Abstract
Background: Sleep‐related breathing disorders (SRBDs) affect as many as 40% of elderly people. The association of SRBDs with structural brain abnormalities remains unclear. In this observational study, we evaluated gray matter changes in the brain associated with sleep abnormalities in volunteers and their relationship with the severity of SRBDs. Methods: One hundred fifty two healthy subjects aged 66.0 ± 0.6 years‐old underwent tridimensional brain MRI and nocturnal polygraphic recording during which apnea/hypopnea index (AHI) and the oxyhemoglobin desaturation index (ODI) were measured. Using voxel‐based morphometry, we investigated the presence of gray matter abnormalities in association with AHI and ODI. Findings: Seventy‐six subjects (50%) had SRBDs defined by an AHI ≥ to 15 and 25 subjects (16%) SRBDs defined by an ODI ≥ 15, in the absence of systematic excessive daytime sleepiness. A significant symmetrical loss of gray matter in the intermediate reticular zone of the bulbopontine area was found to correlate with both AHI and ODI (P < 0.05 corrected for multiple comparisons for cluster significance). Interpretation: This gray matter volume decrease in brain regions involved in breathing/autonomic functions, as well as their correlation with the severity of the disorder, suggests a pathophysiological link between structural changes and SRBDs. Hum Brain Mapp, 2009. © 2008 Wiley‐Liss, Inc.
Keywords: VBM, sleep‐related breathing disorders, brainstem, gray matter volume, hypoxia, elderly
INTRODUCTION
Sleep‐related breathing disorders (SRBDs) include clinical entities such as snoring, obstructive and central sleep apneas [Wolkove et al., 2007]. The most common form of sleep apnea, obstructive sleep apnea syndrome (OSAS) [Parish and Somers, 2004] is frequent in the elderly, with an incidence range of 42–57% [Young et al., 2002], up to 50% in a population without comorbidities [Pavlova et al., 2008]. Recent studies show that OSAS is associated with coronary heart disease, heart failure, cardiac arrhythmias, as well as transient ischemic attacks [Parish and Somers, 2004], and an hazard ratio of 2.52 for stroke was observed in elderly people with a history of severe OSAS [Munoz et al., 2006].
OSAS may induce progressive brain alterations through several mechanisms, such as hypertension [Kono et al., 2007], endothelial dysfunction [Parish and Somers, 2004], episodes of hypoxemia, and reoxygenation cycles [Abramov et al., 2007]. The hypothesis of a hypoxic stress to the brain is supported by neurochemical changes, such as a decrease in creatinine‐containing compounds in the hippocampal areas [Bartlett et al., 2004]. The search for associations between the severity of OSAS and anatomical brain damages has come up with contradictory results ranging from normal structure [O'Donoghue et al., 2005] to scattered defects such as focal gray matter decrease in the left hippocampus [Morrell et al., 2003] or even diffuse gray matter decrease [Macey et al., 2002; Morrell et al., 2003].
To investigate the structural changes that may take place in an apparently normal brain in association with early stages of SRBDs, we conducted an epidemiological study in a population of volunteers aged between 65 and 67 years without a diagnosed sleep disorder. We investigated the presence of early gray matter volume decrease using voxel‐based morphometry (VBM) technique applied to 3D‐MRIs, which could correlate with the apnea/hypopnea index (AHI), and the oxyhemoglobin desaturation index (ODI); we also looked for decreases in brain volumes that could be associated with the severity of OSAS by comparing the group of subjects with mild to moderate SRBDs (ODI ≥ 15) to those with a desaturation index <15.
METHODS
Subjects
The PROgnostic indicator OF cardiovascular and cerebrovascular events (PROOF) study [Barthelemy et al., 2007] is a prospective longitudinal study that includes 1,011 volunteers who were selected from the general population during their 65th year, and that is designed to assess the predictive value of changes in the autonomic nervous system activity for cerebrovascular and cardiovascular diseases, as well as death for any cause. Exclusion criteria were a history of cardiovascular events, neurological disease, diagnosed sleep disorders, Type 1 diabetes, contraindications for MRI, evolutive cancer, hemodialysis, dependent people, and those living in institutions such as nursing homes. One hundred eighty consecutive subjects of the PROOF cohort, who had a sleep polygraphic recording and a tridimensional brain MRI allowing 3D‐brain reconstructions, T2‐weighted and FLAIR MRI scans recorded in the same conditions, were included and constituted the core of the present study. Silent infarcts and other structural abnormalities (meningiomas) detected on brain MRI led to exclude 24 subjects. Thus, the final study group consisted of 156 healthy subjects, free of significant structural brain alterations, except leucoaraiosis that was found in 91 patients. Because of data corruption in 4 subjects, the final sample size of the study was 152. The PROOF study was approved by the local ethical committee (CCPPRB Rhône‐Alpes Loire) and all subjects signed a written informed consent. The sponsor of this study is the University Hospital of Saint Etienne, France (Délégation à la Recherche Clinique).
Blood Pressure
Arterial blood pressure measurements were assessed by 24‐h ambulatory Holter recordings using the auscultatory method (Diasys Integra, Novacor, Rueil‐Malmaison, France). Hypertension was defined as the presence of a antihypertensive medication, or a systolic blood pressure ≥ 130 or a diastolic blood pressure ≥80.
Sleepiness scale score
The presence of excessive daytime sleepiness, as reported by subjects, was assessed through the Epworth Sleepiness Scale score [Johns, 1991].
Polygraphic recordings were performed using a system that included one lead ECG, pulse oxymetry, monitoring of rib cage excursions based on thoracic impedance, body position, and nasal pressure recording for measurement of ventilation (HypnoPTT, Tyco Healthcare, US). All recordings were performed at the subjects' homes. If subjects reported a sleep latency exceeding 2 h or if a recording duration was less than 5 h, a second night of polygraphic monitoring was performed and used to measure sleep‐related variables.
Sleep and sleep‐related variables
An expert investigator blinded to MRI results visually validated and manually scored each recording for apnea/hypopnea events and for runs of increased upper airway resistance. Apnea was defined as the absence of airflow for more than 10 s despite persistent respiratory efforts. Hypopnea was defined as the association of a reduction of 50% or more of the amplitude of respiratory efforts during at least 10 s, together with a fall in oxygen saturation (SaO2) of at least 3% [American Academy of Sleep Medicine Task Force, 1999] or autonomic arousal, according to a decrease in pulse transit time [Smith et al., 1999]. The AHI was defined as the number of episodes of apnea and hypopnea per hour of sleep. Apnea duration, mean and minimal SaO2 were determined for each episode of apnea. Arterial ODI, commonly used to diagnose sleep apnea [Oeverland et al., 2002], was quantified as the number of episodes associated with an oxygen desaturation ≥3% per hour of sleep duration. The absence of rib cage movement associated with an apnea or a hypopnea defined a central respiratory event.
Brain Imaging Analysis
MRI acquisition
All brain scans were acquired on a Siemens 1.0 T scanner. For each subject, a 3D T1‐weighted MRI (MPRage) was acquired with the following parameters: TR = 1,900, TE = 3.95, FOV = 256 × 256, 88 slices per volume with a voxel size of 2 × 2 × 2 mm. T2‐weighted (24 slices of 5.5 mm, TR = 6,620, TE = 123, FOV = 173 × 230, pixel size: 1.5 × 0.9 × 5.5 mm) and FLAIR (24 slices of 5.5 mm, TR = 9,000, TE = 102, FOV = 230 × 173, pixel size: 0.9 × 0.9 × 5.5 mm) scans were also recorded in the same MRI session. 152 subjects had a 3D brain MRI that could be processed by using Statistical Parametric Mapping (SPM2) and the VBM method (Wellcome Department of Cognitive Neurology, London, UK).
Voxel‐based morphometry
All MRIs were analyzed by an experienced radiologist blinded to results of the sleep study. After this step, a final number of 61 MRI scans was considered as having no leucoaraiosis, and therefore as being appropriate for the constitution of the template. To make a proper comparison between all subjects' MRIs, the processing was performed using the optimized protocol [Good et al., 2001] for VBM brain analysis. All scans were first segmented, normalized to a study‐specific template created with the 61 strictly normal MRIs scans, and segmented again. Finally, gray matter volume scans were obtained by using the VBM modulation and a 12‐mm FWHM smoothing.
Statistical Analysis
Two‐tailed unpaired Student's t‐tests were used to compare population characteristics [age, body mass index (BMI), Epworth Scale Score, and whole brain gray matter volume] between males and females, and between subjects with and without SRBDs as defined by ODI and AHI scores. Statistical significance was achieved for P values smaller than 0.05.
Three successive VBM analyses were performed. The first and the second one investigated linear correlations between gray matter volume and AHI or ODI, respectively. In these two analyses, AHI and ODI were entered as “covariates only” in the SPM2 model. The third analysis consisted in group comparisons, with an ODI cutoff value set at 15 events/hour defining two groups.
In each statistical analysis, gender, BMI, ambulatory blood pressure, and total gray matter volume were introduced as covariates. Age, which is also known to influence brain volumes [Good et al., 2001], was not considered as a confounding variable since it was fixed and homogenous in the population. Thresholds for results presentation were set at P < 0.05, corrected for multiple comparisons for cluster significance.
Classical statistical analyses have been done with STATISTICA 6.1 (Statsoft, USA).
RESULTS
In these 152 elderly subjects, the mean age was 66.0 ± 0.6, 143 subjects were right‐handed, 7 were left‐handed, and 2 of them were ambidextrous. Men and women did not significantly differ in terms of age (66.1 ± 0.6 vs. 66.0 ± 0.7 years, ns), BMI (25.5 ± 2.8 vs. 25.4 ± 3.6 kg/m2, ns), or nocturnal respiratory variables (ODI: 10.3 ± 8.8 vs. 8.5 ± 8.6 events/hour, ns; AHI: 21.0 ± 15.0 vs. 16.7 ± 12.9 events/hour, ns). Absolute gray matter volume (0.65 ± 0.05 vs. 0.59 ± 0.04 L, P < 0.001) and Epworth Scale Score (6.6 ± 4.3 vs. 5.2 ± 3.5, P = 0.022) were found significantly higher in men than in women (Table I), this later finding reported by others [Baldwin et al., 2004], suggesting that the phenotype of our population had rather classical characteristics. BMI was significantly higher in subjects with SRBDs (ODI or AHI ≥ 15), as compared to those without them (ODI or AHI < 15, Table II). As expected, AHI and ODI were significantly and strongly correlated (R 2 = 0.71; P < 0.001) and subjects with SRBDs defined by AHI had a significantly higher ODI (Table II). Only one subject had an abnormal central sleep apnea index (17.5 events/hour), but with a predominant obstructive component (AHI = 58 events/hour) and was therefore considered as having mainly obstructive SRBDs. Self‐report of daytime sleepiness, measured by the Epworth Scale Scores, did not show significant differences between SRBDs groups (Table II). Whole brain gray matter volume did not significantly differ between subjects with and without SRBDs (Table II).
Table I.
Characteristics of the population
| Men (n = 55) | Women (n = 97) | Total (N = 152) | P value | ||
|---|---|---|---|---|---|
| Age | (year) | 66.1 (0.6) | 66.0 (0.7) | 66.0 (0.6) | 0.113 |
| BMI | (kg/m2) | 25.5 (2.8) | 25.4 (3.6) | 25.5 (3.3) | 0.845 |
| Handedness | Right‐handed | 51 | 92 | 143 | n.a. |
| Ambidextrous | 1 | 1 | 2 | n.a. | |
| Left‐handed | 3 | 4 | 7 | n.a. | |
| Systolic BPa | (mm Hg) | 122.2 (11.5) | 116.8 (15.1) | 118.8 (14.1) | 0.03 |
| Diastolic BPa | (mm Hg) | 78.6 (6.5) | 74.9 (7.6) | 76.2 (7.4) | <0.01 |
| Hypertensiona | yes/no | 32/22 | 39/56 | 71/78 | |
| yes/no (%) | 59%/41% | 41%/59% | 48%/52% | ||
| Medications | Antiepileptics | 0 | 1 | 1 | n.a. |
| Psychoanaleptics | 1 | 8 | 9 | n.a. | |
| Psycholeptics | 0 | 11 | 11 | n.a. | |
| Serum lipid reducing | 8 | 13 | 21 | n.a. | |
| Sleep data | Epworth Scale Score | 6.7 (4.3) | 5.2 (3.5) | 5.7 (3.9) | 0.022 |
| Epworth Scale Score ≥10 | 31% | 1% | 16% | <0.001 | |
| ODI (event/hour) | 10.3 (8.8) | 8.5 (8.6) | 9.1 (8.7) | 0.063 | |
| AHI (event/hour) | 21.0 (15.0) | 16.7 (12.9) | 18.3 (13.8) | 0.244 | |
| Central AHI (event/hour) | 1.0 (2.4) | 0.7 (1.0) | 0.8 (1.6) | ||
| Gray matter volume | (L) | 0.6 (0.1) | 0.6 (0.0) | 0.6 (0.1) | <0.0001 |
Values are presented as Mean (SD).
P value (Student's unpaired two‐tailed t‐test) between men and women.
Data for blood pressure were only available for 149 subjects (54 males, 95 females).
Table II.
Variables of the study (n = 152 subjects)
| ODI | AHI | |||||
|---|---|---|---|---|---|---|
| <15 | ≥15 (SRBDs) | P | <15 | ≥15 (SRBDs) | P | |
| Number of subjects | 127 | 25 | 76 | 76 | ||
| Sex ratio (M/F) | 44/83 | 11/14 | 24/52 | 31/45 | ||
| Age (year) | 66.0 (0.6) | 66.0 (0.8) | 0.64 | 66.0 (0.6) | 66.1 (0.7) | 0.24 |
| BMI (kg/m2) | 25.1 (3.2) | 27.3 (3.2) | 0.0001 | 24.6 (3.0) | 26.3 (3.4) | 0.0013 |
| Systolic BP (mmHg)a | 117.7 (13.9) | 124.4 (14.1) | 0.03 | 116.2 (20.2) | 121.4 (14.1) | 0.02 |
| Diastolic BP (mmHg)a | 75.9 (7.4) | 78.1 (7.4) | 0.18 | 75.3 (7.7) | 77.2 (7.0) | 0.10 |
| Epworth Scale Score | 5.6 (3.8) | 6.0 (4.6) | 0.64 | 5.5 (3.8) | 5.9 (3.9) | 0.49 |
| Epworth Scale Score ≥ 10 | 16% | 17% | 0.87 | 18% | 16% | 0.67 |
| ODI (event/hour) | 6.2 (4.5) | 24.2 (9.0) | n.a. | 3.7 (3.1) | 14.5 (9.1) | <0.0001 |
| AHI (event/hour) | 14.1 (9.8) | 39.5 (11.5) | <0.0001 | 7.4 (3.8) | 29.2 (11.3) | n.a. |
| AI (events/hour) | 3.3 (4.2) | 12.9 (9.8) | <0.0001 | 1.2 (1.3) | 8.4 (7.6) | <0.0001 |
| HI (events/hour) | 9.7 (6.4) | 23.2 (8.6) | <0.0001 | 5.6 (3.0) | 18.2 (7.4) | <0.0001 |
| cAHI (events/hour) | 0.6 (0.8) | 1.7 (3.5) | <0.001 | 0.3 (0.3) | 1.3 (2.2) | 0.0001 |
| Time below 90% SaO2 (%) | 0.9 (4.4) | 8.1 (11.3) | <0.0001 | 1.1 (5.6) | 3.1 (7.4) | 0.07 |
| SaO2 min | 90.8 (3.2) | 84.8 (7.3) | <0.0001 | 91.4 (3.2) | 88.3 (5.4) | <0.0001 |
| SaO2 mean | 95.7 (2.2) | 94.2 (1.8) | 0.001 | 95.6 (2.7) | 95.3 (1.6) | 0.56 |
| GM volume (L) | 0.6 (0.1) | 0.6 (0.1) | 0.59 | 0.6 (0.1) | 0.6 (0.1) | 0.52 |
Values are presented as Mean (SD).
P value (Student's unpaired two‐tailed t‐test) between SRBDs and non‐SRBDs subjects.
Data for blood pressure were only available for 149 subjects (74 AHI ≥ 15, 75 AHI < 15 and 24 ODI ≥ 15, 125 ODI < 15).
Gray matter decrease in sleep apnea was investigated in correlation with AHI, with ODI, and when comparing subjects with and without SRBDs.
A significant inverse correlation was found between AHI and gray matter volume in the mid/lower pontine area, bilaterally, in a localization consistent with the intermediate reticular zone of the bulbopontine area, between the dorsal nucleus of the vagus nerve, the solitary tract nucleus and the nucleus ambiguus (Fig. 1, Table III). Very similar results were found using the ODI covariate (Fig. 3, Table III). Another significant inverse correlation between gray matter volume and AHI or ODI was found in the cerebellum. Subjects with SRBDS according to an ODI threshold of 15 showed a gray matter decrease in the same bulbopontine area as found in the correlation with AHI or ODI (Fig. 2, Table III).
Figure 1.
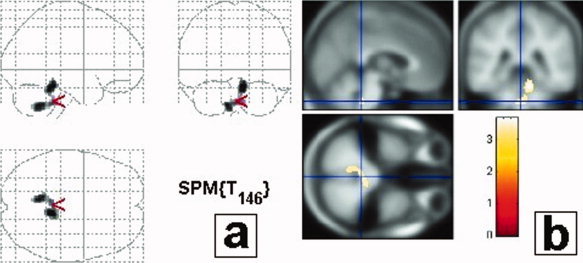
Correlations between gray matter decrease and SRBDs using AHI criteria. An inverse correlation between gray matter volume and apnea hypopnea index (AHI) is present in the brainstem and in the cerebellum. Results are displayed with a P < 0.05 threshold, corrected for multiple comparisons, at the cluster level. Results are presented in neurological convention (i.e., left side of the brain is on the left side of the figure). (a) Projections map (SPM2) showing the correlation between brainstem and SRBDs. No correlation can be seen outside the brainstem and cerebellum. (b) Maximum of correlation between gray matter loss and AHI. Note that the cluster is extended in the upper medulla/inferior pontine region, close to the ventricular surface, bilaterally, almost symmetrically; in a localization consistent with the intermediate reticular zone of the bulbopontine area (see stereotaxic coordinates (MNI) in Table II). The clusters are projected onto the specific template performed for this study from the 61 averaged subjects with normal MRI.
Table III.
Stereotaxic coordinates of peaks of gray matter loss (Montreal Neurological Institute atlas)
| X | y | Z | P (cluster level) | Location | |
|---|---|---|---|---|---|
| Linear correlation between AHI/ODI and gray matter volume | |||||
| AHI | 0 | −38 | −22 | 0.003 | Brainstem |
| −8 | −54 | −44 | 0.003 | Cerebellum | |
| 10 | −40 | −22 | 0.003 | Cerebellum | |
| ODI | −6 | −40 | −42 | <0.001 | Cerebellum |
| 8 | −34 | −40 | <0.001 | Cerebellum | |
| −28 | −48 | −50 | <0.001 | Cerebellum | |
| Group analysis : Subjects with SRBDs (as defined by ODI ≥ 15) versus subjects without SRBDs | |||||
| SRBDs group | −6 | −38 | −40 | 0.002 | Brainstem |
| 10 | −36 | −40 | 0.002 | Cerebellum | |
Figure 3.
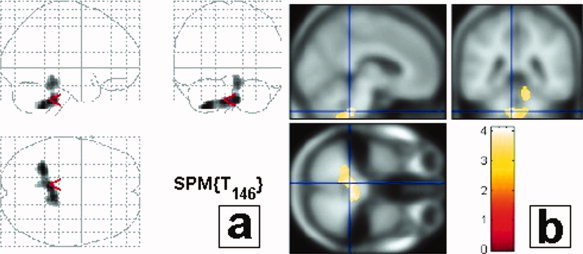
Correlations between gray matter decrease and SRBDs using ODI criteria. An inverse correlation between gray matter volume and oxyhemoglobin desaturation index (ODI) is present in the brainstem with an extension to the cerebellum. Results are displayed with a P < 0.05 threshold, corrected for multiple comparisons, at the cluster level. Results are presented in neurological convention (i.e., left side of the brain is on the left side of the figure). (a) Projections map (SPM2) showing the correlation between brainstem and SRBDs. No correlation can be seen outside the brainstem and cerebellum. (b) Maximum of correlation between gray matter loss and AHI. Note that the cluster is extended in the upper medulla/inferior pontine region, close to the ventricular surface, bilaterally, almost symmetrically; in a localization consistent with the intermediate reticular zone of the bulbopontine area (see stereotaxic coordinates (MNI) in Table II). The clusters are projected onto the specific template performed for this study from the 61 averaged subjects with normal MRI.
Figure 2.
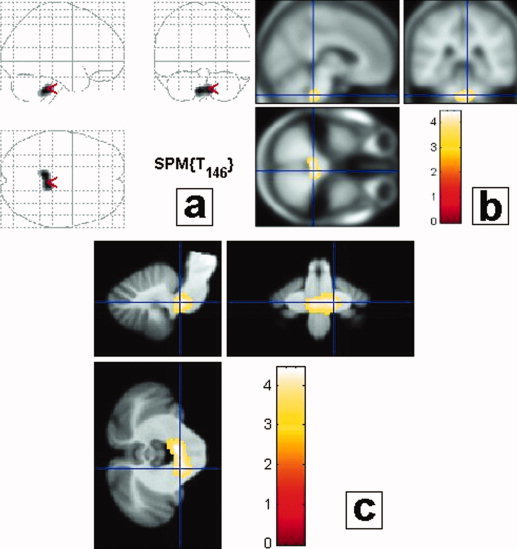
Group analysis: Subjects with severe SRBDs (ODI ≥ 15) versus non‐SRBDs (ODI < 15). Subjects were categorized in two groups, those with severe SRBDs, as defined by ODI ≥ 15 and those without SRBDs (ODI < 15). Results are displayed with a P < 0.05 threshold, corrected for multiple comparisons, at the cluster level (Table II). Gray matter loss in the group of subjects with SRBDs compared to those without SRBDs concerned the middle/inferior part of the lower pontine/upper medulla area. Results are presented in neurological convention (i.e., left side of the brain is on the left side of the figure). (a) Projections map (SPM2) showing a gray matter decrease only in the brainstem. (b) Maximum of correlation between gray matter loss and ODI. Note that the cluster is extended in the upper medulla/inferior pontine region, close to the ventricular surface, bilaterally, symmetrically; in a localization consistent with the intermediate reticular zone of the bulbopontine area (Table II). The clusters are projected onto the specific template performed for this study from the 61 averaged subjects with normal MRI. (c) Maximum of correlation between gray matter loss and ODI (same as b), projected onto the brainstem template from Diedrichsen [2006].
DISCUSSION
To our knowledge, such morphometric changes have never been reported in previous studies investigating the relationships between SRBDs and gray matter volume [Macey et al., 2002; Morrell et al., 2003; O'Donoghue et al., 2005]. These findings are reinforced by the population‐based recruitment of the cohort. The sample size was large enough to exclude a selection bias. The population was homogeneous with respect to age, ruling out the possibility of confounding by age‐related effects, particularly on gray matter volume [Lemaitre et al., 2005] and SRBDs frequency [Young et al., 1993], while other studies generally included populations with a wide age range, including younger patients. Moreover, in terms of sex ratio, our population was representative of a general 66‐year‐old population thus taking into account female contribution to SRBDs, while other studies were restricted to male gender.
Such decreases in gray matter volume were observed while Epworth Scale Scores were normal for the majority of subjects, 83% having an Epworth Scale Score less than 10. Mean ± SD Epworth Scale Score was 5.7 ± 3.9. Such an absence of subjective excessive daytime sleepiness could suggest that we identified an early stage of the disease, probably also explaining why other cortical or subcortical regions were not yet structurally involved. Alternatively, it must be emphasized that our population differs from other studies [Macey et al., 2002; Morrell et al., 2003; O'Donoghue et al., 2005], in that subjects are older and taken from a population‐based sample. For these reasons, a bias related to different populations of subjects cannot be formally ruled out. It might also explain that our subjects did not have sleepiness as assessed on the basis of Epworth scale, either because they are older or because they escape to a disease that occur earlier in the life ([Launois et al., 2007]).
Scattered gray matter loss [Macey et al., 2002] or atrophy limited to the left hippocampus [Morrell et al., 2003] were described as a possible consequence of sleep apnea. In our study, the VBM maps identified loss of gray matter volume in the upper medulla and lower pontine area, close to the ventricular surface and included structures such as the dorsal nucleus of the vagus nerve posteriorly, solitary tract nucleus laterally, and ambiguus nucleus anteriorly with an extension of abnormalities that likely corresponds to the intermediate reticular zone. This localization, nearly exclusively restricted to the brainstem, is highly consistent with previous case‐reports showing a susceptibility of the solitary tract nucleus to sleep apnea in adults [Parenti et al., 2005]. Similar symmetrical necrosis of the brainstem due to prolonged hypoxia, similar to what has been found in our study, have already been described in newborns [Leech and Alvord, 1977] and in adults [Lindenberg, 1963]. Our results, in a large asymptomatic population, may suggest that repeated and severe hypoxic events, such as those observed in SRBDs, may induce structural changes in brainstem centers involved in ventilatory/autonomic regulation [Benarroch, 2007].
In adults, susceptibility to hypoxia has mostly been described in cortical and thalamic areas. Our findings not only differ from those obtained in symptomatic patients presenting evidence of cognitive and executive frontal dysfunction [Beebe and Gozal, 2002; Naegele et al., 2006], but also with prefrontal structural changes described as a consequence of sleep hypoxia [Beebe and Gozal, 2002]. The loss in gray matter volume found in the brainstem, in the absence of clinically patent sleep abnormalities, has not been found in other studies which included patients with diagnosed SRBDs [Morrell et al., 2003]. This loss of gray matter volume would likely suggest silent structural processes in nonsymptomatic subjects who could complete, later in the course of the disease, distant (mainly cortical) symptoms as a consequence of a patent breathing disorder. This possibility may also be supported by the findings that patients had subclinical abnormalities for breathing during the sleep, while they did not have any complaint on sleep on the basis of normal Epworth scores. This morphometric discrepancy between two presentations of the disease can be viewed as an argument in favor of a specific disorder of the elderly [Launois et al., 2007].
The long‐term follow‐up of the present cohort may help to control the anatomical evolution of this disorder, maybe specific to the elderly, and particularly study the emergence of a vascular brain disease. It could also precede the occurrence of clinical features of SRDB, such as induced hypertension, cardiovascular and cerebrovascular events, and other autonomic‐associated disturbances. These observations may help to understand the pathophysiology of SRBDs in elderly, with their intricated autonomic disorders, and their relationships with focal loss of gray matter volume, including in the brainstem.
Acknowledgements
The authors thank Stephanie Mazza for her valuable help and advice for the criticism of the manuscript. They also acknowledge Miss Delphine Maudoux and Mister Arnauld Garcin.
REFERENCES
- Abramov AY,Scorziello A,Duchen MR ( 2007): Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. J Neurosci 27: 1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Sleep Medicine Task Force ( 1999): Sleep‐related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 22: 667–689. [PubMed] [Google Scholar]
- Baldwin CM,Kapur VK,Holberg CJ,Rosen C,Nieto FJ ( 2004): Associations between gender and measures of daytime somnolence in the Sleep Heart Health Study. Sleep 27: 305–311. [DOI] [PubMed] [Google Scholar]
- Barthelemy JC,Pichot V,Dauphinot V,Celle S,Laurent B,Garcin A,Maudoux D,Kerleroux J,Lacour JR,Kossovsky M,Gaspoz JM, Roche F ( 2007): Autonomic nervous system activity and decline as prognostic indicators of cardiovascular and cerebrovascular events: The ‘PROOF’ study. Study design and population sample. Associations with sleep‐related breathing disorders: The ‘SYNAPSE’ study. Neuroepidemiology 29: 18–28. [DOI] [PubMed] [Google Scholar]
- Bartlett DJ,Rae C,Thompson CH,Byth K,Joffe DA,Enright T,Grunstein RR ( 2004): Hippocampal area metabolites relate to severity and cognitive function in obstructive sleep apnea. Sleep Med 5: 593–596. [DOI] [PubMed] [Google Scholar]
- Beebe DW,Gozal D ( 2002): Obstructive sleep apnea and the prefrontal cortex: Towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J Sleep Res 11: 1–16. [DOI] [PubMed] [Google Scholar]
- Benarroch EE ( 2007): Brainstem respiratory control: Substrates of respiratory failure of multiple system atrophy. Mov Disord 22: 155–161. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J ( 2006): A spatially unbiased atlas template of the human cerebellum. Neuroimage 33: 127–138. [DOI] [PubMed] [Google Scholar]
- Good CD,Johnsrude IS,Ashburner J,Henson RN,Friston KJ,Frackowiak RS ( 2001): A voxel‐based morphometric study of ageing in 465 normal adult human brains. Neuroimage 14(1 Part 1): 21–36. [DOI] [PubMed] [Google Scholar]
- Johns MW ( 1991): A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 14: 540–545. [DOI] [PubMed] [Google Scholar]
- Kono M,Tatsumi K,Saibara T,Nakamura A,Tanabe N,Takiguchi Y,Kuriyama T ( 2007): Obstructive sleep apnea syndrome is associated with some components of metabolic syndrome. Chest 131: 1387–1392. [DOI] [PubMed] [Google Scholar]
- Launois SH,Pepin JL,Levy P ( 2007): Sleep apnea in the elderly: A specific entity? Sleep Med Rev 11: 87–97. [DOI] [PubMed] [Google Scholar]
- Leech RW,Alvord EC Jr ( 1977): Anoxic‐ischemic encephalopathy in the human neonatal period. The significance of brain stem involvement. Arch Neurol 34: 109–113. [DOI] [PubMed] [Google Scholar]
- Lemaitre H,Crivello F,Grassiot B,Alperovitch A,Tzourio C,Mazoyer B ( 2005): Age‐ and sex‐related effects on the neuroanatomy of healthy elderly. Neuroimage 26: 900–911. [DOI] [PubMed] [Google Scholar]
- Lindenberg R ( 1963): Patterns of CNS vulnerability in acute hypoxaemia, including anesthetic accidents. Selective vulnerability of the brain in hypoxaemia 184–209. [Google Scholar]
- Macey PM,Henderson LA,Macey KE,Alger JR,Frysinger RC,Woo MA,Harper RK,Yan‐Go FL,Harper RM ( 2002): Brain morphology associated with obstructive sleep apnea. Am J Respir Crit Care Med 166: 1382–1387. [DOI] [PubMed] [Google Scholar]
- Morrell MJ,McRobbie DW,Quest RA,Cummin AR,Ghiassi R,Corfield DR ( 2003): Changes in brain morphology associated with obstructive sleep apnea. Sleep Med 4: 451–454. [DOI] [PubMed] [Google Scholar]
- Munoz R,Duran‐Cantolla J,Martinez‐Vila E,Gallego J,Rubio R,Aizpuru F,De La Torre G ( 2006): Severe sleep apnea and risk of ischemic stroke in the elderly. Stroke 37: 2317–2321. [DOI] [PubMed] [Google Scholar]
- Naegele B,Launois SH,Mazza S,Feuerstein C,Pepin JL,Levy P ( 2006): Which memory processes are affected in patients with obstructive sleep apnea? An evaluation of 3 types of memory. Sleep 29: 533–544. [DOI] [PubMed] [Google Scholar]
- O'Donoghue FJ,Briellmann RS,Rochford PD,Abbott DF,Pell GS,Chan CH,Tarquinio N,Jackson GD,Pierce RJ ( 2005): Cerebral structural changes in severe obstructive sleep apnea. Am J Respir Crit Care Med 171: 1185–1190. [DOI] [PubMed] [Google Scholar]
- Oeverland B,Skatvedt O,Kvaerner KJ,Akre H ( 2002): Pulseoximetry: Sufficient to diagnose severe sleep apnea. Sleep Med 3: 133–138. [DOI] [PubMed] [Google Scholar]
- Parenti A,Macchi V,Snenghi R,Porzionato A,Scaravilli T,Ferrara SD,De Caro R ( 2005): Selective stroke of the solitary tract nuclei in two cases of central sleep apnoea. Clin Neuropathol 24: 239–246. [PubMed] [Google Scholar]
- Parish JM,Somers VK ( 2004): Obstructive sleep apnea and cardiovascular disease. Mayo Clin Proc 79: 1036–1046. [DOI] [PubMed] [Google Scholar]
- Pavlova MK,Duffy JF,Shea SA ( 2008): Polysomnographic respiratory abnormalities in asymptomatic individuals. Sleep 31: 241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RP,Argod J,Pepin JL,Levy PA ( 1999): Pulse transit time: An appraisal of potential clinical applications. Thorax 54: 452–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkove N,Elkholy O,Baltzan M,Palayew M ( 2007): Sleep and aging, Part 1: Sleep disorders commonly found in older people. CMAJ 176: 1299–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young T,Palta M,Dempsey J,Skatrud J,Weber S,Badr S ( 1993): The occurrence of sleep‐disordered breathing among middle‐aged adults. N Engl J Med 328: 1230–1235. [DOI] [PubMed] [Google Scholar]
- Young T,Peppard PE,Gottlieb DJ ( 2002): Epidemiology of obstructive sleep apnea: A population health perspective. Am J Respir Crit Care Med 165: 1217–1239. [DOI] [PubMed] [Google Scholar]


