Abstract
Previous studies showed that neurons in the monkey premotor cortex became active when performing a particular action and also when observing the same action performed by others. These findings suggest a mirror system for action observation. Recently, bimodal neurons, sensitive both to visual and tactile stimulation, were reported in the parietal cortex, suggesting a potential mirror neuron system for observing and experiencing tactile stimulation. Subsequently, a mirror neuron system for observed touch has been suggested. The current study was designed to determine whether the activation of a sensory mirror system during touch observation is affected by possible attributions of the observed touch to oneself (subjective view) or to somebody else (objective view). In the study, healthy volunteers observed video clips of a touched or nontouched hand either in an egocentric or in an allocentric perspective during functional magnetic resonance imaging. Results showed activation of somatosensory cortices when observing the hand being touched in egocentric as well as in the allocentric perspectives. Moreover, somatosensory responses differed depending on the perspective of the observed touch. We discuss the results in terms of a possible mirror neuron system for observed and experienced touch. Hum Brain Mapp 2009. © 2009 Wiley‐Liss, Inc.
Keywords: mirror neuron system, somatosensory cortex, perspective, fMRI, touch
INTRODUCTION
Animal studies have suggested mirror neurons in brain area F5 in monkeys [Rizzolatti et al.,1996a,b]. These neurons discharge when a particular action is performed and also when the monkey observes the same action performed by others. However, they do not respond to the observation of an object alone or when a hand is mimicking an action in the absence of the target [e.g., Rizzolatti et al.,1996a,b]. These findings suggest that a mirror neuron system of action observation may be important for the understanding and imitation of action [Rizzolatti et al.,2001] and for the understanding of intentions [Iacoboni and Dapretto,2006; Iacoboni et al.,2005].
An increasing body of evidence suggests the existence of a mirror system in humans similar to that in monkeys. Results of previous neuroimaging and transcranial magnetic stimulation (TMS) studies suggested the presence of mirror neurons in several cortical areas, in particular the ventral premotor area (homologous to area F5 in monkeys), the superior temporal sulcus (STS), the inferior parietal lobe, and the primary motor cortices [Buccino et al.,2001; Fadiga et al.,1995; Gangitano et al.,2001; Grafton et al.,1996; Hari et al.,1998; Iacoboni et al.,1999]. Several animal studies also found neurons in the parietal cortex responding to both tactile and visual stimulations [e.g., Bremmer et al.,2001; Graziano et al.,2000; Graziano,1999; Iriki et al.,1996]. Recent evidence further suggests that some neurons in the primary somatosensory cortex (SI) code arbitrary visual–tactile associations. Animal studies have shown that neurons in monkey SI may fire both in response to a tactile stimulus, and in response to a visual stimulus previously associated with the tactile stimulus [Zhou and Fuster,1997,2000]. It is possible that this process involves either a local mechanism within SI, or interactions with other cortical areas (parietal cortex), or a combination of both. The interaction with the parietal cortex may involve bimodal neurons, which are sensitive both to vision and touch. Hence, analogous to the mirror system for action observation, a possible mirror system for observing and receiving touch may exist. This mirror system may be based on the activation of sensory cortical areas that are linked to areas containing bimodal neurons. According to the mirror theory [Rizzolatti et al.,2001], observation of touch may be important for the recognition and understanding of touch to form an internal representation of an event and to estimate consequences for action preparation. Thus, a mirror neuron system for tactile observation analogous to action observation could have evolved for rapid assessment and evaluation of how other people may feel or a situation that may be consequential to the observer.
Two previous studies provided evidence for a mirror system for observed touch by demonstrating that observing a body part being touched resulted in neural responses in somatosensory cortices [Blakemore et al.,2005; Keysers et al.,2004]. Keysers et al. [2004] presented subject's video clips of a leg being touched while recording their brain activity in an fMRI experiment. They reported activation in secondary somatosensory cortex (SII) but not in SI. Furthermore, they reported activation in SII both for the observation of touch to human legs and on cylindrical objects. In addition, they manipulated the difficulty of integration of the observed touch into the body schema of the observer (egocentric vs. allocentric perspective) and reported activation in SII irrespective of the perspective of the touched body part. However, this was tested only for SII with a region of interest approach. In contrast, Blakemore et al. [2005] found significant activation of SI and SII when subjects observed touch to a face and a neck. This activation was higher than that elicited by observing touch of an object. Nevertheless, Blakemore et al. used observation of touching a face or a neck (necessarily always in allocentric perspective) and did not examine whether the viewing perspective may have affected the observed activations in the somatosensory cortices. It remains an issue whether a mirror neuron system for observed touch is engaged irrespective of viewing perspectives.
In this study, we hypothesized that neural responses in somatosensory cortices differ when the observed touch relates to the observer's own body relative to when it relates to somebody else. From a psychological point of view, it may be important to distinguish observed touch to one's own body from that perceived to others. For example, being touched to one's own body may signal an adverse situation. Thus, we hypothesized that activation in somatosensory cortices may differ according to the viewing perspective during the observation of touch. To address this issue, we conducted an fMRI study in which subjects observed touching of a hand in an ego‐ or allocentric viewing perspective. While the egocentric (or first‐person‐) perspective allowed the seen touch to be easily integrated in one's own body scheme, the allocentric (or third‐person) perspective minimized attribution to the subject's own body. In contrast to the Keysers et al. [2004] study, subjects in this study were presented with video clips of a hand, instead of a leg, being touched. We chose this paradigm because the cortical representation of the hands in SI is much larger than that of the legs. Thus, we expected the stimuli to induce activation not only in SII but also in SI. Recent studies have shown that viewing one's own body being touched has an impact on somatosensory processing [Taylor‐Clarke et al.,2002]. However, somatosensory activation related to the mirror system may respond only to observation of touch by others. Thus, we expected differential somatosensory activation during the observation of touch in an egocentric versus an allocentric perspective that minimizes the attribution to the observer's own body.
METHODS
Participants
Ten right‐handed healthy volunteers (three males) with a mean age of 27 years (SD 1.75, range 25–30 years) participated in the study. Informed consent was obtained from all subjects. Handedness was examined using the Edinburgh Handedness Inventory [Oldfield,1971]. All subjects had a neurological examination prior to the study. The study adhered to the Declaration of Helsinki and was approved by the local human subjects committee (National Institute of Neurological Disorders and Stroke, USA).
Materials and Design
The experiment used a 2 × 2 factorial design (see Table I). The factors were (1) viewing perspective (egocentric vs. allocentric), and (2) touch observation (a hand being touched vs. a hand not being touched). Further, there was an additional condition with actual touch. For the viewing perspective, subjects watched video clips in which a hand was presented either in an egocentric perspective (i.e., the egocentric condition; 50% trials) such that it matched the orientation of the subjects own hand, or in an allocentric perspective (i.e., the allocentric condition; 50% trials) such that it did not match the orientation of the subject's own resting hand (see Fig. 1). For the factor of touch observation, half of the video clips showed a hand being touched on the index finger repeatedly by a paintbrush (i.e., the touch condition) and in the other half the paintbrush did not touch the hand (i.e., the nontouch condition). The same visual stimuli and motion frequency (1/s) were applied in all video clips across the viewing perspective and observed action. In the nontouch condition, the paintbrush made an identical motion as in the touched condition except that in the former, the brush stroked on the side of the index finger. In all conditions, a right hand was displayed. The motion of the paintbrush was vertical in about 90% of all trials and horizontal in about 10%. Subjects were required to press a key to report whether the number of vertical brushes was equal to 25 at the end of each trial block (see “Procedure” section that follows for details). This was to ensure that subjects were attentively observing the video presentation.
Table I.
Study design

|
Figure 1.
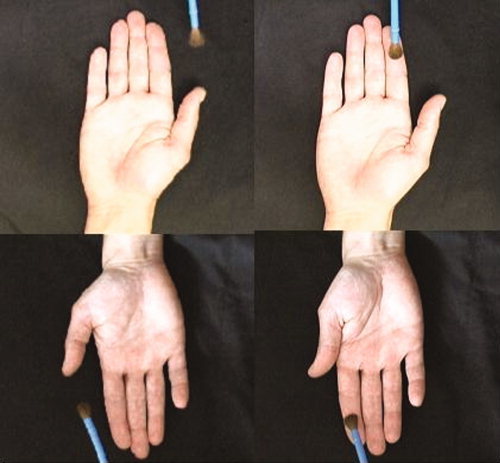
Types of stimuli used in this experiment: On the right is the touch‐condition; on the left is the nontouch condition. The upper panel depicts the hand in egocentric perspective; the lower panel depicts the allocentric perspective. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
In addition to the above four conditions, each subject also received a condition in which the index finger of their right hand was repeatedly touched by a paintbrush during the fMRI scan (i.e., the real touch condition). The manner and frequency of brushing were identical to that shown in the videos. In this condition, no visual display was presented.
Procedure
The experiment consisted of four runs. Each run included all five conditions presented in blocks of trials. Each condition was repeated in two blocks of trials, resulting in 10 trials per block. Each block was 30 s long and followed by a 10‐s resting period. The order of the trial conditions was counterbalanced using a semi Latin square design across the four runs for each subject. To ensure that subjects paid attention to the videos, we instructed the subjects to count all vertical strokes of the paintbrush and to press a “yes” button with their left index finger at the end of each trial block when the number of vertical strokes was equal to or above 25, or press a “no” button with their left middle finger when the number was below 25. The number of brush strokes differed slightly between video clips (±2, pseudorandomized) so that subjects had to attend to each video to provide accurate responses. The behavioral data were analyzed using an ANOVA with repeated measures touch observation (touch vs. nontouch) and perspective (ego‐ vs. allocentric) as within‐subject factors. All trials were included in the analysis, regardless of response accuracy.
FMRI Data Acquisition and Analysis
fMRI data were acquired with a 3T General Electric scanner. T2*‐weighted functional MR images were obtained using axially oriented echo‐planar imaging (TR = 2 s, TE = 28 ms, flip angle = 90°, slice thickness = 5 mm, number of slices = 32 [no gap]). For each subject, data were acquired in four scan runs. The first four volumes of each run were discarded to allow for T1 equilibration effects. For anatomical reference, a T1‐weighted anatomical image was obtained (3D‐SPGR, TR = 24 ms, TE = 8 ms). Visual images were back‐projected to a screen at the end of the scanner bed close to the subject's feet. Subjects viewed the images through a mirror mounted on the birdcage of the receiving coil. In addition to a head strap, foam cushions were placed tightly around the side of the subject's head to minimize head motion.
Data preprocessing and statistical analyses were carried out using SPM5 (Statistical Parametric Mapping, Wellcome Department of Imaging Neuroscience, University College London, London, UK). Individual functional images were realigned to correct for interscan movement using sinc interpolation and subsequently normalized into a standard anatomical space (MNI, Montreal Neurological Institute template) resulting in isotropic 3‐mm voxels as described previously [Friston et al.,1995]. Data were then smoothed with a Gaussian kernel of 6 mm full‐width half maximum.
Statistical parametric maps were calculated using multiple regression with the hemodynamic response function modeled in SPM5. Data analyses were performed at two levels. First, we examined data on the individual subject level by using a fixed effects model (all four runs were concatenated for each subject). Second, the resulting parameter estimates for each regressor at each voxel were then entered into a second‐level analysis with a random effects model. Statistical contrasts (t tests) were performed to examine cortical activation associated with real tactile touch (i.e., real touch–resting baseline), and observing touch versus nontouch in the egocentric and allocentric conditions. To examine common activations during real tactile stimulation and observation of touch, the contrasts between observing touch versus nontouch in the two viewing perspectives were inclusively masked by the contrast (P < 0.05) of real touch minus baseline (i.e., the resting periods between the viewing and touch conditions during which subjects were instructed to relax, not to move, and to fixate on a cross sign displayed at the center of the screen). The resulting images were thresholded at P < 0.05 (corrected for multiple comparisons over the whole brain). In addition, to verify the hypothesis that the postcentral gyrus (SI) and the parietal operculum (SII) may participate in automatic mirror activity (e.g., Blackmore et al.,2005), we report regions of interest that survived a small volume correction (SVC) of P < 0.05 (corrected) for which we had an a priori hypothesis. Thus, a SVC was applied to activations within a sphere of 5 mm radius in the postcentral gyrus (SI) and 5 mm radius in the parietal operculum (SII).
RESULTS
All participants performed the tasks well. None of the subjects reported any difficulty in detecting the horizontal movements of the paintbrush. The overall accuracy of on‐line behavioral responses across conditions was 83% (SD ±14%). There were no significant differences in subjects' performance (i.e., accuracy of stroke count) over the four conditions of action observation.
Relative to the resting baseline, fMRI data showed that the direct tactile stimulation (the real touch condition) induced activation in the left (contralateral) SI and bilateral parietal operculum [SII/parietal ventral area (PV)], as expected (see Fig. 2 and Table II for details). Similarly, observing touch relative to nontouch in both the egocentric and allocentric conditions also showed significant activation in the left SI and right parietal operculum (t test, P < 0.05, corrected, masked with effects of actual touch, see Fig. 3). Based on recent studies of the organization of cytoarchitectonic areas of the parietal operculum [e.g., Eickhof et al.,2007], we refer to the location of activations in the parietal operculum as OP4, a human homolog of primate area PV. Thus, both perspectives of observing touch engaged the somatosensory cortices.
Figure 2.
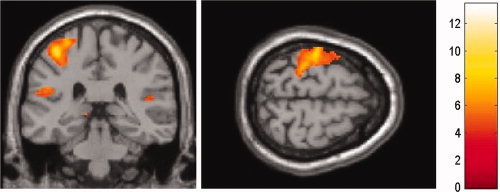
Neural activations due to real tactile stimulation relative to baseline, superimposed on a coronal MR image. Subjects were repetitively touched by a paintbrush on their right hand. The coronal and transversal slices show activations in contralateral SI and bilateral SII. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Table II.
Results of random effects analysis
| Contrast | Brain region | MNI location (x, y, z) | Peak t‐value | Number of voxels | |
|---|---|---|---|---|---|
| Realtouch‐baseline | L SI | −32, −24, 54 | 13.42 | 2,176 | |
| L SII | −46, −22, 16 | 7.91 | 749 | ||
| R SII | 58, −20, 16 | 5.93 | 111 | ||
| Egocentric perspective | Observing touch > nontouch | L SI | −44, −16, 42 | 4.23 | 28 |
| R SII | 66, −12, 26 | 5.74 | 11 | ||
| Allocentric perspective | Observing touch > nontouch | L SI | −44, −16, 42 | 3.60 | 169 |
| R SII | 64, −14, 28 | 5.67 | 11 | ||
| Observing touch > nontouch | Egocentric > allocentric | L SI | −42, −18, 48 | 3.35 | 327 |
| R SII | 64, −12, 30 | 4.76 | 11 | ||
| Allocentric > egocentric | L SI | −44, −34, 62 | 5.82 | 36 | |
| L SII | −50, −32, 24 | 2.80 | 13 |
P < 0.05, corrected, L = left hemisphere, R = right hemisphere.
Figure 3.
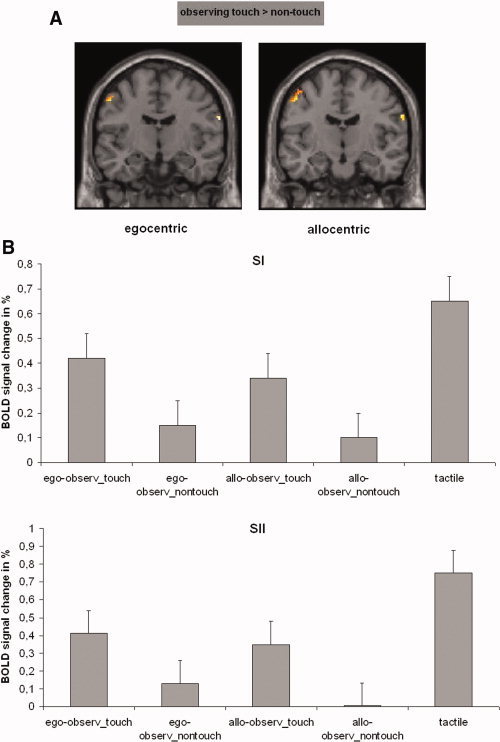
Neural activations of SI and SII while participants observed a touched hand relative to a nontouched hand. (A) Activations are superimposed on the MNI reference brain. Both perspectives yielded comparable neural activations in left SI and right SII. (B) Contrast of parameter estimates for SI and SII. Plots show parameter estimates of the relative activation compared to baseline for the five conditions (ego‐observ_touch = observing touch with egocentric view; ego‐observ_nontouch = observing nontouch with egocentric view; allo‐observ_touch = observing touch with allocentric view; allo‐observ_nontouch = observing nontouch with allocentric view; tactile = real tactile stimulation). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Further analysis of the fMRI data examining the interaction between touch and perspective masked by the effect of actual touch showed that, in contrast to the allocentric observation of touch, observing touch (touch > nontouch) in the egocentric perspective revealed activation of the anterior part of SI (Brodman Area [BA] 3a, 3b), while the allocentric perspective showed significant activation in the posterior portion of SI (BA 2, see Fig. 4 and Table II for details; paired t test, P = 0.05, corrected). This differential activation in SI was similarly observed when unsmoothed data were used (see Fig. 4). Furthermore, the paired t test showed significant activation in the right PV (OP4) for the egocentric perspective. The allocentric perspective revealed activity in the left SII/PV (OP1/OP4) (see Fig. 4 and Table II).
Figure 4.
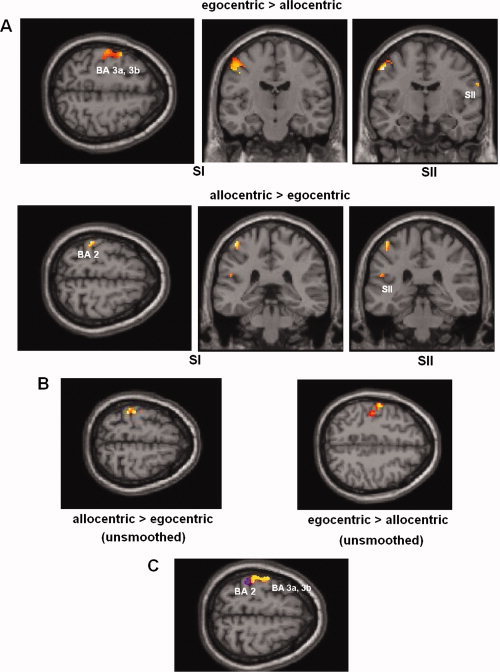
(A) Brain response for observed touch (relative to nontouch) in egocentric perspective compared with neural activations for allocentric perspective, superimposed on the MNI reference brain. Results show significant posterior activation of SI for the allocentric perspective (BA 2) relative to the egocentric perspective (BA 3a, 3b). (B) Same as (A), but here unsmoothed data has been used. Neural activations show comparable results to smoothed data. (C) Overlay of activations. Blue‐colored areas show activations when subjects observe touch in allocentric viewing perspective and yellow regions demonstrate results of the egocentric perspective (green areas show overlap). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
Subjects in this study observed a hand either being touched or not touched by a moving paintbrush. The results showed that the observation of touch relative to nontouch induced activation of contralateral SI and ipsilateral SII, both in the egocentric and the allocentric perspectives. The results are consistent with previous findings regarding activation of SI and SII when observing a touched face [e.g., Blakemore et al.,2005], providing further support for a potential mirror system of sensory observation.
While this study demonstrates that both perspectives of viewing touch are associated with activations in somatosensory cortices, they also point to differences in this activation. When contrasting both perspectives against each other, the egocentric perspective showed activation in the anterior part of SI (BA 3a, 3b). In contrast, the allocentric perspective involved significant activation of the posterior part of SI (BA 2). BA 2 has multimodal receptive fields and connections with the rostral part of the posterior parietal cortex (PPC), which receives visual input from the more caudal parts of the PPC [Iwamura,1998]. The connectivity between these regions may be associated with the activation in BA 2 of SI when observing a hand being touched. However, viewing touch in an egocentric condition may have induced self‐attribution/imagination of being touched oneself. Feeling/imagining being touched oneself may engage a slightly different neural network that requires less multimodal activation in BA 2. Since it is difficult to separate clearly SI in different BAs using fMRI, these interpretations remain speculative. Nevertheless, results of a recent study provide support to the potential of this functional dissociation. Ebisch et al. [2008] used fMRI to examine whether the tactile mirror mechanism applies to the sight of any touch irrespective of the intentionality of the observed touching agent. They found a shared neural circuitry for touch in SII and a significant difference between the sight of an intentional touch compared to an accidental touch in left SI/BA2. The activity in SI/BA2 was correlated with the degree of the seen touch. The authors concluded that this activity in SI may reflect a human tendency to resonate more with an intentional touch agent than with accidentally touched object. In our study, when touch was viewed in an allocentric viewing perspective, brain activation was observed in SI/BA2. These results are consistent with the findings reported in Ebisch et al. [2008], and further suggest that SI/BA2 may differentially respond to nonegocentric body contact.
An alternative explanation for the differential activation of SI and SII/PV with viewing conditions (i.e., egocentric vs. allocentric, and touch vs. nontouch) might relate to mental rotation of the observed hand's orientation, particularly in the allocentric condition [Creem et al.,2001]. Theoretically, subjects in the allocentric condition might have been inclined to rotate the image as it was inconsistent with the subject's own body orientation. This possibility is unlikely since mental rotation of the hand engages primarily frontoparietal regions (not activated in our study] and not the somatosensory cortices [Creem et al.,2001; Creem‐Regehr et al.,2007; Kosslyn et al.,1998; Lamm et al.,2007]. Additionally, the subject's task was simply to count the number of brush strokes, which had no performance‐related incentive for mental rotation.
The results of this study differ from a similar study conducted by Keysers et al. [2004] in which the authors did not observe any activation in SI, although they did report activation in SII for the observation of touch. They also varied the perspective of the presented body part in an egocentric versus an allocentric perspective similar to the present study; however, they did not find any impact of the viewing perspective on somatosensory activations. This difference from our results may be due to differences in the experimental design of the studies. Keysers et al. compared the neural responses when viewing a different body part, the leg being touched versus not touched, and did not observe activation in SI, restricting their analysis on possible impact of viewing perspectives to SII. The lack of activation in SI in the Keysers et al. study may be explained by the fact that they used videos depicting the touch (or nontouch) of legs instead of hands, as we did. Using hands (or faces in the Blakemore et al. study) might have evoked stronger somatosensory activations than using legs [Blakemore et al.,2005]. On‐line behavioral monitoring [i.e., counting brush strokes in the current study; also see Blakemore et al. [2005] and Ebisch et al. [2008] may also have enhanced attentiveness of subjects to the viewing stimuli, an issue that we cannot discuss since the authors did not report behavioral monitoring in their study. Consistent with our results, both Blakemore et al. and Ebisch et al. reported significant activation in SI when observing touch to a face or a hand. However, the experimental design of the Blakemore et al. study did not allow manipulating viewing perspectives, which were explicitly controlled in our investigation. With respect to activations in SII, the present results also differed from previous reports [Blakemore et al.,2005; Keysers et al.,2004] in that previous studies showed bilateral activation of SII/PV, while the present results only showed ipsilateral activation in these regions. Unfortunately, the design of the current study does not provide independent evidence for resolving this difference. Future studies may be able to shed light on the reasons for this difference.
Why was SI/BA2 more strongly activated when observing a touched hand that probably belongs to somebody else? Consistent with Ebisch et al. [2008], we argue that this region may reflect simulation of proprioceptive aspects linked to the seen touch, which is in line with the theories of a mirror neuron network that enables us to understand others. However, recent studies on the mirror neuron system for action observation suggest stronger responses when the observed action is part of the observer's own motor repertoire [e.g., Calvo‐Merino et al.,2006]. Here, touch on a hand is a very familiar situation for all of us. Moreover, it seems difficult to find a specific pool of touch situations relevant only for specific individuals. Thus, the differences in first‐ and third‐person perspectives found in studies on action observation do not seem to be comparable with the observation of touch in the present study. Future studies are necessary to sort out the underlying mechanisms differentiating the effect of observation perspectives on the mirror systems for action versus touch observation.
Further, Keysers et al. as well as Ebisch et al. demonstrated somatosensory activations when touch is observed irrespective of what is being touched, animate subjects or inanimate objects. The present study examined only touch of an animate object (hand). It remains an issue whether the activation of SI/BA2 would be similar irrespective of the animacy of the observed object. In contrast to the mirror system for actions, it is possible that the mirror system for touch is not limited to a social context, consistent with previous results [Ebisch et al.,2008; Keysers et al.,2004].
Mirror neurons for action observation, emotion, and pain have been reported in previous studies [Carr et al.,2003; Rizzolatti et al.,2001; Singer et al.,2004]. In action observation, mirror neurons in premotor cortex were observed to discharge automatically when observing another individual performing goal‐directed action. A similar mirror neuron system for sensory observation may also exist. The activation of the sensory system may be linked to cortical regions containing bimodal neurons in the parietal lobe or in premotor cortex, which are sensitive both for vision and touch [e.g., Bremmer et al.,2001; Iriki et al.,1996]. These neurons may also serve as a mirror system for observed touch similar to the mechanisms operating for the mirror system of observed actions, emotion, and pain. Our results further suggest that the somatosensory mirror system may be automatically, but differentially, activated depending on whether it is in a self‐consistent or self‐inconsistent visual perspective. This may be crucial in designing future studies investigating the automatic nature of the mirror neuron system for sensory observation.
REFERENCES
- Blakemore S‐J,Bristow D,Bird G,Frith C,Ward J ( 2005): Somatosensory activations during the observation of touch and a case of vision‐touch synaesthesia. Brain 128: 1571–1583. [DOI] [PubMed] [Google Scholar]
- Bremmer F,Schlack A,Shah NJ,Zafiris O,Kubischik M,Hoffmann K,Zilles K,Fink GR ( 2001): Polymodal motion processing in posterior parietal and premotor cortex: a human fMRI study strongly implies equivalencies between humans and monkeys. Neuron 29: 287–296. [DOI] [PubMed] [Google Scholar]
- Buccino G,Binkofski F,Fink GR,Fadiga l,Fogassi L,Gallese V,Seitz RJ,Zilles K,Rizzolatti G,Freund HJ ( 2001): Action observation activates premotor and parietal areas in a somatotopic manner: a fMRI study. Eur J Neurosci 13: 400–404. [PubMed] [Google Scholar]
- Calvo‐Merino B,Grèzes J,Glaser DE,Passingham RE,Haggard P ( 2006): Seeing or doing? Influence of visual and motor familiarity in action observation. Curr Biol 16: 1905–1910. [DOI] [PubMed] [Google Scholar]
- Carr L,Iacoboni M,Dubeau MC,Mazziotta JC,Lenzi GL ( 2003): Neural mechanisms of empathy in humans: A relay from neural systems for imitation to limbic areas. Proc Natl Acad Sci USA 100: 5497–5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creem SH,Downs TH,Wraga M,Harrington GS,Proffitt DR,Downs JH 3rd ( 2001): An fMRI study of imagined self‐rotation. Cogn Affect Behav Neurosci 1: 239–249. [DOI] [PubMed] [Google Scholar]
- Creem‐Regehr SH,Neil JA,Yeh HJ ( 2007): Neural correlates of two imagined egocentric transformations. Neuroimage 35: 916–927. [DOI] [PubMed] [Google Scholar]
- Ebisch SJ,Perruci MG,Ferretti A,Del Gratta C,Romani GL,Gallese V ( 2008): The sense of touch: Embodied simulation in a visuotactile mirroring mechanism for observed animate or inanimate touch. J Cogn Neurosci 20: 1–13. [DOI] [PubMed] [Google Scholar]
- Eickhof SB,Grefkes C,Zilles K,Fink GR ( 2007): The somatotopic organization of cytoarchitectonic areas on the human parietal operculum. Cereb Cortex 17: 1800–1811. [DOI] [PubMed] [Google Scholar]
- Fadiga L,Fogassi L,Pavesi G,Rizzolatti G ( 1995): Motor facilitation during action observation: A magnetic stimulation study. J Neurophysiol 73: 2608–2611. [DOI] [PubMed] [Google Scholar]
- Friston KJ,Worsley K,Poline JP,Frith CD,Frackowiak RSJ ( 1995): Statistical parametric maps in functional imaging: A general linear approach. Human Brain Mapp 2: 189–210. [Google Scholar]
- Gangitano M,Mottaghy FM,Pascual‐Leone A ( 2001): Phase‐specific modulation of cortical motor output during movement observation. Neuroreport 12: 1489–1492. [DOI] [PubMed] [Google Scholar]
- Grafton ST,Arbib MA,Fadiga L,Rizzolatti G ( 1996): Localization of grasp representations in humans by positron emission tomography. 2. Observation compared with imagination. Exp Brain Res 112: 103–111. [DOI] [PubMed] [Google Scholar]
- Graziano MS ( 1999): Where is my arm? The relative role of vision and proprioception in the neuronal representation of limb position. Proc Natl Acad Sci USA 96: 1021–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano MS,Cooke DF,Taylor CSR ( 2000): Coding the location of the arm by sight. Science 290: 1782–1786. [DOI] [PubMed] [Google Scholar]
- Hari R,Forss N,Avikainen S,Kirveskari E,Salenius S,Rizzolatti G ( 1998): Activation of human primary motor cortex during action observation: A neuromagnetic study. Proc Natl Acad Sci USA 95: 15061–15065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M,Dapretto M ( 2006): The mirror neuron system and the consequences of its dysfunction. Nat Rev Neurosci 7: 942–951. [DOI] [PubMed] [Google Scholar]
- Iacoboni M,Woods RP,Brass M,Bekkering H,Mazziotta JC,Rizzolatti G ( 1999): Cortical mechanisms of human imitation. Science 286: 2526–2528. [DOI] [PubMed] [Google Scholar]
- Iacoboni M,Molnar‐Szakacs I,Gallese V,Buccino G,Mazziotta JC,Rizzolatti G ( 2005): Grasping the intentions of others with one's own mirror neuron system. PLoS Biol 3: 529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iriki A,Tanaka M,Iwamura Y ( 1996): Coding of modified body schema during tool use by macaque postcentral neurones. Neuroreport 7: 2325–2330. [DOI] [PubMed] [Google Scholar]
- Iwamura Y ( 1998): Hierarchical somatosensory processing. Curr Opin Neurobiol 8: 522–528. [DOI] [PubMed] [Google Scholar]
- Keysers C,Wicker B,Gazzola V,Anton J‐L,Fogassi L,Gallese V ( 2004): A touching sight: SII/PV activation during the observation and experience of touch. Neuron 42: 335–346. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM,DiGirolamo GJ,Thompson WL,Alpert NM ( 1998): Mental rotation of objects versus hands: Neural mechanisms revealed by positron emission tomography. Psychophysiology 35: 51–161. [PubMed] [Google Scholar]
- Lamm C,Windischberger C,Moser E,Bauer H ( 2007): The functional role of dorso‐lateral premotor cortex during mental rotation: An event‐related fMRI study separating cognitive processing steps using a novel task paradigm. Neuroimage 36: 1374–1386. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G,Fadiga L,Gallese V,Fogassi L ( 1996a) Premotor cortex and the recognition of motor actions. Cogn Brain Res 3: 131–141. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G,Fadiga L,Matelli M,Bettinardi V,Paulesu E,Perani D,Fazio F ( 1996b) Localization of grasp representations in humans by PET. 1. Observation versus execution. Exp Brain Res 111: 246–252. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G,Fogassi L,Gallese V ( 2001): Neurophysiological mechanisms underlying the understanding and imitation of action. Nat Rev Neurosci 2: 661–670. [DOI] [PubMed] [Google Scholar]
- Singer T,Seymour B,O'Doherty J,Kaube H,Dolan RJ,Frith CD ( 2004): Empathy for pain involves the affective but not sensory components of pain. Science 303: 1157–1162. [DOI] [PubMed] [Google Scholar]
- Taylor‐Clarke M,Kennett S,Haggard P ( 2002): Vision modulates somatosensory cortical processing. Curr Biol 12: 233–236. [DOI] [PubMed] [Google Scholar]
- Zhou YD,Fuster JM ( 1997): Neuronal activity of somatosensory cortex in a cross‐modal (visuo‐haptic) memory task. Exp Brain Res 116: 551–555. [DOI] [PubMed] [Google Scholar]
- Zhou YD,Fuster JM ( 2000): Visuo‐tactile cross‐modal associations in cortical somatosensory cells. Proc Natl Acad Sci USA 97: 9777–9782. [DOI] [PMC free article] [PubMed] [Google Scholar]


