Abstract
Evidence suggests that motor, sensory, and cognitive training modulates brain structures involved in a specific practice. Functional neuroimaging revealed key brain structures involved in dancing such as the putamen and the premotor cortex. Intensive ballet dance training was expected to modulate the structures of the sensorimotor network, for example, the putamen, premotor cortex, supplementary motor area (SMA), and the corticospinal tracts. We investigated gray (GM) and white matter (WM) volumes, fractional anisotropy (FA), and mean diffusivity (MD) using magnetic resonance‐based morphometry and diffusion tensor imaging in 10 professional female ballet dancers compared with 10 nondancers. In dancers compared with nondancers, decreased GM volumes were observed in the left premotor cortex, SMA, putamen, and superior frontal gyrus, and decreased WM volumes in both corticospinal tracts, both internal capsules, corpus callosum, and left anterior cingulum. FA was lower in the WM underlying the dancers' left and right premotor cortex. There were no significant differences in MD between the groups. Age of dance commencement was negatively correlated with GM and WM volume in the right premotor cortex and internal capsule, respectively, and positively correlated with WM volume in the left precentral gyrus and corpus callosum. Results were not influenced by the significantly lower body mass index of the dancers. The present findings complement the results of functional imaging studies in experts that revealed reduced neural activity in skilled compared with nonskilled subjects. Reductions in brain activity are accompanied by local decreases in GM and WM volumes and decreased FA. Hum Brain Mapp, 2010. © 2009 Wiley‐Liss, Inc.
Keywords: structural neuroplasticity, practice, sports, voxel‐based morphometry, diffusion tensor imaging, ballet dance
INTRODUCTION
Dance is a universal human activity characterized by complex auditory processing of musical stimuli and on‐line synchronization of coordinated body movements according to the perceived auditory patterns. The latter underscores the importance of spatial cognition in dance: Dancers need to continuously track the spatial position of the torso and limbs and do so mainly on the basis of proprioceptive information from the muscles' sensory organs. The complex choreographies particularly in ballet dancing also mean that the spatial perception of the other dancers of the ensemble is vital, for which however the visual sense plays the predominating role.
Human dance has received little attention in neuroscientific research, although the training workload particularly of professional ballet dancers is comparable to that of well researched professional musicians. Several recent studies have addressed some of the functional aspects associated with dancing [Brown et al., 2006; Calvo‐Merino et al., 2005, 2006; Cross et al., 2006]. But developing a complete picture of the neural circuits underlying human dance presents a challenging because of the spatial constraints of the neuroimaging devices and of the sensitivity of these for movement artifacts. Although some work has been carried out in the domain of functional plasticity, there are no studies of structural brain alterations resulting from long‐term intensive dance training.
Structural magnetic resonance imaging (MRI) based on T1‐weighted and diffusion‐weighted pulse sequences combined with computational neuromorphometric procedures have proven to be a valuable methodology for the investigation of the diseased as well as the healthy brain [Ashburner et al., 2003; Chiang et al., 2009; Mori and Zhang, 2006; Thompson et al., 2004]. There is strong evidence from cross‐sectional structural imaging studies that sensory, motor, and cognitive training modulates brain morphology and that these alterations can be measured using structural MRI [Jäncke, 2009a, 2009b; May and Gaser, 2006]. In addition to the seminal work on neuroplasticity done by Ramachandran and colleagues on patients with amputated extremities [Ramachandran, 2005], there are studies that report the influence of decreased and increased sensorimotor practice on brain gray matter (GM) morphology using structural MRI and voxel‐based morphometry (VBM) [Bandettini, 2009; Draganski and May, 2008; May and Gaser, 2006; Peper et al., 2007].
Findings of alterations in white matter (WM) architecture are less consistent. Fractional anisotropy (FA) values, which have been suggested to reflect the extent of fiber integrity [Assaf and Pasternak, 2008; Gulani and Sundgren, 2006], appear to be altered in professional individuals. However, there is an ongoing debate about the direction of the observed effects [Bengtsson et al., 2005; Han et al., 2008; Imfeld et al., 2009; Jäncke et al., 2009; Schmithorst and Wilke, 2002]; that is, whether these structural alterations are the cause or the effect of the exceptional behaviors observed in experts is not entirely answered yet. However, the significant correlations between the magnitude of the specific structural alterations on the one hand and proficiency, time point of training commencement, and training intensity on the other hand suggest that these alterations represent practice‐induced effects [Aydin et al., 2007; Bengtsson et al., 2005; Cannonieri et al., 2007; Maguire et al., 2000].
Effects of physical exercise on brain structure have been reported by a few short‐term longitudinal studies in which untrained subjects learned how to juggle [Boyke et al., 2008; Draganski et al., 2004; Driemeyer et al., 2008]. A general finding of all of these studies was an increase in GM density in the human visual motion processing area (V5) as a consequence of the 3‐months juggling training and a GM density decrease after termination of training. Another study of the same group provided evidence that brain anatomy is also sensitive to short‐term learning in the cognitive domain [Draganski et al., 2006]. This study revealed that learning is associated with increases as well as decreases of GM density in relevant areas. So far, anatomical adaptations in specific athlete groups have rarely been subject to cross‐sectional investigation. In a new study of our group, anatomical alterations were found in the brain of professional male golfers [Jäncke et al., 2009]. In this study, golf‐proficiency specific GM increases were revealed in a fronto‐parietal network including premotor and parietal areas, while WM volume and FA were lower in the corticospinal tract of golf players as compared with the control group. In general, we can conclude from the present body of literature that practice modulates those features of brain anatomy specifically associated with the demands of practice and that these alterations can be measured with structural MRI‐based neuromorphometric methods.
The present study aimed to reveal structural correlates of intensive ballet dance training. We used 3D structural T1‐weighted MRI to investigate GM and WM volumes in cortical and subcortical components of the sensorimotor network, and diffusion tensor imaging (DTI) was performed to investigate coherence and integrity of the fiber bundles subserving sensorimotor functions. Structural neuroplasticity was expected to express itself in GM and WM alterations as well as in FA and mean diffusivity (MD) changes in brain regions associated with sensorimotor functioning. Based on previous work, we expected higher volumes of GM in the primary motor cortex [Gaser and Schlaug, 2003], premotor cortex, supplementary motor area (SMA), and in the putamen [Brown et al., 2006] of the professional ballet dancers as compared with the control group. With respect to WM volume, FA, and MD, however, increased as well as decreased values for these parameters can be hypothesized from previously published data, especially in the corticospinal tract, internal capsule, and corpus callosum [Bengtsson et al., 2005; Jäncke et al., 2009; Schmithorst and Wilke, 2002].
MATERIALS AND METHODS
Participants
We investigated 20 healthy right‐handed subjects divided into two groups of 10 females each. The experimental group, consisting of 10 dancers (mean/standard deviation: 21.5 ± 3.0 years), was compared with a control group, which comprised 10 females who were matched with respect to age (24.3 ± 5.5 years). All dancers were professional ballet dancers. The mean age of dance commencement was 7.3 ± 2.5 years. The average duration of ballet dance training was 14.2 ± 3.3 years with a mean of 35.8 ± 7.8 training hours per week. Control subjects were only included in the study if they had no experience in dance, figure skating, gymnastics, synchronized swimming, equestrian vaulting, playing act, or doing any competitive sport. Body height and weight were assessed to calculate the body mass index (BMI). The subjects reported no past or current neurological, psychiatric, or neuropsychological problems, and denied to take drugs or illegal medication. Subjects were paid for participation. The local ethics committee approved the study and written informed consent was obtained from all participants.
Imaging Data Acquisition
MRI scans were acquired on a 3.0 T Philips Intera whole body scanner (Philips Medical Systems, Best, The Netherlands) equipped with a transmit‐receive body coil and a commercial eight‐element sensitivity encoding (SENSE) head coil array.
T1‐weighted MRI scans
A volumetric 3D T1‐weighted gradient echo sequence (TFE, turbo field echo) scan was obtained with a measured spatial resolution of 1 × 1 × 1.5 mm (acquisition matrix 224 × 224 pixels, 180 slices) and a reconstructed resolution of 0.86 × 0.86 × 0.75 mm (reconstructed matrix 256 × 256 pixels, 180 slices). Further imaging parameters were: Field of view FOV = 220 × 220 mm, echo‐time TE = 2.3 ms, repetition‐time TR = 20 ms, flip‐angle FA = 20°. Total acquisition time was about 8 min.
Diffusion tensor MRI scans
Diffusion‐weighted spin echo echo‐planar (EPI) sequence scans were obtained with a measured spatial resolution of 2.08 × 2.13 × 2.0 mm (acquisition matrix 96 × 96 pixels, 50 slices) and a reconstructed resolution of 1.56 × 1.56 × 2.0 mm (reconstructed matrix 128 × 128 pixels, 50 slices). Further imaging parameters were: Field of view FOV = 200 × 200 mm, echo‐time TE = 50 ms, repetition‐time TR = 10,166 ms, flip‐angle FA = 90°, SENSE factor R = 2.1, b‐value = 1,000 s/mm2. Diffusion was measured in 15 noncollinear directions followed by a nondiffusion‐weighted volume (reference volume). Total acquisition time was about 15 min.
Voxel‐Based Morphometry
To investigate local GM and WM volumes, we applied VBM [Ashburner and Friston, 2000; Good et al., 2001]. The preprocessing steps are implemented in the VBM5 toolbox (http://dbm.neuro.uni-jena.de/vbm/download/) that uses Statistical Parametric Mapping software (SPM5, http://www.fil.ion.ucl.ac.uk/spm/). For the preprocessing of the structural scans, we used customized a priori maps derived from T1‐weighted images of 125 healthy control females scanned on the same scanner with the same pulse sequence (mean/standard deviation: 25.6/6.6 years) to account for the female population in our study. The following preprocessing steps were realized: (1) The coordinate origin of each native image was manually set on the anterior commissure. (2) Intensity inhomogeneity (bias field) correction, tissue class segmentation, and spatial normalization (affine and warping) were performed using the unified segmentation approach [Ashburner and Friston, 2005] and the canonical a priori maps (ICBM 452 T1‐weighted) implemented in SPM5. (3) To enhance tissue class segmentation, Hidden Markov Random Field (HMRF) modulation was applied [Cuadra et al., 2005; Smith et al., 2006, http://dbm.neuro.uni-jena.de/vbm/markov-random-fields/]. (4) To investigate absolute volumes, we multiplied voxelwise the warped images with the Jacobian determinants of the deformations. (5) For customized a priori map creation unmodulated and segmented GM, WM, and CSF images were averaged separately to get customized a priori maps (spatial resolution 1 × 1 × 1 mm, no smoothing). (6) Steps 2–4 were repeated except that the customized a priori maps were used in Step 2. (7) The resulting Jacobian and HMRF modulated and segmented GM and WM images were smoothed with a Gaussian kernel of FWHM = 9 mm and the additional smoothing introduced during the modulation process was about FWHM = 3 mm.
The two groups were compared with the general linear model implemented in SPM5 software. Global GM and WM volumes were used as nuisance variables in analyses of covariance of local GM and WM volumes, respectively. Although strong a priori hypotheses were posited, the statistical extent threshold was corrected for multiple comparisons combined with a nonstationary smoothness correction [Hayasaka and Nichols, 2004; Hayasaka et al., 2004]. We used cluster extent familywise error (FWE) correction with P = 0.01 and a height threshold of P = 0.001 (uncorrected) for GM and WM volumes. Although our sample size is somewhat smaller than the minimal size proposed by Hayasaka et al. [ 2004], the amount of smoothness in our data is about four times larger than the minimum FWHM kernel size proposed by these authors compensating for some missing degrees of freedom in our study.
Diffusion‐Tensor Imaging
To analyze interconnectivity of sensorimotor areas, measured by means of FA and MD, we preprocessed the diffusion‐weighted images with the tools of Tract‐Based Spatial Statistics (TBSS) [Behrens et al., 2003] using the diffusion toolbox (FDT). This toolbox is part of the FSL [Smith et al., 2006] software implemented in the functional magnetic resonance imaging of the brain (FMRIB) software library (http://www.fmrib.ox.ac.uk/fsl/) to create FA and MD maps. The following steps were realized: (1) Head movement and eddy current correction was applied using FDT. (2) A brain mask of the reference volume (no diffusion) was created using the brain extraction tool. (3) Tensors were fitted to the data using DTIFIT to generate FA and MD maps. (4) From Step 4, all following steps were realized with TBSS. FA and MD maps were scaled and converted. (5) Nonlinear registration of all FA and MD maps into standard space was applied. (6) FA and MD images were smoothed with a Gaussian kernel of FWHM = 12 mm. (7) All voxels with FA or MD values smaller than 20% of the mean FA or average MD, respectively, were excluded from the statistical analyses because we were only interested in the diffusion characteristics of WM tissue.
The statistical group comparison of FA and MD data was performed applying the general linear model implemented in SPM5 software. Mean FA and average MD was modeled as a nuisance variable in the analysis of covariance of local FA and local MD, respectively. Although strong a priori hypotheses were posited, the statistical extent threshold was corrected for multiple comparisons combined with a nonstationary smoothness correction [Hayasaka and Nichols, 2004; Hayasaka et al., 2004]. We used cluster extent FWE correction with P = 0.01 and a height threshold of P = 0.001 (uncorrected) for FA and MD maps.
We used two different spatial registration methods, one for the T1‐weighted images and another one for the DTI data because of the fact that DTI images are slightly geometrically distorted compared with T1‐weighted images. Registering the images of these two MR modalities separately might be more accurate than the more common procedure where the transformations are estimated normally from the T1‐weighted images and then applied to the DTI data. Hence, our results reported here are projected into two different reference spaces, one space for GM and WM volumes, and another one for FA and MD.
RESULTS
Demographic Characteristics and Global Brain Measures
The demographic characteristics, whole brain tissue volumes, and global diffusion properties of the professional female ballet dancers and the control subjects are summarized in Table I. There were no significant differences between ballet dancers and control females with respect to age (P = 0.17), total GM volume (P = 0.26), total WM volume (P = 0.13), cerebrospinal fluid volume (P = 0.44), total intracranial volume (P = 0.24), and average MD (P = 0.92). Mean FA showed a statistical trend (P = 0.08) toward lower FA values in dancers. Compared with control females, the ballet dancers had a significantly lower BMI (mean/sd: 18.7 ± 1.53 in dancers; 21.6 ± 2.32 in controls; P = 0.003) and significantly less years of education (mean/sd: 11.1 ± 1.83 years in dancers; 14.3 ± 2.56 years in controls; P = 0.01).
Table I.
Demographic characteristics and global brain tissue parameters of the professional ballet dancers and the control females
| Variable | Professional ballet dancers | Control subjects | Probability two‐tailed | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | S.D. | Min. | Max. | Mean | S.D. | Min. | Max. | ||
| Age (years) | 21.9 | 3.03 | 18.3 | 27.8 | 24.8 | 5.50 | 18.5 | 37.8 | P = 0.17 |
| Body‐mass index (BMI) | 18.7 | 1.53 | 16.7 | 21.9 | 21.6 | 2.32 | 18.6 | 25.8 | P = 0.003 |
| Education (years) | 11.1 | 1.83 | 9.0 | 13.0 | 14.3 | 2.56 | 12.0 | 22.5 | P = 0.01 |
| Age of dance commencement | 7.3 | 2.50 | 4.0 | 13.0 | — | — | — | — | — |
| Intensity of training (h/week) | 35.8 | 7.83 | 20.0 | 45.0 | — | — | — | — | — |
| Total GM volume (cm3) | 717.5 | 81.21 | 625.5 | 844.8 | 753.9 | 55.65 | 661.4 | 851.7 | P = 0.26 |
| Total WM volume (cm3) | 405.0 | 48.89 | 338.5 | 488.4 | 432.7 | 22.97 | 390.7 | 462.8 | P = 0.13 |
| Total CSF volume (cm3) | 434.6 | 76.58 | 377.5 | 568.1 | 457.0 | 23.99 | 413.1 | 484.3 | P = 0.44 |
| Total IC volume (cm3) | 1566.3 | 152.66 | 1318.1 | 1741.6 | 1639.1 | 77.19 | 1558.0 | 1764.2 | P = 0.24 |
| Mean fractional anisotropy | 0.141 | 0.0041 | 0.130 | 0.145 | 0.144 | 0.0050 | 0.138 | 0.156 | P = 0.08 |
| Average mean diffusivity (mm2/s) | 0.000917 | 0.000026 | −0.0042 | 0.0046 | 0.000916 | 0.000029 | −0.0041 | 0.0046 | P = 0.92 |
There were significant differences in body‐mass index and years of education as well as a trend toward significance (0.05 < P < 0.1) in mean fractional anisotropy between the groups revealed by t‐tests for independent samples. Note that the minima of average mean diffusivity are negative because the tool used to compute these values does not impose that the tensors are positive.
CSF, cerebrospinal fluid; GM, gray matter; IC, intracranial; Max., maximum; Min., minimum; S.D., standard deviation; WM, white matter.
Voxelwise Group Comparisons
Given the lower BMI values in the ballet dancer group—a finding that is in line with previous studies [Stokic et al., 2005]—and that nutrition/malnutrition itself has been shown to have an impact on brain structure [Isaacs et al., 2008; Pannacciulli et al., 2006; Taki et al., 2008], we controlled for the BMI in two additional analyses. We regressed voxelwise the BMI against GM and WM volumes as well as against FA and MD, and added the BMI as a further nuisance variable to our ANCOVA models. However, the BMI neither affected the GM and WM volumes nor the FA and MD as revealed by both regressions and ANCOVA models. Hence, we did not include the BMI as a covariate in our statistical models with the effect of preserving statistical power.
The resulting voxelwise statistical group comparisons revealed clusters with decreased GM and WM volumes, and decreased FA in areas associated with sensorimotor functions in the brains of professional ballet dancers as compared with the control group (Fig. 1 and Table II). In dancers compared with nondancers, decreased GM volumes were found exclusively in the left hemisphere in the premotor cortex, the SMA, the putamen, and in the superior frontal gyrus, the latter cluster is located anterior to the premotor cortex. Decreased WM volumes were observed in both corticospinal tracts, both internal capsules, the corpus callosum, and in the left anterior cingulum of the dancers compared with nondancers. FA was lower in the WM underlying the dancers' left and right premotor cortex, left middle frontal gyrus, and right frontal operculum. There were no significant MD differences between the two groups.
Figure 1.
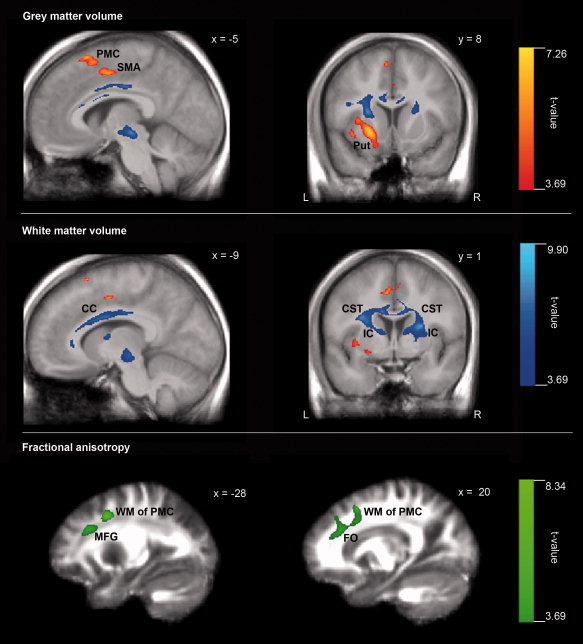
Local structural brain differences between professional female ballet dancers and control females. Decreased gray matter (GM) volume in ballet dancers are shown in red, decreased white matter (WM) volume in blue, and decreased fractional anisotropy (FA) in green. Statistical parametric maps were height‐thresholded with P = 0.001 (uncorrected) and cluster extent familywise error (FWE) corrected with P = 0.01. Additionally, clusters were corrected for nonstationarity of smoothness. Note that the size of the spots in Figure 1 deviates from the cluster size reported in Table II. This difference results from the nonstationary smoothness correction that is only implemented for the number (list) of clusters yet. The correction of the statistical image, that is, the maximum intensity projection itself, is not still possible due to technical problems in its implementation; hence the uncorrected cluster sizes are shown in the figure. L, left; R, right; x,y, x‐ and y‐coordinate in Montreal Neurological Institute (MNI) space.
Table II.
Local structural brain differences between professional female ballet dancers and control females
| Measure (contrast) | Anatomical location | Hem. | MNI coordinates | Number of voxels (k) | t‐value (df = 16) FWE = 0.01 | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Gray matter volume | Premotor cortex (PMC) | left | −7 | 18 | 62 | 3,063 | 7.26 |
| (dancers < controls) | left | −5 | 12 | 55 | 5.28 | ||
| left | −4 | 24 | 57 | 4.81 | |||
| Putamen (Put) | left | −22 | 8 | −5 | 5,177 | 7.15 | |
| left | −34 | 4 | 0 | 5.51 | |||
| left | −37 | 11 | −8 | 4.84 | |||
| Supplementary motor area (SMA) | left | −6 | −2 | 46 | 2,161 | 6.49 | |
| right | 1 | 9 | 38 | 4.13 | |||
| right | 5 | 2 | 52 | 4.11 | |||
| Superior frontal gyrus (SFG) | left | −13 | 28 | 45 | 3,224 | 6.02 | |
| left | −24 | 30 | 43 | 4.91 | |||
| left | −28 | 38 | 40 | 4.44 | |||
| White matter volume | Corticospinal tracts (CST) extending | left | −27 | −10 | 14 | 39,268 | 9.90 |
| (dancers < controls) | into both internal capsules (IC) and | right | 25 | −5 | 10 | 8.89 | |
| corpus callosum (CC) | left | −44 | −5 | 29 | 7.92 | ||
| Anterior cingulum (Cing) | left | −15 | 35 | 30 | 2,726 | 8.11 | |
| left | −16 | 25 | 35 | 5.10 | |||
| Fractional anisotropy | Premotor cortex* (WM of PMC) | left | −28 | 7 | 42 | 4,771 | 8.34 |
| (dancers < controls) | Premotor cortex* (WM of PMC) | right | 20 | 16 | 50 | 8,417 | 6.27 |
| right | 18 | 32 | 30 | 5.99 | |||
| right | 23 | 16 | 40 | 4.84 | |||
| Middle frontal gyrus (MFG) | left | −31 | 29 | 29 | 3,119 | 5.61 | |
| left | −18 | 29 | 28 | 4.26 | |||
| Frontal operculum (FO) | right | 36 | 24 | 13 | 3,140 | 4.80 | |
| right | 33 | 38 | 10 | 4.55 | |||
| right | 36 | 16 | 12 | 4.52 | |||
Statistical parametric maps were height‐thresholded with P = 0.001 (uncorrected) and cluster extent familywise error (FWE) corrected with P = 0.01. Additionally, clusters were corrected for nonstationarity of smoothness. Note that the size of the spots in Figure 1 deviates from the cluster size reported in Table II. This difference results from the nonstationary smoothness correction that is only implemented for the number (list) of clusters yet. The correction of the statistical image, i.e., the maximum intensity projection itself, is not still possible due to technical problems in its implementation; hence the uncorrected cluster sizes are shown in the figure.
df, degrees of freedom; FWE, familywise error cluster extent correction; Hem., hemisphere; MNI; Montreal Neurological Institute; P, error probability.
White matter underlying premotor cortex.
There were no clusters neither with significantly increased GM or WM volumes nor with increased FA and decreased MD in professional ballet dancers compared with control females. Additionally, age of dance commencement was negatively correlated with GM and WM volume in the right premotor cortex and internal capsule, respectively, and positively correlated with WM volume in the left precentral gyrus and corpus callosum. No significant correlations were found neither between age of commencement and FA/MD values nor between years of dance training and any of the morphological measures.
Note that the size of the spots in Figure 1 deviates from the cluster size reported in Table II. This difference results from the nonstationary smoothness correction that is only implemented for the list of clusters. At the moment, a correction for the statistical parametric maps (i.e., the maximum intensity projection) is not yet possible due to technical problems in its implementation.
DISCUSSION
As hypothesized, we found differences in structural characteristics within the sensorimotor neural network between professional female ballet dancers and a control group. Concerning the direction of these structural alterations, our finding is surprising in so far as that compared with the control group; the professional ballet dancers revealed locally restricted decreases in GM and WM volume as well as decreases in FA in areas associated with motor brain functions. In dancers compared with nondancers, decreased GM volumes were found exclusively in the left hemisphere in the premotor cortex, the SMA, the putamen, and in the superior frontal gyrus anterior to the premotor cortex. Decreased WM volumes were observed in both corticospinal tracts, both internal capsules, the corpus callosum, and in the left anterior cingulum of dancers compared with nondancers,. FA was lower in the WM underlying the dancers' left and right premotor cortex. Age of dance commencement was negatively correlated with GM and WM volume in the right premotor cortex and internal capsule, respectively, and positively correlated with WM volume in the left precentral gyrus and corpus callosum. In contrast to the group comparisons that are based on 20 subjects, the correlations are based only on the 10 dancers. With respect to the topology of the clusters found in the group comparison and the clusters revealed by the correlations, there is a minimal overlap between the results of both analyses. This overlap is restricted to several WM voxels located in the internal capsule and the corpus callosum.
It is worth noting that the structural brain differences found in the present study are in line with the results of the first functional imaging study in dancers. Brown et al. used positron emission tomography (PET) to investigate the neural correlates of human dance such as the entrainment of dance steps to music, motions to metric rhythm, and spatial cognitions related to dance. This study revealed strong bilateral activations in amateur tango dancers in the putamen when motions to metric rhythm were contrasted with a rest condition as well as when they were contrasted with a motions to nonmetric rhythm condition. This suggests the involvement of the putamen in the voluntary control of metric movements [Brown et al., 2006], which become highly automated in professional dancers. Therefore, the structural alterations in the putamen found bilaterally in our study may be related to greater expertise of the professional ballet dancers in metric motions compared with females without any dancing experience. Furthermore, contrasting the metric dance condition with rest revealed, in addition to the activation in the putamina, activations in the primary motor cortex, the premotor cortex, and the SMA and CMA [Brown et al., 2006]. Taken together, this PET finding proves that these brain structures are really involved in human dance. Hence, the structurally altered sensorimotor brain structures in ballet dancers, which were found in the present study, might represent the neural correlates of increased performance in organizing body movements into spatial patterns, controlling the body posture precisely, synchronizing their movements with regular and irregular rhythms, integrating proprioceptive information from several muscles and joints in order to generate a representation of the body in space, and in coordinating the body nearly perfectly, ranging from gross to very precise fine motor movements. These skills are central in ballet dancing and are therefore intensively trained.
Training‐Induced Modulation of Gray and White Matter Volume
Decreased GM volumes in sensorimotor areas of dancers are unexpected findings. The majority of studies in the research field of structural neuroplasticity provided strong evidence for a positive correlation between motor or cognitive performance and GM density/volume [Aydin et al., 2007; Boyke et al., 2008; Cannonieri et al., 2007; Draganski et al., 2004, 2006; Gaser and Schlaug, 2003], whereas only very few studies reported the inverse relationship, that is, better performance associated with smaller GM volumes [Draganski et al., 2006; Maguire et al., 2000]. The reasons for our discrepant findings are difficult to explain given current knowledge about structural neuroplasticity. Several suggestions are made here.
Based on what has been learned from functional studies, skillfulness in a particular domain is associated with reduced neural activity (compared to nonskilled subjects) in brain areas involved in the control of the expertise task [Del Percio et al., 2008; Haslinger et al., 2004; Jancke et al., 2000; Krings et al., 2000; Meister et al., 2005]. One could hypothesize that this expertise‐related reduction of neurophysiological activation is accompanied by a local reduction of GM and WM densities after years of practice. Although not demonstrated yet, it is nevertheless possible that the establishment of optimal neurophysiological activation patterns is paralleled by an optimization of the underlying hardware, for example, by pruning back the hardware to the essential synapses, dendrites and axonal connections. But recent findings are not entirely reconcilable with this idea. The study by Gaser and Schlaug [ 2003] reported a positive correlation between musicianship (which is related to practice hours) and GM densities in particular brain areas, and several further studies have demonstrated that the amount of time spent for practicing the expert task is positively correlated with GM density [Aydin et al., 2007; Cannonieri et al., 2007; Maguire et al., 2000; Mechelli et al., 2004].
A further possibility is that practice‐induced anatomical alterations depend on the stage of practicing. It may be, for instance, that anatomical changes are present during early practice stages while no further alterations take place at later practice stages. There may even be a retroregression at later stages for the reasons mentioned above. There are indeed a few studies that at least partly support this notion. For example, a recent study by Driemeyer et al. [ 2008] demonstrated GM increases during early stages of juggling practice with no further GM increase after a particular time point. In addition, professional golfers and nonprofessional golfers did not differ in terms of anatomical measures, although the professional golfers practiced about eight times more [Jäncke et al., 2009]. On the other hand, strong differences were revealed between nonprofessional golfers and novice golfers—supporting the idea that strong alterations are induced at early training stages. Even though a stagnation of structural adaptations may be evident in later training stages, so far there is no support from previous literature for a decrease in GM volume that falls even below baseline.
A third possibility not yet subject to discussion is that of interactions between task characteristics and the induced anatomical changes. Anatomical changes may be induced by practice of one particular task, whereas another task may not induce any changes at all. It is also conceivable that practice in one task leads to GM increases, while practice in another tasks results in GM decreases. These interactions may be further complicated by variables such as duration of practice, stage of practice, kind of training strategy (massed vs. distributed), or other biological circumstances influencing the training of a particular task. Currently, there are no data available that would help to disentangle these potential influences on structural brain alterations in the context of practice. But from a neurophysiological and neuroanatomical point of view, inter‐regional differences in practice‐induced plasticity merit further considerations. Different brain regions show substantial differences in terms of the macro‐ and micro‐structure (e.g., relative size of the different layers in cortical columns; different parts included in the columns, etc.) [Geyer, 2004], and can thus be regarded as functionally different modules.
For the expertise group studied here (ballet dancers), we suggest that the kind of training and the particular biological circumstances associated with practice are the most plausible variables in explaining the unexpected changes of GM volume. All dancers started early in life with ballet training. From this early age on, dancers are known to actively control their body weight and shape by means on specific diets. This results in lower BMI values as compared with controls [Stokic et al., 2005; van Marken Lichtenbelt et al., 1995], a finding that we also observed in our study. The BMI values calculated for the dancers in this study are distributed around the lower margin of BMI values regarded as being normal and close to BMI values reported for females with anorexia nervosa [Connan et al., 2006; Suchan et al., 2009], although a lower mean BMI was reported in other anorexia nervosa studies [e.g., Olmos et al., 2009; Yamashita et al., 2009]. It is important to note that our ballet dancers are considered as a group of athletes with exceptional and unique motor and sensory abilities and not as a subclinical population. With respect to the body height, there were no significant differences between the dancers and nondancers, that is, the difference in BMI is driven solely by the weight. Therefore, we also analyzed the influence of the weight individually (in addition to the BMI) and found no weight‐effects on GM, WM, FA, and MD, neither when weight was used as an additional covariate in the ANCOVA models (group comparisons) nor in regression analyses.
There are some studies reporting small, but partly significant cerebral atrophies in underweighted subjects [Dolan et al., 1988; Krieg et al., 1988, 1989; Swayze et al., 1996, 2003]. However, these studies did not report local anatomical changes related to being underweight except for one recently published study that found reduced bilateral hippocampal volume in females with anorexia nervosa [Connan et al., 2006]. Thus, it is unlikely that the almost underweight dancers show reduced anatomical measures simply due to their specific diet history. But, it is possible that the diet‐specific influences on brain maturation and plasticity have interacted with the particular training influences in specifically those brain areas involved in the control of ballet dancing. Given the complexity of such interaction effects between malnutrition and training on brain anatomy, including the BMI as a linear covariate in our statistical models may have been not sensitive enough to detect such influences if they really exist.
A further possible explanation is related to the characteristics of our control group. The intense dancing practice ballet dancers start off with very early in life most likely affects classical schooling. As a matter of fact, our control subjects were enrolled slightly (but significantly) longer in educational programs. Some of the brain areas for which we found significant between‐group differences are not only related to sensorimotor control processes, but also to the neural control of higher cognitive functions. The thickness of the dorsal frontal cortex, for instance, has been shown to correlate strongly with measured intelligence in children and adolescents [Lerch et al., 2006; Shaw et al., 2006]. The anterior prefrontal cortex, on the other hand, is involved in prospective memory and generating plans for upcoming behavior [Reynolds et al., 2008]. The cingulum and the SMA have also been associated with various cognitive control processes such as attention switching and supervisory control [Alexander et al., 2007; Zastrow et al., 2009]. Finally, the hippocampus is known to be the main bottleneck structure for explicit memory processes [Eichenbaum, 2001]. Since we know from recent research that even a 3‐month long preparation for medical exams causes structural alterations in the cortical architecture (including the hippocampus) [Draganski et al., 2006], it is possible that the shorter duration of school education accounts for the reduced GM volumes in this brain area.
Based on the negative correlations between age of dance commencement and GM and WM volumes found in the right premotor cortex and internal capsule, respectively, our data suggest that the detected anatomical GM and WM volume differences are a consequence of the relatively long and intensive experience of being a professional dancer and of the concomitant circumstances, and do not support the idea of genetically driven anatomical predispositions for particular traits.
Training‐Induced Modulation of Fractional Anisotropy
There is considerable discrepancy with respect to FA values in the context of specific behavioral expertise [Bengtsson et al., 2005; Han et al., 2008; Imfeld et al., 2009; Jäncke et al., 2009; Schmithorst and Wilke, 2002]. The studies by Schmithorst and Wilke [ 2002] and Bengtsson et al. [ 2005] have reported both decreased and increased FA values to be associated with more skilled behavior. Also, in the study of Bengtsson et al. it is puzzling that a positive correlation between the amount of piano practice and FA is reported along with generally lower mean FA values in skilled pianist as compared with controls. The reasons for these inconsistent results are manifold and this issue has attracted little further discussion. FA reflects the proportion of axial and radial diffusion of water molecules in neural WM. High FA values are measured in case of strong axial water diffusion (diffusion along the WM fibers). Therefore, reduced FA values in the group of dancers as compared with the control group suggest (1) increased radial diffusion, or (2) decreased axial diffusion, or (3) a mix of both. Increased radial diffusion would indicate between‐group differences with respect to myelin and axonal membrane structure [Beaulieu, 2002], for example, increases in membrane permeability for water molecules. Also, the axonal diameter has been shown to influence radial diffusion values (larger axonal diameter is associated with stronger radial diffusion) [Beaulieu, 2002]. Decreased axial diffusion is attributed to the growth of axonal neurofibrils, such as microtubules and neurofilaments [Kinoshita et al., 1999], which normally occurs during development [Haynes et al., 2005] but may also play a role in the context of practice.
Reduced FA, that is less axial and/or more radial diffusion, can be observed in cases where several fibers cross, bend, or twist within an individual imaging voxel. DTI can only resolve a single fiber orientation within each voxel due to the constraints of the tensor model. Diffusion spectrum imaging (DSI, also called q‐space or q‐ball imaging) is able to resolve several fiber directions within an individual voxel [Tuch, 2004]. Therefore, DSI will help to shed light on the question of whether reduced FA in sensorimotor‐related brain regions of sensorimotor experts, as observed in the present study, reflect increased fiber crossing, bending, and/or twisting. Whether (increased) crossing, bending, and/or twisting of fibers can be considered as features of (increased) connectivity are still unknown and needs to be investigated in the future, preferentially in an animal model.
But given that the detailed mechanisms underlying the modulation of directional diffusion coefficients are only insufficiently understood, our considerations are speculative. To resolve this controversial issue, further research is needed to clarify the influence of practice on the individual WM components and the influence of these components on FA and on diffusion characteristics in general.
Different Imaging Modalities, Cellular Events, and Physiological Consequences
When we compared the regions that showed FA alterations with the regions that showed WM volume changes, the following topological features are recognized. There is a minimal overlap in the regions that showed WM and FA differences. Generally, the regions with FA changes are predominantly located below the cortical sheet, that is, immediately below the transition zone between GM and WM, whereas the WM volume changes are located predominantly in regions where compact WM tissue occurs, that is, in the semioval center. However, this pattern of WM alterations we observed in the present study should be interpreted cautiously and need further investigations.
The macroscopic changes may be attributed to an increase in cell size, synaptogenesis, genesis of glial or even neural cells, or changes in spine density, blood flow, or interstitial fluid. Our interpretations regarding molecular and cellular events underlying the observed effects are in part speculative. However, given that current knowledge regarding cellular and physiological mechanisms is indeed marginal, it is simply impossible to provide convincing micro‐scale explanations on the basis of our MR signal. This knowledge is necessary in order to make inferences about the possible physiological consequences of these molecular and cellular alterations. Still, the alternative explanations we offer in the discussion section are derived from previous research and even if we cannot prove that these explanations are true, they may at least be viewed as hypotheses to be tested in future studies.
CONCLUSION
Here, we demonstrate that professional ballet dancers show anatomical differences compared with nondancers in brain regions involved in motor control processes. However, although these anatomical differences are clearly evident on a macrostructural level, the microstructural underpinnings as well as the physiological consequences of these alterations are still unknown and cannot be investigated by structural neuroimaging. Further experiments are needed to compare imaging results with histological data for identification of the structural basis of these (possibly) training‐dependent structural changes, both at the microscopic and macroscopic levels. This would provide the basis for more substantial interpretation of findings obtained with “coarse” methods such as MRI. One example of a paradigm would be to study mice or rats before and after acquiring specific skills and to examine whether any histological changes in their brains correlate with anatomical features in their brains as measured with high‐resolution structural MRI.
Acknowledgements
The authors thank the professional ballet dancers and the control subjects for participating in their study and they acknowledge the help by Jacqueline Kamm in data acquisition.
REFERENCES
- Alexander MP, Stuss DT, Picton T, Shallice T, Gillingham S ( 2007): Regional frontal injuries cause distinct impairments in cognitive control. Neurology 68: 1515–1523. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ ( 2000): Voxel‐based morphometry—The methods. Neuroimage 11( 6 Part 1): 805–821. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ ( 2005): Unified segmentation. Neuroimage 26: 839–851. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Csernansk JG, Davatzikos C, Fox NC, Frisoni GB, Thompson PM ( 2003): Computer‐assisted imaging to assess brain structure in healthy and diseased brains. Lancet Neurol 2: 79–88. [DOI] [PubMed] [Google Scholar]
- Assaf Y, Pasternak O ( 2008): Diffusion tensor imaging (DTI)‐based white matter mapping in brain research: A review. J Mol Neurosci 34: 51–61. [DOI] [PubMed] [Google Scholar]
- Aydin K, Ucar A, Oguz KK, Okur OO, Agayev A, Unal Z, Yilmaz S, Ozturk C ( 2007): Increased gray matter density in the parietal cortex of mathematicians: A voxel‐based morphometry study. AJNR Am J Neuroradiol 28: 1859–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandettini PA ( 2009): What's new in neuroimaging methods? Ann N Y Acad Sci 1156 (The Year in Cognitive Neuroscience 2009): 260–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C ( 2002): The basis of anisotropic water diffusion in the nervous system—A technical review. NMR Biomed 15: 435–455. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, Johansen‐Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM ( 2003): Characterization and propagation of uncertainty in diffusion‐weighted MR imaging. Magn Reson Med 50: 1077–1088. [DOI] [PubMed] [Google Scholar]
- Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullen F ( 2005): Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci 8: 1148–1150. [DOI] [PubMed] [Google Scholar]
- Boyke J, Driemeyer J, Gaser C, Buchel C, May A ( 2008): Training‐induced brain structure changes in the elderly. J Neurosci 28: 7031–7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S, Martinez MJ, Parsons LM ( 2006): The neural basis of human dance. Cereb Cortex 16: 1157–1167. [DOI] [PubMed] [Google Scholar]
- Calvo‐Merino B, Glaser DE, Grezes J, Passingham RE, Haggard P ( 2005): Action observation and acquired motor skills: An FMRI study with expert dancers. Cereb Cortex 15: 1243–1249. [DOI] [PubMed] [Google Scholar]
- Calvo‐Merino B, Grezes J, Glaser DE, Passingham RE, Haggard P ( 2006): Seeing or doing? Influence of visual and motor familiarity in action observation. Curr Biol 16: 1905–1910. [DOI] [PubMed] [Google Scholar]
- Cannonieri GC, Bonilha L, Fernandes PT, Cendes F, Li LM ( 2007): Practice and perfect: Length of training and structural brain changes in experienced typists. Neuroreport 18: 1063–1066. [DOI] [PubMed] [Google Scholar]
- Chiang M‐C, Barysheva M, Shattuck DW, Lee AD, Madsen SK, Avedissian C, Klunder AD, Toga AW, McMahon KL, de Zubicaray GI, Wright MJ, Srivastava A, Balov N, Thompson PM ( 2009): Genetics of brain fiber architecture and intellectual performance. J Neurosci 29: 2212–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connan F, Murphy F, Connor SE, Rich P, Murphy T, Bara‐Carill N, Landau S, Krljes S, Ng V, Williams S, Morris RG, Campbell IC, Treasure J ( 2006): Hippocampal volume and cognitive function in anorexia nervosa. Psychiatry Res 146: 117–125. [DOI] [PubMed] [Google Scholar]
- Cross ES, Hamilton AF, Grafton ST ( 2006): Building a motor simulation de novo: Observation of dance by dancers. Neuroimage 31: 1257–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadra MB, Cammoun L, Butz T, Cuisenaire O, Thiran JP ( 2005): Comparison and validation of tissue modelization and statistical classification methods in T1‐weighted MR brain images. IEEE Trans Med Imaging 24: 1548–1565. [DOI] [PubMed] [Google Scholar]
- Del Percio C, Rossini PM, Marzano N, Iacoboni M, Infarinato F, Aschieri P, Lino A, Fiore A, Toran G, Babiloni C, Eusebi F ( 2008): Is there a “neural efficiency” in athletes? A high‐resolution EEG study. Neuroimage 42: 1544–1553. [DOI] [PubMed] [Google Scholar]
- Dolan RJ, Mitchell J, Wakeling A ( 1988): Structural brain changes in patients with anorexia nervosa. Psychol Med 18: 349–353. [DOI] [PubMed] [Google Scholar]
- Draganski B, May A ( 2008): Training‐induced structural changes in the adult human brain. Behav Brain Res 192: 137–142. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A ( 2004): Neuroplasticity: Changes in grey matter induced by training. Nature 427: 311–312. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Kempermann G, Kuhn HG, Winkler J, Buchel C, May A ( 2006): Temporal and spatial dynamics of brain structure changes during extensive learning. J Neurosci 26: 6314–6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driemeyer J, Boyke J, Gaser C, Buchel C, May A ( 2008): Changes in gray matter induced by learning—Revisited. PLoS One 3: e2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H ( 2001): The hippocampus and declarative memory: Cognitive mechanisms and neural codes. Behav Brain Res 127: 199–207. [DOI] [PubMed] [Google Scholar]
- Gaser C, Schlaug G ( 2003): Brain structures differ between musicians and non‐musicians. J Neurosci 23: 9240–9245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer S ( 2004): The Microstructural Border Between the Motor and the Cognitive Domain in the Human Cerebral Cortex. Berlin: Springer. [DOI] [PubMed] [Google Scholar]
- Good CD, Ashburner J, Frackowiak RS ( 2001): Computational neuroanatomy: New perspectives for neuroradiology. Rev Neurol (Paris) 157( 8–9 Part 1): 797–806. [PubMed] [Google Scholar]
- Gulani V, Sundgren PC ( 2006): Diffusion tensor magnetic resonance imaging. J Neuroophthalmol 26: 51–60. [DOI] [PubMed] [Google Scholar]
- Han Y, Yang H, Lv YT, Zhu CZ, He Y, Tang HH, Gong QY, Luo YJ, Zang YF, Dong Q ( 2008): Gray matter density and white matter integrity in pianists' brain: A combined structural and diffusion tensor MRI study. Neurosci Lett 459: 3–6. [DOI] [PubMed] [Google Scholar]
- Haslinger B, Erhard P, Altenmuller E, Hennenlotter A, Schwaiger M, Grafin von Einsiedel H, Rummeny E, Conrad B, Ceballos‐Baumann AO ( 2004): Reduced recruitment of motor association areas during bimanual coordination in concert pianists. Hum Brain Mapp 22: 206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayasaka S, Nichols TE ( 2004): Combining voxel intensity and cluster extent with permutation test framework. Neuroimage 23: 54–63. [DOI] [PubMed] [Google Scholar]
- Hayasaka S, Phan KL, Liberzon I, Worsley KJ, Nichols TE ( 2004): Nonstationary cluster‐size inference with random field and permutation methods. Neuroimage 22: 676–687. [DOI] [PubMed] [Google Scholar]
- Haynes RL, Borenstein NS, Desilva TM, Folkerth RD, Liu LG, Volpe JJ, Kinney HC ( 2005): Axonal development in the cerebral white matter of the human fetus and infant. J Comp Neurol 484: 156–167. [DOI] [PubMed] [Google Scholar]
- Imfeld A, Oechslin MS, Meyer M, Loenneker T, Jancke L ( 2009): White matter plasticity in the corticospinal tract of musicians: A diffusion tensor imaging study. Neuroimage 46: 600–607. [DOI] [PubMed] [Google Scholar]
- Isaacs EB, Gadian DG, Sabatini S, Chong WK, Quinn BT, Fischl BR, Lucas A ( 2008): The effect of early human diet on caudate volumes and IQ. Pediatr Res 63: 308–314. [DOI] [PubMed] [Google Scholar]
- Jäncke L ( 2009a): The plastic human brain. Restor Neurol Neurosci 27: 1–18. [DOI] [PubMed] [Google Scholar]
- Jäncke L ( 2009b): Music drives brain plasticity. F1000 Biol Reports doi:10.3410/B1‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäncke L, Shah NJ, Peters M ( 2000): Cortical activations in primary and secondary motor areas for complex bimanual movements in professional pianists. Brain Res 10: 177–183. [DOI] [PubMed] [Google Scholar]
- Jäncke L, Koeneke S, Hoppe A, Rominger C, Hänggi J ( 2009): The architecture of the Golfer's brain. PLoS One 4: e4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita Y, Ohnishi A, Kohshi K, Yokota A ( 1999): Apparent diffusion coefficient on rat brain and nerves intoxicated with methylmercury. Environ Res 80: 348–354. [DOI] [PubMed] [Google Scholar]
- Krieg JC, Pirke KM, Lauer C, Backmund H ( 1988): Endocrine, metabolic, and cranial computed tomographic findings in anorexia nervosa. Biol Psychiatry 23: 377–387. [DOI] [PubMed] [Google Scholar]
- Krieg JC, Lauer C, Leinsinger G, Pahl J, Schreiber W, Pirke KM, Moser EA ( 1989): Brain morphology and regional cerebral blood flow in anorexia nervosa. Biol Psychiatry 25: 1041–1048. [DOI] [PubMed] [Google Scholar]
- Krings T, Topper R, Foltys H, Erberich S, Sparing R, Willmes K, Thron A ( 2000): Cortical activation patterns during complex motor tasks in piano players and control subjects. A functional magnetic resonance imaging study. Neurosci Lett 278: 189–193. [DOI] [PubMed] [Google Scholar]
- Lerch JP, Worsley K, Shaw WP, Greenstein DK, Lenroot RK, Giedd J, Evans AC ( 2006): Mapping anatomical correlations across cerebral cortex (MACACC) using cortical thickness from MRI. Neuroimage 31: 993–1003. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, Frith CD ( 2000): Navigation‐related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci USA 97: 4398–4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May A, Gaser C ( 2006): Magnetic resonance‐based morphometry: A window into structural plasticity of the brain. Curr Opin Neurol 19: 407–411; 10.1097/01.wco.0000236622.91495.21. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Crinion JT, Noppeney U, O'Doherty J, Ashburner J, Frackowiak RS, Price CJ ( 2004): Neurolinguistics: Structural plasticity in the bilingual brain. Nature 431: 757. [DOI] [PubMed] [Google Scholar]
- Meister I, Krings T, Foltys H, Boroojerdi B, Muller M, Topper R, Thron A ( 2005): Effects of long‐term practice and task complexity in musicians and nonmusicians performing simple and complex motor tasks: Implications for cortical motor organization. Hum Brain Mapp 25: 345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Zhang J ( 2006): Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron 51: 527–539. [DOI] [PubMed] [Google Scholar]
- Olmos JM, Valero C, Barrio AGd, Amado JA, Hern·ndez JL, Menéndez‐Arango J, Gonzalez‐Macias J ( 2009): Time course of bone loss in patients with anorexia nervosa. Int J Eat Disord doi:10.1002/eat.20731 [DOI] [PubMed] [Google Scholar]
- Pannacciulli N, Del Parigi A, Chen K, Le DS, Reiman EM, Tataranni PA ( 2006): Brain abnormalities in human obesity: A voxel‐based morphometric study. Neuroimage 31: 1419–1425. [DOI] [PubMed] [Google Scholar]
- Peper JS, Brouwer RM, Boomsma DI, Kahn RS, Hulshoff Pol HE ( 2007): Genetic influences on human brain structure: A review of brain imaging studies in twins. Hum Brain Mapp 28: 464–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran VS ( 2005): Plasticity and functional recovery in neurology. Clin Med 5: 368–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JR, West R, Braver T ( 2008): Distinct neural circuits support transient and sustained processes in prospective memory and working memory. Cereb Cortex 19: 1208–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M ( 2002): Differences in white matter architecture between musicians and non‐musicians: A diffusion tensor imaging study. Neurosci Lett 321: 57–60. [DOI] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, Evans A, Rapoport J, Giedd J ( 2006): Intellectual ability and cortical development in children and adolescents. Nature 440: 676–679. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen‐Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE ( 2006): Tract‐based spatial statistics: Voxelwise analysis of multi‐subject diffusion data. Neuroimage 31: 1487–1505. [DOI] [PubMed] [Google Scholar]
- Stokic E, Srdic B, Barak O ( 2005): Body mass index, body fat mass and the occurrence of amenorrhea in ballet dancers. Gynecol Endocrinol 20: 195–199. [DOI] [PubMed] [Google Scholar]
- Suchan B, Busch M, Schulte D, Groenermeyer D, Herpertz S, Vocks S: Reduction of gray matter density in the extrastriate body area in women with anorexia nervosa. Behav Brain Res doi:10.1016/j.bbr.2009.08.035 [DOI] [PubMed] [Google Scholar]
- Swayze VW II, Andersen A, Arndt S, Rajarethinam R, Fleming F, Sato Y, Andreasen NC ( 1996): Reversibility of brain tissue loss in anorexia nervosa assessed with a computerized Talairach 3‐D proportional grid. Psychol Med 26: 381–390. [DOI] [PubMed] [Google Scholar]
- Swayze VW II, Andersen AE, Andreasen NC, Arndt S, Sato Y, Ziebell S ( 2003): Brain tissue volume segmentation in patients with anorexia nervosa before and after weight normalization. Int J Eat Disord 33: 33–44. [DOI] [PubMed] [Google Scholar]
- Taki Y, Kinomura S, Sato K, Inoue K, Goto R, Okada K, Uchida S, Kawashima R, Fukuda H ( 2008): Relationship between body mass index and gray matter volume in 1,428 healthy individuals. Obesity (Silver Spring) 16: 119–124. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Sowell ER, Gogtay N, Giedd JN, Rapoport JL, de Zubicaray GI, Janke AL, Rose SE, Semple J, Doddrell DM, Wang Y, van Erp TGM, Cannon TD, Toga AW ( 2004): Mapping cortical change in Alzheimer's disease, brain development, and schizophrenia. Neuroimage 23( Suppl 1): S2–S18. [DOI] [PubMed] [Google Scholar]
- Tuch DS ( 2004): Q‐ball imaging. Magn Reson Med 52: 1358–1372. [DOI] [PubMed] [Google Scholar]
- van Marken Lichtenbelt WD, Fogelholm M, Ottenheijm R, Westerterp KR ( 1995): Physical activity, body composition and bone density in ballet dancers. Br J Nutr 74: 439–451. [DOI] [PubMed] [Google Scholar]
- Yamashita S, Kawai K, Yamanaka T, Inoo T, Yokoyama H, Morita C, Takii M, Kubo C ( 2009): BMI, body composition, and the energy requirement for body weight gain in patients with anorexia nervosa. Int J Eat Disord doi:10.1002/eat.20700 [DOI] [PubMed] [Google Scholar]
- Zastrow A, Kaiser S, Stippich C, Walther S, Herzog W, Tchanturia K, Belger A, Weisbrod M, Treasure J, Friederich HC ( 2009): Neural correlates of impaired cognitive‐behavioral flexibility in anorexia nervosa. Am J Psychiatry 166: 608–616. [DOI] [PubMed] [Google Scholar]


