Abstract
Background. Problems with cognitive flexibility have been observed in patients with attention deficit hyperactivity disorder (ADHD) and in patients with conduct disorder (CD), characterized by the violation of societal rules and the rights of others. Functional magnetic resonance imaging (fMRI) of cognitive switching, however, has only been investigated in patients with ADHD, including comorbidity with CD, finding frontostriatal and temporoparietal underactivation. This study investigates disorder‐specific functional abnormalities during cognitive flexibility between medication‐naïve children and adolescents with noncomorbid CD and those with noncomorbid ADHD compared to healthy controls. Methods. Event‐related fMRI was used to compare brain activation of 14 boys with noncomorbid, childhood‐onset CD, 14 boys with noncomorbid ADHD, and 20 healthy comparison boys during a visual–spatial Switch task. Results. Behaviorally, children with ADHD compared to children with CD had significantly slower reaction times to switch compared to repeat trials. The fMRI comparison showed that the patients with ADHD compared to both controls and patients with CD showed underactivation in right and left inferior prefrontal cortex. No disorder‐specific brain underactivation was observed in patients with CD. Only when compared with controls alone, the disruptive behavior group showed reduced activation in bilateral temporoparietal and occipital brain regions. Conclusions. The findings extend previous evidence for disorder‐specific underactivation in patients with ADHD compared to patients with CD in inferior prefrontal cortex during tasks of inhibitory control to the domain of cognitive flexibility. Inferior prefrontal underactivation thus appears to be a disorder‐specific neurofunctional biomarker for ADHD when compared with patients with CD. Hum Brain Mapp, 2010. © 2010 Wiley‐Liss, Inc.
Keywords: attention deficit hyperactivity disorder, conduct disorder, switching, cognitive flexibility, inferior prefrontal cortex
INTRODUCTION
Attention deficit hyperactivity disorder (ADHD) is characterized by symptoms of inattention, impulsiveness, and hyperactivity (DSM IV) [American Psychiatric Association, 1994]. ADHD is associated with neuropsychological deficits in tasks of inhibitory control, including motor and interference inhibition, but also cognitive switching, measuring the inhibition of previously valid stimulus‐response associations [Rubia et al., 2007a, b; Rubia et al., 2001; Willcutt et al., 2005]. Although many functional magnetic resonance imaging (fMRI) studies have used tasks of motor and interference inhibition, finding predominantly frontostriatal underactivations [Dickstein et al., 2006; Durston et al., 2003, 2006; Rubia et al., 1999, 2005], to our knowledge, only a previous study from our own group has investigated the neural substrates of cognitive switching in ADHD patients, reporting reduced activation in bilateral inferior prefrontal and temporoparietal brain regions [Smith et al., 2006].
Conduct disorder (CD) is defined by the violation of the rights of others and societal rules (DSM IV) [American Psychiatric Association, 1994]; it overlaps clinically, behaviorally, and cognitively with ADHD. About 30% of children with CD have comorbidity with ADHD, which can reach up to 50%, when oppositional defiant disorder (ODD) is also present [Banaschewski et al., 2005; Spencer et al., 1999]. Comorbid patients are often considered severe cases of ADHD [Banaschewski et al., 2005], and the notion of a separate neurobiological basis for CD has been debated [Taylor et al., 1991]. Structural and functional studies have shown abnormalities of the paralimbic system that comprises the orbitofrontal cortex, superior temporal lobe, and underlying limbic brain regions, known to mediate emotion and motivation [Blair et al., 2006]. Thus, structural studies have found reduced volume and gray matter of the temporal and orbitofrontal cortices in children with CD [Huebner et al., 2008; Kruesi et al., 2004]. Limbic volume reductions have been observed in the hippocampus, the insula, and the amygdala [Huebner et al., 2008; Sterzer et al., 2004].
There is evidence that the patients with CD, like ADHD patients, are impaired in tasks of cognitive flexibility such as switching and reversal tasks [Banaschewski et al., 2005; Blair et al., 2006; Lueger and Gill, 1990; Toupin et al., 2000]. The majority of fMRI studies in CD, however, have been on emotion‐processing tasks, finding abnormal activation in limbic and paralimbic regions. Thus, children with CD have shown to have more pronounced deactivation in right dorsal anterior cingulate gyrus during the viewing of pictures with negative valence, which was interpreted by the authors as reduced inhibition of emotional behavior [Sterzer et al., 2005]. The anterior cingulate deactivation furthermore correlated negatively with the aggressive behavior scores and remained when controlling for attention scores [Sterzer et al., 2005]. In two more recent studies, children with CD and callous‐unemotional traits showed reduced amygdala and hippocampus activation during the processing of fearful facial expressions compared to healthy children [Jones et al., 2009; Marsh et al., 2008]. Only one fMRI study, however, has investigated the neurofunctional substrates of cognitive flexibility in the CD subtype of children with psychopathic traits during a reversal task, finding abnormally enhanced ventromedial orbitofrontal activation during errors in the task [Finger et al., 2008].
Few studies have compared the neurofunctional substrates between the two disorders. A series of studies from our group found disorder‐specific underactivation in noncomorbid patients with ADHD when compared with healthy boys and boys with noncomorbid CD in left, right, or bilateral inferior prefrontal cortices during cognitive control tasks, including motor and interference inhibition, attention allocation, and sustained attention [Rubia et al., 2008, 2009b, c]. We also observed enhanced, presumably compensatory activation in the cerebellum in children with ADHD that was reduced in children with CD [Rubia et al., 2009c]. CD patients, on the other hand, demonstrated disorder‐specific activation reductions of the paralimbic system, such as the orbitofrontal cortex during reward [Rubia et al., 2009c], the insula, hippocampus, and anterior cingulate during sustained attention [Rubia et al., 2009c] and the superior temporal lobe during motor inhibition [Rubia et al., 2008]. Two studies compared the CD subgroup of severely disruptive children with psychopathic traits with children with ADHD as well as controls; one found enhanced orbitofrontal activation compared to healthy and ADHD children during errors in a reversal task [Finger et al., 2008] and the other reduced amygdala activation during fear [Marsh et al., 2008]. There was also reduced functional connectivity between the amygdala and ventrolateral orbitofrontal cortex in the callous‐unemotional group compared to healthy and ADHD children, which correlated with symptom severity [Marsh et al., 2008].
In this study, given evidence for shared deficits in functions of cognitive flexibility in the two disorders, we wanted to explore differences and commonalities in the neural substrates of cognitive flexibility during a visual–spatial switching task between carefully selected medication‐naïve children with noncomorbid combined hyperactive‐inattentive subtype of ADHD and with noncomorbid childhood‐onset CD. A switching task was chosen, given the evidence for deficits in switching task performance in both disorders [Banaschewski et al., 2005; Blair et al., 2006; Lueger and Gill, 1990; Toupin et al., 2000; Willcutt et al., 2005]. Furthermore, cognitive switching has been shown to activate ventrolateral prefrontal brain regions [Derrfuss et al., 2005; Rubia et al., 2006; Smith et al., 2004] that are known to be impaired in children with ADHD [Dickstein et al., 2006; Rubia et al., 1999, 2005b, 2008; Smith et al., 2006] with some evidence for impairment in children with CD during tasks of cognitive flexibility [Finger et al., 2008]. We included only the childhood‐onset CD subtype, given evidence for more severe neuropsychological and behavioral impairments [Frick and Ellis, 1999]. Given our previous evidence for reduced inferior prefrontal activation in children with ADHD during this task [Smith et al., 2006] and, furthermore, for disorder‐specific underactivation of inferior prefrontal activation in patients with ADHD compared to patients with CD during other tasks of cognitive control [Rubia et al., 2008, 2009b, c], we hypothesized that ADHD children would also show disorder‐specific bilateral inferior prefrontal underactivation compared to patients with CD during cognitive switching. Based on previous evidence for disorder‐specific abnormalities in areas of the paralimbic system in patients with CD behaviors compared to ADHD patients during tasks of cognitive control [Rubia et al., 2008, 2009b, c] and of reversal learning [Finger et al., 2008], we hypothesized that patients with CD would show underactivations in areas of the paralimbic system such as the orbitofrontal, cingulate, and superior temporal cortices during task performance.
METHODS
Subjects
Forty‐eight male, right‐handed adolescent boys between 9 and 17 years participated in the study. Patients were 28 adolescents with a clinical diagnosis of either CD (N = 14) or ADHD, combined hyperactive‐inattentive subtype (N = 14) (see Table I), recruited from parent support groups, clinics, and advertisement. Clinical diagnosis of combined subtype of ADHD without the diagnosis of CD/ODD and of CD without any clinical ADHD diagnosis (DSM IV) [American Psychiatric Association, 1994] was established through interviews with a child psychiatrist using the standardized structured Maudsley diagnostic interview [Goldberg and Murray, 2002]. Exclusion criteria were comorbidity with other psychiatric disorders, learning disability, specific reading disorder, neurological abnormalities, epilepsy, drug or substance abuse (including nicotine), and previous exposure to stimulant medication. All patients with ADHD scored above threshold on the hyperactivity/inattention scale of the Strength and Difficulty Questionnaire (SDQ) [Goodman and Scott, 1999], whereas all patients with CD scored above threshold on the conduct problems scale of the SDQ (behavioral ratings range from 1 to 10) (see Table I). All patients with CD had an early onset of CD below the age of 10 and also met criteria for ODD. All the patients exhibited overt antisocial behavior (tantrums, fighting, destructiveness, aggression, etc) rather than just covert behavior (lying, stealing, deceptiveness, and truanting) and hence fit criteria for the impulsive‐aggressive subtype, although this categorization was not undertaken formally.
Table I.
Multiple univariate ANOVA group comparisons for age, IQ, and the measures on the SDQ (Strength and Difficulty Questionnaire) for problems with hyperactivity/inattention and conduct disorder
| Psychometric measurement | Healthy controls (N = 20) Mean (SD) | ADHD (N = 14) Mean (SD) | CD (N = 14) Mean (SD) | ANOVA F (df = 2) | P value | Between groups |
|---|---|---|---|---|---|---|
| Age in years, months | 13, 5 (1, 9) | 13, 3 (1, 10) | 12, 6 (2, 3) | 1 | n.s. | |
| IQ estimate | 105 (14) | 100 (14) | 100 (13) | 0.9 | n.s. | |
| SDQ hyperactiviy/inattention | 3 (1) | 9 (2) | 7 (3) | 27 | <0.0001 | C < ADHD** |
| C > CD** | ||||||
| CD < ADHD* | ||||||
| SDQ conduct | 1 (1) | 4 (2) | 8 (2) | 38 | <0.0001 | C < ADHD** |
| C < CD** | ||||||
| CD > ADHD** |
Between‐group post hoc comparisons correcting for multiple testing using LSD;
P < 0.05;
P < 0.01.
Control subjects were 20 children and adolescents with no history of prior diagnosis of ADHD, any other mental or neurological disorder, or of neurotropic medication or drug and substance abuse. They scored below threshold on the SDQ total scale and subcomponent scales of hyperactivity/inattention and conduct problems (see Table I).
All participants scored above cut‐off in the Raven's Standard Progressive Matrices Intelligence Questionnaire (IQ) [Raven, 1960].
Written informed consent/assent from parents was given for all participants, and the study was approved by the local Ethical Committee.
The ADHD group has previously been compared to a larger group of 27 healthy controls [Smith et al., 2006].
One‐way ANOVA showed no significant group differences in age or IQ estimate (see Table I). As expected, groups differed significantly in the hyperactivity/inattention and conduct problems subscale scores of the SDQ. Post hoc t‐test [corrected using least significance difference (LSD)] revealed that ADHD boys scored significantly higher than both controls and CD boys on the hyperactivity/inattention subscale. CD boys scored significantly higher on the conduct problems subscale than both control and ADHD boys (see Table I).
fMRI Paradigm: Switch Task
The 6‐min task was explained to the participants and practiced once before scanning. The task was then presented to the subjects in the scanner via a mirror from a liquid crystal diode projector, and a key pad was used for recording responses onto a PC.
A modified version of the Meiran switch task [Meiran, 1996] was used for this rapid, mixed trial, event‐related fMRI design. The task requires cognitive switching between two spatial dimensions, with minimal working memory load, and is described in detail elsewhere [Rubia et al., 2006; Smith et al., 2004, 2006]. A target dot appeared in one of four corners of a grid with an arrow in the middle of the grid (mean ITI was 2.4 s). If the central arrow was horizontal, the subject had to indicate whether the target was on the left or right side of the grid (left or right button); if the central arrow was vertical, subjects had to indicate whether the target was in the lower or upper half of the grid (up or down button). During switch trials (21%; N = 32), the central arrow changed position, which occurred after every four to six repeat trials (79%; N = 120), that is, at least four repetition times apart for adequate separation of the hemodynamic response (see Fig. 1).
Figure 1.
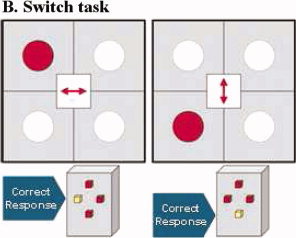
Visual‐spatial Switch task. Subjects have to respond according to the arrow direction in the middle of the grid and switch their response according to the horizontal dimension (indicate whether the dot is left or right of the grid) or the horizontal dimension (indicate whether the dot is up or down of the grid). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The main dependent variable for task performance is the Switch and Repeat reaction times and errors, both of which are typically higher in the Switch than the Repeat condition and has been denominated the Switch reaction time cost and Switch error cost.
The event‐related fMRI analysis subtracts activation associated with repeat trials (where the arrow positions did not change) from activation associated with switch trials (where arrow positions changed and subjects had to make a visual–spatial switch) (switch–repeat) [Rubia, 2006; Smith et al., 2004, 2006].
Subjects Practiced the Switch Task Once Prior to Scanning.
Analysis of performance data
Repeated measures ANOVA with trial type (switch, repeat) as within‐subject variable and group as dependent variable was used for the main variables of the task (reaction times and errors).
fMRI image acquisition
Gradient‐echo echoplanar MR imaging (EPI) data were acquired on a GE Signa 1.5T Horizon LX System (General Electric, Milwaukee, WI) at the Maudsley Hospital, London. Consistent image quality was ensured by a semiautomated quality control procedure. A quadrature birdcage head coil was used for RF transmission and reception. In each of 16 noncontiguous planes parallel to the anterior–posterior commissural, 152 T2*‐weighted MR images depicting BOLD (Blood Oxygen Level Dependent) contrast covering the whole brain were acquired with TE = 40 ms, TR = 1.8 s, flip angle = 90°, in‐plane resolution = 3.1 mm, slice thickness = 7 mm, and slice‐skip = 0.7 mm. This EPI dataset provided almost complete brain coverage.
fMRI image analysis
The fMRI data were analyzed with the XBAM software, version 4, developed at the Institute of Psychiatry (http://www.brainmap.co.uk). This method of analysis makes no normality assumptions, which are usually violated in fMRI data, but instead uses median statistics to control outlier effects and permutation rather than normal theory based inference. Furthermore, the most common test statistic is computed by standardizing for individual difference in residual noise before embarking on second level, multisubject testing using robust permutation‐based methods. This allows a mixed effects approach to analysis—an approach that has recently been recommended following a detailed analysis of the validity and impact of normal theory based inference in fMRI in large number of subjects [Thirion et al., 2007].
Individual level analysis
fMRI data were realigned to minimize motion‐related artefacts [Bullmore et al., 1999] and smoothed using a Gaussian filter (full‐width half maximum, 7.3 mm). Time‐series analysis of individual subject activation was performed using XBAM, with a wavelet‐based resampling method previously described [Bullmore et al., 2001]. Briefly, we first convolved the experimental condition (Switch–Repeat trials), with two Poisson model functions (delays of 4 and 8 s). We calculated the weighted sum of these two convolutions that gave the best fit (least‐squares) to the time series at each voxel. A goodness‐of‐fit statistic (the SSQ‐ratio) was then computed at each voxel consisting of the ratio of the sum of squares of deviations from the mean intensity value due to the model (fitted‐time series), divided by the sum of squares due to the residuals (original time series minus model time series). The appropriate null distribution for assessing significance of any given SSQ‐ratio was established using the wavelet‐based data resampling method [Bullmore et al., 2001] and applying the model‐fitting process to the resampled data. This process was repeated 20 times at each voxel and the data combined over all voxels, resulting in 20 null parametric maps of SSQ‐ratio for each subject, which were combined to give the overall null distribution of SSQ‐ratio. The same permutation strategy was applied at each voxel to preserve spatial correlation structure in the data. Instead of relying on asymptotic distributions such as t or F that assume data normality, we use data‐driven, permutation‐based methods with minimal distributional assumptions that have been shown to be more suitable for fMRI data analysis in samples sizes similar to the ours [Zhang et al., 2009]. Individual SSQ‐ratio maps were transformed into standard space, first by rigid body transformation of the fMRI data into a high‐resolution inversion recovery image of the same subject and then by affine transformation onto a Talairach template [Talairach and Tournoux, 1988].
Group level analysis
A generic activation group map was produced for the experimental condition by calculating the median observed SSQ‐ratio over all subjects at each voxel in standard space and testing them against the null distribution of median SSQ‐ratios computed from the identically transformed wavelet resampled (permuted) data [Brammer et al., 1997]. Then, thresholding at any required level of significance proceeds exactly as for normal t or F tests where the observed statistic is tested against the appropriate critical value from a theoretical rather than a data derived distribution. For cluster‐level testing, the images are first thresholded at a voxel‐wise P‐value of 0.05 to give maximum sensitivity and to avoid type II errors and then grouped (using simple 3D contiguity) into 3D clusters. Next, a cluster‐mass threshold was computed from the distribution of cluster masses in the wavelet‐permuted data such that the final expected number of type I error clusters under the null hypothesis was <1 per whole brain. In this analysis, less than one error clusters were observed at a voxel‐wise P‐level of P < 0.05 and a cluster P‐level of <0.05. Cluster mass rather than a cluster extent threshold was used to minimize discrimination against possible small, strongly responding foci of activation [Bullmore et al., 1999].
ANOVA group difference analysis
Analysis of variance was conducted to test for differences between the three groups using a randomization‐based test for voxel‐ and cluster‐wise activation differences [Bullmore et al., 1999, 2001]. First, the difference between the mean SSQ‐ratio values in each group was calculated at each voxel. The mean ratio was then recalculated 1,000 times at each voxel following random permutation of group membership, and the difference in SSQ‐ratios was calculated after each permutation. The same set of random numbers was used for the permutation at each voxel to preserve spatial correlations in each permuted map. The probability of the original SSQ‐ratio difference under the null hypothesis of no effect of group membership is the number of times we observed an SSQ‐ratio difference as large or larger than the original difference during the permutation process, divided by the total number of permutations. If this value exceeded our threshold for voxel‐level activation, activated voxels were used to identify activation clusters, which were subjected to cluster analysis. For these between‐group comparisons, a P value of P < 0.05 for voxel and P < 0.05 for cluster comparisons was used. Then, statistical measures of BOLD response for each participant were extracted in each of the significant clusters of the one way ANOVA analysis and post hoc t‐tests (corrected using LSDs) were conducted on these measures to identify comparisons between the different groups.
RESULTS
Task Performance
Repeated measures ANOVA with trial type as within‐subject variable and group as dependent variable showed that within all subjects there was a significant linear effect of trial type on errors and reaction times, with all subjects making more errors and having slower reaction times during the switch compared to the repeat trials [F(1,45) = 45; P < 0.0001 for both reaction time and errors].
There was no significant interaction effect for group by error [F(2,45) = 0.5, P < 0.6]. There was, however, a significant interaction effect for group by mean reaction time [F(2,45) = 30, P < 0.009]. Post hoc t‐tests (LSD corrected) showed that this was due to a higher switch effect on reaction times (i.e., Switch reaction time cost) for ADHD compared to CD boys (P < 0.01). No other group differences were observed. The findings show that switching was harder for patients with ADHD than for patients with CD, taking a higher toll on reaction times (see Table II).
Table II.
Main variables of the Switch task by group
| Measure | Controls (N = 20) | ADHD (N = 14) | CD (N = 14) | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| MRT repeat (ms) | 689 | 139 | 777 | 106 | 675 | 180 |
| MRT switch (ms) | 799 | 133 | 861 | 113 | 686 | 113 |
| Error repeat (%) | 2 | 2 | 3 | 4 | 6 | 6 |
| Error switch (%) | 6 | 4 | 8 | 9 | 8 | 9 |
Note: MRT, mean reaction time.
Brain Activation
Movement
The head coil we use is relatively close fitting, and head motion is therefore inherently difficult. We also used chin straps and head pads to further minimize movement. All subjects were within acceptable limits for head movement (below 1.5 mm). Repeated measures ANOVA showed no significant group or group × motion effects in the extent of three‐dimensional motion in x, y, and z translation and rotation.
Within‐Group Activations
Within‐group activations for the contrast of Switch–Repeat are shown in Figure 2.
Figure 2.
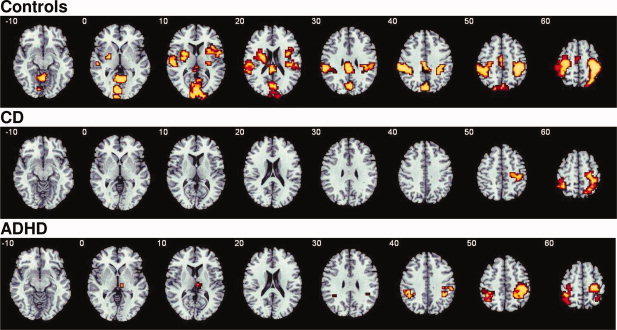
Axial slices for the group activation maps for the three groups for the contrast of Switch versus Repeat trials (P < 0.05 for voxel and cluster levels). Talairach z‐coordinates are indicated for slice distance (in mm) from the intercomissural line. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Controls activated right‐inferior prefrontal cortex, reaching into insula and putamen and in left‐inferior prefrontal cortex, reaching into caudate, putamen, globus pallidus, and insula. They also activated left and right‐superior temporal/inferior parietal lobes, posterior cingulate, precuneus, and occipital regions, supplementary motor area (SMA), and the cerebellar vermis.
Patients with CD activated right medial frontal, pre and postcentral gyri, and inferior/superior parietal lobes, and left‐inferior parietal and pre and postcentral gyri.
Patients with ADHD activated bilateral pre‐ and postcentral and inferior parietal gyri and right thalamus
ANOVA Group Effects
There was a significant group effect for the ANOVA analysis in two clusters: a cluster in right lateral inferior prefrontal cortex, reaching into premotor cortex [BA 47/45/6; Talairach coordinates (Tal. Coord.): 54;11;−2; 30 voxels, P < 0.03] and a cluster in left‐inferior/dorsolateral prefrontal cortex [BA 45/46, Tal. Coord. −40;30;26; 37 voxels, P < 0.05] (see Fig. 3). Post hoc analyses (LSD corrected) showed that the right‐inferior prefrontal activation cluster was significantly reduced in patients in ADHD compared to controls (P < 0.001) and compared to patients with CD (P < 0.04). The cluster in left‐inferior prefrontal cortex was significantly reduced in patients with ADHD compared to both controls (P < 0.012) and to patients with CD (P < 0.021) (see Fig. 3).
Figure 3.
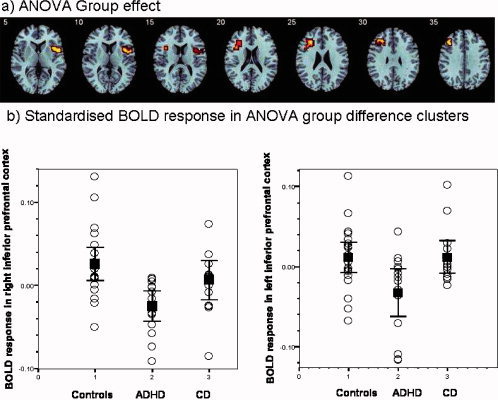
(a) Axial sections showing the ANOVA between‐group difference effects in brain activation for the Switch task (Switch–Repeat trials) (P < 0.05 for voxel and cluster levels). Talairach z‐coordinates are indicated for slice distance (in mm) from the intercommissural line. (b) Below shown is the standardised BOLD response in the left and right inferior prefrontal brain areas of ANOVA group effects that differed between groups for the individual subjects of the three groups as well as the means and the confidence intervals for each group. The activation clusters in right and left inferior prefrontal cortex were reduced in patients with ADHD compared to patients with CD and healthy controls (who did not differ from each other). The left (right) side of the image to the left (right) side of the brain. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
To explore whether children with CD would show brain activation abnormalities when compared separately with either healthy controls or ADHD children alone in two‐group ANOVA comparisons, which might have been missed by the three‐group ANOVA analysis, we compared children with CD separately with healthy comparison children and with children with ADHD at a more conservative P‐value of P < 0.02 to adjust for multiple testing.
Children with CD showed significant reduced activation compared to healthy children in right‐inferior parietal lobe, reaching into precentral gyrus (BA 40; Tal. coord.: 43;−33; 42; 95 voxels) in left‐superior temporal/inferior parietal lobe (BA 38/40; Tal. Coord. −54; −30; 26; 73 voxels) and in left precuneus and cuneus (BA 7/18/19; Tal Coord.: −11; −89; 2; 187 voxels) (see Fig. 4a). No brain regions showed increased activation in patients with CD compared to healthy children.
Figure 4.
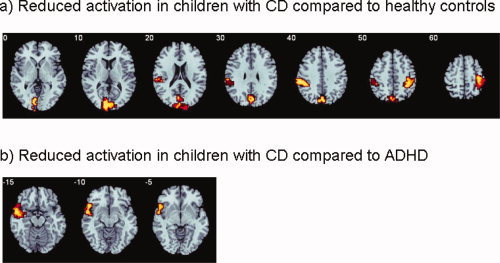
Axial sections showing the ANOVA between‐group difference effects in brain activation for the Switch task (Switch–Repeat trials; P < 0.05 for voxel and P < 0.02 for cluster levels). Talairach z‐coordinates are indicated for slice distance (in mm) from the intercommissural line. (a) Reduced activation in patients with conduct disorder compared to healthy controls in precunues, left superior temporal/inferior parietal, and right inferior parietal lobes. No areas of increased activation were observed in patients with CD compared to healthy controls. (b) Reduced activation in left ventrolateral and superior temporal cortex in patients with ADHD compared to patients with CD. No areas of increased activation were observed in patients with CD compared to ADHD patients. The left (right) side of the image corresponds to the left (right) side of the brain. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Children with CD showed no significant underactivation when compared with patients with ADHD. ADHD patients, however, showed a significant underactivation compared to patients with CD at the junction between left‐ventrolateral prefrontal and superior temporal gyri (BA 47/38; Tal.coord. −43; 4; −24; 149 voxels) (see Fig. 4b).
DISCUSSION
Patients with ADHD and those with CD did not significantly differ from controls in their switch‐task measures. Patients with ADHD, however, had a significantly greater Switch reaction time cost compared to patients with CD, suggesting that the hyperactive/inattentive group found the task more difficult than the disruptive group. The three‐group ANOVA fMRI comparison showed that only patients with ADHD showed disorder‐specific underactivation in right and left inferior prefrontal cortex compared to both healthy controls and patients with CD. Patients with CD showed no disorder‐specific deficits. Only when compared with healthy controls alone, patients with CD showed underactivations in left‐superior temporal, bilateral inferior parietal, and occipital brain regions. The results extend previous findings of disorder‐specific underactivation in inferior prefrontal cortex in ADHD patients compared to patients with CD during tasks of cognitive control to the domain of cognitive flexibility. Together with these previous findings, these data suggest that inferior prefrontal underactivation appears to be a specific neurofunctional biomarker of ADHD when compared with patients with CD that is pervasive across tasks of cognitive control.
Although all participants were slower and made more errors during Switch trials, the Switch reaction time cost was most pronounced in ADHD compared to CD patients. In line with these findings, we have previously found enhanced Switch costs during the same task in younger children with ADHD [Rubia et al., 2007b], but not in younger children with CD [Hobson, 2008]. Meta‐analyses show that children with ADHD have difficulties with cognitive switching in more difficult switching tasks such as the Wisconsin Card Sorting task (WCST) [Willcutt et al., 2005]. In this study, we may not have found switching deficits in the ADHD children compared to controls, because the fMRI adaptation was easier than neuropsychological task versions, due to a higher number of repeat trials interspersed with switch trials to allow for hemodynamic separability of switch trials. Furthermore, the children of this study were older, in the adolescent range, compared to previously tested children in neuropsychological studies [Rubia et al., 2007b]. In children with CD, the evidence for cognitive switching deficits is more controversial. No deficits were found in simpler visual spatial switching tasks [Blair, 2001], and the largest study did not find deficits in the more difficult WCST [Klorman et al., 1999]. Deficits seem to be more consistently observed during reversal tasks that involve reward processing [Aronowitz et al., 1994; Budhani and Blair, 2005; Fisher and Blair, 1998], known to be mediated by the orbitofrontal cortex [Rolls, 2000], rather than switching tasks without reward elements, that are mediated by the inferior prefrontal cortex [Derrfuss et al., 2005; Rubia et al., 2006; Smith et al., 2004].
The fMRI findings in part support this interpretation of dissociative pathologies of inferior prefrontal underactivation in ADHD and orbitofrontal dysfunction in CD. The visual–spatial switch task used in this study, known to be mediated by inferior prefrontal cortex and its connection to parietal and striatal regions [Rubia et al., 2006; Rubia et al., in press; Smith et al., 2004, 2006; Woolley et al., 2008], only elicited performance deficits and brain underactivation in inferior prefrontal cortex in the ADHD compared to the healthy or CD groups. The disorder‐specific underactivation in patients with ADHD in left and right inferior prefrontal lobes is in line with previous findings. Left and right inferior prefrontal cortex has consistently been found to be underactivated in children with ADHD compared to healthy controls during the same switch task [Smith et al., 2006] as well as during other tasks of inhibitory control [Dickstein et al., 2006; Durston et al., 2006; Pliszka et al., 2006; Rubia et al., 1999, 2005]. Furthermore, left and right inferior prefrontal underactivation was disorder‐specific in partly overlapping groups of ADHD patients when compared with controls and CD patients during other executive function tasks, of motor response inhibition [Rubia et al., 2008], interference inhibition [Rubia et al., 2009b] and sustained attention [Rubia et al., 2009c]. Right and left inferior prefrontal cortex was also specifically underactivated in ADHD children when compared with healthy and OCD children during the same switching task and a response inhibition task [Rubia et al., in press]. The findings of this study therefore extend our previous evidence for disorder‐specificity of inferior prefrontal dysfunction in patients with ADHD compared to CD to yet another domain of cognitive control and hence suggest that inferior prefrontal dysfunction may be a disorder‐specific neurofunctional biomarker of ADHD that is pervasive across cognitive control tasks.
CD patients showed no disorder‐specific underactivations in the three‐group comparison. This is not in line with our hypothesis of paralimbic dysfunctions in CD. It differs from our previous findings of disorder‐specific underactivations in the same patients with CD when compared with a larger group of ADHD patients and controls in the orbitofrontal cortex during reward, in limbic and paralimbic regions during sustained attention [Rubia et al., 2009c], and in superior temporal and inferior parietal lobes during inhibition failures [Rubia et al., 2008]. However, we also did not observe disorder‐specific brain abnormalities in patients with CD compared to healthy and ADHD participants during a Simon task of interference inhibition and during attention allocation [Rubia et al., 2009b]. Like with cognitive switching, interference inhibition in the Simon task and attention allocation seem not to be key dysfunctions in patients with CD [Banaschewski et al., 2005; Herba et al., 2006]. It is thus likely that paralimbic abnormalities in patients with CD are only observed during tasks that are most sensitive to the pathology. This would be in line with the observations of paralimbic abnormalities in severely disruptive patients with psychopathic traits compared to ADHD patients during functions that have consistently been shown to be impaired in these children such as a reward reversal [Finger et al., 2008] and emotion processing [Marsh et al., 2008]. It thus appears that disorder‐specific brain dysfunctions in paralimbic brain regions in patients with CD may only be observed in tasks that have most consistently been shown to be sensitive to CD such as sustained attention, motor inhibition, reward‐related functions, and emotion processing.
When we compared patients with CD to the healthy control group alone, however, we observed brain dysfunctions in the disruptive group in left superior temporal, bilateral parietal, and occipital brain regions. The fact that this was only observed in comparison to healthy controls but not to ADHD patients suggests that the two patient groups shared these dysfunction regions. The temporal lobe has been found to be reduced in size in children with CD [Huebner et al., 2008; Kruesi et al., 2004] and been associated with aggression [Herzberg and Fenwick, 1988]. The parietal lobes are thought to mediate visual–spatial executive attention, necessary for cognitive switching [Rushworth, 2006; Smith et al., 2004]. Precuneus and cuneus were also underactivated in CD patients compared to controls during the switch trials and are important areas for visual–spatial attention to saliency [Mesulam et al., 2001; Small et al., 2003]. We have previously found superior temporal and inferior parietal regions as well as precuneus and posterior cingulate gyri to be underactivated in patients with CD compared to healthy controls (but, like in this study, shared with ADHD) during other attention functions that require visual–spatial attention to saliency such as error monitoring during inhibition failures [Rubia et al., 2008] and interference inhibition [Rubia et al., 2009b]. A weakness of the activation in the temporoparietal junction could possibly be related to visual–spatial executive attention deficits in patients with CD. Shared abnormal precuneus activation has also been observed in children with psychopathic traits and with ADHD during errors in a reversal task [Finger et al., 2008]. Reduced temporoparietal and precuneus/posterior cingulate activation is a consistent finding in ADHD patients during tasks that require visual–spatial attention to saliency such as reward, error monitoring, and oddball tasks [Rubia et al., 2005, 2007a, 2008, 2009a, b, c; Stevens et al., 2007; Tamm et al., 2006], and it appears that this deficit may be shared with CD.
A limitation of this study is the relatively small subject numbers. A strength, however, is the careful selection of representative patient subgroups of each disorder, of childhood‐onset CD and of the hyperactive‐inattentive combined subtype of ADHD, who differed from each other in conduct and ADHD problems. CD is considered a highly heterogenous disorder [Frick and Ellis, 1999] and, in this study, we have tested the childhood‐onset CD subtype to maximize homogeneity. Other subtypes, however, have been suggested, such as those associated with predominant psychopathic and those with predominant impulsive‐aggressive traits [Frick and Ellis, 1999]. Although not formally assessed, patients of this study exhibited overt rather than just covert antisocial behavior and hence would fit criteria for the impulsive‐aggressive subtype. We did not test for psychopathic traits, as our sample would have been too small for further subtype analyses. It is conceivable, however, that disruptive patients with psychopathic traits show different neurobiological abnormalities than those with predominant impulsive‐aggressive traits during cognitive switching. A typical design feature of cognitive switching tasks is that switch trials are typically less in number than repeat trials. The reduced number of switch compared to repeat trials in this task design makes switch trials also rare, oddball trials compared to the more frequent repeat trials and may hence coactivate brain systems of visual–spatial attention to saliency. Abnormal activation in relation to switch trials in the patient groups could therefore potentially at least in part also reflects abnormalities in visual–spatial attention allocation systems.
In conclusion, we show, in this fMRI study of cognitive switching, that only children with ADHD have disorder‐specific dysfunctions when compared with patients with CD, in right and left inferior prefrontal cortices. This extends our previous findings of disorder specificity of these brain regions in ADHD patients when compared with patients with CD during three other executive function tasks, of sustained attention, and of motor and interference inhibition [Rubia et al., 2008, 2009b, c] and when compared with patients with OCD during the same task [Rubia et al., in press]. The findings of this study, therefore, together with the previous evidence, confirm and extend disorder‐specific neurofunctional deficits in ADHD patients in the inferior prefrontal cortex during cognitive control tasks. Replication in larger samples and comparisons with other childhood disorders, however, are necessary to further establish the disorder‐specificity of this dysfunction. This study represents a further step toward the clarification of the specificity of the underlying pathophysiology of these two behaviorally overlapping disorders. Disentangling the underlying pathophysiology of these two disorders will hopefully aid to a more objective diagnosis and help with the development of disorder‐tailored treatment.
REFERENCES
- American Psychiatric Association ( 1994): Diagnostic and Statistical Manual of Mental Disorders. Association. AP, editor. Washington, DC. [Google Scholar]
- Aronowitz B, Liebowitz M, Hollander E, Fazzini E, Durlachmisteli C, Frenkel M, Mosovich S, Garfinkel R, Saoud J, Delbene D ( 1994): Neuropsychiatric and neuropsychological findings in conduct disorder and attention‐deficit hyperactivity disorder. J Neuropsychiatry Clin Neurosci 6: 245–249. [DOI] [PubMed] [Google Scholar]
- Banaschewski T, Hollis C, Oosterlaan J, Roeyers H, Rubia K, Willcutt E, Taylor E ( 2005): Towards an understanding of unique and shared pathways in the psychopathophysiology of ADHD. Dev Sci 8: 132–140. [DOI] [PubMed] [Google Scholar]
- Blair RJR ( 2001): Neurocognitive models of aggression, the antisocial personality disorders, and psychopathy. J Neurol Neurosurg Psychiatry 71: 727–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR, Peschardt KS, Budhani S, Mitchell DGV, Pine DS ( 2006): The development of psychopathy. J Child Psychol Psychiatry 47: 262–275. [DOI] [PubMed] [Google Scholar]
- Brammer MJ, Bullmore ET, Simmons A, Williams SC, Grasby PM, Howard RJ, Woodruff PW, Rabe‐Hesketh S ( 1997): Generic brain activation mapping in functional magnetic resonance imaging: A nonparametric approach. Magn Reson Imaging 15: 763–770. [DOI] [PubMed] [Google Scholar]
- Budhani S, Blair RJR ( 2005): Response reversal and children with psychopathic tendencies: Success is a function of salience of contingency change. J Child Psychol Psychiatry 46: 972–981. [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Suckling J, Overmeyer S, Rabe‐Hesketh S, Taylor E, Brammer MJ ( 1999): Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Trans Med Imaging 18: 32–42. [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Long C, Suckling J, Fadili J, Calvert G, Zelaya F, Carpenter TA, Brammer M ( 2001): Colored noise and computational inference in neurophysiological (fMRI) time series analysis: Resampling methods in time and wavelet domains. Human Brain Mapping 12: 61–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrfuss J, Brass M, Neumann J, von Cramon DY ( 2005): Involvement of the inferior frontal junction in cognitive control: Meta‐analyses of switching and Stroop studies. Human Brain Mapping 25: 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein SG, Bannon K, Castellanos FX, Milham MP ( 2006): The neural correlates of attention deficit hyperactivity disorder: An ALE meta‐analysis. J Child Psychol Psychiatry 47: 1051–1062. [DOI] [PubMed] [Google Scholar]
- Durston S, Tottenham NT, Thomas KM, Davidson MC, Eigsti IM, Yang YH, Ulug AM, Casey BJ ( 2003): Differential patterns of striatal activation in young children with and without ADHD. Biol Psychiatry 53: 871–878. [DOI] [PubMed] [Google Scholar]
- Durston S, Mulder M, Casey BJ, Ziermans T, van Engeland H ( 2006): Activation in ventral prefrontal cortex is sensitive to genetic vulnerability for attention‐deficit hyperactivity disorder. Biol Psychiatry 60: 1062–1070. [DOI] [PubMed] [Google Scholar]
- Finger EC, Marsh AA, Mitchell DG, Reid ME, Sims C, Budhani S, Kosson DS, Chen G, Towbin KE, Leibenluft E, Pine DS, Blair JR ( 2008): Abnormal ventromedial prefrontal cortex function in children with psychopathic traits during reversal learning. Arch Gen Psychiatry 65: 586–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher L, Blair RJR ( 1998): Cognitive impairment and its relationship to psychopathic tendencies in children with emotional and behavioral difficulties. J Abnorm Child Psychol 26: 511–519. [DOI] [PubMed] [Google Scholar]
- Frick PJ, Ellis M ( 1999): Callous‐unemotional traits and subtypes of conduct disorder. Clin Child Fam Psychol Rev 2: 149–168. [DOI] [PubMed] [Google Scholar]
- Goldberg D, Murray R, editors ( 2002): Maudsley Handbook of Practical Psychiatry, 4th ed Oxford: Oxford University Press. [Google Scholar]
- Goodman R, Scott S ( 1999): Comparing the strengths and difficulties questionnaire and the child behavior checklist: Is small beautiful? J Abnorm Child Psychol 27: 17–24. [DOI] [PubMed] [Google Scholar]
- Herba CM, Tranah T, Rubia K, Yule W ( 2006): Conduct problems in adolescence: Three domains of inhibition and effect of gender. Dev Neuropsychol 30: 659–695. [DOI] [PubMed] [Google Scholar]
- Herzberg JL, Fenwick PB ( 1988): The aetiology of aggression in temporal‐lobe epilepsy. Br J Psychiatry 153: 50–55. [DOI] [PubMed] [Google Scholar]
- Hobson C ( 2008): The neuropsychological profile relating to behaviour problems in adolescents. London: King's College; 359 p. [Google Scholar]
- Huebner T, Vloet TD, Marx I, Konrad K, Fink GR, Herpertz SC, Herpertz‐Dahlmann B ( 2008): Morphometric brain abnormalities in boys with conduct disorder. J Am Acad Child Adolesc Psychiatry 47: 540–547. [DOI] [PubMed] [Google Scholar]
- Klorman R, Hazel‐Fernandez LA, Shaywitz SE, Fletcher JM, Marchione KE, Holahan JM, Stuebing KK, Shaywitz BA ( 1999): Executive functioning deficits in attention‐deficit hyperactivity disorder are independent of oppositional defiant or reading disorder. J Am Acad Child Adolesc Psychiatry 38: 1148–1155. [DOI] [PubMed] [Google Scholar]
- Kruesi MJP, Casanova MF, Mannheim G, Johnson‐Bilder A ( 2004): Reduced temporal lobe volume in early onset conduct disorder. Psychiatry Res‐Neuroimag 132: 1–11. [DOI] [PubMed] [Google Scholar]
- Lueger RJ, Gill KJ ( 1990): Frontal‐lobe cognitive dysfunction in conduct disorder adolescents. J Clin Psychol 46: 696–706. [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Michell DGV, Sims C, Kosson DS, Towbin KE, Leibenluft E, Pine DS, Blair RJS ( 2008): Reduced amygdala resonse to fearful expressions in children and adolescents with callous‐unemotional traits and disruptive behaviour disorders. Am J Psychiatry 165: 712–720. [DOI] [PubMed] [Google Scholar]
- Meiran N ( 1996): Reconfiguration of processing mode prior to task performance. J Exp Psychol: Learn Mem Cogn 22: 1423–1442. [Google Scholar]
- Mesulam MM, Nobre AC, Kim YH, Parrish TB, Gitelman DR ( 2001): Heterogeneity of cingulate contributions to spatial attention. Neuroimage 13: 1065–1072. [DOI] [PubMed] [Google Scholar]
- Pliszka SR, Glahn DC, Semrud‐Clikeman M, Franklin C, Perez R, Xiong JJ ( 2006): Neuroimaging of inhibitory control areas in children with attention deficit hyperactivity disorder who were treatment naive or in long‐term treatment. Am J Psychiatry 163: 1052–1060. [DOI] [PubMed] [Google Scholar]
- Raven J ( 1960): Guide to the Standard Progressive Matrices, London. London: HK Lewis. [Google Scholar]
- Rolls ET ( 2000): The orbitofrontal cortex and reward. Cerebral Cortex 10: 284–294. [DOI] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, Bullmore ET ( 1999): Hypofrontality in attention deficit hyperactivity disorder during higher‐order motor control: A study with functional MRI. Am J Psychiatry 156: 891–896. [DOI] [PubMed] [Google Scholar]
- Rubia K, Taylor E, Smith AB, Oksanen H, Overmeyer S, Newman S ( 2001): Neuropsychological analyses of impulsiveness in childhood hyperactivity. Br J Psychiatry 179: 138–143. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Toone B, Taylor E ( 2005): Abnormal brain activation during inhibition and error detection in medication‐naive adolescents with ADHD. Am J Psychiatry 162: 1067–1075. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, Brammer M ( 2006): Progressive increase of frontostriatal brain activation from childhood to adulthood during event‐related tasks of cognitive control. Human Brain Mapp 27: 973–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Taylor E ( 2007a) Temporal lobe dysfunction in medication‐naive boys with attention‐deficit/hyperactivity disorder during attention allocation and its relation to response variability. Biol Psychiatry 62: 999–1006. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer M, Taylor E ( 2007b) Performance of children with attention deficit hyperactivity disorder (ADHD) on a test battery for impulsiveness. Child Neuropsychol 30: 659–695. [DOI] [PubMed] [Google Scholar]
- Rubia K, Halari R, Smith AB, Mohammed M, Scott S, Giampietro V, Taylor E, Brammer MJ ( 2008): Dissociated functional brain abnormalities of inhibition in boys with pure conduct disorder and in boys with pure attention deficit hyperactivity disorder. Am J Psychiatry 165: 889–897. [DOI] [PubMed] [Google Scholar]
- Rubia K, Cubillo A, Smith AB, Woolley J, Heyman I, Brammer MJ: Disorder‐specific dysfunction in right inferior prefrontal cortex during two inhibition tasks in boys with attention‐deficit/hyperactivity disorder (ADHD) compared to boys with obsessive‐compulsive disorder (OCD). Hum Brain Mapp (in press). DOI: 10.1002/hbm.20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Halari R, Christakou A, Taylor E ( 2009a): Impulsiveness as a disturbance of timing: Brain dysfunction in ADHD during temporal processes and normalisation with a dopaminergic agonist. Philos Trans R Soc (Biol Sci) 346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Halari R, Smith A, Mohammed M, Scott S, Brammer M ( 2009b): Shared and disorder‐specific prefrontal abnormalities in boys with pure attention‐deficit/hyperactivity disorder compared to boys with pure CD during interference inhibition and attention allocation. J Child Psychol Psychiatry 50: 669–678. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith A, Halari R, Matukura F, Mohammad M, Taylor E, Brammer M ( 2009c) Disorder‐specific dissociation of orbitofrontal dysfunction in boys with pure conduct disorder during reward and ventrolateral prefrontal dysfunction in boys with pure attention‐deficit/hyperactivity disorder during sustained attention. Am J Psychiatry 166: 83–94. [DOI] [PubMed] [Google Scholar]
- Rushworth M ( 2006): Combining neuroimaging techniques to examine frontal and parietal cortical interactions in the control of attention and action. Neurosci Res 55: S5–S5. [Google Scholar]
- Small DM, Gitelman DR, Gregory MD, Nobre AC, Parrish TB, Mesulam MM ( 2003): The posterior cingulate and medial prefrontal cortex mediate the anticipatory allocation of spatial attention. Neuroimage 18: 633–641. [DOI] [PubMed] [Google Scholar]
- Smith AB, Taylor E, Brammer M, Rubia K ( 2004): Neural correlates of switching set as measured in fast, event‐related functional magnetic resonance imaging. Human Brain Mapp 21: 247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AB, Taylor E, Brammer M, Toone B, Rubia K ( 2006): Task‐specific hypoactivation in prefrontal and temporoparietal brain regions during motor inhibition and task switching in medication‐naive children and adolescents with attention deficit hyperactivity disorder. Am J Psychiatry 163: 1044–1051. [DOI] [PubMed] [Google Scholar]
- Spencer T, Biederman J, Wilens T ( 1999): Attention‐deficit/hyperactivity disorder and comorbidity. Pediatric Clin N Am 46: 915–927, vii. [DOI] [PubMed] [Google Scholar]
- Sterzer P, Stadler C, Krebs A, Kleinschmidt A, Poustka F ( 2005): Abnormal neural responses to emotional visual stimuli in adolescents with conduct disorder. Biol Psychiatry 57: 7–15. [DOI] [PubMed] [Google Scholar]
- Stevens MC, Pearlson GD, Kiehl KA ( 2007): An FMRI auditory oddball study of combined‐subtype attention deficit hyperactivity disorder. Am J Psychiatry 164: 1737–1749. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P ( 1988): Co‐Planar Stereotaxic Atlas of the Brain. New York: Thieme. [Google Scholar]
- Tamm L, Menon V, Reiss AL ( 2006): Parietal attentional system aberrations during target detection in adolescents with attention deficit hyperactivity disorder: Event‐related fMRI evidence. Am J Psychiatry 163: 1033–1043. [DOI] [PubMed] [Google Scholar]
- Taylor E, Sandberg S, Thorley G, Giles S ( 1991): The Epidemiology of Childhood Hyperactivity. Maudsley Monographs Series 33. Oxford, UK: Oxford University Press. [Google Scholar]
- Toupin J, Dery M, Pauze R, Mercier H, Fortin L ( 2000): Cognitive and familial contributions to conduct disorder in children. J Child Psychol Psychiatry 41: 333–344. [PubMed] [Google Scholar]
- Thirion B, Pinel P, Meriaux S, Roche A, Dehaene S, Poline JB ( 2007): Analysis of a large fMRI cohort: Statistical and methodological issues for group analyses. Neuroimage 35: 105–120. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF ( 2005): Validity of the executive function theory of attention‐deficit/hyperactivity disorder: A meta‐analytic review. Biol Psychiatry 57: 1336–1346. [DOI] [PubMed] [Google Scholar]
- Woolley J, Heyman I, Brammer M, Frampton I, McGuire PK, Rubia K ( 2008): Brain activation in paediatric obsessive‐compulsive disorder during tasks of inhibitory control. Br J Psychiatry 192: 25–31. [DOI] [PubMed] [Google Scholar]
- Zhang H, Nichols TE, Johnson TD ( 2009): Cluster mass inference via random field theory. Neuroimage 44: 51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]


