Abstract
Gender differences have been well established in verbal and spatial abilities but few studies have examined if these differences also extend into the domain of working memory in terms of behavioural differences and brain activation. The conclusions that can be drawn from these studies are not clear cut but suggest that even though gender differences might not be apparent from behavioural measures, the underlying neural substrate associated with working memory might be different in men and women. Previous research suggests activation in a network of frontal and parietal regions during working memory tasks. This study aimed to investigate gender differences in patterns of brain activation during a verbal version of the N‐back working memory task, which incorporates the effects of increased demands on working memory. A total of 50 healthy subjects, aged 18 to 58 years, that were equally split by gender were recruited matched for age, levels of education and ethnicity. All subjects underwent functional magnetic resonance imaging. We found that men and women performed equally well in terms of accuracy and response times, while using similar brain regions to the same degree. Our observations indicate that verbal working memory is not affected by gender at the behavioural or neural level, and support the findings of a recent meta‐analysis by Hyde ([ 2005]: Sex Roles 53:717–725) that gender differences are generally smaller than intra‐gender differences in many cognitive domains. Hum Brain Mapp, 2009. © 2009 Wiley‐Liss, Inc.
Keywords: working memory, gender, functional imaging
INTRODUCTION
Gender differences have been consistently found in tasks of verbal ability, where women outperform men and in test of visuo‐spatial skills where the opposite is true [Vecci and Girelli, 1998, Weiss et al., 2003, Crucian and Berenbaum, 1998, Lawton and Hatcher, 2005]. Previous functional imaging studies have revealed gender differences in the lateralization of hippocampal activation during a spatial memory task [Frings et al., 2006] and in prefrontal activation during spatial working memory [Schweinsburg et al., 2006]. This study focuses on whether there are gender differences in the patterns of brain activation in verbal working memory.
Very few studies have looked at gender differences in verbal working memory. The earliest by Speck et al. [ 2000] used the N‐back sequential verbal working memory task to obtain functional magnetic resonance imaging data (fMRI) from nine men and eight women. In the N‐back task, participants are presented with a series of visual stimuli and are asked to indicate whether the one currently displayed matches that seen in the previous 1, 2, or 3 trials. In the study by Speck et al. [ 2000] the visual stimuli employed were digits and women were found to have longer reaction times but higher accuracy than men in task performance. Men showed bilateral or right brain activation in prefrontal and parietal cortices whereas women predominantly activated the left hemisphere. Goldstein et al. [ 2005] found that women showed increased activation in middle and ventral prefrontal regions compared with men.
Bell et al. [ 2006] used a different working memory task to examine working memory in 23 men and 10 women. In this task, participants were shown a 5‐digit number and were instructed to memorise it. Four seconds later, an array of 10 digits was displayed and participants were asked to indicate whether the digit previously seen was included in the array. This task places considerably fewer demands on working memory than the N‐Back task. No gender differences were found in reaction time or accuracy. Compared with women, men activated a greater number of pixels in the right superior parietal gyrus and right inferior occipital gyrus, and had a greater blood‐oxygenation level‐dependent (BOLD) signal magnitude in the left inferior parietal lobe. The results of the above studies suggest that working memory tasks for stimulus identity engage mostly right‐sided parietal cortical regions to a greater extent in men than in women. Haut and Barch [ 2006] focused on whether one could identify gender differences in lateralization of brain activation in verbal versus nonverbal episodic and working memory tasks. They found little evidence for gender differences; both men and women showed greater left‐sided activation during verbal tasks while processing of nonverbal material (faces in this case) resulted in greater right‐sided activation. However, the effect of task difficulty and any gender differences in the hemodynamic response to increasing monitoring and maintenance demands are unclear. Koch et al. [ 2007] examined the effect of negative mood induction (using unpleasant odours) on the neural correlates of performing the N‐back task in 21 men and 19 women. In this paradigm men activated more parietal and prefrontal regions whereas women showed greater activation in the amygdala and orbitofrontal regions.
Methodological issues limit the validity of existing evidence with regards to working memory. Specifically the sample size in the study by Speck et al. [ 2000] is very small while Bell et al. [ 2006] examined twice as many men than women. Small sample sizes and imbalanced sampling in terms of gender distribution are susceptible to issues of power and spurious findings. This is further supported by the lack of gender differences in the study by Haut and Brach [ 2006] who included the largest sample to date; 49 and 61 healthy controls for each of the fMRI experiments they conducted. Gender differences in working memory may be influenced by emotional interference which however introduces a further level of complexity.
In this study we used the N‐back sequential letter task to investigate gender differences in behavioural measures and neural response as measured by fMRI in verbal working memory. The N‐back task allows for the examination of potential gender differences at increasing working memory demands and elicits a robust and replicable pattern of brain activation as seen in a recent meta‐analysis by Owen et al. [ 2005]. In addition, we employed a relatively large sample for an imaging study to avoid confounding issues regarding power to detect differences. On the basis of the evidence available at the time we initiated this study. Our initial tentative hypothesis was that men would show greater activation in frontal and parietal regions than women.
MATERIALS AND METHODS
Subjects
Functional magnetic resonance imaging data were obtained from 50 healthy volunteers. The mean age for men was 34.36 (SD = 13.24) years and for women 33.13 (SD = 12.31) years. They were equally split by gender and matched for age (t = 0.327, df = 48, P = 0.74) and level of education (χ2 = 2.72, P = 0.43). Participants were recruited by advert in the local press and were included if they (a) had no personal history of mental health problems, substance use or head injury as established based on the Structured Clinical Interview for DSM‐IV‐TR Axis I Disorders [First et al., 2002], (b) had no medical disorders, (c) did not take any prescribed medication, (d) had no family history of mental illness or hereditary neurological disorders, and (e) had no metallic objects in their body. Because of artefacts, data from four women were excluded from the analysis. All subjects were right‐handed. The protocol was approved by the Ethics Committee of the Institute of Psychiatry, King's College London; written informed consent was obtained from each subject.
fMRI Design
A blocked periodic design, incorporating alternating active and control conditions, was used during the N‐back task. In all conditions participants were asked to respond by button press when they saw a target letter. In the “control” or 0‐back condition the target letter was designated (letter “X”). In the three active conditions, 1, 2, and 3 back, the target letter was defined as any letter that was identical to the one presented in the preceding 1, 2, or 3 trials, respectively. In each condition, a series of 14 letters in yellow font were visually presented on a blue screen for 2 s each by means of a prismatic mirror and responses were monitored. There were 18 epochs in all, each lasting 30 s with the total experiment time of 9 min. The ratio of target to nontarget letters presented per block ranged from 2:12 to 4:10. The ratio of target to non‐target letters presented in total was 49:203. The order of the tasks was pseudo‐randomised to avoid any systematic order effects. Reaction time to target letters, total number of correct responses, errors of omission, and errors of commission were recorded.
Image Acquisition
Gradient echo echoplanar MR images were acquired at study entry and study end point using a 1.5 Tesla GE Neurovascular Sigma MR system (General Electric, Milwaukee, WI) fitted with 40 mT/m high‐speed gradients. In each of the 36 noncontiguous planes parallel to the inter‐commissural (AC‐PC) plane, T2*‐weighted MR images depicting blood‐oxygenation level‐dependent (BOLD) contrast were acquired (TE = 40 ms, TR = 3,000 ms, slice thickness = 3 mm, slice gap = 0.3 mm, flip angle = 90°). 180 images were collected during the 9‐min N‐back task. A quadrature birdcage head coil was used for radio frequency (RF) transmission and reception. Subject head motion was limited using foam padding and a forehead strap mounted to the head coil. A high‐resolution gradient echo‐planar imaging dataset was also acquired during the imaging session for subsequent co‐registration.
Image Pre‐Processing
All images were pre‐processed and analysed using Matlab (version 6, The Mathworks Inc, Natick, MA) and SPM2 software (Statistical Parametric Mapping, the Wellcome Department of Cognitive Neurology, London). Images were realigned to correct for movement and normalised into MNI (Montreal Neurologic Institute) space by using an EPI template. Resultant files were checked using the display function. Translation and rotation graphics for each subject were reviewed and scans were excluded if translation >4 mm or rotation >4 degrees. Four female participants were excluded on the basis of this analysis. The transformed data set for each of the remaining subject was smoothed with an isotropic Gaussian filter (full width at half maximum = 8 mm) to compensate for normal variation in anatomy across subjects.
Image Analysis
Analysis of the N‐back task utilised a random effects procedure and a parametric model to identify brain areas where activation was positively correlated with increasing memory load. This model consisted of a boxcar regressor with three levels (1‐back, 2‐back, 3‐back) with 0‐back as an implicit baseline and convolved with the hemodynamic response function. Images were scaled to remove global signal changes and the time series high pass filtered (128 s) to remove‐low frequency artefacts. Activation maps were obtained for each individual and examined using separate one sample t‐tests for males and females. A random effects analysis was utilised to explore gender differences using a two‐sample t‐test. The height threshold was set at P < 0.001 (uncorrected) and activations passing a cluster‐level threshold of P < 0.05 (corrected) were considered significant.
RESULTS
Behavioural Results
Total number of correct responses (accuracy), errors of omission and errors of commission in the n‐back task were analysed in a multivariate repeated measures ANOVA with gender as a between‐subjects factor and memory load (0, 1, 2, or 3‐back) as a within‐subjects factor. All participants showed decreases in number of correct responses and increases in the number of errors with increased memory load (F = 2123.39, df = 7,35, P < 0.0001), but no significant gender differences were observed (F = 1.20, df = 3,39, P = 0.32) and no significant gender by load interaction (F = 1.25, df = 7,35, P = 0.29). see Figure 1.
Figure 1.
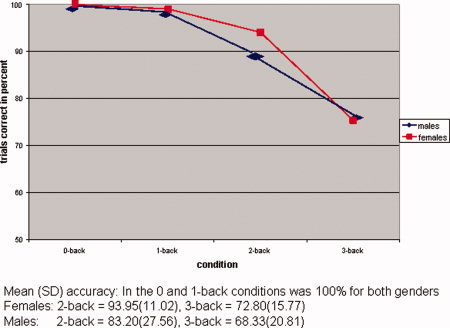
Differences in accuracy according to condition and gender. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Power calculations were carried out based on the number of correct responses to exclude the possibility that our sample was underpowered. To detect gender differences in this study with power of 80% and a P value of 0.05, sample sizes for each gender would have to be much larger (2375 for 3‐back). Response times (in seconds) were also analysed using a repeated measures ANOVA with gender as a between‐subjects factors and memory load (1, 2, or 3‐back) as a within‐subjects factor. Response times were found to increase with memory load (F = 22.46, df = 3, 46, P = 0.0001) (see Fig. 2). No significant gender differences were observed (F = 0.66, df = 1, P = 0.42) or gender by load interaction (F = 0.27, df = 3, 46, P = 0.84).
Figure 2.

Mean response times according to condition and gender. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Power calculations revealed that that these findings were robust; in order to identify possible gender differences with a power of 80% and a P value of 0.05, sample sizes of larger than 650 would be required.
fMRI Results
The pattern of activation that was observed for the n‐back task for both males and females was remarkably similar.(see Fig. 3) Bilateral activation was observed in the superior frontal gyrus (BA 6), middle frontal gyrus (BA 9 and 10), inferior frontal gyrus (BA 47) and the inferior parietal lobule (BA 40). Detailed co‐ordinates of regional peak activations are shown in Table I and illustrated in Figures 4 and 5.
Figure 3.

Overlap in brain activation between males (blue) and females (red) during the N‐back.
Table I.
Distribution of brain activation while performing the N‐back in men (n = 25) and women (n = 21)
| Talairach co‐ordinates (in mm) | ||||||
|---|---|---|---|---|---|---|
| Region | BA | X | Y | Z | Cluster size | t‐value |
| Men | ||||||
| Superior frontal gyrus | 6 | 26 | 12 | 54 | 884 | 8.37 |
| −6 | 22 | 46 | 643 | 6.93 | ||
| Middle frontal gyrus | 9 | 42 | 36 | 32 | 711 | 6.40 |
| 10 | 32 | 64 | 8 | 247 | 5.55 | |
| Inferior frontal gyrus | 47 | −34 | 22 | −4 | 248 | 5.45 |
| Superior parietal lobule | 7 | 14 | −68 | 52 | 814 | 7.39 |
| −28 | 0 | 58 | 631 | 6.77 | ||
| Inferior parietal lobule | 40 | −48 | −48 | 50 | 625 | 6.59 |
| Women | ||||||
| Superior frontal gyrus | 6 | 34 | 4 | 56 | 266 | 7.20 |
| −4 | 14 | 54 | 217 | 7.02 | ||
| Middle frontal gyrus | 9 | 54 | 22 | 30 | 721 | 5.70 |
| 9 | −56 | 20 | 32 | 630 | 9.27 | |
| Inferior frontal gyrus | 47 | −41 | 32 | ‐8 | 245 | 6.41 |
| Superior parietal lobule | 7 | 44 | −54 | 58 | 611 | 7.03 |
BA, Brodman area; X, sagittal; Y, coronal; Z, axial.
Figure 4.

Main effect of task in males during the N‐back (height threshold P < 0.001 uncorrected; cluster‐level P < 0.05 corrected).
Figure 5.

Main effect of task in females during the N‐back (height threshold P < 0.001 uncorrected; cluster‐level P < 0.05 corrected).
DISCUSSION
This study examined gender differences in working memory using the N‐back sequential letter task. We found that men and women showed similar performance in terms of accuracy and response times. Both men and women showed decreased accuracy and increased response times with increased memory load. Similarly, no gender differences were found in patterns of brain activity. The similarity in the behavioural performance of men and women was also reflected in their brain activity.
Our results do not support previous findings of poor performance in women in the N‐back [Speck et al., 2000] or gender differences in pattern of brain activation [Bell et al., 2006; Goldstein et al., 2005; Speck et al., 2000]. We believe that the main reason for the difference between this and the previous studies is our larger and equally balanced by gender sample size, which may be more “representative” of the effect of gender in verbal working memory. We note that both Speck et al. [ 2000] and Bell et al. [ 2006] have used digits rather than letters as visual stimuli but all three studies (including ours) are testing identity‐matching processes. According to the meta‐analysis of working memory tasks by Owen et al. [ 2005], the patterns of activation in identity matching tasks do not appear sensitive to the actual nature (digit or letter) of the visual stimulus. More recent findings by Haut and Barch [ 2006] support this notion.
Gender differences do not seem to be present in verbal working memory, where men and women seem to use the same brain regions and perform equally well. A recent meta‐analysis by Hyde [ 2005] found that intra‐gender differences are generally much larger than differences between men and women in many domains and based on this study this seems to apply both for performance and neural response to verbal working memory.
There is evidence for hormonal influence on brain activity with increased levels of progesterone reducing functional asymmetries during task performance [Fernandez et al., 2003; Hausmann et al., 2002]. Similarly differential engagement of verbal working memory networks may be more evident in the presence of emotional interference [Koch et al., 2007]. We did not examine this here and the possibility of hormonal effect and emotional load on patterns of brain activation during verbal working memory may need to be addressed in future studies.
REFERENCES
- Bell EC,Wilson MC,Wilman AH,Dave S,Silverman PH ( 2006): Males and females differ in brain activation during cognitive tasks. Neuroimage 30: 529–538. [DOI] [PubMed] [Google Scholar]
- Crucian GP, and Berenbaum SA ( 1998): Sex differences in right hemisphere tasks. Brain Cogn 36: 377–389. [DOI] [PubMed] [Google Scholar]
- Fernandez G,Weis S,Stoffel‐Wagner B,Tendolkar I,Reuber M,Beyenburg S,Klaver P,Fell J,de Greiff A,Ruhlmann J,Reul J,Elger CE ( 2003): Menstrual cycle‐dependent neural plasticity in the adult human brain is hormone, task, and region specific. J Neurosci 23: 3790–3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB,Spitzer RL,Gibbon M,Williams JBW ( 2002): Structured Clinical Interview for DSM‐IV‐TR Axis I Disorders, Research Version, Non‐patient Edition. (SCID‐I/NP). New York: Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- Frings L,Wagner K,Unterrainer J,Spreer J,Halsband U,Schulze‐Bonhage A ( 2006): Gender‐related differences in lateralization of hippocampal activation and cognitive strategy. Neuroreport 17: 417–421. [DOI] [PubMed] [Google Scholar]
- Goldstein JM,Jerram M,Poldrack R,Anagnoson R,Breiter HC,Makris N,Goodman JM,Tsuang MT,Seidman LJ ( 2005). Sex differences in prefrontal cortical brain activity during fMRI of auditory verbal working memory. Neuropsychology 19: 509–519. [DOI] [PubMed] [Google Scholar]
- Hausmann M,Becker C,Gather U,Gunturkun O ( 2002): Functional cerebral asymmetries during the menstrual cycle: A cross‐sectional and longitudinal analysis. Neuropsychologia 40: 808–816. [DOI] [PubMed] [Google Scholar]
- Haut KM,Barch DM ( 2006): Sex influences on material‐sensitive functional lateralization in working and episodic memory: Men and women are not all that different. Neuroimage 32: 411–422. [DOI] [PubMed] [Google Scholar]
- Hyde JC ( 2005): The gender similarities hypothesis. Am Psychol 60: 581–592. [DOI] [PubMed] [Google Scholar]
- Lawton C,Hatcher D ( 2005): Gender differences in integration of images in viseospatial memory. Sex Roles 53: 717–725. [Google Scholar]
- Koch K,Pauly K,Kellermann T,Seiferth NY,Reske M,Backes V,Stocker T,Shah NJ,Amunts K,Kircher T,Schneider F,Habel U ( 2007). Gender differences in the cognitive control of emotion: An fMRI study. Neuropsychologia 45: 2744–2754. [DOI] [PubMed] [Google Scholar]
- Owen AM,McMillan KM,Laird AR,Bullmore E ( 2005): N‐back working memory paradigm: A meta‐analysis of normative functional neuroimaging studies. Hum Brain Mapp 25: 46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck O,Ernst T,Braun J,Koch C,Miller E,Chang L ( 2000): Gender differences in the functional organization of the brain for working memory. Neuroreport 11: 2581–2585. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD,Nagel BJ,Tapert SF ( 2005): fMRI reveals alteration of spatial working memory networks across adolescence. J Int Neuropsychol Soc 11: 631–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecci T,Girelli L ( 1998): Gender differences in visuo‐spatial processing: The importance of distinguishing between passive storage and active manipulation. Acta Psychol 99: 1–16. [DOI] [PubMed] [Google Scholar]
- Weiss EM,Kemmler G,Deisenhammer EA,Fleischhacker WW,Delazer M ( 2003): Sex differences in cognitive functions. Pers Indiv Differ 35: 863–875. [Google Scholar]


