Abstract
One of the most important factors controlling material specific processing in the human brain is language dominance, i.e. hemispheric specialization in semantic processes. Although previous studies have shown that lateralized long‐term memory processes in the medial temporal lobes are modified in subjects with atypical (right) language dominance, the effect of language dominance on the neural basis of working memory (WM) has remained unknown. Here, we used functional MRI (fMRI) to study the impact of language dominance on the neural representation of WM. We conducted an n‐back task in three different load conditions and with both verbal and nonverbal (spatial) material in matched groups of left and right language dominant subjects. This approach allowed us to investigate regions showing significant interactions between language dominance and material. Overall, right dominant subjects showed an increased inter‐individual variability of WM‐related activations. Verbal WM involved more pronounced activation of the left fusiform cortex in left dominant subjects and of the right inferior parietal lobule in the right dominant group. Spatial WM, on the other hand, induced activation of right hemispheric regions in left dominant subjects, but no specific activations in right dominant subjects. Taken together, these findings indicate that the neural basis of verbal WM processes depends on language dominance and is more mutable in right dominant subjects. The increased variability in right dominant subjects strongly suggests that a standard network of material‐dependent WM processes exists in left dominant subjects, and that right dominant subjects use variable alternative networks. Hum Brain Mapp, 2009. © 2008 Wiley‐Liss, Inc.
Keywords: working memory, language dominance, fMRI
INTRODUCTION
Working memory (WM) refers to the ability to maintain and manipulate information for a short time interval [Baddeley, 1992]. Previous neuroimaging studies found evidence for domain‐specific organization of the neural basis of WM maintenance [Pasternak and Greenlee, 2005; Postle, 2006a; Wager and Smith, 2003]. Most importantly, maintenance of nonverbal visual stimuli, for example spatial locations, has been distinguished from maintenance of verbal material. Activation of the frontal eye field during short‐term maintenance of spatial items has been interpreted as reflecting rehearsal processes based on covert eye movements [e.g., Courtney et al., 1998; Postle, 2006b]. In addition, activation of regions in the occipito‐parietal “where”‐stream, which plays a crucial role for processing of spatial visual information [Awh et al., 1999; Ungerleider and Haxby, 1994; Ungerleider and Mishkin, 1982], might underlie rehearsal of spatial items based on shifts of attention. It has been suggested that both strategies rely on top‐down control signals from prefrontal regions, which act via selection of relevant representations [Curtis and D'Esposito, 2003; D'Esposito et al., 1995; Rose et al., 2005]. Verbal WM, on the other hand, is closely linked to brain regions devoted to language processing. Left inferior parietal regions, closely adjacent to Wernicke's area, play a crucial role for short‐term phonological representations of verbal material, and Broca's area is relevant for active subvocal rehearsal [Awh et al., 1996; Jonides et al., 1998; Paulesu et al., 1993; Smith and Jonides, 1999].
The attribution of specific WM processes to brain regions depends on various physiological and pathological factors. As a result, altered representations may occur either due to pathological alterations in neurological or psychiatric patients [e.g., AuDuong et al., 2005; Harvey et al., 2005; Walter et al., 2007] or due to physiological differences between individuals. Examples of physiological factors which were shown to affect WM representation include age [Nordahl et al., 2006; Stern et al., 2007], sex, menstrual cycle, and sexual hormones [Schoning et al., 2007]. Hemispheric language specialization is another obvious factor influencing the neural architecture of information processing. Although the vast majority of right‐handed subjects process semantic information predominantly in their left hemisphere, there is a higher incidence of atypical language dominance in left‐handed subjects [Knecht et al., 2000]. Even in right‐handed subjects, lesions in the language dominant hemisphere may lead to a shift of language dominance, depending on lesion characteristics and the age at which the lesion occurred [Brown and Hecaen, 1976; Helmstaedter et al., 1997]. Importantly, while the neural representation of language in the left hemisphere is relatively invariant, it appears to be more variable in subjects with atypical language dominance [Tzourio‐Mazoyer et al., 2004].
In a previous study, we investigated the effect of language dominance on material‐specific long‐term memory processes in the medial temporal lobes (MTL) of healthy subjects [Weber et al., 2007]. In left dominant subjects, we found that memory for words and faces was lateralized to the left and right MTL, respectively; memory for pictures did not show lateralization. With a shift of language dominance to the right hemisphere, lateralized activity in the MTL changed to the contralateral hemispheres as well. These results support a close connection between lateralized activation of the MTL and hemispheric activation during language tasks [Weber et al., 2006] and suggest that MTL processes are specifically prone to switch hemisphere with language dominance. On the other hand, Jansen et al. [ 2005] studied lateralized activity in the cerebellum during a word generation paradigm and observed a shift with language dominance as well, suggesting that hemispheric shifts are not restricted to the MTL. It has been an open question whether language dominance also affects the lateralization of WM processes.
Here, we investigate the effect of language dominance on WM for verbal and nonverbal items. We conducted an n‐back functional MRI (fMRI) paradigm with verbal and spatial items in subjects with left and right language dominance as determined by an established fMRI language paradigm [Fernandez et al., 2001]. The application of a paradigm with three different load levels (0‐back, 1‐back, and 2‐back) allowed us to differentiate activation of material‐specific brain regions from load effects.
MATERIALS AND METHODS
Subjects
Thirty‐two healthy subjects (15 female; mean age ± SD.: 27.7 ± 5.8 years) participated in this study. They were recruited from the University of Bonn as well as via newspaper. The study was approved by the local medical ethics committee, and all subjects gave written informed consent. Prior to participation in the WM experiment, all subjects had conducted an fMRI‐based semantic language paradigm, which is described in detail in the Supplementary Material. This paradigm revealed that 24 of these subjects had left‐hemispheric (typical) language dominance, whereas eight subjects were found to have right hemispheric (atypical) language dominance. From the 24 subjects with typical language dominance, 20 (83.3%) were right‐handed (according to the Edinburgh Handedness Inventory), three (12.5%) were ambidextrous, and one (4.2%) was left‐handed. From the eight subjects with atypical language dominance, two were right‐handed (25%), four were ambidextrous (50%), and two were left‐handed (25%). A larger group of left than right dominant subjects conducted the task to allow for matching between groups: from the 24 left dominant subjects, eight were selected to match the eight right dominant subjects with respect to age, sex, and performance in the WM paradigm (see Results). First, we calculated the averaged number of errors across the different positions for each subject. Then, we selected for each subject with atypical language lateralization a matching subject with typical language lateralization with the same sex and a similar number of errors. Finally, age was matched between the entire groups of eight typical and eight atypical subjects. Thus, only eight left‐lateralized subjects entered the analysis of WM lateralization (marked black in the Supplementary Table I) to avoid any bias due to different group sizes. Matching was performed blinded, i.e. without regard to the pattern of brain activation. An overview of all subjects is given in the Supplementary Table I. Language dominance was assessed by fMRI using an established paradigm [Fernandez et al., 2001; see Supplementary Material]. All subjects were free from cerebral abnormality as assessed on their brain T1‐weighted magnetic resonance images.
Experimental Paradigm
We used an n‐back task with verbal and nonverbal (spatial) material and three different load conditions. We first give an overview of the experiment and then describe details such as presentation times etc. Figure 1 provides a graphical depiction of the paradigm. Material and load conditions were randomly alternated between blocks (eight blocks in each material and load condition, resulting in a total of 48 blocks). In each condition, subjects first saw a slide indicating the condition: In the zero‐back condition, subjects were presented a target stimulus which they had to remember; this target was different in each block. In the one‐back and two‐back conditions, they were presented the words “1‐back” or “2‐back”, respectively. Afterwards, subjects were presented slides with words or white dots in various positions on the screen. In the zero‐back condition, subjects had to press a button with the index finger of their right hand whenever they saw the target item again. In the “1‐back” (“2‐back”) condition, they pressed the button whenever the currently presented item matched the item presented in the last trial (in the last but one trial).
Figure 1.
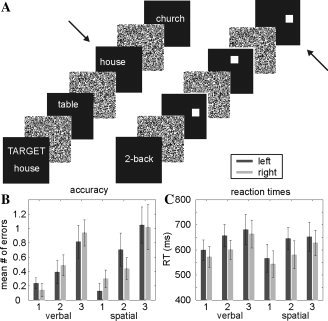
Working‐memory paradigm and behavioral data. (A) Example of the task in the verbal “0‐back” (left) and the spatial “2‐back” (right) condition. Arrows indicate items where subjects were required to press a button. Behavioral performance in the groups of left and right dominant subjects was matched, as indicated by similar accuracy (in terms of errors per block; B) and reaction times (C). Although there was a significant effect of load, there were no effects of material (verbal or spatial) or group (left or right dominant).
The slide indicating the condition was presented for 5 s. Afterwards, each item was presented for 800 ms with an inter‐trial interval of 1,200 ms, during which an image of white noise was presented to avoid after‐images. Each block consisted of 15 items, five of which fulfilled the respective target criterion and thus required a button press. After the block, a fixation cross was presented for 5 s. This resulted in a block length of 40 s (15 × 0.8 = 12 s stimulus presentation +15 × 1.2 = 18 s inter‐trial interval +5 s condition slide +5 s fixation phase between blocks) and a total length of the experiment of 32 min. Words were 180 common German nouns, ranging in length from 5 to 11 letters, presented on a random position on the screen to match eye movements with the spatial condition. Spatial stimuli were constructed using an (invisible) underlying 4 × 4 grid of possible positions; in each block, only eight nonadjacent positions were presented. Stimuli were presented using Presentation software (Version 0.71, Neurobehavioral Systems, Albany, CA) via MRI‐compatible liquid‐crystal display goggles (Nordic Neuro Lab, Bergen, Norway), and responses were obtained through a fiber optic magnetic resonance‐compatible control pad.
MRI Data acquisition
Thirty‐five axial slices were collected at 1.5T (Avanto, Siemens, Erlangen, Germany). We collected 670 T2*‐weighted, gradient echo EPI‐scans, including five initial scans that were discarded to achieve steady‐state magnetization (slice thickness: 3 mm; interslice gap: 0.3 mm; matrix size: 64 × 64; field of view: 192 mm; echo time: 40 ms; repetition time: 2,950 ms).
Data Analysis
MRIs were pre‐processed in SPM2 and SPM5 (http://www.fil.ion.ucl.ac.uk/spm/) using standard pre‐processing steps including realignment, unwarping, normalization, and smoothing with an 8‐mm Gaussian kernel. Pre‐processed data were fitted by the convolution of multiple regressors with a canonical hemodynamic response function to obtain parameter estimates for each condition covariate. All figures with fMRI results are displayed using neurological convention (left hemisphere on the left side of the figure). To identify significant activations, we used an uncorrected threshold of P < 0.001 and a minimum cluster size of five contiguous voxels. As the number of supra‐threshold voxels in individual subjects depends on intraindividual variability across trials (with higher variability resulting in a smaller number of significantly activated voxels), we calculated this measure to differentiate between intra‐individual and inter‐individual variability (between subjects). This was done using the same threshold as for the group analysis (P < 0.001, minimum of five contiguous voxels).
We used six regressors (three regressors for the different load conditions in each material), each covering the entire block length of 40 s (blocked design). Using the “full‐factorial design” feature of SPM5, we calculated a three‐way ANOVA with “material” and “load” as repeated measures and “language dominance” as independent variable. This approach was chosen to investigate domain‐specific effects (i.e., more pronounced activation during the verbal vs. spatial condition and vice versa) both independently for the group of left and right dominant subjects, as well as the difference between the groups (corresponding to a “material” × “language dominance” interaction). Then, the following contrasts were defined: First, main effects of “language dominance” (left > right and right > left); second, main effects of “material” (“verbal > spatial” and “spatial > verbal”) in the left and right dominant group separately. Third, material‐specific processing as a function of language dominance was analyzed, i.e. the “material” × “language dominance” interaction. For example, to find regions where left dominant subjects showed a stronger “verbal > spatial” effect than right dominant subjects, we assigned the following contrast weights: Verballeft: +1; spatialleft: −1; verbalright: −1; spatialright: +1. The result of this contrast was, for instance, inclusively masked (threshold P < 0.05) with the results from the contrast verballeft > spatialleft to restrict it to regions showing a significant effect in the left language dominant subgroup. (This approach also allowed us to distinguish regions where left dominant subjects showed a stronger “verbal > spatial” effect than right dominant subjects from regions where right dominant subjects showed a stronger “spatial> verbal” effect than left dominant subjects: Both contrasts are based on the same interaction, but the latter one was masked with the results from the contrast spatialright > verbalright). Finally, we calculated load effects in each group separately for each material condition (using contrast weights of −1, 0, +1 for the 0‐back, 1‐back and 2‐back conditions) and the interaction of load effects and language dominance (e.g., assigning contrast weights verballeft, 0‐back = −1; verballeft, 1‐back = 0; verballeft, 2‐back = +1; verbalright, 0‐back = +1; verbalright, 1‐back = 0; verbalright, 2‐back = −1 to find regions where left dominant subjects showed a stronger load effect in the verbal condition than right dominant subjects).
RESULTS
Behavioral Data and Language Dominance
As shown in Figure 1B and Table I, there was no significant difference between the performance in the groups of left and right language dominant subjects. A three‐way ANOVA with “language dominance” (left or right) as independent variable and “material” (verbal or spatial) and “load” (0‐back, 1‐back and 2‐back) as repeated measures revealed a highly significant effect of “load” (F 2,2 = 14.007; P < 0.0001), but no effects of (or interaction with) the other factors (all P > 0.1). Similarly, a three‐way ANOVA of reaction times using the same factors revealed a highly significant effect of “load” (F 2,2 = 12.179; P = 0.001), but no effect of (or interaction with) any other factor (all P > 0.2). Both language dominance groups contained four male and four female subjects, and age was not different between the groups (mean and std of age: left: 28.9 ± 6.9 years; right: 25.1 ± 3.3 years; t 14 = 1.418; P > 0.1). These results confirmed that task difficulty increased with load as expected, that there was no effect of material on difficulty, and that the subjects with left and right hemispheric language dominance performed equally well.
Table I.
Behavioral data
| Material | Verbal | Spatial | ||||
|---|---|---|---|---|---|---|
| Load | 0 | 1 | 2 | 0 | 1 | 2 |
| Accuracy | ||||||
| Left | 0.14 ± 0.26 | 0.48 ± 0.41 | 0.94 ± 0.51 | 0.30 ± 0.45 | 0.44 ± 0.43 | 1.00 ± 0.89 |
| Right | 0.23 ± 0.21 | 0.39 ± 0.46 | 0.81 ± 0.65 | 0.13 ± 0.31 | 0.70 ± 0.65 | 1.00 ± 0.71 |
| RTs (ms) | ||||||
| Left | 572 ± 115 | 600 ± 108 | 663 ± 158 | 544 ± 148 | 580 ± 157 | 628 ± 143 |
| Right | 599 ± 114 | 656 ± 127 | 681 ± 172 | 567 ± 158 | 645 ± 123 | 651 ± 166 |
Mean and standard deviation of accuracy (average number of errors per block of 15 items) and reaction times (RTs).
Lateralization indices between the two groups were significantly different in each of the three ROIs, as indicated by two‐tailed t‐tests (Broca's region: t 14 = 10.51; P < 10−7; Wernicke's region: t 14 = 12.42; P < 10−8; prefrontal cortex: t 14 = 13.25; P < 10−8). All LIs in the left dominant subjects were positive, and all LIs in the right dominant subjects were negative (see Supplementary Table I for an overview of all subjects). Moreover, to test whether mixed speech dominance was present [i.e., divergent lateralization within prefrontal, Broca's, and Wernicke's areas; Benke et al., 2006; Ries et al., 2004; Rutten et al., 2002], we calculated correlation coefficients between the LIs in the three regions both in the sample investigated in our study and in a more extended group of 132 healthy subjects. We found that for both groups, LIs were highly correlated (group of 32 subjects from the current study: Broca‐Wernicke: Spearman's correlation coefficient R = 0.682; P < 10−4; Wernicke‐prefrontal: R = 0.628; P < 10−3; Broca‐prefrontal: R = 0.718; P < 10−5; extended group of 132 subjects: Broca‐Wernicke: R = 0.517; Wernicke‐prefrontal: R = 0.569; Broca‐prefrontal: R = 0.526; each P < 10−5). The event of crossed language dominance is very rarely seen in patients with cerebral pathologies (<1% in a recent study by [Lee et al., 2008]. The occurrence of crossed language dominance in healthy subjects is not known but intuitively should be even more uncommon than in patients. Thus, while we cannot exclude that crossed language dominance occurred in individual subjects, overall correlations between LIs were highly significant.
FMRI Data: Main Effects of Language Dominance
Figure 2A,B depict brain regions showing significant differences as a function of language dominance. Supplementary Table II contains a complete list of significantly activated regions for all contrasts. The group of left dominant subjects showed a significantly stronger activation of the visual cortex (Fig. 2A), while the group of right dominant subjects showed significant activations in a widely distributed networks of various brain regions (with a preponderance of the right temporal lobe; Fig. 2B). These results suggest a more distributed representation of WM networks in the right dominant group, consistent with a more variable recruitment of brain areas in this group (for a quantitative analysis of variability see below).
Figure 2.
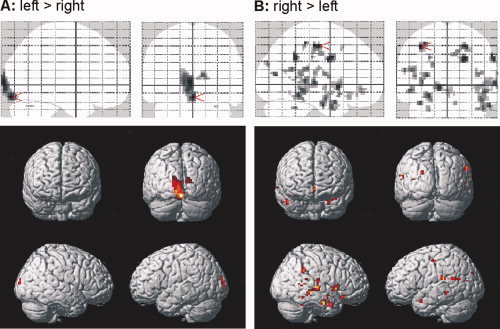
Activity during working memory as a function of language dominance. Comparison of task performance (irrespective of memory load and material). Brain regions indicating more activity in the left (A) and right (B) dominant group. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
FMRI Data: Verbal WM
Next, material‐specific effects were assessed. We first analyzed the effects separately in each group and then compared the two groups. In the group of left dominant subjects, performance of the task in the verbal as compared to the spatial condition activated the left temporolateral cortex (Wernicke's area), left inferior frontal regions (Broca's area), the bilateral inferior temporal (fusiform) cortex, and bilateral occipital regions (Fig. 3A). In the group of right dominant subjects, the same contrast yielded suprathreshold activations in the right primary visual cortex and the right inferior temporal gyrus (Fig. 3B). Overall activation was reduced in the right dominant group. In principle, this reduced activation could be either due to a decreased activation in the individual subjects, or due to an increased variability of the locus of activation in the right dominant group. To differentiate between these possibilities, we calculated the numbers of significantly activated voxels as a function of language dominance in each individual subject. This analysis revealed that the overall amount of activation was not affected by language dominance (mean ± std of the number of significantly activated voxels: left: 880.38 ± 788.69; right: 875.6250 ± 550.3261; two‐tailed t‐test: t 14 = 0.0140; P = 0.99), indicating that the group difference was due to an increased between‐subject variability in the recruitment of specific regions in the right as compared to the left group, consistent with the results for general task‐dependent activity reported above.
Figure 3.
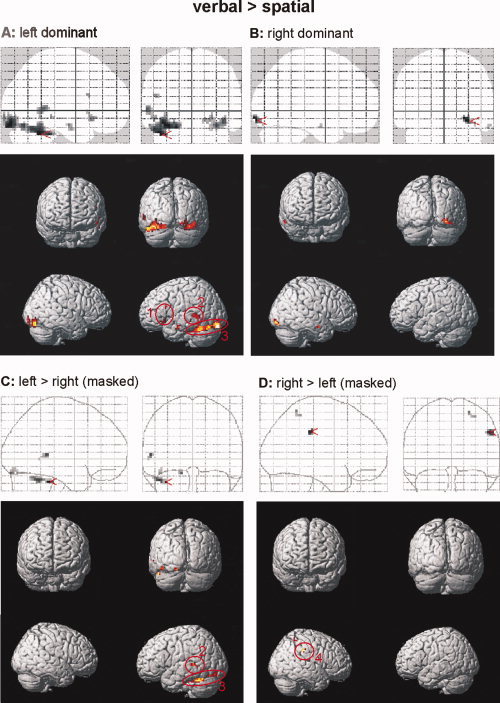
Verbal working memory as a function of language dominance. Verbal as compared to spatial working memory in left (A) and right (B) language dominant subjects. (C) Regions showing more pronounced activity in the left than in the right dominant group [inclusively masked by the results of (A)]. 1: Broca's area; 2: Wernicke's area; 3: inferior temporal cortex. (D) Regions showing more pronounced activity in the right than in the left dominant group [inclusively masked by the results of (B)]. 4: Inferior parietal lobule. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
To directly investigate differences in the neural representation of verbal WM related to language dominance, we analyzed regions showing a significant “material” × “language dominance” interaction. Figure 3C indicates areas where the “verbal > spatial” contrast was larger for left than right dominant subjects, inclusively masked with the “verballeft > spatialleft” contrast (Fig. 3A). This contrast yielded significant clusters in the left temporolateral cortex (Wernicke's area) and in the left inferior temporal (fusiform) cortex. The reverse contrast (stronger “verbal > spatial” effects in the right dominant group, inclusively masked with the “verbalright > spatialright” contrast) revealed increased right hemispheric activation in the right inferior and superior parietal lobule (Fig. 3D). Taken together, these results support a shift of lateralized activity during verbal WM with language dominance.
FMRI Data: Spatial WM
Next, we investigated the effect of language dominance on the neural architecture of spatial WM. Figure 4A,B depict regions showing a significantly stronger activation for spatial as compared to verbal material in the two groups. This contrast mainly activated the dorsal visual stream in the left dominant subjects (Fig. 4A). In the group of right dominant subjects, similar regions were activated, though weaker (Fig. 4B). Again, the extension of activation was very similar between groups (mean ± std of the number of significantly activated voxels: left: 9,945.00 ± 1,048.00; right: 9,941.25 ± 766.05; two‐tailed t‐test: t 14 = 0.0008; P = 0.99), indicating that the representation of spatial WM processes in the right dominant group was more variable, but similarly extended in each individual subject. To find significant differences between the two groups, we again investigated the “material” × “language dominance” interaction, inclusively masked with the results from the language dominance groups separately (Fig. 4C). We found that activation was significantly stronger in regions of the right inferior and superior parietal lobules for left than right dominant subjects, whereas the inverse interaction (more activation in the “spatial > verbal” contrast for right than left dominant subjects, inclusively masked by the regions from Fig. 4B) did not yield any significant results.
Figure 4.
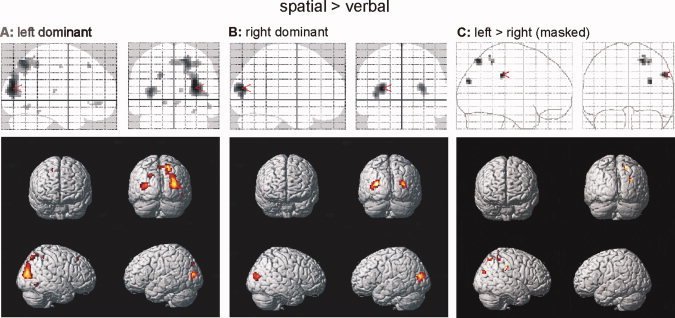
Spatial working memory as a function of language dominance. Spatial as compared to verbal working memory in left (A) and right (B) language dominant subjects. (C) Regions showing more pronounced activity in the left than in the right dominant group [inclusively masked by the results of (A)]. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
FMRI Data: Effects of Load
Finally, we analyzed effects of increasing WM load. In the group of left dominant subjects, an increasing load engaged a wide network of bilateral parietal and frontal regions in the verbal condition (Fig. 5A), and a similar network in the spatial condition (Fig. 5B). In the group of right dominant subjects, a similar network as in the left dominant group was recruited with increasing load (Fig. 5C,D); again, overall activation was weaker than in the left dominant group. To directly compare the two language dominance groups, we calculated a “load” × “language dominance” interaction (separately for the verbal and spatial condition), again inclusively masked with the results from the respective language dominance group. We found that left dominant subjects showed more pronounced activation of left prefrontal regions both for the verbal (Fig. 5E) and the spatial condition (Fig. 5F), plus activation of the right middle temporal gyrus in the verbal condition. The inverse contrast only yielded suprathreshold voxels in the spatial condition (in the right inferior temporal gyrus; Fig. 5G), but not in the verbal condition.
Figure 5.
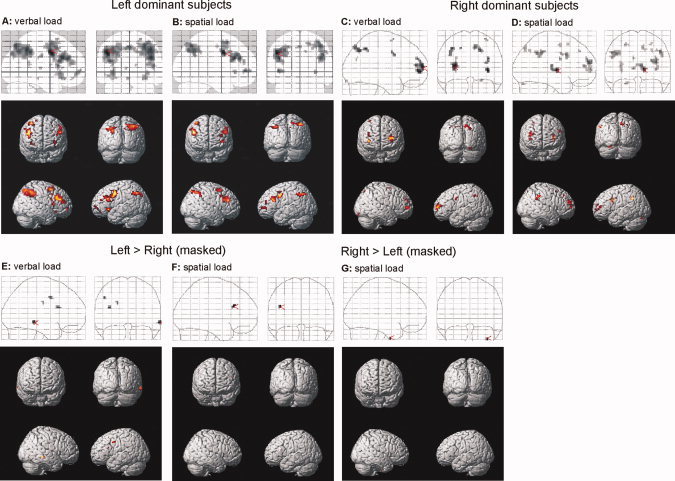
Load effects. Increasing activation for left dominant subjects with load in verbal (A) and spatial (B) working memory. (C), (D) Load effects for verbal and spatial material in right dominant subjects. “Load” × “Language dominance” interactions: Regions showing increased activation for left dominant subjects in the verbal (E) and spatial (F) condition. (G) Regions showing increased load effects in the spatial condition for right dominant subjects (there were no supra‐threshold voxels where load effects were more pronounced for right dominant subjects in the verbal condition). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
We investigated the effect of language dominance on verbal and spatial WM processes. Our study yielded two main results: First, verbal (but not spatial) WM processing involved shifts of lateralized activity as a function of language dominance; second, right dominant subjects showed generally more variable activations than left dominant subjects. We will first describe the neural regions underlying material‐specific effects in the left dominant group and then turn to the specific organization of this network in the right dominant group.
The material‐dependent pattern of activation observed in the group of left dominant subjects is consistent with previous reports. In the verbal condition, we found significantly stronger activation in Broca's and Wernicke's area and in the inferior temporal cortex (Fig. 3A). These results are consistent with previous findings on the neural substrates of verbal WM showing that regions which are engaged in language functions in general are also important for WM maintenance of verbal items [e.g., Awh et al., 1996; Paulesu et al., 1993; Smith and Jonides, 1999]. The inferior temporal cortex as the final processing area of the ventral visual stream comprises object‐selective regions such as the fusiform face area or the parahippocampal place area [Downing et al., 2006]. Accordingly, it supports the representation of specific object categories such as faces, houses, or places, but also written words [Kiehl et al., 1999; Malach et al., 2002; Ranganath et al., 2004; Reddy and Kanwisher, 2006]. The activation of the inferior temporal cortex during verbal WM is likely due to the fact that concrete verbal stimuli, such as the ones used in our study, induce a more pronounced reactivation of object‐related information; on the other hand, even sublexical sign strings have also been shown to elicit activity in inferior temporal cortices [Binder et al., 2006]. Apart from results from neuroimaging studies, intracranial EEG recordings in human epilepsy patients also revealed specific activity within this region upon presentation of words [Allison et al., 1994; Nobre et al., 1994]. The relevance of this region for verbal WM has been shown by a recent study of Fiebach et al. [ 2006], who reported load‐dependent activation in the left inferior temporal cortex during maintenance of words.
In the spatial condition, we observed increased activation of occipital regions of the dorsal visual stream in both left and right dominant subjects (Fig. 4A,B). This region is reliably activated during tasks which involve maintenance of spatial orientations [Awh et al., 1999; Ungerleider and Haxby, 1994; Ungerleider and Mishkin, 1982]. Most recently, an MEG study found increased gamma band activity in the dorsal visual stream during WM maintenance of face orientations [Jokisch and Jensen, 2007]. Moreover, a necessary role of these structures for spatial WM can be inferred from studies on patients with lesions in this region [Müller and Knight, 2006]. No clear laterality effects were observed in either group. This is consistent with previous findings that lateralization of activity in spatial WM maintenance depends on the location of the object within the visual field [Postle et al., 2004]. In our study, spatial stimuli were presented at various locations in both visual hemifields during each block, so that no lateralization would be expected. On the other hand, t‐values of active voxels in the middle occipital gyrus were slightly higher in the right hemisphere for left dominant subjects and in the left hemisphere of right dominant subjects (see Supplementary Table I), suggesting a possible dependence on language dominance which was explored more directly in the “language dominance” × “material” interaction (Fig. 4C; see below).
In right dominant subjects, the neural basis of verbal WM was significantly altered. Due to an increased inter‐individual variability of the neural correlates of WM, less consistent activations were found in the group analysis. The larger variability in right dominant subjects is most impressively demonstrated by a group comparison of the networks recruited in the entire task, i.e. collapsed across material and load conditions. Although the left dominant group showed an increased activation of early visual perceptual areas (Fig. 2A), in the right dominant group a highly dispersed network of small clusters covering basically the entire brain (with a preponderance in the temporal lobe) was observed (Fig. 2B). This network likely corresponds to the increased variability of right‐hemispheric language organization observed in atypical language dominant subjects [Tzourio‐Mazoyer et al., 2004]. The relative invariance of WM representation in left dominant subjects suggests that a “standard” WM network exists in subjects with typical language dominance which is probably best suited to support material‐specific WM processes.
These differences likely reflect a different neural mechanism in the task, despite identical performance (see Fig. 1). Early perceptual areas are generally assumed to be relatively “hard‐wired” and less prone to reorganization and neural plasticity, although plasticity can occur on a cellular and synaptic level [Karmarkar and Dan, 2006]. The reduced activation of the primary visual cortex in right dominant subjects found in our study may thus be explained by the necessity to recruit more flexible higher‐level areas and rely less on standardized processing streams. The scattered activations found in the right dominant group suggest that very variable brain regions are used in different subjects. However, we found that the extent of activation in individual subjects did not differ between the two groups. This indicates that variability occurs between subjects, but that within‐subject variability across the experiment was similar between groups.
Language dominance had a significant impact on verbal WM processes in the left inferior temporal cortex, as revealed by the “language dominance” × “material” interaction (Fig. 3C). Interestingly, while bilateral inferior temporal regions were activated in the left dominant group (Fig. 3A), only the left inferior temporal cortex was significantly stronger activated than in right dominant subjects. In addition, Wernicke's area showed more pronounced activation in left dominant subjects, while activation in Broca's area was not significantly different. Possibly, this indicates that passive storage of verbal material, which is closely linked to Wernicke's area [Jonides et al., 1998] was more important for this task than active articulatory rehearsal processes located in Broca's area [see also Ravizza et al., 2004]. Right dominant subjects, in contrast, showed a stronger activation of the right inferior parietal lobule, a region related to semantic search and ambiguity resolution [Ketteler et al., 2008]. It is possible that increased activity within this region for right dominant subjects indicates more complex semantic strategies in this group, although this idea needs to be tested further. Taken together, these findings show that atypical language dominance induced a shift of verbal WM processes from Wernicke's area and the left inferior temporal cortex to right hemispheric regions, most importantly the right inferior parietal lobule. Thus, similar to material‐dependent processing in long‐term memory [Weber et al., 2007], verbal processes in WM are also correlated with language dominance, although atypical language dominance does not induce a shift to corresponding regions in the right hemisphere.
The neural basis of spatial WM also depended on language dominance. Although we observed bilateral activations in the dorsal visual stream in both groups, a significantly stronger activation by left dominant subjects was only observed in the right dorsal stream (Fig. 4C). Thus, in left dominant subjects, verbal, and spatial material is more strongly lateralized to the left and right hemisphere, respectively. No region survived the significance threshold in the reverse contrast; in other words, there was no region where right dominant subjects showed more pronounced activation in the “spatial > verbal” contrast than left dominant subjects. This suggests that a “standard” network also for spatial WM exists in left dominant subjects, and that right dominant subjects make use of variable alternative networks, but do not shift activity to corresponding regions of the contralateral hemisphere.
Finally, increasing load in both material conditions activated a large network of prefrontal and parietal regions (see Fig. 5), consistent with a large number of previous studies [Altamura et al., 2007; Braver et al., 1997; Druzgal and D'Esposito, 2003; Jansma et al., 2000; Linden et al., 2003; Narajanan et al., 2005]. These load‐sensitive regions were different from the material‐specific areas in the fusiform and occipito‐parietal cortices. A similar pattern has been observed in previous studies [Mecklinger et al., 2000; Rypma et al., 1999] indicating material‐independent WM processes in the prefrontal cortex. Again, the fact that we only observed supra‐threshold activation in the “load” × “language dominance” interaction in the verbal condition for left > right dominant subjects (but not for right > left dominant subjects) suggests that right dominant subjects activated more variable regions with increasing load.
Several limitations of our approach which might complicate the interpretation of our results need to be mentioned. First, language dominance was assessed using a semantic language task with fMRI. This paradigm was previously validated in a group of 12 epilepsy patients undergoing presurgical investigation, of which six conducted a sodium amytal procedure [“Wada‐test”; Fernandez et al., 2001]. Although this study reported a complete correspondence between the results from the Wada test and the semantic language fMRI paradigm, the transferability of these results to healthy subjects has never been shown. Because of the invasiveness of the Wada approach, it can in principle only be conducted for clinical purposes, but not in healthy subjects. On the other hand, different methods to assess language lateralization in healthy subjects have yielded converging results: Both fMRI and transcranial Doppler measurements revealed similar rates of 5% of right‐handed and 30% left‐handed subjects with atypical language dominance; transcranial Doppler showed a high correlation with both fMRI [Deppe et al., 2000] and Wada [Knecht et al., 1998].
A second, more specific issue concerns the use of a language paradigm based on the comparison of activation during a semantic and a syntactic language task. Initial studies comparing lateralization based on semantic fMRI paradigms with results from the Wada test found a high correlation [e.g., Binder et al., 1996]. Although this result was supported by some more recent studies [Baciu et al., 2005; Binder et al., 2008; Seghier et al., 2004], others found that semantic language paradigms resulted in less lateralized activation patterns than syntactic or phonological paradigms [Humphries et al., 2006; Kang et al., 1999] and may thus lead to an overestimation of the incidence of atypical (bilateral or right hemispheric) language dominance. This is an important issue during presurgical language determination, which requires the exact determination of both left and right lateralized patients. Therefore, using extensive test batteries is probably advisable in a clinical setting [Ramsey et al., 2001]. On the other hand, the aim of the current study was to identify differences in the neural basis of WM processing in healthy subjects with left or right language dominance. In this situation, the false assignment of subjects with typical language dominance into the group of atypical dominant subjects due to the use of a semantic language paradigm would result in a blurring between the differences in left and right dominant groups and rather impede the detection of effects of language lateralization than induce false positive findings. In other words, the differences between left and right dominant subjects might be even more pronounced than observed in our study.
However, a false assignment of subjects with typical language dominance to the atypical group might complicate the interpretation of our finding of an increased variability of WM‐related activations in the atypical group. We argue that a false assignment of subjects with typical language dominance to the atypical group is rather unlikely with our paradigm: In a recent study, language lateralization as assessed with the Wada test was compared with the lateralization indices based on the semantic fMRI paradigm in a group of 65 subjects [Wellmer et al., 2008]. Figure 5 of this article shows that the temporo‐parietal ROI (Wernicke's area) has the highest specificity for the definition of atypical language dominance. Lateralization indices <−0.25 in this region were never associated with typical language dominance, but with atypical language dominance in seven cases, indicating relatively low sensitivity but 100% specificity for the detection of atypical language dominance. In our group of eight subjects defined as “atypical”, all LIs in this area were <−0.29, indicating that these subjects indeed exhibit atypical language dominance (see Supplementary Table I).
In general, frontal regions show a higher correlation with Wada results than temporal areas [Benke et al., 2006; Lehéricy et al., 2000]. However, it has been suggested that left inferior frontal activation during semantic language paradigms may not be due to language processes in the narrow sense, but rather due to retrieval, selection and evaluation in the semantic executive system [Poldrack et al., 1999]. In our sample, lateralization indices in the different ROIs were highly correlated, arguing against the possibility that the assignment of subjects to language dominance groups was dominated by the results from the frontal ROI.
Taken together, these findings support the hypothesis that the recruitment of neural regions during WM processes depends on language dominance. The increased variability in the right dominant group indicates that atypical language dominance is not just a contralateral reflection of typical (left‐dominant) language dominance, but follows a completely different organization. Although all subjects were healthy, without visible morphological brain lesions, and performed equally well in the left and right dominant group, our results strongly suggest that a relatively invariant “standard” network is used in the group of left dominant subjects, and variable “alternative” networks are recruited in the group of right dominant subjects.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supplementary Methods
Supplementary Table 1
Supplementary Table 2: Complete overview of activated regions in the different contrasts.
Acknowledgements
The authors thank Klaus Fliessbach for help with the statistics, Jenny Faber for providing language lateralization data, and Christian Hoppe for support with the paradigm.
REFERENCES
- Allison T,McCarthy G,Nobre A,Puce A,Belger A ( 1994): Human extrastriate visual cortex and the perception of faces, words, numbers, and colors. Cereb Cortex 4: 544–554. [DOI] [PubMed] [Google Scholar]
- Altamura M,Elvevag B,Blasi G,Bertolino A,Callicott JH,Weinberger DR,Mattay V,Goldberg T ( 2007): Dissociating the effects of Sternberg working memory demands in prefrontal cortex. Psychiatry Res 154: 103–114. [DOI] [PubMed] [Google Scholar]
- Au Duong MV,Boulanouar K,Audoin B,Treseras S,Ibarrola D,Malikova I,Confort‐Gouny S,Celsis P,Pelletier J,Cozzone PJ,Ranjeva JP ( 2005): Modulation of effective connectivity inside the working memory network in patients at the earliest stage of multiple sclerosis. Neuroimage 24: 533–538. [DOI] [PubMed] [Google Scholar]
- Awh E,Jonides J,Smith EE,Schumacher EH,Koeppe RA,Katz S ( 1996): Dissociation of storage and rehearsal in verbal working memory: Evidence from positron emission tomography. Psychol Sci 7: 25–31. [Google Scholar]
- Awh E,Jonides J,Smith EE,Buxton RB,Frank LR,Love T,Wong EC;Gmeindl L ( 1999): Rehearsal in spatial working memory: evidence from neuroimaging. Psychol Sci 10: 433–437. [Google Scholar]
- Baciu MV,Watson JM,Maccotta L,McDermott KB,Buckner RL,Gilliam FG,Ojemann JG ( 2005): Evaluating functional MRI procedures for assessing hemispheric language dominance in neurosurgical patients. Neuroradiology 47: 835–844. [DOI] [PubMed] [Google Scholar]
- Baddeley A ( 1992): Working memory. Science 255: 556–559. [DOI] [PubMed] [Google Scholar]
- Benke T,Köylü B,Visani P,Karner E,Brenneis C,Bartha L,Trinka E,Trieb T,Felber S,Bauer G,Chemelli A,Willmes K ( 2006): Language lateralization in temporal lobe epilepsy: A comparison between fMRI and the Wada Test. Epilepsia 47: 1308–1319. [DOI] [PubMed] [Google Scholar]
- Binder JR,Swanson SJ,Hammeke TA,Morris GL,Mueller WM,Fischer M,Benbadis S,Frost JA,Rao SM,Haughton VM ( 1996): Determination of language dominance using functional MRI: A comparison with the Wada test. Neurology 46: 978–984. [DOI] [PubMed] [Google Scholar]
- Binder JR,Medler DA,Westbury CF,Liebenthal E,Buchanan L ( 2006): Tuning of the human left fusiform gyrus to sublexical orthographic structure. Neuroimage 33: 739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR,Swanson SJ,Hammeke TA,Sabsevitz DS ( 2008): A comparison of five fMRI protocols for mapping speech comprehension systems. Epilepsia (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS,Cohen JD,Nystrom LE,Jonides J,Smith EE,Noll DC ( 1997): A parametric study of prefrontal cortex involvement in human working memory. Neuroimage 5: 49–62. [DOI] [PubMed] [Google Scholar]
- Brown JW,Hecaen H ( 1976): Lateralization and language representation. Neurology 26: 183–189. [DOI] [PubMed] [Google Scholar]
- Courtney SM,Petit L,Maisog JM,Ungerleider LG,Haxby JV ( 1998): An area specialized for spatial working memory in human frontal cortex. Science 279: 1347–1351. [DOI] [PubMed] [Google Scholar]
- Curtis CE,D'Esposito M ( 2003): Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci 7: 415–423. [DOI] [PubMed] [Google Scholar]
- Deppe M,Knecht S,Papke K,Lohmann H,Fleischer H,Heindel W,Ringelstein EB,Henningsen H ( 2000): Assessment of hemispheric language lateralization: A comparison between fMRI and fTCD. J Cereb Blood Flow Metab 20: 263–268. [DOI] [PubMed] [Google Scholar]
- D'Esposito M,Detre JA,Alsop DC,Shin RK,Atlas S,Grossman M ( 1995): The neural basis of the central executive system of working memory. Nature 378: 279–281. [DOI] [PubMed] [Google Scholar]
- Downing PE,Chan AW,Peelen MV,Dodds CM,Kanwisher N ( 2006): Domain specificity in visual cortex. Cereb Cortex 16: 1453–1461. [DOI] [PubMed] [Google Scholar]
- Druzgal TJ,D'Esposito M ( 2003): Dissecting contributions of prefrontal cortex and fusiform face area to face working memory. J Cogn Neurosci 15: 771–784. [DOI] [PubMed] [Google Scholar]
- Fernandez G,de Greiff A,von Oertzen J,Reuber M,Lun S,Klaver P,Ruhlmann J,Reul J,Elger CE ( 2001): Language mapping in less than 15 minutes: Real‐time functional MRI during routine clinical investigation. Neuroimage 14: 585–594. [DOI] [PubMed] [Google Scholar]
- Fiebach CJ,Rissman J,D'Esposito M ( 2006): Modulation of inferotemporal cortex activation during verbal working memory maintenance. Neuron 51: 251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PO,Fossati P,Pochon JB,Levy R,Lebastard G,Lehericy S,Allilaire JF,Dubois B ( 2005): Cognitive control and brain resources in major depression: An fMRI study using the n‐back task. Neuroimage 26: 860–869. [DOI] [PubMed] [Google Scholar]
- Helmstaedter C,Kurthen M,Linke DB,Elger CE ( 1997): Patterns of language dominance in focal left and right hemisphere epilepsies: Relation to MRI findings, EEG, sex, and age at onset of epilepsy. Brain Cogn 33: 135–150. [DOI] [PubMed] [Google Scholar]
- Humphries C,Binder JR,Medler DA,Liebenthal E ( 2006): Syntactic and semantic modulation of neural activity during auditory sentence comprehension. J Cogn Neurosi 18: 665–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen A,Flöel A,Van Randenborgh J,Konrad C,Rotte M,Förster AF,Deppe M,Knecht S ( 2005): Crossed cerebro‐cerebellar language dominance. Hum Brain Mapp 24: 165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansma JM,Ramsey NF,Coppola R,Kahn RS ( 2000): Specific versus nonspecific brain activity in a parametric N‐back task. Neuroimage 12: 688–697. [DOI] [PubMed] [Google Scholar]
- Jokisch D,Jensen O ( 2007): Modulation of γ and α activity during a working memory task engaging the dorsal or ventral stream. J Neurosci 27: 3244–3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonides J,Schumacher EH,Smith EE,Koeppe RA,Awh E,Reuter‐Lorenz PA,Marshuetz C,Willis CR ( 1998): The role of parietal cortex in verbal working memory. J Neurosci 18: 5026–5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang AM,Constable RT,Gore JC,Avrutin S ( 1999): An event‐related fMRI study of implicit phrase‐level syntactic and semantic processing. Neuroimage 10: 555–561. [DOI] [PubMed] [Google Scholar]
- Karmarkar UR,Dan Y ( 2006): Experience‐dependent plasticity in adult visual cortex. Neuron 52: 577–585. [DOI] [PubMed] [Google Scholar]
- Ketteler D,Kastrau F,Vohn R,Huber W ( 2008): The subcortical role of language processing. High level linguistic features such as ambiguity‐resolution and the human brain; an fMRI study. Neuroimage 39: 2002–2009. [DOI] [PubMed] [Google Scholar]
- Kiehl KA,Liddle PF,Smith AM,Mendrek A,Forster BB,Hare RD ( 1999): Neural pathways involved in the processing of concrete and abstract words. Hum Brain Mapp 7: 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht S,Deppe M,Ebner A,Henningsen H,Huber T,Jokeit H,Ringelstein EB ( 1998): Noninvasive determination of language lateralization by functional transcranial Doppler sonography: A comparison with the Wada test. Stroke 29: 82–86. [DOI] [PubMed] [Google Scholar]
- Knecht S,Drager B,Deppe M,Bobe L,Lohmann H,Floel A,Ringelstein EB,Henningsen H ( 2000): Handedness and hemispheric language dominance in healthy humans. Brain 123: 2512–2518. [DOI] [PubMed] [Google Scholar]
- Lee D,Swanson SJ,Sabsevitz DS,Hammeke TA,Scott Winstanley F,Possing ET,Binder JR ( 2008): Functional MRI and Wada studies in patients with interhemispheric dissociation of language functions. Epilepsy Behav 13: 350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehéricy S,Cohen L,Bazin B,Samson S,Giacomini E,Rougetet R,Hertz‐Pannier L,Le Bihan D,Marsault C,Baulac M ( 2000): Functional MR evaluation of temporal and frontal language dominance compared with the Wada test. Neurology 54: 1625–1633. [DOI] [PubMed] [Google Scholar]
- Linden DE,Bittner RA,Muckli L,Waltz JA,Kriegeskorte N,Goebel R,Singer W,Munk MH ( 2003): Cortical capacity constraints for visual working memory: Dissociation of fMRI load effects in a fronto‐parietal network. Neuroimage 20: 1518–1530. [DOI] [PubMed] [Google Scholar]
- Malach R,Levy I,Hasson U ( 2002): The topography of high‐order human object areas. Trends Cogn Sci 6: 176–184. [DOI] [PubMed] [Google Scholar]
- Mecklinger A,Bosch V,Gruenewald C,Bentin S,von Cramon DY ( 2000): What have Klingon letters and faces in common? An fMRI study on content‐specific working memory systems. Hum Brain Mapp 11: 146–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller NG,Knight RT ( 2006): The functional neuroanatomy of working memory: Contributions of human brain lesion studies. Neuroscience 139: 51–58. [DOI] [PubMed] [Google Scholar]
- Narayanan NS,Prabhakaran V,Bunge SA,Christoff K,Fine EM,Gabrieli JD ( 2005): The role of the prefrontal cortex in the maintenance of verbal working memory: An event‐related FMRI analysis. Neuropsychology 19: 223–232. [DOI] [PubMed] [Google Scholar]
- Nobre AC,Allison T,McCarthy G ( 1994): Word recognition in the human inferior temporal lobe. Nature 372: 260–263. [DOI] [PubMed] [Google Scholar]
- Nordahl CW,Ranganath C,Yonelinas AP,Decarli C,Fletcher E,Jagust WJ ( 2006): White matter changes compromise prefrontal cortex function in healthy elderly individuals. J Cogn Neurosci 18: 418–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak T,Greenlee MW ( 2005): Working memory in primate sensory systems. Nat Rev Neurosci 6: 97–107. [DOI] [PubMed] [Google Scholar]
- Paulesu E,Frith CD,Frackowiak RS ( 1993): The neural correlates of the verbal component of working memory. Nature 362: 342–345. [DOI] [PubMed] [Google Scholar]
- Poldrack RA,Wagner AD,Prull MW,Desmond JE,Glover GH,Gabrieli JD ( 1999): Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage 10: 15–35. [DOI] [PubMed] [Google Scholar]
- Postle BR,Awh E,Jonides J,Smith EE,D'Esposito M ( 2004): The where and how of attention‐based rehearsal in spatial working memory. Brain Res Cogn Brain Res 20: 194–205. [DOI] [PubMed] [Google Scholar]
- Postle BR ( 2006a): Working memory as an emergent property of the mind and brain. Neuroscience 139: 23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle BR ( 2006b): Distraction‐spanning sustained activity during delayed recognition of locations. Neuroimage 30: 950–962. [DOI] [PubMed] [Google Scholar]
- Ramsey NF,Sommer IE,Rutten GJ,Kahn RS ( 2001): Combined analysis of language tasks in fMRI improves assessment of hemispheric dominance for language functions in individual subjects. Neuroimage 13: 719–733. [DOI] [PubMed] [Google Scholar]
- Ranganath C,DeGutis J,D'Esposito M ( 2004): Category‐specific modulation of inferior temporal activity during working memory encoding and maintenance. Brain Res Cogn Brain Res 20: 37–45. [DOI] [PubMed] [Google Scholar]
- Ravizza SM,Delgado MR,Chein JM,Becker JT,Fiez JA ( 2004): Functional dissociations within the inferior parietal cortex in verbal working memory. Neuroimage 22: 562–573. [DOI] [PubMed] [Google Scholar]
- Reddy L,Kanwisher N ( 2006): Coding of visual objects in the ventral stream. Curr Opin Neurobiol 16: 408–414. [DOI] [PubMed] [Google Scholar]
- Ries ML,Boop FA,Griebel ML,Zou P,Phillips NS,Johnson SC,Williams JP,Helton KJ,Ogg RJ ( 2004): Functional MRI and Wada determination of language lateralization: A case of crossed dominance. Epilepsia 45: 85–89. [DOI] [PubMed] [Google Scholar]
- Rose M,Schmid C,Winzen A,Sommer T,Büchel C ( 2005): The functional and temporal characteristics of top‐down modulation in visual selection. Cereb Cortex 15: 1290–1298. [DOI] [PubMed] [Google Scholar]
- Rutten GJ,Ramsey NF,van Rijen PC,Alpherts WC,van Veelen CW ( 2002): FMRI‐determined language lateralization in patients with unilateral or mixed language dominance according to the Wada test. Neuroimage 17: 447–460. [DOI] [PubMed] [Google Scholar]
- Rypma B,Prabhakaran V,Desmond JE,Glover GH,Gabrieli JD ( 1999): Load‐dependent roles of frontal brain regions in the maintenance of working memory. Neuroimage 9: 216–226. [DOI] [PubMed] [Google Scholar]
- Schoning S,Engelien A,Kugel H,Schafer S,Schiffbauer H,Zwitserlood P,Pletziger E,Beizai P,Kersting A,Ohrmann P,Greb RR,Lehmann W,Heindel W,Arolt V,Konrad C ( 2007): Functional anatomy of visuo‐spatial working memory during mental rotation is influenced by sex, menstrual cycle, and sex steroid hormones. Neuropsychologia 45: 3203–3214. [DOI] [PubMed] [Google Scholar]
- Seghier ML,Lazeyras F,Pegna AJ,Annoni JM,Zimine I,Mayer E,Michel CM,Khateb A ( 2004): Variability of fMRI activation during a phonological and semantic language task in healthy subjects. Hum Brain Mapp 23: 140–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE,Jonides J ( 1999): Storage and executive processes in the frontal lobes. Science 283: 1657–1661. [DOI] [PubMed] [Google Scholar]
- Stern Y,Zarahn E,Habeck C,Holtzer R,Rakitin BC,Kumar A,Flynn J,Steffener J,Brown T ( 2008): A common neural network for cognitive reserve in verbal and object working memory in young but not old. Cereb Cortex 18: 959–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N,Josse G,Crivello F,Mazoyer B ( 2004): Interindividual variability in the hemispheric organization for speech. Neuroimage 21: 422–435. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG,Haxby JV ( 1994): ‘What’ and ‘where’ in the human brain. Curr Opin Neurobiol 4: 157–165. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG,Mishkin M ( 1982): In: Ingle DJ,Goodale MA, Mansfield RJW, editors. Analysis of Visual Behavior. Cambridge, MA: MIT Press; pp 549–586. [Google Scholar]
- Wager TD,Smith EE ( 2003): Neuroimaging studies of working memory: A meta‐analysis. Cogn Affect Behav Neurosci 3: 255–274. [DOI] [PubMed] [Google Scholar]
- Walter H,Vasic N,Hose A,Spitzer M,Wolf RC ( 2007): Working memory dysfunction in schizophrenia compared to healthy controls and patients with depression: Evidence from event‐related fMRI. Neuroimage 35: 1551–1561. [DOI] [PubMed] [Google Scholar]
- Weber B,Wellmer J,Reuber M,Mormann F,Weis S,Urbach H,Ruhlmann J,Elger CE,Fernández G ( 2006): Left hippocampal pathology is associated with atypical language lateralization in patients with focal epilepsy. Brain 129: 346–351. [DOI] [PubMed] [Google Scholar]
- Weber B,Fliessbach K,Lange N,Kugler F,Elger CE ( 2007): Material‐specific memory processing is related to language dominance. Neuroimage 37: 611–617. [DOI] [PubMed] [Google Scholar]
- Wellmer J,Weber B,Weis S,Klaver P,Urbach H,Reul J,Fernandez G,Elger CE ( 2008): Strongly lateralized activation in language fMRI of atypical dominant patients‐Implications for presurgical work‐up. Epilepsy Res 80: 67–76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supplementary Methods
Supplementary Table 1
Supplementary Table 2: Complete overview of activated regions in the different contrasts.


