Abstract
Recent studies have demonstrated large amplitude spontaneous fluctuations in functional‐MRI (fMRI) signals in humans in the resting state. Importantly, these spontaneous fluctuations in blood‐oxygenation‐level‐dependent (BOLD) signal are often synchronized over distant parts of the brain, a phenomenon termed functional‐connectivity. Functional‐connectivity is widely assumed to reflect interregional coherence of fluctuations in activity of the underlying neuronal networks. Despite the large body of human imaging literature on spontaneous activity and functional‐connectivity in the resting state, the link to underlying neural activity remains tenuous. Through simultaneous fMRI and intracortical neurophysiological recording, we demonstrate correlation between slow fluctuations in BOLD signals and concurrent fluctuations in the underlying locally measured neuronal activity. This correlation varied with time‐lag of BOLD relative to neuronal activity, resembling a traditional hemodynamic response function with peaks at ∼6 s lag of BOLD signal. The correlations were reliably detected when the neuronal signal consisted of either the spiking rate of a small group of neurons, or relative power changes in the multi‐unit activity band, and particularly in the local field potential gamma band. Analysis of correlation between the voxel‐by‐voxel fMRI time‐series and the neuronal activity measured within one cortical site showed patterns of correlation that slowly traversed cortex. BOLD fluctuations in widespread areas in visual cortex of both hemispheres were significantly correlated with neuronal activity from a single recording site in V1. To the extent that our V1 findings can be generalized to other cortical areas, fMRI‐based functional‐connectivity between remote regions in the resting state can be linked to synchronization of slow fluctuations in the underlying neuronal signals. Hum Brain Mapp 2008. © 2008 Wiley‐Liss, Inc.
Keywords: BOLD, functional MRI, neurophysiology, resting state, spontaneous activity, functional connectivity, monkey, visual cortex
INTRODUCTION
Recent advances in imaging sciences have provided platforms that make it possible to investigate how human brain activity correlates with cognition. The majority of functional brain imaging studies in humans relies on functional MRI (fMRI) and on imaging the blood oxygenation level‐dependent (BOLD) signal in particular [Bandettini et al., 1992; Kwong et al., 1992; Ogawa et al., 1990, 1992]. FMRI relies on metabolic and hemodynamic changes to infer underlying local changes in neuronal activity. The fMRI signal may therefore be considered only an indirect measure of neuronal activity. Understanding how metabolic and hemodynamic signals are derived from the underlying neuronal activity is therefore essential for using fMRI as a tool to study brain function.
Recent studies have established a link between increases in neuronal activity and localized increases in cerebral blood flow [CBF; Mathiesen et al., 1998] and BOLD [Logothetis et al., 2001] signals. Increases in neuronal activity induce an increase in the local CBF, which exceeds the increase in oxygen consumption [Fox and Raichle, 1986; Hoge et al., 1999], resulting in lowered deoxyhemoglobin content and increased BOLD signals [Buxton et al., 2004]. More recently, decreases in fMRI signals relative to baseline were investigated, and were shown to be associated with decreases in CBF and oxygen consumption [Shmuel et al., 2002; Stefanovic et al., 2004; Uludag et al., 2004, Pasley et al., 2007] and correlated with decreases in neuronal activity [Shmuel et al., 2006]. Despite these and other advances, neurovascular coupling mechanisms in the cerebral cortex are not yet fully understood, and interpreting functional brain imaging signals in terms of neuronal activity remains difficult [Lauritzen, 2005].
The relationship between BOLD and neural events during the resting state is of particular current interest. While early human fMRI studies considered large spontaneous signal fluctuations typically seen during rest to be uninteresting “noise”, a great deal of recent work has focused on measuring and interpreting these signals [Biswal et al., 1995; Fox and Raichle, 2007]. These spontaneous fluctuations in fMRI signals are reminiscent of previously demonstrated spontaneous fluctuations in cortical neuronal signals obtained from cats [Arieli et al., 1996] and monkeys [Leopold et al., 2003]. They appear highly structured in their spatial correlations, and are therein thought to reflect potentially meaningful underlying neural processes. Such ongoing spontaneous activity signals have been shown, for example, to influence cortical evoked responses and to even account for variability in the somatomotor cortex BOLD responses following button presses [Fox et al., 2006].
Importantly, fluctuations in fMRI signals at rest are correlated over large parts of the human brain [Biswal et al., 1995; Greicius et al., 2003; Fox et al., 2005; Nir et al., 2006]. Two widely distributed brain networks can be identified on the basis of both spontaneous correlations within each network and negative correlations between them [Greicius et al., 2003; Fox et al., 2005]. One of these networks encompasses cortical regions involved in focused attention and working memory, while the other covers areas that are routinely deactivated during attention demanding cognitive tasks. Additionally, smaller networks of regions that routinely respond together to external stimuli or cognitive tasks show correlated activity in the resting state [Fox and Raichle, 2007]. A recent fMRI study in anesthetized monkeys showed that four different networks can be identified in the monkey cortex, based on correlated spontaneous fluctuations within each system [Vincent et al., 2007]. It has been suggested that task‐driven neuronal responses and behavior are reflections of this dynamic, ongoing, functional organization of the brain [Fox et al., 2005]. Given these findings, it has been hypothesized that rather than being an epiphenomenon, fluctuations in the resting state have an important role in brain function [Fox and Raichle, 2007].
While the spontaneous fluctuations in fMRI signals and functional connectivity at rest have been attracting growing attention from the scientific community, the extent to which they reflect underlying neuronal processes is unclear. Some evidence has suggested that the spontaneous BOLD fluctuations are indeed related to underlying neural activity. A few studies using EEG measured in concert with fMRI acquisition showed that in subjects at rest with eyes closed, posterior EEG alpha power is inversely related to BOLD signal in regions of occipital, superior temporal, inferior frontal, and cingulate cortex [Goldman et al., 2002; Laufs et al., 2006; Moosmann et al., 2003]. Consistent with this observation, NIRS‐EEG measurements demonstrated a positive cross‐correlation in occipital cortex between alpha activity and concentration changes of deoxygenated hemoglobin. Power in a 17‐23 Hz range of beta activity is positively correlated with activity in retrosplenial, temporo‐parietal, and dorsomedial prefrontal cortices, constituting the “default mode” regions [Laufs et al., 2003]. The distributed patterns of fMRI activity that were correlated with power in different EEG bands overlapped strongly with those of functional connectivity, i.e., intrinsic covariations of regional activity at rest [Laufs et al., 2003]. Previous experiments in anesthetized monkeys revealed consistent positive impulse response function when correlating spontaneous fluctuations in local‐field potentials (LFP) and the local fMRI signal around the electrode [Logothetis et al., 2001, Fig. 4a]. Using neurophysiological recordings in monkeys, Leopold et al. [ 2003] demonstrated widespread, slow correlations in the local field γ range power. They proposed that such fluctuations might make a significant contribution to the high amplitude fluctuations observed in the time course of resting state signals, as well as the undesirable variability that plagues the repeated presentation of the same stimulus during functional imaging. In contrast, other recent studies have emphasized fluctuations of non‐neuronal origin arising from physiological fluctuations, e.g. vascular vaso‐motion [Mayhew et al., 1996] respiration [Birn et al., 2006; Wise et al., 2004], and imaging system noise and MRI artifacts [Gretton et al., 2006]. Therefore, the degree to which spontaneous BOLD fluctuations of the type observed in functional connectivity studies reflects fluctuations in the underlying neuronal activity remains unclear.
Figure 4.
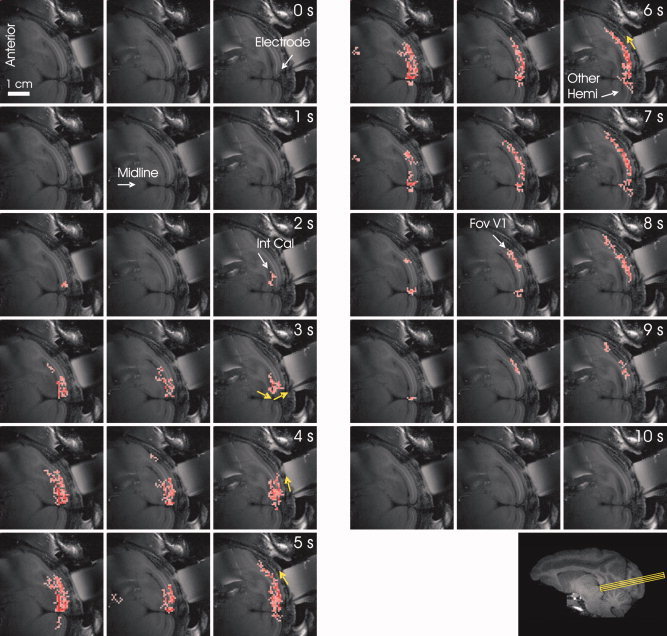
Spatio‐temporal evolution of the correlation between fluctuations in BOLD and neuronal activity. The figure presents the correlation between the slow relative fluctuations in power averaged over frequencies of the broadband neuronal signal recorded at the tip of the electrode (yellow arrow) and the fluctuations in BOLD measured voxel‐by‐voxel. The labels “0 s”, “1 s”, … “10 s” mark the lag between BOLD and neurophysiological signals used for the computation of the corresponding correlation images. Pink colored voxels showed statistically significant positive correlation between the neurophysiological and BOLD signals for the corresponding time‐lag (t‐test, averaging over the correlation obtained time‐segment by time‐segment, P < 0.01). The white arrows point to the tip of the electrode, the midline, the internal calcarine sulcus (“Int Cal”), to the hemisphere contra‐lateral to the electrode (“Other Hemi”) and to the central visual field representation in V1 (“Fov V1”). The yellow arrows point to the direction of propagation of the correlation along the gray matter.
The present study uses a combined techniques approach, where neural and fMRI signals were simultaneously recorded from the brains of anesthetized monkeys. We explored whether spontaneous variation in the BOLD signal in time was correlated with spontaneous fluctuations in neuronal spiking activity, and with relative changes in different frequency bands of the LFP. We show that spontaneous neural signals consistently correlate with the BOLD fluctuations. In considering the correlation independently for each voxel, we describe the temporal features and spatial extent of covariation between the electrical and hemodynamic signals. Preliminary results were presented by Leopold et al. [ 2002] and Shmuel et al. [ 2006, 2007].
MATERIALS AND METHODS
The procedures used for surgery, anesthesia, and simultaneous fMRI and electrical recordings have been described in detail elsewhere [Logothetis et al., 2001]. These procedures are outlined briefly below, whereas new methodological aspects are described in detail.
Animals and Experiments
Data were obtained in nine sessions from seven healthy, anesthetized monkeys (macaca mulatta, 6‐9 kg). The study was approved by the local authorities (Regierungspraesidium) and was in full compliance with the guidelines of the European Community for the care and use of laboratory animals. The monkey eyes were kept open in seven out of nine sessions, in which a uniform and unchanging gray field was presented. The data for these experiments were acquired during 40 s baseline epochs at the beginning of longer scans, in which visual stimuli were presented after these baseline epochs. During each repetition, the first 10 seconds were discarded, leaving multiple 30 s long segments for analyzing correlated fluctuations. We included two additional experiments, one with eyes closed in darkness, and the other with the eyes open in darkness. In these two experiments (Fig. 5), 30 minutes of uninterrupted spontaneous fluctuations in fMRI signals and neuronal activity were recorded and compared.
Figure 5.
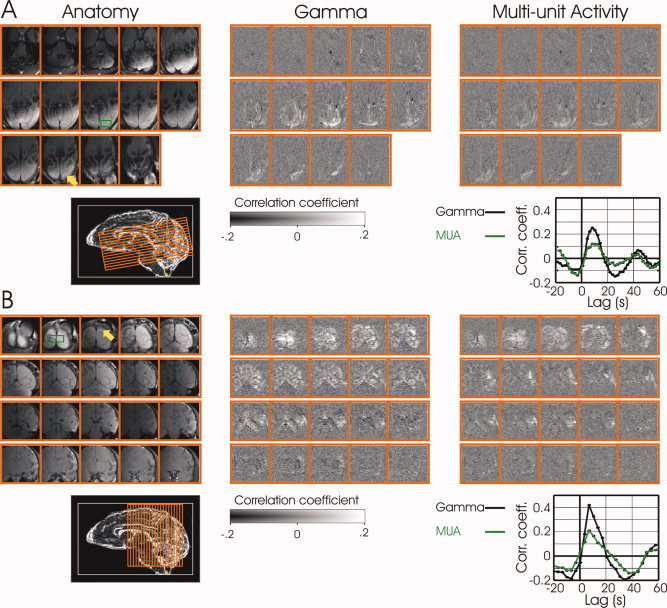
Spatial extent of correlation between the neurophysiological signals in V1 and spontaneous fluctuations in fMRI signals collected continuously over 30 minutes. (A) Correlation maps are shown for horizontal (14 slices) acquisition. The image at the top‐left corner is from the bottom most slice. The image at the bottom‐right corner is from the top‐most slice. (B) Correlation maps are shown for coronal (20 slices) acquisition from a different animal. The image at the top‐left corner is from the most posterior slice. Electrode positions are signified by yellow arrows in the anatomical scans (left). Regions of interest, from which the average BOLD time courses were computed for the correlation, are shown by green rectangles. Middle and rightmost panels show the correlation maps for the gamma and MUA neural signals, respectively. The cross correlation between the average BOLD ROI time course and each of the neural signals is shown in the inset on the far right for each session. Both monkeys were scanned in darkness, though the animal in (A) had its eyes open, while the animal in (B) had its eyes closed. fMRI parameters: field of view 9.6 × 9.6 cm2, matrix 96 × 96, FA 40°, 2 segments, TR 1000 and 1750 ms per segment in (A) and (B) respectively, TE 20 ms.
fMRI Data Acquisition
To localize the electrode, anatomical images were obtained first, using a T1 weighted Gradient‐Echo Fast‐Imaging (GEFI) sequence (45 slices, slice thickness 0.5 mm, field of view 96 × 96 mm2, matrix 512 × 384 reconstructed to 512 × 512, flip angle (FA) 20°, TR/TE 2,000/9 ms). Three adjacent axial oblique slices were selected around the electrode and orthogonal to the cortical surface below the chamber. T1‐weighted high‐resolution anatomical images were obtained from the selected slices using the IR‐RARE pulse sequence (inversion recovery, four segments, field of view 96 × 48 mm2, matrix 256 × 128, resolution 0.375 × 0.375 mm2, slice thickness 2 mm). T2*‐weighted functional images were acquired from the same three slices using a gradient‐echo echo‐planar‐imaging sequence with a field of view of 96 × 48 mm2 and in‐plane resolution of 0.75 × 0.75 mm2. Images were acquired in four segments, with a total readout time of 20.48 ms per segment, TR (time between consecutive excitations in the same slice) of 250 ms, and an acquisition time of 1,000 ms per acquired volume. A TE of 20 ms (T2* of gray matter = 36 ms) and a flip angle of 20‐25° were used. To minimize the effects of inflow and the signal from large draining vessels, we applied flip angles that were smaller than the computed Ernst angle by approximately 10°.
Neurophysiology simultaneous with fMRI
Neurophysiology was carried out simultaneously with fMRI [Logothetis et al., 2001; Shmuel et al., 2006], in epochs in which no stimuli were presented. Figure 1A presents an anatomical image from the posterior part of a monkey brain, with an MR compatible recording chamber attached to the skull above the operculum. The tip of the electrode was in the middle layers of V1. The position of the tip of the electrode within the cortex was estimated using this high‐resolution anatomical GEFI image and similar images obtained in the other experiments. The relative cortical depth of the electrode normalized to the cortical thickness from pia (relative depth 0) to white matter (relative depth 1) was 0.5353 ± 0.1448 (mean ± SD). No statistically significant dependence of the results on cortical depth of recordings was observed in our data.
Figure 1.
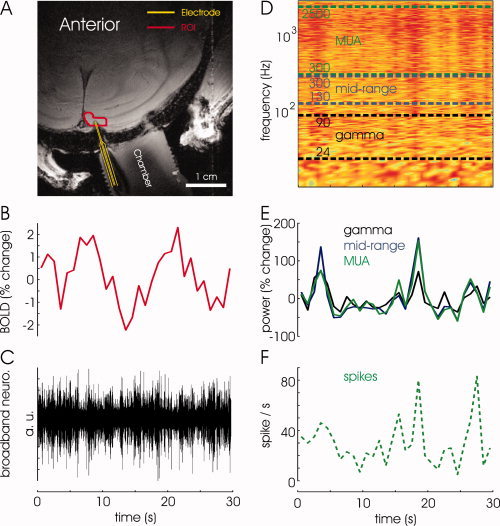
fMRI simultaneous with neurophysiology, and data analysis. (A) An anatomical GEFI image from the posterior part of a monkey brain. The yellow curve delineates the electrode. The red curve encompasses the region of interest used for sampling spontaneous fMRI BOLD signal. (B) Time course of BOLD signal, averaged over voxels in the region of interest. The fMRI signal was acquired at a sampling rate of 1 Hz. (C) The de‐noised broadband neurophysiological signal, originally acquired at a sampling rate of 21 kHz and decimated to 7 kHz. (D) To establish a common time base, a spectrogram was computed from the broadband neurophysiological signal at a resolution of one data point per 1 s, and the relative power changes were averaged over the frequencies of interest to yield a measure of fluctuations in neuronal activity at this resolution. (E) Fluctuations in neuronal activity in the γ, mid‐range, and MUA bands, obtained by averaging relative power over the frequencies in the corresponding ranges from the spectrogram. (F) The spike rate obtained by counting spikes originating from a small number of neurons in the close vicinity (∼50 μ) of the electrode.
Neuronal activity was acquired by digitizing the broadband electrophysiological signal at a sampling rate of 21 kHz (decimated offline to 7 kHz). Offline elimination of residual gradient interference [Logothetis et al., 2001] was applied.
Data Analysis
To use fMRI signals that reached steady state, the first 10 volumes corresponding to the first 10 s of each scan were discarded. A region of interest (ROI) bound to the gray‐matter around the electrode was used to sample spontaneous fMRI BOLD signal (Fig. 1A). To obtain relative changes in fMRI signal, the time‐course of spontaneous fluctuations at rest was normalized by dividing it by the mean of these fluctuations in a scan‐by‐scan and voxel‐by‐voxel manner. The time‐course of relative fluctuations in fMRI signal was averaged over the voxels within the ROI (mean volume across sessions: 6.9 × 2.2 × 6.0 ± 2.9 mm3). fMRI data were acquired at a sampling rate of 1 Hz (Fig. 1B).
To directly compare the time course of the neural and fMRI signals, we first established a common time base. Three types of signals were extracted from the broadband de‐noised neuronal signal. First, we obtained the comprehensive neuronal signal, the LFP in the γ band, mid‐range band, and multi‐unit activity (MUA). To this end, we applied Fourier decomposition separately to nonoverlapping 1‐s segments of the broadband de‐noised electrophysiological signal (Fig. 1C). The resulting spectrogram (Fig. 1D) showed power as a function of frequency and time in successive 1 s epochs. It therefore had a temporal resolution of one data point every 1 s, similar to that of the fMRI data. The global neuronal activity was estimated by averaging the fractional change in power over the entire range of frequencies (1‐2,500 Hz). The LFP in the γ band, the mid‐range band activity, and multi‐unit activity (MUA) were estimated by averaging the fractional change in power over the ranges of 24‐90 Hz, 130‐300 Hz, and 300‐2,500 Hz respectively (Fig. 1D,E). Since these signals were averaged from the spectrogram, their temporal resolution was 1 data point per 1 s, similar to that of the BOLD time course (Fig. 1B). Second, in a separate analysis, we extracted band‐limited power (BLP) traces from the broadband signal by employing the following steps. The recorded broadband signal traces were band‐pass filtered into the frequency ranges used for the estimation of power described above. These band‐limited signals were then full‐wave rectified, isolating amplitude fluctuations over time in the resulting BLP signals. (Note that formally these signals correspond to the square root of the signal power.) The BLP signal was then decimated (i.e. low pass filtered and then resampled) to match the sampling rate of the fMRI data. Third, we identified spikes that were estimated to originate from neurons in the close vicinity (that is, within approximately 50 μm) of the electrode. These action potentials were counted to result in the spike rate at a temporal resolution of 1 s (Fig. 1F).
RESULTS
To investigate possible statistical similarities of the slow fluctuations in BOLD and neuronal activity, we first analyzed the distribution of relative changes and frequency components of these fluctuations. Figure 2A presents the distribution of fluctuations in BOLD signal at rest (in red) relative to the mean of these fluctuations, and the corresponding distributions of relative changes in power in the γ, mid‐range, and MUA frequency bands. The bulk relative changes in BOLD and in slow fluctuations in neuronal activity were within ±4% and −80% to +140%, respectively, demonstrating an approximate 20 fold difference between the two types of signals. The distributions corresponding to neuronal activity in the γ, mid‐range, and MUA bands were similar to each other. To compare the frequencies of slow fluctuations in these signals, we computed the relative amplitudes (square root of power) of their Fourier components. Consistent with the difference in scale between the variation of relative changes in neuronal activity and BOLD signals shown in Figure 2A, the absolute amplitude prior to normalization was 0.051 ± 0.02, 1.70 ± 0.49, 2.16 ± 0.59, and 1.52 ± 0.42 for BOLD, γ, mid‐range, and MUA, respectively (mean ± SD, averaged over animals and frequencies in the range of 0.033‐0.5 Hz). Figure 2B shows the normalized amplitude distributions corresponding to the spontaneous fluctuations in the BOLD signal and in relative changes in neurophysiological activity, allowing the comparison of their frequency components. An increase in power can be seen at 0.1 Hz and at even lower frequencies of fluctuations in BOLD, as observed previously in human studies [Fox et al., 2007], assuring that the monkey model is well suited for investigating this phenomenon. Another prominent peak can be seen at 0.4 Hz, indicating that there are relatively greater signal fluctuations at that frequency, which is the exact frequency of the artificial respiration used in the experiments. The occurrence of a peak in the BOLD signal at the respiratory frequency suggests that part of what is detected as spontaneous fluctuations in fMRI signal is unrelated to neuronal activity; therefore, a careful analysis of the relationship between spontaneous fluctuations in fMRI and neuronal activity is essential. The green curve shows the Fourier amplitudes of the slow fluctuations in relative power averaged from the spectrogram over frequencies of the multi‐unit activity band. As expected, no peak can be seen here that corresponds to the respiration frequency. Slight increases in power can be observed at 0.1 Hz and lower frequencies. Similar results were obtained when computing the spectral distribution of relative fluctuations of power in the mid‐range band (blue curve1). Whereas the normalized distribution of Fourier amplitudes of the slow fluctuations in γ band activity (in black) showed similarity to those of the MUA and mid‐range bands at frequencies higher than 0.1 Hz, it included larger increases in amplitude at frequencies lower than 0.1 Hz and thereby resembled the BOLD spectrum to a larger degree.
Figure 2.
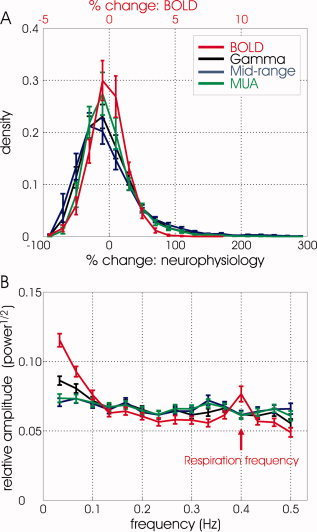
Distribution of relative changes and frequencies of slow fluctuations in BOLD and neuronal signals. (A) Distribution of relative changes of spontaneous fluctuations in BOLD signal and power in the γ, mid‐range, and MUA bands averaged from all data sets. Each of the corresponding histograms obtained from each experiment was first normalized, such that its integral was equal to 1. This normalization was followed by averaging the histograms over all the data‐sets. Note the scale difference between the relative changes in BOLD and those in neurophysiological activity. (B) Normalized distributions of Fourier amplitudes of the relative spontaneous fluctuations in the BOLD signal and the slow fluctuations in neuronal activity (relative power averaged over the frequency range of the γ, mid‐range, and MUA bands). The corresponding distributions of Fourier amplitudes of these fluctuations were normalized such that their integrals over the range of frequencies of 0.033‐0.5 Hz were equal to 1. The red arrow points at the frequency of the artificial respiration used in the experiments. The curves in (A) and (B) show the mean ± SEM distributions averaged over 7 experiments.
The frequency ranges that were used for averaging the power over the MUA, mid‐range, and γ bands did not include 0.1 Hz. What is shown to fluctuate at slow frequencies including 0.1 Hz in Figure 2B is the overall activity, or envelope of oscillations in these bands, separately averaged over these ranges of frequencies from the spectrogram.
To test for covariation in the fMRI and various neuronal signals, multiple nonoverlapping 15 s long segments of relative changes in power of neuronal activity were compared to segments of BOLD time‐course of identical duration. The segments of BOLD signal were at temporal lags in the range of 0‐15 s relative to the segment of neuronal activity they were compared to. Figure 3A shows the correlation as a function of lag from each experiment (grey curves) and the correlation function averaged over seven experiments in five different monkeys (red curve). The vertical axis represents the Spearman's correlation coefficient between BOLD and the mean (averaged over frequencies) relative fluctuations in power of the simultaneously acquired broadband neurophysiological signal. The horizontal axis represents the lag between the two correlated signals, with positive lags standing for BOLD lagging behind the neuronal activity. Statistically significant correlation between the fluctuations in BOLD and the fluctuations in neuronal activity were observed, for lags in the range of 3 to 8 s (P < 0.01, two‐tailed t‐test). The peak in correlation appears at a lag of approximately 5.5 s. As a control for false positive detection of correlation, we shuffled the segments of BOLD and neuronal activity and therefore broke the simultaneity between these acquired signals. Figure 3B shows the result of the exact same data and computation as that presented in Figure 3A, with the difference being that the correlation was computed between segments which were not acquired simultaneously (only segments from the same experiment were compared). No correlation can be observed between the two signals when the simultaneity condition does not hold, indicating that it is not plausible for the detected correlation to be a result of false positive detection.
Figure 3.
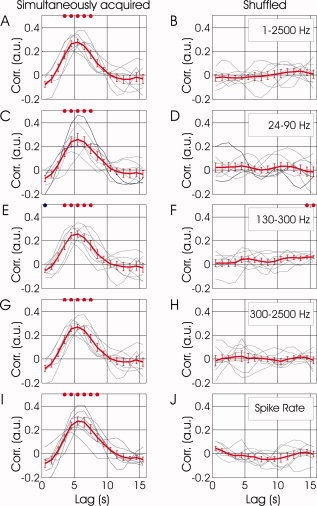
Covariation between spontaneous fluctuations in fMRI and neuronal signals as a function of temporal lag. (A) The grey curves show the correlation as a function of lag from each experiment. The red curve presents the correlation function averaged over seven experiments in five different monkeys (mean ± SEM). The vertical axis represents the Spearman's correlation coefficient between BOLD and the fluctuations in relative (fractional change) power averaged over frequencies of the denoised broadband neurophysiological signal acquired simultaneously with fMRI. The horizontal axis represents the lag between the two correlated signals, with positive lags standing for BOLD lagging behind the neuronal activity. (B) Correlation between the same signals as presented in (A), computed after breaking the simultaneity condition by shuffling the segments of BOLD and neuronal activity obtained within each experiment. (C‐D, E‐F, G‐H) present correlation functions in the format used for (A) and (B), for the LFP γ, mid‐range, and MUA bands, respectively. (I) and (J) present similar correlation functions for fluctuations in spiking activity, estimated by counting identified action potentials over 1s epochs rather than using frequency‐based analysis.
To investigate correlations between fluctuations in different bands of neuronal oscillations and the fluctuations in the fMRI signal, we repeated the analysis used for Figure 3A,B, while incorporating neuronal activity bound to different bands of frequency. Figure 3C demonstrates the mean correlation between the fluctuations in BOLD and in neuronal activity averaged over the γ band. Positive correlation can be observed for the range of 3 to 8 s (P < 0.01, two‐tailed t‐test), with a peak around a 5.5 s lag (see also Fig. 4a in Logothetis et al., 2001). This positive correlation diminished when analyzing signals that were not recorded simultaneously (Fig. 3D). Similar levels of correlation were found for the mid‐range band of frequencies (Fig. 3E,F), and for the multi‐unit band of frequencies (Fig. 3G,H). Lastly, we repeated the analysis by counting identified action potentials rather than using frequency‐based analysis of the neuronal signal. Figure 3I shows that the fluctuations in BOLD correlate with the fluctuations in the locally measured spike rate. In summary, the spontaneous fluctuations in BOLD were correlated with fluctuation in neuronal activity in the γ band and higher frequencies.
To investigate the spatio‐temporal characteristics of the relationship between the fluctuations in BOLD and neuronal activity, we computed the Spearmann's correlation coefficient between the fluctuations in the broadband neuronal signal recorded at the tip of the electrode and the fluctuations in BOLD measured voxel‐by‐voxel. Multiple nonoverlapping 20 s long segments of relative changes in power of neuronal activity were compared to segments of BOLD time‐course of identical duration, that were at temporal lags in the range of 0‐10 s relative to the segment of neuronal activity they were compared to. Figure 4 shows the spatio‐temporal pattern of the cross‐correlation. At a 0 s lag, no correlation could be observed. At a 3 s lag, a seed of correlated activity seemed to develop in the vicinity of the electrode. The fMRI based spontaneous activity that was correlated with the neuronal signal reached its largest volume and spatial extent along the grey matter at 6 s lag. Later on, it decayed gradually, until no correlation could be seen at 10 s lag. Note that the voxels that demonstrated time‐course correlated to that of the neurophysiological signal were within the gray matter. In addition, rather than a static pattern that developed and decayed around the electrode, a slow wave like spatio‐temporal pattern seemed to emerge, with the correlation starting at the more peripheral visual field representation of V1 in the internal calcarine sulcus (3 s), then progressing medially along the grey matter to be centered on the electrode (4 s), and laterally along the most superficial cortex towards foveal V1 (7‐9 s). Also note the positive correlation between the neuronal signal recorded in one hemisphere and the fluctuations in BOLD in V1 in the other hemisphere (5‐7 s). Overall, a complex pattern of spatio‐temporal correlation could be seen, mostly centered around the electrode and with the spatial extent peaking at an approximate lag of 6 s.
The results presented above were obtained using a surface coil, allowing high SNR only in the posterior visual cortical areas. To further investigate the spatial extent of correlation between the neurophysiological signal in V1 and spontaneous fluctuations in fMRI signals we used a larger coil to image a larger fraction of the monkey brain (Fig. 5). In these experiments, the correlation between the neural and BOLD signals was computed over periods of 30 minutes during which data of spontaneous fluctuations in fMRI signals and neuronal activity were collected continuously. Figures 5A and B demonstrate, as before, a high degree of correlation between the fluctuations of neuronal activity in the gamma band in V1 and spontaneous fluctuations in fMRI signal over the posterior cortex, but now extending more than a centimeter rostral to the V1 electrode position. Note that significant correlation with the neural signal was not limited to voxels in the hemisphere in which the electrode was introduced; instead, spontaneous fluctuations in the visual cortex in both hemispheres were correlated with fluctuations in neuronal activity detected in one location in V1. A similar widespread spatial pattern of correlation in the cortex was also observed with spontaneous fluctuations of the MUA, although with lower overall correlation strength. The insets in A and B show the correlation between each of the neural signal types and the average BOLD time course in a region of interest (green rectangle).
DISCUSSION
The Observed Findings Reflect Correlation Between Neuronal Activity and BOLD Signals
Although human fMRI studies have routinely used spontaneous fluctuations in fMRI signal to evaluate functional connectivity at rest, and have found that these fluctuations can influence perception and behavior, the relation of these fluctuations to the underlying neuronal activity has been tenuous. Here we demonstrate significant correlations between slow fluctuations in neuronal activity and fMRI signal measured both locally around the microelectrode, as well as over large parts of the visual cortex. The observed correlations cannot be attributed to physiological fluctuations that are independent of neuronal activity, such as the influence of respiration on the hemodynamic system [Wise et al., 2004; Birn et al., 2006]. In our anesthetized monkey experiments, the respiration rate was regulated at 24 cycles/min, clearly observed as increased amplitude in BOLD fluctuations at 0.4 Hz (Fig. 2B). There were no peaks in this frequency in the simultaneously recorded neuronal signals, providing a convenient way to rule out the unlikely correlation between neural activity and respiration. The correlation also cannot be attributed solely to vascular vaso‐motion [Mayhew et al., 1996]. Whereas BOLD changes due to vaso‐motion may take place, it is implausible that they are the ones to drive fluctuations in neuronal activity. Supporting this notion, positive correlation was obtained between fluctuations in neuronal activity and lagging BOLD signals. Moreover, positive correlations were observed between the neurophysiological signal recorded in one hemisphere, and the fMRI signal obtained from the two hemispheres (Figs 4 and 5). Given that the arterial systems of the two hemispheres are virtually independent, with connection only through the circle Willis, it is implausible that the correlations we showed here are caused by fluctuations in CBF that are purely due to vaso‐motion. It is also implausible that the observed correlation can be attributed to artifacts due to image reconstruction and the rippling effect [Gretton et al., 2006]. While the phenomena described by Gretton et al. caused dependencies between fMRI signals measured at short distances (<4 mm), the correlations described here reached distances of approximately 30 mm along the read‐out direction (Figs. 4 and 5) 15 mm along the phase encoding direction (see Fig. 5). Our analysis controlled explicitly for false positive detection by demonstrating flat correlation functions for fMRI and neuronal signals that were not acquired simultaneously. Lastly, the correlation between fMRI signals and neuronal activity was limited to gray matter regions (Figs. 4 and 5). Overall, while contributions with non‐neuronal origin are possible, they are unlikely to underlie the correlation between neurophysiological and fMRI signals we observed.
Implications for Functional Connectivity at Rest
Figure 6 puts the detected correlation between BOLD signal and the locally measured neuronal activity in the context of the functional connectivity at rest, reported in many human fMRI studies [Biswal et al., 1995; See review by Fox and Raichle, 2007]. It shows two hypothetical remote cortical areas, A1 and A2. We assume that A1 and A2 show BOLD‐fMRI measured functional connectivity at rest (a). Does that mean that the neuronal activities in these areas are synchronized? Assuming that the correlation we detected in V1 holds true for other cortical areas, the spontaneous fluctuations in BOLD signal in both A1 and A2 correlate with the local neuronal activity in each of these areas (b, c). Incorporating the assumed fMRI‐based functional connectivity between these areas at rest (a), we can conclude by induction that the neuronal activities in A1 and A2 are functionally connected (d). Therefore, this analysis indicates that the measurement of fMRI functional connectivity reflects BOLD changes that are driven by actual functional connectivity between slow fluctuations in neuronal activity within the two areas. However, all three correlation measures involved in the model account for only part of the variability in the corresponding time‐courses. Therefore, while this model is indicated by the data, it is not proven by it.
Figure 6.
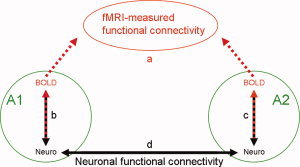
Can fMRI‐based functional connectivity be interpreted as neuronal functional connectivity? The green circles represent two hypothetical remote cortical areas, A1 and A2. The red dashed arrows stand for BOLD‐fMRI measurements within these regions at rest. We assume that these measurements show inter‐regional coherent fluctuations and therefore functional connectivity (a). The red‐dark dashed arrows represent correlations between fluctuations in neuronal and BOLD signals within each area (b, c). The dark arrow stands for neuronal functional connectivity between A1 and A2, indicated by induction (d).
In Figures 4 and 5 we demonstrated correlation between the neuronal activity measured in V1 in one hemisphere, and synchronized fluctuations in fMRI signal in both hemispheres. Similar to these findings, Vincent et al. [ 2007] showed that the time course of BOLD fluctuations in a subset of V1 in one hemisphere is correlated with regions in the contralateral V1. Given the absence of direct inter‐hemispheric connections involving V1 away from the vertical meridian, the correlations in contralateral V1 must be mediated by polysynaptic pathways, which might involve higher visual areas or the thalamus.
CONCLUSION
Our study demonstrated correlations between delayed BOLD time series and fluctuations in power of neuronal activity in the local field potential γ band, multi‐unit activity and spiking activity. fMRI signals in large parts of the visual cortex in both hemispheres were correlated with the fluctuations in neuronal activity measured at a single site in V1. We conclude that, most plausibly, fMRI‐based functional connectivity in the resting state reflects neuronal interplay between remote cortical regions.
Acknowledgements
The data analyzed in this study were obtained in the Lab of Nikos K. Logothetis (MPI für biologische Kybernetik, Tübingen, Germany). The experimental part of this work was supported by NKL through personnel and funds of the Max Planck Society. AS was supported by a long term fellowship from the European Molecular Biology Organization.
Footnotes
We verified that the normalized Fourier amplitudes of the relative fluctuations in power averaged over the broadband range of frequencies were similar to those of the relative power averaged over the MUA band (not shown). This similarity is expected since the MUA frequency range constitutes 88% of that of the broadband signal. Therefore, the weighting of the MUA range makes this range dominant in the computations related to the broadband signal.
REFERENCES
- Arieli A,Sterkin A,Grinvald A,Aertsen A ( 1996): Dynamics of ongoing activity: Explanation of the large variability in evoked cortical responses. Science 273: 1868–1871. [DOI] [PubMed] [Google Scholar]
- Bandettini PA,Wong EC,Hinks RS,Tikofsky RS,Hyde JS ( 1992): Time course EPI of human brain function during task activation. Magn Reson Med 25: 390–397. [DOI] [PubMed] [Google Scholar]
- Birn RM,Diamond JB,Smith MA,Bandettini PA ( 2006): Separating respiratory‐variation‐related fluctuations from neuronal‐activity‐related fluctuations in fMRI. Neuroimage 31: 1536–1548. [DOI] [PubMed] [Google Scholar]
- Biswal B,Yetkin FZ,Haughton VM,Hyde JS ( 1995): Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magn Reson Med 34: 537–541. [DOI] [PubMed] [Google Scholar]
- Buxton RB,Uludag K,Dubowitz DJ,Liu TT ( 2004): Modeling the hemodynamic response to brain activation. Neuroimage 23 ( Suppl 1): S220–S233. Review. [DOI] [PubMed] [Google Scholar]
- Fox MD,Raichle ME ( 2007): Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8: 700–711. [DOI] [PubMed] [Google Scholar]
- Fox PT,Raichle ME ( 1986): Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci USA 83: 1140–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD,Snyder AZ,Vincent JL,Corbetta M,Van Essen DC,Raichle ME ( 2005): The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102: 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD,Snyder AZ,Zacks JM,Raichle ME ( 2006): Coherent spontaneous activity accounts for trial‐to‐trial variability in human evoked brain responses. Nat Neurosci 9: 23–25. [DOI] [PubMed] [Google Scholar]
- Goldman RI,Stern JM,Engel JJ,Cohen MS ( 2002): Simultaneous EEG and fMRI of the alpha rhythm. NeuroReport 13: 2487–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD,Krasnow B,Reiss AL,Menon V ( 2003): Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 100: 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gretton A,Belitski A,Murayama Y,Schölkopf B,Logothetis N ( 2006): The effect of artifacts on dependence measurement in fMRI. Magn Reson Imaging 24: 401–409. [DOI] [PubMed] [Google Scholar]
- Hoge RD,Atkinson J,Gill B,Crelier GR,Marrett S,Pike GB ( 1999): Investigation of BOLD signal dependence on cerebral blood flow and oxygen consumption: The deoxyhemoglobin dilution model. Magn Reson Med 42: 849–863. [DOI] [PubMed] [Google Scholar]
- Kwong KK,Belliveau JW,Chesler DA,Goldberg IE,Weisskoff RM,Poncelet BP,Kennedy DN,Hoppel BE,Cohen MS,Turner R,Cheng H,Brady TJ,Rosen BR ( 1992): Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci USA 89: 5675–5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen M ( 2005): Reading vascular changes in brain imaging: Is dendritic calcium the key? Nat Rev Neurosci 6: 77–85. [DOI] [PubMed] [Google Scholar]
- Laufs H,Kleinschmidt A,Beyerle A,Eger E,Salek‐Haddadi A,Preibisch C,Krakow K ( 2003): EEG‐correlated fMRI of human alpha activity. Neuroimage 19: 1463–1476. [DOI] [PubMed] [Google Scholar]
- Laufs H,Holt JL,Elfont R,Krams M,Paul JS,Krakow K,Kleinschmidt A ( 2006): Where the BOLD signal goes when alpha EEG leaves. Neuroimage 31: 1408–1418. [DOI] [PubMed] [Google Scholar]
- Leopold DA,Logothetis NK ( 2002): Visualizing global brain networks in the monkey using combined fMRI and electrophysiology. Society for Neuroscience abstract number 325.7. [Google Scholar]
- Leopold DA,Murayama Y,Logothetis NK ( 2003): Very slow activity fluctuations in monkey visual cortex: Implications for functional brain imaging. Cereb Cortex 13: 423–433. [DOI] [PubMed] [Google Scholar]
- Logothetis NK,Pauls J,Augath M,Trinath T,Oeltermann A ( 2001): Neurophysiological investigation of the basis of the fMRI signal. Nature 412: 150–157. [DOI] [PubMed] [Google Scholar]
- Mathiesen C,Caesar K,Akgoren N,Lauritzen M ( 1998): Modification of activity‐dependent increases of cerebral blood flow by excitatory synaptic activity and spikes in rat cerebellar cortex. J Physiol 512: 555–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhew JE,Askew S,Zheng Y,Porrill J,Westby GW,Redgrave P,Rector DM,Harper RM ( 1996): Cerebral vasomotion: A 0.1‐Hz oscillation in reflected light imaging of neural activity. Neuroimage 4: 183–193. [DOI] [PubMed] [Google Scholar]
- Moosmann M,Ritter P,Krastel I,Brink A,Thees S,Blankenburg F,Taskin B,Obrig H,Villringer A ( 2003): Correlates of alpha rhythm in functional magnetic resonance imaging and near infrared spectroscopy. Neuroimage 20: 145–158. [DOI] [PubMed] [Google Scholar]
- Nir Y,Hasson U,Levy I,Yeshurun Y,Malach R ( 2006): Widespread functional connectivity and fMRI fluctuations in human visual cortex in the absence of visual stimulation. Neuroimage 30: 1313–1324. [DOI] [PubMed] [Google Scholar]
- Ogawa S,Lee TM,Kay AR,Tank DW ( 1990): Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA 87: 9868–9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S,Tank DW,Menon R,Ellermann JM,Kim SG,Merkle H,Ugurbil K ( 1992): Intrinsic signal changes accompanying sensory stimulation: Functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci USA 89: 5951–5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasley BN,Inglis BA,Freeman RD ( 2007): Analysis of oxygen metabolism implies a neural origin for the negative BOLD response in human visual cortex. Neuroimage 36: 269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuel A,Yacoub E,Pfeuffer J,Van De Moortele PF,Adriany G,Hu X,Ugurbil K ( 2002): Sustained negative BOLD, blood flow and oxygen consumption response and its coupling to the positive response in the human brain. Neuron 36: 1195–1210. [DOI] [PubMed] [Google Scholar]
- Shmuel A,Augath M,Oeltermann A,Logothetis NK ( 2006): Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat Neurosci 9: 569–577. [DOI] [PubMed] [Google Scholar]
- Shmuel A,Augath M,Oeltermann A,Logothetis NK ( 2007): Spontaneous fluctuations in functional MRI signal reflect fluctuations in the underlying local neuronal activity. Neuroimage: 13th Annual Meeting of the Organization for Human Brain Mapping 36: S58. [Google Scholar]
- Stefanovic B,Warnking JM,Pike GB ( 2004): Hemodynamic and metabolic responses to neuronal inhibition. Neuroimage 22: 771–778. [DOI] [PubMed] [Google Scholar]
- Uludag K,Dubowitz DJ,Yoder EJ,Restom K,Liu TT,Buxton RB ( 2004): Coupling of cerebral blood flow and oxygen consumption during physiological activation and deactivation measured with fMRI. Neuroimage 23: 148–155. [DOI] [PubMed] [Google Scholar]
- Vincent JL,Patel GH,Fox MD,Snyder AZ,Baker JT,Van Essen DC,Zempel JM,Snyder LH,Corbetta M,Raichle ME ( 2007): Intrinsic functional architecture in the anaesthetized monkey brain. Nature 447: 83–86. [DOI] [PubMed] [Google Scholar]
- Wise RJS,Ide K,Poulin MJ,Tracey I ( 2004): Resting state fluctuations in arterial carbon dioxide induce significant low frequency variations in BOLD signal. Neuroimage 21: 1652–1664. [DOI] [PubMed] [Google Scholar]


