Abstract
Humans extract behaviorally significant meaning from a situation by integrating meanings from multiple components of a complex daily environment. To determine the neural underpinnings of this ability, the authors performed functional magnetic resonance imaging of healthy subjects while the latter viewed naturalistic scenes of two people and an object, including a threatening situation of a person being attacked by an offender with an object. The authors used a two‐factorial design: the object was either aversive or nonaversive, and the offender's action was either directed to the person or elsewhere. This allowed the authors to examine the neural response to object aversiveness and person‐directed intention separately. A task unrelated to threat was also used to address incidental (i.e., subconscious or unintentional) detection. Assuming individual differences in incidental threat detection, the authors used a functional connectivity analysis using principal components analysis of intersubject variability. The left lateral orbitofrontal cortex and medial prefrontal cortex (MPFC) were specifically activated in response to a threatening situation. The threat‐related component of intersubject variability was extracted from these data and showed a significant correlation with personality scores. There was also a correlation between threat‐related intersubject variability and activation for object aversiveness in the left temporal pole and lateral orbitofrontal cortex; person‐directed intention in the left superior frontal gyrus; threatening situations in the left MPFC; and independently for both factors in the right MPFC. Results demonstrate independent processing of object aversiveness and person‐directed intention in the left temporal‐orbitofrontal and superior frontal networks, respectively, and their integration into situational meaning in the MPFC. Hum Brain Mapp, 2009. © 2008 Wiley‐Liss, Inc.
Keywords: perception, social perception, anxiety, magnetic resonance imaging, echo‐planar imaging, multivariate analysis, principal component analysis, prefrontal cortex, temporal lobe, personality
INTRODUCTION
Humans almost effortlessly extract significant situational meaning (e.g., threat, reward) from a complex daily environment. Such behavioral meaning is usually conveyed not directly by a single component, but by an association of meanings of many components of the environment [De Graef et al., 1990; Friedman, 1979]. For example, to accurately interpret an attack on a person by an offender with a knife as a threatening situation requires the integration of the aversiveness of the knife and the offender's intention to hurt the victim. This same integrative mechanism allows a person to perceive a situation as not threatening when the same “offender” hands some other object to the person or appropriately uses the same knife to cut food. Thus, the meaning of each environmental component is likely processed via a distinct mechanism, and the meanings of multiple components may then be integrated into situational meaning via another distinct mechanism.
Despite the critical importance of this sophisticated ability to instantly extract situational meaning from a complex environment, its neural underpinning has yet to be determined. Although some previous functional imaging studies presented subjects with a complex environment conveying situational meanings, top–down intentional, rather than incidental, detection processes were examined by instructing subjects to pay attention to the situational meaning [Iacoboni et al., 2005; Han et al., 2008]. Other studies addressed the detection process of integrated meaning; however, meaning was extracted by integrating one's own behavior and situation, rather than the components of the environment [Berthoz et al., 2006; King et al., 2006].
To determine the neural underpinnings of the ability to incidentally extract situational meaning from a complex environment, we performed functional magnetic resonance imaging (fMRI) of healthy subjects as they viewed naturalistic scenes of a situation involving two people and an object. We presented four variations of the scenario, in which the object was either aversive or nonaversive and one person directed the motion of the object towards the other person or elsewhere, in a two‐factor design, which allowed us to assess object aversiveness and person‐directed intention separately as main effects and situational threat as the interaction (Fig. 1a–d). Importantly, the study was designed so that the detection of the threatening situation would be incidental, rather than intentional; encounter with the threatening situation would be unexpected by the subjects; and the cognitive task would be unrelated to the threatening situation per se. We chose this task because it was expected to be more or less significant to any subject, and the meaning would be extracted by integrating meanings from distinct environmental components (i.e., object aversiveness and person‐directed intention).
Figure 1.
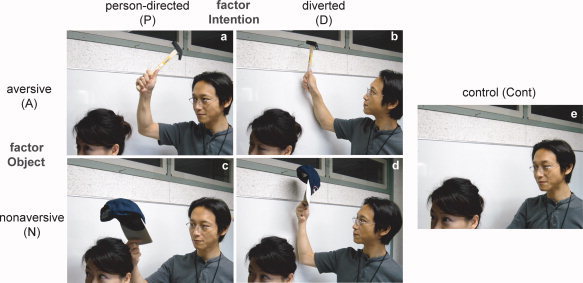
Examples of stimuli. A stimulus set comprised five visually similar pictures of a daily situation involving two actors. One actor had an object/tool in his/her hand in four pictures for the conditions of interest: (a) aversive person‐directed (AP), (b) aversive diverted (AD), (c) nonaversive person‐directed (NP), and (d) nonaversive diverted (ND). The four pictures conformed to a two‐factor design composed of the factors object (aversive, A, or nonaversive, N) and intention (person‐directed, P, or diverted from the person, D). In the control condition ((e) Cont), neither actor had an object/tool, which served as a distracter for the judgment task and a baseline condition for the analysis.
This study design lends itself to intersubject variability in the degree of neural response because the degree of recruitment of threat‐processing mechanisms likely varies greatly during incidental, rather than intentional, detection. Threat‐related brain activity is correlated with affect‐related personality traits [Nitschke et al., 2006; Simmons et al., 2006; Simpson et al., 2001] and risk‐taking tendencies [Eshel et al., 2007]. Individual variability decreases the statistical sensitivity in conventional intersubject fMRI analysis, which regards individual differences as variance [Holmes and Friston, 1998; Wei et al., 2004]. Therefore, we used a complementary approach using intersubject variability as a signal, rather than noise (see Sugiura et al. [2007]). By using principal components analysis (PCA) to find patterns of intersubject variability in regions of interest (ROIs) and to identify a network of cortical regions that systematically show the pattern, we can identify regions of large‐scale variability and extract the rest of the network by examining covariation in activation across remote regions.
This approach also allowed us to associate intersubject variability in neural response with personality traits. We examined the correlation between the threat‐related component of response variability and the scores of the Temperament and Character Inventory (TCI), which includes four temperament dimensions (novelty seeking, harm avoidance, reward dependence, and persistence) and three character dimensions (self‐directedness, cooperativeness, and self‐transcendence; [Cloninger et al., 1993]). We predicted that the degree of threat‐related response would be positively correlated with the harm avoidance (HA) score because it measures the tendency for behavioral inhibition in a threatening situation.
MATERIALS AND METHODS
Subjects
The experimental protocol was approved by the ethics committee of Tohoku University, Sendai, Japan. Written informed consent was obtained from each subject. Forty‐one healthy, right‐handed volunteers (36 males, 5 females; aged 18–24 years) participated. All subjects had normal vision and none had a history of neurological or psychiatric illness. Handedness was evaluated using the Edinburgh Handedness Inventory [Oldfield, 1971]. Exclusion because of psychiatric illness, including drug and alcohol abuse/dependence, was based on an interview with each subject. Each subject completed the Japanese version of the TCI [Kijima et al., 1996].
Since two subjects made excessive head motions (>3 mm), one subject suspected that the purpose of the experiment might be related to threat perception (therefore, encountering the threatening situations was not incidental for this subject), and the task performance of six subjects was dubious (<80% correct responses or mention of drowsiness at the post‐fMRI interview); data from 32 subjects (27 males, 5 females) were analyzed.
Stimuli and Tasks
The visual stimuli consisted of 25 sets of pictures, each depicting a situation involving two actors. Each set was composed of four situational pictures for the conditions of interest (Fig. 1a–d), and one for a control (Cont; Fig. 1e). The four situational pictures composed a two‐factor design as follows: object (aversive, A, or nonaversive, N) and intention (person‐directed, P, or diverted, D). Aversive objects included a knife, hammer, gun, large stone, or chemicals, whereas nonaversive objects included a brush, cap, stopwatch, football, or eyedrop. Under condition P, the object was used to have a direct effect on the other actor, whereas under condition D, the object was used for a normal, goal‐directed action (e.g., using a knife to cut bread). No object was shown in either actor's hands under condition Cont. Five situations using each set of stimuli were acted out by the same pair of actors, arbitrarily selected from two male and three female actors. Each picture was photographed using a digital camera, carefully controlling visual features, except for the experimentally manipulated factors. A mosaic picture generated from each control picture was also included for baseline; however, the estimated activation for this condition was not used in the analysis because some subjects reported that they imagined the situation or remembered the preceding pictures from the color or large‐scale construction of the mosaic picture. The 25 sets of stimuli were selected from 35 candidates by a screening test with 10 subjects. For the 25 selected sets, each AP picture was judged to be threatening by >80% of the subjects, and the other pictures (i.e., AD, NP, AD, and Cont) were judged to be safe by >60% of the subjects. In total, 150 pictures (including mosaic pictures) were presented in random order. Each picture was presented for 2 s, and the interstimulus interval varied from 6.5 to 9.5 s. Each picture was projected onto a semilucent screen attached to the head coil of the MRI scanner, which was viewed via a mirror.
Before the experiment, each subject was informed that he/she would be presented with pictures describing a daily situation with two actors and was instructed to judge whether either actor had an object in his/her hand (or no evidence of having an object when the hands were not visible). When either actor had an object, the subject pressed a button with the right index finger as quickly as possible; otherwise, the subject used the right middle finger.
fMRI Measurements
Thirty‐four transaxial‐gradient echo images (echo time = 50 ms, flip angle = 90°, slice thickness = 3 mm, slice gap = 0.99 mm, field of view = 192 mm, matrix = 64 × 64) covering the whole cerebrum were acquired at a repetition time of 3 s using an echoplanar image (EPI) sequence and a Siemens Symphony (1.5 T; Siemens, Erlangen, Germany) MR scanner. In the case of a few relatively large brains, the lower part of the cerebellum was not scanned because we did not expect threat‐related activation in that area. After 12 dummy scans for stabilization of the T1‐saturation effect and to familiarize the subjects with the MRI environment, 400 volumes were acquired. A T1‐weighted anatomical image for spatial normalization was acquired using SP‐RAGE on a separate occasion for each subject.
Post‐MRI Debriefing and Self‐Evaluation of Threat
Following a conventional debriefing about the general impressions of the task outside of the MRI scanner, each subject was encouraged to guess the purpose of the task to explore the possibility that the subject may have predicted the intent of the experiment, thereby making detection intentional, rather than incidental. Each subject was then asked if he/she found some of the presented situations dangerous to ensure that the subject perceived some of the situations as threatening. After the interview, each subject was presented with the situational pictures for the conditions of interest used during the task and was asked to evaluate the degree of threat for each picture using a 9‐point scale (0 to 8). Because we intended to use this evaluation as a measure of average threat for each condition, rather than that of each subject's sensitivity to threat, each subject was instructed to give the most threatening situation a score of 8 points.
Image Preprocessing
The following preprocessing procedures were performed using the Statistical Parametric Mapping (SPM2) software (Wellcome Department of Imaging Neuroscience, London, UK) and MATLAB (MathWorks, Natick, MA, USA): the adjustment of acquisition timing across slices, correction for head motion, coregistration for an anatomical image, spatial normalization using the anatomical image and the MNI template, and smoothing using a Gaussian kernel with a full‐width at half‐maximum of 10 mm.
We used SPM2, rather than SPM5, because the former was the latest version when we performed the experiment. Since we identified no component of the update from SPM2 to SPM5 that significantly affected the validity of the preprocessing or statistics procedures in our approach, we found no scientific reason to reanalyze the data.
Conventional Subtraction Analysis
A conventional two‐level approach for event‐related fMRI data was used in SPM2. A voxel‐by‐voxel multiple regression analysis of expected signal changes for the six conditions (AP, AD, NP, ND, Cont, and mosaic picture), which were constructed using the hemodynamic response function provided by SPM2, was applied to the preprocessed images of each subject [Friston et al., 1995]. Statistical inference on contrasts of parameter estimates was then performed at the second‐level between‐subject (random effects) model using one‐sample t‐tests. Although our design conforms to a two‐factorial design, we did not use a conventional ANOVA approach with F‐tests because the purpose of the analysis was to test a priori hypotheses of signed differential activation.
First, activation under each of the four conditions of interest relative to the Cont was tested. Neural responses to object aversiveness (main effect of object) and person‐directed intention (main effect of intention) were then tested using contrast A–N (i.e., [AP + AD]–[NP + ND]) and contrast P–D (i.e., [AP + NP]–[AD + ND]), respectively. Cortical activation specifically related to situational threat (AP) was tested using the contrast (AP–AD)–(NP–ND) and masked by the contrast AP–AD and by AP–NP to confirm that activation was specific to AP. For these contrasts, the statistical threshold was set at P < 0.001 for height and corrected to P < 0.05 for multiple comparisons using the cluster size [Friston et al., 1994]. The masks were set at a threshold of P < 0.05 for height. Finally, the activation profile at each activation peak was investigated to test four simple effects (i.e., AP–NP, AD–ND, AP–AD, and NP–ND) and their interaction ([AP–AD]–[NP–ND]) using the threshold of P < 0.05 without correction for multiple comparisons.
Network Identification Using Intersubject Variation in Activation
To overcome intersubject variability as described in preceding text, PCA was used with intersubject variation of the activated areas in the conventional subtraction analysis (i.e., regions of interest, ROIs) under each of the four conditions of interest (see Sugiura et al. [2007]) for details of this approach and Sugiura et al. [2006] for another example). That is, activation under the four conditions of interest relative to Cont in the peak voxels of the two ROIs (see Results) constituted the multivariate factor (four conditions × two ROIs = eight variables), and the 32 subjects constituted the observations, resulting in a matrix of 32 × 8. To perform PCA, this data matrix was normalized to the mean of each variable over the subjects of zero, and singular value decomposition was applied [Friston and Büchel, 2004] in MATLAB. The eigenvariate, eigenvector, and eigenscore corresponded to the principal component, loading, and principal component score, respectively. The number of retained eigenvariates was determined using the scree test [Cattell, 1966]. The scree test, rather than the Kaiser criterion, which was used in the original proposal of this approach [Sugiura et al., 2007], was used because the former is reasonably more conservative than the latter when there are many variates (c.f., [Sugiura et al., 2006]).
Here, each observed eigenvariate represents a pattern of intersubject variation in the neural response in the two ROIs in the four conditions (i.e., eight variables); these were substantiated by the eigenvector and eigenscore. The eigenvector is a value given to each ROI in each condition that indicates the degree to which the pattern of intersubject variation was expressed in the ROI under the condition; the sign of the value has no particular meaning, but opposite signs mean inverse intersubject patterns. The eigenscore represents the degree of each subject's neural response relative to the average for all subjects; a large eigenscore denotes a large neural response when a corresponding element of the eigenvector is positive. We can infer the meaning of each eigenvariate from the eigenvector by knowing the contribution of each ROI under each condition and can get a sense of the pattern of intersubject variation by visualizing the eigenscores.
We associated the psychological (or physiological) measures of the subjects with each eigenvariate by examining their correlation with the eigenscores. Such an association, indicated by a significant correlation, facilitates the interpretation of the eigenvariates. It also ensures psychological (or physiological) significance for the eigenvariates, given that it entails the rejection of the null hypothesis that the intersubject variability represented by the eigenvariate is noise. We tested the correlation between eigenscores and TCI scores.
Importantly, we also associated each eigenvariate with the activation of cortical regions other than the ROIs using the same fMRI data set. This procedure conforms to the analysis of functional connectivity [Friston and Büchel, 2004] and allows us to identify a large‐scale cortical network, represented by the eigenvariate, by using the voxel‐by‐voxel approach [Sugiura et al., 2007]. When the eigenvariate represented individual differences in threat‐related responses, a correlation between the threat‐related neural response and the eigenscore would be expected in the cortical network relevant to threat processing; in fact, the identification of such cortical networks was the primary reason that we used this approach. We entered the eigenscores into a voxel‐by‐voxel multiple regression model of the relevant contrast images using SPM2 (i.e., a further physiologically informed second‐level analysis); by which we can extract cortical areas where the degree of differential activation (i.e., contrast image) has significant correlation with the entered eigenscores. The cortical networks that were associated with the processing of object aversiveness and person‐directed intention were identified using the images from contrast A–N and contrast P–D, respectively. Cortical networks that were positively and negatively correlated with the eigenscores were extracted by testing for significant regressions. The statistical threshold was the same as that used for the conventional subtraction analysis.
To investigate ad hoc an activation profile of each voxel showing the peak statistical value (comparable to the activation peak), differences in the degree of correlation were tested between conditions by examining the correlation between eigenscores and differential activation between conditions. For example, greater positive correlations under the AP condition than the AD condition were assessed as positive correlations between eigenscores and the contrast AP–AD. In this way, contrasts for the four simple effects (i.e., AP–NP, AD–ND, AP–AD, and NP–ND) and their interaction (i.e., [AP–AD]–[NP–ND]) were tested (thresholds at P < 0.05, without correction for multiple comparisons).
RESULTS
Behavioral Data
The percentages of correct responses and mean reaction times of judgments during the fMRI measurements were analyzed using two‐way ANOVA with the effects of condition (5 stimulus types) and subject (32 subjects). Only the effect of condition was tested. The main effect of condition was significant in both the percentage of correct responses and mean reaction time (Fig. 2a,b; F[4,124] = 14.88 and F[4,124] = 81.67, respectively; P < 0.05). The follow‐up pair‐wise comparisons indicated that the percentage of correct responses was higher and the mean reaction time was shorter under the AP, AD, and NP conditions than under the ND and Cont conditions, and the mean reaction time was significantly shorter under the ND condition than under the Cont condition (P < 0.05, Tukey test).
Figure 2.
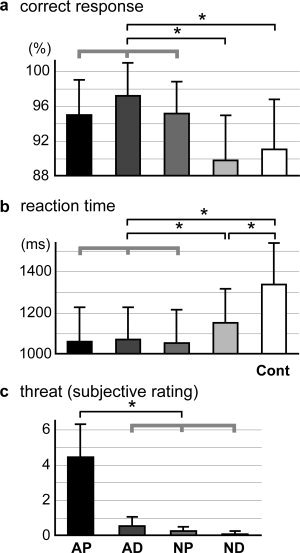
Behavioral data. The mean percent correct responses (a) and mean reaction times (b) for the judgment as to whether either actor had an object in his/her hand during the fMRI experiment are shown for the five picture conditions. (c) The mean scores for the degree of threat of the situations self‐evaluated after the fMRI experiments (9‐point scale) are given for the four conditions of interest. Asterisks on the lines at the top of the graph indicate significant differences between conditions (P < 0.05, Tukey test after ANOVA). Thick gray lines indicate that the behavioral data in the three conditions were comparable (no significant difference) and significantly different from the other condition(s). Error bars indicate standard deviations.
During the debriefing after fMRI, all of the subjects except one reported that although they found some situations threatening, they did not realize the relevance of the threatening situations to the purpose of the experiment. For the self‐evaluated threat of the situational pictures, the effect of condition was significant (Fig. 2c, two‐way ANOVA; F[3,93] = 173.4; P < 0.05), and the situations under the AP condition were evaluated as significantly more threatening than those under the other conditions (P < 0.05, Tukey test).
Conventional Subtraction Analysis
Compared to the Cont condition, statistically significant activation (P < 0.001, corrected to P < 0.05 for multiple comparisons) was observed only in the AP condition in the left medial prefrontal and lateral orbitofrontal cortices (see Fig. 3 and Table I). The activation profile showed that activation of the left medial prefrontal cortex was specific to the AP condition in that the simple effects of AP–AD and AP–NP and their interaction ([AP–AD]–[NP–ND]) were significant (P < 0.05, paired t‐test). Activation of the left lateral orbitofrontal cortex exhibited the same tendency, but a statistically significant difference was observed only for AP–NP.
Figure 3.
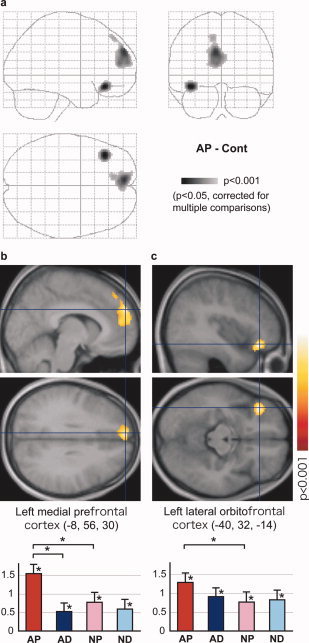
Activation in the contrast: AP–Cont. (a) Glass brain presentation of SPM2; top‐left, top‐right, and bottom‐left panels show projections from the right, back, and top, respectively. The left medial prefrontal (b) and orbitofrontal (c) activations are superimposed on sagittal (top) and transaxial (bottom) sections of the mean image for the normalized T1‐weighted anatomical images of all subjects. The graphs show activation profiles (parameter estimates relative to the Cont condition and their standard deviations at peak activation). Asterisks at the bars and the lines at the top of the graph indicate significant (P < 0.05, paired t‐test) differential activation relative to the Cont condition and between conditions of interest (comparisons for simple effects only), respectively. Error bars indicate the standard errors of the mean.
Table 1.
Activation for the contrast: AP–Cont
| Structure | Coordinate | t‐value | Cluster size (mm3) | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Medial prefrontal cortex | L | −8 | 56 | 30 | 6.11 | 6,216 |
| Lateral orbitofrontal cortex | L | −40 | 32 | −14 | 7.02 | 1,872 |
The coordinate and t‐value of peak activation and cluster size are shown for each activated area. L: left.
No significant activation was identified in the contrasts for the main effects (A–N and P–D) or those for their interaction (AP‐specific activation) at the statistical threshold for the voxel‐by‐voxel analysis (i.e., when corrected for multiple comparisons).
Network Identification Using Intersubject Variation in Activation
PCA was applied to intersubject variation in activation in the left medial prefrontal and lateral orbitofrontal cortices under each of the four conditions of interest. Of the eight eigenvariates generated, the first three were retained after the scree test (Fig. 4a). A large positive or negative element of eigenvector specifically for threat‐related conditions (i.e., AP, AD, or NP) was observed for the third eigenvariate (Fig. 4b). The eigenvector of the third eigenvariate appeared to have large values specifically for the AP condition in the left medial prefrontal cortex and to object aversiveness in the left lateral orbitofrontal cortex. The patterns of the eigenvector suggested that the third eigenvariate represented individual differences in threat‐related responses. More specifically, the left medial prefrontal cortex responded to threatening situations and the left lateral orbitofrontal cortex responded to object aversiveness. Accordingly, we focused on the third eigenvariate for subsequent analyses.
Figure 4.
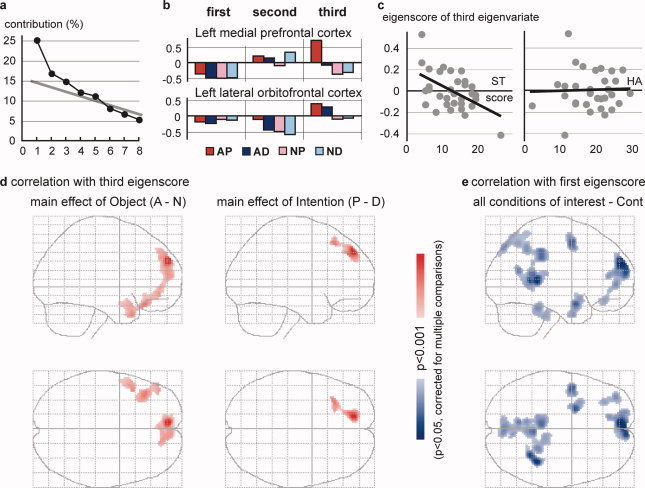
Results of PCA for intersubject variability of activation (relative to Cont) for the four conditions of interest in the two regions of interest (ROIs; the left medial prefrontal [−8, 56, 30] and lateral orbitofrontal [−40, 32, −14] cortices). (a) Results of the scree test. Three eigenvariates (principal components) were retained. (b) Eigenvector (loadings) of the four conditions for the two ROIs. (c) Plots of the third eigenscores against the ST and HA scores of the TCI for all subjects. The regression lines are shown. (d) Significant correlations (slope of the regression line) between the third eigenscores and the differential activations of the contrasts A–N (left panel) and P–D (right panel). (e) Significant correlations between the first eigenscores and the mean activation of all conditions of interest (relative to Cont). Cortical areas showing significant correlations are shown in a glass brain presentation (see legend for Fig. 3a).
The eigenscore of the third eigenvariate was significantly correlated with the self‐transcendence (ST) score of the TCI (r = −0.51, P = 0.0026; P < 0.05, by Bonferroni correction for multiple comparisons; Fig. 4c). Although the data for two extreme subjects appeared to affect the results, the correlation was still significant after removing the data for either subject (r = −0.49, P = 0.0099 and r = −0.40, P = 0.025). No significant correlation was observed for the scores of other dimensions in the TCI, including the HA (Fig. 4c).
A significant positive correlation (slope of the regression line) between the eigenscores of the third eigenvariate and differential activation was observed in distinct cortical networks for the contrasts A–N and P–D (Fig. 4d). For the A–N contrast, significant correlations were observed in the bilateral medial prefrontal cortex, left lateral orbitofrontal cortex, and temporal pole (Table II, Fig. 5a). In these regions, except for the left medial prefrontal cortex, the correlation was significant for the simple contrasts AP–NP and AD–ND (i.e., regardless of person‐directed intention). In the right medial prefrontal cortex, the correlation was also significant for the simple contrasts AP–AD and NP–ND (i.e., effect of person‐directed intention). In the left medial prefrontal cortex, in which a significant cortical response was identified specifically for threatening situations (AP) in conventional subtraction, a significant correlation was observed for the simple contrasts AP–NP and AP–AD and the contrast for their interaction ([AP–AD]–[NP–ND]; i.e., specifically for the AP condition).
Table II.
Correlation between the third eigenscore and activation related to object aversiveness (contrast: A–N)
| Structure | Coordinate | t‐value | Cluster size | Region of interest analysis (t‐value) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | (mm3) | AP–NP | AD–ND | AP–AD | NP–ND | Interaction | |||
| Medial prefrontal cortex | L | −8 | 56 | 30 | 8.19 | 7480* | 7.63 | 4.24 | 3.89 | ||
| R | 4 | 58 | 12 | 4.98 | * | 4.06 | 3.18 | 2.15 | 1.74 | ||
| Lateral orbitofrontal cortex | L | −42 | 30 | −20 | 5.79 | 2208 | 4.04 | 3.06 | |||
| Temporal pole | L | −44 | 14 | −32 | 5.40 | 1320 | 2.96 | 4.56 | |||
The coordinates and t‐value of peak voxel and cluster size are shown for each area where estimated activation significantly correlated with the third eigenscore; here, “correlation” denotes the slope of the regression line. The results of the region of interest analyses, that is, t‐values for significant (P < 0.05) correlations between the third eigenscore and contrasts for four simple effects (AP–NP, AD–ND, AP–AD, and NP–ND) and their interaction [(AP–AD)–(NP–ND)] are also shown for each peak. L: left, R: right.
: The peaks are in the same cluster.
Figure 5.
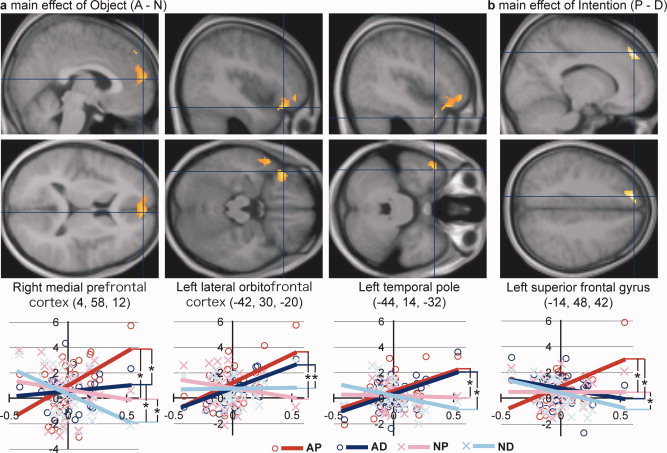
Detailed presentation of the significant correlations between the third eigenscores and the differential activations of contrasts A–N (a) and P–D (b) (as presented in Fig. 4d). Activation (significant correlation) was superimposed on sagittal and transaxial sections (top and middle panels) of the mean image on the normalized T1‐weighted anatomical images for all subjects. Activation (parameter estimate) relative to the Cont condition is plotted against the third eigenscore for each subject for each of the four conditions of interest, and the regression line is shown in different color for each condition (bottom panel). Asterisks between the regression lines indicate significant differences in the slopes of the regression lines, as listed in Tables II and III.
In the analysis of the P–D contrast, a significant positive correlation was observed in the left superior frontal gyrus and sulcus (Table III, Fig. 5b). In the left superior frontal gyrus, the correlation was significant for the simple contrasts AP–AD and NP–ND (i.e., regardless of object aversiveness).
Table III.
Correlation between the third eigenscore and activation related to person‐directed intention (contrast: P–D)
| Structure | Coordinate | t‐value | Cluster size | Region of interest analysis (t‐value) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | (mm3) | AP–NP | AD–ND | AP–AD | NP–ND | Interaction | |||
| Superior frontal gyrus | L | −14 | 48 | 42 | 5.49 | 1,800* | 2.94 | 4.23 | 1.98 | ||
| Superior frontal sulcus | L | −32 | 26 | 50 | 4.87 | * | 2.79 | 3.88 | |||
Notes for the table are the same as those for Table II.
Although the first and second eigenvariates were not likely relevant to individual threat‐related sensitivity (Fig. 4b), we performed ad hoc analyses of these eigenvariates to investigate their possible meaning. No significant correlation with the TCI score was observed for the eigenscores of either eigenvariate. Voxel‐by‐voxel multiple regression analyses using the eigenscores were performed for the contrast of the mean over the four conditions of interest (All) against the Cont because the first eigenvariate had negative eigenscores under all four conditions of interest in the left medial prefrontal cortex and the second eigenvariate exhibited a similar pattern in the orbitofrontal cortex. For the first eigenvariate, a significant negative correlation was observed in several cortical areas, including the medial prefrontal and posterior cortices, the left premotor area, the orbitoinsular junction, and the frontal pole (Fig. 4e). Although several cortical regions exhibited significant correlations for the second eigenvariate at a voxel‐level threshold of P < 0.001, including positive and negative correlations in the left medial prefrontal and orbitofrontal cortices, respectively, no area survived the correction for multiple comparisons by cluster size.
DISCUSSION
Participants considered the AP condition significantly more threatening than the other conditions, as expected, and activation specific to this condition occurred in the left medial prefrontal and lateral orbitofrontal cortices. This suggests the involvement of these regions in detecting threatening situations. However, as suspected, no statistically significant activation was observed in most of the contrasts in the voxel‐by‐voxel analysis, probably because of intersubject variability.
Therefore, we applied PCA to this intersubject variability and extracted three eigenvariates, of which the third was regarded as threat‐related variability because it was expressed only in the threat‐related conditions (i.e., AP and AD). In the left lateral orbitofrontal cortex and temporal pole, the score of the third eigenvariate was positively correlated with the cortical response to object aversiveness (i.e., contrast A–N), indicating a link between individual sensitivity to threat and neural responses to object aversiveness in these regions. A positive correlation between the third eigenscore and the cortical response to person‐directed intention (i.e., contrast P–D) was observed in the left superior frontal gyrus. These results suggest that object aversiveness and person‐directed intention are extracted independently by the left temporal‐orbitofrontal network and the left superior frontal sulcus, respectively.
There was a positive correlation between the third eigenscore and cortical response in the left medial prefrontal cortex specifically for the AP condition. Consistent with the AP‐specific activation observed for this region in the conventional subtraction analysis, this result suggests the role of this region in detecting situational threat perceived by integrating object aversiveness and person‐directed intention. In the right medial prefrontal cortex, the positive correlation with the third eigenscore was significant for responses to object aversiveness and for response to person‐directed intention independently. These results indicate that the medial prefrontal cortex is involved in the extraction of situational threat by integrating object aversiveness and person‐directed intention, whereas the left and right activation foci have different functional characteristics. Hereafter, we discuss the behavioral and fMRI data in detail, together with previous findings.
Task Performance and Threat
Behavioral performance was significantly better in terms of accuracy and speed under conditions that contained a threat‐related factor (i.e., AP, AD, and NP) than under the ND or Cont conditions. This suggests that either object aversiveness or person‐directed intention facilitated task execution and provides behavioral evidence that both of the threat‐related signals were processed, although the threat was incidental and the task was irrelevant to the threat. Importantly, the observed across‐condition patterns of behavioral performance show that any differential effects reflected in behavioral performance, such as task difficulty, cannot explain the neural responses to object aversiveness or person‐directed intention, which were detected as main effects, or as a specific response to AP.
Individual Differences in Threat Perception
The eigenscore of the third eigenvariate was negatively correlated with the ST score of the TCI. Although ST was originally assumed to be a positive personality trait such as self‐forgetfulness and spirituality [Cloninger et al., 1993], high scores in this personality trait have been implicated in some pathological states, arguably attributable to the consequences of low sensitivity to significant situational meaning, such as non‐drug‐dependent HIV‐positivity [Fassino et al., 2004] and pathological gambling [Martinotti et al., 2006]. The negative correlation with ST score may thus support the relationship between the high third eigenscore and high sensitivity to threat.
Interestingly, no significant correlation was found with the HA score. This may be because our third eigenscore represented intersubject variability in the detection process of threat given in a third‐person perspective, rather than that in the behavioral response (inhibition) to the threatening situation given in a first‐person perspective, which the HA dimension mainly addresses [Cloninger, 1993].
Processing of Object Meaning
The roles of the left orbitofrontal cortex and temporal pole are not likely limited to the processing of object aversiveness, but might be generalized to the extraction of significant meaning from the environment. These regions are also conjointly sensitive to stimuli conveying positive affective values [Azim et al., 2005; Hennenlotter et al., 2005].
The left temporal pole has been implicated in the acquisition of the meaning of a stimulus depending on associative or contextual information [Ganis and Kutas, 2003; Humphries et al., 2005]. The identification of a specific individual, which entails the retrieval of associated biographical information, also involves the temporal pole [Nakamura et al., 2001; Sugiura et al., 2001]. However, these previous studies addressed the role of these regions in the extraction of meaning conveyed by a specific component, rather than a situation.
The left lateral orbitofrontal cortex seems to respond to the behavioral significance of the meaning. This region responds to the anticipation of aversive events [Breiter et al., 2001; Chua et al., 1999] as well as many types of induced emotion (for review, see Steele and Lawrie, 2004]. The orbitofrontal cortex is generally thought to integrate the affective value of the stimulus and the behavioral response [Kringelbach, 2005]. Furthermore, given the proposed role of this region in the inhibition of inappropriate responses [Elliot and Deakin, 2005], activation of a region may reflect suppression of behavioral responses inherent to object aversiveness.
Processing of Person‐Directed Intention
The involvement of the left superior frontal gyrus in the processing of person‐directed intention is supported by previous findings. Although this region may be a part of the medial prefrontal cortex, which encompasses a large complex of functional modules for social cognition [Amodio and Frith, 2006], previous functional imaging studies that have reported activation close to this specific region typically used tasks that required the processing of person‐directed intention or emotion [Berthoz et al., 2006; Mitchell et al., 2005; Walter et al., 2004; Wicker et al., 2003].
Integrating the Meaning of Environmental Components Into Situational Meaning
The importance of the medial prefrontal cortex in threat perception is supported by the fact that the activation of this region reflects the awareness of and behavioral response to threat. Top–down modulation of anxiety is exhibited in this region [Erk et al., 2006; Kalisch et al., 2005]. Alexthymic individuals, who has impaired emotional self‐awareness, had less activation in this region [Moriguchi et al., 2006]. Beneficial decision making in the Iowa Gambling Test were related to greater activation of this region [Northoff et al., 2006].
The observed distinct functional characteristics of the left and right medial prefrontal activation peaks, which occupied slightly dorsal and ventral locations, respectively, appear to be consistent with the proposed functional inhomogeneity of the medial prefrontal cortex [Amodio and Frith, 2006]. We reviewed previous imaging studies that reported activation peaks 10 mm or less distant in MNI coordinates from one of our two activation peaks. Those near our right ventral peak (4, 58, 12) were typically reported in studies that addressed a relatively simple affect‐stimulus relationship [Kampe et al., 2003; Kensinger and Schacter, 2005; Spiers and Maguire, 2006; Völlm et al., 2006], suggesting that this region responds to any type of behaviorally significant meaning. In contrast, activation peaks close to our left dorsal peak (−8, 56, 30) were usually reported in studies that addressed behavioral significance in social [Mitchell et al., 2005; Sassa et al., 2007; Wakusawa et al., 2007] or temporal [Akitsuki et al., 2003] contexts, suggesting that this region plays an integrative role in a strict sense that includes the construction of situational meaning by integrating the meanings of multiple environmental components.
Independent and Integrative Networks for the Extraction of Situational Meaning
The networks involved in the independent processing of the two threat‐related signals and their integration, which we functionally identified, appear to have an anatomical basis, at least in monkeys. The temporal pole is reciprocally connected to higher visual object‐processing areas and the lateral orbitofrontal areas [Kondo et al., 2003; Nakamura and Kubota, 1996] and thus likely support the object‐aversiveness network. The superior frontal cortex receives input from the superior temporal sulcus and premotor cortices [Barbas et al., 1999], which play roles in the visual processing of body action and action intention [Perrett et al., 1989; Umilta et al., 2001], thus reasonably accommodating the person‐directed intention network. Both the orbitofrontal and superior frontal cortices are connected to the polar part of the medial prefrontal cortex [Barbas et al., 1999], which may provide an anatomical basis for integrating threat‐related signals from the two distinct networks.
The threat‐related brain networks that we identified were reasonably different than those identified in previous studies of the anticipation of aversive events. The involvement of the lateral orbitofrontal and medial prefrontal cortices in processing object aversiveness reported herein is consistent with previous studies [Breiter et al., 2001; Chua et al., 1999; Nitschke et al., 2006; Ploghaus et al., 1999; Simpson et al., 2001]. The involvement of the temporal pole and the superior frontal gyrus has not been reported previously, probably because ours was the first study to use the incidental threat‐perception model in an experimental setting and to analyze the factors of person‐directed intention. In contrast, the activation of the amygdala, insula, and anterior cingulate cortex [Breiter et al., 2001; Büchel et al. 1998; Chua et al., 1999; LaBar et al., 1998; Ploghaus et al., 1999; Simmons et al., 2006] was not observed. The lack of activation in these limbic or paralimbic structures may be because the threatening situation was virtual, given in a third‐person perspective, or incidental to the task, thus providing minimal affective significance. We speculate that these structures may respond to the affective aspect of threat or behaviorally significant meaning, whereas the lateral orbitofrontal and medial prefrontal cortices may process their cognitive aspect.
First and Second Eigenvariates
The mean activation of the four conditions of interest was negatively correlated with the eigenscore of the first eigenvariate in the medial prefrontal, medial posterior, and left premotor cortices (Fig. 4e). Given that the medial prefrontal and posterior cortices are deactivated by goal‐directed tasks in proportion to attentional load [McKiernan et al., 2003], this eigenvariate may be related to the differential attentional demand between the conditions of interest and the control. The involvement of the left premotor cortex may be related to the recruitment of action knowledge for tool or action observation [Chao and Martin, 2000; Johnson‐Frey et al., 2003] during the conditions of interest, but not during the control; the degree of recruitment may also be modulated by the attentional load.
The correlation of the second eigenvariate with the eigenscore in several cortical regions, including the two ROIs, did not survive the correction for multiple comparisons. Together with the inconsistent across‐condition patterns of the loadings between the two ROIs (Fig. 4b), it is reasonable to assume that the second eigenvariate represents intersubject variability in regional cortical activation limited to the two ROIs, rather than variability in physiologically meaningful large‐scale cortical networks.
CONCLUSIONS
We demonstrated the neural mechanisms for the incidental extraction and integration of meaning from distinct environmental components to detect situational meaning. Using PCA to address intersubject variability in activation was important to overcome individual differences in response. The left medial prefrontal and lateral orbitofrontal cortices were identified as specifically responsive to threatening situations (AP condition) in the conventional subtraction analysis. Intersubject variability was extracted using PCA, and its relevance to individual sensitivity to situational meaning was suggested by the observed correlation with the ST scores of the TCI. The functional connectivity analysis using this variability indicated that object aversiveness and person‐directed intention are processed separately in the left temporal pole and lateral orbitofrontal cortex and in the network including the left superior frontal sulcus, respectively. While both threat‐related signals from these networks join in the polar part of the medial prefrontal cortex, the right and left regions seem to play different roles: the right region responds to any type of behavioral significance, whereas the left region integrates the signals to construct situational meaning.
Acknowledgements
We thank Ai Fukushima and Wataru Suzuki for helpful suggestions on the manuscript.
REFERENCES
- Akitsuki Y,Sugiura M,Watanabe J,Yamashita K,Sassa Y,Awata S,Matsuoka H,Maeda Y,Matsue Y,Fukuda H, Kawashima, R ( 2003): Context‐dependent cortical activation in response to financial reward and penalty: An event‐related fMRI study. Neuroimage 19: 1674–1685. [DOI] [PubMed] [Google Scholar]
- Amodio DM,Frith CD ( 2006): Meeting of minds: The medial frontal cortex and social cognition. Nat Rev Neurosci 7: 268–277. [DOI] [PubMed] [Google Scholar]
- Azim E,Mobbs D,Jo B,Menon V,Reiss AL ( 2005): Sex differences in brain activation elicited by humor. Proc Natl Acad Sci USA 102: 16496–16501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H,Ghashghaei H,Dombrowski SM,Rempel‐Clower NL ( 1999): Medial prefrontal cortices are unified by common connections with superior temporal cortices and distinguished by input from memory‐related areas in the rhesus monkey. J Comp Neurol 410: 343–367. [DOI] [PubMed] [Google Scholar]
- Berthoz S,Grezes J,Armony JL,Passingham RE,Dolan RJ ( 2006): Affective response to one's own moral violations. Neuroimage 31: 945–950. [DOI] [PubMed] [Google Scholar]
- Breiter HC,Aharon I,Kahneman D,Dale A,Shizgal P ( 2001): Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron 30: 619–639. [DOI] [PubMed] [Google Scholar]
- Büchel C,Morris J,Dolan RJ,Friston KJ ( 1998): Brain systems mediating aversive conditioning: An event‐related fMRI study. Neuron 20: 947–957. [DOI] [PubMed] [Google Scholar]
- Cattell RB ( 1966): The scree test for the number of factors. Multivariate Behav Res 1: 140–161. [DOI] [PubMed] [Google Scholar]
- Chao LL,Martin A ( 2000): Representation of manipulable man‐made objects in the dorsal stream. Neuroimage 12: 478–484. [DOI] [PubMed] [Google Scholar]
- Chua P,Krams M,Toni I,Passingham R,Dolan R ( 1999): A functional anatomy of anticipatory anxiety. Neuroimage 9: 563–571. [DOI] [PubMed] [Google Scholar]
- Cloninger CR,Svrakic DM,Przybeck TR ( 1993): A psychobiological model of temperament and character. Arch Gen Psychiatry 50: 975–990. [DOI] [PubMed] [Google Scholar]
- De Graef P,Christiaens D,d'Ydewalle G ( 1990): Perceptual effects of scene context on object identification. Psychol Res 52: 317–329. [DOI] [PubMed] [Google Scholar]
- Elliott R,Deakin B ( 2005): Role of the orbitofrontal cortex in reinforcement processing and inhibitory control: Evidence from functional magnetic resonance imaging studies in healthy human subjects. Int Rev Neurobiol 65: 89–116. [DOI] [PubMed] [Google Scholar]
- Erk S,Abler B,Walter H ( 2006): Cognitive modulation of emotion anticipation. Eur J Neurosci 24: 1227–1236. [DOI] [PubMed] [Google Scholar]
- Eshel N,Nelson EE,Blair RJ,Pine DS,Ernst M ( 2007): Neural substrates of choice selection in adults and adolescents: Development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychologia 45: 1270–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassino S,Leombruni P,Amianto F,Abbate‐Daga G ( 2004): Personality profile of HIV outpatients: Preliminary results and remarks on clinical management. Psychother Psychosom 73: 361–365. [DOI] [PubMed] [Google Scholar]
- Friedman A ( 1979): Framing pictures: The role of knowledge in automatized encoding and memory for gist. J Exp Psychol Gen 108: 316–355. [DOI] [PubMed] [Google Scholar]
- Friston KJ,Büchel C ( 2004): Functional connectivity: Eigenimages and multivariate analysis In: Frackowiak RSJ,Friston KJ,Frith CD, Dolan RJ, Price CJ, Zeki S, Ashburner J, Penny W, editors. Human Brain Function. San Diego: Academic Press; pp 999–1018. [Google Scholar]
- Friston KJ,Worsley KJ,Frackowiak RSJ,Mazziotta JC,Evans AC ( 1994): Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp 1: 214–220. [DOI] [PubMed] [Google Scholar]
- Friston KJ,Holmes AP,Worsley KJ,Poline JB,Frith C,Frackowiak RSJ ( 1995): Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp 2: 189–210. [Google Scholar]
- Ganis G,Kutas M ( 2003): An electrophysiological study of scene effects on object identification. Brain Res Cogn Brain Res 16: 123–144. [DOI] [PubMed] [Google Scholar]
- Han S,Gao X,Humphreys GW,Ge J ( 2008): Neural processing of threat cues in social environments. Hum Brain Mapp 29: 945–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennenlotter A,Schroeder U,Erhard P,Castrop F,Haslinger B,Stoecker D,Lange KW,Ceballos‐Baumann AO ( 2005): A common neural basis for receptive and expressive communication of pleasant facial affect. Neuroimage 26: 581–591. [DOI] [PubMed] [Google Scholar]
- Holmes AP,Friston KJ ( 1998): Generalisability, random effects and population inference. Neuroimage 7: S754. [Google Scholar]
- Humphries C,Love T,Swinney D,Hickok G ( 2005): Response of anterior temporal cortex to syntactic and prosodic manipulations during sentence processing. Hum Brain Mapp 26: 128–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M,Molnar‐Szakacs I,Gallese V,Buccino G,Mazziotta JC,Rizzolatti G ( 2005): Grasping the intentions of others with one's own mirror neuron system. PLoS Biol 3: e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson‐Frey SH,Maloof FR,Newman‐Norlund R,Farrer C,Inati S,Grafton ST ( 2003): Actions or hand‐object interactions? Human inferior frontal cortex and action observation. Neuron 39: 1053–1058. [DOI] [PubMed] [Google Scholar]
- Kalisch R,Wiech K,Critchley HD,Seymour B,O'Doherty JP,Oakley DA,Allen P,Dolan RJ ( 2005): Anxiety reduction through detachment: Subjective, physiological, and neural effects. J Cogn Neurosci 17: 874–883. [DOI] [PubMed] [Google Scholar]
- Kampe KK,Frith CD,Frith U ( 2003): “Hey John”: Signals conveying communicative intention toward the self activate brain regions associated with “mentalizing,” regardless of modality. J Neurosci 23: 5258–5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA,Schacter DL ( 2005): Emotional content and reality‐monitoring ability: fMRI evidence for the influences of encoding processes. Neuropsychologia 43: 1429–1443. [DOI] [PubMed] [Google Scholar]
- Kijima N,Saito R,Takeuchi M,Yoshino A,Ono Y,Kato M,Kitamura T ( 1996): Cloninger's seven‐factor model of temperament and character and Japanese version of Temperament and Character Inventory (TCI). Kikan Seishinka Shindangaku 7: 379–399. [Google Scholar]
- King JA,Blair RJ,Mitchell DG,Dolan RJ,Burgess N ( 2006): Doing the right thing: A common neural circuit for appropriate violent or compassionate behavior. Neuroimage 30: 1069–1076. [DOI] [PubMed] [Google Scholar]
- Kondo H,Saleem KS,Price JL ( 2003): Differential connections of the temporal pole with the orbital and medial prefrontal networks in macaque monkeys. J Comp Neurol 465: 499–523. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML ( 2005): The human orbitofrontal cortex: Linking reward to hedonic experience. Nat Rev Neurosci 6: 691–702. [DOI] [PubMed] [Google Scholar]
- LaBar KS,Gatenby JC,Gore JC,LeDoux JE,Phelps EA ( 1998): Human amygdala activation during conditioned fear acquisition and extinction: A mixed‐trial fMRI study. Neuron 20: 937–945. [DOI] [PubMed] [Google Scholar]
- Martinotti G,Andreoli S,Giametta E,Poli V,Bria P,Janiri L ( 2006): The dimensional assessment of personality in pathologic and social gamblers: The role of novelty seeking and self‐transcendence. Compr Psychiatry 47: 350–356. [DOI] [PubMed] [Google Scholar]
- McKiernan KA,Kaufman JN,Kucera‐Thompson J,Binder JR ( 2003): A parametric manipulation of factors affecting task‐induced deactivation in functional neuroimaging. J Cogn Neurosci 15: 394–408. [DOI] [PubMed] [Google Scholar]
- Mitchell JP,Banaji MR,Macrae CN ( 2005): The link between social cognition and self‐referential thought in the medial prefrontal cortex. J Cogn Neurosci 17: 1306–1315. [DOI] [PubMed] [Google Scholar]
- Moriguchi Y,Ohnishi T,Lane RD,Maeda M,Mori T,Nemoto K,Matsuda H,Komaki G ( 2006): Impaired self‐awareness and theory of mind: An fMRI study of mentalizing in alexithymia. Neuroimage 32: 1472–1482. [DOI] [PubMed] [Google Scholar]
- Nakamura K,Kubota K ( 1996): The primate temporal pole: Its putative role in object recognition and memory. Behav Brain Res 77: 53–77. [DOI] [PubMed] [Google Scholar]
- Nakamura K,Kawashima R,Sugiura M,Kato T,Nakamura A,Hatano K,Nagumo S,Kubota K,Fukuda H,Ito K,Kojima S ( 2001): Neural substrates for recognition of familiar voices: A PET study. Neuropsychologia 39: 1047–1054. [DOI] [PubMed] [Google Scholar]
- Nitschke JB,Sarinopoulos I,Mackiewicz KL,Schaefer HS,Davidson RJ ( 2006): Functional neuroanatomy of aversion and its anticipation. Neuroimage 29: 106–116. [DOI] [PubMed] [Google Scholar]
- Northoff G,Grimm S,Boeker H,Schmidt C,Bermpohl F,Heinzel A,Hell D,Boesiger P ( 2006): Affective judgment and beneficial decision making: Ventromedial prefrontal activity correlates with performance in the Iowa Gambling Task. Hum Brain Mapp 27: 572–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Perrett DI,Harries MH,Bevan R,Thomas S,Benson PJ,Mistlin AJ,Chitty AJ ( 1989): Frameworks of analysis for the neural representation of animate objects and actions. J Exp Biol 146: 87–113. [DOI] [PubMed] [Google Scholar]
- Ploghaus A,Tracey I,Gati JS,Clare S,Menon RS,Matthews PM,Rawlins JN ( 1999): Dissociating pain from its anticipation in the human brain. Science 284: 1979–1981. [DOI] [PubMed] [Google Scholar]
- Sassa Y,Sugiura M,Jeong H,Horie K,Sato S,Kawashima R ( 2007): Cortical mechanism of communicative speech production. Neuroimage 37: 985–992. [DOI] [PubMed] [Google Scholar]
- Simmons A,Strigo I,Matthews SC,Paulus MP,Stein MB ( 2006): Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety‐prone subjects. Biol Psychiatry 60: 402–409. [DOI] [PubMed] [Google Scholar]
- Simpson JR Jr,Drevets WC,Snyder AZ,Gusnard DA,Raichle ME ( 2001): Emotion‐induced changes in human medial prefrontal cortex: II during anticipatory anxiety. Proc Natl Acad Sci USA 98: 688–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiers HJ,Maguire EA ( 2006): Spontaneous mentalizing during an interactive real world task: An fMRI study. Neuropsychologia 44: 1674–1682. [DOI] [PubMed] [Google Scholar]
- Steele JD,Lawrie SM ( 2004): Segregation of cognitive and emotional function in the prefrontal cortex: A stereotactic meta‐analysis. Neuroimage 21: 868–875. [DOI] [PubMed] [Google Scholar]
- Sugiura M,Kawashima R,Nakamura K,Sato N,Nakamura A,Kato T,Hatano K,Schormann T,Zilles K,Sato K,Ito K,Fukuda H ( 2001): Activation reduction in anterior temporal cortices during repeated recognition of faces of personal acquaintances. Neuroimage 13: 877–890. [DOI] [PubMed] [Google Scholar]
- Sugiura M,Sassa Y,Jeong H,Miura N,Akitsuki Y,Horie K,Sato S,Kawashima R ( 2006): Multiple brain networks for visual self‐recognition with different sensitivity for motion and body part. Neuroimage 32: 1905–1917. [DOI] [PubMed] [Google Scholar]
- Sugiura M,Friston KJ,Willmes K,Shah NJ,Zilles K,Fink GR ( 2007): Analysis of intersubject variability in activation: An application to the incidental episodic retrieval during recognition test. Hum Brain Mapp 28: 49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umilta MA,Koelher E,Gallese V,Fogassi L,Fadiga L,Keysers C,Rizzolatti G ( 2001): I know what you are doing: A neurophysiological study. Neuron 31: 155–165. [DOI] [PubMed] [Google Scholar]
- Völlm BA,Taylor AN,Richardson P,Corcoran R,Stirling J,McKie S,Deakin JF,Elliott R ( 2006): Neuronal correlates of theory of mind and empathy: A functional magnetic resonance imaging study in a nonverbal task. Neuroimage 29: 90–98. [DOI] [PubMed] [Google Scholar]
- Wakusawa K,Sugiura M,Sassa Y,Jeong H,Horie K,Sato S,Kawashima R ( 2007): Comprehension of implicit meanings in involving irony in social situations: An fMRI Study. Neuroimage 37: 1417–1426. [DOI] [PubMed] [Google Scholar]
- Walter H,Adenzato M,Ciaramidaro A,Enrici I,Pia L,Bara BG ( 2004): Understanding intentions in social interaction: The role of the anterior paracingulate cortex. J Cogn Neurosci 16: 1854–1863. [DOI] [PubMed] [Google Scholar]
- Wei X,Yoo SS,Dickey CC,Zou KH,Guttmann CR,Panych LP ( 2004): Functional MRI of auditory verbal working memory: Long‐term reproducibility analysis. Neuroimage 21: 1000–1008. [DOI] [PubMed] [Google Scholar]
- Wicker B,Perrett DI,Baron‐Cohen S,Decety J ( 2003): Being the target of another's emotion: A PET study. Neuropsychologia 41: 139–146. [DOI] [PubMed] [Google Scholar]


