Abstract
Recent imaging studies have evidenced various cerebral patterns dependent on educational level during cognitive tasks in neurodegenerative diseases. Determining relationships between educational status and cerebral activation during cognitive demands in physiological conditions may help to better understand the role of education on cognitive efficacy and functional reorganisation in pathological conditions. We proposed to analyse by functional MRI (fMRI) the relationship between educational status and cerebral activation during various attentional requests in healthy young adults. Twenty healthy young adults completed four successive conditions of a Go/No‐go test of increasing complexity under fMRI. An effect of education was observed on attentional performances. Both in‐scanner response times and cerebral activation increased during the Go/No‐go paradigm. Healthy subjects with higher education exhibited higher activity in cerebellum and lower activity in medial prefrontal and inferior parietal regions compared with the healthy subjects with lower educational levels while performing the conditions of Go/No‐go task. Our data evidence the influence of education on automatized strategies in healthy adults by modulating a functional balance of activation between cerebral cortex and cerebellar regions during attentional processes. Hum Brain Mapp 2009. © 2008 Wiley‐Liss, Inc.
Keywords: cognition, fMRI, education
INTRODUCTION
Accumulating evidence indicates beneficial effects of cognitive enrichment, as indicated by level of education, complexity of work environment or nature of leisure activities, in protecting against the development of age‐associated dementia (for review see Milgram et al.,2006]. Among these factors of enrichment, the influence of educational level and leisure activities have been demonstrated on the incidence of Alzheimer's disease (AD) patients [Fabrigoule et al.,1995; Stern et al.,1994; Wilson et al.,2002]. In AD patients, an inverse relationship between education and resting cortical perfusion deficit has been found [Stern et al.,1992,1993,1994,1995] and an effect of the educational level was demonstrated on both cognitive performances and brain activity [Stern et al.,1999,2000]. The specific cognitive compensatory phenomenon depending on the educational level and called cognitive reserve may temporarily protect patients with a high education from disabling cognitive symptoms [Le Carret et al.,2005; Wilson et al.,2002].
Additionally, past studies of normal aging showed the importance of educational level earlier in life on cognitive ability at a later time, suggesting that cognitive challenges experienced during life, accumulate reserve and allow cognitive function to be maintained at older age. Such protective effect of the educational level on cognitive performances has been shown in elderly healthy subjects during memory tasks [Hultsch et al.,1999; Scarmeas et al.,2003; Springer et al.,2005]. Moreover, a recent MRI study during memory tasks has revealed that topography of cerebral recruitment is age‐dependent and varied according to cognitive reserve [Stern et al.,2005]. Staff et al. [2004] showed that education level and occupational attainment specifically contribute to cerebral reserve independently of morphological parameters such as intracranial volume. These results lead to the hypothesis of differential cognitive and cerebral strategies occurring in elderly subjects with different educational level suggesting that cognitive reserve could be an expression of the ability of compensatory reallocation of brain function to withstand a greater degree of age‐related changes while maintaining intact functioning.
The question arises whether cognitive reserve could act differentially in healthy young and older people affecting specific cognitive processes, such as memory and attention for instance. At present, few studies were performed in healthy young adults, but they strongly suggest that cognitive reserve is associated with neuropsychological differences in memory processing [Stern et al.,2003]. The correlation between educational level and frontal or temporal activity were inversed in elderly subjects when compared with young adults [Springer et al.,2005]. Indeed, although attentional processes are known to interact with numerous domains of cognition particularly with memory, at present none of the studies has investigated the role of education on the attentional processing in healthy young adults. The purpose of the present work was to explore whether different ways of attentional demand processing are associated with different educational levels in healthy young adults.
With the assumption that people with higher cognitive reserve might recruit brain networks with greater capacity and/or efficacy while coping with increased task difficulty than people with lower cognitive reserve, we aimed to investigate the neuronal activity in healthy young adults with different educational levels while performing a cognitive task of increasing attentional load. We hypothesized that educational dependent changes in brain activation during a cognitive task, may reflect the efficacy in cognitive processing and strategies. This postulates that task‐related neural processing in individuals would be a function of educational level.
Using fMRI and a Go/No‐go paradigm, a cognitive test measuring attentional and inhibition capacities, we proposed to investigate whether differential cognitive or cerebral strategies, which depend on education are already present in healthy young adults during attentional requests. Moreover, in order to know if these strategies depend on cognitive resources, we designed the Go/No‐go test according to three successive conditions with various attentional load of increasing complexity (TA, IG, and CG). In addition, the cognitive paradigm included a condition of reversing instruction (RG) for which we hypothesized a similar level of complexity than IG, to assess education‐related cerebral strategies in healthy young adults for a comparable level of attentional load.
MATERIALS AND METHODS
Subjects
Twenty right‐handed healthy subjects, 11 men and 9 women, were included in the study (mean age: 32.45 ± 9.77 years; mean education: 14.4 ± 3.55 years). According to the Declaration of Helsinki, all subjects had given written informed consent before entering the study, which was approved by the local ethics committee. Subjects with any neurological diseases or known psychiatric illnesses, history of head trauma or drug abuse were not included in this study.
fMRI Protocol
Prior to any evaluation all subjects underwent a fMRI session for which we used the Go/No‐go paradigm with increasing difficulty under four successive conditions. For the first condition, the tonic alertness (TA) task, the subject had to click a button each time a figure (round) appeared on a screen. The second condition, the Initial Go/No‐go (IG), included two figures, a target (round) and a distracter (triangle). The instruction was to push a button only when the round figure appeared. Under the third condition, the Reversal Go/No‐go (RG), the target and the distracter were reversed. The triangle became the relevant visual stimulus whereas the round figure should be ignored. For the last condition, the Complex Go/No‐go (CG), the test included two relevant targets (rhombus, hexagon) for five distracters (square, rectangle, trapezoid, cross and parallelogram). The protocol consisted of four series, one for each Go/No‐go condition. A series included four activation block periods interleaved with four rest periods, each period lasting 24 s. An activation block period corresponded to 70% of target figures and 30% of distracter figures (except for the first condition). Figures were presented during 1250 ms with inter‐stimulus intervals of 250, 500, or 750 ms. The experimental designs are schematically presented in Figures 1 and 2. The same pseudo‐random sequence was used along the four Go/No‐go conditions. Figures appeared in black on a white screen. During the rest period, subjects passively viewed the white screen. All the subjects performed the same sequence for the four conditions of the paradigm. The stimuli were delivered by an Apple Macintosh Powerbook with Psyscope software and displayed via a video system on a projection screen, which could be seen by the subject in the magnet by means of a mirror placed on the head coil. Instruction of each condition was presented on the white screen before the task was performed by the subjects. Subjects were instructed to respond to targets by pressing a moss with their last three fingers of their right hand. Response times and number of errors were recorded via a button box system designed for the study. Anova with repeated measures (P < 0.05) and the Spearman rank‐order coefficient (P < 0.05; r ≥ 0.30) were used for in pairs comparisons between in‐scanner response time Go/No‐go conditions and for correlations between in‐scanner response times and educational level, respectively.
Figure 1.
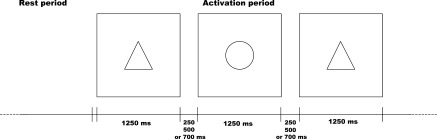
fMRI paradigm 1250 ms: delay for the presentation of the figures. 250, 500, or 750 ms: delay between presentation of figures. (ms, milliseconds).
Figure 2.
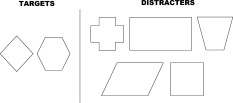
Items of the complex Go/No‐go condition.
fMRI Acquisition
MRI data were collected on a 1.5 T Gyroscan ACS NT Power Track 6000 (Philips Medical System, Best, Netherlands). Functional scans were acquired using a T2*‐weighted single shot gradient echo, echo‐planar sequence (TR/TE = 3000/60 ms, Flip angle = 90°, Matrix = 64 × 64; FOV = 230 mm). Each scan included 30 slices parallel to the AC‐PC (Anterior Commissure ‐ Posterior Commissure) covering the whole cortex (no gap, thickness 3.5 mm). For each series, 64 dynamic scans were acquired with three dummy scans to reach steady‐state magnetization. A high‐resolution T1‐weighted scan was also acquired to obtain a morphological reference (TR/TE = 266/25 ms, Matrix = 256 × 256, Field of view = 230 mm) including 30 slices whose characteristics were identical to the functional ones.
fMRI Analysis
All data were analysed using SPM2 (Statistical Parameter Mapping, Wellcome Department of Imaging Neuroscience, London, UK) and MATLAB 7.0.1 (The Mathworks, Natick, MA).
For each subject and each of the four Go/No‐go condition series, the dynamic scans were realigned to the first scan to correct for head movement, normalized to the standard Montreal Neurological Institute space (MNI) and spatially filtered by applying a 10 × 10 × 3.5 mm3 Gaussian kernel.
High‐pass filtering (cut‐off 128 s) was performed to remove low frequency artefacts as signal drift, and cardiac and respiratory effects. Then, a general linear model was used to model the data [Friston et al.,1995], the functional time series were assessed by a boxcar model convoluted with an assumed hemodynamic response. After estimation of the model parameters, a linear contrast, activation vs rest, was applied to the defined parameters to test the effect of each condition. These results in four Go/No‐go condition contrasts by participants (i) TA‐Rest (ii) IG‐Rest (iii) RG‐Rest (IV) CG‐Rest, which were entered in a second‐level random effect analysis to allow inference at the population level. First, one sample t‐test was used to identify the activation pattern of each condition. Then, the increase in complexity for the Go/No‐go paradigm was investigated by between‐task contrasts (paired t‐tests) between Go/No‐go condition contrasts as follow IG > TA; CG > IG, and CG > TA. In addition, the paired t‐test RG > IG was also assessed in order to investigate cerebral activation without attentional load differences. For the one sample t‐tests, clusters of activated voxels were identified with a global height threshold of P < 0.0001 (uncorrected for multiple comparisons) and an extent threshold of 20 voxels. For the paired t‐tests, clusters of activated voxels were identified with a global height threshold of P < 0.002 (uncorrected for multiple comparisons) and an extent threshold of 10 voxels. Anatomical localization was performed using AAL atlas [Tzourio‐Mazoyer et al.,2002] and Brodmann's areas were assigned to activate clusters after converting the MNI coordinates to the Talairach coordinates.
Additionally, the relationships between BOLD response with in‐scanner performances and BOLD response with educational level were investigated by linear regression. The four Go/No‐go condition contrasts from all subjects were entered in two second‐level simple regression models in SPM2 with in‐scanner performances and number of years of education included as regressors respectively. The threshold for significant positive or negative correlation across the brain was set to P < 0.002 (uncorrected) and an extent threshold of 10 voxels. Because of problems with the response collection device, one subject was excluded from the regression analysis concerning response times.
Neuropsychological Assessment
All subjects underwent a neuropsychological assessment of several cognitive functions outside the fMRI context. Neuropsychological testing was carried out on the same day as MRI acquisition by a trained neuropsychologist, and was proposed after the fMRI protocol in order not to influence the performance of the subjects for the fMRI evaluation. Subjects had never before any neuropsychological testing. The neuropsychological assessment included all the tests of the brief repeatable battery (BRB) [Rao et al.,1990], which is widely used in the assessment of cognitive impairment in multiple sclerosis. The BRB included a 12‐word version of the Selective Reminding Test that evaluates short (Long Term Storage, SRT‐LTS, and Consistent Long Term Retrieval, SRT‐CLTR) and long term (Delayed Recall, SRT‐DR) verbal memory; the Spatial Recall Test that evaluates short‐term (SPART) and long‐term (Delayed Recall, SPART‐DR) visuospatial memory; the oral version of the Symbol Digit Modalities Test (SDMT) investigating sustained and complex attention, information processing speed, and working memory; the 60‐trial versions of the Paced Auditory Serial Addition Task (3‐s and 2‐s versions (PASAT 3s, PASAT 2s) which evaluate sustained and complex attention, information processing speed, and working memory; and the Word List Generation test (WLG) assessing semantic verbal fluency. The neuropsychological evaluation also included the Similarities subtest of the Wechsler adult intelligence scale‐revised (Similarities), evaluating conceptualisation [Wechsler et al.,1981] and the Stroop test [Amieva et al.,2002], evaluating the difficulty in inhibiting an automatic response to word reading. The scores of the neuropsychological tests were the numbers of correct answers with the exception of the Stroop test, which was scored as the ratio between the time of the interference Stroop task and the time of the colour naming task. The Spearman rank‐order coefficient was used to test the correlation between the scores of the neuropsychological tests and the educational level (P < 0.05; r ≥ 0.30). As two subjects presented missing values for the neuropsychological assessment, they were not included in the Spearman analysis according to education. No correlation was performed between neuropsychological data and brain activation.
RESULTS
Behavioural Results
The neuropsychological performances of healthy subjects, tested after the fMRI session, are presented in Table I. All the subjects presented normal cognitive values compared with values obtained from a population of healthy subjects [Deloire et al.,2005] according to age, sex and educational level. Significant correlations were found between education and four cognitive tests of the neuropsychological battery: SDMT (P = 0.01; r = 0.62), PASAT 3s (P = 0.01; r = 0.63), PASAT 2s (P = 0.009; r = 0.66) and Similarities sub‐test of the WAIS‐R (P = 0.02; r = 0.64).
Table I.
Neuropsychological scores of healthy subjects (n =18) and their correlation with educational level
| Neuropsychological tests | Scores | Spearman correlation with educational level | |
|---|---|---|---|
| R | P‐value | ||
| SRT‐LTS | 59.78 ± 6.92 | 0.20 | 0.50 |
| SRT‐CLTR | 55.17 ± 9.78 | 0.13 | 0.69 |
| SRT‐DR | 11.50 ± 0.86 | 0.10 | 0.69 |
| SPART | 24.28 ± 3.04 | −0.17 | 0.35 |
| SPART‐DR | 8.17 ± 2.31 | 0.07 | 0.84 |
| SDMT | 59.06 ± 10.34 | 0.62 | 0.01 |
| PASAT 3s | 48.78 ± 7.53 | 0.63 | 0.01 |
| PASAT 2s | 37.72 ± 9.75 | 0.66 | 0.009 |
| WLG | 28.22 ± 6.58 | 0.41 | 0.12 |
| Similarities | 22.06 ± 4.74 | 0.64 | 0.02 |
| Stroop | 1.73 ± 0.92 | 0.13 | 0.69 |
For all tests, the results are expressed as mean ± standard deviation number of correct answers except for Stroop test (ratio time to the interference Stroop task/time to the colour naming task). P‐values over 0.05 were considered as nonsignificant. SRT, selective reminding test evaluating short term verbal memory (LTS, long term storage; CLTR, consistent long term retrieval); SRT‐DR, delayed recall evaluation long term verbal memory; SPART, spatial recall test evaluating short term visuospatial memory; SPART‐DR, delayed recall evaluating long term visuospatial memory; SDMT, symbol digit modalities test evaluating attention, information processing speed and working memory; PASAT, paced auditory serial addition test (3 and 2‐s versions) evaluating attention, information processing speed, and working memory; WLG, word list generation test evaluating verbal fluency; similarities subtest of the Wechsler adult intelligence scale–revised evaluating conceptualisation; Stroop test evaluating inhibition. Two subjects presenting missing values for the neuropsychological assessment were not included in the Spearman correlation according to education.
In‐Scanner Performances
The mean (SD) of the response times (ms) for TA condition was 343.36 (68.53), for IG 415.57 (44.25), for RG 422.33 (49.54) and for CG 530.40 (74.14). Taking into account the very low level of the number of errors (mean ± SD) made by subjects for all the conditions of the Go/No‐go task (IG: 1.21 ± 1.36, RG: 2 ± 1.45, and CG: 0.6 ± 1.27), only response times, and more precisely, only response times corresponding to good answers, were considered as the index of in‐scanner subject performances. Response times significantly increased along the Go/No‐go conditions (P < 0.0001), except for comparison RG > IG (P = 0.54).
Response times significantly correlated with education for IG (P = 0.01; r = −0.61), and RG conditions (P = 0.04; r = −0.44).
Cerebral Activation
The results of the one‐sample t‐tests for each condition of the Go/No‐go task are presented in Figure 3.
Figure 3.
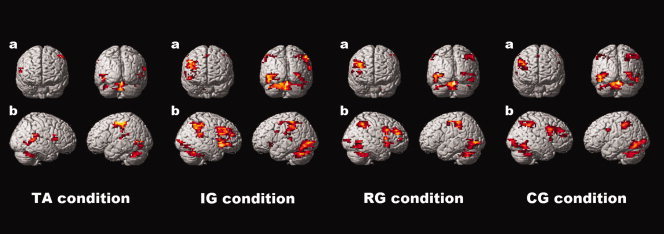
One sample t‐test for each condition of the Go/No‐go task. Global height threshold of P < 0.0001 (uncorrected for multiple comparisons) and an extent threshold of 20 voxels.
The in pairs comparisons between conditions and with respect to the TA condition are presented in Table II and displayed in Figure 4.
Table II.
Comparison of cerebral activation between Go/No‐go conditions (n = 20)
| Region, gyrus | BA | Hemisphere | x | y | z | Z |
|---|---|---|---|---|---|---|
| IG > TA | ||||||
| Occipital gyrus | 18,19 | L | −30 | −85 | −17 | 4.20 |
| Cerebellum hemisphere | L | −30 | −44 | −27 | 4.16 | |
| DLPFC | 9, 46 | R | 41 | 20 | 22 | 3.90 |
| CG > IG | ||||||
| Superior Parietal | 7 | R | 22 | −85 | 32 | 4.86 |
| CG > TA | ||||||
| Superior Parietal | 7 | R | 29 | −71 | 42 | 3.88 |
| Temporo‐Occipital | 19,37 | L | −42 | −67 | −20 | 3.76 |
| Premotor area | R | 41 | 6 | 28 | 3.69 | |
| Occipital gyrus | 18,19 | R | 32 | −85 | −11 | 3.56 |
| Occipital gyrus | 18,19 | L | −33 | −92 | −5 | 3.35 |
BA, Brodmann's area; R, right; L, left; TA, tonic alertness task; IG, initial Go/No‐go; RG, reversal Go/No‐go; CG, complex Go/No‐go. No supplementary activation was observed for the comparison RG > IG nor for RG > TA.
Figure 4.
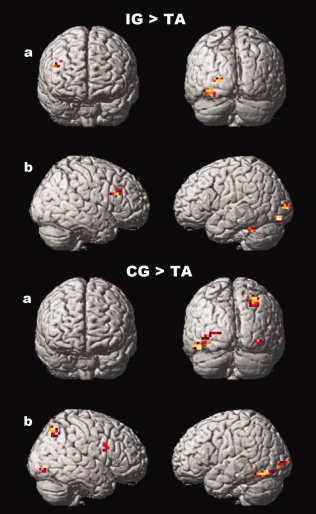
Supplementary activation in 20 healthy subjects for IG compared with TA (top panel) and CG compared with TA (bottom panel). (a) 3‐D coronal views. (b) 3‐D sagittal views. Global height threshold of P < 0.002 (uncorrected for multiple comparisons) and an extent threshold of 10 voxels.
Activation obtained for IG compared with TA is presented in Figure 4 (top panel). Activation concerned the right dorsolateral prefrontal cortex (DLPFC) (BA 9, 46), the left occipital gyrus (BA 18, 19) and the left cerebellum hemisphere.
Activation obtained for CG compared with IG concerned the right superior parietal lobe (BA 7)
Figure 4 (bottom panel) presents activation obtained for CG compared with TA. Activation concerned the right premotor area, the right superior parietal lobe (BA 7), the left temporo‐occipital gyrus (BA 19, 37) and the bilateral occipital gyrus (BA 18, 19).
No supplementary activation was found in neither RG compared with TA nor in RG compared with IG.
Regression Analysis
As the educational level and response times were strongly correlated, we can not perform a multivariate analysis with these two variables as regressors. Therefore, the regression analysis was performed for each variable, i.e., educational level and response times, independently.
With in‐scanner performances (response times)
The cerebral regions correlated with in‐scanner response times are presented in Table III and displayed in Figure 5 (top panel).
Table III.
Positive and negative correlations between cerebral activities and performance (n = 19)
| Region, gyrus | BA | Hemisphere | x | y | z | Z |
|---|---|---|---|---|---|---|
| Positive correlations | ||||||
| TA condition | ||||||
| Superior Temporal | 38 | R | 38 | 3 | −9 | 4.01 |
| Superior Temporal | 38 | L | −40 | −3 | −16 | 3.96 |
| Superior Parietal | 7 | R | 34 | −37 | 44 | 3.86 |
| Superior Parietal | 7 | R | 29 | −27 | 17 | 3.83 |
| DLPFC | 9,46 | L | −24 | 24 | 32 | 3.71 |
| Inferior Parietal | 40 | L | −52 | −24 | 14 | 3.60 |
| IG condition | ||||||
| SMA | 6 | R | 22 | −10 | 40 | 3.98 |
| Insula | L | −27 | 1 | 3 | 3.40 | |
| RG condition | ||||||
| Inferior Parietal | 40 | L | −42 | −27 | 11 | 3.88 |
| CG condition | ||||||
| Inferior Parietal | 40 | L | −40 | −24 | 17 | 3.31 |
| Negative correlations | ||||||
| IG condition | ||||||
| Vermis | R | 7 | −89 | −23 | 3.47 | |
| RG condition | ||||||
| Cerebellar hemisphere | L | −20 | −58 | −19 | 3.50 | |
BA, Brodmann's area; R, right; L, left; TA, tonic alertness task; IG, initial Go/No‐go; RG, reversal Go/No‐go; CG, complex Go/No‐go. One subject was excluded from regression analysis because of problems with the response collection device.
Figure 5.
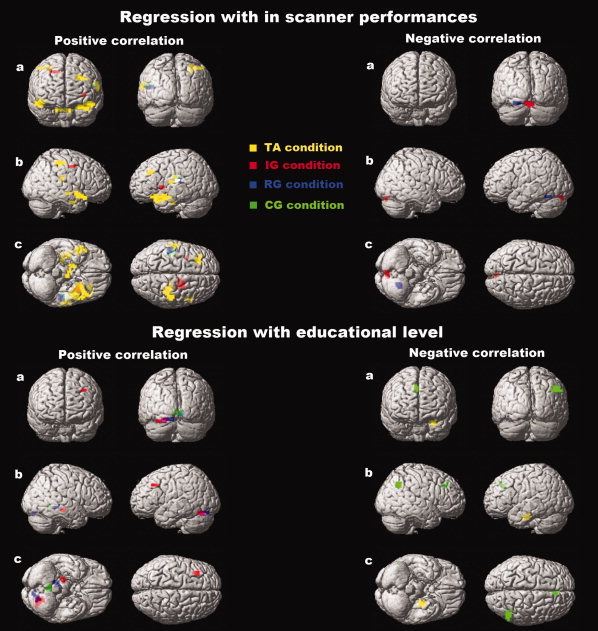
Cerebral regions correlating with Go/No‐go performances (n = 19) (top panel) or with educational level (n = 20) (bottom panel) in healthy subjects. Faster response times and higher educational levels correlate with cerebellar activity. Lower response times and lower educational levels correlate with cortical activity. (a) 3‐D coronal view. (b) 3‐D sagittal view. (c) 3‐D axial view. Orange colour, seen on the axial view of the positive regression with in‐scanner performances, reflects common cerebral regions activated for both TA (yellow) and IG (red) conditions. Purple colour, seen on the coronal, sagittal and axial views of the positive regression with educational level, reflects common cerebral regions activated for both IG (red) and RG (blue) conditions. White colour, seen on the sagittal view of the positive regression with in‐scanner performances, reflects commune cerebral regions activated for TA (yellow), RG (blue) and CG (green) conditions. Neither the green colour nor the yellow colour was obtained by a blend of TA (yellow) and RG (blue), or IG (red) and CG (green), respectively. A deeper localisation of the cerebral activation related to a Go/No‐go condition leads to an intensity reduction of the corresponding colour. Global height threshold of P < 0.002 (uncorrected for multiple comparisons) and an extent threshold of 10 voxels.
Positive correlation.
For TA, the activity of the left DLPFC (BA 9, 46), the right superior parietal lobe (BA 7), the left inferior parietal lobe (BA 40) and the right inferior temporal pole (BA 38) increased with response times.
For IG, the activity in the supplementary motor area (BA 6) increased with response times
For RG and CG, the activity in the left inferior parietal lobe (BA 40) increased with response times.
Negative correlation.
For IG and RG, the decrease in response times was associated with greater activity in the cerebellum, respectively the vermis and the left cerebellar hemisphere.
No correlation was found between performance and activation for TA or CG.
With educational level
The cerebral regions correlating with years of education are presented in Table IV and displayed in Figure 5 (bottom panel).
Table IV.
Positive and negative correlations between cerebral activities and education (n = 20)
| Region, gyrus | BA | Hemisphere | x | y | z | Z |
|---|---|---|---|---|---|---|
| Positive correlations | ||||||
| IG condition | ||||||
| DLPFC | 9,46 | L | −27 | 20 | 34 | 4.16 |
| Cerebellar hemisphere | L | −20 | −75 | −23 | 3.60 | |
| Parahippocampal gyrus | R | 21 | −24 | −14 | 3.26 | |
| RG condition | ||||||
| Occipital gyrus | 18,19 | R | 13 | −37 | −8 | 3.78 |
| Cerebellar hemisphere | L | −15 | −75 | −20 | 3.65 | |
| CG condition | ||||||
| Vermis | R | 4 | −51 | −6 | 3.91 | |
| Negative correlations | ||||||
| TA condition | ||||||
| Parahippocampal gyrus | L | −24 | −17 | −26 | 4.51 | |
| CG condition | ||||||
| Inferior Parietal | 40 | R | 47 | −64 | 27 | 3.54 |
| Anterior Cingulum | 24,32 | R | 4 | 34 | 38 | 3.21 |
BA, Brodmann areas; R, right; L, left; TA, tonic alertness task; IG, initial Go/No‐go; RG, reversal Go/No‐go; CG, complex Go/No‐go.
Positive correlation.
No positive correlation was found between education and TA activation.
For IG, activities in the left DLPFC (BA 9, 46) and the right parahippocampal gyrus increased with educational level.
For RG, the activity in the right occipital gyrus (BA 18, 19) increased with educational level.
In addition, for IG, RG and CG, positive correlations were found between education and cerebellar activity, respectively in the left cerebellar hemisphere (IG and RG) and the vermis (CG).
Negative correlation.
For TA, a lower level of education was associated with a greater activation in the left parahippocampal gyrus.
No negative correlation was found between education and activation for IG or RG.
For CG, a lower level of education was associated with greater activity in the right anterior cingulum (BA 24, 32) and the right inferior parietal lobe (BA 7, 40).
The slices of the cerebellum presenting the different activation for each condition of the Go/No‐go task according to in‐scanner performances or educational level are displayed in Figure 6.
Figure 6.
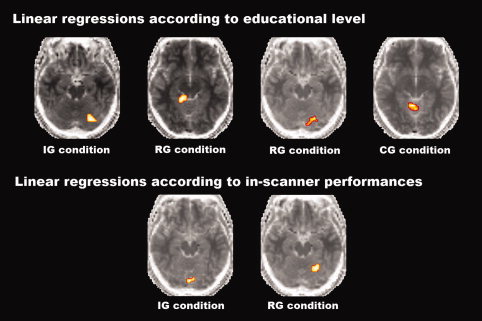
Slices of the cerebellum presenting the different activations for each condition of the Go/No‐go task according to in‐scanner performances or educational level. Global height threshold of P < 0.002 (uncorrected for multiple comparisons) and an extent threshold of 10 voxels.
DISCUSSION
The present study demonstrates that neural substrates underlying attentional processing are modulated according to educational level. Indeed, in healthy young adults the higher the educational level, the more the mobilisation of attentional resources leads to a higher activation of the cerebellum and a lower activation of the cortical areas including medial prefrontal and inferior parietal cortices compared with subjects with lower educational level during the Go/No‐go task. Particularly for the condition that requires the greatest attentional requests within the increasing complexity Go/No‐go paradigm, our data indicate that education influences attentional strategies of healthy young adults by modulating a functional balance of cerebral activation between cortical and cerebellar regions.
Relevance of the Go/No‐Go Paradigm
As expected, we found an effect of the educational level on neuropsychological performances outside the scanner. Main correlations were found between education and all of the attentional tests of the neuropsychological battery (SDMT, PASAT3s, and PASAT2s). Interestingly, no correlation was found between education and neuropsychological tests assessing memory capacities (SRT and SPART). Moreover, no correlation was found between educational level and the Stroop test score, which evaluates inhibition capacities of the subjects. The observation of significant correlations between educational level and in‐scanner response times, taken as marker of the subjects performances, for two conditions of the Go/No‐go paradigm, i.e., IG and RG, argues that education preferentially influences attentional but not inhibition parts of this task. However, no correlation was found between educational level and in‐scanner response times for the easier (TA) and the more difficult task (CG). We can hypothesize that the lack of correlation between CG performance and educational status accounted for a ceiling effect of education, all the subjects becoming equal for this most complex condition.
Independent of their educational status, subjects present in each condition of the increasing attentional load i.e., TA, IG, CG of Go/No‐go paradigm a significant increase in response times compared with previous ones, but as expected, no significant increase in response times was found for the comparison IG vs. RG. Moreover, no supplementary cerebral activation was found in RG compared with IG. The lack of significant differences for both in‐scanner response times and brain activity between these two conditions of the cognitive paradigm lends credence to our initial hypothesis of a non‐specific increase in complexity for the reversal of the instruction in healthy young adults, RG vs IG. These differences between tasks were not due to different motor treatment, all the conditions of the Go/No‐go paradigm using the same motor responses.
The increase of in‐scanner response times from TA to IG and CG conditions in all subjects may be accounted for a longer cognitive treatment while performing the successive cognitive tasks suggesting an increasing difficulty along TA, IG, and CG. This appears to be associated at the neural level with the engagement of a wider neuronal network, which encompass dedicated attentional areas, the right dorsolateral prefrontal cortex (DLPFC) (BA 9, 46), the left occipital gyrus (BA 18,19) and the left cerebellum for IG compared with TA and the right superior parietal lobe (BA 7) for IG compared with CG. The DLPFC is known to be involved in attentional processes [Milham et al.,2003] as the superior parietal lobe, which is known to play a significant role in attention shifting and detecting specific or salient targets [Behrmann et al.,2004]. Likewise, a previous study underlined the contribution of the cerebellum in attentional processes [Allen et al.,1997]. The results of the present study, i.e., the increase in response times associated with a more distributed neuronal networks including areas classically involved in attentional processing under the Go/No‐go conditions, provide strong evidence that a supplementary attentional treatment occurs in response to a significant increase in the complexity of the given cognitive task.
Effect of Performance and Educational Level on Cerebral Activation
Longer response times correlated exclusively with cortical areas activity, i.e., frontal (BA 9, 46) and temporal regions (BA 38) for TA condition, and inferior parietal regions (BA 40) for all but one conditions of the cognitive paradigm (TA, RG and CG). On the contrary, faster response times correlated with greater activation in cerebellar regions. Indeed, a high level of cognitive performance is associated with a decrease in activation in the inferior parietal region, with this lower parietal activation being counterbalanced by a higher activation in the left cerebellar hemisphere.
Interestingly, this differential involvement of cortical and cerebellar activation was also found according to educational level. Indeed, lower education is associated with preferential cortical activation (i.e., right inferior parietal and anterior cingulum regions) (CG) whereas higher educational level is associated with preferential cerebellar recruitment during the more complex tasks (IG, RG, and CG). Thus, involvement of the inferior parietal region seems to be associated with both lower in‐scanner cognitive performances and lower educational levels, whereas higher educational levels and higher in‐scanner cognitive performances are both associated with a greater involvement of cerebellar regions, suggesting an influence of educational status on the relationship between cortical regions and cerebellum. Altogether these results strongly support the hypothesis of a balance between cortical, i.e., prefrontal and parietal, and cerebellar regions recruitment in attentional processing depending on educational status of healthy young subjects. This hypothesis is reinforced by the evidence that, for the two conditions with a comparable attentional load (i.e., IG and RG), a similar pattern of cerebellar activation was found according to higher performance and higher educational levels, while a similar pattern of cortical activation was found according to lower performance and lower educational status The cerebellum is generally associated with motor automaticity [Jenkins et al.,1994; Jueptner and Weiller,1998; Wu et al.,2004], but a recent study also showed its involvement in cognitive automaticity [Puttemans et al.,2005] suggesting the involvement of the cerebellum in automaticity procedures as a more general activation strategy. Moreover, previous data also underlined the contribution of the cerebellum in cognitive processes [Desmond and Fiez,1998; Tavano et al.,2007] and particularly in attentional processes [Allen et al.,1997; Gottwald et al.,2003]. Indeed, Gottwald et al. demonstrated the critical role of the cerebellum for divided attention using also a Go/No‐go paradigm. Allen et al. have demonstrated cerebellar activity during a “focused‐attention” task in the left superior posterior cerebellum by using fMRI. Moreover, during shifting‐attention tasks, a significative cerebellar activity was highlighted in the right lateral hemisphere. Although we observed in our study some activation in the superior posterior regions of the cerebellum, confirming in part results of Allen et al., however none of these activation was localised in the right side of the cerebellum. Some studies interested in attention deficit hyperactivity disorder (ADHD) have shown smaller posterior inferior vermis lobules in children with ADHD [Berquin et al.,1998; Castellanos et al.,2001]. It seems likely that the posterior inferior vermal areas are part of frontal‐subcortical networks relevant for the executive aspects of attention. On the other hand, orienting and spatial shifting of attention could be involved a network including posterior superior vermal areas and the parietal cortex [Mostofski et al.,1998]. These results and ours are congruent to highlight the involvement of the vermal areas in attentional processes.
Taken together these observations support the hypothesis that in high educational subjects the cerebellum may assume the most automatized parts of attentional requests that are needed to perform this cognitive task in order to spare cortical regions. The experiments by Allen [Allen et al.,2005] have demonstrated the presence of cerebellar‐parietal and cerebellar‐prefrontal functional connectivity. Our results suggest that this functional relation strength between cortical, i.e., prefrontal and parietal regions, and the cerebellum are related to education. We hypothesised that healthy subjects with a higher educational status would tend to set up automatized strategies economizing cortical regions involvement, and these regions becoming more easily mobilised with increasing attentional requests. Moreover, experiments by Springer [Springer et al.,2005] have also demonstrated a negative correlation between brain activity in frontal areas and educational level in both young subjects during a recognition task of pictures and words. These and our results support the assumption that such a brain activity‐related educational modulation in young adults is not dependent of the cognitive domain. In pathological conditions, when brain damage induces the recruitment of new cortical areas to perform an attentional task at the same performance level prior to the disease, it may be hypothesized a higher facility for high educated patients to mobilise greater cortical activation in order to counteract the effect of tissue damage.
Education level is used in this study to reflect cognitive training. We are aware that other variables might be useful to measure like cognitive demand in daily life. Another possible limitation of this study is that no training procedure was performed before the fMRI protocol. We cannot exclude the possibility that at least a part of the results could reflect the efficient learning capacity in highly educated subjects.
CONCLUSION
Overall, the neural substrates underlying attentional processes clearly appeared to be modulated by the educational level. Moreover, the results achieved in the present study offer the support for the hypothesis that at the neural level, a functional interaction exists between cortical areas, i.e., prefrontal and parietal cortices, and cerebellum supervised by educational level in healthy young subjects, which obviously meet the results in both neurological diseases and normal aging studies. This effect may be subtended by regular and frequent confrontation of the subject with a significant cognitive environment leading to stronger learning of appropriate cognitive strategies. By demonstrating the influence of education on the functional relationship between cortical regions and the cerebellum, we provide strong evidence that education may differently influence automatized strategies in healthy young adults by modulating the specific involvement of the two parts of this functional balance according to the environmental cognitive solicitations. In pathological conditions affecting young people such as in multiple sclerosis, which correspond to a new cerebral environment, it may be useful to determine the impact of tissue damage on this cortico‐cerebellar functional balance and to follow the beneficial role of education on the potential new equilibrium.
Acknowledgements
We acknowledge Isabelle Hesling, Willy Mayo and Luc Letenneur for their help in reviewing the manuscript.
REFERENCES
- Allen G,Buxton RB,Wong EC,Courchesne E ( 1997): Attentional activation of the cerebellum independent of motor involvement. Science 275: 1940–1943. [DOI] [PubMed] [Google Scholar]
- Allen G,McColl R,Barnard H,Ringe WK,Fleckenstein J,Cullum CM ( 2005): Magnetic resonance imaging of cerebellar‐prefrontal and cerebellar‐parietal functional connectivity. NeuroImage 28: 39–48. [DOI] [PubMed] [Google Scholar]
- Amieva H,Lafont S,Auriacombe S,Le Carret N,Dartigues FJ,Orgogozo MJ,Colette F ( 2002): Inhibitory breakdown and dementia of the Alzheimer type: A general phenomenon? J Clin Exp Neuropsychol 24: 503–516. [DOI] [PubMed] [Google Scholar]
- Behrmann M,Geng J,Shomstein S ( 2004): Parietal cortex and attention. Curr Opin Neurobiol 14: 212–217. [DOI] [PubMed] [Google Scholar]
- Berquin PC.,Giedd JN,Jacobsen LK,Hamburger SD,Krain BA.,Rapoport JL,Castellanos FX ( 1998): Cerebellum in attention‐deficit hyperactivity disorder. Neurology 50: 1087–1093. [DOI] [PubMed] [Google Scholar]
- Castellanos FX,Giedd JN,Berquin PC,Walter JM,Sharp W,Tran T,Vaituzius C,Blumenthal JD,Nelson J,Bastain TM,Zijdenbos A,Evans AC,Rapoport JL ( 2001): Quantitative brain magnetic resonance imaging in girls with attention‐deficit/hyperactvity disorder. Arch Gen Psychiatry 58: 289–295. [DOI] [PubMed] [Google Scholar]
- Deloire MS,Salort E,Bonnet M,Arimone Y,Boudineau M,Amieva H,Barroso B,Ouallet JC,Pachai C,Galliaud E,Petry KG,Dousset V,Fabrigoule C,Brochet B ( 2005): Cognitive impairment as marker of diffuse brain abnormalities in early relapsing remitting multiple sclerosis. J Neurol Neurosurg Psychiatry 76: 519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond JE,Fiez JA ( 1998): Neuroimaging studies of the cerebellum: Language, learning and memory. Trends Cogn Sci 2: 355–362. [DOI] [PubMed] [Google Scholar]
- Fabrigoule C,Letenneur L,Dartigues JF,Zarrouk M,Commenges D,Barberger‐Gateau P ( 1995): Social and leisure activities and risk of dementia: A prospective longitudinal study. J Am Geriatr Soc 43: 485–490. [DOI] [PubMed] [Google Scholar]
- Friston KJ,Holmes AP,Worsley KJ,Poline JP,Frith CD,Frackowiak RSJ ( 1995): Statistical Parametric Maps in Functional Imaging: A Genaral Linear Approach. Hum Brain Mapp 2: 189–210. [Google Scholar]
- Gottwald B,Mihajlovic Z,Wilde B,Mehdorn HM ( 2003): Does the cerebellum contribute to specific aspects of attention? Neuropsychologia 41: 1452–1460. [DOI] [PubMed] [Google Scholar]
- Hultsch DF,Hertzog C,Small BJ,Dixon RA ( 1999): Use it or lose it: Engaged lifestyle as a buffer of cognitive decline in aging? Psychol Aging 14: 245–263. [DOI] [PubMed] [Google Scholar]
- Jenkins IH,Brooks DJ,Nixon PD,Frackowiak RSJ,Passingham RE ( 1994): Motor sequence learning: A study with positron emission tomography. J Neurosci 14: 3775–3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jueptner M,Weiller C ( 1998): A review of differences between basal ganglia and cerebellar control of movements as revealed by functional imaging studies. Brain 121: 1437–1449. [DOI] [PubMed] [Google Scholar]
- Le Carret N,Auriacombe S,Letenneur L,Bergua V,Dartigues JF,Fabrigoule C ( 2005): Influence of education on the pattern of cognitive deterioration in AD patients: The cognitive reserve hypothesis. Brain and Cognition 57: 120–126. [DOI] [PubMed] [Google Scholar]
- Milgram NW,Siwak‐Tapp CT,Araujo J, Head E (2006): Neuroprotective effects of cognitive enrichment. Ageing Res Rev 5: 354–369. [DOI] [PubMed] [Google Scholar]
- Milham MP,Banich MT,Claus ED,Cohen NJ ( 2003): Practice‐related effects demonstrate complementary roles of anterior cingulate and prefrontal cortices in attentional control. NeuroImage 18: 483–493. [DOI] [PubMed] [Google Scholar]
- Mostofski SH,Reiss AL,Lockhart P,Denckla MB ( 1998): Evaluation of cerebellar size in attention‐deficit hyperactivity disorder. J Child Neurol 13: 434–439. [DOI] [PubMed] [Google Scholar]
- Puttemans V,Wenderoth N,Swinnen SP ( 2005): Changes in brain activation during the acquisition of a multifrequency bimanual coordination task: From the cognitive stage to advanced levels of automaticity. J Neurosci 25: 4270–4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SM ( 1990): Cognitive Function Study Group, National Multiple Sclerosis society A Manual for the Brief Repeatable Battery of Neuropsychological Tests in Multiple Sclerosis. New York: National Multiple Sclerosis Society. [Google Scholar]
- Scarmeas N,Zarahn E,Anderson KE,Hilton J,Flynn J,Van Heertum RL,Sackeim HA,Stern Y ( 2003): Cognitive reserve modulates functional brain responses during memory tasks: A PET study in healthy young and elderly subjects. NeuroImage 19: 1215–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer MV,McIntosh AR,Winocur G,Grady CL ( 2005): The relation between brain activity during memory tasks and years of education in young and older adults. Neuropsychology 19: 181–192. [DOI] [PubMed] [Google Scholar]
- Staff RT,Murray AD,Deary IJ,Whalley LJ ( 2004): What provides cerebral reserve? Brain 127: 1191–1199. [DOI] [PubMed] [Google Scholar]
- Stern Y,Alexander GE,Prohovnik I,Mayeux R ( 1992): Inverse relationship between education and parietotemporal perfusion deficit in Alzheimer's disease. Ann Neurol 32: 371–375. [DOI] [PubMed] [Google Scholar]
- Stern Y,Richards M,Sano M,Mayeux R ( 1993): Comparison of cognitive changes in patients with Alzheimer's and Parkinson's disease. Arch Neurol 50: 1040–1045. [DOI] [PubMed] [Google Scholar]
- Stern Y,Gurland B,Tatemichi TK,Tang MX,Wilder D,Mayeux R ( 1994): Influence of education and occupation on the incidence of Alzheimer's disease. JAMA 271: 1004–1010. [PubMed] [Google Scholar]
- Stern Y,Tang MX,Denaro J,Mayeux R ( 1995): Increased risk of mortality in Alzheimer's disease patients with more advanced educational and occupational attainment. Ann Neurol 37: 590–595. [DOI] [PubMed] [Google Scholar]
- Stern Y,Albert S,Tang M,Tsai WY ( 1999): Rate of memory decline in AD is related to education and occupation: Cognitive reserve? Neurology 45: 1161–1168. [DOI] [PubMed] [Google Scholar]
- Stern Y,Moeller JR,Anderson KE,Luber B,Zubin NR,DiMauro AA,Park A,Campbell CE,Marder K,Bell K,Van Heertum R,Sackeim HA ( 2000): Different brain networks mediate task performance in normal aging and AD: Defining compensation. Neurology 55: 1291–1297. [DOI] [PubMed] [Google Scholar]
- Stern Y,Zarahn E,Hilton HJ,Flynn J,DeLaPaz R,Rakitin B ( 2003): Exploring the neural basis of cognitive reserve. J Clin Exp Neuropsychol 25: 691–701. [DOI] [PubMed] [Google Scholar]
- Stern Y,Habeck C,Moeller J,Scarmeas N,Anderson KE,Hilton J,Flynn J,Sackeim H,Van Heertum R ( 2005): Brain networks associated with cognitive reserve in healthy young and old adults. Cereb Cortex 15: 394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavano A,Grasso R,Gagliardi C,Triulzi F,Bresolin N,Fabbro F,Borgatti R ( 2007): Disorders of cognitive and affective development in cerebellar malformations. Brain 130: 2646–2460. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N,Landeau B,Papathanassiou D,Crivello F,Etarrd O,Delacroix N,Mazoyer B,Joliot M ( 2002): Automated anatomical labelling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. NeuroImage 15: 273–289. [DOI] [PubMed] [Google Scholar]
- Wechsler DA ( 1981): Manual for the Wechsler Adult Intelligence Scale Revised. New York: The Psychological Corporation. [Google Scholar]
- Wilson RS,Bennett DA,Bienias JL,Aggarwal NT,Mendes De Leon CF,Morris MC,Schneider JA,Evans DA ( 2002): Cognitive activity and incident AD in a population‐based sample of older persons. Neurology 59: 1910–1914. [DOI] [PubMed] [Google Scholar]
- Wu T,Kansaku K,Hallett M ( 2004): How self‐initiated memorized movements become automatic: A fMRI study. J Neurophysiol 91: 1690–1698. [DOI] [PubMed] [Google Scholar]


