Abstract
Acute psychosocial stress in humans triggers the release of glucocorticoids (GCs) and influences performance in declarative and working memory (WM) tasks. These memory systems rely on the hippocampus and prefrontal cortex (PFC), where GC‐binding receptors are present. Previous studies revealed contradictory results regarding effects of acute stress on WM‐related brain activity. We combined functional magnetic resonance imaging with a standardized psychosocial stress protocol to investigate the effects of acute mental stress on brain activity during encoding, maintenance, and retrieval of WM. Participants (41 healthy young men) underwent either a stress or a control procedure before performing a WM task. Stress increased salivary cortisol levels and tended to increase WM accuracy. Neurally, stress‐induced increases in cortical activity were evident in PFC and posterior parietal cortex (PPC) during WM maintenance. Furthermore, hippocampal activity was modulated by stress during encoding and retrieval with increases in the right anterior hippocampus during WM encoding and decreases in the left posterior hippocampus during retrieval. Our study demonstrates that stress increases activity in PFC and PPC specifically during maintenance of items in WM, whereas effects on hippocampal activity are restricted to encoding and retrieval. The finding that psychosocial stress can increase and decrease activity in two different hippocampal areas may be relevant for understanding the often‐reported phase‐dependent opposing behavioral effects of stress on long‐term memory. Hum Brain Mapp, 2010. © 2010 Wiley‐Liss, Inc.
Keywords: fMRI, glucocorticoids, hippocampus, posterior parietal cortex, prefrontal cortex, Trier Social Stress Test
INTRODUCTION
It is a well‐known fact from everyday life, as well as from laboratory research, that cognitive processes in general and especially memory are affected by various conditions of acute mental stress (see Lupien et al. [ 2007], for a review). One of the mechanisms through which stress exerts its influence on memory function is an elevation in cortisol level [Wolf, 2008]. Cortisol‐binding glucocorticoid (GC) receptors are located with high density in two memory‐related brain regions: the hippocampus and the prefrontal cortex (PFC) [Perlman et al., 2007; Watzka et al., 2000; Webster et al., 2002]. Accordingly, stress effects have been described for mainly hippocampus‐dependent declarative memory [e.g., Buchanan et al., 2006; de Quervain, 2000, 2003; Kuhlmann et al., 2005a] and mainly PFC‐dependent working memory (WM) processes [Elzinga and Roelofs, 2005; Lupien et al., 1999; Oei et al., 2006; Schoofs et al., 2008; Young et al., 1999]. While for declarative memory the direction of the stress effects depends mainly on the relative time point of stress application [enhancing effects when applied around encoding, deleterious effects when applied before retrieval; Lupien et al., 2007; Smeets et al., 2008; Wolf, 2008], evidence from WM tasks is heterogeneous. Age, gender, task difficulty, and complexity as well as the kind of dependent variable used to assess stress effects seem to be the main mediating factors [Porcelli et al., 2008; Schoofs et al., 2008; van Stegeren, 2009].
So far, only two neuroimaging studies with contradictory results have examined the neural mechanisms underlying the effects of acute stress on WM function. Using a physical stressor (cold‐water hand‐immersion) and a Sternberg task, Porcelli et al. [ 2008] revealed stress‐induced activity increases in PFC during blocks of high WM demand trials in a group of men and women. Qin et al. [ 2009] employed a numerical N‐back task and induced a relatively small physical stress response in women by presenting short movie clips containing extreme violence. They observed reduced activity in bilateral dorsolateral prefrontal Brodmann area (BA) 46 during blocks of high WM demand trials when compared to nonstressed control participants. Neither of these studies found any behavioral effects of the applied stressors nor were they able to separately analyze different WM phases.
The effects of strong acute mental stress on WM‐related brain activity still remain to be investigated. The questions of whether stress influences brain activity during all phases of WM (encoding, maintenance, and retrieval) and, if so, does it do so in a similar manner in all cases are also unresolved. To study these questions, we combined a standardized and highly effective psychosocial stress protocol with a subsequent visual WM task where encoding, maintenance, and retrieval of memory could be investigated. Event‐related functional magnetic resonance imaging (fMRI) was used to capture potential neural correlates of memory phase‐dependent effects of stress in the human brain. Based on the previous literature [Porcelli et al., 2008; Qin et al., 2009], we expected that stressed and nonstressed participants would differ in neural activity in areas related to WM maintenance [PFC and posterior parietal cortex (PPC); Fletcher and Henson, 2001; Purves et al., 2008]. We further hypothesised that stressed participants would show an increase in hippocampal activity during memory encoding, corresponding to improved encoding and subsequent memory previously reported for declarative memory tasks [Otten et al., 2001]. According to findings related to effects of GC administration on declarative memory retrieval [Oei et al., 2007], this should contrast with a stress‐induced decrease of neural activity in the hippocampus during retrieval, reflecting the often‐reported impairments of declarative memory performance.
MATERIALS AND METHODS
Participants
Forty‐three right‐handed healthy young men (age range: 20–36 years, mean age: 26.5 years) took part in this study. To exclude age‐ or sex‐related differences in hormonal balance and stress effects as confounds, all participants were male, under the age of 40 and had a body mass index between 18 and 25 [Wolf et al., 2001]. All participants had normal or corrected‐to‐normal vision and no history of neurological or mental disorders. They were asked to come well rested and were also requested not to eat, drink, or smoke 1 h before the experiment. The study was conducted in accordance with the Declaration of Helsinki [ 2000], and all procedures were carried out with the adequate understanding and written informed consent of the participants. Ethics approval was obtained from the ethics committee of the German Psychological Society (DGPs). The participants received €32 for their attendance. The data of two participants were excluded from further analyses (one reported dizziness during scanning and another misunderstood the instructions regarding the passive condition) leaving a total dataset of 41 participants for statistical analyses.
Design
We combined a standardized psychosocial stress protocol leading to robust increases in cortisol with a subsequent visual WM task. Behavioral, physiological, subjective, and fMRI blood oxygenation level‐dependent (BOLD) data were collected to capture stress effects. Participants were randomly assigned to the stress (20 participants) or control group (21 participants).
Psychosocial Stress
We used the Trier Social Stress Test [TSST; Kirschbaum et al., 1993], which is a standardized and well‐established treatment to induce psychosocial stress in a laboratory setting. After an anticipatory preparation period, participants had to perform a free speech in front of a committee (fictitious job interview), followed by a mental arithmetic task (counting backwards from 2,043 in steps of 17). Each of the three periods lasted 5 min while participants were video‐ and voice‐recorded for potential postanalysis. This protocol is a combination of social‐evaluative threat and an uncontrollable situation, which is consistently associated with a significant cortisol increase in saliva and blood [Dickerson and Kemeny, 2004]. The uncontrollable and evaluative aspects were omitted in the control condition, where participants had to perform a free speech (about a recently experienced motion picture or book) and an easy mental arithmetic task (counting forwards from 0 in steps of 15) in an empty room without committee and recording [Kuhlmann et al., 2005b].
Working Memory Task
A WM task similar to that used by Gazzaley et al. [ 2005] was employed (see Fig. 1). Stimuli were gray‐scale images of scenes (landscapes) and faces with neutral expressions, which were presented against a gray background. Images were categorized in landscapes with or without buildings and female or male faces. Only images of the same category and high similarity were presented within a given trial to keep the task challenging. The size (width = 9.5° visual angle, height = 10.7°) and mean luminance of the images were held constant, and the participants were instructed to keep their gaze on the central fixation cross throughout the whole experiment.
Figure 1.
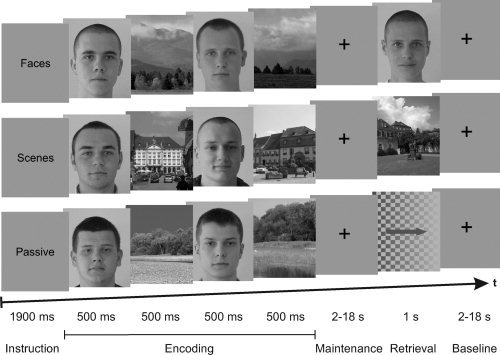
Working memory task. Each trial began with an instruction to either remember the faces and ignore the scenes or remember the scenes and ignore the faces or passively view both. This was followed by the encoding phase, where four stimuli (two faces, two scenes) were presented in a randomized order. After a variable maintenance interval, a probe stimulus was presented in the two active conditions and participants had to determine via button press whether it matched one of the previously shown sample stimuli, which was the case in 50% of the trials. In passive viewing trials, an arrow heading left or right was presented superimposed on a checkerboard with a decreasing luminance contrast gradient in the direction of the arrow, and participants had to press the button corresponding to the direction of the arrow. A variable baseline period followed before the onset of the next trial. The order of different trials was randomized.
There were three different conditions in the task that were presented in random order. At the beginning of the two active WM conditions, participants were instructed to either remember the faces and ignore the scenes (“Faces”) or remember the scenes and ignore the faces (“Scenes”). In the non‐WM condition, subjects were instructed to passively view the faces and scenes (“Passive”) and no later retrieval occurred. Four stimuli (two faces, two scenes) were presented in a randomized order in the following encoding phase. Each image was shown for 500 ms and separated from the next by a 200‐ms blank screen. Thereafter, a delay period ranging from 2 to 18 s (in randomized steps of 4 s) followed, before a probe stimulus was presented for a period of 1 s. Depending on the respective instruction, the probe was either a face, a scene, or—in passive viewing trials—an arrow heading left or right superimposed on a checkerboard with a decreasing luminance contrast gradient in the direction of the arrow. Participants had to determine via button press whether the probe matched one of the sample stimuli presented in the encoding phase (index finger) or not (middle finger), each of which was the case in 50% of the trials. In the passive condition, participants had to press with the finger corresponding to the direction of the arrow. After retrieval, a baseline period followed, which also lasted for 2–18 s in a complementary manner to the delay period, resulting in a constant total trial length of 26 s. The purpose of the variable lengths of the maintenance and baseline periods was to statistically decorrelate the encoding‐ and retrieval‐related BOLD‐signals. The whole task comprised 20 trials for each of the three conditions, leading to a total number of 60 trials and a total duration of ∼26 min. Participants' button presses were registered with an MRI‐compatible optical response keypad (“LUMItouch,” Photon Control, Burnaby, BC, Canada), which was placed at the participants' right hand. The experimental control software was programmed using Cogent 2000 (Cogent 2000 team, FIL and ICN, UCL, London, United Kingdom) and Cogent Graphics (John Romaya, LON, Wellcome Trust Centre for Neuroimaging, UCL, London, United Kingdom). The images used as stimuli were either produced by ourselves or kindly made available by Francesc Tarrés (Department of Signal Theory and Communications, Technical University of Catalonia, Barcelona, Spain), Peter Peer [Computer Vision Laboratory, University of Ljubljana, Ljubljana, Slovenia; Solina et al., 2003], the Psychological Image Collection at Sterling (PICS; Department of Psychology, University of Sterling, Sterling, United Kingdom) or the internet portal “Lichtbildwerkstatt.net.”
Procedure
Participants arrived at 14:30 h or 17:00 h and were informed about the course of the experiment and the task. They performed a 5‐min training session inside the MRI scanner (off‐state) with a separate set of stimuli and were then taken to a different room where the TSST or the control condition was conducted for about 15 min. Following this, scanning started and the participants performed the WM task in the MRI scanner. Stimuli were presented via back projection with a D‐ILA projector (DLA‐G15E, JVC Professional Europe, Friedberg, Germany) onto a semiluminescent screen. This screen was located behind the participants' head at the aperture of the scanner bore and was viewed via a mirror located above the head coil.
Physiological (salivary levels of free cortisol and α‐amylase as a marker of sympathetic nervous system activity) and subjective measures (rating scales) of the effects of the TSST and the control condition, respectively, were collected at three times: before the start of the TSST/control condition (baseline, t0), directly afterwards (t1), and after completion of the WM task (t2). Saliva was collected using Salivette collection devices (Sarstedt AG, Nümbrecht, Germany), which were stored afterwards at −20°C until biochemical analysis. Affective responses were assessed with the German version of the Multidimensional Mood State Questionnaire [MDBF; Steyer et al., 1997], which consists of 24 items with a five‐point rating scale each. These 24 items rely on three underlying dimensions: good mood–bad mood, awake–tired, calm–nervous. While at t1 and t2, saliva collection and MDBF completion took place at exactly the same time, t0 was different for these two measures: saliva was collected directly before the start of the TSST (t0), whereas the MDBF was completed during the anticipatory preparation period of the TSST (t0*).
MRI Data Acquisition
During execution of the WM task, a Siemens MAGNETOM Sonata MRI scanner (Siemens AG, Erlangen, Germany) with a field strength of 1.5 Tesla and a gradient system of 40 mT/m field strength in combination with a standard one‐channel head coil was used to obtain 658 T2*‐weighted gradient echo planar imaging (EPI) volumes with BOLD contrast [time to repeat (TR) = 2.5 s; time to echo (TE) = 50 ms; flip angle α = 90°]. These volumes consisted of 29 axial slices with a gap of 0.75 mm in‐between recorded in ascending order. Each slice had a matrix size of 64 × 64 voxels with a voxel size of 3 × 3 × 3 mm3, resulting in a field‐of‐view of 192 mm. After completion of the task, a T1‐weighted scan (176 contiguous slices, each slice 448 × 512 voxels, voxel size = 1 × 1 × 1 mm3) was conducted to collect a high‐resolution structural volume of each participant. A magnetization‐prepared rapid acquisition gradient echo (MPRAGE) sequence was employed with TR = 1.97 s, TE = 3.93 ms, and α = 15°.
Data Analysis
Physiological data
Salivary levels of free cortisol were measured using a luminescence immunoassay (IBL GmbH, Hamburg, Germany). Inter‐ and intra‐assay variations are below 15%. α‐amylase was analyzed with a quantitative enzyme kinetic method. Within‐ and between‐group differences of 40 participants (one participant had to be excluded from the analysis of physiological and MDBF data because of missing data) were analyzed with a two‐factorial mixed‐effects analysis of variance (ANOVA) with the factors group (stress or control) and time of measurement. This analysis as well as that of the subjective and behavioral data was performed using SPSS 15.0 (SPSS GmbH, Munich, Germany) at a significance threshold of p ≤ 0.05. Significant effects were followed by paired and unpaired post‐hoc t‐tests (Bonferroni‐corrected).
Subjective data
The 24 ratings of the MDBF were mapped onto the three underlying dimensions good mood–bad mood, calm–nervous, and awake–tired. The resulting values on each dimension were analyzed with a two‐factorial mixed‐effects ANOVA with the factors group (stress or control) and time of measurement.
Behavioral data
For every participant, mean reaction times (RTs) of trials with a correct answer were calculated for each condition (face, scene, and passive). These mean RT values and the accuracy (percentage of correct responses) were analyzed with a two‐factorial mixed‐effects ANOVA with the factors group (stress or control) and condition (face, scene, or passive).
MRI data
The MRI data were analyzed with SPM5 software (FIL, Welcome Trust Centre for Neuroimaging, UCL, London, UK). Prior to preprocessing of the functional EPI data, the first five scans were discarded to account for T1 equilibration effects. The remaining 653 images were spatially realigned and unwarped to compensate for motions of the participants' heads during data acquisition. The time series of each voxel was temporally realigned to the acquisition time of the middle slice to correct for acquisition time differences between slices. After spatial registration of the structural T1‐weighted volume with a mean image of the EPI data, all volumes were spatially normalized to the MNI reference brain (Montreal Neurological Institute, Quebec, Canada) by applying a unified tissue segmentation and normalization algorithm. The EPI data were then spatially smoothed with a three‐dimensional Gaussian kernel of 8 mm full‐width‐at‐half‐maximum to accommodate interindividual anatomical variability and to increase signal‐to‐noise ratio.
Statistical analysis of the fMRI data was conducted using a mixed‐effects model. At the single‐subject level (“first level”), regressors were built for the nine events of interest (encoding, maintenance, and retrieval periods for “Face,” “Scene,” and “Passive” trials, respectively) and one regressor for error trials. These regressors are convolutions of a box‐car or stick function (depending on the duration of the respective event: box‐car function for encoding and maintenance, stick function for retrieval) with a canonical synthetic hemodynamic response function time‐locked to the respective onsets of the different events. The general linear model was used to calculate regression coefficients (betas) for each regressor at each measured voxel in the brain and linear contrasts between these betas. To investigate effects of stress on WM phases, we made comparisons between the two active conditions (where subjects had to encode, maintain, and retrieve faces or scenes) on the one hand and the passive condition (where no encoding, maintenance, or retrieval of information was required) and the baseline condition (fixation) on the other hand for each of the three trial phases. The resulting contrast estimates served as summary statistics and were taken to the between‐subject level (“second level”) to calculate within‐ and between‐group effects using one‐ or two‐sample t‐tests, respectively. At the first level, data were high‐pass filtered at 1/128 Hz to account for nonphysiological slow drifts in the measured signal and modelled for temporal autocorrelation across scans with an AR(1) model. The significance threshold used for all statistical analyses was p ≤ 0.05 (familywise error corrected for multiple comparisons). Because of the differing hypotheses regarding stress effects on activity in different brain areas (i.e., the hippocampus for encoding and retrieval and PFC and PPC for maintenance), all analyses were performed separately for the two hypothesis‐related regions of interest (ROI) using two anatomical masks. The first mask encompassed the hippocampus bilaterally (ROI‐size = 975 voxels). The second mask contained cortical areas in bilateral PFC (BAs 9–11 and 44–47) and PPC (BAs 7 and 40) (ROI‐size = 14,424 voxels) known to be related to WM and executive functions [Fletcher and Henson, 2001; Purves et al., 2008]. The masks used for the ROI analyses were derived from the WFU Pickatlas [Maldjian et al., 2003, 2004]. The correction for multiple comparisons was performed for all voxels tested within the respective ROI (i.e., 975 voxels for the hippocampus and 14,424 voxels for PFC and PPC areas). An additional exploratory analysis was performed for the whole brain. Coordinates provided in the Results section are in MNI space. Effect maxima were plotted as a function of group and condition and were tested for group differences with post‐hoc t‐tests (Bonferroni‐corrected).
RESULTS
Physiological and Mood Effects
As can be seen in Figure 2, the cortisol data showed a strong response to acute psychosocial stress as implemented by the TSST. The results of the ANOVA yielded a significant effect of the factors group [F (1, 38) = 10.196, p < 0.01] and time of measurement [F (2, 76) = 29.373, p < 0.001], as well as a significant interaction of these two factors [F (2, 76) = 33.355, p < 0.001]. Post‐hoc t‐tests revealed that within the stress group, absolute values in salivary levels of free cortisol were significantly higher at t1 [T (19) = −7.546, p < 0.001] and t2 [T (19) = −4.814, p < 0.001] as compared to t0. Between groups, significant differences were observed at t1 [T (38) = 3.652, p < 0.001] and t2 [T (38) = 5.415, p < 0.001] with higher values in the stress group. α‐amylase values showed a numeric increase at t1 in the stress group, which returned to baseline values at t2. There were however no significant differences in comparison to the control group.
Figure 2.
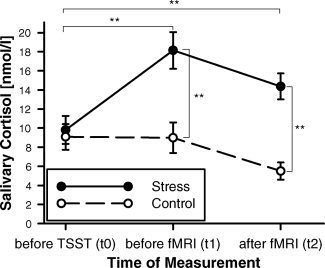
Salivary cortisol data of the two groups of participants at three different times of measurement. Salivary levels of free cortisol were significantly increased in the stress group at t1 (after the TSST) and t2 (after the WM task) as compared to t0 (prior to stress) and to t1 and t2 in the control group. **p < 0.01 (paired and unpaired t‐tests, Bonferroni‐corrected).
The subjective ratings (data not shown) exhibited a pattern of results comparable to the cortisol data. The ANOVA revealed a significant effect of the factor time for the calmness [F (2, 76) = 16.72, p < 0.001] and the wakefulness scale [F (1.467, 76) = 30.378, p < 0.001] as well as a significant effect of the factor group for the scales of good mood–bad mood [F (1, 38) = 5.134, p < 0.05] and calmness [F (1, 38) = 10.476, p < 0.01]. The time × group interaction of both factors had a significant effect on the values of the calmness scale [F (2, 76) = 7.302, p < 0.01]. Post‐hoc t‐tests showed that in comparison to the control group, stressed participants gave significantly lower ratings on the scales of mood and calmness at t0* [T (32.632) = −1.887, p < 0.05; T (39) = −4.466, p < 0.001] and at t1 [T (38) = −2.957, p < 0.01; T (38) = −2.82, p < 0.01]. This indicates lower ratings of mood and calmness in the anticipatory preparation period and immediately after the TSST in the stress group.
Behavioral Data
Table I presents mean reaction time and accuracy data obtained in the WM task during scanning. The type of trial (“Face,” “Scene,” “Passive”) had a significant effect on reaction times [F (2, 78) = 57.995, p < 0.001] and accuracy of the responses [F (2, 78) = 23.567, p < 0.001]. This was due to faster and more accurate responses for the passive condition, where nothing had to be remembered. There was a trend for a significant effect of stress on performance accuracy [F (1, 39) = 3.44, p = 0.071] with higher accuracy in the stress group. Reaction times were numerically increased by stress; this missed however significance [F (1, 39) = 0.97, p = 0.331]. We found no evidence for an interaction effect of the factors task condition and stress on any of the behavioral measures. The length of the maintenance interval did not differentially affect the behavioral measures in any of the two groups of subjects.
Table I.
Mean reaction time (RT) and accuracy data (% correct)
| Group | RT (ms) (σn) | % correct (σn) | ||||
|---|---|---|---|---|---|---|
| Face | Scene | Passive | Face | Scene | Passive | |
| Stress | 1,051 (67) | 1,140 (78) | 773 (49) | 96 (0.9) | 93.7 (1.4) | 99.3 (0.5) |
| Control | 953 (49) | 1,033 (63) | 757 (41) | 93 (1.5) | 90 (1.9) | 99.5 (0.5) |
σn = standard error.
fMRI Data: PFC and PPC
The ROI analysis in PFC and PPC revealed stress‐induced modulation of neural activity during the maintenance period only. We found several sites where participants of the stress group exhibited significantly stronger activation than the control group during the maintenance period. In left dorsolateral prefrontal BA 9 (x = −45, y = 12, z = 21; Z = 4.53; 22 voxels), right ventrolateral prefrontal BA 47 (x = 45, y = 36, z = −9; Z = 4.01; 18 voxels) as well as in posterior parietal BAs 7 (x = 15, y = −57, z = 45; Z = 4.51; 27 voxels) and 40 (x = −33, y = −48, z = 33; Z = 4.47; 13 voxels), the difference in measured BOLD signal between active and passive conditions was significantly stronger in the stress as compared to the control group. While stressed participants showed consistently higher activity levels during active than passive trials, control participants showed exactly the reverse pattern of activity (see Fig. 3). A post‐hoc multivariate analysis of variance (MANOVA) of the data presented in Figure 3 revealed a significant effect of the factor task condition [“active” or “passive”; F (4, 36) = 3.859, p < 0.01] as well as a significant interaction of the factors task condition and group [“stress” or “control”; F (4, 36) = 25.237, p < 0.001]. However, no evidence for differences in neural activity within prefrontal or posterior parietal areas was found between the two groups during memory encoding or retrieval.
Figure 3.
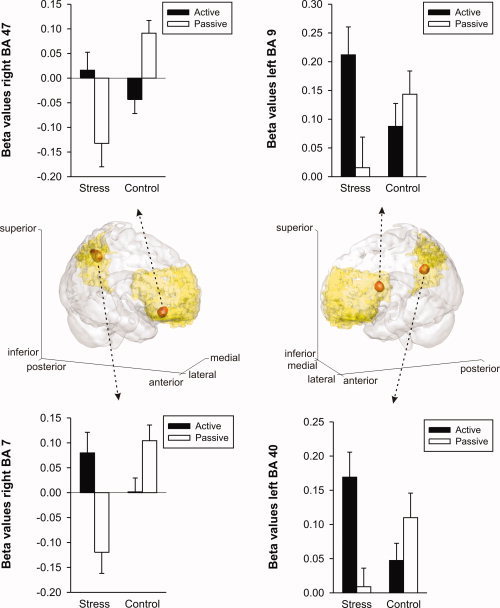
Prefrontal and parietal activity differences between the stress and the control group during maintenance of memory. Middle row: 3D transparent “glass brain” renderings showing (in red) the sites of group differences in cortical activity within PFC and PPC (significance threshold: p ≤ 0.05, familywise error corrected for multiple comparisons). Shaded in yellow are the regions of interest (ROI) comprising prefrontal (BAs 9, 10, 11, 44, 45, 46, and 47) and posterior parietal cortical areas (BAs 7 and 40). Upper and lower row: Estimated betas of the peak voxels as a function of condition (active/passive) and group (stress/control). Error bars represent ±1 standard error. In all four areas, stressed participants show stronger activity during active as compared to passive trials, while this activity pattern is reversed in control participants.
fMRI Data: Hippocampus
The ROI analysis in the hippocampus revealed stress‐induced modulations of neural activity during encoding and retrieval only (see Fig. 4). During the encoding period, the difference in measured BOLD signal between the two active conditions—where stimuli had to be remembered—and the passive condition was significantly higher for the stress group as compared to the control group in the right anterior hippocampus (x = 33, y = −9, z = −21; Z = 3.58; 2 voxels). As can be seen in Figure 4c (left side), this effect was due to a numeric increase of neural activity in the active condition and a significant decrease in activity during the passive condition (post‐hoc t‐test: T (39) = 2.359, p < 0.05). Compared to baseline, participants of the control group showed a similar increase of neural activity in the passive and the two active conditions. In contrast, stressed participants exhibited an increase of hippocampal activity only during the active, but not passive, conditions. In other words, stressed participants show bigger differences in neural activity between trials where stimuli had to be remembered (active) and trials where remembering was not necessary (passive).
Figure 4.
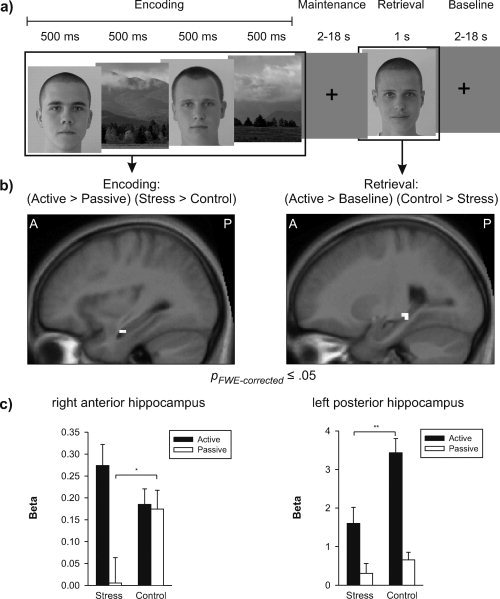
Hippocampal activity differences between the stress and the control group during memory encoding (left side) and memory retrieval (right side): (a) Illustration of memory phase entering into analysis; (b) sagittal slices of an anatomical mean image of all participants depicting sites of hippocampal activity differences (significance threshold: p ≤ 0.05, familywise error corrected for multiple comparisons). Left side: bigger difference between the activity during the encoding phases of active and passive trials in the stress as compared to the control group in right anterior hippocampus (x = 33, y = −9, z = −21; Z = 3.58; 2 voxels). Right side: bigger difference between the activity during retrieval phases of active trials and baseline in the control as compared to the stress group in left posterior hippocampus (x = −21, y = −33, z = −9; Z = 3.61; 5 voxels). (c) Estimated betas of the peak voxels of the clusters shown in (b) as a function of condition (active/passive) and group (stress/control). Error bars represent ±1 standard error. During encoding, stress leads to a numeric increase in anterior hippocampal activity in active trials and a significant decrease in passive trials. During retrieval, stress leads to a significant decrease of posterior hippocampal activity during active trials. *p < 0.05, **p < 0.01 (unpaired t‐tests, Bonferroni‐corrected).
To investigate retrieval‐related activity, we compared the BOLD signal during retrieval in active and passive trials between the two groups. Here, the increase in activity in left posterior hippocampus (x = −21, y = −33, z = −9; Z = 3.61; 5 voxels) during the active conditions compared to baseline activity was significantly bigger in the control than in the stress group (see Fig. 4c, right side). A post‐hoc t‐test revealed a significant difference between the two groups regarding active trials with a smaller signal in stressed participants [T (39) = 3.311, p < 0.01]. No evidence was found for a difference in hippocampal activity between the two groups with respect to retrieval during passive trials. Note that there were also no group differences in hippocampal activity between active or passive trials during the maintenance period. Furthermore, the length of the maintenance period did not affect hippocampal activity during retrieval in any of the two groups nor did it interact with the group differences in hippocampal activity. To test the assumption that stress affects the two mentioned parts of the hippocampus differently during WM encoding and retrieval, we performed a post‐hoc three‐way ANOVA on the effect maxima with the factors hippocampal region (right anterior, left posterior), group (stress, control), and WM phase (encoding, retrieval). This analysis revealed a significant interaction of these three factors [F (1, 39) = 4.026, P = 0.05] indicating that stress differentially affects the right anterior and left posterior hippocampus during encoding and retrieval.
Explorative whole brain analyses for all memory phases and contrasts revealed one additional locus of differential cortical activity. During the maintenance period of active trials, control participants showed significantly stronger activity in the right middle temporal gyrus (x = 60, y = −12, z = −6; Z = 5.14; 3 voxels) than stressed participants when compared to baseline activity. Neither the ROI nor the whole brain analyses revealed any significant differences between “Face” and “Scene” trials or any correlations between behavioral measures and BOLD signal with respect to any of the experimental hypotheses.
fMRI: Attention
In an additional analysis, neural activity in the fusiform face area and parahippocampal place area was gauged for all three trial types to ascertain that participants complied with task instructions and attended the faces and scenes only in active, but not in passive trials. Neural activity in the fusiform face area and parahippocampal place area was higher for trials where faces or scenes, respectively, had to be encoded as compared to the passive condition (data not shown). This suggests that passive trials did not attract the same amount of attention as WM trials and that faces and scenes were attended according to the instructions. We found no statistical evidence for differences between stressed and nonstressed participants in these effects.
DISCUSSION
By combining a well‐established and highly effective psychosocial stress protocol with a subsequent WM task and fMRI, we were able to reveal a stress‐related enhancement of prefrontal and posterior parietal activity during the maintenance period of WM. Further, we found increases and decreases of neural activity in different parts of the hippocampus during encoding and retrieval as a consequence of psychosocial stress.
Endocrine, Affective, and Behavioral Data
The significant group differences in salivary cortisol and subjective mood ratings replicate results of many prior studies (see Dickerson and Kemeny [ 2004], for a review) and confirm that the employed psychosocial stress protocol (TSST) successfully induced a neuroendocrine and subjective stress response. Cortisol levels were elevated before as well as immediately after the scanning session.
Similar to previous studies [Porcelli et al., 2008; Qin et al., 2009; Schoofs et al., 2008], we found only a weak effect of stress on behavioral measures in the WM task. This may be explained by the fact that the stressor always affects both encoding and retrieval in WM tasks. Since previous studies regarding declarative memory have shown that stress applied before or after encoding improves memory performance, but deteriorates it when applied before retrieval (see Het et al. [ 2005] and Lupien et al. [ 2007], for reviews), it is possible that these opposite effects on behavioral measures of memory performance only led to a trend of improved accuracy under stress in our WM task where encoding and retrieval cannot be temporally separated. Additionally, a ceiling effect in task difficulty (as indicated by accuracy levels above 90% in both groups of participants) might have occluded more pronounced behavioral stress effects. Oei et al. [ 2006], for example, showed that stress impairs WM performance only at high loads, but not at low loads in a Sternberg paradigm.
fMRI Data
In contrast to behavioral measures and previous block‐design fMRI studies, which cannot disentangle the effects of stress on WM encoding, maintenance, and retrieval, event‐related fMRI data enable a separate analysis of these memory phases. However, due to the nature of WM, it is impossible to completely separate direct effects of acute stress on cortical activity related to WM maintenance and retrieval from indirect ones that result from stress effects on WM encoding and subsequently altered maintenance and retrieval.
PFC and PPC
Comparable to our results, increases in the extent of prefrontal cortical activity during blocks of high WM demand trials have been described in a recent fMRI study employing cold stress [Porcelli et al., 2008]. Additionally, stress induced by mental calculation or the TSST has been shown to increase lateral prefrontal cortical metabolism and perfusion [Kern et al., 2008; Wang et al., 2005]. Here, we show that this increased activity is restricted to the maintenance phase and not present during encoding or retrieval. Whether these effects are due to the action of cortisol or rather reflect the action of an acute stress‐induced increase in catecholamines cannot be answered in the present study. However, our results suggest that adrenergic effects were weak. Further, if an adrenergic activation contributed to the present findings, behavioral effects should have been more pronounced in the first half of the experiment, which was not the case in our subjects (data not shown). Our findings are in contrast to a study by Qin et al. [ 2009] who found a stress‐induced decrease in WM‐related PFC activity in women. Note, however, that their stress procedure (aversive movie clips) did not induce an increase, but merely attenuated a decrease of salivary cortisol in comparison to a control group (neutral movie clips).
Prior neuroimaging studies employing WM tasks have shown increases in dorso‐ and ventrolateral prefrontal as well as left parietal cortical activity with increasing WM load or in subjects with low WM performance [Braver et al., 1997; Rypma et al., 2002; Veltmann et al., 2003]. Shaw et al. [ 2009] reported elevated activity in PFC and PPC during WM maintenance in patients with post‐traumatic stress disorder and interpreted this finding as indicative of inefficient allocation of resources. A recent fMRI study by Liston et al. [ 2009] revealed a disruption of functional connectivity within a frontoparietal network that mediates attention shifts by chronic psychosocial stress. Taken together with data from pharmacological fMRI studies, where it was shown that the procholinergic drug physostigmine reduced neural activity in the PFC and reaction times in a WM task [Furey et al., 2000], this suggests that lower prefrontal cortical activity may represent a correlate of efficient WM performance. We suggest that the combination of stress‐induced increases in cortical activity in several prefrontal and posterior parietal areas and of a trend for a stress‐induced improvement in behavioral accuracy as found here indicates that stressed participants overcompensated stress‐related difficulties in task performance. Preceding stress may have made the WM task more difficult by increasing the overall load on cognitive functions so that stressed participants had to put more effort in task completion (hence the increased cortical activity). The increase in effort may have led to changes in cognitive strategy resulting in slightly longer reaction times, but higher accuracy.
Hippocampus
Even though hippocampal activations have so far been primarily found in declarative memory studies, they have also been reported during encoding and retrieval in WM tasks and are not restricted to declarative memory tasks [Karlsgodt et al., 2005]. Our results revealed that psychosocial stress affects WM‐related activity in hippocampal regions during encoding and retrieval, with opposing effects in both phases. Effects of exogenously or endogenously raised cortisol levels on neural activity during WM encoding have not been investigated before. Here, we show for the first time increased anterior hippocampal activity in stressed participants during encoding of contents into WM. The decreased neural activity in posterior hippocampus, which we found during retrieval, is in line with results from declarative memory tasks with pharmacological manipulations. There, exogenously raised cortisol levels were found to induce decreases in neural activity in posterior parts of the hippocampus during declarative memory retrieval [de Quervain et al., 2003; Oei et al., 2007]. Kukolja et al. [ 2008] recently reported a negative effect of endogenously raised cortisol levels on hippocampal activity during recognition memory retrieval in older participants. Another study found cortisol‐related deactivations in the limbic system, including the hippocampal formation, during a stressful mental arithmetic task with negative feedback as compared to the same task without negative feedback [Pruessner et al., 2008]. Henckens et al. [ 2009] revealed a stress‐induced negative correlation between hippocampal activity during declarative memory encoding and later memory performance. Our finding of a relative stress‐induced increase in right anterior hippocampal activity during WM encoding and a decrease in left posterior hippocampal activity during retrieval suggests that stress not only affects different hippocampal areas depending on the memory phase but also increases and decreases hippocampal activity. Such bidirectional effects have similarities with behavioral observations, showing that stress effects on declarative memory performance vary depending on the relative time of stress application [Lupien et al., 2007; Smeets et al., 2008; Wolf, 2008]. Although there are some differences between WM and declarative memory, this may suggest a contribution of the hippocampal activity pattern to the previously observed behavioral stress effects.
What are the mechanisms responsible for the stress‐induced differences in anterior hippocampal activity during the encoding period and in the posterior hippocampus during the retrieval period? Our data suggest that the differential effects during encoding are driven by both a numeric increase of hippocampal activity during presentation of the “to be remembered” stimuli during active trials and a significant decrease during presentation of the “not to be remembered” stimuli in passive trials. Even though these stimuli did not need to be encoded, participants in the control group showed similar increases of hippocampal activity during active and passive trials. In contrast, the hippocampal activity in stressed participants did not rise during passive trials. This finding fits well with the abovementioned increases in PFC and PPC activity, which are indicative of increased effort to perform the task. If task difficulty increases under stress, participants are forced to better differentiate between task‐relevant and ‐irrelevant information and to activate their memory resources only when necessary, thereby increasing the overall signal‐to‐noise ratio and minimizing possible interference between several memory representations. On the contrary, control participants might have had more spare resources to use for processing task‐irrelevant stimuli.
In contrast to this stress‐induced increase in encoding‐related hippocampal activity during active as compared to passive trials, the mechanism observed at retrieval was a stress‐induced decrease of hippocampal activity when retrieval was necessary in active trials. In addition to the abovementioned studies, decreased hippocampal activity during memory retrieval (word recognition) is, for example, also seen in old age [Dennis et al., 2008].
The fact that we found differences in hippocampal activity in an anterior site during memory encoding and—comparable to de Quervain et al. [ 2003] and Oei et al. [ 2007]—in a posterior site during retrieval fits well with the HIPER model of hippocampal activity for declarative memory tasks [Lepage et al., 1998]. This model is based on an extensive review of 52 PET studies concerning hippocampal activity during declarative memory encoding and retrieval and suggests that during encoding, mainly anterior parts of the hippocampus are active, while during retrieval, predominantly posterior hippocampal regions are activated. However, a more recent meta‐analysis does not suggest such a clear distinction [Henson, 2005]. Others have found a functional segregation within the hippocampus depending on stimulus novelty with the anterior hippocampus being responsive to novel stimuli and the posterior hippocampus showing stronger responses with increasing stimulus familiarity [Strange et al., 1999]. Our data are also in line with such an interpretation, since stimuli were novel during encoding and partly (in 50% of the cases) familiar during retrieval.
CONCLUSION
Our results suggest that increased prefrontal and parietal neural activity may represent a correlate of stress‐induced difficulties in WM processes and their compensation. We further propose that stress‐induced increases and decreases of neural activity in different hippocampal locations during encoding and retrieval may represent one possible neural mechanism of the often observed bidirectional effects of psychosocial stress on memory performance. Future studies should investigate whether a similar mechanism holds true for declarative memory tasks.
Acknowledgements
We thank Jale Özyurt (Carl von Ossietzky Universität Oldenburg) for helpful comments on an earlier version of the manuscript and Daniela Schoofs (Ruhr‐Universität Bochum) for introduction to the psychosocial stress protocol.
REFERENCES
- Braver TS,Cohen JD,Nystrom LE,Jonides J,Smith EE,Noll DC ( 1997): A parametric study of prefrontal cortex involvement in human working memory. Neuroimage 5: 49–62. [DOI] [PubMed] [Google Scholar]
- Buchanan TW,Tranel D,Adolphs R ( 2006): Impaired memory retrieval correlates with individual differences in cortisol response but not autonomic response. Learn Mem 13: 382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Declaration of Helsinki ( 2000): 52nd WMA General Assembly, Edinburgh, Scotland, October 2000.
- Dennis NA,Kim H,Cabezal R ( 2008): Age‐related differences in brain activity during true and false memory retrieval. J Cogn Neurosci 20: 1390–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Quervain DJ,Roozendaal B,Nitsch RM,McGaugh JL,Hock C ( 2000): Acute cortisone administration impairs retrieval of long‐term declarative memory in humans. Nat Neurosci 3: 313–314. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ,Henke K,Aerni A,Treyer V,McGaugh JL,Berthold T,Nitsch RM,Buck A,Roozendaal B,Hock C ( 2003): Glucocorticoid‐induced impairment of declarative memory retrieval is associated with reduced blood flow in the medial temporal lobe. Eur J Neurosci 17: 1296–1302. [DOI] [PubMed] [Google Scholar]
- Dickerson SS,Kemeny ME ( 2004): Acute stressors and cortisol response: A theoretical integration and synthesis of laboratory research. Psychol Bull 130: 355–391. [DOI] [PubMed] [Google Scholar]
- Elzinga BM,Roelofs K ( 2005): Cortisol‐induced impairments of working memory require acute sympathetic activation. Behav Neurosci 119: 98–103. [DOI] [PubMed] [Google Scholar]
- Fletcher PC,Henson RN ( 2001): Frontal lobes and human memory: Insights from functional neuroimaging. Brain 124: 849–881. [DOI] [PubMed] [Google Scholar]
- Furey ML,Pietrini P,Haxby JV ( 2000): Cholinergic enhancement and increased selectivity of perceptual processing during working memory. Science 290: 2315–2319. [DOI] [PubMed] [Google Scholar]
- Gazzaley A,Cooney JW,McEvoy K,Knight RT,D'Esposito M ( 2005): Top–down enhancement and suppression of the magnitude and speed of neural activity. J Cogn Neurosci 17: 507–517. [DOI] [PubMed] [Google Scholar]
- Henckens MJAG,Hermans EJ,Pu Z,Joels M,Fernández G ( 2009): Stressed memories: How acute stress affects memory formation in humans. J Neurosci 29: 10111–10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson R ( 2005): A mini‐review of fMRI studies of human medial temporal lobe activity associated with recognition memory. Q J Exp Psychol B 58: 340–360. [DOI] [PubMed] [Google Scholar]
- Het S,Ramlow G,Wolf OT ( 2005): A meta‐analytic review of the effects of acute cortisol administration on human memory. Psychoneuroendocrinology 30: 771–784. [DOI] [PubMed] [Google Scholar]
- Karlsgodt KH,Shirinyan D,van Ep TGM,Cohen MS,Cannon TD ( 2005): Hippocampal activations during encoding and retrieval in a verbal working memory paradigm. Neuroimage 25: 1224–1231. [DOI] [PubMed] [Google Scholar]
- Kern S,Oakes TR,Stone CK,McAuliff EM,Kirschbaum C,Davidson RJ ( 2008): Glucose metabolic changes in the prefrontal cortex are associated with HPA axis response to a psychosocial stressor. Psychoneuroendocrinology 33: 517–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C,Pirke K‐M,Hellhammer DH ( 1993): The “Trier Social Stress Test”—A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 28: 76–81. [DOI] [PubMed] [Google Scholar]
- Kuhlmann S,Kirschbaum C,Wolf OT ( 2005a) Effects of oral cortisol treatment in healthy young women on memory retrieval of negative and neutral words. Neurobiol Learn Mem 83: 158–162. [DOI] [PubMed] [Google Scholar]
- Kuhlmann S,Piel M,Wolf OT ( 2005b) Impaired memory retrieval after psychosocial stress in healthy young men. J Neurosci 25: 2977–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukolja J,Thiel CM,Wolf OT,Fink GR ( 2008): Increased cortisol levels in cognitively challenging situations are beneficial in young but not older subjects. Psychopharmacology (Berl) 201: 293–304. [DOI] [PubMed] [Google Scholar]
- Lepage M,Habib R,Tulving E ( 1998): Hippocampal PET activations of memory encoding and retrieval: The HIPER model. Hippocampus 8: 313–322. [DOI] [PubMed] [Google Scholar]
- Liston C,McEwen BS,Casey BJ ( 2009): Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc Natl Acad Sci USA 106: 912–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ,Gillin CJ,Hauger RL ( 1999): Working memory is more sensitive than declarative memory to the acute effects of corticosteroids: A dose‐response study in humans. Behav Neurosci 113: 420–430. [DOI] [PubMed] [Google Scholar]
- Lupien SJ,Maheu F,Tu M,Fiocco A,Schramek TE ( 2007): The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition. Brain Cogn 65: 209–237. [DOI] [PubMed] [Google Scholar]
- Maldjian JA,Laurienti PJ,Burdette JB,Kraft RA ( 2003): An automated method for neuroanatomic and cytoarchitectonic atlas‐based interrogation of fMRI data sets. Neuroimage 19: 1233–1239. [DOI] [PubMed] [Google Scholar]
- Maldjian JA,Laurienti PJ,Burdette JH ( 2004): Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage 21: 450–455. [DOI] [PubMed] [Google Scholar]
- Oei NYL,Everaerd WTAM,Elzinga BM,van Well S,Bermond B ( 2006): Psychosocial stress impairs working memory at high loads: An association with cortisol levels and memory retrieval. Stress 9: 133–141. [DOI] [PubMed] [Google Scholar]
- Oei NYL,Elzinga BM,Wolf OT,de Ruiter MB,Damoiseaux JS,Kuijer JPA,Veltmann DJ,Scheltens P,Rombouts SARB ( 2007): Glucocorticoids decrease hippocampal and prefrontal activation during declarative memory retrieval in young men. Brain Imaging Behav 1: 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten LJ,Henson RN,Rugg MD ( 2001): Depth of processing effects on neural correlates of memory encoding: Relationship between findings from across‐ and within‐task comparisons. Brain 124: 399–412. [DOI] [PubMed] [Google Scholar]
- Perlman WR,Webster MJ,Herman MM,Kleinman JE,Weickert CS ( 2007): Age‐related differences in glucocorticoid receptor mRNA levels in the human brain. Neurobiol Aging 28: 447–458. [DOI] [PubMed] [Google Scholar]
- Porcelli AJ,Cruz D,Wenberg K,Patterson MD,Biswal BB,Rypma B ( 2008): The effects of acute stress on human prefrontal working memory systems. Physiol Behav 95: 282–289. [DOI] [PubMed] [Google Scholar]
- Pruessner JC,Dedovic K,Khalili‐Mahani N,Engert V,Pruessner M,Buss C,Renwick R,Dagher A,Meaney MJ,Lupien S ( 2008): Deactivation of the limbic system during acute psychosocial stress: Evidence from positron emission tomography and functional magnetic resonance imaging studies. Biol Psychiatry 63: 234–240. [DOI] [PubMed] [Google Scholar]
- Purves D,Brannon EM,Cabeza R,Huettel SA,LaBar KS,Platt ML,Woldorff MG ( 2008): Principles of Cognitive Neuroscience. Sunderland: Sinauer Associates. [Google Scholar]
- Qin S,Hermans EJ,van Marle HJF,Luo J,Fernández G ( 2009): Acute psychosocial stress reduces working memory‐related activity in the dorsolateral prefrontal cortex. Biol Psychiatry 66: 25–32. [DOI] [PubMed] [Google Scholar]
- Rypma B,Berger JS,D'Esposito M ( 2002): The influence of working memory demand and subject performance on prefrontal cortical activity. J Cogn Neurosci 14: 721–731. [DOI] [PubMed] [Google Scholar]
- Schoofs D,Preuß D,Wolf OT ( 2008): Psychosocial stress induces working memory impairments in an N‐back paradigm. Psychoneuroendocrinology 33: 643–653. [DOI] [PubMed] [Google Scholar]
- Shaw ME,Moores KA,Clark RC,McFarlane AC,Strother SC,Bryant RA,Brown GC,Taylor JD ( 2009): Functional connectivity reveals inefficient working memory systems in post‐traumatic stress disorder. Psychiatry Res Neuroimaging 172: 235–241. [DOI] [PubMed] [Google Scholar]
- Smeets T,Otgaar H,Candel I,Wolf OT ( 2008): True or false? Memory is differently affected by stress‐induced cortisol elevations and sympathetic activity at consolidation and retrieval. Psychoneuroendocrinology 33: 1378–1386. [DOI] [PubMed] [Google Scholar]
- Solina F,Peer P,Batagelj B,Juvan S,Kovac J ( 2003): Color‐based face detection in the “15 seconds of fame” art installation. In: Proceedings of Mirage 2003, INRIA Rocquencourt, France, March 10–11, 2003. pp 38–47. See also: CVL face database: http://www.lrv.fri.uni-lj.si/facedb.html.
- Steyer R,Schwenkmezger P,Notz P,Eid M ( 1997): Der Mehrdimensionale Befindlichkeitsfragebogen (MDBF). Göttingen: Hogrefe. [Google Scholar]
- Strange BA,Fletcher PC,Henson RNA,Friston KJ,Dolan RJ ( 1999): Segregating the functions of human hippocampus. Proc Natl Acad Sci USA 96: 4034–4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Stegeren AH ( 2009): Imaging stress effects on memory: A review of neuroimaging studies. Can J Psychiatry 54: 16–27. [DOI] [PubMed] [Google Scholar]
- Veltmann DJ,Rombouts SARB,Dolan RJ ( 2003): Maintenance versus manipulation in verbal working memory revisited: An fMRI study. Neuroimage 18: 247–256. [DOI] [PubMed] [Google Scholar]
- Wang J,Rao H,Wetmore GS,Furlan PM,Korczykowski M,Dinges DF,Detre JA ( 2005): Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proc Natl Acad Sci USA 102: 17804–17809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watzka M,Bidlingmaier F,Beyenburg S,Henke RT,Clusmann H,Elger CE,Schramm J,Klingmüller D,Stoffel‐Wagner B ( 2000): Corticosteroid receptor mRNA expression in the brains of patients with epilepsy. Steroids 65: 895–901. [DOI] [PubMed] [Google Scholar]
- Webster MJ,Knable MB,O'Grady J,Orthmann J,Weickert CS ( 2002): Regional specificity of brain glucocorticoid receptor mRNA alterations in subjects with schizophrenia and mood disorders. Mol Psychiatry 7: 985–994. [DOI] [PubMed] [Google Scholar]
- Wolf OT ( 2008): The influence of stress hormones on emotional memory: Relevance for psychopathology. Acta Psychol (Amst) 127: 513–531. [DOI] [PubMed] [Google Scholar]
- Wolf OT,Schommer NC,Hellhammer DH,McEwen BS,Kirschbaum C ( 2001) The relationship between stress induced cortisol levels and memory differs between men and women. Psychoneuroendocrinology 26: 711–720. [DOI] [PubMed] [Google Scholar]
- Young AH,Sahakian BJ,Robbins TW,Cowen PJ ( 1999): The effects of chronic administration of hydrocortisone on cognitive function in normal male volunteers. Psychopharmacology (Berl) 145: 260–266. [DOI] [PubMed] [Google Scholar]


