Abstract
Objectives: Various studies indicate that serotonin regulates impulsivity and the inhibitory control of aggression. Aggression is also known to be modified by sex hormones, which exert influence on serotonergic neurotransmission. The present study aimed to elucidate potential interactions between human aggression, the inhibitory serotonergic 5‐HT1A receptor, and sex hormones. Experimental Design: Thirty‐three healthy volunteers (16 women, aged 26.24 ± 5.5 yr) completed a validated questionnaire incorporating five dimensions of aggression. Subsequently, all subjects underwent positron emission tomography with the radioligand [carbonyl‐11C]WAY‐100635 to quantify 5‐HT1A binding potentials (BPNDs) in the prefrontal cortex, limbic areas, and midbrain. Also, plasma levels of testosterone, 17ß‐estradiol and sex hormone‐binding globulin (SHBG) were measured. Relations between aggression scores, regional 5‐HT1A BPNDs, and hormone levels were analyzed using correlations, multivariate analyses of variance, and linear regressions. Principal Observations: Statistical analyses revealed higher 5‐HT1A receptor BPNDs in subjects exhibiting higher aggression scores in prefrontal (all P < 0.041) and anterior cingulate cortices (P = 0.016). More aggressive subjects were also characterized by lower SHBG levels (P = 0.015). Moreover, higher SHBG levels were associated with lower 5‐HT1A BPNDs in frontal (P = 0.048) and cingulate cortices (all P < 0.013) and in the amygdala (P = 0.03). Conclusions: The present study provides first‐time evidence for a specific interrelation between the 5‐HT1A receptor distribution, sex hormones, and aggression in humans. Our findings point to a reduced down‐stream control due to higher amounts or activities of frontal 5‐HT1A receptors in more aggressive subjects, which is presumably modulated by sex hormones. Hum Brain Mapp 30:2558–2570, 2009. © 2008 Wiley‐Liss, Inc.
Keywords: positron emission tomography, serotonergic 1A receptor, serotonin, aggression, sex hormones, prefrontal cortex
INTRODUCTION
The serotonergic system has been implicated in the regulation of impulsivity and aggressive behavior [reviewed in Ferrari et al., 2005; Nelson and Trainor, 2007]. On the one hand, reduced serotonin (5‐HT) levels have been frequently linked to higher levels of aggression in rodents, nonhuman primates, and humans [Brown et al., 1979; Chiavegatto et al., 2001; Kantak et al., 1980; Mehlman et al., 1994; Valzelli et al., 1981]. Moreover, selective serotonin reuptake inhibitors (SSRIs) have been shown to exhibit both antidepressant and aggression‐lowering effects [Coccaro and Kavoussi, 1997; Kasper and Olié, 2002; New et al., 2004; Pinna et al., 2003; Reist et al., 2003]. On the other hand, antidepressants that block the degradation of serotonin and other amines including norepinephrine by inhibiting monoamine‐oxidase A (MAO‐A), turned out to increase aggression in rodents [Datla and Bhattacharya, 1990; Florvall et al., 1978]. This is in line with highly aggressive behaviors of MAO‐A knockout mice [Cases et al., 1995], and behavioral anomalies of persons exhibiting a MAO‐A point mutation [Brunner et al., 1993].
Aggressive behavior might thus depend on a more complex involvement of serotonin‐mediated neurotransmission, particularly when considering area‐specific effects [de Boer and Koolhaas, 2005; Miczek et al., 2007]. In the cortex, antagonists at the excitatory 5‐HT2 receptor reduced aggression in mice [Sakaue et al., 2002; White et al., 1991], whereas agonists at the inhibitory 5‐HT1A and/or 5‐HT1B receptors decreased aggressive behavior probably via presynaptic autoreceptors in the raphe nuclei (RN) in rodents [Bannai et al., 2007; De Almeida and Lucion, 1997; de Boer et al., 2000; Miczek et al., 1998]. Rats that were bred on low aggression showed a higher 5‐HT1A receptor expression in the midbrain [Popova et al., 2005], yet a decrease in 5‐HT1A receptor mRNA in the frontal cortex [Popova et al., 2007].
In humans, 5‐HT2 receptor antagonists also decreased aggressive behavior [Cherek and Lane, 2001]. Parsey et al. [2002] found negative correlations between life‐time history of aggression and the inhibitory 5‐HT1A receptor binding potentials in the RN, but surprisingly also in frontal areas, using positron emission tomography (PET) and the radioligand [11C]WAY‐100635 in 25 healthy humans. Conversely, pointing to a rather enhanced inhibition of prefrontal areas in aggression due to serotonergic neurotransmission, patients suffering from impulsive personality disorder exhibited a lower prefrontal glucose metabolism after a serotonin releasing challenge compared to controls [Siever et al., 1999; Soloff et al., 2003].
Other factors that might have contributed to these partly conflicting findings are aggression‐related actions of sex hormones [reviewed in Archer, 2006; Soma et al., 2008], because the impact of both serotonin and sex hormones on aggression appeared to be interrelated [Birger et al., 2003; Higley et al., 1996]. Moreover, interventional studies in rodents suggested that sex hormones modulate aggressive behavior via the serotonergic system, specifically via 5‐HT1A and 5‐HT1B receptors [Cologer‐Clifford et al., 1997; Ricci et al., 2006; Simon et al., 1998]. Recent imaging studies in the living human brain revealed activations of the prefrontal cortex and limbic areas in aggression‐related processing [reviewed in Bufkin and Luttrell, 2005]. These activations were more persistent after administration of sex hormones [Hermans et al., 2008], however, a direct involvement of 5‐HT1A receptor‐mediated neurotransmission has to be shown in humans.
Taken together, previous studies gave rise to the hypothesis that serotonin modulates aggressive behavior partially via the inhibitory 5‐HT1A receptor in selective brain areas such as the prefrontal cortex. Further, both aggression and the 5‐HT1A receptor distribution are suggested to be influenced by the amount of sex hormones. To test these assumptions, we wanted to elucidate whether aggressive traits, measured by sensitive neuropsychological testings, are associated with the regional distribution of pre‐ and postsynaptic 5‐HT1A receptors in specific prefrontal, limbic, and midbrain areas using in vivo PET. We also measured plasma levels of sex hormones to investigate the assumed regulatory influence of sex hormones on aggression, and on the regional 5‐HT1A receptor distribution.
METHODS
Subjects
Thirty‐six healthy young subjects (18 women and 18 men, aged 26.17 yr ± 5.3 SD) in a narrow age range and with similar educational levels (≥12 yr of education) were recruited via advertisement at the Medical University of Vienna, Austria. All subjects underwent a medical examination at the screening visit including medical history, electrocardiogram, and routine laboratory tests. Exclusion criteria were history of severe disease, any psychiatric or neurological disorder, and drug abuse. One woman and one man had to be excluded after PET‐scanning from further analysis because the respective integral of the time activity curve in the reference region was an outlier [mean ± 2 SD, Bantick et al., 2004; Rabiner et al., 2002]. Further, one woman had to be excluded because of failure to complete the questionnaire, leaving 33 subjects (aged 26.24 yr ± 5.5 SD) for statistical analysis. PET‐ and aggression‐data acquisition and analyses were done by investigators blinded to the study question. All females were measured in the follicular phase to exclude potential effects of menstrual cycle phase on the 5‐HT1A‐receptor distribution [Bethea et al., 2002; Flügge et al., 1999; Parsey et al., 2002], which in healthy women appeared to be rather minor and restricted to the RN [Jovanovic et al., 2006]. Demographic details and hormone plasma levels are given in Table I. All subjects provided written informed consent and received reimbursement after participation. The study was approved by the Ethics Committee of the Medical University of Vienna.
Table 1.
Demographic parameters and hormone plasma levels of subjects included in the analyses (n = 33)
| Mean ± SD | Minimum | Maximum | |
|---|---|---|---|
| Age, yr | 26.24 ± 5.5 | 20 | 47 |
| Body mass index, kg/m2 | 23.13 ± 3.3 | 16.9 | 36.3 |
| Sex‐hormone binding globulin (SHBG), ng/l | 48.04 ± 27.5 | 12.3 | 131.4 |
| Testosterone in males (bioavailable), μg/l | 3.12 ± 1.2 | 1.7 | 6.8 |
| Testosterone in females (bioavailable), μg/l | 0.12 ± 0.05 | 0.04 | 0.24 |
| 17ß‐estradiol in males (bioavailable), ng/l | 19.77 ± 5.8 | 12 | 30 |
| 17ß‐estradiol in females (bioavailable), ng/l | 27.81 ± 15.4 | 12 | 59 |
Aggression Score
Aggression‐related personality traits and the disposition to aggressive behavior were assessed by the validated Questionnaire for Measuring Factors of Aggression [German, Hampel and Selg, 1975] on the day of the PET measurements. This test, frequently used in similar studies [Gietl et al., 2007; Rujescu et al., 2008], comprises in line with the Buss and Durkee Hostility Inventory [Buss and Durkee, 1957] 77 items on five dimensions of aggression including (1) spontaneous verbal or physical aggression towards others, (2) reactive aggression, attacks in response to an event, (3) impulsiveness with the qualities rage and anger, (4) self‐aggression including depressive symptoms and self‐harm, and (5) inhibition of aggressive thoughts, acts, and impulsivity. The sum of the first three subscores (1–3) gives a total aggression score without self‐aggressive behaviors. In addition, several items were included to test whether subjects answer straightforward (frankness). As main outcome measures, test evaluation resulted in one total score and five subscores for the five aggression‐related dimensions with a higher score indicating higher ratings on spontaneous aggression, reactive aggression, impulsiveness, self‐aggression, and inhibition of aggression, respectively. No significant differences (independent t tests/Mann‐Whitney tests) or correlations (Pearson/Spearman) between age or sex and aggression scores were found (P > 0.05).
In our study‐sample of healthy individuals with a high educational level and social status (most subjects were students of medicine), nearly all subjects exhibited a generally low level of aggression. Therefore, aggression scores were non‐normally distributed and hardly continuous over a large range. Because this might conflict with linear regression theory [Zou et al., 2003], we considered to use as secondary outcome measures a dichotomous division of aggression scores. Therefore, two group percentiles (“higher” vs. “lower” aggression) were separated on the basis of median values. Given that n = 33, separating two percentiles was rated as best approach.
Positron Emission Tomography (PET)
All subjects underwent PET in an advance high‐resolution full‐ring scanner (General Electric Medical Systems, Milwaukee, WI) with the selective radioligand [carbonyl‐11C]WAY‐100635 to measure the distribution of the 5‐HT1A receptor subtype in selected brain regions of interest (ROIs) as previously described by our group [Gerstl et al., 2008; Lanzenberger et al., 2007; Spindelegger et al., in press; Stein et al., in press]. The radiotracer was prepared in a fully automated PET synthesizer (GE Healthcare, Uppsala, Sweden) as published by our group in detail [Wadsak et al., 2007]. Heads were fixed and adjusted in parallel to the orbitomeatal line with the neocortex and full cerebellum in the field of view. Image acquisition included a two‐dimensional 5‐min transmission scan for correction of tissue attenuation using a retractable 68Ge ring source before dynamic three‐dimensional scans lasting 90 min. Measurements started with a bolus injection of [carbonyl‐11C]WAY‐100635 in phosphate‐buffered saline (mean injected activity: 5.54 MBq/kg body weight ± 0.9 SD; mean specific activity: 136 GBq/μmol ± 118 SD; mean radiochemical purity: 97.7% ± 1.3 SD; mean weight of WAY‐100635: 2.05 μg ± 4 SD; mean weight of unlabeled WAY‐100635: 3.6 μg ± 4.7 SD). Dynamic scans were obtained 15 times each 1 min followed by 15 times each 5 min, resulting in 30 consecutive time frames. Thereof 35 contiguous brain slices (matrix 128 × 128, thickness 4.25 mm) were reconstructed using an iterative filtered back‐projection algorithm (FORE‐ITER) with a spatial resolution of 4.36‐mm full‐width at half maximum at the center of the field of view.
Magnetic Resonance Imaging
Structural magnetic resonance imaging (MRI) was performed with a 3 Tesla whole‐body MEDSPEC S300 MR‐scanner (Bruker BioSpin, Ettlingen, Germany) using a magnetization‐prepared rapid gradient‐echo (MPRAGE, T1 weighted) sequence (128 slices, 256 × 256 matrix, slice thickness 1.56 mm, voxel size 0.78 × 0.86 mm). Coregistration between high‐resolution MR and PET images (Fig. 1) was done using SPM5 (The Wellcome Department of Imaging Neuroscience, University College London; http://www.fil.ion.ucl.ac.uk/spm/).
Figure 1.
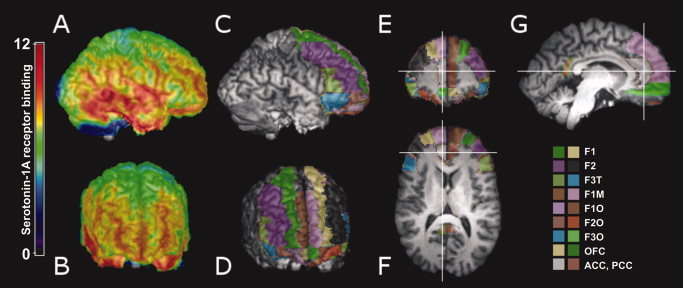
Exemplary parametric 5‐HT1A receptor binding potential map (A, B) and automated delineation of regions of interest (ROIs, C–G) drawn on an individual coregistered magnetic resonance image. F1, superior frontal cortex; F2, middle frontal cortex; F3T, triangular part of the inferior frontal cortex; F1M, medial part of the superior frontal cortex; F1O, orbital part of the superior frontal cortex; F2O, orbital part of the middle frontal cortex; F3O, orbital part of the inferior frontal cortex; OFC, orbitofrontal cortex; ACC, anterior cingulate cortex; PCC, posterior cingulate cortex. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Kinetic Modeling and ROI Analysis
As outcome measure of the 5‐HT1A receptor distribution, the regional 5‐HT1A receptor binding potential [BPND; Innis et al., 2007] was calculated using the kinetic modeling tool implemented in PMOD 2.95 [PMOD Technologies, Zurich, Switzerland; see Mikolajczyk et al., 1998]. Dynamic PET scans were spatially normalized to a tracer‐specific template in MNI stereotactic space corresponding to a ROI‐template based on automated anatomical labeling [Tzourio‐Mazoyer et al., 2002]. Using the 30 time‐frames of the dynamic PET data, decay‐corrected time activity curves were obtained within thirteen relevant ROIs. Quantification of the 5‐HT1A receptor distribution was carried out by use of the Simplified Reference Tissue Model 2 [Lammertsma and Hume, 1996; Wu and Carson, 2002] with the cerebellum as reference region due to a very low 5‐HT1A receptor density in this area [Burnet et al., 1997; Hall et al., 1997]. On the basis of previous imaging studies on aggression [Bufkin and Luttrell, 2005] and 5‐HT1A receptor density analyses [Rabiner et al., 2002; Varnäs et al., 2004], the following (bilateral) ROIs were selected including on the postsynaptic side the dorsolateral, ventromedial, and orbital prefrontal cortex as well as limbic areas (Fig. 1): (1) superior frontal cortex (F1), (2) middle frontal cortex (F2), (3) triangular part of the inferior frontal cortex (F3T), (4) medial part of the superior frontal cortex (F1M), (5) orbital part of the superior frontal cortex (F1O), (6) orbital part of the middle frontal cortex (F2O), (7) orbital part of the inferior frontal cortex (F3O), (8) medial orbital part of the superior frontal cortex (OFC), (9) anterior cingulate cortex (ACC), (10) posterior cingulate cortex (PCC), (11) amygdala (AMY), and (12) caput hippocampi (all characterized by rather high 5‐HT1A BPNDs). On the presynaptic side, (13) the RN were delineated. In addition, (14) the cerebellum served as reference region. Right and left ROIs were combined to improve signal‐to‐noise ratio.
Hormone Measurements
In the mornings before PET scans, blood samples were collected to perform standard screenings (e.g., control of self‐reported cycle phase in women). In addition, plasma levels of 17ß‐estradiol, testosterone, and the sex hormone‐binding globulin (SHBG) were assessed to check for potential interactions with the 5‐HT1A receptor distribution and aggression scores [Bethea et al., 2002; Cologer‐Clifford et al., 1997; Higley et al., 1996; Ricci et al., 2006; Rubinow et al., 1998; Simon et al., 1998]. SHBG is a glycoprotein that binds to and is indirectly regulated by sex hormones. SHBG also determines the amount of circulating testosterone and estrogen and can be regarded as an integrative marker for the availability of sex hormones. For analyses, bioavailable amounts of 17ß‐estradiol and testosterone were used. Therefore, values of free 17ß‐estradiol and testosterone can be estimated by division by 24.6 and 23.4, respectively [Vermeulen et al., 1999]. Details of hormonal plasma levels are given in Table I.
Statistical Analysis
To detect potential interactions between serotonergic neurotransmission and different dimensions of aggression, raw aggression scores were compared with regional 5‐HT1A receptor BPNDs using bivariate Spearman correlation coefficients (two tailed) and a Bonferroni adjustment for multiple comparisons. Secondary, due to non‐normal distributions, aggression scores were divided a priori into two groups each (higher vs. lower ratings) based on median values (Table II). Therewith, multivariate analyses of variance (MANOVA) with regional 5‐HT1A receptor BPNDs as dependent variables (13 levels) and either one of the aggression subscores (total aggression or self‐aggression or inhibition of aggression) as main factor (each two levels) were calculated. To adjust for potential confounding variables, radiochemical parameters (injected activity, specific activity, radiochemical purity, and weight of precursor WAY‐100635), sex, and age [Jovanovic et al., 2008; Parsey et al., 2002] were included into the model. Radiochemical parameters revealed no significant associations and were therefore excluded from further analyses. Subsequently, independent t tests were performed to assess regional BPND differences between groups.
Table 2.
Aggression scores between groups according to the Questionnaire for Measuring Factors of Aggression (Hampel and Selg, 1975)
| Mean ± SD | P a (1 vs. 2) | |||
|---|---|---|---|---|
| Group 1 (low ratings) | Group 2 (high ratings) | Total | ||
| Total aggression (1‐3), maximum = 45 | 3.56 ± 2.1 | 10.27 ± 5 | 6.58 ± 5 | <0.0001 |
| Women/men/all | 10/8/18 | 6/9/15 | 17/16/33 | NS |
| Spontaneous (1), maximum = 19 | 0.78 ± 0.9 | 2.8 ± 1.4 | 1.7 ± 1.5 | <0.0001 |
| Reactive (2), maximum = 13 | 0.78 ± 0.9 | 2.73 ± 2.8 | 1.67 ± 2.2 | 0.005 |
| Impulsiveness (3), maximum = 13 | 1.94 ± 1.6 | 4.73 ± 2 | 3.21 ± 2.3 | 0.0001 |
| Frankness, maximum = 10 | 5.4 ± 2.6 | 6.7 ± 2.1 | 6 ± 2.4 | NS |
| Self‐aggression (4), maximum = 13 | 0.33 ± 0.5 | 4.83 ± 3 | 1.97 ± 2.8 | <0.0001 |
| Women/men/all | 10/11/21 | 6/6/12 | 17/16/33 | NS |
| Frankness, maximum = 10 | 5.43 ± 2.7 | 7 ± 1.5 | 6 ± 2.4 | NS |
| Inhibition of aggression (5), maximum = 10 | 2.72 ± 1.3 | 6.18 ± 1 | 4.5 ± 2.1 | <0.0001 |
| Women/men/all | 7/9/16 | 9/8/17 | 17/16/33 | NS |
| Frankness maximum = 10 | 5.75 ± 2.5 | 6.24 ± 2.4 | 6 ± 2.4 | NS |
Two independent sample tests (Mann‐Whitney).
To test whether aggression scores were associated with sex hormones, bivariate correlations were conducted with plasma levels of SHBG (pooled), 17ß‐estradiol (sex specific), and testosterone (sex specific) using Spearman correlation coefficients (two tailed) and a Bonferroni adjustment for multiple comparisons. Secondary, dichotomous aggression score groups were compared with sex hormones using independent t tests (two tailed) and the dependent variables SHBG (pooled), 17ß‐estradiol (sex specific), and testosterone (sex specific), corrected by a Bonferroni adjustment for multiple comparisons.
Regional 5‐HT1A BPNDs were related to sex hormones by linear regression models (forward inclusion) with regional 5‐HT1A receptor BPNDs as dependent variables and levels of SHBG, or 17ß‐estradiol, or testosterone, as fixed factors. Age and sex were entered into the model to adjust for potential confounding. Regarding 17ß‐estradiol and testosterone, analyses were performed for women and men separately.
Normal distribution, equality of covariance matrices, and homogeneity of variances considering 5‐HT1A receptor BPND and sex hormone data were assumed according to the Kolmogorov‐Smirnov test (P > 0.05), the Box's test (P > 0.05), and the Levene's test (P > 0.05). Statistical analyses were performed using SPSS 12 (SPSS, Chicago, IL).
RESULTS
Correlations Between Aggression Scores and 5‐HT1A
Significant correlations that survived the Bonferroni correction for multiple comparisons (P < 0.0038) were observed between total aggression scores and regional BPNDs in the ACC (r = 0.512, P = 0.002). Significant correlations did not reach the Bonferroni threshold in the superior (r = 0.47, P = 0.006) and middle (r = 0.46, P = 0.008) frontal cortices, in the triangular (r = 0.39, P = 0.024) and orbital (r = 0.49, P = 0.004) parts of the inferior frontal cortex, in the medial part of the superior (r = 0.42, P = 0.016) and in the orbital part of the middle (r = 0.37, P = 0.032) frontal cortices as well as in the orbitofrontal cortex (r = 0.44, P = 0.01).
Even more robust Bonferroni‐corrected correlations were found when separating the individual components “spontaneous aggression” and “impulsiveness” from the total aggression score (Table III for r and P values; Fig. 2 for examples). The component “reactive aggression” as well as scores on self‐aggression and inhibition of aggression showed no correlations in any ROI (all P > 0.05, data not shown).
Table 3.
Statistics of Spearman correlations between aggression subscores and regional 5‐HT1A receptor BPNDs
| Total aggression score | ||||
|---|---|---|---|---|
| Spontaneous aggression | Impulsiveness | |||
| r | P | r | P | |
| Superior frontal cortex | 0.41 | 0.018 | 0.59 | 0.0003 |
| Middle frontal cortex | 0.33 | 0.063 | 0.41 | 0.018 |
| Triangular part of the inferior frontal cortex | 0.35 | 0.047 | 0.58 | 0.0004 |
| Medial part of the superior frontal cortex | 0.32 | 0.068 | 0.53 | 0.0016 |
| Orbital part of the superior frontal cortex | 0.36 | 0.04 | 0.58 | 0.0004 |
| Orbital part of the middle frontal cortex | 0.29 | 0.1 | 0.48 | 0.0047 |
| Orbital part of the inferior frontal cortex | 0.35 | 0.048 | 0.6 | 0.0002 |
| Orbitofrontal cortex | 0.32 | 0.066 | 0.55 | 0.0009 |
| Anterior cingulate cortex | 0.25 | 0.16 | 0.4 | 0.023 |
| Posterior cingulate cortex | 0.08 | 0.65 | 0.22 | 0.22 |
| Amygdala | 0.32 | 0.07 | 0.56 | 0.0007 |
| Hippocampus | 0.09 | 0.61 | 0.17 | 0.34 |
| Raphe nuclei | 0.03 | 0.86 | 0.05 | 0.78 |
Figure 2.
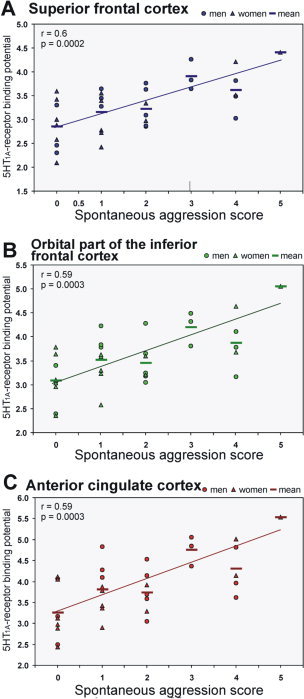
Correlations between the 5‐HT1A receptor binding potential and the “spontaneous aggression” component of the total aggression score in the frontal superior cortex (F1, A), in the orbital part of the frontal inferior cortex (F3O, B), and in the anterior cingulate cortex (ACC, C). Lines give regression fit, r and P values according to Spearman correlation coefficients triangles = women, circles = men, bars = means; n = 33. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Aggression Score Subgroups and 5‐HT1A
Secondary, MANOVA detected significant effects of total aggression score subgroups (Pillai's trace = 0.68, F (13, 19) = 3.1, P = 0.013) and self‐aggression score subgroups (Pillai's trace = 0.64, F (13, 19) = 2.63, P = 0.027) on the regional 5‐HT1A receptor BPNDs. No significant interactions were found between other aggression scores subgroups or potentially confounding variables (radiochemical parameters, age, sex) and regional 5‐HT1A BPNDs (P > 0.05).
Considering total aggression, subsequent t tests revealed significant mean differences between groups (high vs. low ratings) in the following ROIs (Fig. 3A): superior (F1, r = 0.38, P = 0.029) and middle (F2, r = 0.38, P = 0.033) frontal cortices, medial part of the superior frontal cortex (F1M, r = 0.37, P = 0.033), orbital part of the middle (F2O, r = 0.36, P = 0.041) and inferior (F3O, r = 0.45, P = 0.009) frontal cortices, orbitofrontal cortex (OFC, r = 0.41, P = 0.017), and ACC (r = 0.42, P = 0.016). In these areas on average, a 13.25% higher mean 5‐HT1A receptor BPND corresponded to the more aggressive subgroup. No group‐differences were found in the other ROIs including the hippocampus. Note that mean 5‐HT1A receptor BPND in the RN contrasted to those in frontal areas, namely that the high‐aggressive subgroup exhibited a lower mean 5‐HT1A receptor BPND, however, without statistical significance.
Figure 3.
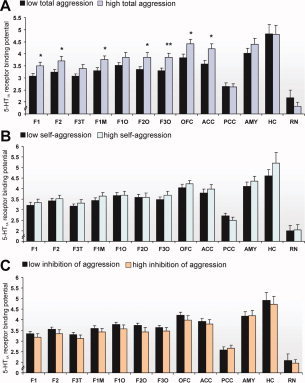
Regional 5‐HT1A receptor binding potentials in high‐ vs. low‐aggression subgroups. (A) total aggression score, (B) self‐aggression score, (C) inhibition of aggression score. Asterisks indicate levels of significance according to independent t tests (*P < 0.05; **P < 0.01). Bars display means, error bars give standard error (SE), n = 33. AMY, amygdalae; HC, hippocampus; RN, raphe nuclei; further abbreviations explained in the legend of Figure 1. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Regarding self‐aggression, post hoc independent t tests failed to reach significance in any region (P > 0.05), yet a somewhat similar trend, meaning higher mean 5‐HT1A BPNDs in the more (self‐)aggressive subgroup, can be seen (Fig. 3B). Rather opposite associations, i.e., lower regional 5‐HT1A BPNDs in the high‐rating subgroup, were observed when evaluating inhibition of aggression (Fig. 3C), however, data did not reach the level of significance in MANOVA.
Correlations Between Aggression Scores and Sex Hormones
Correlation analyses revealed inverse associations between inhibition of aggression scores and SHBG levels, which withstood the Bonferroni correction for multiple comparisons (r = −0.43, P = 0.012). Without correction, significant inverse correlations were observed between the “spontaneous aggression” component of the total aggression score and SHBG (r = −0.36, P = 0.039). The other components did not correlate with SHBG levels (P > 0.05). Strong Bonferroni‐corrected positive correlations were found in men comparing inhibition of aggression with 17ß‐estradiol (r = 0.73, P = 0.001) and testosterone (r = 0.58, P = 0.016). No further correlations were seen.
Aggression Score Subgroups and Sex Hormones
Secondary, independent t tests revealed a significantly lower mean plasma SHBG level in subjects exhibiting higher scores on the “spontaneous aggression” component of the total aggression score, which withstood the Bonferroni correction (t = 2.59, P = 0.015, Fig. 4). A similar trend was seen between the “reactive aggression” component groups of the total aggression score (t = 1.99, P = 0.058, Fig. 4). No differences were found between other aggression score subgroups (P > 0.05).
Figure 4.
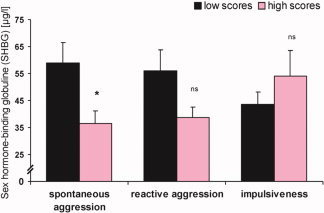
Plasma levels of sex hormone‐binding globulin in high‐ vs. low‐aggression subgroups in the components of the total aggression score (“spontaneous aggression,” “reactive aggression,” and “impulsiveness”). Asterisks indicate levels of significance according to t tests (*P < 0.05). Bars display means, error bars give standard error of the median (SE), n = 33. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Mean plasma levels of 17ß‐estradiol (t = −2.35, P = 0.002, Bonferroni‐corrected) and testosterone (t = −3.85, P = 0.033, uncorrected) were significantly higher in men exhibiting higher scores of inhibition of aggression. In women, a significantly higher mean testosterone level was seen in the subgroup with higher scores on “spontaneous aggression” as component of the total aggression score (t = −2.39, P = 0.031, uncorrected). No other effects were observed (P > 0.05).
Sex Hormones and 5‐HT1A
Linear regression analyses revealed significant inverse associations of SHBG and the 5‐HT1A receptor BPNDs in the orbital part of the inferior frontal cortex (β = −0.35, t = −2.06, P = 0.048), in the ACC (β = −0.43, t = −2.65, P = 0.013) and PCC (β = −0.54, t = −3.53, P = 0.001; Fig. 5), as well as in the AMY (β = −0.38, t = −2.28, P = 0.03). Similar trends were seen in the other prefrontal cortices (P < 0.1) but not in the hippocampus and RN (P > 0.1).
Figure 5.
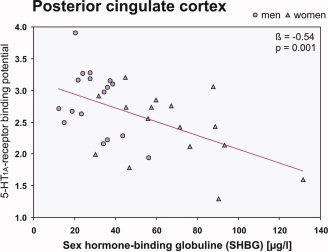
Inverse association between plasma levels of sex hormone‐binding globulin (SHBG) and the 5‐HT1A receptor BPNDs in the posterior cingulate cortex. Line gives regression fit, β and P values according to linear regression, triangles = women, circles = men; n = 33. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
In the RN, 17ß‐estradiol levels were significantly associated with the 5‐HT1A receptor BPNDs in women (β = 0.54, t = 3.45, P = 0.002). Testosterone levels were found to be significantly related to 5‐HT1A receptor BPNDs in frontal areas in women, namely in the superior (β = 0.61, t = 2.91, P = 0.012) and middle frontal cortices (β = 0.64, t = 3.09, P = 0.008), in the triangular (β = 0.65, t = 3.24, P = 0.006, Fig. 6A) and orbital parts of the inferior cortex (β = 0.59, t = 2.72, P = 0.017), in the medial part of the superior (β = 0.61, t = 2.9, P = 0.012) and orbital part of the middle frontal cortex (β = 0.68, t = 3.46, P = 0.004, Fig. 6B), in the orbitofrontal cortex (β = 0.59, t = 2.53, P = 0.024), as well as in the ACC (β = 0.63, t = 3.06, P = 0.008). In men, testosterone was significantly associated only in the PCC (β = 0.49, t = 2.15, P = 0.049). In this area, a similar trend was also seen in women (P = 0.09). No further associations were found (P > 0.05).
Figure 6.
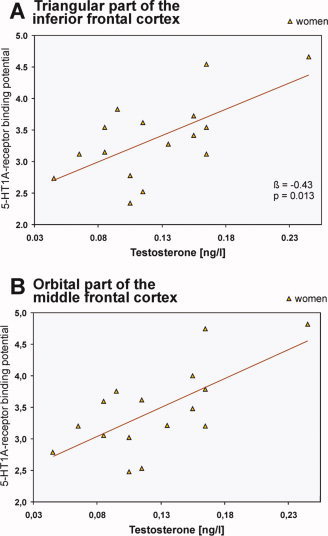
Association between plasma levels of testosterone and the 5‐HT1A receptor BPNDs in women in the triangular part of the inferior frontal (A) and in the orbital part of the middle frontal cortices (B). Lines give regression fit, β and P values according to linear regressions; n = 16. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
This study demonstrates for the first time a selective interrelation between the 5‐HT1A receptor distribution, sex hormones, and aggression scores in humans, as previously hypothesized [Birger et al., 2003; Davidson et al., 2000]. Using PET and the radioligand [11C]WAY‐100635 in 33 healthy subjects, we found higher total aggression scores in subjects exhibiting a higher density or affinity of postsynaptic 5‐HT1A receptors in the dorsolateral and ventromedial prefrontal cortex, in the orbitofrontal cortex, and in the ACC. Moreover, a higher rating on the “spontaneous aggression” component of the total aggression score was characterized by lower SHBG levels. Third, higher SHBG plasma levels corresponded to lower postsynaptic 5‐HT1A receptor affinities or densities in the prefrontal cortex and even more pronounced in the ACC and PCC and in the AMY. In women only, strong correlations were also found regarding levels of testosterone and frontal areas.
Aggression and Frontal Serotonin
It is well established that the outcome variable “binding potential” (= BMax/KD) measured with PET and the radioligand [11C]WAY‐100635 stands for the density or affinity of the 5‐HT1A receptor in the respective area [detailed in a consensus statement, Innis et al., 2007; Pike et al., 1996]. Thus, our findings point to a higher density or affinity of inhibitory 5‐HT1A receptors in frontal areas in subjects exhibiting higher aggression scores. As a consequence, it seems likely that higher aggression goes along with an increased inhibition or hypofunction of prefrontal and anterior cingulate cortices during serotonergic neurotransmission. Considering the suggested crucial role of higher‐order cortices like the prefrontal cortex in the control of impulsive affective states [Davidson et al., 2000], this might result in intensified emotional behaviors. For example, lesions of the prefrontal cortex due to stroke [Chan et al., 2006] or war injuries [Grafman et al., 1996] have been reported to increase aggressive behavior in humans. Further support for this hypothesis arose from PET studies ascribing prefronto‐limbic circuits an essential role in allowing or inhibiting aggressive behaviors. In patients with impulsive personality disorder, a drastically reduced glucose metabolism was found in the prefrontal and anterior cingulate cortices after administration of the serotonin‐releaser fenfluramine compared to controls, thus suggesting an impaired (pre)frontal activation by serotonin [Siever et al., 1999; Soloff et al., 2003]. A study including 41 murderers likewise observed prefrontal hypo‐ and subcortical hyperactivation using PET [Raine et al., 1997, 1998]. When trying to reduce pathologic aggressive behavior, treatments with SSRIs have commonly shown to be potent agents [New et al., 2004]. This provided further evidence of a positive association between aggression and the postsynaptic 5‐HT1A receptor, because SSRI treatment in anxiety patients were found to reduce 5‐HT1A receptor densities or affinities in the posterior and subgenual part of the ACC and hippocampus and as trend also in the orbitofrontal cortex according to a recent study of our group [Spindelegger et al., in press]. It seems therefore likely that a loss of inhibitory prefrontal control over limbic centers, due to 5‐HT1A‐mediated neurotransmission, might induce exaggerated affective responses including enhanced aggressive behavior [Davidson et al., 2000].
Pre‐ and Postsynaptic 5‐HT1A Receptors
The current results, namely a higher 5‐HT1A receptor distribution in the prefrontal and anterior cingulate cortices in subjects with higher aggression scores, are in line with a recent animal study using polymerase chain reaction in genetically defined aggressive rats [Popova et al., 2007]. However, they contradict a previous PET study investigating 25 human subjects [Parsey et al., 2002]. We observed an inverse correlation between aggression and the 5‐HT1A receptor BPND in the ventromedial prefrontal, orbitofrontal, and anterior cingulate cortices, but also in the AMY and RN using also the selective radiotracer [11C]WAY‐100635 [Parsey et al., 2002]. Note that in the current study, we observed a congruent pattern in the RN, which might have failed to reach significance due to partial volume effects. A similar negative 5‐HT1A correlation in the RN was also claimed by other studies in rodents [Popova, 2006]. However, in addition to the relatively smaller number of subjects, Parsey et al. [2002] used a questionnaire designed to measure life‐time history of aggressive events, which might not reflect the same separated dimensions of aggression as measured by the sensitive questionnaire used in our study [Hampel and Selg, 1975]. Moreover, the age range was quite large and statistical results were considerably weaker than ours. Similar limitations may also explain the negative findings of Rabiner et al. [2002], who correlated the 5‐HT1A receptor distribution with global scores of personality questionnaires in a database of 61 healthy, exclusively male subjects but did not find any significant associations.
Our findings are in line with studies suggesting a different role of pre‐ and postsynaptic 5‐HT1A receptors in mediating aggression‐related serotonergic neurotransmission [de Boer and Koolhaas, 2005; Miczek et al., 2007]. It was found that certain high‐affinity 5‐HT1A receptor agents, such as ipsapirone and S‐15535, exert antiaggressive effects most likely via a selective agonistic action at somatodendritic autoreceptors in the RN, but no or a rather antagonistic action on postsynaptic 5‐HT1A receptors in central target areas as measured in rodents [de Boer et al., 1999; Millan et al., 1994; Scott et al., 1994]. Thus, corresponding to our results, a functionally distinct role of presynaptic [i.e., in the RN, Hoyer et al., 2002] and postsynaptic [i.e., in neocortical areas, Hoyer et al., 2002] 5‐HT1A receptors should be assumed in consistency with the literature.
Aggression, Serotonin, and Sex Hormones
Considering sex hormones, we found higher aggression scores to be significantly associated with lower SHBG levels. Moreover, we observed inverse associations between plasma levels of SHBG and the 5‐HT1A receptor density or affinity in frontal and limbic areas. Because SHBG determines the amount of circulating testosterone and estrogen, it should display reciprocal associations compared to testosterone and 17ß‐estradiol. We observed such an inverse relationship regarding testosterone and aggression as well as testosterone and 5‐HT1A in women. This on the one hand further underlines the well‐documented modulatory role of sex steroid hormones in aggression. For example, levels of both estrogens and testosterone have been shown to control maternal aggression, whereas primarily testosterone exerts regulatory influence on intermale aggression in rats [reviewed in Albert et al., 1992; Lonstein and Gammie, 2002]. Because higher testosterone levels are also associated with more aggressive behaviors in mammals including humans [reviewed in Archer, 2006], our positive relationship between levels of bioavailable sex hormones, particularly testosterone, and aggression scores in men are quite as expected.
On the other hand, our study provides first evidence for the putative link between serotonin and sex hormones in the modulation of human aggressive behavior [Birger et al., 2003]. It was frequently suggested that aggression‐related actions of sex hormones are at least in part be mediated by serotonergic neurotransmission [Cosgrove et al., 2007; Rubinow et al., 1998]. Beside a variety of studies on sexual dimorphisms in the serotonergic system [Cosgrove et al., 2007], several studies in rodents and primates have investigated how sex‐steroids modulate serotonergic transmission. Regarding the 5‐HT1A receptor, it has been suggested in both rodents [Flügge et al., 1999] and humans [Spindelegger et al., 2008] that fluctuating levels of sex hormones during the menstrual cycle are accompanied by changing 5‐HT1A receptor distributions. Moreover, as shown in nonhuman primates, a long‐term administration of estrogen leads to down‐regulating of both pre‐ and postsynaptic 5‐HT1A receptor affinity or density [Lu and Bethea, 2002; Pecins‐Thompson and Bethea, 1999]. This regulatory effect of estrogen might be reflected by our results, since we observed a significant positive correlation between 17ß‐estradiol levels and the 5‐HT1A receptor BPNDs in the RN in women. Here, specific transcription factors might be implemented in the underlying molecular machinery, which was first demonstrated experimentally in nonhuman primates for MAO. According to Zhang et al. [2006] and Gundlah et al. [2002], ovarian steroids have been found to activate MAO gene expression via estrogen receptors. Taken together, extensive interrelation between sex hormones and serotonergic neurotransmission might play a crucial role in modulating aggressive behavior. Interestingly, androgenic and estrogenic metabolites of testosterone were found to distinctively modulate 5‐HT1A and B receptor agonist effects in the control of aggressive behavior in mice and hamsters in the lateral septum and medial preoptic area [Cologer‐Clifford et al., 1996; Simon et al., 1998]. This is in line with our findings in humans when considering selective, area‐specific associations between aggression, sex hormones, and the 5‐HT1A receptor BPNDs.
Strengths and Limitations
As a limitation of our study, the subject population primarily consisted of medical students which might have affected the generalizability of results. In addition, aggressive behavior was not assessed in an experimental setting but in a self‐reported, yet detailed and validated questionnaire that was designed to differentiate between distinct dimensions of aggression. However, the sensitive acquisition of aggression‐related items resulted in statistically robust data. Further strengths of our study lie in the large and balanced sample size including both sexes, the advanced PET imaging with [11C]WAY‐100635, as well as adjustment for potential confounders and sensitive hormone measures.
CONCLUSION AND OUTLOOK
Our study provides good evidence that aggressive behavior is closely associated with and presumably controlled by serotonergic neurotransmission in humans, particularly via postsynaptic 5‐HT1A receptors in frontal areas. These interactions might be further modulated by a specific regulatory effect of sex hormones on the serotonergic system, because plasma hormone levels were related to both aggression and 5‐HT1A receptor BPNDs. Future prospective trials should now examine the proposed underlying mechanisms. Specifically, it should be clarified how sex hormones might up‐ or down‐regulate serotonergic receptors, transporters, and signalling with regard to aggressive behavior.
Acknowledgements
The authors thank Alexander Holik, Johannes Tauscher, Robert Dudczak, Herbert Bauer, Alexander Becherer, Christian Bieglmayer for their support.
REFERENCES
- Archer J ( 2006): Testosterone and human aggression: An evaluation of the challenge hypothesis. Neurosci Biobehav Rev 30: 319–345. [DOI] [PubMed] [Google Scholar]
- Albert DJ,Jonik RH,Walsh ML ( 1992): Hormone‐dependent aggression in male and female rats: Experiential, hormonal, and neural foundations. Neurosci Biobehav Rev 16: 177–192. [DOI] [PubMed] [Google Scholar]
- Bannai M,Fish EW,Faccidomo S,Miczek KA ( 2007): Anti‐aggressive effects of agonists at 5‐HT1B receptors in the dorsal raphe nucleus of mice. Psychopharmacology 193: 295–304. [DOI] [PubMed] [Google Scholar]
- Bantick RA,Montgomery AJ,Bench CJ,Choudhry T,Malek N,McKenna PJ,Quested DJ,Deakin JFW,Grasby PM ( 2004): A positron emission tomography study of the 5‐HT1A receptor in schizophrenia and during clozapine treatment. J Psychopharmacol 18: 346–354. [DOI] [PubMed] [Google Scholar]
- Bethea CL,Lu NZ,Gundlah C,Streicher JM ( 2002): Diverse actions of ovarian steroids in the serotonin neural system. Front Neuroendocrinol 23: 41–100. [DOI] [PubMed] [Google Scholar]
- Birger M,Swartz M,Cohen D,Alesh Ya,Grishpan C,Kotelr M ( 2003): Aggression: The testosterone‐serotonin link. Isr Med Assoc J 5: 653–658. [PubMed] [Google Scholar]
- Brown GL,Goodwin FK,Ballenger JC,Goyer PF,Major LF ( 1979): Aggression in humans correlates with cerebrospinal fluid amine metabolites. Psychiatry Res 1: 131–139. [DOI] [PubMed] [Google Scholar]
- Brunner HG,Nelen M,Breakefield XO,Ropers HH,van Oost BA ( 1993): Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science 262: 578–580. [DOI] [PubMed] [Google Scholar]
- Bufkin JL,Luttrell VR ( 2005): Neuroimaging studies of aggressive and violent behavior: Current findings and implications for criminology and criminal justice. Trauma Violence Abuse 6: 176–191. [DOI] [PubMed] [Google Scholar]
- Burnet PW,Eastwood SL,Harrison PJ ( 1997): [3H]WAY‐100635 for 5‐HT1A receptor autoradiography in human brain: A comparison with [3H]8‐OH‐DPAT and demonstration of increased binding in the frontal cortex in schizophrenia. Neurochem Int 30: 565–574. [DOI] [PubMed] [Google Scholar]
- Buss AH,Durkee A ( 1957): An inventory for assessing different kinds of hostility. J Consult Psychol 21: 343–349. [DOI] [PubMed] [Google Scholar]
- Cases O,Seif I,Grimsby J,Gaspar P,Chen K,Pournin S,Mueller U,Aguet M,Babinet C,Shih JC ( 1995): Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science 268: 1763–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KL,Campayo A,Moser DJ,Arndt S,Robinson RG ( 2006): Aggressive behavior in patients with stroke: Association with psychopathology and results of antidepressant treatment on aggression. Arch Phys Med Rehabil 87: 793–798. [DOI] [PubMed] [Google Scholar]
- Cherek DR,Lane SD ( 2001): Acute effects of d‐fenfluramine on simultaneous measures of aggressive escape and impulsive responses of adult males with and without a history of conduct disorder. Psychopharmacology 157: 221–227. [DOI] [PubMed] [Google Scholar]
- Chiavegatto S,Dawson VL,Mamounas LA,Koliatsos VE,Dawson TM,Nelson RJ ( 2001): Brain serotonin dysfunction accounts for aggression in male mice lacking neuronal nitric oxide synthase. Proc Natl Acad Sci USA 98: 1277–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccaro EF,Kavoussi RJ ( 1997): Fluoxetine and impulsive aggressive behavior in personality‐disordered subjects. Arch Gen Psychiatry 54: 1081–1088. [DOI] [PubMed] [Google Scholar]
- Cologer‐Clifford A,Simon NG,Lu SF,Smoluk SA ( 1997): Serotonin agonist‐induced decreases in intermale aggression are dependent on brain region and receptor subtype. Pharmacol Biochem Behav 58: 425–430. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP,Mazure CM,Staley JK ( 2007): Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry 62: 847–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datla KP,Bhattacharya SK ( 1990): Effect of selective monoamine oxidase A and B inhibitors on footshock induced aggression in paired rats. Indian J Exp Biol 28: 742–745. [PubMed] [Google Scholar]
- Davidson RJ,Putnam KM,Larson CL ( 2000): Dysfunction in the neural circuitry of emotion regulation–a possible prelude to violence. Science 289: 591–594. [DOI] [PubMed] [Google Scholar]
- De Almeida RM,Lucion AB ( 1997): 8‐OH‐DPAT in the median raphe, dorsal periaqueductal gray and corticomedial amygdala nucleus decreases, but in the medial septal area it can increase maternal aggressive behavior in rats. Psychopharmacology 134: 392–400. [DOI] [PubMed] [Google Scholar]
- de Boer SF,Koolhaas JM ( 2005): 5‐HT1A and 5‐HT1B receptor agonists and aggression: A pharmacological challenge of the serotonin deficiency hypothesis. Euro J Pharmacol 526: 125–139. [DOI] [PubMed] [Google Scholar]
- de Boer SF,Lesourd M,Mocaer E,Koolhaas JM ( 1999): Selective antiaggressive effects of alnespirone in resident‐intruder test are mediated via 5‐hydroxytryptamine1A receptors: A comparative pharmacological study with 8‐hydroxy‐2‐dipropylaminotetralin, ipsapirone, buspirone, eltoprazine, and WAY‐100635. J Pharmacol Exp Ther 288: 1125–1133. [PubMed] [Google Scholar]
- de Boer SF,Lesourd M,Mocaer E,Koolhaas JM ( 2000): Somatodendritic 5‐HT(1A) autoreceptors mediate the anti‐aggressive actions of 5‐HT(1A) receptor agonists in rats: An ethopharmacological study with S‐15535, alnespirone, and WAY‐100635. Neuropsychopharmacology 23: 20–33. [DOI] [PubMed] [Google Scholar]
- Ferrari PF,Palanza P,Parmigiani S,de Almeida RMM,Miczek KA ( 2005): Serotonin and aggressive behavior in rodents and nonhuman primates: Predispositions and plasticity. Euro J Pharmacol 526: 259–273. [DOI] [PubMed] [Google Scholar]
- Florvall L,Ask AL,Ogren SO,Ross SB ( 1978): Selective monoamine oxidase inhibitors. I. Compounds related to 4‐aminophenethylamine. J Med Chem 21: 56–63. [DOI] [PubMed] [Google Scholar]
- Flügge G,Pfender D,Rudolph S,Jarry H,Fuchs E ( 1999): 5HT1A‐receptor binding in the brain of cyclic and ovariectomized female rats. J Neuroendocrinol 11: 243–249. [DOI] [PubMed] [Google Scholar]
- Gerstl F,Windischberger C,Mitterhauser M,Wadsak W,Holik A,Kletter K,Moser E,Kasper S,Lanzenberger R ( 2008): Multimodal imaging of human early visual cortex by combining functional and molecular measurements with fMRI and PET. Neuroimage 41: 204–211. [DOI] [PubMed] [Google Scholar]
- Gietl A,Giegling I,Hartmann AM,Schneider B,Schnabel A,Maurer K,Moller H‐J,Rujescu D ( 2007): ABCG1 gene variants in suicidal behavior and aggression‐related traits. Euro Neuropsychopharmacol 17: 410–416. [DOI] [PubMed] [Google Scholar]
- Grafman J,Schwab K,Warden D,Pridgen A,Brown HR,Salazar AM ( 1996): Frontal lobe injuries, violence, and aggression: A report of the Vietnam Head Injury Study. Neurology 46: 1231–1238. [DOI] [PubMed] [Google Scholar]
- Gundlah C,Lu NZ,Bethea CL ( 2002): Ovarian steroid regulation of monoamine oxidase‐A and ‐B mRNAs in the macaque dorsal raphe and hypothalamic nuclei. Psychopharmacology 160: 271–282. [DOI] [PubMed] [Google Scholar]
- Hall H,Lundkvist C,Halldin C,Farde L,Pike VW,McCarron JA,Fletcher A,Cliffe IA,Barf T,Wikstrom H,Sedvall G ( 1997): Autoradiographic localization of 5‐HT1A receptors in the post‐mortem human brain using [3H]WAY‐100635 and [11C]way‐100635. Brain Res 745: 96–108. [DOI] [PubMed] [Google Scholar]
- Hampel R,Selg H ( 1975): Fragebogen zur Erfassung von Aggressivitätsfaktoren. Göttingen: Hogrefe Verlag. [Google Scholar]
- Hermans EJ,Ramsey NF,van Honk J ( 2008): Exogenous testosterone enhances responsiveness to social threat in the neural circuitry of social aggression in humans. Biol Psychiatry 63: 263–270. [DOI] [PubMed] [Google Scholar]
- Higley JD,Mehlman PT,Poland RE,Taub DM,Vickers J,Suomi SJ,Linnoila M ( 1996): CSF testosterone and 5‐HIAA correlate with different types of aggressive behaviors. Biol Psychiatry 40: 1067–1082. [DOI] [PubMed] [Google Scholar]
- Hoyer D,Hannon JP,Martin GR ( 2002): Molecular, pharmacological and functional diversity of 5‐HT receptors. Pharmacol Biochem Behav 71: 533–554. [DOI] [PubMed] [Google Scholar]
- Innis RB,Cunningham VJ,Delforge J,Fujita M,Gjedde A,Gunn RN,Holden J,Houle S,Huang SC,Ichise M,Iida H,Ito H,Kimura Y,Koeppe RA,Knudsen GM,Knuuti J,Lammertsma AA,Laruelle M,Logan J,Maguire RP,Mintun MA,Morris ED,Parsey R,Price JC,Slifstein M,Sossi V,Suhara T,Votaw JR,Wong DF,Carson RE ( 2007): Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 27: 1533–1539. [DOI] [PubMed] [Google Scholar]
- Jovanovic H,Cerin A,Karlsson P,Lundberg J,Halldin C,Nordstrom AL ( 2006): A PET study of 5‐HT1A receptors at different phases of the menstrual cycle in women with premenstrual dysphoria. Psychiatry Res 148: 185–193. [DOI] [PubMed] [Google Scholar]
- Kantak KM,Hegstrand LR,Whitman J,Eichelman B ( 1980): Effects of dietary supplements and a tryptophan‐free diet on aggressive behavior in rats. Pharmacol Biochem Behav 12: 173–179. [DOI] [PubMed] [Google Scholar]
- Kasper S,Olié JP ( 2002): A meta‐analysis of randomized controlled trials of tianeptine versus SSRI in the short‐term treatment of depression. Eur Psychiatry 17( Suppl 3): 331–340. [DOI] [PubMed] [Google Scholar]
- Lammertsma AA,Hume SP ( 1996): Simplified reference tissue model for PET receptor studies. Neuroimage 4: 153–158. [DOI] [PubMed] [Google Scholar]
- Lanzenberger RR,Mitterhauser M,Spindelegger C,Wadsak W,Klein N,Mien L‐K,Holik A,Attarbaschi T,Mossaheb N,Sacher J,Geiss‐Granadia T,Kletter K,Kasper S,Tauscher J ( 2007): Reduced serotonin‐1A receptor binding in social anxiety disorder. Biol Psychiatry 61: 1081–1089. [DOI] [PubMed] [Google Scholar]
- Lonstein JS,Gammie SC ( 2002): Sensory, hormonal, and neural control of maternal aggression in laboratory rodents. Neurosci Biobehav Rev 26: 869–888. [DOI] [PubMed] [Google Scholar]
- Lu NZ,Bethea CL ( 2002): Ovarian steroid regulation of 5‐HT1A receptor binding and G protein activation in female monkeys. Neuropsychopharmacology 27: 12–24. [DOI] [PubMed] [Google Scholar]
- Mehlman PT,Higley JD,Faucher I,Lilly AA,Taub DM,Vickers J,Suomi SJ,Linnoila M ( 1994): Low CSF 5‐HIAA concentrations and severe aggression and impaired impulse control in nonhuman primates. Am J Psychiatry 151: 1485–1491. [DOI] [PubMed] [Google Scholar]
- Miczek KA,Hussain S,Faccidomo S ( 1998): Alcohol‐heightened aggression in mice: Attenuation by 5‐HT1A receptor agonists. Psychopharmacology 139: 160–168. [DOI] [PubMed] [Google Scholar]
- Miczek KA,de Almeida RMM,Kravitz EA,Rissman EF,de Boer SF,Raine A ( 2007): Neurobiology of escalated aggression and violence. J Neurosci 27: 11803–11806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikolajczyk K,Szabatin M,Rudnicki P,Grodzki M,Burger C ( 1998): A JAVA environment for medical image data analysis: Initial application for brain PET quantitation. Medical Inform 23: 207–214. [DOI] [PubMed] [Google Scholar]
- Millan MJ,Canton H,Gobert A,Lejeune F,Rivet JM,Bervoets K,Brocco M,Widdowson P,Mennini T,Audinot V ( 1994): Novel benzodioxopiperazines acting as antagonists at postsynaptic 5‐HT1A receptors and as agonists at 5‐HT1A autoreceptors: A comparative pharmacological characterization with proposed 5‐HT1A antagonists. J Pharmacol Exp Ther 268: 337–352. [PubMed] [Google Scholar]
- Nelson RJ,Trainor BC ( 2007): Neural mechanisms of aggression. Nature reviews. Neuroscience 8: 536–546. [DOI] [PubMed] [Google Scholar]
- New AS,Buchsbaum MS,Hazlett EA,Goodman M,Koenigsberg HW,Lo J,Iskander L,Newmark R,Brand J,O'Flynn K,Siever LJ ( 2004): Fluoxetine increases relative metabolic rate in prefrontal cortex in impulsive aggression. Psychopharmacology 176: 451–458. [DOI] [PubMed] [Google Scholar]
- Parsey RV,Oquendo MA,Simpson NR,Ogden RT,Van Heertum R,Arango V,Mann JJ ( 2002): Effects of sex, age, and aggressive traits in man on brain serotonin 5‐HT1A receptor binding potential measured by PET using [C‐11]WAY‐100635. Brain Res 954: 173–182. [DOI] [PubMed] [Google Scholar]
- Pecins‐Thompson M,Bethea CL ( 1999): Ovarian steroid regulation of serotonin‐1A autoreceptor messenger RNA expression in the dorsal raphe of rhesus macaques. Neuroscience 89: 267–277. [DOI] [PubMed] [Google Scholar]
- Pike VW,McCarron JA,Lammertsma AA,Osman S,Hume SP,Sargent PA,Bench CJ,Cliffe IA,Fletcher A,Grasby PM ( 1996): Exquisite delineation of 5‐HT1A receptors in human brain with PET and [carbonyl‐11 C]WAY‐100635. Eur J Pharmacol 301: R5–R7. [DOI] [PubMed] [Google Scholar]
- Pinna G,Dong E,Matsumoto K,Costa E,Guidotti A ( 2003): In socially isolated mice, the reversal of brain allopregnanolone down‐regulation mediates the anti‐aggressive action of fluoxetine. Proc Natl Acad Sci USA 100: 2035–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova NK ( 2006): From genes to aggressive behavior: The role of serotonergic system. Bioessays 28: 495–503. [DOI] [PubMed] [Google Scholar]
- Popova NK,Naumenko VS,Plyusnina IZ,Kulikov AV ( 2005): Reduction in 5‐HT1A receptor density, 5‐HT1A mRNA expression, and functional correlates for 5‐HT1A receptors in genetically defined aggressive rats. J Neurosci Res 80: 286–292. [DOI] [PubMed] [Google Scholar]
- Popova NK,Naumenko VS,Plyusnina IZ ( 2007): Involvement of brain serotonin 5‐HT1A receptors in genetic predisposition to aggressive behavior. Neurosci Behav Physiol 37: 631–635. [DOI] [PubMed] [Google Scholar]
- Rabiner EA,Messa C,Sargent PA,Husted‐Kjaer K,Montgomery A,Lawrence AD,Bench CJ,Gunn RN,Cowen P,Grasby PM ( 2002): A database of [(11)C]WAY‐100635 binding to 5‐HT(1A) receptors in normal male volunteers: Normative data and relationship to methodological, demographic, physiological, and behavioral variables. Neuroimage 15: 620–632. [DOI] [PubMed] [Google Scholar]
- Raine A,Buchsbaum M,LaCasse L ( 1997): Brain abnormalities in murderers indicated by positron emission tomography. Biol Psychiatry 42: 495–508. [DOI] [PubMed] [Google Scholar]
- Raine A,Meloy JR,Bihrle S,Stoddard J,LaCasse L,Buchsbaum MS ( 1998): Reduced prefrontal and increased subcortical brain functioning assessed using positron emission tomography in predatory and affective murderers. Behav Sci Law 16: 319–332. [DOI] [PubMed] [Google Scholar]
- Reist C,Nakamura K,Sagart E,Sokolski KN,Fujimoto KA ( 2003): Impulsive aggressive behavior: Open‐label treatment with citalopram. J Clin Psychiatry 64: 81–85. [PubMed] [Google Scholar]
- Ricci LA,Rasakham K,Grimes JM,Melloni RH ( 2006): Serotonin‐1A receptor activity and expression modulate adolescent anabolic/androgenic steroid‐induced aggression in hamsters. Pharmacol Biochem Behav 85: 1–11. [DOI] [PubMed] [Google Scholar]
- Rubinow DR,Schmidt PJ,Roca CA ( 1998): Estrogen‐serotonin interactions: Implications for affective regulation. Biol Psychiatry 44: 839–850. [DOI] [PubMed] [Google Scholar]
- Rujescu D,Giegling I,Mandelli L,Schneider B,Hartmann AM,Schnabel A,Maurer K,Mueller H Jr,Serretti A ( 2008): NOS‐I and ‐III gene variants are differentially associated with facets of suicidal behavior and aggression‐related traits. Am J Med Genet B Neuropsychiatr Genet 147: 42–48. [DOI] [PubMed] [Google Scholar]
- Sakaue M,Ago Y,Sowa C,Sakamoto Y,Nishihara B,Koyama Y,Baba A,Matsuda T ( 2002): Modulation by 5‐hT2A receptors of aggressive behavior in isolated mice. Jpn J Pharmacol 89: 89–92. [DOI] [PubMed] [Google Scholar]
- Scott PA,Chou JM,Tang H,Frazer A ( 1994): Differential induction of 5‐HT1A‐mediated responses in vivo by three chemically dissimilar 5‐HT1A agonists. J Pharmacol Exp Ther 270: 198–208. [PubMed] [Google Scholar]
- Siever LJ,Buchsbaum MS,New AS,Spiegel‐Cohen J,Wei T,Hazlett EA,Sevin E,Nunn M,Mitropoulou V ( 1999): d,l‐fenfluramine response in impulsive personality disorder assessed with [18F]fluorodeoxyglucose positron emission tomography. Neuropsychopharmacology 20: 413–423. [DOI] [PubMed] [Google Scholar]
- Simon NG,Cologer‐Clifford A,Lu SF,McKenna SE,Hu S ( 1998): Testosterone and its metabolites modulate 5HT1A and 5HT1B agonist effects on intermale aggression. Neurosci Biobehav Rev 23: 325–336. [DOI] [PubMed] [Google Scholar]
- Soloff PH,Kelly TM,Strotmeyer SJ,Malone KM,Mann JJ ( 2003): Impulsivity, gender, and response to fenfluramine challenge in borderline personality disorder. Psychiatry Res 119: 11–24. [DOI] [PubMed] [Google Scholar]
- Soma KK,Scotti M‐AL,Newman AEM,Charlier TD,Demas GE ( 2008): Novel mechanisms for neuroendocrine regulation of aggression. Front Neuroendocrinol 29: 476–489. [DOI] [PubMed] [Google Scholar]
- Spindelegger C,Mitterhauser M,Stein P,Mien L‐K,Moser U,Fink M,Wadsak W,Kletter K,Kasper S,Lanzenberger R ( 2008): Progesterone and estradiol plasma levels modulate serotonin1A binding in the human brain. Euro Neuropsychopharmacol 18( Suppl 1): 28. [Google Scholar]
- Spindelegger C,Lanzenberger R,Wadsak W,Mien LK,Stein P,Mitterhauser M,Moser U,Holik A,Pezawas L,Kletter K,Kasper S: Influence of escitalopram treatment on 5‐HT(1A) receptor binding in limbic regions in patients with anxiety disorders. Mol Psychiatry 2008. Mar 25. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Stein P,Savli M,Wadsak W,Mitterhauser M,Fink M,Spindelegger C,Mien L,Moser U,Dudczak R,Kletter K,Kasper S,Lanzenberger R. The serotonin‐1A receptor distribution in healthy men and women measured by PET and [carbonyl‐11C]WAY‐100635. Eur J Nucl Med Mol Imaging 2008. Jun 10. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N,Landeau B,Papathanassiou D,Crivello F,Etard O,Delcroix N,Mazoyer B,Joliot M ( 2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15: 273–289. [DOI] [PubMed] [Google Scholar]
- Valzelli L,Bernasconi S,Garattini S ( 1981): p‐Chlorophenylalanine‐induced muricidal aggression in male and female laboratory rats. Neuropsychobiology 7: 315–320. [DOI] [PubMed] [Google Scholar]
- Varnäs K,Halldin C,Hall HK ( 2004): Autoradiographic distribution of serotonin transporters and receptor subtypes in human brain. Hum Brain Mapp 22: 246–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen A,Verdonck L,Kaufman JM ( 1999): A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 84: 3666–3672. [DOI] [PubMed] [Google Scholar]
- Wadsak W,Mien L‐K,Ettlinger DE,Lanzenberger R,Haeusler D,Dudczak R,Kletter K,Mitterhauser M ( 2007): Simple and fully automated preparation of [carbonyl‐11C]WAY‐100635. Radiochimica acta 95: 417–422. [Google Scholar]
- White SM,Kucharik RF,Moyer JA ( 1991): Effects of serotonergic agents on isolation‐induced aggression. Pharmacol Biochem Behav 39: 729–736. [DOI] [PubMed] [Google Scholar]
- Wu Y,Carson RE ( 2002): Noise reduction in the simplified reference tissue model for neuroreceptor functional imaging. J Cereb Blood Flow Metab 22: 1440–1452. [DOI] [PubMed] [Google Scholar]
- Zhang Z,Chen K,Shih JC,Teng CT ( 2006): Estrogen‐related receptors‐stimulated monoamine oxidase B promoter activity is down‐regulated by estrogen receptors. Mol Endocrinol 20: 1547–1561. [DOI] [PubMed] [Google Scholar]
- Zou KH,Tuncali K,Silverman SG ( 2003): Correlation and simple linear regression. Radiology 227: 617–622. [DOI] [PubMed] [Google Scholar]


