Abstract
Brain activity is associated with physiological changes, which alter the optical properties of tissue. These changes can be detected by near‐infrared spectroscopy (NIRS). Aim of the study was to determine changes in cerebral oxygenation in response to stimulation in the visual cortex in newborn infants during spontaneous sleep in the first days of life. We used an in‐house developed multichannel NIRS imaging instrument, the MCP‐II, to measure changes in concentration of oxyhemoglobin (O2Hb) and deoxyhemoglobin (HHb) in specific brain areas. In 10 out of 15 subjects, a significant increase in O2Hb and/or a significant decrease in HHb were found in one or more channels over the occipital cortex. During stimulation, O2Hb increased by a mean of 0.98 μmol/l, HHb decreased by a mean 0.17 μmol/l, and total‐Hb increased by a mean of 0.81 μmol/l. The hemodynamic response to visual stimulation in the occipital cortex in newborn infants is similar to adults. The increase in O2Hb and the simultaneous decrease in HHb during stimulation suggest an increase in cerebral blood flow (CBF) that overcompensates for the increased oxygen consumption (CMRO2) in the activated cortical area. Hum Brain Mapp, 2008. © 2007 Wiley‐Liss, Inc.
Keywords: functional near‐infrared spectroscopy; infants; visual stimulation, hemodynamic response; behavioral state; age dependence
INTRODUCTION
Brain activity is associated with physiological changes, which alter the optical properties of tissue. These changes can be detected by near‐infrared spectroscopy (NIRS). This is a technique that can be used in fragile newborn infants, because it is portable and noninvasive. NIRS has the potential to provide insights into the origin of neonatal brain lesions, as well as into functional development of both the normal and abnormal brain. It is clinically important to assess the normal development of the brain anatomy and function in order to detect abnormalities in the early infantile period [Konishi et al., 2002].
Functional changes in the adult human brain were detected by several groups, mainly measuring responses to visual stimulation over the occipital region [Hoshi, 2003; Hoshi and Tamura, 1993; Kato et al., 1993; Meek et al., 1995; Villringer and Chance, 1997; Villringer et al., 1993; Wolf et al., 2003]. These demonstrated localized increases in oxyhemoglobin (O2Hb) during stimulation with a corresponding decrease in deoxyhemoglobin (HHb) as a typical pattern for an increase in cerebral blood flow (CBF). This was consistent with a positive blood oxygenation level‐dependent (BOLD) functional magnetic resonance imaging (fMRI) signal during activation as found in simultaneous NIRS and fMRI studies [Kleinschmidt et al., 1996; Toronov et al., 2001].
A part of the visual cortex is responsive to visual stimulation from birth in neonates and infants and can be detected using fMRI [Martin et al., 1999]. Born et al. [ 1996, 1998] reported that flickering light stimulation induced a BOLD signal decrease in the occipital region in infants, which was different from the BOLD signal increase in adults, and the localization of the activation was age‐dependent. Furthermore, Yamada et al. [ 1997, 2000] reported an inverse response in infants that is related to the period of rapid formation of synapses in the white matter.
In infants, NIRS has been used to asses the activation of the visual cortex induced by a checkerboard or flashlight stimulation [Hoshi et al., 2000; Meek et al., 1998; Taga et al., 2000, 2003a, b] in awake [Meek et al., 1998; Taga et al., 2003, b] and sleeping infants [Hoshi et al., 2000; Konishi et al., 2002; Kusaka et al., 2004; Taga et al., 2003a] and at different ages ranging from the 32 weeks of gestation until 4 month. Different responses have been seen due to different behavioral state and postnatal age. As pointed out in detail in the discussion section, relatively little and controversial information exists on the hemodynamic responses to visual stimulation in the visual cortex of the neonatal brain [Hoshi et al., 2000; Konishi et al., 2002; Kusaka et al., 2004; Meek et al., 1998; Taga et al., 2003a, b]. Therefore, the idea was to start investigations in a homogeneous and healthy population to increase the body of evidence and in future studies proceed to identify the most important factors affecting the response. The aim of this study was to determine changes in cerebral oxygenation in response to brief visual stimulation in the visual cortex in newborn infants during spontaneous sleep during the first days of life.
MATERIALS AND METHODS
Subjects
We studied 20 healthy (12 male, 8 female) term neonates during the first 10 days of life. Median gestational age was 39 weeks (range 37–42 weeks). The median postnatal age at the time of the measurement was 5.5 days (range 2–9 days). The prenatal and postnatal courses were uncomplicated in all of these infants. The study protocol was approved by the local ethics committee and before each study, written informed parental consent was obtained.
Protocol
Visual stimulation was elicited by red lights (LEDs), flashing at a frequency of 0.5 and 1 Hz, held at approximately 5 cm in front of the eyes. Both eyes were stimulated simultaneously. Stimulation periods of 20 s were alternated by rest periods of approximately 20 s. In each infant, two stimulation “runs,” each consisting of 10 consecutive stimulation and rest periods, were performed with a prolonged rest period of several minutes in between. In half of the infants, the stimulation rate was set at 0.5 Hz during the first run and to 1 Hz during the second run, and vice versa for the other half of the infants. The centre of the sensor was positioned 1 cm above the inion. The sensor was fixed with an elastic strap and covered with dark cloth to prevent external light interference. The measurements were performed during spontaneous sleep after feeding in a dimly lit room. The criteria used for behavioral states of the newborn infant according to Prechtl [ 1974] were closely monitored at the bedside.
Instrumentation
An in‐house developed multichannel NIRS imaging instrument, the MCP‐II, was used. The novelty of this instrument, its algorithms and how this instrument compares to other instruments, is described in detail in the paper of Haensse et al. [ 2005]. In brief, the measuring unit of the MCP‐II allows the simultaneous acquisition of up to 48 channels with a sampling rate of 100 Hz. A Linux notebook was used to record and display the data. The stimulation unit can generate stimulation patterns that were synchronized with the NIRS measurements, for different types of stimuli (e.g., visual, auditory, and tactile). The NIRS sensor contains four light sources and four detectors, covering an area of 2.5 cm by 3.75 cm as shown in Figure 1. Each light source contains light emitting diodes (LEDs) of wavelengths 730 and 830 nm. The light sources are time multiplexed, i.e. only one source is on at a time. After propagation through the tissue, the light is detected by a photodiode. This configuration allows the measurement of O2Hb and HHb concentration changes in up to 16 different locations simultaneously, providing a differentiated regional mapping of hemodynamic changes. For the current study, the 10 light paths shown in Figure 1 were considered. Because of the low intensity, the (incoherent) light is harmless for the newborn's skin and eyes, and tissue heating is negligible. The sensor was molded in soft silicon for comfortable attachment on the neonatal head. The algorithm used to quantify the changes in O2Hb and HHb assumes a homogenous change in a homogenous tissue. Since the tissue is layered and the activation is localized the quantification of O2Hb and HHb is not quantitatively correct, i.e. the size of the change is underestimated. This has to be kept in mind, when looking at our results. However, in functional studies the quantification is not important, because we are mostly interested, whether there is a significant response. Since many other publications are using the same algorithm and have published their values, we supply our values for comparison.
Figure 1.

Position of the light sources (circles S1, S2, S3, S4) and detectors (squares D1, D2, D3, D4) on the head of an infant. The center of the sensor was positioned 1 cm above the inion. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Data Analysis
From the measured light intensities, changes in O2Hb and HHb were calculated using the modified Beer–Lambert law assuming a differential path length factor of 4 [Wyatt et al., 1990]. An example of unprocessed O2Hb and HHb concentrations is shown in Figure 2. In addition to the arterial pulsations, with a frequency around 2.5 Hz (∼150 bpm), large, spontaneous fluctuations with a period approximately 10 s, presumably due to vasomotion, are observed. In order to remove the arterial pulsations and the fluctuations caused by the respiration, the signals were low pass filtered using a fourth‐order Butterworth filter with cut‐off frequency of 0.25 Hz. The filtered signals were then detrended by subtracting a moving average over a 40‐s period from the signals. From the resulting signals, the O2Hb and HHb concentrations during the last 10 s of each artifact free stimulation period were averaged and compared to the concentrations during the 10 s preceding the stimulation. For each channel and stimulation run, the average change in O2Hb and HHb concentrations was calculated and the statistical significance of the difference was assessed using the paired Wilcoxon sign rank test.
Figure 2.
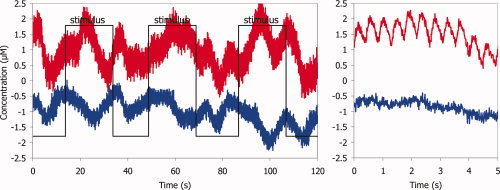
Example of (unprocessed) NIRS signals from infant 7: left side: concentration changes of O2Hb (upper trace) and HHb (lower trace). The square wave indicates when the visual stimulation is on. On the right side: extended time scale with visualisation of pulse wave in O2Hb. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
RESULTS
We obtained data from 15 (10 male, 5 female) infants. Five other infants were not included in the study because we could not obtain sufficiently reliable data due to head movement during measurement. The clinical data and average change in O2Hb and HHb concentration in response to visual stimulation for each neonate are shown in Table I. In 10 out of these 15 subjects, a significant increase in O2Hb was found in one or more channels over the occipital cortex. A significant decrease in HHb was observed in 5 out of 15 infants. A topological representation of the averaged concentration changes in the different channels for a typical measurement is shown in Figure 3. The global average of all significant responses among all channels and subjects is shown in Figure 4. During stimulation, O2Hb increased by a mean of 0.98 μmol/l, HHb decreased by a mean 0.17 μmol/l, and total Hb increased by a mean of 0.81 μmol/l. A color‐coded image of the concentration changes (mmol/l) of (a) O2Hb and (b) HHb due to visual stimulation (data from the same infant as in Fig. 3) is shown in Figure 5. The color coding corresponds to the average concentration change between the last 10 s of each artifact free stimulation period and during the 10 s preceding the stimulation.
Table I.
Clinical data and average change in O2Hb and HHb concentration in response to visual stimulation for each neonate, only given for significant (p < 0.05) changes
| ID | Gender | Birth‐weight | GA at birth (weeks – days) | Age at measurement (days) | State of alertness | Number of channels with significant change (p < 0.01) (1st/2nd run) | ΔO2Hb (μmol/l) | ΔHHb (μmol/l) |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 2780 | 38, 1/7 | 9 | Quiet sleep | 1/1 | +0.54 | −0.28 |
| 2 | M | 2780 | 38, 1/7 | 8 | Quiet sleep | 4/2 | +1.54 | −0.07 |
| 3 | M | 3490 | 41, 1/7 | 5 | Sleep (movements) | 2/0 | +0.38 | −0.14 |
| 4 | M | 3970 | 40 4/7 | 8 | Quiet sleep | 4/0 | +0.80 | −0.24 |
| 5 | M | 3370 | 40, 1/7 | 7 | Superficial sleep | 0/0 | ||
| 7 | F | 4020 | 42, 0/7 | 2 | Quiet sleep | 0/7 | +1.35 | −0.70 |
| 8 | M | 3290 | 38, 0/7 | 4 | Superficial sleep | 1/0 | +1.23 | −0.45 |
| 9 | F | 3890 | 41, 0/7 | 6 | Quiet sleep | 0/0 | ||
| 10 | F | 2550 | 37, 1/7 | 3 | Quiet sleep | 7/5 | +0.97 | −0.06 |
| 13 | M | 3550 | 38, 1/7 | 6 | Indeterminate sleep | 0/0 | ||
| 14 | M | 4130 | 40, 4/7 | 3 | Quiet sleep | 0/0 | ||
| 16 | F | 3640 | 38, 2/7 | 4 | Quiet sleep | 0/4 | +1.03 | −0.21 |
| 17 | F | 3580 | 39, 3/7 | 3 | Active/indeterminate sleep | 0/4 | +0.63 | +0.36 |
| 18 | M | 3500 | 38, 4/7 | 7 | Quiet sleep | 0/0 | ||
| 20 | M | 3210 | 41, 2/7 | 7 | Quiet sleep | 2/0 | +1.40 | −0.01 |
| Mean | 10 M, 5 F | 3450 | 39, 4/7 | 5 | +0.98 | −0.17 |
Figure 3.
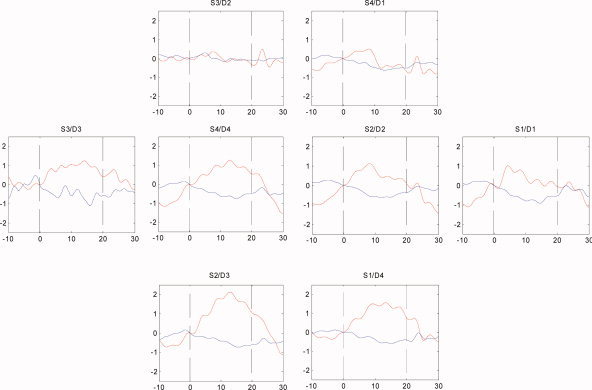
Topological representation of the averaged hemodynamic responses to visual stimulation in infant 7. Each plot corresponds to a light bundle, identified by source (S1, S2, S3, S4) and detector (D1, D2, D3, D4) number, positioned as shown in Figure 1. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Figure 4.
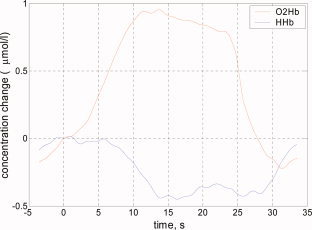
Global average of all significant responses in all infants (upper trace O2Hb, lower trace HHb). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Figure 5.
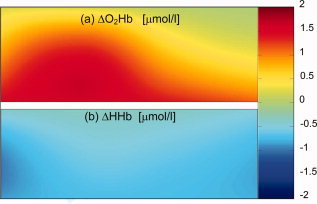
Topographical image of the concentration changes (μmol/l) of (a) O2Hb and (b) HHb due to visual stimulation (data from the same infant as in Fig. 3). The color coding corresponds to the average concentration change between the last 10 s of each artifact free stimulation period and during the 10 s preceding the stimulation. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
We demonstrated a hemodynamic response in the visual cortex in newborn infants using functional NIRS during their first days of life. In our study we found an increase in O2Hb and a decrease in HHb which is similar to the findings in adults but different to the response in infants observed in other studies [Hoshi et al., 2000; Konishi et al., 2002; Kusaka et al., 2004; Meek et al., 1998].
The normal pattern, when adults are studied with NIRS, is an increase in O2Hb and tHb and a decrease in HHb during visual stimulation [Meek et al., 1995; Villringer et al., 1993] which is in agreement with fMRI and PET studies [Fox and Raichle, 1984; Kleinschmidt et al., 1996]. These changes are usually interpreted as an increase in oxygen consumption (CMRO2) in the activated area of the brain, which is closely followed by an increase in CBF. The latter is so large that there is a higher oxygen concentration in the activated tissue than before the activation.
In infants, various changes in the direction of O2Hb and HHb have been observed in the activated area [Meek et al., 1998; Hoshi et al., 2000; Konishi et al., 2002; Taga et al., 2003a b; Kusaka et al., 2004]. Meek et al. [ 1998] found an increase in total Hb (the sum of O2Hb and HHb), which indicates an increase of both CMRO2 and CBF. Other findings were an increase in O2Hb, while changes in HHb differed in the subjects [Hoshi et al., 2000; Taga et al., 2003a] or a decrease in both O2Hb and HHb [Kusaka et al., 2004], which indicates a decrease in CBF, although photostimulation induces neural activation and a higher CMRO2. In the study of Konishi et al. [ 2002], a hemodynamic response was only found in newborns but not in infants aged 1 month [Konishi et al., 2002].
Thus there is a remarkable variation between the results in infants in the literature. The reason for this could be the differences in patterns of photostimulation, behavioral state (natural sleep, sedation, awake), gestational and postnatal age [Kusaka et al., 2004], or different localization of activation. We will consider the influence of the different factors below and compare the results also to those of previous fMRI studies.
Stimulation Pattern
In fMRI and NIRS studies different types of stimuli were used for visual brain function in adults and infants [Born et al., 1998; Colier et al., 2001; Meek et al., 1998; Takahashi et al., 2000; Villringer et al., 1993]. In adults the size of the activated area depends on the type of the stimulus which was maximal for the checkerboard paradigm, but the physiological effect responsible for the observed stimulus dependence could not be clarified [Kruger et al., 1998]. The sensitivity to stimulation at higher frequencies is known to increase in the first month of life [Apkarian, 1993; Fiorentini and Trimarchi, 1992]. In children under the age of 48 weeks postmenstrual age (PMA), the largest activated areas were achieved with stimulation at 1 Hz, whereas in the older children, activation at 8 Hz was stronger than that at 1 Hz [Born et al., 2000]. This is similar to the findings in adults, where rCBF at 1 Hz was undistinguishable from the unstimulated condition [Fox and Raichle, 1984]. In previous NIRS studies on visual brain function in infants different types of stimuli were used such as a checkerboard reversing at a frequency of 5 Hz [Meek et al., 1998; Taga et al., 2003, b] and flashing light at a frequency from 8 to 14 Hz [Hoshi et al., 2000; Konishi et al., 2002; Kusaka et al., 2004; Taga et al., 2003a]. We used flashing red LED goggles with a frequency of 0.5–1 Hz, because according to the visual evoked potential (VEP) studies in preterm infants [Eken, 1996], the hemodynamic response in newborn infants can also be detected at slower stimulation frequencies. This as well as the high signal‐to‐noise ratio of our instrument may be explanations for comparably lower mean concentration changes in O2Hb and HHb in our study. It seems that the stimulus frequency is a significant determinant of the hemodynamic response in the visual cortex, but whether the direction (increase or decrease) of changes in the NIRS parameters depends on the pattern of photostimulation is still unclear.
Age Dependence
When comparing most of the previous NIRS studies to fMRI studies, the results are surprising. Infants older than 8 weeks showed a stimulus induced BOLD signal decrease (=HHb increase) in the visual cortex, whereas infants younger than 7 weeks showed a signal increase (=HHb decrease) [Yamada et al., 2000]. These findings were confirmed by Kusaka et al. [ 2004] and Konishi et al. [ 2002]. It was hypothesized, that the inverse response in infants may represent rapid synapse formation in the visual cortex disproportionate to the oxygen delivery. Martin et al. [ 1999] concluded from the results of their study that the absence of any BOLD contrast signal in neonates must be due to a combination of immature vascular response and low responsiveness of the primary visual area in this age group. This might be an explanation why we found a response only in 10 out of 15 infants.
NIRS studies have been performed within inhomogeneous groups of infants at different ages ranging from 32 weeks of gestation to 4 month postnatal age [Hoshi et al., 2000; Konishi et al., 2002; Kusaka et al., 2004; Meek et al., 1998; Taga et al., 2000, 2003a, b]. It was postulated that the difference in changes in O2Hb and HHb has been due to the different ages but Taga et al. [ 2003a, b] found the same response in newborn infants as well as in infants as young as 2 month of age. These results are in accordance with the increase in O2Hb and decrease in HHb as in our newborn infants during their first 10 days of life. In two NIRS studies in infants, after auditory stimulation there was always an increase in O2Hb, while HHb varied between an increase or decrease [Sakatani et al., 1999; Zaramella et al., 2001]. There may be an age‐dependency in the changes in HHb, but not that much in O2Hb.
Behavioral State
The measurements in our study were performed during spontaneous sleep after feeding. Although in previous studies it was hypothesized that sedation or state of alertness and attention may profoundly affect the hemodynamic response to visual stimulation [Joeri et al., 1996; Lindauer et al., 1993], it has been shown that sedated and unsedated children showed a similar response to visual stimulation [Born et al., 1998; Martin et al., 1999; Yamada et al., 2000]. Taga et al. [ 2003a, b] observed the same hemodynamic response to visual stimulation over the occipital cortex in sleeping newborn infants as well as in awake infants as young as 2 months of age. Thus, it is unlikely that the behavioral state alone is the cause of different responses. However, spontaneous sleep has an effect on the shape and latency of visual evoked potential in infants [Apkarian, 1991; Mercuri et al., 1995] and sleep stages influence the cerebral hemoglobin concentration [Munger et al., 1998]. Born et al. [ 2002] comparing sleeping and awake adults using two different brain mapping techniques found a decrease in CBF in the visual cortex in the group of sleeping adults and a different localization of activation. This comparison of brain activation has not been performed in infants.
Difference in the Localization of Activation
Simultaneous NIRS and fMRI studies have shown that a displacement of less than 1 cm can result in a substantial loss of NIRS signals [Kleinschmidt et al., 1996]. Therefore we were using an imaging sensor, which covers a large area. Spatial distribution of activation by photic stimulation in infants and adults using fMRI measurements was different [Born et al., 1996, 1998]. In adults, the activation area is the whole length of the calcarine fissure. In contrast, in infants, activation area is restricted to the anterolateral part of the fissure. The activated area did not extend to the occipital surface of the brain. In infants, therefore, a decrease in CBF in the measurement area may indicate that blood is being supplied to the activation area that is deeper from the scalp than that in adults [Kusaka et al., 2004]. The measurement area of NIRS is from the surface of the scalp, but it is difficult to specify the region adjacent to the visual cortex on the head surface. For our measurements, we placed the sensor above the inion, where the visual cortex is suspected according to the international 10–20 system [Steinmetz et al., 1989].
NIRS vs BOLD Signal Changes and Cerebral Metabolism after Visual Stimulation
The BOLD signal is mostly related to HHb‐concentration, which depends on the balance of CMRO2 and CBF increase. A BOLD signal decrease means a greater proportional increase in CMRO2 compared to the CBF increase during stimulation. This could be related to the rapid rate of synapse formation in children older than 8 weeks [Huttenlocher et al., 1982; Yamada et al., 1997]. There is no evidence that the process of synapse formation is associated with increased baseline CMRO2. In summary in the previous fMRI and fNIRS studies and also in ours there was no significant change in HHb in half of the infants despite a significant increase in O2Hb. This indicates that the O2Hb is much more reliable indicator of functional activity than HHb or BOLD signal. NIRS has the advantage over fMRI BOLD to provide separate measures of O2Hb and HHb. These two measures are advantageous in separating signals due to increased CMRO2, which is a considerable concern of fMRI studies [Aslin and Mehler, 2005].
Due to the still conflicting results, further studies are needed to investigate the influence of the stimulation pattern, age dependence, and behavioral state on the hemodynamic response after visual stimulation in the visual cortex in the developing brain of newborns and infants.
In conclusion, we have demonstrated that a hemodynamic response to brief visual stimulation can be detected in the visual cortex in sleeping newborn infants in the first 10 days of life. The hemodynamic response to visual stimulation in the occipital cortex in newborn infants is similar to adults. The increase in O2Hb and the simultaneous smaller decrease in HHb during stimulation suggest an increase in CBF that overcompensates for the increased CMRO2 in the activated cortical area.
REFERENCES
- Apkarian P( 1993): Temporal frequency responsivity shows multiple maturational phases: State‐dependent visual evoked potential luminance flicker fusion from birth to 9 months. Vis Neurosci 10: 1007–1018. [DOI] [PubMed] [Google Scholar]
- Aslin R, Mehler J( 2005): Near‐infrared spectroscopy for functional studies of brain activity in human infants: Promise, prospects, and challenges. J Biomed Opt 10: 1–3. [DOI] [PubMed] [Google Scholar]
- Born AP, Rostrup E, Leth H, Peitersen B, Lou HC( 1996): Change of visually induced cortical activation patterns during development. Lancet 347: 543. [DOI] [PubMed] [Google Scholar]
- Born AP, Leth H, Miranda MJ, Rostrup E, Stensgaard A, Peitersen B, Larsson HB, Lou HC( 1998): Visual activation in infants and young children studied by functional magnetic resonance imaging. Pediatr Res 44: 578–583. [DOI] [PubMed] [Google Scholar]
- Born AP, Miranda MJ, Rostrup E, Toft PB, Peitersen B, Larsson HB, Lou HC( 2000): Functional magnetic resonance imaging of the normal and abnormal visual system in early life. Neuropediatrics 31: 24–32. [DOI] [PubMed] [Google Scholar]
- Born AP, Rostrup E, Miranda MJ, Larsson HB, Lou HC( 2002): Visual cortex reactivity in sedated children examined with perfusion MRI (FAIR). Magn Reson Imaging 20: 199–205. [DOI] [PubMed] [Google Scholar]
- Colier WN, Quaresima V, Wenzel R, van der Sluijs MC, Oeseburg B, Ferrari M, Villringer A( 2001): Simultaneous near‐infrared spectroscopy monitoring of left and right occipital areas reveals contra‐lateral hemodynamic changes upon hemi‐field paradigm. Vision Res 41: 97–102. [DOI] [PubMed] [Google Scholar]
- Eken P ( 1996): Cerebral visual impairment in infants with haemorrhagic‐ischaemic lesions of the neonatal brain. Utrecht, Netherlands, Utrecht. [DOI] [PubMed]
- Fiorentini A, Trimarchi C( 1992): Development of temporal properties of pattern electroretinogram and visual evoked potentials in infants. Vision Res 32: 1609–1621. [DOI] [PubMed] [Google Scholar]
- Fox PT, Raichle ME( 1984): Stimulus rate dependence of regional cerebral blood flow in human striate cortex, demonstrated by positron emission tomography. J Neurophysiol 51: 1109–1120. [DOI] [PubMed] [Google Scholar]
- Haensse D, Szabo P, Brown D, Fauchère JC, Niederer P, Bucher HU, Wolf M( 2005): A new multichannel near‐infrared spectrophotometry system for functional studies of the brain of adults and neonates. Opt Exp 13: 4525–4538. [DOI] [PubMed] [Google Scholar]
- Hoshi Y( 2003): Functional near‐infrared optical imaging: utility and limitations in human brain mapping. Psychophysiology 40: 511–520. [DOI] [PubMed] [Google Scholar]
- Hoshi Y, Tamura M( 1993): Dynamic multichannel near infrared optical imaging of human brain activity. Appl Physiol 75: 1842–1846. [DOI] [PubMed] [Google Scholar]
- Hoshi Y, Kohri S, Matsumoto Y, Cho K, Matsuda T, Okajima S, Fujimoto S( 2000): Hemodynamic responses to photic stimulation in neonates. Pediatr Neurol 23: 323–327. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, de Courten C, Garey LJ, Van der Loos H( 1982): Synaptogenesis in human visual cortex—Evidence for synapse elimination during normal development. Neurosci Lett 33: 247–252. [DOI] [PubMed] [Google Scholar]
- Joeri P, Huisman T, Rumpel H, Ekatodramis D, Loenneker T, Martin E( 1996): Comparison of fMRI‐signal changes during visual stimulation, in awake vs phenobarbital sedated volunteers. Mag Res Mater Phys Biol Med IV( Suppl): 176–177. [Google Scholar]
- Kato T, Kamei A, Takashima S, Ozaki T( 1993): Human visual cortical function during photic stimulation monitored by means of near‐infrared spectroscopy. J Cereb Blood Flow Metab 13: 516–520. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt A, Obrig H, Requardt M, Merboldt KD, Dirnagl U, Villringer A, Frahm J ( 1996): Simultaneous recording of cerebral blood oxygenation changes during human brain activation by magnetic resonance imaging and near‐infrared spectroscopy. J Cereb Blood Flow Metab 16: 817–826. [DOI] [PubMed] [Google Scholar]
- Konishi Y, Taga G, Yamada H, Hirasawa K ( 2002): Functional brain imaging using fMRI and optical topography in infancy. Sleep Med 3( Suppl 2): S41–43. [DOI] [PubMed] [Google Scholar]
- Kruger G, Kleinschmidt A, Frahm J ( 1998): Stimulus dependence of oxygenation‐sensitive MRI responses to sustained visual activation. NMR Biomed 11: 75–79. [DOI] [PubMed] [Google Scholar]
- Kusaka T, Kawada K, Okubo K, Nagano K, Namba M, Okada H, Imai T, Isobe K, Itoh S ( 2004): Noninvasive optical imaging in the visual cortex in young infants. Hum Brain Mapp 22: 122–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindauer U, Villringer A, Dirnagl U ( 1993): Characterization of CBF response to somatosensory stimulation: Model and influence of anesthetics. Am J Physiol 264: 1223–1228. [DOI] [PubMed] [Google Scholar]
- Martin E, Joeri P, Loenneker T, Ekatodramis D, Vitacco D, Hennig J, Marcar VL ( 1999): Visual processing in infants and children studied using functional MRI. Pediatr Res 46: 135–140. [DOI] [PubMed] [Google Scholar]
- Meek JH, Elwell CE, Khan MJ, Romaya J, Wyatt JS, Delpy DT, Zeki S ( 1995): Regional changes in cerebral haemodynamics as a result of a visual stimulus measured by near infrared spectroscopy. Proc Biol Sci 261: 351–356. [DOI] [PubMed] [Google Scholar]
- Meek JH, Firbank M, Elwell CE, Atkinson J, Braddick O, Wyatt JS ( 1998): Regional hemodynamic responses to visual stimulation in awake infants. Pediatr Res 43: 840–843. [DOI] [PubMed] [Google Scholar]
- Mercuri E, v. Siebenthal K, Tutuncuoglu S, Guzetta F, Casaer P ( 1995): The effect of behavioral states on visual evoked responses in preterm and full‐term infants. Neuropediatrics 26: 211–213. [DOI] [PubMed] [Google Scholar]
- Munger D, Bucher HU, Duc G ( 1998): Sleep state changes associated with cerebral blood volume changes in healthy term newborn infants. Early Hum Dev 52: 27–42. [DOI] [PubMed] [Google Scholar]
- Prechtl HFR ( 1974): The behavioral states of the newborn infant. Brain Res 76: 185–212. [DOI] [PubMed] [Google Scholar]
- Sakatani K, Chen S, Lichty W, Zuo H, Wang Y ( 1999): Cerebral blood oxygenation changes induced by auditory stimulation in newborn infants measured by near infrared spectroscopy. Early Hum Dev 55: 229–236. [DOI] [PubMed] [Google Scholar]
- Steinmetz H, Furst G, Meyer BU ( 1989): Craniocerebral topography within the international 10–20 system. Electroencephalogr Clin Neurophysiol 72: 499–506. [DOI] [PubMed] [Google Scholar]
- Taga G, Konishi Y, Maki A, Tachibana T, Fujiwara M, Koizumi H ( 2000): Spontaneous oscillation of oxy‐ and deoxy‐hemoglobin changes with a phase difference throughout the occipital cortex of newborn infants observed using non‐invasive optical topography. Neurosci Lett 282: 101–104. [DOI] [PubMed] [Google Scholar]
- Taga G, Asakawa K, Hirasawa K, Konishi Y ( 2003a). Hemodynamic responses to visual stimulation in occipital and frontal cortex of newborn infants: a near‐infrared optical topography study. Early Hum Dev 75( Suppl): S203–S210. [DOI] [PubMed] [Google Scholar]
- Taga G, Asakawa K, Maki A, Konishi Y, Koizumi H ( 2003b): Brain imaging in awake infants by near‐infrared optical topography. Proc Natl Acad Sci USA 100: 10722–10227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Ogata S, Atsumi Y, Yamamoto R, Shiotsuka S, Maki A, Yamashita Y, Yamamoto T, Koizumi H, Hirasawa H, Igawa M ( 2000): Activation of the visual cortex imaged by 24‐channel near‐infrared spectroscopy. J Biomed Opt 5: 93–96. [DOI] [PubMed] [Google Scholar]
- Toronov V, Webb A, Choi JH, Wolf M, Michalos A, Gratton E, Hueber D ( 2001): Investigation of human brain hemodynamics by simultaneous near‐infrared spectroscopy and functional magnetic resonance imaging. Med Phys 28: 521–527. [DOI] [PubMed] [Google Scholar]
- Villringer A, Chance B ( 1997): Non‐invasive optical spectroscopy and imaging of human brain function. Trends Neurosci 20: 435–442. [DOI] [PubMed] [Google Scholar]
- Villringer A, Planck J, Hock C, Schleinkofer L, Dirnagl U ( 1993): Near infrared spectroscopy (NIRS): A new tool to study hemodynamic changes during activation of brain function in human adults. Neurosci Lett 154: 101–104. [DOI] [PubMed] [Google Scholar]
- Wolf M, Wolf U, Choi JH, Toronov V, Paunescu LA, Michalos A, Gratton E ( 2003): Fast cerebral functional signal in the 100‐ms range detected in the visual cortex by frequency‐domain near‐infrared spectrophotometry. Psychophysiology 40: 521–528. [DOI] [PubMed] [Google Scholar]
- Wyatt JS, Cope M, Delpy DT, van der Zee P, Arridge S, Edwards AD, Reynolds EO ( 1990): Measurement of optical path length for cerebral near‐infrared spectroscopy in newborn infants. Dev Neurosci 12: 140–144. [DOI] [PubMed] [Google Scholar]
- Yamada H, Sadato N, Konishi Y, Kimura K, Tanaka M, Yonekura Y, Ishii Y ( 1997): A rapid brain metabolic change in infants detected by fMRI. Neuroreport 8: 3775–3778. [DOI] [PubMed] [Google Scholar]
- Yamada H, Sadato N, Konishi Y, Muramoto S, Kimura K, Tanaka M, Yonekura Y, Ishii Y, Itoh H ( 2000): A milestone for normal development of the infantile brain detected by functional MRI. Neurology 55: 218–223. [DOI] [PubMed] [Google Scholar]
- Zaramella P, Freato F, Amigoni A, Salvadori S, Marangoni P, Suppjei A, Schiavo B, Chiandetti L ( 2001): Brain auditory activation measured by near‐infrared spectroscopy (NIRS) in neonates. Pediatr Res 49: 213–219. [DOI] [PubMed] [Google Scholar]


