Abstract
The present study aimed to investigate cerebral activation following intranasal trigeminal chemosensory stimulation using O15‐H2O‐PET. A total of 12 healthy male participants underwent a PET scan presented with four scanning conditions; two left‐sided intranasal CO2‐stimuli and two matched baseline conditions consisting of odorless air. CO2 was used as it produces burning and stinging sensations. Stimulation started 20 s before intravenous injection of the isotope and lasted for the first 60 s of the 5 min scan time. A comparison between CO2 and baseline showed a pronounced activation of the trigeminal projection area at the base of the postcentral gyrus (primary and secondary somatosensory cortex) which was more intense for the right hemisphere, contralateral to the side of stimulation. In addition, activation was also found in the piriform cortex which is typically activated following odor presentation and thus thought of as primary olfactory cortex. In conclusion, and in line with previously published work, our data suggest that intranasal trigeminal stimulation not only activates somatosensory projection areas, but that it also leads to activation in cerebral areas associated with the processing of olfactory information. This may be interpreted in terms of the intimate relation between the intranasal chemosensory systems. Hum Brain Mapp, 2009. © 2008 Wiley‐Liss, Inc.
Keywords: pain, stinging, nose, anosmia, olfaction, positron emission tomography, 15O‐H2O
INTRODUCTION
Recently a series of imaging studies have investigated the neural correlates of intranasal trigeminal stimulation using functional magnetic resonance imaging (FMRI) [Hummel et al., 2005; Boyle et al., 2007a, b; Iannilli et al., 2007]. Including somatosensory regions, these studies reported activation of the orbitofrontal and piriform cortex in response to the intranasal trigeminal stimulant CO2. These findings were of particular interest as CO2 is odorless and the latter regions are commonly thought of as regions coding for smell. Further, the piriform cortex is often considered to be primary olfactory cortex. The use of FMRI provided these studies with superior spatial resolution but limited reliability of the findings in the orbitofrontal and piriform cortex as the method is highly susceptible to problems of image distortion in these regions [Stenger, 2006]. An alternate imaging method, positron emission tomography (PET), provides excellent signal reliability in known olfactory regions and as such would serve as an optimal technique to either support or challenge the findings that orbitofrontal and piriform cortex are activated during the processing of intranasally induced trigeminal stimulation.
Because of the intimate connections between the olfactory and trigeminal systems [Hummel and Livermore, 2002] and in line with previous work mentioned above it was hypothesized that painful, intranasal stimulation with CO2 should not only activate areas associated with the processing of pain, e.g., the primary and secondary somatosensory cortex the anterior cingulate, the insular cortex [Coghill et al., 1994], supplementary motor cortices [Hummel et al., 2005], or the brainstem [Hummel et al., 2005; Iannilli et al., 2008; Tracey et al., 2002; Zambreanu et al., 2005], but also areas involved in the processing of odorous information such as the piriform cortex, the superior temporal gyri, the orbito‐frontal cortex, or the gyrus rectus [Gottfried, 2006; Kettenmann et al., 2001; Savic, 2002].
MATERIALS AND METHODS
Subjects
The study was approved by the Ethics Committee of the University of Dresden Medical School and by the federal agency for radiation protection (“Bundesamt für Strahlenschutz”). All subjects provided written informed consent. To exclude sex‐related variability [Lundstrom and Hummel, 2006], only men were included (n = 12; age 30 to 58 years; mean age 36 years).
Absence of major nasal pathology was ascertained by detailed otorhinolaryngological examination including nasal endoscopy. All subjects maintained that they were in good health and had normal olfactory function as established by the “Sniffin' Sticks” test battery [Kobal et al., 2000]; all of them were right‐handed as ensured by a handedness survey [Oldfield, 1971]. Thresholds for intranasal pain elicited with gaseous CO2‐stimuli were determined with a staircase paradigm [stimulus duration 200 ms; interstimulus interval 30 s; Lötsch et al., 1997]. This was performed to ascertain that all subjects would perceive CO2 as painful when it was presented later during the PET‐experiments.
Stimulation
Odorless CO2 was chosen for trigeminal stimulation [Fröhlich 1851; Stevens et al., 1982; Thürauf et al., 1991]; it produces sensations like “burning,” “stinging,” or “biting.” Using a stimulator based on the principles of air‐dilution olfactometry [ANAMON; Draeger, Lübeck, Germany; Kobal, 1981] CO2 stimuli (60% v/v) were presented to the left nostril (stimulus duration 1 s, interval 3 s; total flow 6 l/min; relative humidity 80%) embedded in a constant flow of odorless air. The baseline condition consisted of odorless air. Stimuli were delivered intranasally through tubing terminating in a nose piece (inner diameter 4 mm). Stimuli were not presented in synchrony with breathing. Subjects performed velopharyngeal closure to restrict breathing through the mouth [Kobal, 1981].
Imaging Procedure
PET measurements were obtained with an ECAT system (ECAT EXACT HR+, Siemens, Erlangen, Germany). After a 10 min transmission scan (68Ge‐68Ga rod sources), subjects underwent four dynamic 15O‐H2O‐PET‐sessions of 5 min each with a time interval between the scans of at least 15 min. During 2 of the 4 sessions subjects received CO2‐stimuli Stimuli and baseline were applied in random order. Stimulation started 20 s before bolus intravenous injection of 1.7 GBq O15‐H2O and lasted for 80 s. During the other two sessions subjects received odorless air only. Following each task paradigm subjects verbally rated the overall intensity of the stimuli using an 11‐point category scale (zero = no sensation; 5 = moderately strong; and 10 = extremely strong).
An individually rescaled standard input function [van den Hoff et al., 2001] was used to generate parametric maps proportional to local blood flow. The quantification algorithm [van den Hoff et al., 1993] includes corrections for input function delay and shape deviations. Compared with summed images of the initial uptake phase, the resulting parametric maps yield reasonable estimates of absolute flow values. Regional variations in these parametric maps are truly flow‐proportional and the statistical accuracy is superior in comparison with uptake images of the initial phase, thus increasing sensitivity when looking for small alterations of regional perfusion. For further analysis SPM software was used (Wellcome Department of Cognitive Neurology, London, UK). Images were realigned with the first as the reference, and finally spatially normalized into the space defined by Talairach and Tournoux [1988]. Normalized images were smoothed with a Gaussian filter of 12 mm full width at half‐maximum. Statistical parametric maps were derived with a prespecified contrast, comparing regional cerebral blood flow during exposure to CO2 versus the odorless baseline. An uncorrected threshold of P < 0.001 was chosen for tabular and graphical reporting. In areas with a prior anatomical hypothesis, the reporting criterion was P < 0.05 applying a small volume correction [Worsley et al., 1996] for multiple non‐independent comparisons using a 10‐mm radius sphere centered on the piriform cortices (coordinates: −22, 10, −18; 22, 10, −18) and the right anterior cingulate cortex (coordinates: 8, 14, 28).
RESULTS
Subjects rated the CO2 stimuli as moderately painful with an average intensity of 4.8 (SD 1.0; minimum 3, maximum 6.5).
The comparison between CO2 and odorless air showed a pronounced trigeminal activation at the base of the postcentral gyrus bilaterally. Right‐sided activations were more intense than left‐sided activation, contralateral to the side of stimulation. Other predicted intranasal trigeminal areas included the left superior temporal gyrus and anterior cingulate cortex. Significant activations were also located in the bilateral piriform cortices and right supplementary motor area (Fig. 1, Table I). Unpredicted activated regions were the right parietal operculum and left planum polare.
Figure 1.
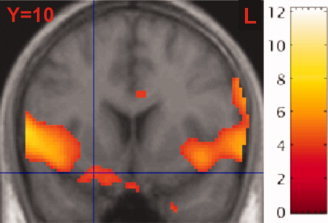
Activated voxels in the condition “CO2 vs. baseline”. Activation is shown with its center at the right‐sided piriform cortex (coordintaes 22/10/‐18). Images and coordinates are reported in neurological convention. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Table 1.
Activated voxels in the condition “CO2 vs. baseline” (main effects)
| Anatomical brain area | Peak coordinates (Talairach‐space) in mm | Statistical Z value | Voxel P value | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Right parietal operculum | 56 | −8 | 12 | 7.67 | 0.000 |
| Right postcentral gyrus | 48 | −12 | 22 | 6.87 | 0.000 |
| Right postcentral gyrus | 54 | −12 | 30 | 6.62 | 0.000 |
| Left superior ternporal gyrus | −66 | −4 | 8 | 6.85 | 0.000 |
| Left planum polare, transition between STG and insula | −40 | 0 | −10 | 5.90 | 0.000 |
| Left postcentral gyrus | −46 | −12 | 36 | 5.76 | 0.000 |
| Bilateral supplementary motor area (SMA) | 8 | −4 | 64 | 4.52 | 0.001 |
| Left anterior cingulate gyrus | −8 | 14 | 28 | 3.70 | 0.006a |
| Right medial temporopolar region and piriform cortex | 22 | 10 | −18 | 3.61 | 0.001a |
| Left piriform cortex | −26 | 2 | −18 | 3.30 | 0.010a |
DISCUSSION
The present PET results support previously reported fMRI‐based finings in that they confirm the activation of both trigeminal and olfactory regions, namely the piriform cortex, in response to the intranasal trigeminal stimulant CO2. Activation of trigeminal regions is consistent with findings by Boyle et al. [2007a] who showed, in independent analyses for each nostril, activity in the contralateral secondary somatosensory cortex, superior temporal gyrus and piriform cortex in response to CO2 stimulation. They are also consistent with activation caused by birhinal [Hummel et al., 2005] and right‐sided stimulation with CO2 [Iannilli et al., 2007]. Both, activation of the supplementary motor area [Hummel et al., 2005] and contralateral activation of the piriform cortex has been previously reported [Boyle et al., 2007b] in response to intranasal trigeminal stimulation, although to the best of our knowledge this is the first time that bilateral activation of the piriform cortex following monorhinal stimulation of CO2 has been found [for neuroanatomical pathways involved see Boyle et al., 2007a]. Our preeminent activation of the bilateral piriform cortex may be attributed to the greater signal acquisition capacity in the region provided by the use of PET versus FMRI [Devlin et al., 2000]. In addition, it may relate to the high stimulus intensity that was used over a relatively long time in comparison to previous FMI studies where trigeminal stimuli were presented for 30 s [Boyle et al., 2007a], whereas this period was 80 s in the present work.
Conversely, in the current study we were unable to support previously reported FMRI findings of orbitofrontal activation in response to an intranasal trigeminal stimulant [Boyle et al., 2007a; Hummel et al., 2005]. The latter regions, considered to be secondary olfactory cortex [Gottfried and Zald, 2005; Zatorre and Jones‐Gotman, 2000] were absent from the results of our analyses. The lack of results in the area may be indicative of image displacement [Stenger, 2006], or alternatively, for differences in the design of the various studies, e.g., time of painful stimulation or the fact that only men were investigated in the present study. Having said that, it should be kept in mind that orbitofrontal cortex activation in response to an intranasal trigeminal stimulant is plausible as neurons in the orbitofrontal cortex receive inputs via the piriform cortex and dorsomedial thalamic nuclei [Ray and Price, 1993]. Both the piriform cortex and the thalamus have been shown to be involved in the processing of intranasal trigeminal stimulants [Boyle et al., 2007b; Hummel et al., 2005] and processing of pain [Casey et al., 1996; Jantsch et al., 2005]. Thus, at this point in time it is unclear which of these two possibilities is accurate. More studies directly comparing the various designs seem to be necessary to decide this question.
The right parietal operculum was also found to be activated in the present study. Previous work also mentioned such activation in the vicinity of the secondary somatosensory cortex in response to painful stimuli [Treede et al., 1999]. In addition, the parietal operculum has been shown to be involved in the orientation toward painful stimuli and in stimulus categorization and representation [Christmann et al., 2007; Dowman, 2007; Ohara et al., 2006]. With regard to the planum polare more research is needed on its possible role in the processing of intranasal trigeminal stimuli—previous studies mostly attributed activation in this area to the higher level processing of auditory information [e.g., Hasson et al., 2007].
In conclusion, our data suggest that intranasal trigeminal stimulation not only activates somatosensory projection areas, but that it also leads to activation in areas associated with the processing of olfactory information. This may be interpreted in terms of the intimate relation between intranasal trigeminal and olfactory activation.
Acknowledgements
We thank Bertold Renner and Gerd Kobal for their kind support regarding the chemosensory stimulator used in this study. Special thanks to the staff of the cyclotron and radiopharmacy, physicists, and technologists of the PET‐Zentrum Dresden‐Rossendorf for provision of 15O‐H2O and for skillful technical support.
REFERENCES
- Boyle JA,Frasnelli J,Gerber J,Heinke M,Hummel T ( 2007a): Cross‐modal integration of intranasal stimuli‐an fMRI study. Neuroscience 149: 223–231. [DOI] [PubMed] [Google Scholar]
- Boyle JA,Heinke M,Gerber J,Frasnelli J,Hummel T ( 2007b): Cerebral activation to intranasal chemosensory trigeminal stimulation. Chem Senses 32: 343–353. [DOI] [PubMed] [Google Scholar]
- Casey KL,Minoshima S,Morrow TJ,Koeppe RA ( 1996): Comparison of human cerebral activation pattern during cutaneous warmth, heat pain, and deep cold pain. J Neurophysiol 76: 571–581. [DOI] [PubMed] [Google Scholar]
- Christmann C,Koeppe C,Braus DF,Ruf M,Flor H ( 2007): A simultaneous EEG‐fMRI study of painful electric stimulation. Neuroimage 34: 1428–1437. [DOI] [PubMed] [Google Scholar]
- Coghill RC,Talbot JD,Evans AC,Meyer E,Gjedde A,Bushnell MC,Duncan GH ( 1994): Distributed processing of pain and vibration by the human brain. J Neurosci 14: 4095–4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin JT,Russell RP,Davis MH,Price CJ,Wilson J,Moss HE,Matthews PM,Tyler LK ( 2000): Susceptibility‐induced loss of signal: Comparing PET and fMRI on a semantic task. Neuroimage 11: 589–600. [DOI] [PubMed] [Google Scholar]
- Dowman R ( 2007): Neural mechanisms of detecting and orienting attention toward unattended threatening somatosensory target stimuli. II. Intensity effects Psychophysiology 44: 420–430. [DOI] [PubMed] [Google Scholar]
- Fröhlich R ( 1851): Ueber einige Modificationen des Geruchsinnes. Akad. Wiss. Wien, math‐nat. CL 6: 322–328. [Google Scholar]
- Gottfried JA ( 2006): Smell: Central nervous processing. Adv Otorhinolaryngol 63: 44–69. [DOI] [PubMed] [Google Scholar]
- Gottfried JA,Zald DH ( 2005): On the scent of human olfactory orbitofrontal cortex: Meta‐analysis and comparison to non‐human primates. Brain Res. Brain Res Rev 50: 287–304. [DOI] [PubMed] [Google Scholar]
- Hasson U,Skipper JI,Nusbaum HC,Small SL ( 2007): Abstract coding of audiovisual speech: Beyond sensory representation. Neuron 56: 1116–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel T,Doty RL,Yousem DM ( 2005): Functional MRI of intranasal chemosensory trigeminal activation. Chem Senses 30(suppl 1): i205–i206. [DOI] [PubMed] [Google Scholar]
- Hummel T,Livermore A ( 2002): Intranasal chemosensory function of the trigeminal nerve and aspects of its relation to olfaction. Int Arch Occup Environ Health 75: 305–313. [DOI] [PubMed] [Google Scholar]
- Iannilli E,Del Gratta C,Gerber JC,Romani GL,Hummel T ( 2008): Trigeminal activation using chemical, electrical, and mechanical stimuli. Pain (submitted). [DOI] [PubMed]
- Iannilli E,Gerber J,Frasnelli J,Hummel T ( 2007): Intranasal trigeminal function in subjects with and without an intact sense of smell. Brain Res 1139: 235–244. [DOI] [PubMed] [Google Scholar]
- Jantsch HH,Kemppainen P,Ringler R,Handwerker HO,Forster C ( 2005): Cortical representation of experimental tooth pain in humans. Pain 118: 390–399. [DOI] [PubMed] [Google Scholar]
- Kettenmann B,Hummel T,Kobal G ( 2001): Functional imaging of olfactory activation in the human brain In: Simon SA,Nicolelis MAL, editors. Methods and Frontiers in Chemosensory Research. Boca Raton, Florida, USA: CRC press; p 477–506. [Google Scholar]
- Kobal G ( 1981): Elektrophysiologische Untersuchungen des menschlichen Geruchssinns. Stuttgart: Thieme Verlag. [Google Scholar]
- Kobal G,Klimek L,Wolfensberger M,Gudziol H,Temmel A,Owen CM,Seeber H,Pauli E,Hummel T ( 2000): Multicenter investigation of 1,036 subjects using a standardized method for the assessment of olfactory function combining tests of odor identification, odor discrimination, and olfactory thresholds. Eur Arch Otorhinolaryngol 257: 205–211. [DOI] [PubMed] [Google Scholar]
- Lötsch J,Nordin S,Hummel T,Murphy C,Kobal G ( 1997): Chronobiology of nasal chemosensitivity: Do odor or trigeminal pain thresholds follow a circadian rhythm? Chem Senses 22: 593–598. [DOI] [PubMed] [Google Scholar]
- Lundstrom JN,Hummel T ( 2006): Sex‐specific hemispheric differences in cortical activation to a bimodal odor. Behav Brain Res 166: 197–203. [DOI] [PubMed] [Google Scholar]
- Mai JK,Assheuer J,Paxinos G ( 2004): Atlas of the Human Brain, 2nd ed San Diego: Academic Press/Elsevier. [Google Scholar]
- Ohara S,Anderson WS,Lawson HC,Lee HT,Lenz FA ( 2006): Endogenous and exogenous modulators of potentials evoked by a painful cutaneous laser (LEPs). Acta Neurochir suppl 99: 77–79. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Ray JP,Price JL ( 1993): The organization of projections from the mediodorsal nucleus of the thalamus to orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol 337: 1–31. [DOI] [PubMed] [Google Scholar]
- Savic I ( 2002): Imaging of brain activation by odorants in humans. Curr Opin Neurobiol 12: 455–461. [DOI] [PubMed] [Google Scholar]
- Stenger AV ( 2006): Technical considerations for BOLD fMRI of the orbitofrontal cortex In: Zald DH,Rauch SL, editors. The Orbitofrontal Cortex. London: Oxford University Press; p 423–446. [Google Scholar]
- Stevens JC,Plantinga A,Cain WS ( 1982): Reduction of odor and nasal pungency associated with aging. Neurobiol Aging 3: 125–132. [DOI] [PubMed] [Google Scholar]
- Talairach J,Tournoux P ( 1988): Co‐planar stereotaxic atlas of the human brain. New York: Thieme. [Google Scholar]
- Thürauf N,Friedel I,Hummel C,Kobal G ( 1991): The mucosal potential elicited by noxious chemical stimuli: Is it a peripheral nociceptive event. Neurosci Lett 128: 297–300. [DOI] [PubMed] [Google Scholar]
- Tracey I,Ploghaus A,Gati JS,Clare S,Smith S,Menon RS,Matthews PM ( 2002): Imaging attentional modulation of pain in the periaqueductal gray in humans. J Neurosci 22: 2748–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treede RD,Kenshalo DR,Gracely RH,Jones AK ( 1999): The cortical representation of pain. Pain 79: 105–111. [DOI] [PubMed] [Google Scholar]
- van den Hoff J,Burchert W,Fricke H,Matzke KH,Meyer GJ,Boerner AR,Knapp WH ( 2001): Quantifizierung der Hirnperfusion mit [15O]‐H2O PET unter Verwendung einer Standard‐Inputfunktion. Nuklearmedizin 40: A29. [Google Scholar]
- van den Hoff J,Burchert W,Müller‐Schauenburg W,Meyer GJ,Hundeshagen H ( 1993): Accurate local blood flow measurements with dynamic PET: Fast determination of input function delay and dispersion by multilinear minimization. J Nucl Med 34: 1770–1777. [PubMed] [Google Scholar]
- Worsley KJ,Marrett S,Neelin P,Vandal AC,Friston KJ,Evans AC ( 1996): A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp 4: 58–73. [DOI] [PubMed] [Google Scholar]
- Zambreanu L,Wise RG,Brooks JC,Iannetti GD,Tracey I ( 2005): A role for the brainstem in central sensitisation in humans. Evidence from functional magnetic resonance imaging. Pain 114: 397–407. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ,Jones‐Gotman M ( 2000): Functional imaging of the chemical senses In: Toga AW,Mazziotta JC, editors. Brain Mapping: The Applications. San Diego: Academic Press; p 403–424. [Google Scholar]


