Abstract
Several electrophysiological studies have provided evidence for the frontal asymmetry of emotion. In this model the motivation to approach is lateralized to the left, whereas the motivation to avoidance is lateralized to the right hemisphere. The aim of the present experiment was to seek evidence for this model by relating electrophysiological and phenomenological indices of frontal asymmetry to a direct measure of cortical excitability. Frontal asymmetrical resting states of the electroencephalogram and motivational tendencies indexed by the Carver and White questionnaire were compared with neural excitability of the left and right primary motor cortex as assessed by transcranial magnetic stimulation in 24 young healthy right‐handed volunteers. In agreement with the model of frontal asymmetry, predominant left over right frontal cortical excitability was associated with enhanced emotional approach relative to emotional avoidance. Moreover, the asymmetries of brain excitability and approach–avoidance motivational predispositions were both reflected by frontal beta (13–30 Hz) electroencephalogram asymmetries. In conclusion, the currently demonstrated interconnections between cortical excitability, electrophysiological activity, and self‐reported emotional tendencies for approach or avoidance support the frontal asymmetry of emotion model and provide novel insights into its biological underpinnings. Hum Brain Mapp 2008. © 2007 Wiley‐Liss, Inc.
Keywords: behavioral approach, behavioral avoidance, beta oscillations, cortical excitability, electroencephalogram, frontal cortex, motor threshold, transcranial magnetic stimulation
INTRODUCTION
One of the most influential cortical centered models in contemporary human affective neuroscience is the frontal asymmetry model of emotion. The model proposes that the left frontal cortex subserves approach‐related emotions whereas the right frontal cortex is involved in avoidance‐related emotions [Allen and Kline, 2004; Davidson, 1992, 2003; Harmon‐Jones, 2003; Schutter et al., 2004]. Importantly, the approach and avoidance dimensions not only account for several basic aspects of personality, including extraversion, introversion, neuroticism, and positive and negative emotionality [Elliot and Thrash, 2002], but also correlate with Eysenck's personality traits of neuroticism and extraversion–introversion [Murris et al., 2005]. More recently, it was shown that even the key factors neuroticism, extraversion, agreeableness, and conscientiousness of the Big Five personality model can also adequately be explained by the approach–avoidance dimensions [Smits and Boeck, 2006]. These findings indicate that the approach–avoidance dimensions are able to capture the core aspects of personality.
Among the many unknowns concerning the underlying physiology of the frontal asymmetry model of emotion is the role of cortical excitability. Another significant question is the functional meaning of the different frequency bandwidths in the electroencephalogram (EEG) spectrum. There is currently debate as to whether anterior resting state alpha (8–12 Hz) oscillations reflect enhanced cortical excitability or reduced cortical activity [Cooper et al., 2003]. In addition, recent EEG studies have introduced beta (13–30 Hz) oscillations as a cortical brain rhythm that may reflect active neural inhibition [Jensen et al., 2005; Wanquier, 1998]. The physiological functions of these fast wave oscillations in the frontal asymmetry model of emotion are therefore in need of further elucidation.
A way of addressing these matters in a more direct fashion than simply by EEG is by means of transcranial magnetic stimulation (TMS). TMS is a noninvasive technique that is capable of both manipulating and measuring cortical physiology by applying magnetic pulses to the scalp [Hallett, 2000]. Several TMS studies in healthy subjects have found support for the frontal asymmetry model of emotion by demonstrating shifts to more approach‐related behavior after applying slow inhibitory repetitive TMS over the right frontal cortex [Schutter et al., 2001; Van Honk et al., 2002], whereas slow inhibitory repetitive TMS over the left frontal cortex has resulted in shifts toward increased avoidance‐related behavior [D'Alfonso et al., 2000; Knoch et al., 2006]. In line with several electrophysiological studies it has been shown that slow inhibitory repetitive TMS over one cerebral hemisphere can induce transient shifts of brain activity to the contralateral side [Nahas et al., 2001; Schutter et al., 2001]. In addition to the use of TMS in the modulation of brain activity, the causal connection existing between cortical excitability and magnetic pulse intensity [Wassermann, 1998] makes TMS a suitable technique to study cortical physiology and to establish relationships between neuronal excitability, brain oscillations, and phenomenology.
Even though the term neural excitability is often used loosely and means different things to different researchers, it has been proposed that on a cellular level excitability refers to specific neuronal populations and that the excitability of a neuron depends on the balance of excitatory and inhibitory inputs [Lui and Lachamp, 2006]. In the current study cortical excitability is defined as the net sum of excitatory (i.e., NMDA) and GABA‐ergic mediated inhibitory synaptic inputs on motor neurons required to activate the corticospinal tract to produce a motor evoked response in the contralateral limb [for a review see Fitzgerald et al., 2006]. The lowest TMS pulse intensity required to evoke a motor response is therefore a quantitative measure of cortical excitability and is called the motor threshold (MT). The physiology of the MT, although not fully understood, likely reflects the excitability of axonal fibers of corticospinal neurons and interneurons that act on the output cells in the motor cortex [Moll et al., 1999]. Although neural excitability of the primary motor cortex (M1) does not necessarily generalize to other cortical areas an interleaved single pulse TMS‐EEG study revealed a very strong relationship between cortical responses obtained from M1 and the prefrontal cortex [Kähkönen et al., 2005]. The aim of the present study was to seek more direct evidence of the physiological mechanisms subserving the frontal asymmetry of emotion by linking EEG measures and self‐reported predispositions for approach/avoidance to a direct measure of cortical excitability. It was hypothesized that greater left compared with right cortical excitability would be accompanied by enhanced emotional approach relative to emotional avoidance, and we explored whether cortical excitability and phenomenology would be reflected in patterns of resting state fast wave EEG oscillations that could provide some novel leads toward understanding the physiological meaning of resting state alpha and beta oscillations.
SUBJECTS AND METHODS
Subjects
Twenty‐four healthy nonsmoking right‐handed volunteers (11 males), aged between 18 and 25 years (mean ± SD, 22.2 ± 1.97) were recruited at Utrecht University, The Netherlands. Participants were medication free, with the exception of the women who all used oral contraceptives. None of the subjects had a history of psychiatric or neurological conditions. Written informed consent was obtained. All volunteers were unaware of the aim of the study and were paid for participation. The study was in accordance with the standards set by the Declaration of Helsinki.
Approach‐ and Avoidance‐Related Motivation
The Carver and White's [1994] orthogonally dimensioned BIS/BAS self‐report scales were administered to measure motivational direction. High BAS scores are associated with approach‐related motivation, whereas high BIS scores are related to avoidance‐related motivation [Sutton and Davidson, 1997]. In combination, BIS and BAS scores reflect the balance between approach‐ and avoidance‐related motivational predispositions.
Electrophysiological Recording
Resting state EEGs were recorded from 32 scalp Ag/AgCl tipped electrodes according to the International 10/20 EEG system (sampling rate: 256 Hz). Electro‐oculogram was recorded by placing Ag/AgCl electrodes on the suborbit and supraorbit of the right eye and on the external canthi of both eyes to correct eye movements. ECI EEG gel was used as conducting medium for both EEG and electro‐oculogram electrodes. EEG recordings were made with the ActiveTwo system (BioSemi, Amsterdam, The Netherlands) relative to the common mode sense. By physically integrating the first amplifier stage with a sintered Ag‐AgCl electrode, extremely low‐noise recordings free of interference can be achieved. The active‐electrode is a sensor with a very low output impedance. The ground consists of the active common mode sense and passive driven right leg electrode that form a feedback loop driving the subject's average potential as close as possible to the analog‐to‐digital converter (i.e., the amplifier “zero”) reference voltage in the A/D‐box. As a result, the ActiveTwo system does not require impedance measurements or gain adjustments (http://www.biosemi.com).
Transcranial Magnetic Stimulation
TMS was performed using a Neotonus (Atlanta) magnetic brain stimulator (maximum output 2300 A peak/1750 VAC peak) and a modified 8‐shaped coil with an iron core (Neotonus, Atlanta) was used with a current magnetic induction field of approximately 2 T.
Procedure
On arrival at the laboratory subjects were screened for contraindications to TMS [Keel et al., 2000] and the procedure was briefly explained to the subjects. After informed consent was obtained participants filled out the Edinburgh handedness inventory [Oldfield, 1971], mean ± SD, 45 ± 2.5, and the Carver and White questionnaire. Next, volunteers were seated in a comfortable dentist chair in a dimly lit room adjacent to the control room and instructed to relax and keep head movements to a minimum. A 4‐min resting state EEG was recorded (eyes open–closed–open–closed) and resting MTs were determined in a randomized counterbalanced fashion for the left and right hemisphere according to the standardized visual thumb movement procedure as described by Pridmore et al. [1998] and Schutter and Van Honk [2006]. Participants were seated upwardly in a dentist chair and asked to relax the arm contralateral to the site of stimulation by placing it on the upper‐leg with the palm of the hand facing upwards. The coil was initially placed on the scalp halfway the vertex and the external auditory meutus. By moving the coil in different directions by approximately 1 cm and gradually increasing TMS intensity (interstimulus interval 0.2 Hz) the site for eliciting reliable thumb twitches (five of five) can be localized. Next, intensity is decreased until five of 10 consecutive pulses induced a visually identifiable twitch. Finally, the coil is moved again over the scalp and 0.2 Hz TMS is applied to look for an additional scalp site that surpasses the 50% thumb movement criterion. If such a site is found, TMS intensity is further decreased according to this 50% criterion, subsequently leading to a reliable estimation of the MT.
Data Reduction and Analysis
To attain a self‐response metric conceptually comparable with EEG and cortical excitability asymmetry, the following asymmetry [(BIS − BAS)/(BIS + BAS)] was calculated for each participant to index the balance between approach‐ and avoidance‐related motivation [Sutton and Davidson, 1997].
EEG data recorded from the left (C3, Fc1, Fc5, F3) and right frontal cortex (C4, Fc2, Fc6, F4) were rereferenced offline to the cz electrode. Although most EEG studies on frontal asymmetry do not include the C3 and C4 electrodes into the asymmetry index there are several reasons for incorporating these electrodes in calculating the asymmetry: (1) the frontal cortex consists of the prefrontal and motor areas that are functionally and anatomically highly intertwined [Fuster, 1997]. In accordance, approach‐ and avoidance‐related motivation are reciprocally connected to motor behavior [Harmon‐Jones, 2006; Wilkowski and Robinson, 2006]; (2) electrophysiological studies have shown that brain responses to TMS applied at individual MT intensities over the motor cortex are strongly correlated to cortical responses over the prefrontal cortex [Kähkönen et al., 2005]; (3) the inclusion of more than one electrode pair results in more reliable estimations of frontal asymmetry. EEG signals were digitally band‐pass filtered (1–30 Hz, 24 dB slope), segmented into 4‐s epochs and corrected for eye movements (Gratton and Coles). EEG signal containing residual muscle movements, or other artifacts greater than +/−50 mV were rejected before further analysis by removal of the epoch for all channels. A fast Fourier transform method (Hamming window: length 10%) was used to estimate spectral power (μV2) in the lower alpha (8–10 Hz), upper alpha (10–12 Hz) and beta (13–30 Hz) frequency bandwidths. Mean spectral power for the left and right frontal cortex was calculated by averaging the five corresponding electrodes. To assess relative hemispheric contribution and to control for non‐neurogenic sources of individual variation including skull thickness and scalp‐to‐cortex distance, asymmetries were calculated for the EEG and MT variables according to the following formula: [(right − left)/(right + left)].
Stepwise linear regression analyses (method: probability of F to enter ≤ 0.05; criteria probability of F to remove ≥ 0.1) were performed to find the solution that would explain the maximum variance for a minimum of predictors. The alpha level was set at 0.05, two‐tailed. In addition, Cohen's effect sizes (f) were calculated according to the formula: f = R 2/(1 − R 2). Although the Cohen's f value should be treated with caution by convention an f = 0.10 represents a small effect, an f = 0.25 stands for a medium effect, and an f = 0.40 is considered a large effect [Cohen, 1988].
RESULTS
The regression analysis wherein the alpha and beta asymmetries were entered as the independent variables to model the balance between approach‐ and avoidance‐related motivational predispositions demonstrated that the beta EEG asymmetry (y 1) could explain the balance between approach‐ and avoidance‐related motivational predispositions (x 1), F(1, 23) = 7.15; P = 0.01 (R 2 = 0.25; f = 0.35), y 1 = −0.35x 1 − 0.78. The lower and upper alpha EEG asymmetry variables could not be modeled and were excluded by the regression analysis. Results of the regression analysis wherein the lower alpha, upper alpha, and beta EEG asymmetries were entered as the independent variables to model cortical excitability asymmetry (y 2) showed that the beta EEG asymmetry (x 2) could best explain the cortical excitability asymmetry, F(1, 23) = 7.75; P = 0.01 (R 2 = 0.26, f = 0.33), y 2 = 0.56x 2 − 0.02. Finally, a linear regression showed that the cortical excitability asymmetry (y 2) was positively associated with approach‐ and avoidance‐related motivational predispositions (x 1), F(1, 23) = 6.28; P = 0.02 (R 2 = 0.22, f = 0.28), y 2 = −0.81x 1 − 0.36. Figure 1 displays the statistically significant relationships.
Figure 1.
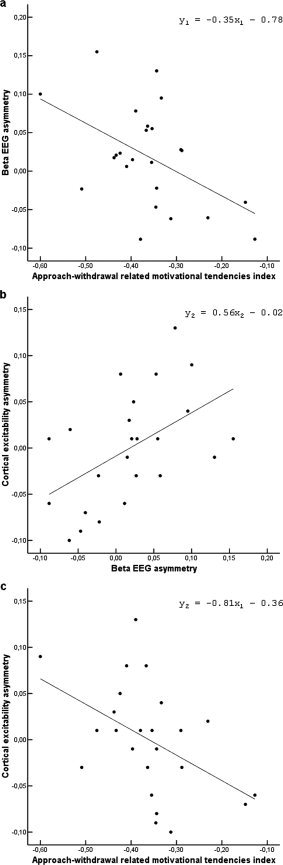
Relatively higher left‐to‐right‐sided cortical excitability was accompanied by increased approach‐related motivational predispositions (a), and reductions in left‐to‐right sided beta EEG asymmetry (b). Relatively higher approach‐to‐avoidance‐related motivational predispositions were correlated with reduced left‐to‐right sided beta asymmetries (c).
DISCUSSION
The frontal asymmetry of emotion is a classic theoretical model supported by several lines of evidence. The model argues for left‐sided involvement in approach‐related motivation and right‐sided involvement in avoidance‐related motivation. The aim of the present experiment was to seek qualitatively new evidence for this model not only by examining relations between surface EEG and motivational predispositions but also by assessing cortical excitability. In agreement with the frontal asymmetry model of emotion, lateralized left‐to‐right frontal cortical excitability was associated with enhanced emotional approach relative to emotional avoidance. Furthermore, hemispheric asymmetries in brain excitability and self‐reported motivational predispositions for approach and avoidance both correlated with frontal beta (13–30 Hz) EEG asymmetry. These findings concur with the proposal that hemispheric asymmetries of neural excitability signify functional cerebral dominance [Triggs et al., 1994] and earlier studies that have successfully used TMS to establish relationships between cortical excitability and personality traits [Spitzer et al., 2004; Wassermann et al., 2001]. Previous EEG studies on frontal asymmetry provide extensive support for left‐sided involvement in emotional approach and right‐sided involvement in emotional avoidance [see Allen and Kline, 2004; Sutton and Davidson, 1997 for a review], yet this is to our knowledge the first study demonstrating a link between hemispheric asymmetries in cortical excitability and frontal asymmetry of emotion. In accordance, recent findings of Bajbouj et al. [2006] in a group of medication‐free patients showed that clinical depression, a disorder characterized by increased avoidance and decreased approach motivational tendencies [Kasch et al., 2002], is associated with higher right‐to‐left sided levels of cortical excitability as determined by MT measurements. It has been suggested that γ‐amino‐butyric acid (GABA) may be intrinsically involved [Bajbouj et al., 2005; Wassermann et al., 2001]. Motor threshold is assumed to represent a measure of membrane excitability of large pyramidal neurons and pharmacological studies have demonstrated that GABA‐ergic agents are capable of modulating membrane excitability by targeting the inhibitory neuronal circuits of the cortex [Ziemann, 2004; Ziemann and Hallett, 2000]. This is further substantiated by the fact that because of the tangential orientation of the magnetic current relative to the brain surface TMS pulses are likely to influence horizontal cortical elements that in addition to the axonal bendings of pyramidal nerves cells include GABA‐ergic interneurons [Amassian et al., 1987; Barker, 1999]. Interestingly, human electrophysiological studies have found “paradoxical” increases in beta oscillations after administrating GABA agonists [Jensen et al., 2005; Wanquier, 1998]. However, evidence in support of beta oscillations reflecting locally active inhibitory processes has been provided by interleaved TMS‐event‐related potentials studies over the motor cortex [Paus et al., 2001; Van der Werf and Paus, 2006]. These studies demonstrated amplitude increases in beta oscillatory activity in response to single TMS pulses over the primary motor cortex which may reflect the onset of cortical idling [Niedermeyer, 1999] or a physiological process related to cortical inhibition [Pfurtscheller et al., 2003]. Although the amplitude changes in beta oscillations were TMS‐induced responses rather than spontaneous resting state beta oscillations, the presently observed linear relationship between the asymmetries of cortical excitability and resting state beta oscillations does seem to support the proposed “active inhibition” interpretation.
The lack of significant associations between cortical excitability, approach–avoidance‐related motivational predispositions, and anterior alpha EEG asymmetry renders it impossible to contribute to the ongoing debate on the physiological meaning of alpha oscillations. It has been suggested that the upper alpha frequency reflects inhibitory mechanisms. Hummel et al. [2002] showed that during active inhibition upper alpha power increases (alpha synchronization) and cortical excitability reduces significantly as measured with TMS. These findings were interpreted in terms of local alpha oscillations reflecting an active inhibition process of neuronal activity. Klimesch et al. [2007] has suggested that alpha synchronization during active inhibition is linked to top down, cognitive control in terms of timing. An important difference between the current and Hummel et al. study is that our data were acquired under resting state conditions and thus arguably reflect a different functional brain state. Moreover, because of the low spatial resolution of EEG scalp recordings, it is conceivable that the frontal electrical distribution of alpha oscillations may have been the result of neural sources located in the posterior regions of the brain. Because alpha oscillations tend to dominate over posterior scalp site regions during resting state recordings, a posterior dipole configuration pointing in the posterior‐to‐anterior direction could explain the currently absent relationship between resting state frontal excitability and alpha oscillations. Moreover, in the present study the vertex (Cz) was used as the reference. Despite the fact that Cz is the most frequently used reference point in asymmetry studies, it may have negative effects on the signal‐to‐noise ratio [Hagemann, 2004]. The vertex is an electrically active site, and on the premise that the frontal sites have lower alpha activity compared with the vertex derivations of the left‐ and right‐sided electrodes against the vertex will consequently yield confounds in the lateral frontal recordings sites. Although this explanation may account for the absent relationships for the alpha asymmetry, it can be readily assumed that this would equally affect frontal beta activity which was not the case. In fact, the results provide additional support for the notion that beta and faster brain oscillations are more likely to reflect true underlying cortical activity [Lubar, 1997]. The current frontal beta asymmetry findings are however in good agreement with a substantial body of behavioral and physiological studies, showing that the current findings were not confounded by the applied reference schema.
Furthermore, it is important to note that even though the electric responses recorded over the primary motor cortex have been shown to correlate with the electric responses over the prefrontal cortex evoked by single pulses of TMS [i.e., Kähkönen et al., 2004, 2005], arguably sharing comparable underlying neurophysiological properties, this assumption is by no means indisputable [for a recent review see Komssi and Kähkönen, 2006]. Even though the chronometrics of the evoked brain potentials for PFC and M1 TMS have been found to be very similar, the TMS‐evoked responses over the PFC are on average 32% smaller when compared with TMS‐evoked responses over the primary motor cortex (M1) [Kähkönen et al., 2004]. These authors speculated that the different responses may arise from differences in neurotransmitter function as the chemical regulation is not uniform across the cortex. Alternatively, it has been proposed that the larger scalp‐to‐cortex distance of PFC (12–22 mm) compared with M1 (10–19 mm) can account for the smaller response [Sommer et al., 2003]. In sum, the generalization of TMS evoked brain responses from M1 to the PFC should be made with caution, but the interleaved TMS‐EEG studies suggest that the physiological properties as measured from the primary motor cortex do provide a useful index for the prefrontal cortex as well.
In conclusion, frontal EEG asymmetry is a well‐established model of approach/avoidance motivational tendencies and this study found interhemispheric differences in motor cortex excitability to be congruent with this model. The current findings therefore provide a significant link between a neurophysiological index of motivation (frontal EEG asymmetry) and the activation threshold of the final neural gateway to behavior (M1 cortical excitability). This study therefore not only provides strong support for the frontal asymmetry model of emotion but also provides a brand new, and relative to frontal EEG, more distal point of entry into the neural substrates of motivation and behavior. As such, motor cortex excitability asymmetry offers a promising new perspective on the neurobiology of emotion.
REFERENCES
- Allen JJB,Kline JP ( 2004): Frontal EEG asymmetry, emotion and psychopathology: The first, and the next 25 years. Biol Psychol 67: 1–5. [DOI] [PubMed] [Google Scholar]
- Amassian VE,Stewart M,Quirk GJ,Rosenthal JL ( 1987): Physiological basis of motor effects of a transient stimulus to cerebral cortex. Neurosurgery 20: 74–93. [PubMed] [Google Scholar]
- Bajbouj M,Lisanby SH,Lang UE,Danker‐Hopfe H,Heuser I,Neu P ( 2006): Evidence for impaired cortical inhibition in patients with unipolar major depression. Biol Psychiatry 59: 395–400. [DOI] [PubMed] [Google Scholar]
- Barker AT ( 1999): The history and principles of magnetic nerve stimulation. Electroencephalogr Clin Neurophysiol Suppl 51: 3–21. [PubMed] [Google Scholar]
- Carver CS,White TL ( 1994): Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. J Pers Soc Psychol 67: 319–333. [Google Scholar]
- Cohen J ( 1988): Statistical Power Analysis for the Behavioral Sciences, 2nd ed Hillsdale, NJ: Erlbaum. [Google Scholar]
- Cooper NR,Croft RJ,Dominey SJJ,Burgess AP,Gruzelier JH ( 2003): Paradox lost? Exploring the role of alpha oscillations during externally vs. internally directed attention and the implications for idling and inhibition hypotheses. Int J Psychophysiol 47: 65–74. [DOI] [PubMed] [Google Scholar]
- D'Alfonso AAL,Van Honk J,Hermans E,Postma A,De Haan EHF ( 2000): Laterality effects in selective attention to threat after repetitive transcranial magnetic stimulation at the prefrontal cortex in female subjects. Neurosci Lett 280: 195–198. [DOI] [PubMed] [Google Scholar]
- Davidson RJ ( 1992): Anterior cerebral asymmetry and the nature of emotion. Brain Cogn 20: 125–151. [DOI] [PubMed] [Google Scholar]
- Davidson RJ ( 2003): Affective neuroscience and psychophysiology: Toward a synthesis. Psychophysiology 40: 655–665. [DOI] [PubMed] [Google Scholar]
- Elliot AJ,Thrash TM ( 2002): Approach and avoidance motivation in personality: Approach and avoidance temperaments and goals. J Pers Soc Psychol 82: 804–818. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB,Fountain S,Daskalakis ZJ ( 2006): A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol 117: 2584–2596. [DOI] [PubMed] [Google Scholar]
- Fuster JM ( 1997): The Prefrontal Cortex: Anatomy, Physiology, and Neuropsychology of the Frontal Lobe, 3rd ed Philadelphia: Lippincott‐Raven. [Google Scholar]
- Hallett M ( 2000): Transcranial magnetic stimulation and the human brain. Nature 406: 147–150. [DOI] [PubMed] [Google Scholar]
- Hagemann D ( 2004): Individual differences in anterior EEG asymmetry: Methodological problems and solutions. Biol Psychol 67: 157–182. [DOI] [PubMed] [Google Scholar]
- Harmon‐Jones E ( 2003): Clarifying the emotive functions of asymmetrical frontal cortical activity. Psychophysiology 40: 838–848. [DOI] [PubMed] [Google Scholar]
- Harmon‐Jones E ( 2006): Unilateral right‐hand contractions cause contralateral alpha power suppression and approach motivational affective experience. Psychophysiology 43: 598–603. [DOI] [PubMed] [Google Scholar]
- Hummel F,Andres F,Altenmuller E,Dichgans J,Gerloff C ( 2002): Inhibitory control of acquired motor programmes in the human brain. Brain 125: 404–420. [DOI] [PubMed] [Google Scholar]
- Jensen O,Goel P,Kopell N,Pohja M,Hari R,Ermentrout B ( 2005): On the human sensorimotor‐cortex beta rhythm: Sources and modeling. Neuroimage 26: 347–355. [DOI] [PubMed] [Google Scholar]
- Kähkönen S,Wilenius J,Komssi S,Ilmoniemi RJ ( 2004): Distinct differences in cortical reactivity of motor and prefrontal cortices to magnetic stimulation. Clin Neurophysiol 115: 583–588. [DOI] [PubMed] [Google Scholar]
- Kähkönen S,Komssi S,Wilenius J,Ilmoniemi RJ ( 2005): Prefrontal TMS produces smaller EEG responses than motor‐cortex TMS: Implications for rTMS treatment in depression. Psychopharmacology (Berl) 181: 16–20. [DOI] [PubMed] [Google Scholar]
- Kasch KL,Rottenberg J,Arnow BA,Gotlib IH ( 2002): Behavioral activation and inhibition systems and the severity and course of depression. J Abnorm Psychol 111: 589–597. [DOI] [PubMed] [Google Scholar]
- Keel JC,Smith MJ,Wassermann EM ( 2000): A safety screening questionnaire for transcranial magnetic stimulation. Clin Neurophysiol 112: 720. [DOI] [PubMed] [Google Scholar]
- Klimesch W,Sauseng P,Hanslmayr S ( 2007): EEG alpha oscillations: The inhibition‐timing hypothesis. Brain Res Rev 53: 63–88. [DOI] [PubMed] [Google Scholar]
- Knoch D,Gianotti LR,Pascual‐Leone A,Treyer V,Regard M,Hohmann M,Brugger P ( 2006): Disruption of right prefrontal cortex by low‐frequency repetitive transcranial magnetic stimulation induces risk‐taking behavior. J Neurosci 26: 6469–6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komssi S,Kähkönen S ( 2006): The novelty value of the combined use of electroencephalography and transcranial magnetic stimulation for neuroscience research. Brain Res Rev 52: 183–192. [DOI] [PubMed] [Google Scholar]
- Lubar JF ( 1997): Neocortical dynamics: Implications for understanding the role of neurofeedback and related techniques for the enhancement of attention. Appl Psychophysiol Biofeedback 22: 111–126. [DOI] [PubMed] [Google Scholar]
- Lui SJ,Lachamp P ( 2006): The activation of excitatory glutamate receptors evokes a long‐lasting increase in the release of GABA from cerebellar stellate cells. J Neurosci 25: 9332–9339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll GH,Heinrich H,Wischer S,Tergau F,Paulus W,Rothenberger A ( 1999): Motor system excitability in healthy children: Developmental aspects from transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol Suppl 51: 243–249. [PubMed] [Google Scholar]
- Muris P,Meesters C,De Kanter E,Timmerman PE ( 2005): Behavioural inhibition and behavioural activation system scales for children: Relationships with Eysenck's personality traits and psychopathological symptoms. Pers Individ Differ 38: 831–841. [Google Scholar]
- Nahas Z,Lomarev M,Roberts DR,Shastri A,Lorberbaum JP,Teneback C,McConnell K,Vincent DJ,Li X,George MS,Bohning DE ( 2001): Unilateral left prefrontal transcranial magnetic stimulation (TMS) produces intensity‐dependent bilateral effects as measured by interleaved BOLD fMRI. Biol Psychiatry 50: 712–720. [DOI] [PubMed] [Google Scholar]
- Niedermeyer E ( 1999): The normal EEG of the waking adult In: Niedermeyer E,Lopes da Silva F, editors. Basic Principles, Clinical Applications, and Related Fields, 4th ed Baltimore: Williams and Wilkins; pp 149–173. [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Paus T,Sipila PK,Strafella AP ( 2001): Synchronization of neuronal activity in the human primary motor cortex by transcranial magnetic stimulation: An EEG study. J Neurophysiol 86: 1983–1990. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G,Woertz M,Supp G,Lopes da Silva FH ( 2003): Early onset of post‐movement beta electroencephalogram synchronization in the supplementary motor area during self‐paced finger movement in man. Neurosci Lett 339: 111–114. [DOI] [PubMed] [Google Scholar]
- Pridmore S,Fernandes Filho JA,Nahas Z,Liberatos C,George MS ( 1998): Motor threshold in transcranial magnetic stimulation: A comparison of a neurophysiological method and a visualization of movement method. J ECT 14: 25–27. [PubMed] [Google Scholar]
- Schutter DJLG,Van Honk J ( 2006): A standardized motor threshold estimation procedure for transcranial magnetic stimulation. J ECT 22: 176–178. [DOI] [PubMed] [Google Scholar]
- Schutter DJLG,Van Honk J,D'Alfonso AAL,Postma A,De Haan EHF ( 2001): Effects of slow rTMS at the right dorsolateral prefrontal cortex on EEG asymmetry and mood. Neuroreport 12: 445–447. [DOI] [PubMed] [Google Scholar]
- Schutter DJLG,De Haan EHF,Van Honk J ( 2004): Anterior asymmetrical alpha activity predicts Iowa gambling performance: Distinctly but reversed. Neuropsychologia 42: 939–943. [DOI] [PubMed] [Google Scholar]
- Smits DJM,Boeck PD ( 2006): From BIS/BAS to the big five. Eur J Pers 20: 255–270. [Google Scholar]
- Sommer J,Steinstrater O,Breitenstein C,Jansen A,Konrad C,Deppe M,Foerster A,Ringelstein EB,Knecht S ( 2003): Interindividual variability in the cortex‐to‐scalp distance. Neuroimage 19: S47. [Google Scholar]
- Spitzer C,Willert C,Grabe HJ,Rizos T,Moller B,Freyberger HJ ( 2004): Dissociation, hemispheric asymmetry, and dysfunction of hemispheric interaction: A transcranial magnetic stimulation approach. J Neuropsychiatry Clin Neurosci 16: 163–169. [DOI] [PubMed] [Google Scholar]
- Sutton SK,Davidson RJ ( 1997): Prefrontal brain asymmetry: A biological substrate of the behavioral approach and inhibition systems. Psychol Sci 8: 204–210. [Google Scholar]
- Triggs WJ,Calvanio R,Macdonell RA,Cros D,Chiappa KH ( 1994): Physiological motor asymmetry in human handedness: Evidence from transcranial magnetic stimulation. Brain Res 636: 270–276. [DOI] [PubMed] [Google Scholar]
- Van der Werf YD,Paus T ( 2006): The neural response to transcranial magnetic stimulation of the human motor cortex. I. Intracortical and cortico‐cortical contributions. Exp Brain Res 175: 231–245. [DOI] [PubMed] [Google Scholar]
- Van Honk J,Schutter DJLG,D'Alfonso AAL,Kessels RPC,De Haan EHF ( 2002): 1 Hz rTMS over the right prefrontal cortex reduces vigilant attention to unmasked but not to masked fearful faces. Biol Psychiatry 52: 312–317. [DOI] [PubMed] [Google Scholar]
- Wanquier A ( 1998): EEG and neuropharmachology In: Niedermeyer E,Lopes da Silva F, editors. Electroencephalography: Basic Principles, Clinical Applications, and Related Fields, 4th ed Baltimore: Williams and Wilkins; pp 660–670. [Google Scholar]
- Wassermann EM ( 1998): Risk and safety of repetitive transcranial magnetic stimulation: Report and suggested guidelines from the international workshop on the safety of repetitive transcranial magnetic stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol 108: 1–16. [DOI] [PubMed] [Google Scholar]
- Wassermann EM,Greenberg BD,Nguyen MB,Murphy DL ( 2001): Motor cortex excitability correlates with an anxiety‐related personality trait. Biol Psychiatry 50: 377–382. [DOI] [PubMed] [Google Scholar]
- Wilkowski BM,Robinson MD ( 2006): Stopping dead in one's tracks: Motor inhibition following incidental evaluations. J Exp Soc Psychol 42: 479–490. [Google Scholar]
- Ziemann U ( 2004): TMS and drugs. Clin Neurophysiol 115: 1717–1729. [DOI] [PubMed] [Google Scholar]
- Ziemann U,Hallett M ( 2000): Basic neurophysiological studies with TMS In: George MS,Belmaker RH, editors. Transcranial Magnetic Stimulation in Neuropsychiatry. Washington: American Psychiatric Press; pp 45–98. [Google Scholar]


