Abstract
Individualism and collectivism refer to cultural values that influence how people construe themselves and their relation to the world. Individualists perceive themselves as stable entities, autonomous from other people and their environment, while collectivists view themselves as dynamic entities, continually defined by their social context and relationships. Despite rich understanding of how individualism and collectivism influence social cognition at a behavioral level, little is known about how these cultural values modulate neural representations underlying social cognition. Using cross‐cultural functional magnetic resonance imaging (fMRI), we examined whether the cultural values of individualism and collectivism modulate neural activity within medial prefrontal cortex (MPFC) during processing of general and contextual self judgments. Here, we show that neural activity within the anterior rostral portion of the MPFC during processing of general and contextual self judgments positively predicts how individualistic or collectivistic a person is across cultures. These results reveal two kinds of neural representations of self (eg, a general self and a contextual self) within MPFC and demonstrate how cultural values of individualism and collectivism shape these neural representations. Hum Brain Mapp, 2009. © 2008 Wiley‐Liss, Inc.
Keywords: fmri, social cognition, culture, self
INTRODUCTION
One of the most enduring enigmas of modern psychology is to understand the nature of the self. Over the past 30 years, anthropologists and cultural psychologists have argued that a fundamental way in which cultural values shape psychological processes is in how people define themselves and their relation to others in their environment [Markus and Kitayama, 1991; Nisbett et al., 2001; Oyserman et al., 2002; Triandis, 1995]. In particular, cultural psychologists have identified two primary styles of self construal across cultures, individualism and collectivism [Markus and Kitayama, 1991; Oyserman et al., 2002; Triandis, 1995]. People who endorse individualistic values think of people as independent of each other and describe individuals using stable personality traits (eg, I am honest) rather than situation‐specific attributes. By contrast, people who endorse collectivistic values think of people as highly interconnected to one another and describe themselves and others as embedded in a specific social context or situations (eg, When talking to my mother, I am honest) rather than using generic trait adjectives. The notion of self‐construal style (SCS) is thought to originate from cultural divergences in Western and East Asian philosophical views of the self [Markus and Kitayama, 1991; Oyserman et al., 2002; Triandis, 1995]. However, people from Western and East Asian cultures have also been shown to demonstrate varying degrees of individualism and collectivism. In particular, recent meta‐analytic evidence indicates that native Japanese are not as collectivistic as Caucasian‐Americans [Oyserman et al., 2002], a phenomenon which likely stems from the adaptive nature of individuals across Western and East Asian nations to endorse cultural values to varying extents, depending on their degree of sensitivity to cultural norms [Oyserman et al., 2002]. Despite rich understanding of the importance of individualism and collectivism at a behavioral level, the impact of SCS on neurobiological processes remains unclear.
Activity within the anterior rostral portion of the medial prefrontal cortex (MPFC) is thought to reflect the neural basis of self‐knowledge [Amodio and Frith, 2006; Gillihan and Farah, 2005; Kelley et al., 2002; Macrae et al., 2004; Northoff et al., 2006]. The anterior rostral portion of MPFC response reliably increases when viewing general trait descriptions about one's self (eg, I am honest) relative to general trait descriptions about others who are familiar, such as Bill Clinton (eg, Bill Clinton is honest) [Amodio and Frith, 2006; Gillihan and Farah, 2005; Kelley et al., 2002; Macrae et al., 2004; Northoff et al., 2006]. A recent neuroimaging study found no difference in MPFC activation in Chinese subjects when viewing general trait descriptions of one's self or a close other, such as one's mother (eg, Your mother is honest), suggesting the possibility of cultural variation in neural representations of general traits for self and other [Zhu et al., 2007]. However, prior studies to date have focused solely on neural representations of one's own and others' personality traits and not on the knowledge of self and others embedded within particular contexts or situations. Moreover, none have directly measured SCS and its relation to neural representations of self. Although it is well established that people vary in their SCS and that MPFC serves as a neural substrate of self‐knowledge, it remains unknown whether SCS affects neural representations of the self.
Here, we used cross‐cultural functional magnetic resonance imaging (fMRI) in the United States and Japan to examine whether MPFC responses during general trait and contextual trait judgments vary as a function of SCS. Based on a prior work demonstrating that individualistic and collectivistic individuals think of themselves in either a general or contextual fashion, respectively, and that activity in MPFC reflects self‐processing, we hypothesized that individualists would show a greater response for general self‐descriptions but that collectivists would show a greater response for contextual self‐descriptions within the anterior rostral portion of the MPFC region.
MATERIALS AND METHODS
Participants
Twenty‐four right‐handed university students with normal or corrected‐to‐normal vision had participated in this study for payment. Half of the 24 participants were native Japanese young adults (7 males, 5 females; M = 24 years, SD = 5.3 years) living in Nagoya, Japan and the remaining half of the participants were Caucasian‐American young adults (7 males, 5 females; M = 21.1 years, SD = 2.27 years) living in Chicago, IL. Informed written consent was obtained from each participant prior to the experiment.
Stimuli
Stimuli consisted of 72 black and white photographs (512 × 384 pixels) consisting of 24 general self‐descriptions, 24 contextual self‐descriptions and 24 self‐descriptions in either italicized or non‐italicized font. Two identical sets of stimuli were created, one written in English and one written in Japanese, to present participants with stimuli in their native language. Accurate translation of materials was verified by a bilingual translator.
Procedure
We employed a mixed block design consisting of 12 blocks within a functional run. There were three types of blocks consisting of either general self‐descriptions, contextual self–descriptions, or self‐descriptions that varied in font style (ie, italicized or plain). Each block consisted of six unique trials of that block type. For each trial, a general self, contextual self or font description was displayed for 4,000 ms. Trials were separated by a centered fixation cross which was presented in a jittered manner ranging from 2,000 to 6000 ms (average duration of ITI for each block = 4,000 ms).
During scanning, participants completed two types of self judgments, a general self task (eg, in general, does this sentence describe you?) and contextual self task (eg, does this sentence describe you when you are talking to your mother?), as well as a font judgment task which served as a control (eg, is this sentence written in italics?) (see Fig. 1). Prior to entering the scanner, participants were shown examples of each type of task and given a practice trial to become familiar with all the three tasks. After scanning, all participants completed the 24‐item Self‐Construal Scale [Singelis, 1994] in their native language to assess each individual participant's degree of endorsement of individualistic and collectivistic values on a Likert scale from 1 (strongly disagree) to 7 (strongly agree).
Figure 1.
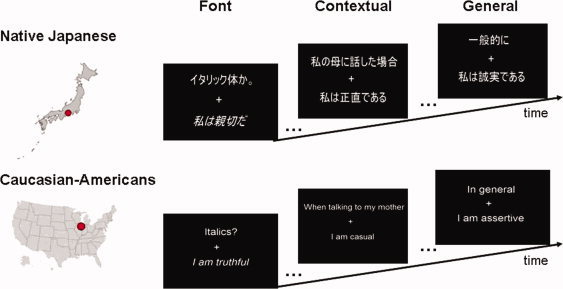
Example of experimental stimuli used in self‐judgment task. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Definition of Individualism and Collectivism
Given prior cultural psychological evidence that cultural affiliation (eg, American or Japanese) and nationality (eg, US or Japan) are not necessarily reliable predictors of individualism and collectivism, respectively [Oyserman et al., 2002]; we did not assume participants' individualism and collectivism solely by their cultural affiliation or nationality (eg, being Caucasian‐American or native Japanese individual) in the current study.
Instead, we defined individualism and collectivism based on participants' self‐report on the Singelis [1994] Self‐construal Scale. Participants' SCS indices were used in the current experiment in two ways. First, we used participants' SCS index to divide participants into two groups: individualists and collectivists. To calculate each participants' SCS index, mean agreement for the twelve individualistic (IND) and twelve collectivistic (COL) items in the Self‐Construal Scale were calculated for each participant. Each participant's SCS index was then calculated using a simple scoring algorithm [SCS index = M of agreement for IND items minus M of agreement for COL items]. Participants with a positive SCS index (ie, > agreement for IND items) were placed in the IND group; participants with a negative SCS index (ie, > agreement for COL items) were placed in the COL group. The IND group consisted of 7 Japanese and 3 Caucasian participants (SCS index, M = 0.56, SD = 0.38); the COL group consisted of 5 Japanese and 9 Caucasian participants (SCS index, M = −0.54, SD = 0.38).
Second, we used participants' SCS indices as a continuous variable to conduct whole‐brain regression analyses to explore the strength of the relationship between SCS and neural activity during general and contextual self‐descriptions.
Imaging Parameters
Functional brain images were acquired at two facilities, the Center for Advanced Magnetic Resonance Imaging (CAMRI) facility located in the Northwestern Medical Hospital in Chicago, IL and the National Institute for Physiological Sciences (NIPS) in Okazaki, Japan.
Scanning at CAMRI occurred on a comparable 3.0‐Tesla Siemens Trio MRI scanner and scanning at NIPS occurred on a 3.0‐Tesla Siemens Allegra MRI scanner. We acquired functional images by using T2*‐weighted, gradient echo, echo planar imaging sequences [repetition time (TR) = 2,000 ms; echo time (TE) = 25 ms; flip angle = 70°; FOV = 20 cm, 64 × 64 matrix; 34 slices; voxel size = 3.0 × 3.0 × 3.0 mm3]. A high‐resolution anatomical T1‐weighted image was also acquired [TR = 2,300 ms; TE = 2.91 ms; flip angle = 9°; FOV = 256 mm; 256 × 256 matrix; 176 slices; voxel size = 1.0 × 1.0 × 1.0 mm3] for each subject.. All stimuli were presented using Presentation software (Neurobehavioral Systems, Albany, CA) and projected onto a half‐transparent viewing screen located behind the head coil. Subjects viewed the projected stimuli through a mirror.
Imaging Processing and Statistical Analysis
Functional images were analyzed using SPM2 software (Wellcome Department of Imaging Neuroscience, London, UK) implemented in Matlab (Mathworks, Cherborn, MA). The first six volumes were discarded due to unsteady magnetization, and all of the remaining volumes were realigned spatially to the first volume and a mean image was created. After a high‐resolution image was coregistered onto the mean image, all volumes were normalized to the Montreal Neurological Institute (MNI) space using a transformation matrix obtained from the normalization process of the high‐resolution image of each individual subject to the MNI template. The normalized images were then spatially smoothed with an 8‐mm Gaussian kernel.
After preprocessing, statistical analysis for each individual subject was conducted using the general linear model [Friston et al., 1995]. At the first level, each block of trials was modeled by convolving with a hemodynamic response function. For each subject, a linear regressor was applied to filter noise. In the subtraction analysis, three task conditions [font, general, and contextual] were modeled separately, including fixation. A whole brain, voxel‐wise random effects analysis was conducted with two factors (cultural group: IND or COL and type of judgment: general self, contextual self, or font). To examine our hypotheses, we examined the following main effect and interaction contrasts of interest: general > contextual and contextual > general; IND > COL and COL > IND; [(INDgeneral + COLcontextual)] – [(INDcontextual + COLgeneral)] with a threshold of P < 0.005, extant threshold = 15 voxels.
Additionally, regions‐of‐interest (ROI) analyses were conducted within our primary region of interest, namely the anterior rostral portion of MPFC, by using the average maxima obtained from a meta‐analysis of 10 prior neuroimaging studies of self‐knowledge as the center of a small volume (defined as a spherical ROI with a 8‐mm radius centered at X = 0, Y = 51, Z = 9) [Amodio and Frith, 2006]. ROI analyses were performed within this spherical ROI using Marsbar software (http://marsbar.sourceforge.net/).
To determine the strength of the relationship between SCS and MPFC activations during self‐judgments, a whole‐brain correlation analysis was performed using the contrast image [contextual minus general] with a SCS index [COL score minus IND score] as the covariate of interest. To test hypotheses about regional specific covariate effects, the estimates were compared using a positive linear contrast with significance levels at P < 0.005, extant threshold = 15 voxels.
Small‐volume correction for multiple comparisons was applied for the anterior rostral portion of MPFC by using the spherical ROI with a 8‐mm radius centered at X = 0, Y = 51, Z = 9 [Amodio and Frith, 2006]. Corrected P values reported later refer to this small volume correction procedure. MNI coordinates were converted to Talairach using a nonlinear transformation (http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach). Brodmann areas and brain regions were identified based on the Talairach Atlas [Talairach and Tournoux, 1988].
Cross‐Site Scanner Comparison Analysis
Given the use of two scanner site facilities (eg, CAMRI and NIPS) to acquire functional images, it is possible that variation in scanner performance between these two facilities may lead to variation in neural activation patterns across the two cultural groups. However, several prior neuroimaging studies have demonstrated several approaches to measuring and minimizing cross‐site variation in fMRI data collected from multicenter fMRI sites [Friedman and Glover, 2006]. Here, we accounted for the possibility of systematic, site‐dependent effects in fMRI sensitivity between the two scanner facilities in two ways. First, functional neuroimaging data was collected from two scanner sites using nearly identical vendor's instrumentation and imaging parameters, which has previously been shown to result in negligible interscanner variability [Friedman and Glover, 2006].
Second, we conducted an interscanner reliability test by scanning a separate cohort of four participants (1 female, 3 males, M = 33.8 years, SD = 9.7 years) at both scanner facilities within 6 months time. At both sites, participants were scanned while completing the self‐judgment task. Prior scanner variability studies have also included a small sample size of participants who were tested at each site on the same functional task [Friedman and Glover, 2006]. A cross‐site scanner comparison of signal‐to‐noise ratio (SNR) [Parrish et al., 2000] and % signal change analysis within the MPFC, defined as a spherical ROI with a 8‐mm radius centered on X = 0, Y = 44, Z = 6, was conducted on functional images acquired during the self‐judgment task. Results indicated no significant difference in SNR, t (3) = −1.04, P = 0.18, or % signal change within MPFC, F (1, 3) = 0.22, P = 0.67, across scanner sites. We conclude that there exists no significant variation in scanner performance between the two scanner facilities used to acquire functional images. Hence, variation in scanner site performance is not a likely explanation for any variability in neural activation observed within the MPFC region between the two cultural groups.
RESULTS
Behavioral Results
Reaction time
Behavioral results indicate a significant main effect of judgment type on reaction time, F (2, 40) = 114.22, P < 0.0001 (see Table I). Participants were fastest when performing font judgments relative to contextual, t (23) = 15.99, P < 0.0001, and general self‐judgments, t (23) = 11.12, P < 0.0001. Participants were also significantly faster in making general self‐judgments relative to contextual self‐judgments, t (23) = 5.52, P < 0.0001. However, there were no significant two‐way interactions between SCS and judgment type, culture of participant and judgment type or three‐way interaction between SCS, culture of participant, and judgment type on reaction time (all Ps > 0.30).
Table I.
Behavioral performance during self‐judgment task (Mean ± SE)
| Individualism (IND) | Collectivism (COL) | |
|---|---|---|
| Proportion agreement | ||
| General | 0.77 (0.05) | 0.77 (0.04) |
| Contextual | 0.62 (0.04) | 0.65 (0.04) |
| Font | 0.49 (0.03) | 0.54 (0.03) |
| Reaction time (ms) | ||
| General | 1,766 (112) | 1,640 (90) |
| Contextual | 2,019 (88) | 1,902 (71) |
| Font | 1,269 (97) | 1,188 (79) |
Proportion agreement
There was a significant main effect of judgment type on proportion agreement during self‐judgments, F (2, 40) = 29.28, P < 0.0001 (see Table I). Overall, participants agreed less with font judgments relative to contextual, t (23) = 3.17, P < 0.004 and general self‐judgments, t (23) = 6.78, P < 0.0001. Participants also agreed more with general self‐judgments relative to contextual self‐judgments, t (23) = 5.48, P < 0.0001. However, there were no significant two‐way interactions between SCS and judgment type, cultural affiliation of participant and judgment type or three‐way interaction between SCS, cultural affiliation of participant, and judgment type on proportion agreement (all Ps > 0.05).
Neuroimaging Results
Main effect of individualism and collectivism
Individualists showed greater activation in bilateral thalamus, right putamen, bilateral cuneus, right insula, bilateral cerebellum, and right superior frontal gyrus during self‐judgments relative to collectivists (Table II). By contrast, collectivists showed greater activation in left middle temporal gyrus during self‐judgments relative to individualists (Table II).
Table II.
fMRI results of main effect of IND and COL
| X | Y | Z | Z score | BA | Brain area |
|---|---|---|---|---|---|
| Individualism (IND) > Collectivism (COL) | |||||
| 9 | −11 | 12 | 3.66 | R thalamus | |
| 30 | −6 | −2 | 3.57 | R putamen | |
| −15 | −17 | 15 | 3.36 | L thalamus | |
| −3 | −49 | 11 | 3.33 | 31 | L cuneus |
| 24 | 6 | 11 | 3.18 | R insula | |
| 15 | −84 | 10 | 3.17 | 18 | R cuneus |
| −36 | −71 | −17 | 3.13 | L cerebellum | |
| 30 | −77 | −16 | 3.08 | R cerebellum | |
| 15 | 65 | 11 | 3.01 | 10 | R superior frontal gyrus |
| Collectivism (COL) > Individualism (IND) | |||||
| −45 | −9 | −15 | 3.09 | 21 | L middle temporal gyrus |
Main effect of type of self judgment
Across all participants, greater activation was found in bilateral primary somatosensory cortex (BA 1–3) as well as primary motor cortex, left cerebellum, and cingulate gyrus in response to general relative to contextual self‐descriptions (see Table III). By contrast, greater activation was found in bilateral middle temporal gyrus, bilateral thalamus, right dorsolateral prefrontal cortex, left insula, right cingulate gyrus, right cerebellum, left cuneus, right middle frontal gyrus, right superior temporal gryus and secondary motor cortex in response to contextual relative to general self‐descriptions (see Table III).
Table III.
fMRI results of main effect of type of self‐judgments
| X | Y | Z | Z score | BA | Brain area |
|---|---|---|---|---|---|
| General > Contextual | |||||
| −50 | −6 | −47 | 3.81 | 4 | L precentral gyrus |
| 56 | −21 | 51 | 3.69 | 1/2/3 | R postcentral gyrus |
| −56 | −18 | 40 | 3.22 | 1/2/3 | L postcentral gyrus |
| −12 | −39 | −18 | 3.08 | L cerebellum | |
| 0 | 34 | −12 | 2.89 | 21 | Cingulate gyrus |
| Contextual > General | |||||
| 56 | −29 | −4 | 4.72 | 21 | R middle temporal gyrus |
| 15 | −23 | 15 | 4.25 | R thalamus | |
| −53 | 2 | −18 | 4.16 | 21 | L middle temporal gyrus |
| −18 | −26 | 12 | 4.06 | L thalamus | |
| 36 | 48 | 22 | 3.86 | 46 | R middle frontal gyrus |
| 3 | 23 | 46 | 3.86 | 8 | R medial frontal gyrus |
| −33 | 14 | −3 | 3.83 | L insula | |
| 6 | −37 | 27 | 3.56 | 39 | R cingulate gyrus |
| 50 | 24 | 7 | 3.55 | 46 | R inferior frontal gyrus |
| 33 | −71 | −27 | 3.53 | R cerebellum | |
| 3 | 36 | 29 | 3.51 | 9 | R medial frontal gyrus |
| 3 | −73 | 4 | 3.38 | 18 | L cuneus |
| 3 | 10 | 24 | 3.25 | 32 | R cingulate gyrus |
| 21 | 52 | −10 | 3.07 | 10 | R middle frontal gyrus |
| 42 | −40 | 10 | 3.00 | 22 | R superior temporal gyrus |
| 39 | −7 | 36 | 3.00 | 6 | R precentral gyrus |
| 24 | 6 | 52 | 2.91 | 6 | R middle frontal gyrus |
Relationship between SCS and self judgment
Consistent with our neural hypotheses, Figure 2A shows the observed significant interaction between SCS and type of self judgment in neural response within the anterior rostral region of MPFC (X = 3, Y = 45, Z = 9; P < 0.05 corrected, see Table IV). Region‐of‐interest analyses indicated that individualists showed significantly greater activation for general self‐descriptions relative to contextual self‐descriptions, t (9) = 2.44, P < 0.05, and font judgments, t (9) = 4.57, P < 0.001, whereas collectivists demonstrated significantly greater activation for contextual self‐descriptions compared to general self‐descriptions, t (13) = 2.96, P < 0.05, and font judgments, t (13) = 4.07, P < 0.001 (Fig. 2 B). A significant interaction between SCS and type of self judgment in neural response was also found in bilateral parahippocampal gyri, right middle temporal gyrus, and left superior occipital gyrus (see Table IV). We did not observe a significant interaction between cultural affiliation and neural response during different judgment tasks within the anterior rostral portion of the MPFC, F (2, 44) = 0.99, P = 0.38.
Figure 2.

(A) fMRI result of interaction contrast [(INDgeneral + COLcontextual) − (INDcontextual + COLgeneral)] reveals increased activity in the anterior rostral portion of medial prefrontal cortex (MPFC) (peak coordinate: X = 3, Y = 45, Z = 9, P < 0.05 corrected). (B) fMRI region‐of‐interest analysis within this MPFC region. Individualists show greater MPFC response for general relative to contextual trait and font descriptions. Collectivists show greater MPFC response for contextual relative to general trait and font descriptions. (C) Scatterplot of the correlation of MPFC activity [Δ for contextual−general] with self‐construal style index [COL score−IND score] for each participant (R = 0.70, P < 0.003).
Table IV.
fMRI results of interaction contrast [(INDgeneral + COLcontextual) − (INDcontextual + COLgeneral)]
| X | Y | Z | Z score | BA | Brain area |
|---|---|---|---|---|---|
| 53 | −27 | −6 | 3.59 | 21 | R middle temporal gyrus |
| −33 | −82 | 24 | 3.43 | 19 | L superior occipital gyrus |
| −24 | −24 | −19 | 3.41 | 35 | L parahippocampal gyrus |
| 3 | 44 | 6 | 2.92 | 32 | R medial prefrontal cortex |
| 18 | −27 | −21 | 2.84 | 35 | R parahippocampal gyrus |
Whole‐brain regression analyses further revealed that degree of MPFC response to self‐judgments (contextual minus general) correlated positively and significantly with degree of SCS (COL minus IND), R = 0.70, P < 0.003 (Fig. 2 C). A significant, positive correlation between neural activity and SCS was also observed within a number of temporal and frontal regions including bilateral parahippocampal gyri as well as left superior, middle, and inferior temporal gyrus, right inferior and superior frontal gyrus, right fusiform, and right cerebellum (see Table V). There was no significant relationship between MPFC response and reaction time or proportion agreement during self judgments (all Ps > 0.05).
Table V.
fMRI results of whole‐brain correlation analysis of self‐judgment contrast image [contextual‐general] with self‐construal style index [COL score–IND score]
| X | Y | Z | Z score | BA | Brain area |
|---|---|---|---|---|---|
| −59 | −37 | 16 | 4.13 | 22 | L superior temporal gyrus |
| 0 | 44 | 6 | 3.75 | 32 | Medial prefrontal cortex |
| −33 | −86 | 24 | 3.59 | 19 | L superior occipital gyrus |
| −24 | −24 | −19 | 3.34 | 35 | L parahippocampal gyrus |
| 33 | −82 | −16 | 3.21 | 19 | R fusiform gyrus |
| 15 | −46 | 8 | 3.13 | 29 | R posterior cingulate gyrus |
| 62 | −1 | −13 | 3.09 | 21 | R middle temporal gyrus |
| 15 | −27 | −24 | 2.99 | 35 | R parahippocampal gyrus |
| −39 | −28 | 21 | 2.70 | 13 | L insula |
DISCUSSION
Our results show that self‐relevant processing within MPFC varies as a function of SCS. People who endorse individualistic cultural values showed greater MPFC activation to general self‐descriptions, whereas people who endorse collectivistic cultural values showed greater MPFC activation to contextual self‐descriptions. Furthermore, degree of agreement with individualistic or collectivistic values predicted MPFC response to general or contextual self‐descriptions, respectively. Behavioral results indicate this modulation of activity within the MPFC region by SCS is not due to either task difficulty or degree of agreement with general or contextual self‐descriptions. Neural results further indicate that MPFC was not activated to a greater extent by either SCS (eg, individualistic or collectivistic) or type of self‐description (eg, general or contextual) alone.
Instead, modulation of neural activity within the anterior rostral portion of the MPFC by SCS likely reflects enhanced explicit general and contextual representations of self‐knowledge in individualists and collectivists, respectively. Prior neuroimaging studies have shown that MPFC plays a unique role in the formation, storage, and retrieval of self‐knowledge. For instance, activity within MPFC has been shown to predict subsequent recognition memory for self‐relevant traits [Macrae et al., 2004] suggesting that the MPFC enhances encoding of self‐relevant information during the formation and storage of self‐knowledge. Greater MPFC response has also been found for familiar photos when participants recognized photos as taken by themselves relative to photos taken by other participants [Cabeza and St. Jacques, 2007] as well as when participants recall autobiographical memories relative to semantic [Cabeza and St. Jacques, 2007] or episodic [Gilboa, 2004] memories. We suggest that heightened activation within MPFC for general self‐descriptions for individualists and contextual self‐descriptions for collectivists reflects the role that SCS plays in how knowledge about the self is formed, and possibly also stored and retrieved. Similarly, in the current study, activation in bilateral parahippocampal gyri to general versus contextual self‐descriptions was also modulated by SCS and positively correlated with degree of individualism and collectivism, respectively. Given the importance of bilateral parahippocampal gyri during memory formation for verbal experiences [Wagner et al., 1998], including those involving the self [Macrae et al., 2004], this finding compliments the notion that knowledge representations of one's self for individualists (eg, general self‐representations) and collectivists (eg, contextual self‐representations) are culturally‐specific at the neural level. Finally, we also observed greater neural activity within right superior frontal gyrus during self judgments in individualists relative to collectivists. We speculate that greater activity within right superior frontal gyrus observed in the current study may reflect evidence of enhanced self‐relevant processing in individualists relative to collectivists. Future studies are needed to further elucidate how cultural values of individualism and collectivism, as well as other group traits that vary across cultures such as social hierarchy and communalism, affect neural processing during social cognition.
An intriguing aspect of the current findings is that participants' cultural values (eg, individualism or collectivism), and not necessarily their cultural affiliation (eg, being Caucasian‐American or native Japanese), modulated neural response within MPFC during self judgments. Our results are consistent with a recent meta‐analysis of cross‐cultural behavioral studies of SCS demonstrating that native Japanese are not necessarily more collectivistic relative to Caucasian‐Americans [Oyserman et al., 2002] but more generally, that cross‐cultural differences in individualism and collectivism are not static [Oyserman and Lee, 2008]. Although some aspects of culture groups remain stable across time, other cultural traits are dynamic, evolving across macro‐ (eg, generations, lifespan) [Chiao and Ambady, 2007; Li, 2003; Mesoudi et al., 2006] and micro‐level (eg, situations) time scales [Oyserman and Lee, 2008; Gardner et al., 1999]. From early philosophical divergence in Western and East Asian concepts of self and its relation to the environment, it is likely that predominant cultural values within Western and East Asian cultural groups, particularly those endorsed by Caucasian‐American and Japanese young adults, have since adapted in response to a number of environmental demands [Oyserman et al., 2002; Oyserman and Lee, 2008]. By extension to the domain of neural functioning, the current results indicate that neural representations of self in Westerners and East Asians are not inherently different, but instead reflect cultural values of individualism and collectivism that are endorsed by the individual, a process that is malleable to influence across multiple time scales. Hence, the current results illustrate the dynamic nature of cultural values across individuals and cultural groups [Hong et al., 2000; Oyserman et al., 2002; Oyserman and Lee, 2008] as well as how such dynamic cultural values shape neural representations [Chiao and Ambady, 2007; Chiao, et al. in press].
Additionally, we observed the influence of cultural values on neural responses within MPFC during self judgments, despite the absence of differences at the behavioral level. Prior cultural psychological studies have also shown limitations of behavioral measures in revealing influences of SCS on mental processes due to cultural differences in the willingness to disclose personal information [Heine et al., 2002; Peng et al., 1997] and what it means to ‘agree’ or ‘disagree’ with a statement [Heine et al., 2002; Peng et al., 1997]. Hence, our results reveal an advantage of examining cultural values such as SCS at the neural level. By examining effects of SCS at the neural level, we were able to measure the influence of SCS on neural representations of self within MPFC, even when such influence was not observable at the behavioral level.
Cultural psychology and social neuroscience have evolved as separate disciplines but in reality, cultural values, beliefs, and practices must be important to social brain functioning. Here, we provide direct evidence that the cultural values of individualism and collectivism influence neural mechanisms underlying the self. The current findings contribute to a growing body of cultural neuroscience research illustrating how functional brain imaging can provide insights into how variation in cultural values may guide the functioning of neural circuitry critical to self‐knowledge and self‐relevant processes. The scope of the impact of SCS on neural circuitry underlying a wider range of self‐relevant processes remains to be elucidated. The present study nevertheless indicates that the degree of activation in the anterior rostral portion of the MPFC during general or contextual self‐judgments reflect how individualistic or collectivistic a person is and therefore, predicts the extent to which a person thinks of him or herself as autonomous of others and his or her environment, or defined by their social context and relationships.
Acknowledgements
The authors thank D.J. Bridge and J. Choi for assistance and D. Medin, K. Paller, M. Grabowecky, J.A. Richeson and S.L. Franconeri for helpful discussion.
REFERENCES
- Amodio DM,Frith CD ( 2006): Meeting of minds: The medical prefrontal cortex and social cognition. Nat Rev Neurosci 7: 268–277. [DOI] [PubMed] [Google Scholar]
- Cabeza R,St. Jacques PL ( 2007): Functional neuroimaging of autobiographical memory. Trends in Cogn Sci 11: 219–277. [DOI] [PubMed] [Google Scholar]
- Chiao JY,Ambady N ( 2007): Cultural neuroscience: Parsing universality and diversity across levels of analysis In: Kitayama S,Cohen D, editors. Handbook of Cultural Psychology, New York: Guilford Press; pp 237–254. [Google Scholar]
- Chiao JY,Li Z,Harada T ( 2008): Cultural neuroscience of consciousness: From visual perception to self‐awareness. J Consciousness Studies, 10–11. [Google Scholar]
- Friedman L,Glover GH,FBIRN consortium ( 2006): Reducing interscanner variability of activation in a multicenter fMRI study: Controlling for signal‐to‐fluctuation‐noise‐ratio (SFNR) differences. Neuroimage 33: 471–481. [DOI] [PubMed] [Google Scholar]
- Friston KJ,Holmes AP,Worlsey KJ,Poline JP,Frith CD,Frackowiak RSJ ( 1995): Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp 2: 189–210. [Google Scholar]
- Gardner WL,Gabriel S,Lee AY ( 1999): “I” value freedom but “we” value relationships: Self construal priming mirrors cultural differences in judgment. Psychol Sci 10: 321–326. [Google Scholar]
- Gilboa A ( 2004): Autobiographical and episodic memory – one and the same? Evidence from prefrontal activation in neuroimaging studies. Neuropyschologia 42: 1336–1349. [DOI] [PubMed] [Google Scholar]
- Gillihan SJ,Farah MJ ( 2005): Is self special? A critical review of evidence from experimental psychology and cognitive neuroscience. Psychol Bull 131: 76–97. [DOI] [PubMed] [Google Scholar]
- Heine SJ,Lehman DR,Peng K,Greenholtz J ( 2002): What's wrong with cross‐cultural comparisons of subjective Likert scales: The reference‐group problem. J Pers Soc Psychol 82: 903–918. [PubMed] [Google Scholar]
- Hong Y,Morris MW,Chiu C,Benet‐Martinez V ( 2000): Multicultural minds: A dynamic constructivist approach to culture and cognition. Am Psych 55: 709–720. [DOI] [PubMed] [Google Scholar]
- Kelley WM,Macrae CN,Wyland CL,Caglar S,Inati S,Heatheron TF ( 2002): Finding the self? An event‐related fMRI study. J Cogn Neurosci 14: 785–794. [DOI] [PubMed] [Google Scholar]
- Li SC ( 2003): Biocultural orchestration of developmental plasticity across levels: The interplay of biology and culture in shaping the mind and behavioral across the life span. Psychol Bull 129: 171–794. [DOI] [PubMed] [Google Scholar]
- Macrae CN,Moran JM,Heatherton TF,Banfield JF,Kelley WM ( 2004): Medial prefrontal activity predicts memory for self. Cereb Cortex 14: 647–654. [DOI] [PubMed] [Google Scholar]
- Markus H,Kitayama S ( 1991): Culture and the self: Implication for cognition, emotion and motivation. Psychol Rev 98: 224–253. [Google Scholar]
- Mesoudi A,Whiten A,Laland KN ( 2006): Towards a unified science of cultural evolution. Behav Brain Sci 29: 329–383. [DOI] [PubMed] [Google Scholar]
- Nisbett R,Peng K,Choi I,Norenzayan A ( 2001): Culture and systems of thought: Holistic versus analytic cognition. Psychol Rev 108: 291–310. [DOI] [PubMed] [Google Scholar]
- Northoff G,Heinzel A,de Greck M,Bermpohl F,Dobrowolny H,Panksepp J ( 2006): Self‐referential processing in our brain‐ a meta‐analysis of imaging studies on the self. Neuroimage 31: 440–457. [DOI] [PubMed] [Google Scholar]
- Oyserman D,Coon HM,Kemmelmeier M ( 2002): Rethinking individualism and collectivism: Evaluation of theoretical assumptions and meta‐analyses. Psychol Bull 128: 3–72. [PubMed] [Google Scholar]
- Oyserman D,Lee SW ( 2008): Priming ‘culture’: Culture as situated cognition In: Kitayama S,Cohen D, editors. Handbook of Cultural Psychology. New York: Guilford Press; pp 255–276. [Google Scholar]
- Parrish TB,Gitelman DR,LaBar KS,Mesulam MM ( 2000): The impact of signal‐to‐noise on functional MRI. Magn Reson Med 44: 925–932. [DOI] [PubMed] [Google Scholar]
- Peng K,Nisbett RE,Wong NYC ( 1997): Validity problems comparing values across cultures and possible solutions. Psychol Methods 2: 329–344. [Google Scholar]
- Singelis TM ( 1994): The measurement of independent and interdependent self‐construals. Pers Soc Psychol Bull 20: 50–591. [Google Scholar]
- Talairach J,Tournoux P ( 1988): Co‐Planar Steretaxic Atlas of the Human Brain: 3‐Dimensional Proportional System—An Approach to Cerebral Imaging. New York: Theime Medical Publishers. [Google Scholar]
- Triandis HC ( 1995): Individualism and Collectivism. Boulder: Westview Press; pp 1–43. [Google Scholar]
- Wagner AD,Schacter DL,Rotte M,Koutstaal W,Maril A,Dale AM,Rosen BR,Buckner RL ( 1998): Building memories: Remembering and forgetting verbal experiences as predicted by brain activity. Science 281: 1188–1191. [DOI] [PubMed] [Google Scholar]
- Zhu Y,Zhang L,Fan J,Han S ( 2007): Neural basis of cultural influence on self‐representation. Neuroimage 34: 1310–1316. [DOI] [PubMed] [Google Scholar]


