balkin TJ,Duyn JH ( 2007): Low frequency BOLD fluctuations during resting wakefulness and light sleep: A simultaneous EEG‐fMRI study. Hum Brain Mapp 29: 671–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingvar DH ( 1979): “Hyperfrontal” distribution of the cerebral grey matter flow in resting wakefulness; on the functional anatomy of the conscious state. Acta Neurol Scand 60: 12–25. [DOI] [PubMed] [Google Scholar]
- Kenet T,Bibitchkov D,Tsodyks M,Grinvald A,Arieli A ( 2003): Spontaneously emerging cortical representations of visual attributes. Nature 425: 954–956. [DOI] [PubMed] [Google Scholar]
- Laufs H,Krakow K,Sterzer P,Eger E,Beyerle A,Salek‐Haddadi A,Kleinschmidt A ( 2003): Electroencephalographic signatures of attentional and cognitive default modes in spontaneous brain activity fluctuations at rest. Proc Natl Acad Sci USA 100: 11053–11058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laureys S,Goldman S,Phillips C,Van Bogaert P,Aerts J,Luxen A,Franck G,Maquet P( 1999a): Impaired effective cortical connectivity in vegetative state: Preliminary investigation using PET. Neuroimage 9: 377–382. [DOI] [PubMed] [Google Scholar]
- Laureys S,Lemaire C,Maquet P,Phillips C,Franck G ( 1999b): Cerebral metabolism during vegetative state and after recovery to consciousness. J Neurol Neurosurg Psychiatry 67: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laureys S,Faymonville ME,Degueldre C,Fiore GD,Damas P,Lambermont B,Janssens N,Aerts J,Franck G,Luxen A,Moonen G,Lamy M,Maquet P( 2000a): Auditory processing in the vegetative state. Brain 123 (Pt 8): 1589–1601. [DOI] [PubMed] [Google Scholar]
- Laureys S,Faymonville ME,Luxen A,Lamy M,Franck G,Maquet P ( 2000b): Restoration of thalamocortical connectivity after recovery from persistent vegetative state. Lancet 355: 1790–1791. [DOI] [PubMed] [Google Scholar]
- Laureys S,Owen AM,Schiff ND ( 2004): Brain function in coma, vegetative state, and related disorders. Lancet Neurol 3: 537–546. [DOI] [PubMed] [Google Scholar]
- Laureys S,Perrin F,Schnakers C,Boly M,Majerus S ( 2005): Residual cognitive function in comatose, vegetative and minimally conscious states. Curr Opin Neurol 18: 726–733. [DOI] [PubMed] [Google Scholar]
- Libet B ( 2006): Reflections on the interaction of the mind and brain. Prog Neurobiol 78(3–5): 322–326. [DOI] [PubMed] [Google Scholar]
- Majerus S,Van der Linden M ( 2000): Wessex Head Injury Matrix and Glasgow/Glasgow‐Liège Coma Scale: A validation and comparison study. Neuropsychol Rehabil 10: 167–184. [Google Scholar]
- Majerus S,Gill‐Thwaites H,Andrews K,Laureys S ( 2005): Behavioral evaluation of consciousness in severe brain damage. Prog Brain Res 150: 397–413. [DOI] [PubMed] [Google Scholar]
- McKiernan KA,D'Angelo BR,Kaufman JN,Binder JR ( 2006): Interrupting the “stream of consciousness”: An fMRI investigation. Neuroimage 29: 1185–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM,Coleman MR,Boly M,Davis MH,Laureys S,Pickard JD ( 2006): Detecting awareness in the vegetative state. Science 313: 1402. [DOI] [PubMed] [Google Scholar]
- Perrin F,Schnakers C,Schabus M,Degueldre C,Goldman S,Bredart S,Faymonville ME,Lamy M,Moonen G,Luxen A,Maquet P,Laureys S ( 2006): Brain response to one's own name in vegetative state, minimally conscious state, and locked‐in syndrome. Arch Neurol 63: 562–569. [DOI] [PubMed] [Google Scholar]
- Raichle ME,MacLeod AM,Snyder AZ,Powers WJ,Gusnard DA,Shulman GL ( 2001): A default mode of brain function. Proc Natl Acad Sci USA 98: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M,McCormick DA,Sejnowski TJ ( 1993): Thalamocortical oscillations in the sleeping and aroused brain. Science 262: 679–685. [DOI] [PubMed] [Google Scholar]
- The Multi‐Society Task Force on PVS ( 1994): Medical aspects of the persistent vegetative state (1). N Engl J Med 330: 1499–1508. [DOI] [PubMed] [Google Scholar]
- Tononi G ( 2004): An information integration theory of consciousness. BMC Neurosci 5: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsodyks M,Kenet T,Grinvald A,Arieli A ( 1999): Linking spontaneous activity of single cortical neurons and the underlying functional architecture. Science 286: 1943–1946. [DOI] [PubMed] [Google Scholar]
- Vincent JL,Patel GH,Fox MD,Snyder AZ,Baker JT,Van Essen DC,Zempel JM,Snyder LH,Corbetta M,Raichle ME ( 2007): Intrinsic functional architecture in the anaesthetized monkey brain. Nature 447: 83–86. [DOI] [PubMed] [Google Scholar]
- White NS,Alkire MT ( 2003): Impaired thalamocortical connectivity in humans during general‐anesthetic‐induced unconsciousness. Neuroimage 19(2 Pt 1): 402–411. [DOI] [PubMed] [Google Scholar]
- Wijdicks EF ( 1995): Determining brain death in adults. Neurology 45: 1003–1011. [DOI] [PubMed] [Google Scholar]
- Wijdicks EF ( 2001): The diagnosis of brain death. N Engl J Med 344: 1215–1221. [DOI] [PubMed] [Google Scholar]


