Abstract
This meta‐analysis explores the location and function of brain areas involved in social cognition, or the capacity to understand people's behavioral intentions, social beliefs, and personality traits. On the basis of over 200 fMRI studies, it tests alternative theoretical proposals that attempt to explain how several brain areas process information relevant for social cognition. The results suggest that inferring temporary states such as goals, intentions, and desires of other people—even when they are false and unjust from our own perspective—strongly engages the temporo‐parietal junction (TPJ). Inferring more enduring dispositions of others and the self, or interpersonal norms and scripts, engages the medial prefrontal cortex (mPFC), although temporal states can also activate the mPFC. Other candidate tasks reflecting general‐purpose brain processes that may potentially subserve social cognition are briefly reviewed, such as sequence learning, causality detection, emotion processing, and executive functioning (action monitoring, attention, dual task monitoring, episodic memory retrieval), but none of them overlaps uniquely with the regions activated during social cognition. Hence, it appears that social cognition particularly engages the TPJ and mPFC regions. The available evidence is consistent with the role of a TPJ‐related mirror system for inferring temporary goals and intentions at a relatively perceptual level of representation, and the mPFC as a module that integrates social information across time and allows reflection and representation of traits and norms, and presumably also of intentionality, at a more abstract cognitive level. Hum Brain Mapp, 2009. © 2008 Wiley‐Liss, Inc.
Keywords: social neuroscience, trait inference, theory of mind, goal inference, intentionality
INTRODUCTION
Functional neuroimaging has played a crucial role in seeking to isolate brain regions specific to social cognition. Social cognition broadly includes the cognitive processes used to understand and store information about other persons including the self, and about interpersonal norms and scripts (or procedures) to navigate efficiently in the social world. Essentially, it requires that perceivers extract and understand the behavioral motives and stable dispositions of themselves and other persons and groups. Humans draw on “social intelligence” to ascribe dangerous behavior to aggressive goals (she wants to hurt me) and traits (she is always so aggressive). To do this, we often refer to the other's thoughts and beliefs, as if we can read their mind. This capacity is known as theory of mind (ToM) or mentalizing. Although many species including primates can accurately predict the goals of their conspecific's behavior, it appears that only humans can separate a mental perspective of their own actions from that of others' actions [Emery, 2005]. When explaining a person's behavior in terms of a goal, desire, or trait, we recognize that this mental representation does not necessarily correspond to our own interpretations or to reality.
Social cognition, and mentalizing in particular, is a high‐level capacity. It has been studied from various theoretical and methodological perspectives, most notably social psychology and social neuroscience. Social psychologists have investigated how we perceive and interpret our social environment including other persons, groups, and the self, how we build social knowledge structures that reflect the norms and values of society, and how this is influenced through conscious and unconscious processing mechanisms, which sometimes lead to biased judgments [e.g., Gilbert and Malone, 1995; Trope and Gaunt, 2000; Van Rooy et al., 2003]. Neuroscientists have analyzed which structures in the brain support the mental processes involved in social cognition. It is commonly assumed that the capacity to mentalize depends on cognitive brain mechanisms that are potentially dedicated specifically to social reasoning. Neurological evidence from studies of brain lesions [Apperly et al., 2004; Wood et al., 2005] and autism [Baron‐Cohen, 2006; Frith and Frith, 1999, 2001] supports this hypothesis. The advance of brain imaging and especially functional magnetic resonance imaging (fMRI) which allows unprecedented precision and validity in the localization of brain activity, provides a powerful tool for increasing our understanding of the neural activity in the brain that is associated with social cognition processes [for an introduction to fMRI, see Huettel et al., 2004].
The main goals of this article are to explore the brain areas that are held responsible for social reasoning, to analyze what specific sub processes or functions are computed in these areas, and to look for other brain systems that may support this social cognition capacity. Although there have already been reviews and perspectives on the neural correlates of social cognition, most of them lack an exhaustive overview of the available evidence. Therefore, this article presents a meta‐analysis on a larger scale of many processes involved in social cognition that have been explored with fMRI since the turn of the millennium. It focuses on “cold” processes of social reasoning, and only touches briefly on “hot” or affective processes.
Because a purely data‐driven approach would undoubtedly miss some important questions and insights that have already been developed earlier, this analysis will start with an overview of recent theoretical insights and perspectives. Functional neuroimaging can provide answers to these theoretical debates and questions because it demonstrates whether two tasks or processes engage common or distinct brain mechanisms. This relies on the key assumption that different areas are related to qualitative differences in psychological processes. However, this assumption is not necessarily correct [Henson, 2006; Saxe et al., 2004a]. Each brain region may contain thousands of neurons with distinct functions that cannot be teased apart with the current techniques. Hence, when two tasks activate the same area, it may well be that the fMRI technique is too rough and that what seems a common area may in fact reflect a distinct location and different processes. Conversely, even if we assume that the same brain area and process is involved, it may be that this region is more strongly recruited for social cognition than for any other process, as if the underlying general neural architecture has become specialized for a particular social function. In conclusion, a single study is unlikely to be decisive, and an exhaustive overview of evidence might potentially provide more reliable answers.
Theoretical Perspectives on Social Cognition
Most social and developmental psychologists conceive social cognition as involving a plethora of different social inferences. Cross‐cutting these finer distinctions, however, one can divide social processes in two major types of mental inferences: (1) inferences of transitory states (goals and intentions) and (2) inferences of enduring characteristics (personality traits and social scripts). It is generally believed in social and development psychology that transitory goal inferences are more perceptual in nature and directly related to observed behaviors, whereas enduring dispositions involve abstract inferences that require a more mature capacity to mentalize.
The division parallels many similar distinctions in social psychology. Perhaps, the most popular view is that observers first identify and categorize a person's behavior (e.g., helpful gesture) and then attribute the corresponding trait to the actor [e.g., he or she is helpful; Gilbert and Malone, 1995; Trope and Gaunt, 2000]. A related perspective is that we first identify the intentions, desires, or motives of an actor spontaneously [Fein, 1996; Hassin et al., 2005; Heider, 1958; Malle, 1999; Read and Miller, 1993] and that this process shapes the trait inferences we subsequently make. For instance, when an actor engages in helpful behavior, we wonder which reasons or motives may have compelled the actor to do so (out of sincere desire to help or to ingratiate?) and this influences whether we make a trait attribution of helpfulness or insincerity [Malle and Knobe, 1997; Malle et al., 2000; Reeder et al., 2002, 2004].
This division is also reflected in children's development of social mentalizing. Infants of 5‐ to 8‐month‐old have a sense of goal‐directed behavior and look longer when the target of a movement changes than when the path towards the same target changes [Woodward, 1998]. At the age of 18 months, children can complete an action that they have seen an adult attempt, but did not finish [Frith and Frith, 1999]. At the age of 3 or 4 years, children describe representational mental states or beliefs of others, distinct from their own, with a limited repertoire of mental concepts, such as desires and perceptions [Saxe et al., 2004a]. However, it is only by the age of 7 or 8 years that they show a marked increase in the use of personality traits and begin to appreciate that people have stable dispositions that help to predict future behaviors across different situations and that should be distinguished from situational pressures [Hagá and Garcia‐Marques, 2007; Rholes et al., 1990].
A mentalizing capacity is perhaps most clearly demonstrated in “false” belief tasks, because this task requires distinguishing between own and other beliefs. A typical example of a false belief task is when, in a verbal or cartoon story, unbeknown to an actor (but in full view to the participant) an object (e.g., sweet) is switched between two boxes (or the boxes are switched), so that after returning, the actor points to the wrong box when asked where the sweet lies. The participant must then identify where the actor (falsely) beliefs the sweet is. For instance, a child's mother moves a chocolate from the green to the blue cupboard while the child is outside playing. The participant is then asked to report the content of the actor's belief (Where does the child think the chocolate is?) or to predict the actor's action (Where will the child look for the chocolate?). Most children before the age of 4 are unable to distinguish the actual location of the chocolate (blue cupboard) from the actor's false beliefs on the former location (green cupboard), and use the actual location to infer the actor's belief. However, children of all ages perform better on false beliefs tasks when the (true) location of the target object is less salient, such as when the chocolate is eaten [Saxe et al., 2004a; Wellman et al., 2001]. Patients with lesions in the temporo‐parietal junction, who generally fail on false belief tasks, are able to indicate the correct box when the actor does not point at the wrong location [Apperly et al., 2004]. These children or patients have difficulties dissociating the actor's false goal from their own correct goal, but less so when their own (true) goal location is absent or less salient.
The diversity in social inferences is consistent with neuroscientists' modular view on the brain, where social cognition is seen as a neural circuit with a set of related and highly intertwined, but separate processes that are each specialized in some aspect of the social mentalizing system. Brain imaging techniques have identified two main areas responsible for human social cognition—the temporo‐partial junction (TPJ) and the medial prefrontal cortex (mPFC; see Fig. 1A,B). According to some authors, mentalizing is a high‐level mental process that is subserved mainly by cortical midline structures, especially the mPFC [e.g., Amodio and Frith, 2006; Gallagher and Frith, 2003]. On the other hand, an increasing number of authors suggest that the TPJ has specific social functions of its own, in particular for identifying the goals or intentions behind behaviors, together with the aid of “mirror neurons” [e.g., Gallese et al., 2004; Keysers and Gazzola, 2007; Saxe and Powell, 2006; Uddin et al., 2007].
Figure 1.
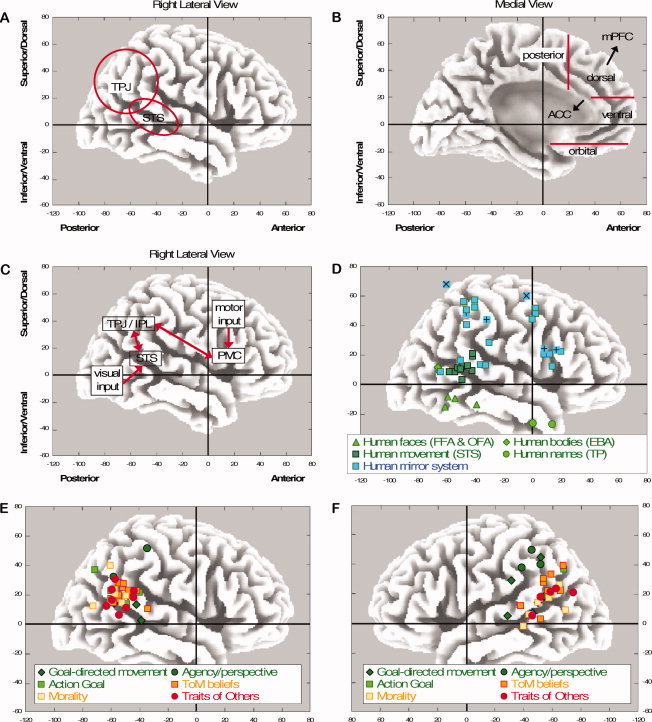
[A,B] The anatomy of the human brain, and the major areas involved in social cognition, placed in x‐y‐z stereotactic atlas. Left‐right (not shown) reflects the anatomical x‐axis, posterior‐anterior at the bottom reflects the anatomical y‐axis, and inferior‐superior (or ventral‐dorsal) on the left reflects the anatomical z‐axis. [C] The mirror system: Visual input in the STS is propagated to the TPJ/IPL, and further to the PMC where it is compared with own action schemas and associated goals. The matched goal behind the action detected at the PMC is sent back to the TPJ/IPL (for goal identification) and STS (for agency identification). [D] How a human, its body (parts), movements and name are represented in the brain in the FFA (Fusiform Face Area), OFA (Occipital Face Area), EBA (Extrastriate Body Area), STS (Superior Temporal Sulcus) and the anterior Temporal Pole, and how the mirror system is recruited for observing (posterior areas) and executing (anterior areas) movements of mouth/face [+], hand/arm [□] or foot [×]. [E,F] The TPJ involved in social inferences of intentionality and traits; right and left lateral view, respectively. The studies involved in D‐F can be identified via the y‐z coordinates in Table I.
I begin with a brief review of the functions of the temporo‐parietal cortex and the mirror system, followed by the functions of the mPFC. This overview is based on the most recent perspectives in this area based on brain imaging data and additionally supported by data from single‐cell recordings and lesion studies. There are probably as many different theoretical perspectives as there are major researchers in the social neuroscience field, so that it is impossible to include all relevant views here. Next, the meta‐analysis is presented and the results are discussed in view of the theoretical claims made earlier. I end with a short discussion of topics that are of interest for social psychology and social neuroscience.
How the Temporo‐Parietal Cortex identifies Action Goals: The Mirror System
Mirror neurons in the motor and associative cortex of humans and monkeys discharge not only when specific actions are executed, but also when these same actions are observed in other animals or humans [Gallese et al., 2004; Keysers and Perrett, 2004]. They allow identifying the underlying goals of a biological movement, by matching the perceived behavior with one's own behavior, and the most common goals associated with it.
This mirror mechanism is a very attractive proposal because it provides a very simple and elegant explanation for automatic inferences of self and other's intentions. How does it work? As depicted in Figure 1C, single‐cell recording studies with monkeys and fMRI studies with humans has revealed that the mirror system consists of a cortical structure involving the superior temporal sulcus (STS), the inferior parietal lobe (IPL) or parietal mirror system, and the premotor cortex (PMC) including the inferior frontal gyrus (IFG) or frontal mirror system [homologous to the F5 in monkeys; Gallese et al., 2004; Iacoboni, 2005; Keysers and Perrett, 2004]. Visual information in the STS is propagated to the IPL (∼60% of the neurons in the monkey), where it is passed to the PMC (∼20% of the neurons). The PMC region is responsible for action execution. There the perceived action, its future path and intention is recognized and identified by its resemblance to one's own actions, and this information is passed back to the IPL [Iacoboni, 2005; Keysers and Perrett, 2004]. Thus, the shared representation of other and self movements and intentions supplements observed visual input with inferences about what is not immediately visible but very likely to occur next. In a sense, the IPL “sees” the intentions behind other's actions by “simulating” or “matching” the actions of others in a shared representation [but see Jacob and Jeannerod, 2005, for a different view]. Perhaps, the mirror system developed this social function on top of an earlier and more basic function for fine‐tuning one's movements on the basis of visual feedback from one's own movements [Keysers and Perrett, 2004].
The mirror system implies that goals and intentions do not require a high‐level propositional or symbolic representation. Instead, a rudimentary coding of the anticipated spatial end‐state of an action may suffice. Single‐cell recordings in macaque monkeys demonstrate that different mirror neurons discharge when a movement (e.g., grasping) is part of different end‐state. Thus, when a monkey reaches for food and brings it to the mouth (e.g., for eating), different mirror neurons are activated than when the monkey places the food aside [e.g., for placing; Fogassi et al., 2005]. Similar goal identifying brain regions have been documented in the IFG of the human brain [Iacoboni et al., 2005]. Yet, some authors hypothesized that the left IPL is connected to the language motor system (Broca area) via the mirror system, so that a more symbolic representation may facilitate reasoning on other's goals and intentions [Iacoboni, 2005; Rizzolatti and Arbib, 1998].
It is important to note that although many mirror neurons in the STS respond to the same degree for other and own movement, a selective number of mirror neurons discharge only to visual information on other's movements, and tend to be inhibited by own movements and kinesthetic information [Keysers and Perrett, 2004]. These viewpoint‐other mirror cells allow the brain to resolve the issue of the identity of the actor. This provides us with a direct and automatic sense of agency or ownership, or the experience that body and movements are one's own or from someone else.
If the IPL mirror area is capable to infer the motor intentions of others on the basis of simple action observations, then it appears very plausible that the IPL or a related parietal mirror region could identify intentions that are of a more complex social nature. As this meta‐analysis will reveal, the TPJ which extends from the STS to the IPL, is the most likely candidate for such a mirror area of social cognition. If true, the TPJ should be involved in making the following inferences:
Inferring the intention underlying a perceived social movement or behavior.
Identifying the agent of a social action as the self, or as distinct from the self.
Distinguishing social intentions of the self from those of someone else, even when they diverge.
The mirror system goes a long way towards explaining action and intention understanding. However, evidence for the involvement of a mirror system in more abstract and long‐term forms of mentalizing is lacking. Instead, the prefrontal cortex seems to be more involved in the processing of long‐term traits of self and others, and interpersonal knowledge on norms and scripts [Keysers and Gazzola, 2007; Uddin et al., 2007].
How the mPFC Identifies Social Beliefs, Traits, and Scripts
Long‐lasting social dispositions and interpersonal knowledge such as personality traits and social rules involve the capacity to remember the behaviors of people over a long stretch of time under multiple circumstances, to recognize the common goal in these behaviors and to link them to the actors' most likely plan of past and future actions. For instance, to infer whether a person is socially skilled, one needs to observe his or her behaviors under easy and difficult circumstances (e.g., talking to one person or before an audience), and infer how he or she reacts under various goals (e.g., under free choice or coercion). There is growing evidence indicating that attributing traits and scripts involves the mPFC. This brain area has a high degree of interconnectivity through connections from the dorso‐lateral prefrontal cortex (PFC), the anterior STS, the TPJ, and other brain regions. Therefore, the mPFC can handle considerable neural input, and this may contribute to the capacity of the mPFC to implement more abstract inferences [e.g., Amodio and Frith, 2006; Leslie et al., 2004; Northoff and Bermpohl, 2004]. Unfortunately, there is as yet no computational account of the mechanisms that underlie this mentalizing ability.
In social psychology, trait inferences have been typically viewed in terms of internal causal attributions that obey the general‐purpose causality principles of contingency or covariation [Kelley, 1967] in much the same way as causal attributions for physical events. These theories emphasize the causal role of the actor in producing the behavior, unless external environmental factors constrain his or her freedom and mitigate his or her causal role. Perceivers automatically attribute the behavior to a correspondent trait of the actor, and discount the inference to the actor on the basis of situational circumstances if present and relevant [although this is sometimes done insufficiently; Gilbert and Malone, 1995; Gilbert et al., 1988; Trope and Gaunt, 2000]. This is typically seen as a high‐level reflective reasoning process involving “an iterative or even simultaneous evaluation of the various hypotheses before reaching a conclusion” [Trope and Gaunt, 2000, p. 353].
In social neuroscience, a number of general‐purpose evaluative and executive brain functions in the medial or lateral areas of the PFC have been proposed as subserving or facilitating trait inferences. Several authors have even suggested that maintaining different representations of self and other's intentions or beliefs is a high‐level capacity mediated by the PFC [e.g., Amodio and Frith, 2006; Decety and Chaminade, 2003; Frith and Frith, 2001; Gallagher and Frith, 2003; Leslie et al., 2004]. The following functions have been proposed and are explored in this meta‐analysis:
Emotional inputs (i.e., from the amygdala and orbital part of the PFC) may aid the formation of positive or negative evaluations about the self or others, which are a key component of trait inferences.
Monitoring one's behavior and in particular inhibiting irrelevant cues [e.g., Amodio and Frith, 2006] may help to discard one's own beliefs and contemplate beliefs from others.
Focusing one's attention selectively on others instead of the self has also been suggested as a crucial process subserving mentalizing [e.g., Leslie et al., 2004].
Working memory may aid in the representation of different perspectives of self and others and in the decoupling of mental states from reality.
Episodic retrieval may aid trait inferences of self and others.
Alternatively, recent neurological evidence suggests that neurons in the mPFC are uniquely oriented to time and fire over extended periods of time and across events [Huey et al., 2006; Wood and Grafman, 2003]. This has led to the hypothesis that the mPFC serves the integration of social information over time, by storing this information in traits or scripts [Huey et al., 2006]. Evidence from single‐cell recordings in rats [Runyan et al., 2004] documented that the mPFC is crucially involved in the learning of long‐term storage of information associating multimodal but temporally disconnected events. The neurons can continuously fire during an interval between an input and a delayed output. The mPFC stores these temporally disconnected events before or in parallel to hippocampal memory (believed to be the typical storage for episodic events). Likewise, Matsumoto and Tanaka (2004) reviewed several single‐cell studies with monkeys and reported that mPFC cells show increasing activation during the initial steps of learning a novel sequence of actions, and this activity avoids rejection of early steps that go unrewarded initially, but when maintained are rewarded in the final steps. A lesion study involving rabbits [McLaughlin et al., 2002] demonstrated that lesions to the mPFC delay trace conditioning (that requires forming associations between intervals that extend several seconds), but not conditioning at shorter intervals. In a lesion study with humans, Wood et al. (2005) documented that patients with lesions in the right ventral PFC (who show socially inappropriate behavior in their everyday life) are impaired to inhibit inappropriate parts of a social script sequence.
This meta‐analysis seeks evidence for these alternative accounts of the social cognition process in the mPFC, and explores to what extent each of them receives support. It does so by identifying the brain areas in the mPFC that subserve social inferences of traits and scripts and comparing them with the areas involved in general‐purpose functions to assess how much they might contribute to social inference. If general‐purpose functions of causality, emotion, and supervisory execution are core components of social reasoning, their activations should overlap considerably with social activations in the mPFC. On the other hand, if they only aid and facilitate social inferences, they will overlap less.
METHOD
The studies reviewed in this article were identified by searches in PubMed, ScienceDirect, and PsychInfo by the term “fMRI” along with at least one of the following terms “person,” “self,” or “social” in the title or abstract (or keywords when available to search). The search was confined between January 2000 and April 2007. For more complete coverage, I inspected several review articles from which I identified additional articles [Amodio and Frith, 2006; Beer and Ochsner, 2006; Decety and Chaminade, 2003; Frith and Frith, 2001; Gallagher and Frith, 2003; Grèzes and Decety, 2001; Matsumoto and Tanaka, 2004; Mitchell, 2006; Northoff and Bermpohl, 2004; Ochsner et al., 2004a; Olsen et al., 2007; Saxe et al., 2004a; Todorov et al., 2006]. In addition, to augment these studies with other functions that might be relevant to social cognition and that might activate the same brain areas, additional fMRI studies which also appeared between January 2000 and April 2007 besides those identified in the general search, were included for the following tasks:
Script sequences learning and response sequences learning (excluding motor sequence learning) identified by searches in PubMed and ScienceDirect by the term “fMRI” along with at least one of the following terms “sequence,” “script,” or “trace” in the title or abstract (or keywords when available to search).
Causal attribution identified by searches in PubMed and ScienceDirect by the term “fMRI” along with at least one of the following terms “cause,” “causal,” or “attribution” in the title or abstract (or keywords when available to search).
Emotional judgment and processing by including the studies listed in the review by Ochsner et al. (2004a).
Response conflict and inhibitory control by including the studies listed in the meta‐analysis by Neumann et al. (2008).
Executive and memory functions of the prefrontal cortex by including the brain coordinates from the meta‐analysis by Gilbert et al. (2006; their article search was confined between January 1999 to October 2004; the peak coordinates are reported in that article).
Studies were included only if they investigated unmedicated healthy children or adults, used fMRI scanning (unless reported otherwise), and reported the coordinates of activations in the space of the MNI template [Collins et al., 1994] or the atlas of Talairach and Tournoux (1988). When activations were reported in MNI space they were transformed into Talairach and Tournoux coordinates, using a nonlinear transformation (http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach) so that all coordinates were in a common stereotaxic framework. I restricted the brain areas to the regions of interest, including the superior temporal sulcus (STS), temporo‐parietal junction (TPJ) bilaterally, lateral prefrontal cortex (PFC) bilaterally, and medial prefrontal cortex (mPFC). For convenience, I included the anterior part of the cingulate cortex in the mPFC throughout this review (see Fig. 1B). I included all the studies that reported a priori defined tasks, even if their peak activation coordinates did not fall within any of these expected regions so that the proportion of studies satisfying the expected localization could be estimated. The reported activations were restricted to significant contrasts involving a comparison between an experimental versus base‐line control task or, in a limited number of studies, a parametric correlation with an experimental variable. If both types of outcomes were available in a single study, then only the coordinates involving the more relevant parametric correlation with the task of interest is reported. The contrast involved either increase or decrease in activation.
Task categories were identified that included components that were most identical among a set of similar studies, to create large enough samples of comparable functions of mental processes. Additional features of the studies were also categorized. The stimulus material was categorizes as visual versus verbal (see Table I), and the instructions were coded either as directing the participants explicitly to the process under study, or not and involving implicit social inferences (see “SSI” in Table I). When the description of tasks, stimuli, and especially instructions was not clear‐cut (e.g., no example or verbatim reports), the characterization by the authors was used for categorization. Classification of all studies proceeded in two steps. The first classification was provisional after the first reading of each article. A second and third classification was made about 1 and 3 months later, after rereading the relevant article sections and a decision was made to maintain or alter the original classification. This second and third reading generally confirmed the initial subdivision of task categories, although a few initial subcategories were collapsed or removed due an insufficient number of studies to permit reliable conclusions. Table I lists all the studies included in this meta‐analysis under the heading of each task category as classified in the final round. As can be seen, each task category incorporates a number of highly similar task components and control conditions.
Table 1.
Major task categories, stimuli, instructions, conditions and corresponding Talairach coordinates
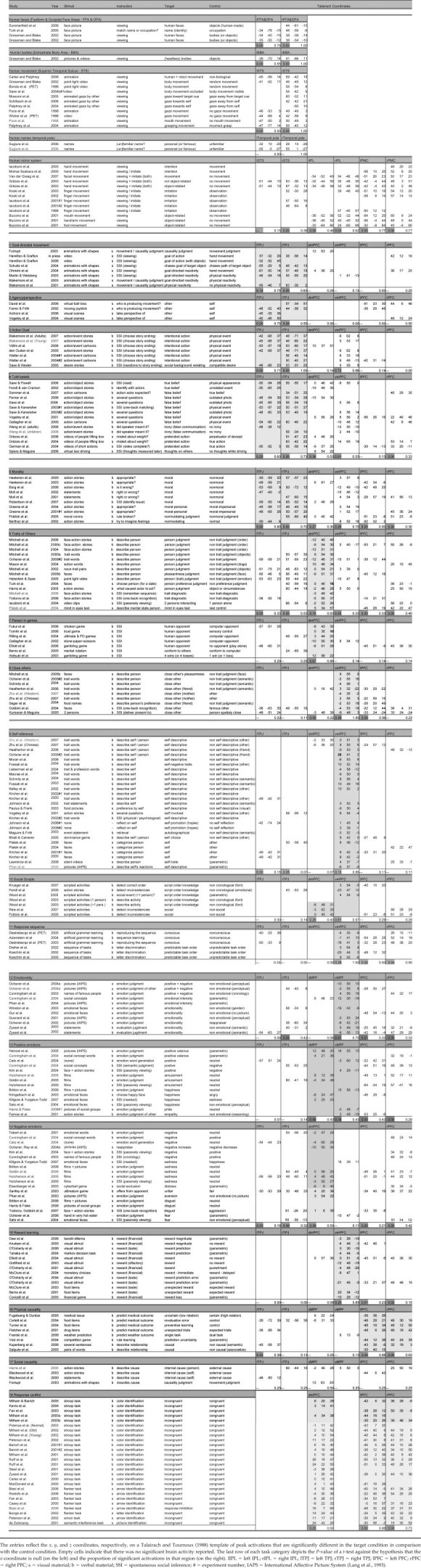
|
Activations were accepted as significant according to the criteria set by each study. To ensure that the activation peaks entered into the meta‐analysis resulted from independent contrasts, the reported coordinates were restricted to one per study and task category. In a limited number of cases, two activations were reported, one for each different task components of interest (e.g., two separate negative emotions) or different sample of participants. The retained coordinates involved the most significant activation peak for each of the regions of interest. Each activation peak was classified as medial when its distance on the x‐axis (left‐right axis) did not exceed 20 mm (although most studies were within a 12 mm distance, see Table I). For simplicity and coherence, a few small exceptions beyond the 20 mm limit were occasionally allowed if the majority of the conditions/studies were in the medial area, and vice versa for the lateral categorization (indicated in bold in Table I). Conventional t‐tests confirmed that the mean x‐coordinate for each task category was never significantly different from zero for the medial localization and significantly different from zero for the lateral localization (see Table I).
Because statistical tests were computed on dichotomous codings (i.e., presence or absence of activation), all tests were nonparametrical. Comparisons between brain areas involved Cochran's Q, and between sets of studies (e.g., task categories) involved Kruskal‐Wallis analysis of variance (ANOVA). Correlations were computed using Goodman‐Kruskal's gamma (Γ). All these tests are implemented in Statistica 7.0.
RESULTS
All the coordinates involved in this meta‐analysis are listed in Table I, and the main results and statistical tests are summarized in Table II. As shown in Table II, Cochran tests revealed that most tasks elicit differential activation across the various regions of interest, consistent with the idea that different areas are preferentially engaged in distinct processes (i.e., tasks) of social cognition. In addition, Kruskal‐Wallis' ANOVAs reveal to what extent these activations are consistent or different throughout similar task categories that span action intentionality (Tasks 1–5), trait inferences and scripts (Tasks 6–10), emotions and other general‐purpose functions (Tasks 11–18).
Table II.
An overview of major task categories and corresponding brain areas
| Task category | lTPJ | rTPJ | dmPFC | vmPFC | lPFC | rPFC | Cochran Q |
|---|---|---|---|---|---|---|---|
| Action intentionality (temporary) | |||||||
| Goal‐directed movement | 0.75 | 0.63 | 0.25 | 0.13 | 0.00 | 0.25 | 0.007 |
| Agency/perspective | 0.75 | 0.50 | 0.00 | 0.00 | 0.50 | 0.50 | 0.202 |
| Action Goal | 0.71 | 1.00 | 0.14 | 0.86 | 0.14 | 0.00 | <.001 |
| ToM beliefs | 0.60 | 0.73 | 0.53 | 0.53 | 0.13 | 0.33 | 0.025 |
| Morality | 0.80 | 0.70 | 0.30 | 0.70 | 0.50 | 0.30 | 0.141 |
| Kruskal‐Wallis ANOVA | 0.859 | 0.401 | 0.192 | 0.009 | 0.059 | 0.418 | |
| Traits and scripts (enduring) | |||||||
| Traits of Others | 0.33 | 0.53 | 0.93 | 0.33 | 0.33 | 0.40 | 0.005 |
| Person in games | 0.29 | 0.29 | 0.86 | 0.14 | 0.14 | 0.14 | 0.035 |
| Close others | 0.22 | 0.11 | 0.22 | 1.00 | 0.56 | 0.22 | <.001 |
| Self‐reference | 0.15 | 0.07 | 0.07 | 0.85 | 0.11 | 0.11 | <.001 |
| Social Scripts | 0.00 | 0.14 | 0.43 | 0.57 | 0.29 | 0.29 | 0.282 |
| Kruskal‐Wallis ANOVA | 0.386 | 0.012 | <.001 | <.001 | 0.083 | 0.276 | |
| Other functions | |||||||
| Response sequence | 0.33 | 0.00 | 0.00 | 1.00 | 0.50 | 0.50 | 0.004 |
| Emotionality | 0.08 | 0.42 | 0.08 | 0.75 | 0.33 | 0.33 | 0.005 |
| Positive emotions | 0.08 | 0.15 | 0.46 | 0.46 | 0.46 | 0.31 | 0.165 |
| Negative emotions | 0.17 | 0.28 | 0.56 | 0.11 | 0.22 | 0.39 | 0.015 |
| Reward learning | 0.00 | 0.00 | 0.08 | 0.85 | 0.23 | 0.15 | <.001 |
| Physical causality | 0.38 | 0.13 | 0.25 | 0.13 | 0.88 | 0.63 | 0.009 |
| Social causality | 0.25 | 0.25 | 0.50 | 0.25 | 0.25 | 0.25 | 0.956 |
| Response conflict | 0.00 | 0.00 | 0.92 | 0.00 | 0.88 | 0.73 | <.001 |
| Kruskal‐Wallis ANOVA | 0.035 | 0.012 | <.001 | <.001 | <.001 | 0.022 | |
Cell entries denote the proportion of studies where activation in that region was identified. The cell entries of the Kruskal‐Wallis ANOVA and Cochran Q (in italic) refer to the significance level P of these tests. Additional Kruskal‐Wallis tests reveal that the left and right TPJ activations do not differ for each of the tasks listed.
Table III lists the nonparametric Goodman‐Kruskal Γ between the presence of activation and (visual‐verbal) material or (explicit‐spontaneous) instructions. These two latter extraneous variables are largely independent, Γ = 0.06, ns. Table III demonstrates that for social inferences (Tasks 1–10), in general, the TPJ is more engaged given visual material and spontaneous instructions, whereas the mPFC as a whole is more engaged for verbal material. However, with respect to instructions, the dorsal part of the PFC is more engaged during spontaneous inferences (in line with the TPJ) while the ventral part is more engaged during explicit inferences. The right PFC is more engaged given visual material and the left PFC is more implicated during explicit instructions (perhaps reflecting a rehearsal of verbal instructions). It is obvious that material and instruction have a significant influence on brain activation beyond the social tasks involved, and this is further discussed in the following sections when appropriate.
Table III.
Gamma correlations between tasks and activation in specific brain areas
| Stimulus type | lTPJ | rTPJ | dmPFC | vmPFC | lPFC | rPFC |
|---|---|---|---|---|---|---|
| Visual–Verbal | ||||||
| Tasks 1–5 (n = 44) | 0.43* | 0.13 | −0.13 | 0.85** | 0.40 | −0.54* |
| Tasks 6–10 (n = 65) | −0.55** | −0.46* | 0.46* | −0.15 | 0.01 | −0.46* |
| Tasks 11–18 (n = 100) | −0.04 | −0.10 | 0.05 | −0.09 | 0.31* | 0.41** |
| All Tasks (n = 209) | −0.04 | −0.11 | 0.10 | 0.25** | 0.14 | −0.07 |
| Explicit–Spontaneous | ||||||
| Tasks 1–5 (n = 44) | 0.47* | 0.63** | 0.13 | −0.10 | −0.52* | −0.40 |
| Tasks 6–10 (n = 65) | 0.55** | 0.30 | 0.75*** | −0.82*** | 0.19 | 0.30 |
| Tasks 11–18 (n = 100) | −1.00 | 0.31 | −0.35 | −0.53* | −0.61** | −0.01 |
| All Tasks (n = 209) | 0.56*** | 0.59*** | 0.11 | −0.35** | −0.45*** | −0.16 |
The more verbal or spontaneous, the stronger a positive correlation is
P < 0.05;
P < 0.01;
P < 0.001.
Where We Aim to: Action Goals and Intentions
Recent fMRI research has revealed that the perception of social information and in particular goal‐directed behavior is supported by (a) incoming information from the visual system that detects and represents biological information, such as human faces, bodies, and movements, and (b) the mirror system that identifies the goals behind these behaviors. Although this issue is not part of this meta‐analysis, it is informative to sketch a relevant background against which social information is processed. Therefore, I first briefly describe this visual and mirror system.
Identifying the social other
Where and how is the visual input on biological or social stimuli and movements represented and analyzed by the human brain? I selected an illustrative fMRI study by Grossman and Blake (2002) as well as other studies that reported several distinct areas from the primary visual system and the temporal cortex that are involved in identifying humans and human movement (for a more extensive review, see Allison et al., 2000). In addition, I included a number of illustrative studies highlighting the brain areas involved in the mirror system for identifying simple movement (see Fig. 1D).
The recognition of human faces involves two regions known as the fusiform and the occipital face area [FFA and OFA; but see also Turk et al., 2005];
The recognition of a stationary body of other people involves the extrastriate body area (EBA), and recent research suggests that one's own body movements (e.g., of hand and foot) can also involve the EBA [Astafiev et al., 2004; Jeannerod, 2004];
More importantly, human motions such as body, gaze, and hand movements involve the superior temporal sulcus (STS). This is most often identified at the right hemisphere (all studies reported in Table I or 100%) and less so at the left hemisphere (55%). It is important to note that fMRI responses to human movement have been shown not only with films of human actors, but also with robots that mimic human movements as well as with point‐lights that display movements with only a handful small dots at the major human joints while all the rest is invisible. Our familiarity with human and biological movement render these moving light dots so compelling that they are perceived as natural movements in comparison with dots moving randomly [Bonda et al., 1996; Grossman and Blake, 2002].
Somewhat separated from the previous regions, the names of known people are represented in the lower anterior part of the temporal lobe. It has been suggested that this region is implicated in a neural circuit for person identity [Sugiura et al., 2006].
The mirror system for identifying simple hand, mouth, face or foot movements (e.g., grasping an object) is composed of the STS, IPL (extending to the superior parietal lobe) and PMC. To elucidate the location of this mirror system in humans, researchers typically sought for brain areas common to action observation and execution (i.e., imitation) as opposed to static positions, for areas that were more strongly recruited during imitating as opposed to viewing movements, or that were more strongly engaged during goal‐directed movements as opposed to movements without goal involvement. Of interest is that the parietal lobe and PMC reveal a rough somatotopic organization, much in line with the classic homunculus of the motor system, with foot movements at superior areas and mouth/face movements at inferior areas, with hand movements in‐between. This somatotopic organization has been revealed also for auditory input [Gazzola et al., 2006]. Two recent studies also identified the IFG (an inferior part of the PMC) as an area involved in identifying the goals of hand movements (e.g., during behaviors like drinking and cleaning) as opposed to action execution alone [Iacoboni et al., 2005; Molnar‐Szakacs et al., 2005]. That the mirror system codes predominantly goals rather than movements themselves, has been demonstrated by the activation of this region during observation of hand movements even when the specific details of the movement are outside the motor vocabulary of the observer: hand actions for participants born without hands [Gazzola et al., 2007a] and robotic actions for typically developed participants [Gazzola et al., 2007b].
All these areas of human visual perception, with the exception of person names, are located in a specific and limited part of the superior temporal and parietal cortex (see Fig. 1D). The human mirror system additionally recruits the PMC, especially for goal identification of behaviors.
Identifying action intention
Contrary to simply action identification which recruits the IPL, social human behavior recruits the TPJ, which stretches between the IPL and STS involved in the visual detection of biological movement (Fig. 1A). As noted in the introduction, there is large agreement that the human visual information detected at a lower level in the STS as well as verbal information, informs the higher‐level analysis at the TPJ. If our suggestion is correct that the TPJ is a key mirror site for social cognition, than the TPJ should be involved in the identification of the goals and intentions of humans. Kruskal‐Wallis ANOVAs in Table II confirm that inferences of action intentionality (Tasks 1–5) consistently engage the TPJ, although the mPFC is also involved in some tasks. The activation of the left and right TPJ does not differ significantly between each other, as also revealed by additional Kruskal‐Wallis tests. This is true for all tasks listed in Table II.
Figure 1E,F depicts the TPJ coordinates of a large set of fMRI studies in which the critical task is to infer intentionality by the actor, either close at the perceptual level by inferring the expected end‐goal or actor (Task 1–2), or at a higher cognitive level by inferring the actor's beliefs on the desired outcome (Task 3–5). As can be seen in Table I, the task stimuli involve mostly verbal and visual material like short stories or sentences, or visual material like cartoons or videos, as well as interactive games with real humans. This is consistent with the notion that the TPJ has a multimodal functionality, that is, it reacts to visual as well as verbal material (e.g., stories).
Goal‐directed Movement: At the most basic perceptual level perhaps, participants are viewing animations of shapes that move in a human‐like manner in comparison to random movements. This is reminiscent of a classic study by Heider and Simmel (1944) in which observers ascribe human intentions to moving circles and triangles (such as chase, follow etc.). Given their human‐like nature, observers perceive them as biological movements that are directed towards a goal, resulting in TPJ activation (75% and 63% of the studies in Table I, for the left and right hemisphere, respectively). It is interesting to note that these animations are so compelling that they create greater activity in the TPJ than goal‐directed hand manipulations of an object (cup, hammer, telephone etc. Ohnishi et al., 2004).
Agency and Perspective: In these studies, participants observe a visual scene or movements involving different persons and identify the relative locations of the objects from the perspective of the self or other, or identify whether the other (versus the self) is initiating the movement. Although these tasks are only somewhat similar and could have been separated in two task distinct categories (if more studies were available), they both require the perceiver to distinguish own action perspective and goal from those of other persons. Nevertheless, these tasks consistently engage the TPJ (75% and 50%), in agreement with several similar PET studies that also reveal the TPJ as major site of brain activation [Chaminade and Decety, 2002; Chaminade et al., 2002; Decety et al., 2002; Farrer et al., 2003; Ruby and Decety, 2001].
-
Action Goals: Participants are requested to identify the likely or desired end state of a story in comparison with mere physical consequences (e.g., a gulf destroying a sand castle at the beach). Although many earlier reviews have tended to confound this task with ToM inferences (see below), we isolated it as theoretically distinct and important, because it only requires identifying the implied goal of an action, without an understanding or insight of the actor's own beliefs. Goal inferences lead to activation in the TPJ (71% and 100%).
ToM Beliefs: At a somewhat higher inferential level, participants must not only realize that others' movements and behaviors involve goals and intentionality, they must also identify these intentions. This requires the understanding that the protagonist acted the way he or she did, only because of a distinct goal in mind, a capacity called theory of mind (ToM). Several tasks have been devised to measure this capacity. In tasks that involve a “true” belief, the observer simply needs to identify the protagonists' belief or intention. However, the ToM capacity becomes most evident when own and other intentions diverge in “false” belief tasks described earlier, or when participants have to detect false from true actions or communications (as in irony) because these tasks require distinguishing between own and other beliefs.
Earlier studies using the false belief task took physical events as control tasks, whereas more recent studies apply carefully matched controls, such as stories involving photos that are “false” in the sense that they became outdated after some critical event [Perner et al., 2006; Saxe et al., 2006]. Similarly, in other tasks, videotapes show actors who on some occasions pretend to lift objects that are heavier than they actually are in comparison with the same action without deceit of the objects' weight [German et al., 2004; Grèzes et al., 2004, 2006; see Table I]. Many of these tasks lead to increased TPJ activation (60% and 73%).
Moral Judgments: The transition from false beliefs to unjust or unfair beliefs or intentions appears to be a close one. This is attested by the fact that the TPJ is also strongly involved in judgments of morality. Participants are given stories or dilemmas with different levels and types of moral injustice (e.g., commissions of unjust actions or omissions of just actions; where a person is critically involved or not; where a moral dilemma is left open or the wrong‐doing has been committed). What is common in all these tasks is the intentional act of wrongdoing. Moral, personal, or norm violating stories elicit more activation in the TPJ than nonmoral, impersonal or normal activities, respectively (80% and 70%). Thus, when confronted with a moral dilemma, we seem to base our decisions on right or wrong intentions to act or effortful action, rather than on a logical analysis of death count and so on. This emphasis on intentionality is also seen in the law (e.g., difference between premeditated murder and accidental manslaughter).
Summary and discussion
The studies in this overview support the contention that the TPJ is crucially involved in the identification and representation of action goals. However, as can be also inspected on Figure 2A, many tasks on intentionality also engage the mPFC. This has led to the view that the mPFC is the key region in mentalizing and aids in the realization of different perspectives for tasks that require decoupling other's perspective from one's own or reality [Frith and Frith, 2001; Gallagher and Frith, 2003]. However, true belief tasks do not require holding in mind diverging beliefs or intentions, and yet they also tend to activate the mPFC [Ferstl and von Cramon, 2002; Saxe and Powell, 2006; see also Table I]. More importantly, Saxe and Powell (2006) found that the TPJ was more strongly activated in relation to beliefs for inferred events (that could be false, e.g., “his dog broke loose so he guessed that the leash had come untied”) versus true events (e.g., “he was sick, felt weak and had a high fever”). In contrast, the mPFC was equally activated in these two conditions. Similarly, Sommer et al. (2007, see Table I) found stronger activation of the TPJ for false beliefs in comparison with true beliefs, while the mPFC was not differentially engaged. Lesion studies with humans have demonstrated that patients with frontal lobe lesions make more mistakes on difficult false belief and deception tasks than patients with other brain lesions, although they make no more errors on the simplest of the false belief tasks [Stuss et al., 2001]. Hence, holding in mind different perspectives seems not the key explanation for the involvement of the mPFC.
Figure 2.
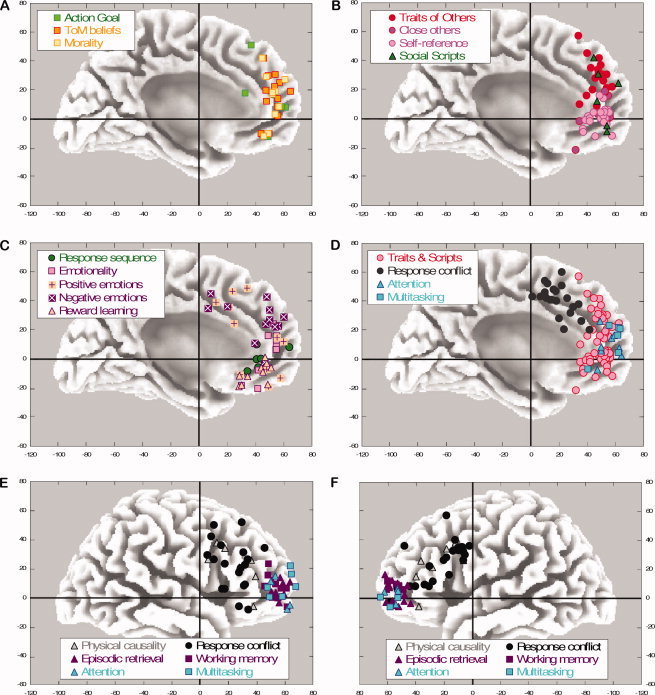
[A] The mPFC involved in social inferences of intentionality. [B] The mPFC involved in social inferences of traits (other and self) and scripts. Social inferences during interpersonal games are included in the trait inferences of others. [C] The mPFC involved in sequence learning and emotional responses. [D] The medial PFC involved in executive functions, in comparison with social inferences of traits and scripts (copied from Fig. 2B). [E,F] The lateral PFC involved in causal learning and executive functions; right and left lateral view, respectively. The studies involved in A‐F can be identified via the y‐z coordinates in Table I.
Closer inspection of the data suggests another reason for the engagement of the mPFC. Inferences on goal‐directed movement and agency (Tasks 1–2, see Table I) are based on visual stimuli, while the other intentionality and belief judgments (Tasks 3–5) are predominantly based on verbal action stories, and this difference is highly significant, Kruskal‐Wallis H (1, N = 44) = 23.17, P < 0.001. More importantly, a nonparametric correlation between the presence of verbal material and the engagement of the mPFC is highly significant, not only for the ventral part as listed in Table III (Γ = 0.85, P < 0.001), but also when the ventral and dorsal parts are taken together (Γ = 0.90, P < 0.001). This suggests that the mPFC is additionally engaged when there is no immediate visual substrate on which to base intentionality judgments.
But why is the mPFC engaged under such circumstances? Perhaps, verbal stories may require more cognitive or complex processing subserved by the mPFC. This is consistent with many perspectives that stress this region as major site of social cognition [Amodio and Frith, 2006; Leslie et al., 2004; Northoff and Bermpohl, 2004]. Alternatively, verbal stories are typically richer in socially relevant context, and therefore may have induced participants not only to infer action goals, but also traits of the actors involved. For instance, by hiding a desired object from view in a false belief scenario, one might be tempted to infer that the actor is unreliable or unfriendly. Social psychology research has since long established that we often make trait inferences automatically with little awareness or intention on the basis of short behavioral descriptions [see Uleman, 1999, for a review]. This interpretation is supported by a significant nonparametric correlation revealing that spontaneous judgments across all social task categories (denoted “SSI” for Tasks 1–10 in Table I) elicit more activation not only in the left and right TPJ (Γ = 0.58 and 0.54, P < 0.001), but also in the dorsal mPFC (Γ = 0.42, P < 0.01) which is believed to subserve trait inferences about others (see below). Because none of these studies were designed to control for spontaneous trait inferences, it is unclear to what extent this interpretation is correct.
Taken together, there is strong evidence that the TPJ is a necessary substrate for inferring the goals of others even if they diverge from one's own, but perhaps the mPFC may be involved in more complex and explicit meta‐representations and distinctions of social inference, when rich verbal material is available or when we (spontaneously) make additional trait inferences about others. The parietal area is known as an association cortex responsible for the analysis and identification of higher‐order information with respect to spatial location (the “where” system). One type of spatial information is the expected end‐point of a movement, that is, its goal. This is a basic capacity of many fast‐moving organisms, which enables perceivers to orient themselves in order to avoid unwanted collisions, or to approach and collaborate with each other efficiently. We can therefore label the TPJ as the “where‐to” system. A recent study by Mitchell (2008) provides support for this idea. Participants performed typical ToM tasks as well as an attentional orienting task. This is a simple task where an arrow cues the participants towards the position of a forthcoming target stimulus. It was found that the same area of the TPJ was implicated in the ToM task as well as in the orientation task when participants were miscued and their expectations on the target location were violated. This shows that this region is involved in a variety of other nonsocial tasks that require participants to (re)orient their attention to a task‐relevant direction or end‐point. Future research may perhaps identify separate sub areas in the TPJ dedicated to social and nonsocial orientation functions.
Who We Are: Inferences of Traits and Social Scripts
As noted in the introduction, enduring social dispositions and interpersonal knowledge, such as personality traits and social rules, involve the capacity to remember the behaviors of people over a long stretch of time under multiple circumstances, to recognize the common goal in these behaviors and to link them to the actors' most likely plan of past and future actions. Permanent characteristics tell us what kind of a person someone is, and social scripts tell us to what kind of group we belong.
It has been hypothesized that the mPFC is crucially involved in the formation of such enduring inferences. The mPFC is a large brain structure that can be functionally subdivided (see Fig. 1B) into a posterior part, a dorsal part (dmPFC) which lies above the 20 mm coordinate of the inferior‐superior z‐axis, a ventral part (vmPFC) which lies below it, and an orbital part which lies below the −15 mm coordinate of the z‐axis. The dorsal and ventral parts are the core regions of social cognition.
Identifying the traits of others and self
In large agreement with most theoretical positions, enduring social judgments uniformly involve the ventral or dorsal part of the mPFC (see Table II). This is attested by a nonsignificant Kruskal‐Wallis H (4, N = 65) = 4.86, ns, when considering these two parts of the mPFC together. The TPJ is also engaged, but only in one task (i.e., inferring traits of others; Kruskal‐Wallis H (4, N = 65) = 12.96, P < 0.05; see also Fig. 1E,F). The tasks that engage the mPFC are depicted in Figure 2B and detailed in Table I.
Trait Inferences: These studies require participants to make judgments in terms of enduring traits (instead of immediate action goals) about actors on the basis of short stories, sentences or on the basis of single trait words (studies that also involve self judgments were excluded from this category, as this might induce a different mind set than mere judgments about others). All these tasks activate the dorsal part of the mPFC, except for the study by Heberlein and Saxe (2005) who made a somewhat unusual comparison between trait and emotion inferences and found an increased activation in the ventral part of the mPFC (although close to the dmPFC). Tasks that require participants to make unrelated judgments (e.g., memory tasks) suggest that trait inferences were also made spontaneously (see “SSI” in Table I) for trait‐implying actions compared to non trait‐implying actions, as the former lead to greater activation in the mPFC [Iacoboni et al., 2004; Mitchell et al., 2006; Todorov et al., 2007].
Interactive Games: Trait impressions of others are presumably also spontaneously made in interactive games because to win a game, it is important to make an accurate guess on the next moves of the opponent. To do so, one must develop an impression on the trust, cooperativeness or competitiveness of the other. Quite often in these tasks, the control condition is a computer instead of a human opponent, which seems to induce other strategy considerations. In other studies, a first win or loss is compared against long‐term wins or losses (which presumably induce more trait inferences). A great majority of these games involve the mPFC (100%; the majority or 86% in the dorsal part).
Close Others: The more ventral part of the mPFC is activated when people make trait inferences about familiar others, such as mother, relatives, and friends (100%). Given that close others are often experienced as very similar to the self, it is perhaps quite plausible that judgments of close others is located in the same brain area as judgments about the self (see later). Similarity to the self was parametrically analyzed by Mitchell et al. (2005b) and their results indicated that when others are judged similar to the self, this strongly activates the vmPFC (see also Table I).
Self‐references: A plethora of studies explored all sorts of inferences about the self, from pure trait ratings and permanent descriptions that are applicable to the self, thinking about one's hopes or memories about the self, to involvement of the self in viewing of one's face, or in interaction games. Although these latter tasks are not directly about traits, it is easily imaginable that while seeing one's face or other self‐relevant material or while thinking about one's hopes and choices, many characteristics and dispositions of the self are spontaneously generated, retrieved, or compared. Of the more than 20 studies listed in Table I, 85% elicit the ventral part of the mPFC. Note also in Figure 2B that all judgments of self and close others are located at the vmPFC and never surpass the 20 mm z‐coordinate (with one exception). Contrary to some alternative theoretical views, no other regions are systematically engaged in the representation, evaluation or description of the self.
Social Scripts: These studies do not focus on a single actor, but rather on social scripts that describe adequate social action for all actors. Participants are asked to check for an incorrect versus correct sequence order within scripted stories. These tasks require activating and employing interpersonal knowledge on social rules and scenarios by checking the correct chronological order. In all these studies, social scripts consistently engage the mPFC, both in the ventral or dorsal part (100%).
Summary and discussion
Undoubtedly, there is a great deal of consistency in the location of trait inferences and social script knowledge, as the amount and consistency of the empirical evidence is impressive. In all these enduring social judgments, the mPFC is almost uniquely engaged. Trait information about unfamiliar others selectively engages the dorsal part of the mPFC, whereas the ventral part is implicated when making trait inferences about familiar others or the self. The dmPFC can thus be considered the neural substrate of trait processing of other people, whereas the vmPFC can be considered the anatomical substrate of core experiences of the self and close others. Knowledge on social scripts involves both parts of the mPFC.
No other regions of interest are reliably implicated (<35%), except for the rTPJ which is also engaged in trait inferences about others (Task 6). This can be explained by the tendency for enduring social judgments (Tasks 6–10) to engage the TPJ more when visual task material is provided, as attested by a significant nonparametric correlation (Γ = 0.45 and 0.55 for right and left TPJ, respectively, P < 0.05). This parallels the opposite effect of temporary goal inferences (Tasks 1–5) discussed earlier where verbal material additionally engages the mPFC. Hence, the conclusion that enduring trait and norm inferences crucially involve the mPFC seems overwhelmingly supported by the empirical data, whereas the TPJ seems additionally engaged for processing visual material about others. This provides support for the view that the understanding of humans as enduring organisms with permanent social and psychological properties such as traits and norms is a crucial common element that engages the mPFC.
Is Social Cognition Subserved by Other Brain Functions?
As we have seen in the introduction, a plethora of hypotheses have been offered on how mentalizing in the mPFC is served by other complex brain functions involving time integration, emotionality, causality, or executive processing (e.g., inhibition of one's own beliefs in favor of other's beliefs by supervisory control or attentional mechanisms; working memory processes in differential perspective taking and the decoupling of mental states from reality; episodic retrieval in the service of self‐references). An analysis of these functions provides us with a sense of how much they are selectively used for social cognition. If the activation of a function falls within the ventral and dorsal areas of mPFC, then there is reason to believe that it is a core process of social cognition. If not, and the overlap is only partial, then this function more likely aids social processes, but is not an integral part of it. The selection of studies on these additional task categories was based on the general literature search and additional searches detailed in the Method section.
Response Sequence: It has been hypothesized that enduring social inferences and knowledge on traits and scripts require the integration of chronological sequences over larger time intervals, and that this time integration is performed in the mPFC [Huey et al., 2006; Wood and Grafman, 2003]. This implies that the mPFC is engaged in several form of sequence learning, not only in social script learning. Given the paucity of fMRI experiments on sequence learning, PET studies on this topic were also included in this analysis (see Table I). Sequence learning tasks do not require to generate motor sequences, but to respond to observed stimulus sequences with an appropriate response. Perhaps, the most well‐known example of a response sequence task is artificial grammar learning [Destrebecqz et al., 2003, 2005]. Participants respond to the spatial locations of stimuli (e.g., left or right whenever the stimulus appears somewhere left or right on the screen), which are presented in a predictable order or sequence of about 15 locations long. After some time, participants may become sensitive to this sequence of responses. Destrebecqz et al. (2003, 2005) measured the participants' brain activation when they are requested to generate the sequence and are consciously aware of it. As shown in Table I and depicted in Figure 2C, these and similar studies identified provide evidence that sequence learning engages the ventral part of the mPFC (100%), although in some studies activation extends to the lateral PFC.
Emotions: What is the role of emotions in “cold” social cognition processes? As shown in Table I and Figure 2C, emotional compared to nonemotional judgments (82%), as well as the experience of positive (79%) and negative (63%) emotions activate the dorsal and ventral regions of the mPFC, although they seem to engage the superior part of the mPFC as well. All these studies use verbal as well as visual material, and explicit or implicit instructions. In addition, other studies examining how people learn to discriminate stimuli that are financially or sensory rewarding as opposed to nonrewarding or punishing also implicate the vmPFC, extending further to the orbital mPFC (85%). As emotions and evaluations on our self and other people are part of how we form traits about them, it is perhaps not surprising to see that both the ventral and dorsal parts of the mPFC are involved. However, emotional responses engage regions that extend also to more orbital and superior regions of the mPFC.
Causality Detection: In traditional theories of social psychology, trait inferences are interpreted in terms of internal causal attributions that obey the classical causality principles of contingency or covariation [Kelley, 1967] in much the same way as causal attributions for physical events. To assess the possible role of covariation and causal reasoning in trait inferences, several studies were explored that require participants to assess causal contingencies of physical (i.e., nonsocial) and social events.
For nonsocial causal attributions, participants learn, for instance, to predict the possibility of a medical outcome (e.g., an allergic reaction) by extracting the covariation between food items and the presence of allergic reactions after reading several medical cases. As can be seen in Figure 2E,F and Table I, quite surprisingly, none of these physical causality tasks engaged the mPFC, but rather the lateral PFC (88% and 63% at the left and right, respectively). The strongest activation is reported at unexpected trial outcomes or when the learning error or uncertainty was greatest (so that learning opportunities at subsequent trials are largest).
In contrast, the location of social causality judgments is less specific. Participants are requested to give the internal (personal) versus external cause of several social behaviors depicted in short stories or animations. Contrary to BlackWood et al. (2003) who found activation in the lateral PFC for stories involving self attributions, Harris et al., (2005) found activation in the mPFC, just as we had seen earlier for trait inferences. Perhaps, this difference is due to the fact that Blackwood used short behavioral sentences, while Harris et al. (2005) provided additional information summarizing the covariation across other people, circumstance, or time [cf., Kelley, 1967]. This may have rendered the whole action description more socially rich, leading to more trait inferences and the activation of the mPFC.
Response Conflict: Many authors speculated that mentalizing is in part directed by the ability to monitor one's behavior and inhibit irrelevant cues. This would allow inhibiting one's own intention beliefs in favor of other people's beliefs in false belief tests [e.g., Amodio and Frith, 2006]. Response Conflict and inhibition is typically investigated in Stroop and flanker tasks. For instance, in a Stroop task, participants report the color of a word in ink, and this task requires inhibition of the semantic meaning of the word when it involves an incongruent color (e.g., the word “red” written in blue ink). The activity of the mPFC reported in these studies (see Fig. 2D) overlaps entirely with the mean peak activity observed in an extensive meta‐analysis on response conflict by Barch et al. (2001; Talairach coordinates 3, 19, 35) and by Neumann et al. (2008). As can be seen in Figure 2D, although action monitoring is located at the midline PFC, it often engages more posterior/superior regions outside the dorsal en ventral regions implicated in social cognition, and also extends in the lateral regions (Fig. 2E,F).
Executive Functions: Other roles for executive functions in social cognition have been put forward, including the capacity to focus one's attention on others versus the self [e.g., Leslie et al., 2004], as well as other executive and memory functions of the PFC, including dual attention and episodic retrieval. To assess these possibilities, I plotted all coordinates on executive brain functions from the recent meta‐analysis by Gilbert et al. (2006) in Figure 2D–F. As can be seen, none of the coordinates of these executive and memory functions are located at the mPFC, except for a small portion of the multitasking and attention studies that tend to be located at a more anterior part of the mPFC, and extend also to the lateral PFC. In a recent study comparing directly mentalizing and attention tasks, Gilbert et al. (2007) confirmed that attention was located more anterior (at the tip of the mPFC) than mentalizing functions. Although a number of fMRI and PET studies revealed a substantial overlap between autobiographic memory [Graham et al., 2003; Maguire and Frith, 2003; Maguire and Mummery, 2003] and self‐references in the vmPFC, the results seem to indicate that this has little to do with episodic memory per se, which shows little overlap. Given the limited number of autobiographic studies identified and the high degree of self‐involvement they share with self‐reference studies, it remains unclear whether autobiographic memory is a separate memory system or whether it is involved in a larger social self system.
Summary and discussion
Of the many additional functions explored in this analysis that involve the PFC, only sequence learning seems to overlap completely within the ventral or dorsal areas of the mPFC implicated in inferences of enduring traits and scripts. This is consistent with the idea put forward by Grafman and colleagues that the mPFC is especially tuned towards the integration of relevant stimuli over time [Huey et al., 2006; Krueger et al., 2007; Wood and Grafman, 2003; Wood et al., 2005]. In the social domain, this takes the form of traits and scripts of social behavior. Thus, there is strong evidence that time integration is a core component of enduring social inferences that is accomplished in the mPFC, although that region is not selectively used for social processing, as it is also implicated in nonsocial time‐related processes.
No other brain function seems to selectively subserve social inferences, because the overlap between their activation and the ventral and dorsal mPFC is limited (with the exception perhaps of autobiographic memory). Because the brain areas implicated in emotional experiences extend beyond the ventral and dorsal parts of the mPFC, it seems more likely that emotionality is a not a subsystem of trait impression formation per se [see also Amodio and Frith, 2006, Heberlein and Saxe, 2005], but rather a different neural circuit (involving also subcortical areas, such as the amygdala) of which the input to the mPFC presumably modulates and enriches trait inferences with affective associations. Interestingly, it is tempting to speculate that the close proximity of the reward system in the orbito‐frontal cortex explains the ventral position of trait inferences of self and familiar others (who typically generate more warmth and emotionality) relative to the more dorsal positions of the same judgments for unknown people.
Given the minimal overlap with the ventral and dorsal mPFC, the current meta‐analysis suggests that physical causality detection, action inhibition, attention and other executive or episodic memory functions may perhaps influence social cognition, but do not play a crucial role in it. This contradicts earlier proposals that endowed these functions a greater role in social cognition [e.g., Amodio and Frith, 2006; Leslie et al., 2004; Northoff and Bermpohl, 2004].
CONCLUSIONS
This overview covers more than 100 fMRI studies on human social cognition as well as about 100 fMRI studies involving potentially related functionalities. Based on past research in social and development psychology, I distinguished two major types of social judgments: (1) Temporary inferences involving the detection and identification of a person's goals and goal‐related beliefs, and (2) enduring inferences of personality traits and interpersonal scripts and norms. This distinction is also revealed in the progressive mastery of social capacities by young children up to the age of eight [Rholes et al., 1990], and in social research on the processing stages from goal identification to trait inference [Malle and Knobe, 1997; Malle et al., 2000; Reeder, et al., 2002, 2004]. For instance, when an actor engages in harmful behavior, we wonder which reasons or motives may have caused this behavior (out of a motive to harm or after provocation?), and this will determine whether we see this person as violent or not. The data in this meta‐analysis are consistent with this framework, and can perhaps be best interpreted as revealing a neural circuit starting from the more posterior TPJ, where immediate goals and desires are inferred, towards the more anterior mPFC associated with inferences of a more explicit nature of temporary goals and of more enduring traits and scripts.
Intentionality and Goals
This review provides strong support for the claim that the TPJ is a necessary substrate for inferring the goals of others, even if they diverge from one's own as in a false belief task. The data document that this neural system is sufficient to identify the direction and goal of behaviors that are visually available, and I therefore labeled the TPJ the “where‐to” system in analogy with the “where” spatial system in the parietal lobe. It is still a matter of debate whether the TPJ is sufficient when behaviors are presented in a verbal (including a mixed cartoon‐like) format, because in these cases the mPFC is also involved. It is possible that the greater complexity of such stimulus material requires additional processing in mPFC for representing explicit meta‐representations. Alternatively, such verbal material is richer in social content and context, which perhaps allows for more spontaneous trait inferences that engage the mPFC. Research that controls for such spontaneous inferences is currently lacking, but is much needed if we want to learn more about the role of the mPFC in inferences of the goals and intentions of others.
The major role played by the TPJ for inferring goals and intentions is consistent with idea of a mirror circuit for identifying intentionality in the human brain as suggested by Keysers and Perrett (2004) and Iacoboni (2005), although this region is more caudal and inferior to the IPL which was identified in studies on the mirror properties of simple movements. As far as I am aware, evidence on the mirror properties of the TPJ for complex social behavior is currently lacking, and research on this crucial topic is needed. Also lacking is evidence on the role of the PMC as the “matching” site of the mirror system for identifying and representing intentions underlying complex social behaviors.
Enduring Traits and Scripts
In contrast to the somewhat equivocal role of the mPFC in immediate goal inferences, the meta‐analysis showed robust empirical evidence consistent with the idea that understanding humans as enduring organisms with permanent social and psychological properties such as traits and norms is a crucial common element that engages the mPFC. This analysis also demonstrated that trait information about unfamiliar others selectively engages the dorsal part of the mPFC, whereas the ventral part is implicated when making trait inferences about familiar others or the self [see also Mitchell et al., 2005b]. Knowledge on social scripts involves both parts of the mPFC. No other regions analyzed in this meta‐analysis are reliably engaged, except the rTPJ during trait inferences of other persons. It was, however, argued, that this is most likely due to the visual information which tends to engage the TPJ.
However, this does not imply that no other functions are subserved by the mPFC. However, of the many brain functions explored, only sequence learning seems to be tied intimately to social cognition, as both areas of activation overlap entirely. This provides support for the idea that the mPFC is crucially involved in the integration of information across time, be it social or not (Huey et al., 2006; Krueger et al., 2007; Wood and Grafman, 2003; Wood et al., 2005). This meta‐analysis also documented a substantial overlap with emotional and reward processing, which confirms the long‐held idea that the mPFC is strongly involved in the integration of different sort of information. However, the data suggest that emotionality is a not a subsystem of trait impression formation per se [see also Amodio and Frith, 2006, Heberlein and Saxe, 2005], but rather that it provides input to the mPFC to modulate and enrich social inferences with affective associations. Other general‐purpose functions engage areas of the PFC that are not activated during social cognition, and are therefore very unlikely to constitute a core process of social inference, contrary to ideas put forward by many authors in the social neuroscience literature [e.g., Amodio and Frith, 2006; Leslie et al., 2004; Northoff and Bermpohl, 2004] and social psychology [cf. Kelley, 1967]. These distinct functions are physical causality, action monitoring and inhibition, divided and directed attention, episodic memory, and working memory.
OPEN QUESTIONS
The amount of research devoted to social cognition in the last decade has been impressive. Nevertheless, a large number of unresolved questions have not yet been answered. Below, I briefly list a number of them that strike me as particularly interesting for future research. Some of these novel questions have recently been taken up in new research.
Different Types of Judgments on Individuals and Groups
Are inferences of goals and traits of other persons subserved by a sequential posterior‐anterior neurological circuit as suggested, and if so, in what ways? To uncover the primacy of one process over the other, we probably need time‐sensitive methods like event‐related potentials (ERPs). Can this sequence explain why humans are so prone to the well‐known fundamental attribution bias, that is, the pervasive tendency to put more weight to the actor in explaining behavior and less to the external environment [Gilbert and Malone, 1995; Trope and Gaunt, 2000]? More generally, can social neuroscience help to unravel the sources of other cognitive heuristics and biases in social cognition?
What are the neurological substrates of other social inferences that have received relatively little attention in social neuroscience, including inferences at the individual level like ulterior motives (which are similar to false beliefs and deceit explored in neurological research, e.g., Grézes et al., 2004), and at the group level like categorization, stereotyping or norm learning [Tomelleri et al., 2007]?
Can we differentiate various types of enduring inferences at the neurological and psychological level? For instance, is the difference between trait inferences of others and self due to actual differences in mPFC processes or rather due to differences in informational sources such as the emotional warmth and closeness generated by familiar others (including the self; from the reward area located at the ventral and orbital mPFC) or greater autobiographic memory [located at the ventral mPFC, see Graham et al., 2003; Maguire and Frith, 2003; Maguire and Mummery, 2003]? As another example, do trait inferences and social script knowledge engage entirely identical brain areas as suggested by this meta‐analysis?
The present analysis suggests that social inferences are processed in a different manner than physical causality. However, which brain areas subserve explicit causality judgments on social events?
Spontaneity of Judgments
This meta‐analysis suggests that spontaneous inferences preferentially engage the TPJ and dorsal mPFC, whereas explicit inferences preferentially engage the ventral mPFC. Many fMRI studies have relied on explicit instructions or questions, which pose less internal validity problems. But in everyday live, we more often make spontaneous social inferences. Can social neuroscience help to identify the processing elements in social cognition that are spontaneous and implicit, versus those that require mental reflection and resources [see also Keysers and Gazzola, 2007]?
Are false beliefs inferred from other people made spontaneously or not [e.g., Apperly et al., 2006], and if so, which areas are engaged?
Are trait inferences made spontaneously, and if so, which areas are involved? Mitchell et al. (2006) found no differences between spontaneous and intentional trait inferences, whereas Van Duynslaeger et al. (2007) found more involvement of the TPJ during spontaneous trait inferences, in line with the present analysis.
Are group inferences made spontaneously? ERP data indicate that categorization of group members is spontaneous and fast, while the attribution of stereotypes depends on minimal processing resources and goals [e.g., Tomelleri et al., 2007].
Social Judgments and Change
Given that social inferences are at times undesirable and derogative (e.g., negative stereotyping of minority groups), it is important to explore not only which conditions give rise to these inferences but also under which conditions they may change. Does change require a minimal amount of empathy in the other's mind? Does it require the deployment of automatic processes or of executive control and inhibition processes, or both? What are the mental brain processes involved in this? As far as I am aware, these questions are only beginning to be raised in social neuroscience [Achtzinger et al., 2007]. Nevertheless, insight in the brain processes that underlie change in social cognition has the potential to drastically alter our understanding on how the brain is responsible for our past successes and failures in attempting to change social minds and social behavior.
Acknowledgements
I am greatly indebted to Maurits van der Molen for his insightful suggestions to an earlier version of this manuscript.
REFERENCES
- Achtziger A,Moratti S,Jaudas A,Rockstroh B,Gollwitzer PM ( 2007): Stereotype inhibition by means of an implementation intention is reflected in the modulation of the N400. Paper Abstract of the ESCON Conference, Brno, Czech Republic.
- Aichhorn M,Perner J,Kronbichler M,Staffen W,Ladurner G ( 2006): Do visual perspective tasks need theory of mind? Neuroimage 30: 1059–1068. [DOI] [PubMed] [Google Scholar]
- Aizenstein HJ,Stenger VA,Cochran J,Clark K,Johnson M,Nebes RD,Carter CS ( 2004): Regional brain activation during concurrent implicit and explicit sequence learning. Cereb Cortex 14: 199–208. [DOI] [PubMed] [Google Scholar]
- Akitsuki Y,Sugiura M,Watanabe J,Yamashita K,Sassa Y,Awata S,Matsuoka H,Maeda Y,Yoshihiko Matsue Y,Fukuda H,Kawashimab R ( 2003): Context‐dependent cortical activation in response to financial reward and penalty: An event‐related fMRI study. Neuroimage 19: 1674–1685. [DOI] [PubMed] [Google Scholar]
- Allison T,Puce A,McCarthy G ( 2000): Social perception from visual cues: Role of the STS region. Trends Cogn Sci 4: 267–278. [DOI] [PubMed] [Google Scholar]
- Amodio DM,Frith CD ( 2006): Meeting of minds: The medial frontal cortex and social cognition. Nat Rev 7: 268–277. [DOI] [PubMed] [Google Scholar]
- Apperly IA,Samson D,Chiavarino D,Humphreys GW ( 2004): Frontal and temporo‐parietal lobe contributions to theory of mind: Neuropsychological evidence from a false‐belief task with reduced language and executive demands. J Cogn Neurosci 16: 177–1784. [DOI] [PubMed] [Google Scholar]
- Apperly IA,Riggs KJ,Simpson A,Chiavarino C,Samson D ( 2006): Is belief reasoning automatic? Psychol Sci 17: 841–844. [DOI] [PubMed] [Google Scholar]
- Astafiev SV,Stanley CM,Shulman GL,Corbetta M ( 2004): Extrastriate body area in human occipital cortex responds to the performance of motor actions. Nat Neurosci 7: 542–548. [DOI] [PubMed] [Google Scholar]
- Banich MT,Milham MP,Atchley R,Cohen NJ,Webb A,Wszalek T,Kramer AF,Liang Z‐P,Wright A,Shenker J,Magin R ( 2001): FMRI studies of Stroop tasks reveal unique roles of anterior and posterior brain systems in attentional selection. J Cogn Neurosci 12: 988–1000. [DOI] [PubMed] [Google Scholar]
- Barch DM,Braver TS,Akbudak E,Conturo T,Ollinger J,Snyder A ( 2001): Anterior cingulate cortex and response conflict: Effects of response modality and processing domain. Cereb Cortex 11: 837–848. [DOI] [PubMed] [Google Scholar]
- Baron‐Cohen S ( 2006): The hyper‐systemizing, assortative mating theory of autism. Prog Neuro‐Psychopharmacol Biol Psychiatry 30: 865–872. [DOI] [PubMed] [Google Scholar]
- Beer JS,Ochsner KN ( 2006): Social cognition: A multi level analysis. Brain Res 1079: 98–105. [DOI] [PubMed] [Google Scholar]
- Berns GS,McClure SM,Pagnoni G,Montague PR ( 2001): Predictability modulates human brain response to reward. J Neurosci 21: 2793–2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns GS,Chappelow S,Zink CF,Pagnoni G,Martin‐Skurski ME,Richards J ( 2005): Neurobiological correlates of social conformity and independence during mental rotation. Biol Psychiatry 58: 245–253. [DOI] [PubMed] [Google Scholar]
- Berthoz S,Armony JL,Blain RJR,Dolan RJ ( 2002): An fMRI study of intentional and unintentional (embarrasing) violations of social norms. Brain 125: 1696–1708. [DOI] [PubMed] [Google Scholar]
- Bhatt M,Camerer CF ( 2005): Self‐referential thinking and equilibrium as states of mind in games: fMRI evidence. Games Econ Behav 52: 424–459. [Google Scholar]
- Blackwood NJ,Howard RJ,FFytche DH,Simmons A,Bentall RP,Murray RM ( 2000): Imaging attentional and attributional bias: An fMRI approach to the paranoid delusion. Psychol Med 30: 873–883. [DOI] [PubMed] [Google Scholar]
- Blackwood NJ,Bentall RP,FFytche DH,Simmons A,Murray RM,Howard RJ, ( 2003): Self‐responsibility and the self‐serving bias: An fMRI investigation of causal attributions. Neuroimage 20: 1076–1085. [DOI] [PubMed] [Google Scholar]
- Blakemore JS,den Ouden H,Choudhury S,Frith C ( 2007): Adolescent development of the neural circuitry for thinking about intentions. Soc Cogn Affect Neurosci 2: 130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ,Fonlupt P,Pachot‐Clouard M,Darmon C,Boyer P,Meltzoff AN,Segebarth C,Decety J ( 2001): How the brain perceives causality: an eventrelated fMRI study. Neuroreport 12: 3741–3746. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ,Boyer P,Pachot‐Clouard M,Meltzoff A,Segebarth C,Decety J ( 2003): The detection of contingency and animacy from simple animations in the human brain. Cereb Cortex 8: 837–844. [DOI] [PubMed] [Google Scholar]
- Blasi G,Goldberg TE,Weickert T,Das S,Kohn F,Zoltick B,Bertolino A,Joseph H,Callicott JH,Weinberger DR,Venkata S,Mattay VR ( 2006): Brain regions underlying response inhibition and interference monitoring and suppression. Eur J Neurosci 12: 1658–1664. [DOI] [PubMed] [Google Scholar]
- Bonda E,Petrides M,Ostry D,Evans A ( 1996): Specific involvement of human parietal systems and the amygdale in the perception of biological motion. J Neurosci 16: 3737–3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg JS,Hynes C,Van Horn J,Grafton S,Sinnott‐Armstrong W ( 2006): Consequences, action, and intention as factors in moral judgments: An fMRI investigation. J Cogn Neurosci 5: 803–817. [DOI] [PubMed] [Google Scholar]
- Britton JC,Phan KL,Taylor SF,Welsh RC,Berridge KC,Liberzon I ( 2006): Neural correlates of social and nonsocial emotions: An fMRI study. Neuroimage 31: 397–409. [DOI] [PubMed] [Google Scholar]
- Buccino G,Binkofski F,Fink GR,Fadiga L,Fogassi L,Gallese V,Seitz RJ,Zilles K,Rizzolatti G,Freund H‐J ( 2001): Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. Eur J Neurosci 13: 400–404. [PubMed] [Google Scholar]
- Bunge SA,Hazeltine E,Scanlon MD,Rosen AC,Gabrieli JDE ( 2002): Dissociable contributions of prefrontal and parietal cortices to response selection. Neuroimage 17: 1562–1571. [DOI] [PubMed] [Google Scholar]
- Carter EJ,Pelphrey KA ( 2006): School‐aged children exhibit domain‐specific responses to biological motion. Soc Neurosci 1: 396–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS,Macdonald AM,Botvinick M,Ross LL,Stenger VA,Noll D,Cohen JD ( 2000): Parsing executive processes: Strategic vs. evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci USA 97: 1944–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ,Thomas KM,Welsh TW,Badgaiyan RD,Eccard CH,Jennings JR,Crone EA ( 2000): Dissociation of response conflict, attentional selection, and expectancy with functional magnetic resonance imaging. Proc Natl Acad Sci USA 97: 8728–8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cato MA,Crosson B,Gökçay D,Soltysik D,Wierenga C,Gopinath K,Himes N,Belanger H,Bauer RM,Fischler IS,Gonzalez‐Rothi L,Briggs RW ( 2004): Processing words with emotional connotation: An fMRI study of time course and laterality in rostral frontal and retroplenial cortices. J Cogn Neurosci 16: 167–177. [DOI] [PubMed] [Google Scholar]
- Chaminade T,Decety J ( 2002): Leader or follower? Involvement of the inferior parietal lobule in agency. Neuroreport 13: 1975–1978. [DOI] [PubMed] [Google Scholar]
- Chaminade T,Meltzoff AN,Decety J ( 2002): Does the end justify the means? A PET exploration of the mechanisms involved in human imitation. Neuroimage 15: 318–328. [DOI] [PubMed] [Google Scholar]
- Collins DL,Neelin P,Peters TM,Evans AC ( 1994): Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Compu Assist Tomogr 18: 192–205. [PubMed] [Google Scholar]
- Coricelli G,Critchley HD,Joffily M,O'Doherty J,Sirigu A,Dolan RJ ( 2005): Regret and it's avoidance: A neuroimaging study of choice behavior. Nat Neurosci 8: 1255–1262. [DOI] [PubMed] [Google Scholar]
- Corlett PR,Aitken MRF,Dickinson A,Shanks DR,Honey GR,Honey RAE,Robbins TW,Bullmore ET,Fletcher PC ( 2004): Prediction error during retrospective revaluation of causal associations in humans: fMRI evidence in favor of an associative model of learning. Neuron 44: 877–888. [DOI] [PubMed] [Google Scholar]
- Cunningham WA,Johnson MK,Gatenby JC,Gore JC,Banaji MR ( 2003): Neural components of social evaluation. J Pers Soc Psychol 85: 639–649. [DOI] [PubMed] [Google Scholar]
- Cunningham WA,Raye CL,Johnson MK ( 2004): Implicit and explicit evaluation: fMRI correlates of valence, emotional intensity, and control in the processing of attitudes. J Cogn Neurosci 16: 1717–1729. [DOI] [PubMed] [Google Scholar]
- David N,Bewernick BH,Cohen MX,Newen A,Lux S,Fink GR,Shah NJ,Vogeley K ( 2006): Neural representations of self versus other: Visual‐spatial perspective taking. J Cogn Neurosci 6: 898–910. [DOI] [PubMed] [Google Scholar]
- Daw ND,O'Doherty JP,Dayan P,Seymour B,Dolan RJ ( 2006): Cortical substrates for exploratory decisions in humans. Nature 441: 876–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zubicaray GI,Wilson SJ,McMahon KL,Muthiah S ( 2001): The semantic interference effect in the picture‐word paradigm: An event‐related fMRI study employing overt responses. Human Brain Mapp 14: 218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J,Chaminade T ( 2003): When the self represents the other: A new cognitive neuroscience view on psychological identification. Conscious Cogn 12: 577–596. [DOI] [PubMed] [Google Scholar]
- Decety J,Chaminade T,Grézes J,Meltzoff AN ( 2002): A PET Exploration of the neural mechanisms involved in reciprocal imitation. Neuroimage 15: 265–272. [DOI] [PubMed] [Google Scholar]
- den Ouden HEM,Frith U,Frith C,Blakemore S‐J ( 2005): Thinking about intentions. Neuroimage 28: 787–796. [DOI] [PubMed] [Google Scholar]
- Destrebecqz A,Peigneux P,Laureys S,Degueldre C,Del Fiore G,Aerts J,Luxen A,van der Linden MVD,Cleeremans A,Maquet P ( 2003): Cerebral correlates of explicit sequence learning. Cogn Brain Res 16: 391–398. [DOI] [PubMed] [Google Scholar]
- Destrebecqz A,Peigneux P,Laureys S.,Degueldre C,Del Fiore GD,Aerts J,Luxen A,Van Der Linden MVD,Cleeremans A,Maquet P ( 2005): The neural correlates of implicit and explicit sequence learning: Interacting networks revealed by the process dissociation procedure. Learn Mem 12: 480–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher J‐C,Koechlin E,Ali SO,Grafman J, ( 2002): The roles of timing and task order during task switching. Neuroimage 17: 95–109. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI,Lieberman MD,Williams KD ( 2003): Does rejection hurt? An fMRI study of social exclusion. Science 302: 290–292. [DOI] [PubMed] [Google Scholar]
- Elliot R,Newman JL,Longe OA,Deakin JFW ( 2004): Instrumental responding for rewards is associated with enhanced neuronal response in subcortical reward systems. Neuroimage 21: 984–990. [DOI] [PubMed] [Google Scholar]
- Elliott R,Völlm B,Drury A,McKie S,Richardson P,Deakin JFW ( 2006): Co‐operating with another player in a financially rewarded guessing game activates regions implicated in theory of mind. Soc Neurosci 1: 385–395. [DOI] [PubMed] [Google Scholar]
- Emery NJ ( 2005): The evolution of social cognition In: Easton A,Emery NJ, editors. Cognitive Neuroscience of Social Behavior. New York: Psychology Press, 115–156 pp. [Google Scholar]
- Fan J,Flombaum JI,McCandliss BD,Thomas KM,Posner MI ( 2003): Cognitive and brain consequences of conflict. Neuroimage 18: 42–57. [DOI] [PubMed] [Google Scholar]
- Farrer C,Frith CD ( 2002): Experiencing oneself vs. another person as being the cause of an action: The neural correlates of the experience of agency. Neuroimage 15: 596–603. [DOI] [PubMed] [Google Scholar]
- Farrer C,Franck N,Georgieff N,Frith CD,Decety J,Jeannerod M ( 2003): Modulating the experience of agency: A positron emission tomography study. Neuroimage 18: 324–333. [DOI] [PubMed] [Google Scholar]
- Farrow TFD,Zheng CAY,Wilkinson ID,Spence SA,Deakin WJF,Tarrier N,Griffiths PD,Woodruff PWR ( 2001): Investigating the functional anatomy of empathy and forgiveness. Neuroreport 12: 2433–2438. [DOI] [PubMed] [Google Scholar]
- Fein S ( 1996): Effects of suspicion on attributional thinking and the correspondence bias. J Pers Soc Psychol 70: 1164–1184. [Google Scholar]
- Ferstl EC,von Cramon DY ( 2002): What does the frontomedian cortex contribute to language processing: Coherence or theory of mind? Neuroimage 17: 1599–1612. [DOI] [PubMed] [Google Scholar]
- Ferstl EC,Rinck M,von Cramon DY ( 2005): Emotional and temporal aspects of situation model processing during text comprehension: An event‐related fMRI study. J Cogn Neurosci 17: 724–739. [DOI] [PubMed] [Google Scholar]
- Fiddick L,Spampinato MV,Grafman J ( 2005): Social contracts and precautions activate different neurological systems: An fMRI investigation of deontic reasoning. Neuroimage 28: 778–786. [DOI] [PubMed] [Google Scholar]
- Fletcher PC,Anderson JM,Shanks DR,Honey R,Carpenter TA,Donovan T,Papadakis N,Bullmore ET, ( 2001): Responses of human frontal cortex to surprising events are predicted by formal associative learning theory. Nat Neurosci 4: 1043–1048. [DOI] [PubMed] [Google Scholar]
- Foerde K,Knowlton BJ,Poldrack RA ( 2006): Modulation of competing memory systems by distraction. Proc Natl Acad Sci USA 103: 11778–11783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogassi L,Ferrari PF,Gesierich B,Rozzi S,Chersi F,Rizzolatti G ( 2005): Parietal lobe: From action organization to intention understanding. Science 308: 662–667. [DOI] [PubMed] [Google Scholar]
- Fonlupt P ( 2003): Perception and judgement of physical causality involve different brain structures. Cogn Brain Res 17: 248–254. [DOI] [PubMed] [Google Scholar]
- Fossati P,Hevenor SJ,Graham SJ,Grady C,Knightly ML,Craik MAF,Mayberg H ( 2003): In search of the emotional self: An fMRI Study using positive and negative emotional words. Am J Psychiatry 160: 1938–1945. [DOI] [PubMed] [Google Scholar]
- Fossati P,Hevenor SJ,Lepage M,Graham SJ,Grady C,Keightley ML,Craik F,Mayberg H ( 2004): Distributed self in episodic memory: Neural correlates of successful retrieval of self‐encoded positive and negative personality traits. Neuroimage 22: 1596–1604. [DOI] [PubMed] [Google Scholar]
- Frith CD,Frith U ( 1999): Interacting minds: A biological basis. Science 286: 1692–1695. [DOI] [PubMed] [Google Scholar]
- Frith U,Frith C. ( 2001): The biological basis of social interaction. Curr Dir Psychol Sci 151–155. [Google Scholar]
- Fugelsang JA,Dunbar KN ( 2005): Brain‐based mechanisms underlying complex causal thinking. Neuropsychologia 43: 1204–1213. [DOI] [PubMed] [Google Scholar]
- Fukui H,Murai T,Shinozaki J,Aso T,Fukuyama H,Hayashi T,Hanakawa T ( 2006): The neural basis of social tactics: An fMRI study. Neuroimage 32: 913–920. [DOI] [PubMed] [Google Scholar]
- Gallagher HL,Frith CD ( 2003): Functional imaging of ‘theory of mind’. Trends Cogn Sci 7: 77–83. [DOI] [PubMed] [Google Scholar]
- Gallagher HL,Happé F,Brunswick N,Fletcher PC,Frith U,Frith CD ( 2000): Reading the mind in cartoons and stories: An fMRI study of ‘theory of mind’ in verbal and nonverbal tasks. Neuropsychologia 38: 11–21. [DOI] [PubMed] [Google Scholar]
- Gallagher HL,Jack AI,Roepstorff A,Frith CD ( 2002): Imaging the intentional stance in a competitive game. Neuroimage 16: 814–821. [DOI] [PubMed] [Google Scholar]
- Gallese V,Keysers C,Rizzolatti G ( 2004): A unifying view of the basis of social cognition. Trends Cogn Sci 8: 396–403. [DOI] [PubMed] [Google Scholar]
- Gazzola V,Aziz‐Zadeh L,Keysers C ( 2006): Empathy and the somatotopic auditory mirror system in humans. Curr Biol 16: 1824–1829. [DOI] [PubMed] [Google Scholar]
- Gazzola V,Worp H,Mulder T.,Wicker B,Rizzolatti G,Keysers C ( 2007a) Aplasics born without hands mirror the goal of hand actions with their feet. Curr Biol 17: 1235–1240. [DOI] [PubMed] [Google Scholar]
- Gazzola V,Rizzolatti G,Wicker B,Keysers C. ( 2007b) The anthropomorphic brain: The mirror neuron system responds to human and robotic actions. Neuroimage 35: 1674–1684. [DOI] [PubMed] [Google Scholar]
- German TP,Niehaus JL,Meghan P,Roarty MP,Giesbrecht B,Miller MB ( 2004): Neural correlates of detecting pretense: Automatic engagement of the intentional stance under covert conditions. J Neurosci 16: 1805–1817. [DOI] [PubMed] [Google Scholar]
- Gilbert DT,Malone PS ( 1995): The correspondence bias. Psychol Bull 117: 21–38. [DOI] [PubMed] [Google Scholar]
- Gilbert DT,Pelham BW,Krull DS ( 1988): On cognitive busyness: When person perceivers meet persons perceived. J Pers Soc Psychol 54: 733–740. [Google Scholar]
- Gilbert SJ,Spengler S,Simons JS,Steele D,Stephen M.,Lawrie SM,Christopher D,Frith CD,Burgess PW ( 2006): Functional specialization within rostral Prefrontal cortex (Area 10): A Meta‐analysis. J Cogn Neurosci 18: 932–948. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ,Williamson IDM,Dumontheil I,Simons JS,Frith CD,Burgess PW ( 2007): Distinct regions of medial rostral prefrontal cortex supporting social and nonsocial functions. Soc Cogn Affect Neurosci 2: 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbini MI,Leibenluft E,Santiago N,Haxby JV ( 2004): Social and emotional attachment in the neural representation of faces. Neuroimage 22: 1628–1635. [DOI] [PubMed] [Google Scholar]
- Goldin PR,Hutcherson CAC,Ochsner KN,Glover GH,Gabrieli JDE,Gross JJ ( 2005): The neural bases of amusement and sadness: A comparison of block contrast and subject‐specific emotion intensity regression approaches. Neuroimage 27: 26–36. [DOI] [PubMed] [Google Scholar]
- Gottfried JA,O'Doherty J,Dolan RJ ( 2003): Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science 301: 1104–1107. [DOI] [PubMed] [Google Scholar]
- Graham KS,Lee ACH,Brett M,Patterson K ( 2003): The neural basis of autobiographical and semantic memory: New evidence from three PET studies. Cogn Affect Behav Neurosci 3: 234–254. [DOI] [PubMed] [Google Scholar]
- Greene JD,Sommerville RB,Nystrom LE,Darley JM,Cohen JD ( 2001): An fMRI investigation of emotional engagement in moral judgment. Science 293: 2105–2108. [DOI] [PubMed] [Google Scholar]
- Greene JD,Nystrom LE,Engell AD,Darley JM,Cohen JD ( 2004): The neural bases of cognitive conflict and control in moral judgment. Neuron 44: 389–400. [DOI] [PubMed] [Google Scholar]
- Grézes J,Decety J ( 2001): Functional anatomy of execution, mental simulation, observation, and verb generation of actions: A meta‐analysis. Human Brain Mapp 12: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grèzes J,Armony JL,Rowe J,Passingham RE ( 2003): Activations related to ‘mirror’ and ‘canonical’ neurones in the human brain: an fMRI study. Neuroimage 18: 928–937. [DOI] [PubMed] [Google Scholar]
- Grézes J,Frith C,Passingham RE ( 2004): Brain mechanisms for inferring deceit in the actions of others. J Neurosci 24: 5500–5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grézes J,Berthoz S,Passingham RE ( 2006): Amygdala activation when one is the target of deceit: Did he lie to you or to someone else? Neuroimage 30: 601–608. [DOI] [PubMed] [Google Scholar]
- Grossman ED,Blake R ( 2002): Brain areas active during visual perception of biological motion. Neuron 35: 1167–1175. [DOI] [PubMed] [Google Scholar]
- Gur RC,Schroeder L,Turner T,McGrath C,Chan RM,Turetsky BI,Alsop D,Maldjian J,Gur RE ( 2002): Brain activation during facial emotion processing. Neuroimage 16: 651–662. [DOI] [PubMed] [Google Scholar]
- Gusnard DA,Akbudak E,Shulman GL,Marcus E,Raichle ME ( 2001): Medial prefrontal cortex and self‐referential mental activity: Relation to a default mode of brain function. Proc Natl Acad Sci USA 98: 4259–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagá S,Garcia‐Marques L ( 2007): Listening to the inner child in person perception: The ontogeny of social information processing dueal architecture. Paper presented at the 9th ESCON meeting, Brno, Czech Republic.
- Hamilton A,Grafton ST ( 2006): Goal representations in human anterior intraparietal sulcus. J Neurosci 25: 1133–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton A,Grafton ST ( 2007): Action outcomes are represented in human inferior fronto‐parietal cortex. Cereb Cortex (in press). [DOI] [PubMed] [Google Scholar]
- Harris LT,Fiske ST ( 2006): Dehumanizing the lowest of the low. Psychol Sci 17: 847–853. [DOI] [PubMed] [Google Scholar]
- Harris LT,Todorov A,Fiske ST ( 2005): Attributions on the brain: Neuro‐imaging dispositional inferences, beyond theory of mind. Neuroimage 28: 763–769. [DOI] [PubMed] [Google Scholar]
- Hassin R,Aarts H,Ferguson MJ ( 2005): Automatic goal inferences. J Exp Soc Psychol 41: 129–140. [Google Scholar]
- Heatherton TF,Wyland CL,Macrae CN,Demos KE,Denny BT,Kelley WM ( 2006): Medial prefrontal activity differentiates self from close others. Soc Cogn Affect Neurosci 1: 18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberlein AS,Saxe RR ( 2005): Dissociation between emotion and personality judgments: Convergent evidence from functional neuroimaging. Neuroimage 28: 770–777. [DOI] [PubMed] [Google Scholar]
- Heekeren HR,Wartenburger I,Schmidt IH,Schwintowski HP,Villringer A ( 2003): An fMRI study of simple ethical decision making. Neuroreport 14: 1215–1219. [DOI] [PubMed] [Google Scholar]
- Heekeren HR,Wartenburger I,Schmidt H,Prehn K,Schwintowski HP,Villringer A ( 2005): Influence of bodily harm on neural correlates of semantic and moral decision‐making. Neuroimage 24: 887–897. [DOI] [PubMed] [Google Scholar]
- Heider F ( 1958): The Psychology of Interpersonal Relations. New York: Wiley. [Google Scholar]
- Heider F,Simmel M ( 1944): An experimental study of apparent behavior. Am J Psychol 57: 243–259. [Google Scholar]
- Heinzel A,Bermpohl F,Niese R,Pfenniga A,Pascual‐Leonea A,Schlaugb G,Northoff G ( 2005): How do we modulate our emotions? Parametric fMRI reveals cortical midline structures as regions specifically involved in the processing of emotional valences. Cogn Brain Res 25: 348–358. [DOI] [PubMed] [Google Scholar]
- Henson R ( 2006): Forward inference using functional neuroimaging: Dissociations versus associations. Trends Cogn Sci 10: 64–67. [DOI] [PubMed] [Google Scholar]
- Huettel SA,Song AW,McCarthy G.( 2004): Functional magnetic resonance imaging. Sunderland, MA: Sinauer Associates. [Google Scholar]
- Huey ED,Krueger F,Grafman J ( 2006): Representations in the human prefrontal cortex. Assoc Psychol Sci 15: 167–171. [Google Scholar]
- Hutcherson CA,Goldin PR,Ochsner KN,Gabrieli JD,Barrett LF,Gross JJ ( 2005): Attention and emotion: Does rating emotion alter neural responses to amusing and sad films? Neuroimage 27: 656–668. [DOI] [PubMed] [Google Scholar]
- Iacoboni M ( 2005): Neural mechanisms of imitation. Curr Opin Neurobiol 15: 632–637. [DOI] [PubMed] [Google Scholar]
- Iacoboni M,Woods RP,Brass M.Bekkering H,Mazziotta JC,Rizzolatti G ( 1999): Cortical mechanisms of human imitation. Science 286: 2526–2527. [DOI] [PubMed] [Google Scholar]
- Iacoboni M,Koski LM,Brass M,Bekkering H,Woods RP,Dubeau M‐C,Mazziotta JC,Rizzolatti G ( 2001): Reafferent copies of imitated actions in the right superior temporal cortex. Proc Natl Acad Sci USA 98: 13995–13999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M,Lieberman MD,Knowlton BJ,Molnar‐Szakacs I,Moritz M,Throop JC,Fiske AP ( 2004): Watching social interactions produces dorsomedial prefrontal and medial parietal BOLD fMRI signal increases compared to a resting baseline. Neuroimage 21: 1167–1173. [DOI] [PubMed] [Google Scholar]
- Iacoboni M,Molnar‐Szakacs I,Gallese V,Buccino G,Mazziotta JC,Rizzolatti G ( 2005): Grasping the intentions of others with one's own mirror neuron system. PLoS Biol 3: e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob P,Jeannerod M ( 2005): The motor theory of social cognition: A critique. Trends Cogn Sci 9: 21–25. [DOI] [PubMed] [Google Scholar]
- Jeannerod M ( 2004): Visual and action cues contribute to the self‐other distinction. Nat Neurosci 7: 422–423. [DOI] [PubMed] [Google Scholar]
- Johnson SC,Baxter LC,Wilder LS,Pipe JG,Heiserman JE,Prigatano GP ( 2002): Neural correlates of self‐reflection. Brain 125: 1808–1814. [DOI] [PubMed] [Google Scholar]
- Johnson MK,Raye CL,Mitchell KJ,Touryan SR,Greene EJ,Nolen‐Hoeksema S ( 2006): Dissociating medial frontal and posterior cingulate activity during self‐reflection. Soc Cogn Affect Neurosci 1: 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley HH ( 1967): Attribution in social psychology. Nebraska Symp Motiv 15: 192–238. [Google Scholar]
- Kelley WM,Macrae CN,Wyland CL,Caglar S,Inati S,Heatherton TF ( 2002): Finding the self? An event‐related fMRI study. J Cogn Neurosci 14: 785–794. [DOI] [PubMed] [Google Scholar]
- Kerns JG,Cohen JD,MacDonald AW III,Cho RY,Stenger VA,Carter CS ( 2004): Anterior cingulate conflict monitoring and adjustments in control. Science 303: 1023–1026. [DOI] [PubMed] [Google Scholar]
- Keysers C,Gazzola V ( 2007): Integrating simulation and theory of mind: From self to social cognition. Trends Cogn Sci 11: 194–196. [DOI] [PubMed] [Google Scholar]
- Keysers C,Perrett DI ( 2004): Demystifying social cognition: A Hebbian perspective. Trends Cogn Sci 8: 501–507. [DOI] [PubMed] [Google Scholar]
- Killgore WDS,Yurgelun‐Todd DA ( 2007): Unconscious processing of facial affect in children and adolescents. Soc Neurosci 2: 28–47. [DOI] [PubMed] [Google Scholar]
- Kim H,Somerville LH,Johnstone T,Polis S,Alexander AL,Shin LM,Whalen PJ ( 2004): Contextual modulation of amygdala responsivity to surprised faces. J Cogn Neurosci 16: 1730–1745. [DOI] [PubMed] [Google Scholar]
- Kircher TTJ,Senior C,Phillips ML,Benson PJ,Bullmore ET,Brammer M,Simmons A,Williams SCR,Bartels M,David AS ( 2000): Towards a functional neuroanatomy of self processing: Effects of faces and words. Cogn Brain Res 10: 133–144. [DOI] [PubMed] [Google Scholar]
- Kircher TTJ,Brammer M,Bullmore E,Simmons A,Bartels M,David AS ( 2002): The neural correlates of intentional and incidental self processing. Neuropsychologia 40: 683–692. [DOI] [PubMed] [Google Scholar]
- Koechlin E,Corrado G,Pietrini P,Grafman J ( 2000): Dissociating the role of the medial and lateral anterior prefrontal cortex in human planning. Proc Natl Acad Sci USA 97: 7651–7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin E,Danek A,Burnod Y,Grafman J ( 2002): Medial prefrontal and subcortical mechanisms underlying the acquisition of motor and cognitive action sequences in humans. Neuron 35: 371–381. [DOI] [PubMed] [Google Scholar]
- Koski L,Wohlschläger A,Bekkering H,Woods RP,Dubeau M‐C,Mazziotta JC,Iacoboni M ( 2002): Modulation of motor and premotor activity during imitation of target‐directed actions. Cereb Cortex 12: 847–855. [DOI] [PubMed] [Google Scholar]
- Koski L,Iacoboni M,Dubeau M‐C,Woods RP,Mazziotta JC ( 2003): Modulation of cortical activity during different imitative behaviors. J Neurophysiol 89: 460–471. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML,Rolls ET ( 2003): Neural correlates of rapid reversal learning in a simple model of human social interaction. Neuroimage 20: 1371–1383. [DOI] [PubMed] [Google Scholar]
- Krueger F,Moll J,Zahn JR,Heinecke A,Grafman J ( 2007): Event frequency modulates the processing of daily life activities in human medial prefrontal cortex. Cereb Cortex 17: 2346–2353. [DOI] [PubMed] [Google Scholar]
- Kumaran D,Maguire EA ( 2005): The human hippocampus: Cognitive maps or relational memory? J Neurosci 25: 7254–7259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperberg GR,Lakshmanan BM,Caplan DN,Holcomb PJ ( 2006): Making sense of discourse: An fMRI study of causal inferencing across sentences. Neuroimage 33: 343–361. [DOI] [PubMed] [Google Scholar]
- Lang PJ,Greenwald MK,Bradley MM,Hamm AO ( 1993): Looking at pictures: Affective visceral and bheavioral reactions. Psychophysiology 30: 261–273. [DOI] [PubMed] [Google Scholar]
- Lawrence EJ,Shaw P,Giampietro VP,Surguladze S,Brammer MJ,David AS ( 2006): The role of shared representations in social perception and empathy: An fMRI study. Neuroimage 29: 1173–1184. [DOI] [PubMed] [Google Scholar]
- Leslie AM,Friedman O,German TP ( 2004): Core mechanisms in ‘theory of mind’. Trends Cogn Sci 8: 528–533. [DOI] [PubMed] [Google Scholar]
- Lieberman MD,Jarcho JM,Satpute AB ( 2004): Evidence‐based and intuition‐based self‐knowledge: An fMRI study. J Pers Soc Psychol 87: 421–435. [DOI] [PubMed] [Google Scholar]
- MacDonald AW III,Cohen JD,Stenger VA,Carter CS ( 2000): Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 288: 1835–1838. [DOI] [PubMed] [Google Scholar]
- Macrae CN,Moran JM,Heatherton TF,Banfield JF,Kelley WM ( 2004): Medial prefrontal activity predicts memory for self. Cereb Cortex 14: 647–654. [DOI] [PubMed] [Google Scholar]
- Maguire EA,Frith CD ( 2003): Aging affects the engagement of the hippocampus during autobiographical memory retrieval. Brain 126: 1511–1523. [DOI] [PubMed] [Google Scholar]
- Maguire EA,Mummery CJ ( 2003): Differential modulation of a common memory retrieval network revealed by positron emission tomography. Hippocampus 9: 54–61. [DOI] [PubMed] [Google Scholar]
- Malle BF ( 1999): How people explain behavior: A new theoretical framework. Pers Soc Psychol Rev 3: 21–43. [DOI] [PubMed] [Google Scholar]
- Malle BF,Knobe J ( 1997): The folk concept of intentionality. J Exp Soc Psychol 33: 101–121. [Google Scholar]
- Malle BF,Knobe J,O'Laughlin MJ,Pearce GE,Nelson SE ( 2000): Conceptual structure and social functions of behavior explanations: Beyond person‐situation attributions. J Pers Soc Psychol 79: 309–326. [DOI] [PubMed] [Google Scholar]
- Martin A,Weisberg J ( 2003): Neural foundations for understanding social and mechanical concepts. Cogn Neuropsychol 20: 575–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF,Banfield JF,Macrae CN ( 2004): Thinking about actions: The neural substrates of person knowledge. Cereb Cortex 14: 209–214. [DOI] [PubMed] [Google Scholar]
- Matsumoto K,Tanaka K ( 2004): The role of the medial prefrontal cortex in achieving goals. Curr Opin Neurobiol 14: 178–185. [DOI] [PubMed] [Google Scholar]
- McClure SM,Berns GS,Montague PR ( 2003): Temporal prediction errors in a passive learning task activate human striatum. Neuron 38: 339–346. [DOI] [PubMed] [Google Scholar]
- McClure SM,Laibson DL,Loewenstein G,Cohen JD ( 2004): Separate neural systems value immediate and delayed monetary rewards. Science 306: 503–507. [DOI] [PubMed] [Google Scholar]
- McLaughlin J,Skaggs H,Churchwell J,Powell DA ( 2002): Medial prefrontal cortex and Pavlovian conditioning: Trace versus delay conditioning. Behav Neurosci 116: 37–47. [PubMed] [Google Scholar]
- Milham MP,Banich1 MT,Webb A,Barad V,Cohen NJ,Wszalek T,Kramer AF ( 2001): The relative involvement of anterior cingulate and prefrontal cortex in attentional control depends on nature of conflict. Cogn Brain Res 12: 467–473. [DOI] [PubMed] [Google Scholar]
- Milham MP,Erickson KI,Banich MT,Kramer AF,Webb A,Wszalek T,Cohen NJ ( 2002): Attentional control in the aging brain: Insights from an fMRI study of the Stroop task. Brain Cogn 49: 277–296. [DOI] [PubMed] [Google Scholar]
- Milham MP,Banich MT,Barad V ( 2003a): Competition for priority in processing increases prefrontal cortex's involvement in top‐down control: An event‐related fMRI study of the stroop task. Cogn Brain Res 17: 212–222. [DOI] [PubMed] [Google Scholar]
- Milham MP,Banich MT,Claus ED,Cohen NJ ( 2003b): Practice‐related effects demonstrate complementary roles of anterior cingulate and prefrontal cortices in attentional control. Neuroimage 18: 483–493. [DOI] [PubMed] [Google Scholar]
- Milham,Banich MT ( 2005): Anterior cingulate cortex: An fMRI analysis of conflict specificity and functional differentiation. Human Brain Mapp 25: 328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP ( 2008): Mentalizing and Marr: An information processing approach to the study of social cognition. Brain Res 1079: 66–75. [DOI] [PubMed] [Google Scholar]
- Mitchell JP ( 2008): Activity in right temporo‐parietal junction is not selective for theory of mind. Cereb Cortex 18: 262–271. [DOI] [PubMed] [Google Scholar]
- Mitchell JP,Heatherton TF,Macrae CN ( 2002): Distinct neural systems subserve person and object knowledge. Proc Natl Acad Sci USA 99: 15238–15243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP,Macrae CN,Banaji MR ( 2004): Encoding‐specific effects of social cognition on the neural correlates of subsequent memory. J Neurosci 24: 4912–4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP,Banaji MR,Macrae CN ( 2005a) General and specific contribution of the medial prefrontal cortex to knowledge about mental states. Neuroimage 28: 757–762. [DOI] [PubMed] [Google Scholar]
- Mitchell JP,Banaji MR,Macrae CN ( 2005b) The link between social cognition and self‐referential thought in the medial prefrontal cortex. J Cogn Neurosci 17: 1306–1315. [DOI] [PubMed] [Google Scholar]
- Mitchell JP,Macrae CN,Banaji MR ( 2005c) Forming impression of people versus inanimate objects: Social‐cognitive processing in the medial prefrontal cortex. Neuroimage 26: 251–257. [DOI] [PubMed] [Google Scholar]
- Mitchell JP,Cloutier J,Banaji MR,Macrae CN ( 2006): Medial prefrontal dissociations during processing of trait diagnostic and nondiagnostic person information. Soc Cogn Affect Neurosci 1: 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J,Eslinger PJ,De Oliveira‐Souza R ( 2001): Frontopolar and anterior temporal cortex activation in a moral judgment task. Ar Qneuropsiquiatr 59: 657–664. [DOI] [PubMed] [Google Scholar]
- Moll J,de Oliveira‐Souza R,Bramati IE,Grafman J ( 2002): Functional networks in emotional moral and nonmoral social judgments. Neuroimage 16: 696–703. [DOI] [PubMed] [Google Scholar]
- Molnar‐Szakacs I,Iacoboni M,Koski L,Mazziotta JC ( 2005): Functional segregation within Pars Opercularis of the inferior frontal gyrus: Evidence from fMRI Studies of imitation and action observation. Cereb Cortex 15: 986–994. [DOI] [PubMed] [Google Scholar]
- Moran JM,Macrae CN,Heatherton TF,Wyland CL,Kelley WM ( 2006): Neuroanatomical evidence for distinct cognitive and affective components of self. J Cogn Neurosci 18: 1586–1594. [DOI] [PubMed] [Google Scholar]
- Mosconi MW,Peter B.,Mack PB,McCarthy G,Pelphrey KA ( 2005): Taking an “intentional stance” on eye‐gaze shifts: A functional neuroimaging study of social perception in children. Neuroimage 27: 247–252. [DOI] [PubMed] [Google Scholar]
- Neumann J,van Cramon DY,Lohmann G ( 2008): Model‐based clustering of meta‐analytic functional imaging data. Human Brain Mapp 29: 177–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G,Bermpohl F ( 2004): Cortical midline structures and the self. Trends Cogn Sci 8: 102–107. [DOI] [PubMed] [Google Scholar]
- O'Doherty J,Kringelbach ML,Rolls ET,Hornak JC,Andrews C ( 2001): Abstract reward and punishment representations in the human orbitofrontal cortex. Neurosci 4: 95–102. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP,Dayan P,Friston K,Critchley H,Dolan RJ ( 2003): Temporal difference models and reward‐related learning in the human brain. Neuron 28: 329–337. [DOI] [PubMed] [Google Scholar]
- O'Doherty J,Dayan P,Schultz J,Deichmann R,Friston K,Dolan RJ ( 2004): Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science 304: 452–454. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP,Buchanan TW,Seymour B,Raymond J,Dolan RJ ( 2006): Predictive neural coding of reward preference involves dissociable responses in human ventral midbrain and ventral striatum. Neuron 49: 157–166. [DOI] [PubMed] [Google Scholar]
- Ochsner KN,Bunge SA,Gross JJ,Gabrieli JDE ( 2002): Rethinking feelings: fMRI study of the cognitive regulation of emotion. J Cogn Neurosci 14: 1215–1229. [DOI] [PubMed] [Google Scholar]
- Ochsner KN,Knierim K,Ludlow DH,Hanelin J,Ramachandran T,Glover G,Mackey SC ( 2004a): Reflecting upon feelings: An fMRI study of neural systems supporting the attribution of emotion to self and other. J Cogn Neurosci 16: 1746–1772. [DOI] [PubMed] [Google Scholar]
- Ochsner KN,Ray RD,Cooper JC,Robertson ER,Chopra S,Gabrieli JDE,Gross JJ ( 2004b): For better or for worse: Neural systems supporting the cognitive down‐ and up‐regulation of negative emotion. Neuroimage 23: 483–499. [DOI] [PubMed] [Google Scholar]
- Ochsner KN,Beer JS,Robertson ER,Cooper JC,Gabrieli JDE,Kihsltrom JF,D'Esposito M ( 2005): The neural correlates of direct and reflected self‐knowledge. Neuroimage 28: 797–814. [DOI] [PubMed] [Google Scholar]
- Ochsner KN,Ludlow DH,Knierim K,Hanelin J,Ramachandran T,Glover CC,Mackey SC ( 2006): Neural correlates of individual differences in pain‐related fear and anxiety. Pain 120: 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi T,Moriguchi Y,Matsuda H,Mori T,Hirakata M,Imabayashi E,Hirao K,Nemoto K,Kaga M,Inagaki M,Yamada M,Uno A ( 2004): The neural network for the mirror system and mentalizing in normally developed children: An fMRI study. Neuroreport, 15: 1483–1487. [DOI] [PubMed] [Google Scholar]
- Olsen IR,Plotzker A,Ezzyat Y ( 2007): The enigmatic temporal pole: A review of findings on social and emotional processing. Brain 130: 1718–1731. [DOI] [PubMed] [Google Scholar]
- Paulus MP,Frank LR ( 2003): Ventromedial prefrontal cortex activation is critical for preference judgments. Neuroreport 14: 1311–1315. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA,Morris JP,McCarthy G ( 2004): Grasping the intentions of others: The perceived intentionality of an action influences activity in the superior temporal sulcus during social perception. J Cogn Neurosci 16: 1706–1716. [DOI] [PubMed] [Google Scholar]
- Perner J,Aichhorn M,Kronbichler M,Staffen W,Ladurner G ( 2006): Thinking of mental and other representations: The role of left and right temporo‐parietal junction. Soc Neurosci 1: 245–258. [DOI] [PubMed] [Google Scholar]
- Peterson BS,Kane MJ,Alexander GM,Lacadie C,Skudlarski P,Leung H‐C,May J,Gore JC ( 2002): An event‐related functional MRI study comparing interference effects in the Simon and Stroop tasks. Cogn Brain Res 13: 427–440. [DOI] [PubMed] [Google Scholar]
- Phan KL,Taylor SF,Welsh RC,Decker LR,Noll DC,Nichols TE,Britton JC,Liberzon I ( 2003): Activation of the medial prefrontal cortex and extended amygdala by individual ratings of emotional arousal: An fMRI study. Biol Psychiatry 53: 211–215. [DOI] [PubMed] [Google Scholar]
- Phan KL,Taylor SF,Welsh RC,Ho SH,Britton JC,Liberzon I ( 2004): Neural correlates of individual ratings of emotional salience: A trial‐related fMRI study. Neuroimage 21: 768–780. [DOI] [PubMed] [Google Scholar]
- Phan KL,Fitzgerald DA,Nathan PJ,Moore GJ,Uhde TW,Tancer ME ( 2005): Neural substrates for voluntary suppression of negative affect: A functional magnetic resonance imaging study. Biol Psychiatry 57: 210–219. [DOI] [PubMed] [Google Scholar]
- Platek SM,Keenan JP,Gallup GG Jr,Mohamed FB ( 2004): Where am I? The neurological correlates of self and other. Cogn Brain Res 19: 114–122. [DOI] [PubMed] [Google Scholar]
- Platek SM,Loughead JW,Ruben C.,Gur RC,Busch S,Ruparel K,Phend N,Panyavin IS,Langleben DD ( 2006): Neural substrates for functionally discriminating self‐face from personally familiar faces. Human Brain Mapp 27: 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MN,Leung H‐C,Blumberg HP,Peterson BS,Fulbright RK,Lacadie CM,Skudlarski P,Gore JC ( 2003): An fMRI Stroop task study of ventromedial prefrontal cortical function in pathological gamblers. Am J Psychiatry 160: 1990–1994. [DOI] [PubMed] [Google Scholar]
- Puce A,Allison T,Bentin S,Gore JC,McCarthy G ( 1998): Temporal cortex activation in humans viewing eye and mouth movements. J Neurosci 18: 2188–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read SJ,Miller LC ( 1993) Rapist or “regular guy”: Explanatory coherence in the construction of mental models of others. Pers Soc Psychol Bull 19: 526–541. [Google Scholar]
- Reeder GD,Kumar S,Hesson‐McInnis MS,Trafimow D ( 2002): Inferences about the morality of an aggressor: The role of perceived motive. J Pers Soc Psychol 83: 789–803. [DOI] [PubMed] [Google Scholar]
- Reeder GD,Vonk R,Ronk MJ,Ham J,Lawrence M ( 2004): Dispositional attribution: Multiple inferences about motive‐related traits. J Pers Soc Psychol 86: 530–544. [DOI] [PubMed] [Google Scholar]
- Reis DL,Brackett MA,Shamosh NA,Kiehl KA,Salovey P,Gray JR ( 2007): Emotional intelligence predicts individual differences in social exchange reasoning. Neuroimage 35: 1385–1391. [DOI] [PubMed] [Google Scholar]
- Rholes WS,Ruble DN,Newman LS ( 1990): Children's understanding of self and other: Developmental and motivational aspects of perceiving persons in terms of invariant dispositions In Sorrentino RM,Higgins ET, editors.The Handbook of Motivation and Cognition: Foundations of Social Behavior, Vol. II Hillsdale, NJ: Lawrence Erlbaum; pp 369–407. [Google Scholar]
- Rilling JK,Sanfey AG,Aronson JA,Nystrom LE,Cohen JD ( 2004): The neural correlates of theory of mind within interpersonal interactions. Neuroimage 22: 1694–1703. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G,Arbib MA ( 1998): Language within our grasp. Lang Mirror Neurons 21: 188–194. [DOI] [PubMed] [Google Scholar]
- Robertson D,Snarey J,Ousley O,Keith Harenski K,Bowmand FD,Gilkey R,Kilts C ( 2007): The neural processing of moral sensitivity to issues of justice and care. Neuropsychologia 45: 755–766. [DOI] [PubMed] [Google Scholar]
- Roelofs A,van Turennout M,Coles MGH ( 2006): Anterior cingulate cortex activity can be independent of response conflict in Stroop‐like tasks. Proc Natl Acad Sci 103: 13884–13889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby P,Decety J ( 2001): Effect of subjective perspective taking during simulation of action: A PET investigation of agency. Nat Sci 4: 546–550. [DOI] [PubMed] [Google Scholar]
- Ruff CC,Woodward TS,Laurens KR,Liddle PF ( 2001): The role of the anterior cingulate cortex in conflict processing: evidence from reverse Stroop interference. Neuroimage 14: 1150–1158. [DOI] [PubMed] [Google Scholar]
- Runyan JD,Moore AN,Dash PK ( 2004): A role for prefrontal cortex in memory storage for trace fear conditioning. J Neurosci 24: 1288–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfey AG,Rilling JK,Aronson JA,Nystrom LE,Cohen JD ( 2003): The neural basis of economic decision‐making in the ultimatum game. Science 300: 1755–1758. [DOI] [PubMed] [Google Scholar]
- Sato W,Kochiyama T,Yoshikawa S,Naito E,a Matsumura M ( 2004): Enhanced neural activity in response to dynamic facial expressions of emotion: an fMRI study. Cogn Brain Res 20: 81–91. [DOI] [PubMed] [Google Scholar]
- Satpute AB,Fenker DB,Waldmann MR,Tabibnia G,Holyoak KJ,Lieberman MD ( 2005): An fMRI study of causal judgments. Eur J Neurosci 22: 1233–1238. [DOI] [PubMed] [Google Scholar]
- Saxe R,Kanwisher N ( 2003): People thinking about thinking people: The role of the temporo‐parietal junction in “theory of mind”. Neuroimage 19: 1835–1842. [DOI] [PubMed] [Google Scholar]
- Saxe R,Powell LJ ( 2006): It's the thought that counts. Psychol Sci 17: 692–699. [DOI] [PubMed] [Google Scholar]
- Saxe R,Wexler A ( 2005): Making sense of another mind: The role of the right temporo‐parietal junction. Neuropsychologia 43: 1391–1399. [DOI] [PubMed] [Google Scholar]
- Saxe R,Carey S,Kanwisher N ( 2004a): Understanding other minds: Linking developmental psychology and functional neuroimaging. Annu Rev Psychol 55: 87–124. [DOI] [PubMed] [Google Scholar]
- Saxe R,Xiao D‐K,Kovacsc G,Perrett DI,Kanwisher N ( 2004b) A region of right posterior superior temporal sulcus responds to observed intentional actions. Neuropsychologia 42: 1435–1446. [DOI] [PubMed] [Google Scholar]
- Saxe R,Schulz LE,Jiang YV ( 2006): Reading minds versus following rules: Dissociating theory of mind and executive control in the brain. Soc Neurosci 1: 284–298. [DOI] [PubMed] [Google Scholar]
- Schilbach L,Wohlschlaeger AM,Kraemer NC,Newen A,Shah NJ,Fink GR,Vogeley K ( 2006): Being with virtual others: Neural correlates of social interaction. Neuropsychologia 44: 718–730. [DOI] [PubMed] [Google Scholar]
- Schmitz TW,Kawahara‐Baccus TN,Johnson SC ( 2004): Metacognitive evaluation, self‐relevance, and the right prefrontal cortex. Neuroimage 22: 941–947. [DOI] [PubMed] [Google Scholar]
- Schultz J,Imamizu H,Kawato M,Frith CD ( 2004): Activation of the human superior temporal gyrus during observation of goal attribution by intentional objects. J Cogn Neurosci 16: 1695–1705. [DOI] [PubMed] [Google Scholar]
- Seger CA,Stone MS,Keenan JP ( 2004): Cortical activations during judgments about the self and an other person. Neuropsychologia 42: 1168–1177. [DOI] [PubMed] [Google Scholar]
- Sommer M,Döhnel K,Sodian B,Meinhardt J,Thoermer C,Hajak G ( 2007): Neural correlates of true and false belief reasoning. Neuroimage 35: 1378–1384. [DOI] [PubMed] [Google Scholar]
- Spiers HJ,Maguire EA ( 2006): Spontaneous mentalizing during an interactive real world task: An fMRI study. Neuropsychologia 44: 1674–1682. [DOI] [PubMed] [Google Scholar]
- Steel CC,Haworth EJ,Peters E,Hemsley DR,Sharma T,Gray JA,Pickering A,Gregory L,Simmons A,Bullmore ET,Williams SCR ( 2001): Neuroimaging correlates of negative priming. Neuroreport 12: 3619–3624. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Gallup GG.Jr ,Alexander MP ( 2001): The frontal lobes are necessary for “theory of mind”. Brain 124: 279–286. [DOI] [PubMed] [Google Scholar]
- Sugiura M,Sassa Y,Watanabe J,Akitsuki Y,Maeda Y,Matsue Y,Fukuda H,Kawashima R ( 2006): Cortical mechanisms of person representation: Recognition of famous and personally familiar names. Neuroimage 31: 853–860. [DOI] [PubMed] [Google Scholar]
- Summerfield C,Egner T,Greene M,Koechlin E,Mangels J,Hirsch J ( 2006): Predictive codes for forthcoming perception in the frontal cortex. Science 314: 1311–1314. [DOI] [PubMed] [Google Scholar]
- Tabert MH,Borod JC,Tang CY,Lange G,Wei TC,Johnson R,Nusbaum AO,Buchsbaum MS ( 2001): Differential amygdala activation during emotional decision and recognition memory tasks using unpleasant words: An fMRI study. Neuropsychologia 39: 556–573. [DOI] [PubMed] [Google Scholar]
- Talairach J,Tournoux P ( 1988): Co‐planar Stereotaxic Atlas of the Human Brain. Stuttgart: Thieme. [Google Scholar]
- Tanaka SC,Doya K,Okada G,Ueda K,Okamoto Y,Yamawaki S ( 2004): Prediction of immediate and future rewards differentially recruits cortico‐basal ganglia loops. Nat Neurosci 7: 887–893. [DOI] [PubMed] [Google Scholar]
- Todorov A,Harris LT,Fiske ST ( 2006): Toward socially inspired social neuroscience. Brain Res 1079: 76–85. [DOI] [PubMed] [Google Scholar]
- Todorov A,Gobbini MI,Evans KK,Haxby JV ( 2007): Spontaneous retrieval of affective person knowledge in face perception. Neuropsychologia 45: 163–173. [DOI] [PubMed] [Google Scholar]
- Tomelleri S,Castelli L,Ito TA ( 2007): Gender and race on the brain: Electrophysiological studies about the automaticity of social categorization and stereotyping. Paper Abstract of the ESCON Conference, Brno, Czech Republic.
- Tomlin D,Kayali MA,King‐Casas B,Anen C,Camerer CF,Quartz SR,Montague PR ( 2006): Agent‐specific responses in the cingulate cortex during economic exchanges. Science 312: 1047–1050. [DOI] [PubMed] [Google Scholar]
- Trope Y,Gaunt R ( 2000): Processing alternative explanations of behavior: Correction or integration? J Pers Soc Psychol 79: 344–354. [DOI] [PubMed] [Google Scholar]
- Turk DJ,Banfield JF,Walling BR,Heatherton TF,Grafton ST,Handy TC,Gazzaniga MS,Macrae CN ( 2004): From facial cue to dinner for two: The neural substrates of personal choice. Neuroimage 22: 1281–1290. [DOI] [PubMed] [Google Scholar]
- Turk DJ,Banfield JF,Walling BR,Heatherton TF,Grafton ST,Handy TC,Gazzaniga MS,Macrae CN ( 2005): From facial cue to dinner for two: the neural substrates of personal choice. Neuroimage 22: 1281–1290. [DOI] [PubMed] [Google Scholar]
- Turner DC,Aitken MRF,Shanks DR,Sahakian BJ,Robbins TW,Schwarzbauer C,Fletcher PC ( 2004): The Role of the lateral frontal cortex in causal associative learning: Exploring preventative and super‐learning. Cereb Cortex 14: 872–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ,Iacoboni M,Lange C,Keenan JP ( 2007): The self and social cogntion: The role of cortical midline structures and mirror neurons. Trends in Cogn Sci 11: 153–157. [DOI] [PubMed] [Google Scholar]
- Uleman JS ( 1999): Spontaneous versus intentional inferences in impression formation In: Chaiken S,Trope Y, editors. Dual‐Process Theories in Social Psychology. New York, NY: The Guilford Press; pp 141–160. [Google Scholar]
- van der Gaag C,Minderaa RB,Keysers C ( 2007): Facial expressions: What the mirror neuron system can and cannot tell us. Soc Neurosci 2: 179–222. [DOI] [PubMed] [Google Scholar]
- Van Duynslaeger M,Van Overwalle F,Verstraeten E ( 2007): Electrophysiological time course and brain areas of spontaneous and intentional trait inferences. Soc Cogn Affect Neurosci 2: 174–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rooy D,Van Overwalle F,Vanhoomissen T,Labiouse C,French R ( 2003): A recurrent connectionist model of group biases. Psychol Rev 110: 536–563. [DOI] [PubMed] [Google Scholar]
- Vogeley K,Bussfeld P,Newen A,Herrmann S,Happé F,Falkai P,Maier W,Shah NJ,Fink GR,Zilles K ( 2001): Mind reading: Neural mechanisms of theory of mind and self‐perspective. Neuroimage 14: 170–181. [DOI] [PubMed] [Google Scholar]
- Vogeley K,May M,Ritzl A,Falkai PK,Zilles K,Fink GR ( 2004): Neural correlates of first‐person perspective as one constituent of human self‐consciousness. J Cogn Neurosci 16: 817–827. [DOI] [PubMed] [Google Scholar]
- Vollm BA,Taylor ANW,Richardson P,Corcoran R,Stirling J,McKie S,Deakin JFW,Elliott R ( 2006): Neuronal correlates of theory of mind and empathy: A functional magnetic resonance imaging study in a nonverbal task. Neuroimage 29: 90–98. [DOI] [PubMed] [Google Scholar]
- Volz KG,Schubotz RI,von Cramon DY ( 2004): Why am I unsure? Internal and external attributions of uncertainty dissociated by fMRI. Neuroimage 21: 848–857. [DOI] [PubMed] [Google Scholar]
- Walter H,Adenzato M,Ciaramidaro A,Enrici I,Pia L,Bara BG ( 2004): Understanding intentions in social interaction: The role of the anterior paracingulate cortex. J Cogn Neurosci 16: 1854–1863. [DOI] [PubMed] [Google Scholar]
- Wang AT,Lee SS,Sigman M,Dapretto M ( 2006): Developmental changes in the neural basis of interpreting communicative intent. Soc Cogn Affect Neurosci 1: 107–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman HM,Cross D,Watson J ( 2001): Meta‐analysis of theory‐of‐mind development: The truth about false belief. Child Dev 72: 655–684. [DOI] [PubMed] [Google Scholar]
- Wicker B,Michel F,Henaff M‐A,Decety J ( 1998): Brain regions involved in the perception of gaze: A PET study. Neuroimage 8: 221–227. [DOI] [PubMed] [Google Scholar]
- Winston JS,Strange BA,O'Doherty J,Dolan RJ ( 2003): Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nat Neurosci 5: 277–283. [DOI] [PubMed] [Google Scholar]
- Wood JN,Grafman J ( 2003): Human prefrontal cortex: Processing and representational perspectives. Nature 4: 139–147. [DOI] [PubMed] [Google Scholar]
- Wood JN,Knutson KM,Grafman J ( 2005): Psychological structure and neural correlates of event knowledge. Cereb Cortex 15: 1155–1161. [DOI] [PubMed] [Google Scholar]
- Woodward AL ( 1998): Infants selectively encode the goal object of an actor's reach. Cognition 69: 1–34. [DOI] [PubMed] [Google Scholar]
- Zhu Y,Zhang L,Fan J,Han S ( 2007): Neural basis of cultural influence on self‐representation. Neuroimage 34: 1310–1316. [DOI] [PubMed] [Google Scholar]
- Zysset S,Müller K,Lohmann G,von Cramon DY ( 2001): Color‐word matching stroop task: Separating interference and response conflict. Neuroimage 13: 29–36. [DOI] [PubMed] [Google Scholar]
- Zysset S,Huber O,Ferstl E,von Cramon DY ( 2002): The anterior frontomedian cortex and evaluative judgment: An fMRI study. Neuroimage 15: 983–991. [DOI] [PubMed] [Google Scholar]
- Zysset S,Huber O,Samson A,Ferstl EC,von Cramon DY ( 2003): Functional specialization within the anterior medial prefrontal cortex: A functional magnetic resonance imaging study with human subjects. Neuroscience 335: 183–186. [DOI] [PubMed] [Google Scholar]


