Abstract
Patients with major depressive disorder (MDD) often show a tendency to strongly introspect and reflect upon their self, which has been described as increased self‐focus. Although subcortical‐cortical midline structures have been associated with reflection and introspection of oneself in healthy subjects, the neural correlates of the abnormally increased attribution of negative emotions to oneself, i.e. negative self‐attribution, as hallmark of the increased self‐focus in MDD remain unclear. The aim of the study was, therefore, to investigate the neural correlates during judgment of self‐relatedness of positive and negative emotional stimuli thereby testing for emotional self‐attribution. Using fMRI, we investigated 27 acute MDD patients and compared them with 25 healthy subjects employing a paradigm that focused on judgment of self‐relatedness when compared with mere perception of the very same emotional stimuli. Behaviourally, patients with MDD showed significantly higher degrees of self‐relatedness of specifically negative emotional stimuli when compared with healthy subjects. Neurally, patients with MDD showed significantly lower signal intensities in various subcortical and cortical midline regions like the dorsomedial prefrontal cortex (DMPFC), supragenual anterior cingulate cortex, precuneus, ventral striatum (VS), and the dorsomedial thalamus (DMT). Signal changes in the DMPFC correlated with depression severity and hopelessness whereas those in the VS and the DMT were related to judgment of self‐relatedness of negative emotional stimuli. In conclusion, we present first evidence that the abnormally increased negative self‐attribution as hallmark of the increased self‐focus in MDD might be mediated by altered neural activity in subcortical‐cortical midline structures. Hum Brain Mapp, 2009. © 2008 Wiley‐Liss, Inc.
Keywords: major depressive disorder, fMRI, neuroimaging, self‐focus, self‐relatedness
INTRODUCTION
Patients with major depressive disorder (MDD) can be characterized by multiple self‐abnormalities including ruminations, self‐blame, and increased association of their self with negative emotions (Gruenbaum et al., 2005; Ingram, 1990; Northoff, 2007; Rimes and Watkins, 2005; Treynor, 2003). Clinically, these different abnormalities of the self may be subsumed under the concept of increased self‐focus (Ingram, 1990; Northoff, 2007). One hallmark of such increased self‐focus is the abnormal attribution of negative emotions to patients' self which may consequently be described as increased self‐attribution of negative emotions, i.e., emotional or negative self‐attribution.
Despite these well‐known clinical phenomena, the underlying neural networks and the pathophysiological mechanisms remain unclear. The topic of self has recently become a focus in functional neuroimaging. Imaging studies in healthy subjects demonstrated recruitment of the dorsomedial prefrontal cortex (DMPFC) and other cortical and subcortical midline structures like the supragenual anterior cingulate cortex (SACC), the precuneus, the dorsomedial thalamus (DMT), and the ventral striatum (VS) during self‐related stimuli (Northoff and Bermpohl, 2004; Northoff et al., 2006; Schmitz and Johnson, 2006). These subcortical‐cortical midline regions are supposed to mediate introspective processes like reflection, evaluation, and recollection upon oneself (Damasio, 1999; deGreck et al., 2008; Keenan et al., 2001; Kelley et al., 2002; Mitchell et al., 2006; Northoff et al., 2006; Northoff, 2007; Northoff, 2008; Ochsner et al., 2005; Schmitz and Johnson, 2007; Uddin et al., 2007). These introspective processes and hence the self have specifically been associated with high resting state neural activity in the subcortical‐cortical midline regions (Northoff et al., 2006, Schneider et al., in press, Mason et al., 2007, D'Argembeau et al., 2005). Lateral cortical regions have been associated with other aspects of the self. The insula, for instance, is supposed to be implicated in the bodily‐vegetative aspects of the self (Craig, 2002, 2003, 2004) and the ventro‐ and dorsolateral prefrontal cortex (DLPFC) in processing recognition of oneself (Keenan et al., 2001; Northoff et al., 2006).
Interestingly, the very same regions have also been observed to be abnormal in MDD. For instance, several studies demonstrated altered neural activity in subcortical‐cortical midline regions like the DMPFC, the SACC, the precuneus, the DMT, and the VS in MDD during various kinds of emotional‐cognitive stimulation (Fitzgerald et al., 2008; Grimm et al., 2008; Mayberg, 2002, 2003; Phillips et al., 2003; Siegle et al., 2006; Steele et al., 2007). Furthermore, there is strong evidence that these subcortical‐cortical midline regions might be crucial in the so‐called default‐mode network (Raichle et al., 2001, 2005, Buckner et al., 2008) that show increased neural activity during the resting state in MDD (Greicius et al., 2007; Grimm et al., 2008; Mayberg, 2002, 2003; Philips et al., 2003 for review). Such increased resting state neural activity should then lead to decreased activity as induced by external stimuli, i.e., stimulus‐induced activity. This has indeed been demonstrated in a recent study of ours with regard to emotional stimuli though unfortunately not in association with self‐relatedness (see Grimm et al., 2008).
In contrast to these studies, so far no studies reported neural abnormalities of the self during emotional‐cognitive stimulation in MDD. The aim of our study was to investigate the neural mechanisms underlying the abnormalities of the self in MDD, e.g., the ability to reflect upon and introspect the relation of positive and negative emotions to oneself as it is for instance required when attributing emotions to oneself. We hypothesized that the apparently abnormally high resting state neural activity in these patients should lead to lower stimulus‐induced signal changes by self‐relatedness in subcortical‐cortical midline regions. One would consequently expect that the tendency of patients with MDD to strongly reflect upon oneself, i.e., the increased self‐focus, and particularly the increased self‐attribution of negative emotions may be directly modulated by decreased signal changes in subcortical‐cortical midline regions. To test these hypotheses, we investigated 27 patients with acute MDD during the judgment of self‐relatedness of positive and negative emotional stimuli thus investigating self‐attribution of emotions as hallmark of what can be clinically described as increased self‐focus.
METHODS AND MATERIALS
Subjects
Subjects with an acute MDD episode (DSM‐IV, American Psychiatric Association, 1994) were recruited from the inpatient department of Psychiatry at the University of Zurich. Eligibility screening procedures included the 21‐item Hamilton Depression Rating Scale (HDRS) (Hamilton, 1960), the 21‐item Beck Depression Inventory (BDI) (Beck et al., 1961), the 20‐item Beck Hopelessness Scale (BHS) (Beck et al., 1974), which includes many items about oneself, and clinical laboratory tests. Diagnoses of depression were made by the participants' treating psychiatrists. Inclusion criteria were a score of at least 18 on the HDRS and the BDI. Exclusion criteria were major medical illnesses, histories of seizures, head trauma with loss of consciousness, abnormal clinical laboratory tests, and pregnancy. In addition, patients who were actively suicidal, met criteria for any psychiatric disorder other than MDD, had a history of substance abuse or electroconvulsive therapy in the previous 6 mo, or had a history of substance dependence were excluded from the study. Healthy subjects without any psychiatric, neurologic, or medical illness were self‐referred from online study advertisements. The study was approved by the University of Zurichs' Institutional Review Board, and all subjects gave written informed consent before screening. All subjects were right handed as assessed with the Edingburgh Handedness Inventory (Oldfield et al., 1970). After applying the exclusion criteria above, fMRI scans from 27 depressed subjects and 25 healthy control subjects were processed. Of these scans, two could not be included in the analysis owing to structural abnormalities in the 3D T1‐weighted anatomical scan (two depressed subjects). This resulted in usable fMRI data on 25 subjects with depression and 25 healthy control subjects.
Pictorial Stimuli
Subjects viewed full‐color pictures selected from the International Affective Picture System (IAPS) (Lang et al., 1999) with positive (IAPS norm ratings: 7.32 ± 2.06) and negative (IAPS norm ratings: 2.24 ± 2.67) valence. The picture sets were counterbalanced across all subjects as well as within each subject according to the two categories of valence as well as according to dominance, intensity, human faces, and human figures. We used IAPS stimuli that were successfully applied in previous studies (Grimm et al., 2006, 2008) and also validated with regard to self‐relatedness in two separate groups of healthy subjects (Northoff et al., in press). The pictures were generated by Presentation® (Neurobehavioral Systems, Albany, CA) and rear projected onto a projection screen positioned at the head end of the MRI scanner bore. Subjects viewed the screen through a mirror mounted on the head coil and responded by pushing a fiber‐optic light sensitive keypress.
Experimental Design
The fMRI design was “event related”” with positive and negative stimuli alternating with a fixation control condition. The IAPS pictures were presented for 4 s. A total of 150 pictures was presented twice: once for judgment and once for passive viewing (PV). In case of the judgment condition, subjects had to judge the pictures with regard to their self‐relatedness [“picture judgment” (“PJ”)]; this was indicated by the letter “B” (i.e., German term for judgment or evaluation) in one corner of the picture. Pictures had to be judged as either self‐related or not (yes‐no option).
During the viewing condition, subjects had to passively view the picture (“PV”), which was indicated by the letter “E” in one corner of the picture. Here, subjects had to arbitrarily press a button without making any judgment. Responses and reaction times were recorded. After the presentation of each picture, a resting period followed, where a fixation cross was presented for 6–8 s (6.0, 6.5, 7.0, 7.5, 8.0 s). This allowed the subjects to recover from emotional stimulation and, in addition, served as a baseline condition to distinguish between positive and negative BOLD responses (Stark and Squire, 2001). A total of 300 trials was presented in six runs; 150 trials were presented for PJ and 150 trials for PV. The different task conditions were pseudorandomized within and across the six runs and their order counterbalanced across all subjects. Immediately after the fMRI session pictures were presented for a second time. Each of the 160 pictures (including 10 new ones for distraction) was followed by a task period which consisted of a concern rating (subjects had to rate whether they felt affected by the picture), dominance rating, intensity rating, valence rating, and self‐relatedness rating. All responses were given using a scale ranging from 1 to 9. The 10 new emotional pictures were matched in valence, intensity, and dominance with those presented in fMRI. The mean of each of the five ratings was calculated for each subject. Analysis of postscanning ratings was conducted separately for positive and negative pictures. Postscanning ratings were conducted with 16 healthy controls and 15 depressed subjects, because some of the subjects were too exhausted after the fMRI‐scan to continue the investigation.
Functional Imaging
Measurements were performed on a Philips Intera 3T whole‐body MR unit equipped with an eight‐channel Philips SENSE head coil. Functional time series were acquired with a sensitivity encoded (Pruessmann et al., 1999) single‐shot echo‐planar sequence (SENSE‐sshEPI). The following acquisition parameters were used in the fMRI protocol: echo time = 35 ms, field of view = 22 cm, acquisition matrix = 80 × 80, interpolated to 128 × 128, voxel size: 2.75 × 2.75 × 4 mm3, SENSE acceleration factor R = 2.0. Using a midsaggital scout image, 32 contiguous axial slices were placed along the anterior‐posterior commissure plane covering the entire brain with a TR = 3000 ms (θ = 82°). The first three acquisitions were discarded due to T1 saturation effects.
Statistical Analysis
Behavioral data
Reaction times and judgments (self‐relatedness rating) were analyzed in a multivariate analysis of variance (ANOVA) with the factors group (healthy subjects/patients with MDD), valence (positive/negative pictures), and task (PJ/PV). Postscanning ratings of concern, dominance, valence, intensity, and self‐relatedness were analyzed in a group × valence ANOVA. We performed multivariate ANOVAS where we included the different substance—classes of psychotropic medication (SSRIs, tricyclica, benzodiazepines, etc.) as well as age as covariates whereas the dosage of the medication was not considered in the analysis.
fMRI data
fMRI data were analyzed using MATLAB 6.5.1 (The Mathworks, Natick, MA) and SPM2 (Statistical parametric mapping software, SPM; Wellcome Department of Imaging Neuroscience, London, UK; http://www.fil.ion.ucl.ac.uk). For each subject, a design matrix was defined modeling judgment of self‐relatedness (PJ) and perception (PV) as separate events. In addition to these two events the baseline condition was included in the design matrix and modeled separately, independent of the other events. In addition, for each experimental run, the six parameters obtained in the realignment procedure were included as regressors in the design matrix.
For the fMRI data group analyses the contrast images from the analysis of the individual subjects were analyzed using two‐sample t tests to compare signal changes in the above mentioned conditions between healthy and MDD subjects. Activations are reported at a level of significance P < 0.001, uncorrected and a cluster threshold of greater than 5. All group comparisons included age and psychotropic medication as co‐variates.
For the regions of interest (ROI) analyses of peak voxels, coordinates that were obtained in contrasts of the between‐group analyses (Table I) were selected. ROI were functionally defined by centering spheres on the respective peak voxels with a radius of 3 mm. Analyses were carried out for the DMPFC (−8, 36, 12), SACC (12, 30, 28), precuneus (−4, −72, 38), VS (10, 10, 6), bilateral DMT (−10, −14, 10; 12, −14, 14), left DLPFC (−54, 6, 42), and insula (−42, 16, 4). For the ROI analyses, % signal changes for the different conditions were extracted for each subject separately using Marsbar (http://www.marsbar.sourceforge.net/). For each event % signal changes were calculated relative to the mean signal intensity of this ROI across the whole experiment. Time‐course analyses were performed applying a finite impulse response model, which does not make an assumption on the resulting signal changes after stimulus presentation. Parameter estimates were calculated for 8 time bins of 1TR (= 3 s) length for each regressor of the design matrix.
Table I.
Differences between healthy and depressed subjects in self‐related judgement
| Region | Side | PJ > PV | |
|---|---|---|---|
| H > MDD | MDD > H | ||
| DMPFC | L | −8, 36, 12 | 3.28 |
| SACC | R | 12, 30, 28 | 3.78 |
| Precuneus | L | −4, −72, 38 | 3.70 |
| DMT | L | −10, −14, 10 | 4.22 |
| R | 12, −14, 14 | 4.58 | |
| VS | R | 10, 10, 6 | 3.84 |
| DLPFC | L | −54, 6, 42 | 3.60 |
| Insula | L | −42, 16, 4 | 3.20 |
The global height threshold for between‐group comparisons (healthy vs. MDD) was set to P < 0.001 uncorrected, the extent threshold to k = 5 voxels for the contrast. The values in the table represent maximum z values with peak voxel coordinates in the MNI stereotactic space.
PJ, picture judgment; PV, passive viewing; H, healthy subjects; MDD, major depressive disorder patients; DMPFC, dorsomedial prefrontal cortex; SACC, supragenual anterior cingulate cortex; DMT, dorsomedial thalamus; VS, ventral striatum; DLPFC, dorsolateral prefrontal cortex.
To detect the association of signal changes in response to self‐related judgment with psychopathological parameters and postscanning ratings, the correlation between the different psychopathological components of the BDI (3‐factor solution: anhedonia/inhibition, negative self‐concept, somatic complaints; Schotte et al., 1997), the individual scores of the BHS, the individual scores of postscanning ratings and signal changes in the ROI was analyzed in a post hoc, ROI analysis using Marsbar (see above). Subjects' individual scores were correlated with signal changes during judgment of self‐relatedness (PJ) > perception (PV) using Pearson correlation analysis.
RESULTS
Subjects
The control group had a mean age of 32.4 with 12 women and 13 men. The depressed group had a mean age of 37.0 with 9 women and 16 men. Groups did not differ significantly in age (t test P = 0.09) or in gender distribution (χ2 P = 0.39). The mean HDRS score was 26.8 (SD 7.1), the mean BDI score 26.6 (SD 9.1), and the mean BHS score 31.08 (SD 5.2) in the depressed group, indicating that patients were severely depressed and showed self‐abnormalities with increased attribution of negative emotions to oneself as hallmark of an increased self‐focus. The mean duration of the current episode was 8.1 weeks (SD 8.4), the number of previous depressive episodes 2.2 (SD 1.5). Regarding exposure to psychotropic medications, 2 of the 25 depressed subjects were not taking any when investigated. Twenty‐three depressed subjects were taking one or more medications from the following classes: antidepressants (19 subjects; Selective serotonin reuptake inhibitors (SSRIs): 15 subjects; Tricyclic antidepressants (TCAs): 5 subjects), antipsychotics (5 subjects), anxiolytics (6 subjects), and mood stabilizers (Lithium: 3 subjects). None of the control subjects was taking any psychotropic medications at the time of the investigation.
Behavioral Data
Intrascanner ratings (reaction times and self‐related judgments)
There was a significant effect of group (F(1) = 216.61, P < 0.001), task (F(1) = 2419.21, P < 0.001), and valence (F(1) = 29.07, P < 0.001) on reaction times, whereas there were no interactions between these factors. Post hoc t tests demonstrated faster reaction times in healthy subjects in PV (t = −12.52, P < 0.001) as well as in PJ (t = −8.05, P < 0.001). Reaction times were faster in positive pictures (t = 4.57, P < 0.001) and PV (t = −48.99, P < 0.001). Concerning the self‐related judgments there was a significant group effect (F(1) = 120.62, P < 0.001) and valence effect (F(1) = 20.21, P < 0.001), but again no interaction effects. Differences in intrascanning self‐related judgment between groups concerned positive (t = 7.67, P < 0.001) and negative pictures (t = 8.75, P < 0.001), which both were rated significantly more self‐related by the patients with MDD. Inclusion of age and psychotropic medication as covariates did not have any influence on the results from group comparisons. The results are indicative of a consistent psychomotor impairment and show an increased tendency to relate emotional stimuli to the own self in patients with MDD thus supporting the assumption of an increased negative self‐attribution as hallmark of an increased self‐focus.
Postscanning ratings
There was a significant effect of participant group (healthy, MDD) on ratings of intensity (F(1) = 20.52, P < 0.001). Picture valence (positive, negative) had a significant effect on ratings of concern (F(1) = 173.45, P < 0.001), valence (F(1) = 4,776.14, P < 0.001), intensity (F(1) = 99.78, P < 0.001), and self‐relatedness (F(1) = 602.39, P < 0.001). There was a significant interaction effect between the factors group and picture valence for intensity (F(1) = 7.89, P < 0.005) and self‐relatedness (F(1) = 10.41, P < 0.001). Post hoc t tests demonstrated that negative pictures were rated significantly more intense (t = −5.37, P < 0.001) and self‐related (t = −3.63, P < 0.001) by the depressive patients, whereas there were no differences between groups for ratings of intensity and self‐relatedness of positive pictures (Fig. 1). Inclusion of age and psychotropic medication as covariates did not have any influence on the results from group comparisons. Results support the assumption of an increased self‐attribution specifically with regard to negative emotions in MDD.
Figure 1.
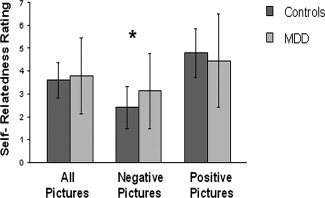
Self‐relatedness ratings of positive and negative pictures in healthy and depressed subjects. Performance in postscanning self‐relatedness ratings for all pictures, negative pictures, and positive pictures. Patients with MDD rated negative pictures to be significantly more self‐related, although there were no differences in positive pictures. *P < 0.05. MDD, major depressive disorder.
fMRI Data
Effect of self‐related judgment
To elucidate the effects of self‐related judgment, we compared PJ > PV between subjects with MDD and healthy subjects. Patients with MDD showed significantly lower signal intensities in the DMPFC, SACC, precuneus, bilateral DMT, VS, left DLPFC, and insula. Locations of responses are summarized in Table I. Higher signal intensities in patients with MDD could only be obtained if the threshold was lowered to P < 0.005 uncorrected and were found in the right precentral cortex and left parietal cortex. Bar diagrams and time curves show clear significant differences in signal changes during PJ, whereas signal changes during PV did not differ significantly between subjects with MDD and healthy subjects (Fig. 2). Inclusion of age, gender, and psychotropic medication as covariates did not have any influence on the results from group comparisons. Taken together, fMRI results demonstrate abnormal neural activity in subcortical‐cortical midline regions (and lateral cortical regions like DLPFC and insula) specifically during judgment of self‐relatedness as distinguished from mere perception of the very same stimuli.
Figure 2.

Comparisons between healthy subjects and depressed patients concerning judgment of self‐relatedness and PV (PJ > PV). SPM images show statistical parametric (T) maps for comparisons between healthy subjects and patients with MDD, overlaid on a single subject's normalized brain in the MNI stereotactic space (P < 0.001; uncorrected; k > 5). The saggital view represents the left hemisphere in the upper panel and the right hemisphere in the lower panel. Bar diagrams show % signal changes in judgment of self‐relatedness (PJ) and passive viewing (PV) in healthy controls and patients with MDD. Time curves show % signal changes in judgment of self‐relatedness in healthy controls and patients with MDD. Time curves and bar diagrams represent the % signal changes in the precuneus (−4, −72, 38), DMPFC (−8, 36, 12), DMT (12, −14, 14), and SACC (12, 30, 28) for healthy controls and MDD. PJ, picture judgment; PV, picture viewing; DMPFC, dorsomedial prefrontal cortex; DMT, dorsomedial thalamus; SACC, supragenual anterior cingulate cortex; MDD, major depressive disorder. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
To investigate parametric dependence of neural activity on self‐relatedness, postscanning ratings of self‐relatedness for positive and negative pictures were separately correlated with signal intensities in the contrast PJ > PV. In subjects with MDD, this analysis revealed a significant positive correlation of self‐relatedness ratings for negative emotional pictures in the bilateral DMT (left DMT: r = 0.62, P < 0.05; right DMT: r = 0.61, P < 0.05) and VS (r = 0.67, P < 0.01). Healthy subjects, in contrast, showed a different correlation pattern with no significant correlations in both VS (r = −0.44, P > 0.05) and bilateral DMT (r = 0.19, P > 0.05); further the direction of the relationship was reversed in the case of the VS (Fig. 3). Signal changes in the precuneus also correlated differently with self‐relatedness ratings of negative pictures in healthy subjects (r = 0.59, P < 0.05) and subjects with MDD (r = 0.35, P > 0.05). During positive emotional pictures, subject with MDD showed a significant positive correlation of self‐relatedness ratings with signal changes in the insula (r = 0.56, P < 0.05) whereas healthy subjects showed a reversed correlation pattern (r = −0.33, P > 0.05). Inclusion of intensity, valence, dominance, and concern ratings as well medication dosage did not have any influence on correlation results in positive and negative pictures. Taken together, these results support the involvement of subcortical midline regions in modulating abnormal self‐relatedness in negative emotions in MDD.
Figure 3.
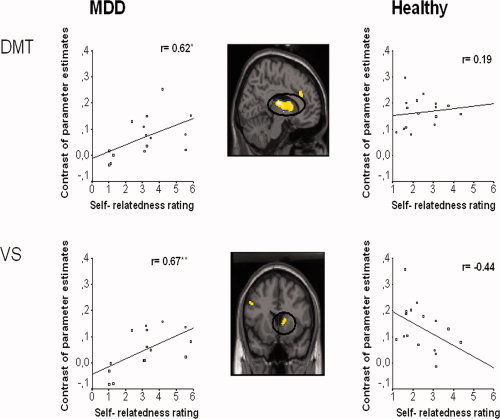
Correlation of postscanning subjective ratings for self‐relatedness in negative emotional pictures with BOLD signals obtained in the contrast PJ > PV in healthy and subjects with MDD. SPM images shows statistical parametric (T) maps for the comparison between healthy subjects and patients with MDD, overlaid on a single subject's normalized brain in the MNI stereotactic space (P < 0.001; uncorrected; k > 5) during PJ > PV. The saggital view represents the left hemisphere. The scatter plots show subjects' self‐relatedness ratings for negative pictures on x‐axis and % signal change in left DMT (−10, −14, 10) and VS (10, 10, 6) during PJ > PV on y‐axis. Scatter plots are presented for both healthy and depressed subjects (**P < 0.01; *P < 0.05). DMT, dorsomedial thalamus; VS, ventral striatum; MDD, major depressive disorder. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Correlation of signal changes with psychopathological symptoms
To demonstrate the psychopathological relevance of altered neural activity in MDD, contrast estimates of PJ > PV were correlated with patients total BDI scores as well as with BDI subscores and BHS scores. There was a significant correlation between subjective ratings of depression of patients with MDD with signal changes in the DMPFC (r = −0.66, P < 0.01). Also, the subscore for “negative self‐concept” (r = −0.62, P < 0.01) as well as the one for anhedonia/inhibition” (r = −0.57, P < 0.01) correlated significantly with signal changes in the DMPFC (Fig. 4). We also observed significant correlation of DMPFC signal changes with scores in the BHS (r = −0.58, P < 0.05) that includes many items about self‐relatedness and emotions. Taken together, these correlation results point out a special role of the DMPFC in mediating abnormal self‐relatedness in MDD and thus these patients' abnormally negative concept of their own self.
Figure 4.

Correlation of depression symptom severity (BDI) with BOLD signals obtained in the contrast PJ > PV in patients with MDD. The SPM image shows a statistical parametric (T) map for the comparison between healthy subjects and patients with MDD, overlaid on a single subject's normalized brain in the MNI stereotactic space (P < 0.001; uncorrected; k > 5) during PJ > PV. The saggital view represents the left hemisphere. The scatter plot shows BDI subscore ratings for negative self‐concept on x‐axis and % signal change in DMPFC (−8, 36, 12) during PJ > PV on y‐axis. The scatter plot is presented for depressed subjects (**P < 0.01). DMPFC, dorsomedial prefrontal cortex; BDI, Beck Depression Inventory, MDD, major depressive disorder. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
We report neural abnormalities during self‐relatedness in MDD. Behaviorally, patients with MDD showed significantly higher degrees of self‐relatedness of negative emotional stimuli and thus increased self‐attribution of negative emotions, which is well in accordance with the clinical observation of increased reflection and introspection of oneself, i.e., an increased self‐focus. Neuronally, judgment of self‐relatedness (when compared with mere perception of the very same stimuli) was associated with reduced signal changes in cortical and subcortical midline regions (DMPFC, SACC, Precuneus, VS, DMT) that are supposed to mediate the reflection and introspection of oneself. This is well in accordance with our hypothesis of reduced signal changes that may be traced back to abnormally high resting state activity in these regions. Reduced signal changes in subcortical midline structures (VS, DMT) correlated with abnormally high ratings of self‐relatedness of negative emotional pictures whereas signal changes in the DMPFC correlated with psychopathological symptoms (BDI, BHS) of self, a negative self‐concept, and anhedonia. This corroborates our hypothesis that the increased negative self‐attribution in MDD may be modulated by reduced subcortical‐cortical midline signal changes. Taken together, our results provide first evidence for association of the increased negative self‐attribution in MDD with abnormal neural activity in subcortical‐cortical midline regions.
The DMPFC has often been observed to show reduced neural activity during predominantly cognitive or cognitive‐emotional tasks in MDD (Fitzgerald et al., 2008; Mayberg, 2003; Phillipps et al., 2003). In healthy subjects, the DMPFC has been strongly associated with self‐relatedness (Kelley et al., 2002; Northoff et al., 2006; Ochsner et al., 2005) and particularly the reflection and introspection of oneself including self‐awareness, self‐reflection, self‐evaluation, and self‐recollection (Ochsner et al., 2005; Schmitz and Johnson, 2007). This and the often observed increased negative self‐attribution in MDD led us to suggest that the DMPFC may show altered activity specifically during judgment of self‐relatedness in MDD mirroring negative self‐attribution. Our results demonstrated significantly reduced signal intensities during judgment of self‐relatedness in MDD. Most interestingly, this was specific for the judgment period while during mere perception of the very same stimuli no significant differences were observed between both groups in this region. This indicates that abnormal stimulus‐induced activity in DMPFC may be specifically related to judgment of the self and thus emotional self‐attribution. More specifically, reduced stimulus‐induced activity in DMPFC seems to be specifically elicited by reflection and introspection of the self as required in judgment of self‐relatedness rather than mere perception without reflection and introspection.
The apparent involvement of the DMPFC in self‐abnormalities in MDD is further supported by the significant correlation between DMPFC signal intensities and the BDI/BHS scores as subjective measures of depression in general and self‐abnormalities in particular. The lower DMPFC signal intensities during judgment of self‐relatedness, the more severe MDD patients judged their symptoms (BDI total) and the more self‐abnormalities (BDI subscore for negative self‐concept and BHS) they experienced. These results indicate that psychopathological symptoms in MDD, such as the increased negative attribution as hallmark of the increased self‐focus, may be modulated by abnormal neural activity in the DMPFC.
In addition to the DMPFC, we also observed abnormalities in various other cortical midline regions. The SACC has been associated with monitoring of self‐relatedness, which may be crucial in allowing to reflect about oneself (Northoff et al., 2006) and has often been observed to be abnormal in MDD (Fitzgerald et al., 2008; Mayberg, 2002, 2003; Phillips et al., 2003). Our observation of SACC deficits in MDD during judgment of self‐relatedness may indicate altered monitoring of self‐relatedness, which psychologically may lead to increased self‐relatedness with less detachment specifically from negative emotional stimuli. The precuneus has been associated with retrieval of autobiographical and thus self‐relevant information (Northoff et al., 2006) in healthy subjects. Patients with MDD showed alterations in the precuneus, which may indicate altered retrieval of episodic or autobiographical memories.
We also observed subcortical midline regions to be altered in MDD. Patients with MDD showed significantly reduced signal changes in the DMT and the VS during judgment of self‐relatedness. These regions have been shown to be specifically implicated in associating self and emotions in healthy subjects (Northoff et al., in press; Phan et al., 2004). Although the very same regions have also been implicated in MDD (Epstein et al., 2006; Fitzgerald et al., 2008; Grimm et al., 2008; Mayberg, 2002, 2003), it remained unclear whether they are also involved in mediating the abnormally increased negative self‐attribution and thus the increased self‐focus in these patients. Our results lend evidence to their involvement in the increased negative self‐attribution by showing reduced signal changes during judgment of self‐relatedness, which also correlated parametrically with ratings of negative emotions. This indicates that subcortical midline regions seem to be crucial in associating self and especially negative emotions in MDD.
Neurally, the observation of reduced signal changes in subcortical‐cortical midline regions like the DMPFC, SACC, DMT, and precuneus seem to be paradoxical. Increased self‐focus should go along with increased neural activity rather than reduced signal changes as observed here. This, however, is to neglect that self‐related stimuli do certainly not induce neural activity from zero in subcortical‐cortical midline regions but modulate a preexisting state of high resting state activity in these regions. Hence, the degree of stimulus‐induced signal changes during self‐relatedness may strongly depend upon the degree of resting state activity. There is strong evidence that resting state activity in subcortical‐cortical midline regions is abnormally high in MDD (Greicius et al., 2007; Grimm et al., 2008; Mayberg, 2002, 2003; Phillips et al., 2003). If true, this should lead to reduced stimulus‐induced signal changes in these patients, which has indeed been observed during emotional stimulation (Grimm et al., 2008). Studies in healthy subjects indicate that the degree of resting state activity in these regions may mirror the degree of self‐relatedness (Mason et al., 2007; Schneider et al., in press; see D'Argembeau et al., 2005). Abnormally high resting state activity in MDD should then be accompanied by increased negative self‐attribution and thus an increased self‐focus, which can indeed be observed both clinically and behaviourally. If the increased self‐focus is indeed related to apparently abnormally high resting state activity, one would expect reduced signal changes in subcortical‐cortical midline regions in these patients. This is exactly what we observed here and which was further confirmed by our parametric analysis. However, our results provide at best indirect evidence because we neither investigated resting state activity itself nor did we focus on those regions that were specifically active during the resting state. Hence, further studies with different experimental designs and analyses are necessary that provide a less indirect relationship between resting state activity and self‐relatedness.
It should also be mentioned that other cortical regions also showed altered neuronal activity including the DLPFC and the Insula. The DLPFC has been associated with self‐relatedness, particularly its cognitive components like self‐recognition and self‐manipulation (Northoff et al., 2006; Ochsner et al., 2005; Mitchell et al., 2006). We also demonstrate altered neuronal activity in left DLPFC during judgment of self‐relatedness, which contributes in further characterizing the dysfunction in this region in MDD (Grimm et al., 2008). Altered neuronal activity during judgment of self‐relatedness was also observed in the insula, which has often been shown to be deficient in MDD (Bar et al., 2004; Fitzgerald et al., 2008). This dysfunction might be related to possible alterations in interoceptive processing as the presumed main function of this region in healthy subjects (Craig, 2002, 2003, 2004; Critchley et al., 2005).
Several limitations in our study should be acknowledged. First, we did not investigate unmedicated MDD patients so that antidepressant medication may have confounded our results. Including medication dosage as covariate in our various analyses did not alter the obtained results though. Second, one may criticize that we investigated only one particular symptom of the increased self‐focus, the increased negative self‐attribution, whereas our design does not allow to make inferences about other symptoms of the increased self‐focus like ruminations, self‐blame, etc., and the distinction between analytical and experiential self‐focus (Rimes and Watkins, 2005). Because this is certainly true more specific paradigms need to be developed to test for the distinct components of the increased self‐focus in MDD. One may also be concerned about our control condition, the perception of the very same stimuli as used in the judgment condition. It should be obvious, that we remain unable to exclude other types of processing than self‐related processing in the perception condition. For instance it may be argued that the task we call “picture viewing” will almost always involve some kind of judgment. Indeed, some kind of unconscious or preconscious judgment that might implicate implicit self‐related processing cannot be completely ruled out. Studies that focus on implicit versus explicit self‐relatedness are necessary to investigate whether both modes recruit subcortical‐cortical midline structures or whether these regions are specifically associated with explicit self‐related processing such as introspection and reflection of self‐relatedness. Finally, the issue of task difficulty needs to be mentioned. Though subjects had to press a button even during perception, both perception and judgment show different task difficulties with only judgment requiring a cognitive effort whereas perception does not. This difference in task difficulty applied, however, equally to both groups healthy and subjects with MDD and is therefore cancelled out when comparing both groups. We also have to mention that we cannot distinguish between state and trait markers in our sample because we investigated the patients only in the acute depressed state. One may for instance imagine that the apparently high resting state neural activity in midline regions could be a trait marker whereas the stimulus‐induced activity in these regions may be state rather than trait marker. Finally, the relationship of self‐relatedness to other cognitive functions associated with alterations in the same regions in MDD remains unclear (Grimm et al., 2008). Future studies may consequently focus on the interaction between self‐relatedness and cognitive functions to delineate the exact psychological function responsible for the reduced signal changes in these regions. One may criticize the lack of neutral stimuli in our design, which makes it impossible to investigate the interaction between emotion and self‐relatedness. Because our main focus was on judgment of self‐relatedness as distinguished from mere perception, we refrained from including neutral stimuli, which would have overextended the paradigm.
In conclusion, our study provides first evidence of altered neural activity in a subcortical‐cortical midline network during judgment of self‐relatedness in MDD. These changes may be crucial in mediating patients' abnormally increased self‐attribution of negative emotions as hallmark of what clinically can be conceptualized as increased self‐focus.
REFERENCES
- American Psychiatric Association ( 1994): Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association. [Google Scholar]
- Bar KJ,Greiner W,Jochum T,Friedrich M,Wagner G,Sauer H ( 2004): The influence of major depression and its treatment on heart rate variability and pupillary light reflex parameters. J Affect Disord 82: 245–252. [DOI] [PubMed] [Google Scholar]
- Beck AT,Ward CH,Mendelson M,Mock J,Erbaugh J ( 1961): An inventory for measuring depression. Arch Gen Psychiatry 4: 561–571. [DOI] [PubMed] [Google Scholar]
- Beck AT,Weissman A,Lester D,Trexler L ( 1974): The measurement of pessimism: the hopelessness scale. J Consult Clin Psychol 42: 861–865. [DOI] [PubMed] [Google Scholar]
- Buckner RL,Andrews‐Hanna JR,Schacter DL ( 2008): The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124: 1–38. [DOI] [PubMed] [Google Scholar]
- Craig AD ( 2002): How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 3: 655–666. [DOI] [PubMed] [Google Scholar]
- Craig AD ( 2003): Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol 13: 500–505. [DOI] [PubMed] [Google Scholar]
- Craig AD ( 2004): Human feelings: why are some more aware than others? Trends Cogn Sci 8: 239–241. [DOI] [PubMed] [Google Scholar]
- Critchley HD,Rotshtein P,Nagai Y,O'Doherty J,Mathias CJ,Dolan RJ ( 2005): Activity in the human brain predicting differential heart rate responses to emotional facial expressions. NeuroImage 24: 751–762. [DOI] [PubMed] [Google Scholar]
- Damasio AR ( 1999): How the brain creates the mind. Sci Am 281: 112–117. [DOI] [PubMed] [Google Scholar]
- D'Argembeau A,Collette F,Van der Linden M,Laureys S,Del Fiore G,Degueldre C,Luxen A,Salmon E ( 2005): Self‐referential reflective activity and its relationship with rest: a PET study. NeuroImage 25: 616–624. [DOI] [PubMed] [Google Scholar]
- de Greck M,Supady A,Thiemann R,Tempelmann C,Bogerts B,Forschner L,Ploetz KV,Northoff G ( 2008): Is our self based on reward? Self‐relatedness recruits neural activity in the reward system. NeuroImage 39: 2066–2075. [DOI] [PubMed] [Google Scholar]
- Epstein J,Pan H,Kocsis JH,Yang Y,Butler T,Chusid J,Hochberg H,Murrough J,Strohmayer E,Stern E,Silbersweig DA( 2006): Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. Am J Psychiatry 163: 1784–1790. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB,Laird AR,Maller J,Daskalakis ZJ ( 2008): A meta‐analytic study of changes in brain activation in depression. Hum Brain Mapp 29: 683–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD,Flores BH,Menon V,Glover GH,Solvason HB,Kenna H,Reiss AL,Schatzberg AF ( 2007): Resting‐state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry 62: 429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm S,Schmidt CF,Bermpohl F,Heinzel A,Dahlem Y,Wyss M,Hell D,Boesiger P,Boeker H,Northoff G ( 2006): Segregated neural representation of distinct emotion dimensions in the prefrontal cortex—An fMRI study. NeuroImage 30: 325–340. [DOI] [PubMed] [Google Scholar]
- Grimm S,Beck J,Schuepbach D,Hell D,Boesiger P,Bermpohl F,Niehaus L,Boeker H,Northoff G ( 2008): Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: An fMRI study in severe major depressive disorder. Biol Psychiatry 63: 369–376. [DOI] [PubMed] [Google Scholar]
- Grimm S,Boesiger P,Beck J,Schuepbach D,Bermpohl F,Walter M,Ernst J,Hell D,Boeker H,Northoff G ( 2008): Altered negative BOLD‐responses in the default‐mode network during emotion processing in depressed subjects. Neuropsychopharmacology (in press). [DOI] [PubMed] [Google Scholar]
- Grunebaum MF,Keilp J,Li S,Ellis SP,Burke AK,Oquendo MA,Mann JJ ( 2005): Symptom components of standard depression scales and past suicidal behavior. J Affect Disord 87: 73–82. [DOI] [PubMed] [Google Scholar]
- Hamilton M ( 1960): A rating scale for depression. J Neurol Neurosurg Psychiatry 23: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram RE ( 1990): Self‐focused attention in clinical disorders: Review and a conceptual model. Psychol Bull 107: 156–176. [DOI] [PubMed] [Google Scholar]
- Keenan JP,Nelson A,O'Connor M,Pascual‐Leone ( 2001): A neurology: Self‐recognition and the right hemisphere. Nature 409: 305. [DOI] [PubMed] [Google Scholar]
- Kelley WM,Macrae CN,Wyland CL,Caglar S,Inati S,Heatherton TF ( 2002): Finding the self? An event‐related fMRI study. J Cogn Neurosci 14: 785–794. [DOI] [PubMed] [Google Scholar]
- Lang PJ,Bradley MM,Cuthbert BN ( 1999): International Affective Picture System (IAPS). The Center for Research in Psychophysiology, University of Florida.
- Mason MF,Norton MI,Van Horn JD,Wegner DM,Grafton ST,Macrae CN ( 2007): Wandering minds: the default network and stimulus‐independent thought. Science 5810: 393–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg H ( 2002): Depression, II: Localization of pathophysiology. Am J Psychiatry 159: 1979. [DOI] [PubMed] [Google Scholar]
- Mayberg HS ( 2003): Modulating dysfunctional limbic‐cortical circuits in depression: towards development of brain‐based algorithms for diagnosis and optimised treatment. Br Med Bull 65: 193–207. [DOI] [PubMed] [Google Scholar]
- Mitchell JP,Macrae CN,Banaji MR ( 2006): Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron 50: 655–663. [DOI] [PubMed] [Google Scholar]
- Northoff G ( 2007): Psychopathology and pathophysiology of the self in depression—Neuropsychiatric hypothesis. J Affect Disord 104: 1–14. [DOI] [PubMed] [Google Scholar]
- Northoff G ( 2008): What kind of neural coding and self does Hurley's shared circuit model presuppose? Behav Brain Sci2008; 31: 33–34. [Google Scholar]
- Northoff G,Bermpohl F ( 2004): Cortical midline structures and the self. Trends Cogn Sci 8: 102–107. [DOI] [PubMed] [Google Scholar]
- Northoff G,Heinzel A,de Greck M,Bermpohl F,Dobrowolny H,Panksepp J ( 2006): Self‐referential processing in our brain—A meta‐analysis of imaging studies on the self. NeuroImage 31: 440–457. [DOI] [PubMed] [Google Scholar]
- Northoff G,Schneider F,Rotte M,Matthiae C,Tempelmann C,Wiebking C,Bermpohl F,Heinzel A,Danos P,Heinze HJ,Bogerts B,Walter M,Panksepp J: Differential parametric modulation of selfrelatedness and emotions in different brain regions. Hum Brain Mapp (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN,Beer JS,Robertson ER,Cooper JC,Gabrieli JD,Kihsltrom JF,D'Esposito M ( 2005): The neural correlates of direct and reflected self‐knowledge. NeuroImage 28: 797–814. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Phan KL,Wager TD,Taylor SF,Liberzon I ( 2004): Functional neuroimaging studies of human emotions. CNS Spectr 9: 258–266. [DOI] [PubMed] [Google Scholar]
- Phillips ML,Drevets WC,Rauch SL,Lane R ( 2003): Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry 54: 515–528. [DOI] [PubMed] [Google Scholar]
- Pruessmann KP,Weiger M,Scheidegger MB,Boesiger P ( 1999): SENSE: sensitivity encoding for fast MRI. Magn Reson Med 42: 952–962. [PubMed] [Google Scholar]
- Raichle ME,Gusnard DA ( 2005): Intrinsic brain activity sets the stage for expression of motivated behavior. J Comp Neurol 493: 167–176. [DOI] [PubMed] [Google Scholar]
- Raichle ME,MacLeod AM,Snyder AZ,Powers WJ,Gusnard DA,Shulman GL ( 2001): A default mode of brain function. Proc Natl Acad Sci USA 98: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimes KA,Watkins E ( 2005): The effects of self‐focused rumination on global negative self‐judgements in depression. Behav Res Ther 43: 1673–1681. [DOI] [PubMed] [Google Scholar]
- Schmitz TW,Johnson SC ( 2006): Self‐appraisal decisions evoke dissociated dorsal—Ventral aMPFC networks. NeuroImage 30: 1050–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider F,Bermpohl F,Heinzel A,Rotte M,Walter M,Tempelmann C,Wiebking C,Dobrowolny H,Heinze HJ,Northoff G: The resting brain and our self: Self‐relatedness modulates resting state neural activity in cortical midline structures. Neuroscience (in press). [DOI] [PubMed] [Google Scholar]
- Schotte CKW,Maes M,Cluydts R,De Doncker D,Cosyns P ( 1997): Construct validity of the Beck Depression Inventory in a depressive population. J Affect Disord 46: 115–125. [DOI] [PubMed] [Google Scholar]
- Siegle GJ,Carter CS,Thase ME ( 2006): Use of fMRI to predict recovery from unipolar depression with cognitive behavior therapy. Am J Psychiatry 163: 735–738. [DOI] [PubMed] [Google Scholar]
- Stark CE,Squire LR ( 2001): When zero is not zero: The problem of ambiguous baseline conditions in fMRI. Proc Natl Acad Sci USA 98: 12760–12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele JD,Kumar P,Ebmeier KP ( 2007): Blunted response to feedback information in depressive illness. Brain 130: 2367–2374. [DOI] [PubMed] [Google Scholar]
- Taylor SF,Phan KL,Decker LR,Liberzon I ( 2003): Subjective rating of emotionally salient stimuli modulates neural activity. NeuroImage 18: 650–659. [DOI] [PubMed] [Google Scholar]
- Treynor E ( 2003): Rumination reconsidered: A psychometric analysis. Cogn Ther Res 27: 247–259. [Google Scholar]
- Uddin LQ,Iacoboni M,Lange C,Keenan JP ( 2007): The self and social cognition: The role of cortical midline structures and mirror neurons. Trends Cogn Sci 11: 153–157. [DOI] [PubMed] [Google Scholar]


