Abstract
The dorsomedial prefrontal cortex (DMPFC) has been shown to be involved in attending different states, all including a strong emotional component. It remains unclear, though, whether neural activity in the DMPFC is predominantly determined by either a particular domain, as emotional stimuli, or by a specific process, as attention. Here, we used functional magnetic resonance imaging to test the alternative hypotheses of domain‐ versus process‐specificity in DMPFC. Subjects had to perceive pictures from three different domains, sexual, emotional, and neutral stimuli, in both a nonattended, i.e., unexpected, and attended, i.e., expected mode. Our results show DMPFC activation during attended, i.e., expected stimulus perception when compared with nonattended, i.e., unexpected stimuli perception. DMPFC activation and corresponding behavioral changes (reaction time, subjective ratings) were observed in all three domains, sexual, emotional, and neutral stimuli. As opposed to those process‐specific effects that were found predominantly in posterior DMPFC, a process by domain interaction was found to be characteristic for more anterior parts of the DMPFC. Taken together, our findings favour the hypothesis that neural activity in the posterior DMPFC is determined by a specific process, i.e., attending stimuli, and thus characterized by process‐specificity rather than by a particular domain, i.e., sexual, emotional, or neutral stimuli, reflecting domain‐specificity. This suggests that the anterior and posterior DMPFC is involved in the process of attending mental states while remaining more (posterior DMPFC) or less (anterior DMPFC) independent of the type or domain of the respective stimulus. Hum Brain Mapp, 2009. © 2007 Wiley‐Liss, Inc.
Keywords: expectancy, emotion, fMRI, prefrontal cortex
INTRODUCTION
Daily experience shows that perception and processing of emotions is not a static process. It strongly depends on many factors, especially on our expectations and thus our attention, how we are about to perceive the subsequent stimulus and what it is like to experience it. Being, for example, confronted with a strong emotional stimulus with prior warning evokes a different reaction as a stimulus we were not anticipating. The same can be observed in the case of sexual stimuli where the mere anticipation or expectation may change subsequent perception and processing of the actual stimulus. Previous neuroimaging and neuropsychological research has explored the functional neuroanatomy of attending and expecting mental states like emotional or sexual states. This research has identified the medial prefrontal cortex, and in particular the dorsal medial prefrontal cortex (DMPFC), as one of the key regions in processing mental states. For example, the DMPFC has been shown to be activated in various conditions like emotional judgment [Gusnard et al.,2001; Lane et al.,1997; Northoff et al.,2004; Phan et al.,2002], moral judgment [Greene and Haidt,2002; Moll et al.,2005], theory of mind tasks [Frith,2002; Frith and Frith,1999,2003; Gallagher and Frith,2003; Kampe et al.,2003], memory retrieval tasks [Fossati et al.,2003; Lou et al.,2004; Macrae et al.,2004; Maddock et al.,2003], self‐related processing [Kelley et al.,2002; Northoff and Bermpohl,2004; Northoff et al.,2006; Wicker et al.,2003; Zysset et al.,2002], mentalizing tasks [Mitchell et al.,2005], verbal decision tasks [Johnson et al.,2002; Kjaer et al.,2002], sexual stimuli [Beauregard et al.,2001; Ferretti et al.,2005; Karama et al.,2002; Park et al.,2001; Stoléru et al.,1999], or face recognition [Platek et al.,2004]. The involvement of the DMPFC in a variety of different tasks (judgment, retrieval, recognition, decision) and in different domains (verbal, facial, social, moral, emotional, sexual) raises the question for its functional organization: is neural activity in the DMPFC instantiated by a particular task, i.e., process, remaining independent of the different domains or is it rather determined by a specific domain rather than by a particular task, i.e., process?
Different models of functional organization have been discussed, especially in the case of the dorsolateral prefrontal cortex (DLPFC). Goldman‐Rakic and others [Courtney et al.,1998; Goldman‐Rakic,2000; Levy and Goldman‐Rakic,2000; Mottaghy et al.,2002; Ungerleider et al.,1998] argue for what they call domain‐specificity. Domain specificity reflects functional organization with regard to a certain class of stimuli or content, i.e., the domain (such as verbal or spatial in the case of working memory) independently of the required task or process. It should be noted that the domain‐specific hypothesis is not a modality‐specific hypothesis since several sensory modalities may feed into one domain as it is, for example, the case in the verbal domain. By contrast, process specificity suggests a functional organization along specific psychological processes (such as storage and manipulation of information in working memory) independently of the class of stimuli or contents, i.e., the domains [D'Esposito et al.,2000; Fuster,2001; Miller et al.,2002; Nieder and Miller,2003; Nyberg et al.,2003; Owen,2000; Wig et al.,2004; Yovel and Kanwisher,2004].
What does domain‐ and process‐specificity mean in the context of the DMPFC? Here, domain‐specificity may refer to a particular content or material of mental states like emotional or nonemotional contents reflecting different domains. For instance, several imaging studies demonstrated involvement of the DMPFC in emotional contents when compared with nonemotional contents [Murphy et al.,2003; Phan et al.,2002]. Because this was observed in different types of tasks or processes like perception and judgment of emotion [Grimm et al.,2006; Gusnard et al.,2001; Northoff et al.,2004], one may assume domain‐dependence and process‐unspecificity in the DMPFC whose neural activity may thus be determined by emotional contents.
However, other studies demonstrated involvement of the DMPFC in the cognitive regulation of emotion processing [Beauregard et al.,2001; Blood and Zatorre,2001; Hornak et al.,2004; Kalisch et al.,2006; Ochsner et al.,2002,2004; Phillips et al.,2003; Price,1999]. Phan et al. [2002] therefore conclude that the DMPFC is engaged in implicit cognitive aspects of emotion processing that are common across emotional tasks. Recent results indicate that this implicit cognitive function may be the specific attentional modulation of emotional stimulus processing [Bermpohl et al.,2006a; Fichtenholtz et al.,2004; Keightley et al.,2003; Lane et al.,1999; Liberzon et al.,2000; Nitschke et al.,2006; Pessoa et al.,2002; Ueda et al.,2003; Winston et al.,2003]. This suggests that neural activity in the DMPFC is characterized by a specific process within a particular domain. As opposed to this, interaction of domain and process one may further suspect a cooccurence of effects of both domain and process without an interaction, thus leaving neural activity in DMPFC to be modulated either by say emotion or attention in a more general manner.
Finally, parts of DMPFC may be involved in the process of attending different types of states (verbal, moral, social, emotional, sexual; see above) or in processing attended stimuli as opposed to unattended without showing modulation of neural activity by emotions per se—in this case one would assume process‐specificity and domain‐independence. (Fig. 1a).
Figure 1.
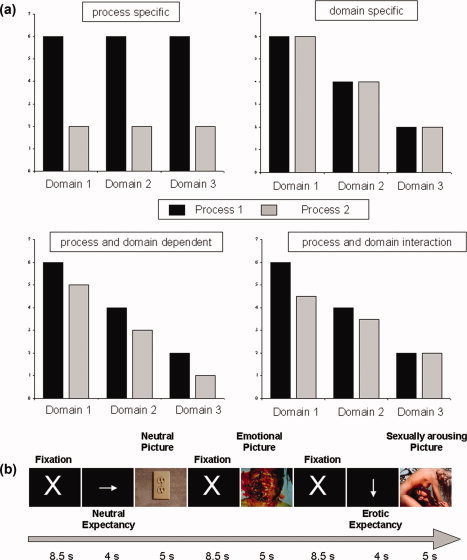
fMRI paradigm and hypotheses. (a) Hypotheses about signal strength for different processes and domains depending on the basic mechanism. Upper panel: Process specificity (left): Signal strength differs between processes, but not between domains. Domain specificity (right): Signal strength differs between domains, but not between processes. Lower panel: Process and domain‐dependence: Signal strength differs between domains as well as between domains either with (right) or without (left) an interaction of effects of process and domain. (b) Paradigm: after presentation of a fixation cross for 8.5 s, serving as a experimental baseline, pictures taken from the IAPS were shown for 5 s, either instantly or after a 4 s lasting cue, indicating the type of picture—emotional, sexual arousing, or neutral—following. Subjects were instructed to build up expectancy according to the type of cue. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
These different types of functional modulation could, however, occur in different subregions of DMPFC. As pointed out by recent studies [Amodio and Frith,2006; Steele and Lawrie,2004], a rostro‐caudal distribution of regions which are primarily modulated by either emotional domains or cognitive processes seems to better characterise actual functioning in DMPFC.
Analogous to a distinction of separate divisions of anterior cingulate cortex [Bush et al.,2000; Devinsky et al.,1995] cognitive processes were shown to dominate modulation of more posterior parts of DMPFC, whereas studies on emotional processes found modulation of neuronal activations in predominantly anterior parts of DMPFC [Phan et al.,2002; Steele and Lawrie,2004].
The general aim of our study was to investigate and test these conflicting hypotheses of functional organization in DMPFC using functional magnetic resonance imaging (fMRI) and behavioral parameters (subjective rating) as markers. We thereby adapted an expectancy paradigm previously used by Bermpohl et al. [2006], which seems particularly suitable to investigate the functional organization of the DMPFC because it can include both different processes and domains. The different processes include anticipatory cognitive processes without picture perception (i.e., expectancy period), stimulus processing (i.e., unexpected picture viewing), and interactive processes between cognitive and stimulus processes (i.e., expected picture viewing). The different domains include sexual, emotional, and neutral pictures. More specifically, we first tested for a specific process, i.e., process‐specificity, to be associated with neural activity in the DMPFC; we investigated the impact of preceding attention, i.e., expectancy, on subsequent stimulus perception [Chawla et al.,1999; Kastner et al.,1999] and compared attended, i.e., expected, and nonattended, i.e., unexpected stimuli. This was done in three different domains—sexual, emotional, and neutral stimuli—to test for domain‐dependence. In the case of domain‐dependence, DMPFC neural activity should be determined by a particular stimulus domain, as for example emotional stimuli as distinguished from sexual and neutral stimuli domains (see also Fig. 1a). Whereas in the case of process‐specificity, DMPFC neural activity should be solely determined by a specific process, i.e., attending the respective stimulus as induced by expectancy (see also Fig. 1a). Finally, effects of domain and process may apply at the same time, so that DMPFC neural activity may be modulated by both preceding attention, i.e., expectancy, and the different stimuli domains, i.e., sexual, emotional, and neutral, which may occur independently for both process and domain or as process by domain interaction (see Fig. 1a).
METHODS
Subjects
For our main study (Study 1), we investigated 21 healthy subjects (10 female, 11 male, age: 23.7 ± 2.1, mean ± standard deviation) without any neurological, medical, or psychiatric disease. To validate effects of preceding expectancy (see below for details), data from another study [Walter et al., in press] was analyzed. In this control study, 13 different healthy subjects (13 males; age: 38.71 ± 6.76; mean ± standard deviation) also without any neurological, medical, or psychiatric disease had been included.
After detailed explanation of the study design and potential risks, all subjects of both studies gave written informed consent. Both studies were approved by the institutional review board of the Otto‐von‐Guericke University of Magdeburg.
Paradigm
Study 1 (see also Fig. 1b)
Subjects were instructed to passively view 256 photographs from the International Affective Picture System [Lang et al.,2005] which were presented for a duration of 5 s. Picture sets were counterbalanced across subjects as well as within each subject according to the three categories sexual arousing, nonsexual emotional, and neutral. Sexual arousing pictures were pictures showing naked people or people engaged in sexual activities; these stimuli have been shown to induce neural activity in those regions (hypothalamus, amygdala, ventromedial prefrontal cortex) that are supposed to be associated with sexual arousal [Heinzel et al.,2006; Walter et al., in press]. Emotional pictures were those supposed to produce emotions without any sexual component like smiling babies while neutral pictures included nonemotional and nonsexual scenes like a book. Emotional and erotic picture types were matched with respect to mean values (mean ± SD) provided with the IAPS dataset for arousal (neutral pictures 3.41 ± 0.88, emotional pictures 5.94 ± 0.73, erotic pictures 5.95 ± 0.73) and emotional valence (neutral pictures 5.03 ± 0.28, emotional pictures 4.81 ± 2.64, erotic pictures 4.58 ± 2.29).
The paradigm consisted of 256 stimuli, 128 of them emotional but not erotic, another 64 stimuli showing erotic contents and another 64 neutral stimuli. To provide a sufficient number of stimuli within each picture category (erotic, emotional, or neutral) 25% of the used pictures for each category were displayed twice, but never twice in the same run to exclude repetition effects within runs. Stimuli were distributed over eight runs of each 32 stimuli with four runs (Runs 1–4) consisting of 16 emotional and 16 neutral pictures, and the other four runs (Runs 5–8) consisting of 16 emotional and 16 sexual pictures with runs being presented in a randomised order. Subjects were instructed to passively view the pictures and to make an immediate button press to ensure a constant level of attention during picture viewing. Reaction times from picture onset to button press were measured.
Half of the pictures were preceded by an expectancy period with a duration of 4 s, in which the type (sexual, emotional, or neutral) of the following picture was indicated by a white arrow on a dark background pointing to different directions. Following Kastner et al. [1999], an upward pointing arrow was followed by a nonsexual emotional picture, a downward pointing arrow by a sexual arousing and a rightward pointing arrow by a neutral picture. The other half of the pictures were presented instantly after a fixation cross without a preceding expectancy period. As pointed out in previous studies [Bermpohl et al.,2006a], this allowed to measure the neural activity associated with mere stimulus perception without any specific or general influences of any kind of preceding expectancy period. Expected and unexpected pictures (see below) were balanced for the number of sexual arousing, nonsexual emotional and neutral pictures, the total number of pictures, and IAPS values.
Each picture presentation was followed by a fixation cross which was presented for 8.5 s. The nonpictorial stimuli (arrows, fixation cross) were of equal size and color and were centred on a black background. During the fMRI session, pictures were projected automatically via a computer and a forward projection system on a screen placed at the end of the subject's gurney. Subjects lay supine in the scanner and viewed the screen through a mirror positioned on the head coil. Subjects were asked to keep their eyes open and fixate the middle of the screen in front of them. They were asked not to move finger, head, or body during picture viewing with the exception of the button press for the response. Prior to the experimental session, subjects were familiarized with the paradigm by completing a test run.
Control study for effects during picture expectancy
In a second study (Study 2, consisting of the healthy control group from Walter et al., [in press]), a similar paradigm was applied which enabled us to further explore potential effects of preceding expectancy on results from our our study. This paradigm, which is also described in Heinzel et al. [2006] and Walter et al. [in press], included varying durations for expectancy periods from 4–6 s (in steps of 0.5s, mean durations counterbalanced across stimulus types) hence introducing jittered ISI's. Second one third of the expectancy periods were followed by fixation periods to decrease colinearity of expectancy and picture events. Effect of expectancy periods on neural activity as compared to rest was then compared between both studies to estimate potential effects of preceding expectancy in DMPFC that might have been underestimated by our experimental design in Study 1.
Behavioral Monitoring and Analysis
Reaction times were defined as the time between the onset of the picture screen (IAPS photograph) and the subsequent button press. Average reaction times were compared using paired t‐tests. Subjective rating of pictures was conducted outside the scanner after the fMRI session. The very same pictures as presented in fMRI were presented in a new and randomized order; using a visual analogue scale ranging from 1 to 9 subjects had to evaluate sexual intensity and emotional intensity and valence of each picture. Emotional valence was assessed using the question “How unpleasant/pleasant is that picture?” and ranged on a continuum from “negative” (1) to “positive” (9). Emotional and sexual intensity were assessed using the question “How emotionally/sexually intense is this picture?” and ranged on a continuum from “low” (1) to “high” (9). (All questions translated from German). We were aware that emotional responses might attenuate when pictures are seen for a second time outside the scanner [Ishai et al.,2004]. However, this potential habituation effect applied equally to all picture conditions and was not expected to affect the differences between conditions [Anderson et al.,2004] especially as our focus was set to effects between expected and unexpected pictures which consisted of equal numbers of stimuli from all three domains. Because of problems with data acquisition during post scanning tests complete ratings exist only for 18 of the 21 subjects and intra scan reaction times were complete only for 15 subjects. ANOVA's were performed with factors domain (neutral, emotional or erotic pictures) and process (expected or unexpected) for the three posthoc ratings as well as reaction times of button press during the fMRI scan. Ratings and reaction times were then compared between expected and unexpected conditions (for all pictures as well as for sexual, emotional, and neutral pictures separately) using paired t‐tests.
fMRI—Data Aquistion and Analysis
Scanning procedure
Data acquisition was conducted on a 1.5 Tesla General Electric Signa scanner using a standard headcoil. Imaging procedures included collection of (a) structural high resolution images (rf‐spoiled GRASS sequence 60 slices sagittal, 2.8 mm thickness), (b) T1 weighted anatomic images coplanar with the functional images (23 slices, aligned to the plane connecting the anterior and posterior commissure axis covering the whole head in oblique axial orientation), (c) inversion recovery T1 weighted echo planar images coplanar with the functional images, and (d) echo planar functional images sensitive to BOLD contrast (eight runs with each 257 sequential acquisitions, 23 slices with 3.125 mm in‐plane resolution, 5 mm thickness, 1 mm gap; T2* weighted gradient echo sequence: TR 2s, TE 40 ms). By a mounted mirror on the headcoil a screen was visible, on which stimuli were projected using a LCD projector while functional images were acquired.
Image preprocessing and statistical analysis
Image processing and statistical analyses were carried out using MATLAB 6.5.1 and SPM2 [Friston et al.,1994]. The first seven images of each run were discarded because of T1 saturation effects. A remaining total of 2,000 (8 × 250) volume images were realigned to the first image to correct for head movement between scans, mean‐adjusted by proportional scaling, resliced, and normalized into standard stereotactic space (resulting in an isotrophic 3 mm resolution). Image normalization was performed using the MNI (Montreal Neurological Institute) template provided by SPM. Spatial transformation included both linear and nonlinear dimensions and used a nonlinear sampling algorithm [Friston et al.,1994]. Data were thereafter expressed in terms of standard stereotactic coordinates in the x, y, and z axes. Transformed functional data sets from each subject were smoothed with a Gaussian kernel of 8 mm (full‐width half‐maximum) for the group analysis to meet the statistical requirements of the General Linear Model and to compensate for normal variation in individual brain size, shape, and sulcal/gyral anatomy across subjects. Subject‐specific low frequency drifts in signal were removed by a high pass filter of 128 s.
Definition and estimation of the statistical design followed a methodology previously used by Bermpohl et al. [2006a,b] as well as by Herwig et al. [2007a,b,c] or Abler et al. [2007]. For each subject a design matrix was defined modelling unexpected and expected viewing of sexual, emotional, and neutral IAPS pictures and the baseline condition (i.e., fixation cross) as well as expectancy periods as separate events. Convolution of regressor specific onset vectors used a canonical hemodynamic response function as provided by SPM. After estimation of all model parameters, specific effects were tested by applying appropriate linear contrasts to the parameter estimates for each condition resulting in a t‐statistic for each voxel. These individual results were taken to the second level analysis conducting random effects, one sample t‐tests to make an inference on a general population. The threshold for significant signal changes was set to P < 0.05, FWE corrected, cluster size >10.
To analyse the effects of the preceding expectancy on subsequent stimulus perception, we compared all expected to all unexpected pictures. This was first done for all expected and all unexpected pictures as well as separately for expected and unexpected sexual, emotional, and neutral pictures in a second step.
To control for overlapping effects of expectancy and viewing of expected pictures, the main contrast [expected > unexpected pictures] was exclusively masked by the contrast [expectancy periods > fixation periods]. Second, the specificity of activations during viewing of expected pictures was tested by exclusively masking significant effects of the above described main contrast with both potential activations during unexpected picture viewing as well as deactivations when compared with fixation periods. The second contrast [fixation > unexpected pictures] was entered into the mask to exclude that effects in the main contrast may be due to relative signal decreases during unexpected picture viewing.
In a next step, domain effects were excluded for resulting voxels from the main contrast by excluding those voxels that show differential activations during viewing of stimuli any of the three picture domains as compared to rest (fixation). The exclusive mask for the main contrast applied here therefore consisted of the contrasts of each picture category compared to fixation, e.g., [emotional pictures > fixation] and [emotional pictures < fixation]. We report voxels that survived a mask consisting of all four domain specific contrasts, as well as results from individual masking analyses only using the two contrasts [picture < fixation] and [picture > fixation] for each picture category.
To account for possible differences in neuronal effects of emotional stimuli in those runs that consisted of emotional and neutral stimuli as compared to runs consisting of emotional and erotic stimuli, we created separate masks for emotional stimuli in Runs 1–4 and Runs 5–8 therefore having four instead of three domain driven contrast groups.
All exclusive masking analyses used an uncorrected P‐value of P < 0.05 for their masks.
The distinction of emotional stimuli from Runs 1–4 from those in Runs 5–8 was done over the whole course of our analysis to account for the fact that psychological as well as neuronal effects of emotional stimuli have to be considered highly context dependent and therefore fitting of corresponding regressors should not be confounded by context dependent differences.
However, because of this approach we were able to search for commonalities independent of the experimental context, entering four separate contrasts of expected vs. unexpected stimuli of the respective domain (having two “domains” for emotional stimuli) into a conjunction analysis. For this conjunction of four contrasts per subject on a random effects level, the statistical threshold was lowered to P < 0.001 uncorrected.
In the same manner, a context dependent effect for emotional stimuli was considered by serial subtractions that sought for stronger effects of preceding expectancy on subsequent emotional as compared to neutral stimuli, therefore indicating an interaction of domain and process [Bermpohl et al.,2006a]. The serial subtraction applied t‐tests on contrasts of [expected > unexpected stimuli] for the different stimulus types. These respective contrasts for erotic pictures were compared to those of emotional pictures in Runs 5–8 and in a second serial subtraction those of emotional pictures in Runs 1–4 were compared to those of neutral pictures. Here, common effects of the underlying interactions of domain and process were assed by conjoining both serial subtractions to generalize effects of interactions over different domains. For this second conjunction revealing common effects of process x domain interaction the level of statistical significance was lowered to an uncorrected P < 0.005.
For all three types of analyses “glass brain” projections are shown in transversal, coronal or sagital orientation.
For the purpose of visualization percent, signal changes for all experimental conditions were calculated for a region of interest that was constituted by voxels lying in a 10 mm sphere centered over the peak of activations in the DMPFC for the underlying main contrast [all expected > all unexpected pictures] using MarsBaR [Brett et al.,2002]. For reasons described above effects were plotted separately for Runs 1–4 and 5–8.
Finally, to visualize regional subspecialization within DMPFC, smaller ROI's (5 mm spheres) were aligned in a rostro‐caudal direction following the dorsomedial cortical curvature.
ROI's were placed 5mm bilaterally from the midline with following y,z coordinates: (20, 60); (30, 55); (38, 50); (45, 45); (50, 38); (55, 30); and (60, 20). For these subregions, main effects of domain and process were estimated subtracting corresponding percent signal changes from conditions representing domains (neutral, emotional, sexual) or processes (expected or unexpected pictures). While for all runs process effects were calculated subtracting signal changes of unexpected from those of expected pictures, domain effects were calculated subtracting signal changes during neutral pictures from signal changes during emotional pictures in Runs 1–4 and subtracting signal changes for emotional pictures from those induced by erotic pictures both in Runs 5–8. Plots show mean values for all runs and for left and right spheres placed ± 5 mm laterally the midline. For separate analysis of left and right spheres as well as for Runs 1–4 and 5–8, these additional plots were generated and are added to the supplementary Figure 3 (supplementary material online).
RESULTS
Behavioral Data
ANOVA for reaction times of button press during fMRI sessions revealed significant effects for the factors process (levels: expected or unexpected; df: 1.14; P < 0.001, F = 15.79) and domain (levels: erotic, emotional, or neutral; df: 2.13; P < 0.028, F = 4.76) while no significant interaction could be found. Subjects responded significantly faster to expected than to unexpected emotional (T = 3.49, P < 0.003) neutral (T = 3.88, P < 0.001) or erotic (T = 4.04) pictures (Fig. 2). Further responses to erotic pictures were significantly slower than those to neutral pictures (T = 3.20, P < 0.006) or responses to emotional pictures (T = 2.37, P < 0.033), while no significant difference of reaction times existed between neutral and emotional pictures.
Figure 2.
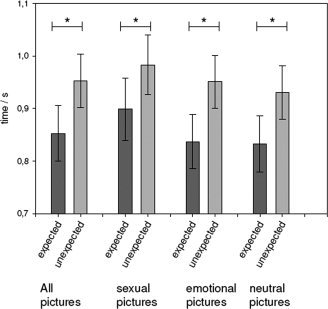
Behavioral results—Reaction times. Bars represent times required for button press after picture presentation for the different picture categories (means in seconds ± SEM). Values are also presented for the main effect on reaction times over all picture types, asterisk indicates differences significant in a pairedt‐test at P < 0.01; for details see results section.
ANOVA of behavioral ratings of emotional intensity and emotional valence revealed significant main effects (P < 0.001) for both factors (domain‐intensity: F = 92.37, domain‐valence: F = 29.10; df: 2.16; process‐intensity: F = 22.80, process‐valence: F = 273.23; df: 1.17) as well as significant (P < 0.001) interactions of both factors (intensity: F = 45.58, valence: F = 110.65; df: 2.16).
Although emotional and erotic stimuli were a priori matched by their IAPS standard values, emotional intensities were rated higher in erotic pictures as compared to emotional pictures (T = 12.77, P < 0.001) and further higher in emotional compared to neutral pictures (T = 14.86, P < 0.001). Emotional intensities were rated higher for expected than for unexpected emotional (T = 6.79, P < 0.001) and erotic pictures (T = 2.91, P < 0.01) but higher for unexpected than for expected neutral pictures (T = 3.20, P < 0.005).
Emotional valences for erotic pictures were significantly lower than for neutral pictures (T = 6.20, P < 0.001) but higher than for emotional pictures (T = 3.09, P < 0.007). Further emotional valences were rated higher for unexpected compared to expected emotional (T = 15.66, P < 0.001) and unexpected compared to expected erotic stimuli (T = 4.75, P < 0.001) while no significant difference existed between expected and unexpected neutral pictures.
ANOVA of sexual intensities revealed significant effects only for the factor domain (df: 2.16; P < 0.001, F = 74.65) with erotic pictures being more sexually intense than emotional (T = 11.86, P < 0.001) or neutral pictures (T = 12.08, P < 0.001).
FMRI Data
Control for effects of preceding expectancy
The main effect of preceding expectancy on subsequent pictures was assessed by the main contrast [expected > unexpected pictures] which was controlled for effects during the preceding expectancy period (Fig. 3a) by exclusively masking with the contrast [expectancy > fixation]. This analysis revealed significant effects (P < 0.05, FWE corrected) in DMPFC at a posterior (x, y, z = −6, 30, 57; Z = 6.23) and an anterior location (x, y, z = −6, 54, 39; Z = 6.35) as well as left inferior frontal gyrus (IFG) (x, y, z = −48, 21, −12; Z = 5.68).
Figure 3.
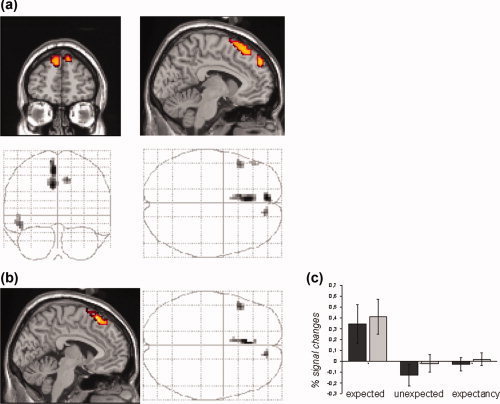
Modulation of stimulus perception by preceding attention. (a) Active voxels showing greater effects for expected compared to unexpected picture viewing but no activations during preceding expectancy periods when compared with rest. Activations indicate significant effects for the contrast [all expected pictures > all unexpected pictures], exclusively masked by the contrast [all expectancy periods > rest]. (b) Effects of expected picture viewing controlled for effects of unexpected picture viewing: the main contrast [all expected pictures > all unexpected pictures] was exclusively masked by the contrasts [all unexpected pictures < rest] and [rest > all unexpected pictures]. The resulting voxels thus reflect those activations during expected picture viewing which were neither observed during unexpected picture viewing nor due to relative deactivations during the latter. Significant voxels in (a) and (b) reflect effects at an FWE‐corrected P‐level of 0.05, k > 10 voxels and an uncorrected mask P‐value of 0.05. (c) Signal changes in DMPFC. Effects of expected and unexpected pictures as well as of preceding expectancy periods are plotted for the DMPFC. Bars represent percentage signal changes (±SEM) for stimuli in runs with emotional and neutral stimuli (dark gray) and for runs with erotic and emotional stimuli (bright gray). For both type of runs, effects were clearly restricted to activations during expected picture viewing. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Specificity of effects for expected pictures
To test if activations in these areas are specific for expected pictures as opposed to gradually stronger activations during general effects of picture viewing, the second mask applied to our main contrast consisted of the contrast [unexpected pictures > fixation] and to further of the inverse contrast [fixation > unexpected pictures] to exclude effects of our main contrast that are due to relative deactivations during the unexpected picture condition. Significant activations of this analysis (P < 0.05, FEW‐corrected) were found in DMPFC with a dorsal peak activation (x, y, z = −3, 27, 60; Z = 6.06) and a more anterior peak (x, y, z = 12, 51, 45; Z = 5.68) and in left IFG with the same peak location (Fig. 3b).
Effects of expected and unexpected pictures as well as of preceding expectancy periods are plotted in Figure 3c for a region of interest (ROI) located in posterior DMPFC (sphere 10 mm; x, y, z = 0, 30, 57) and show similar effects for runs with emotional and neutral stimuli (Runs 1–4) and runs with emotional and erotic stimuli (Runs 5–8) when analysed separately.
Exclusion of domain effects
In a next step, main effects of domain dependence were excluded for voxels revealed by our main contrast [expected pictures > unexpected pictures]. The exclusive mask applied here, thus, consisted of corresponding contrasts of domain specific picture subtypes, i.e., neutral, erotic and emotional pictures in Runs 1–4 and Runs 5–8 compared to fixation. As shown in Figure 4, this analysis revealed domain independent effects of expected picture viewing in DMPFC with a posterior (x, y, z = −6, 27, 63; Z = 7.34) intermediate (x, y, z = −6, 42, 57; Z = 5.39) and an additional peak extending to right superior frontal gyrus (x, y, z = 15, 57, 39; Z = 6.23) at an FEW‐corrected P < 0.05. When only contrasts of one picture type compared to fixation where used as exclusive masks, comparable results were obtained, however, additional effects in left IFG survived these masking analyses (see supplementary Fig. 1 provided online).
Figure 4.
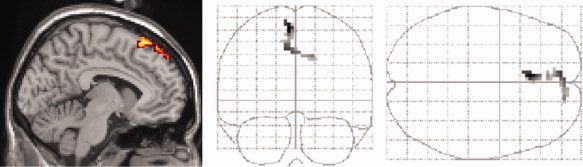
Exclusion of domain effects. Exclusion of domain effects within activated voxels for the main contrast [all expected pictures > all unexpected pictures] by exclusively masking resulting voxels with domain specific contrasts: [erotic picture viewing < > rest], [emotional picture viewing < > rest] and [neutral picture viewing < > rest]. Activated voxels reflect significant effects at P < 0.05 (FWE‐corrected, k >10 voxels) for a mask P‐value of 0.05, uncorrected. For separate masking analyses for each domain see supplementary Figure 1. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Main effects of process in different domains
Next to further support this domain independence, instead of masking with domain dependent contrasts, clusters with common effects of expected picture viewing for pictures in different domains were sought by conjoining the four separate contrasts [expected pictures > unexpected pictures] obtained for the four experimental picture types (neutral, erotic, emotional in Runs 1–4 and in Runs 5–8). This conjunction analysis revealed one major cluster located in dorsal DMPFC (x, y, z = −6, 27, 60; Z = 3.98) when the level of statistical threshold was lowered to P < 0.001, uncorrected (Fig. 5a).
Figure 5.
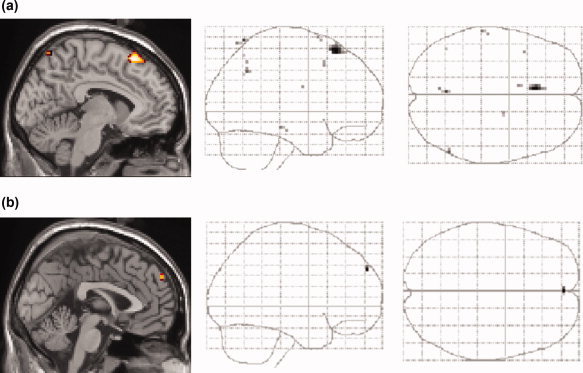
Main effects and interactions of process and domains. (a) Main effects of preceding expectancy on subsequent picture periods as revealed by a conjunction (P < 0.001, uncorrected) of the following four contrasts: (1) [all expected neutral pictures > all unexpected neutral pictures], (2) [all expected erotic pictures > all unexpected erotic pictures], (3 and 4) [all expected emotional pictures > all unexpected emotional pictures] for pictures in Runs 1–4 (neutral and emotional pictures) and 5–8 (emotional and erotic pictures), respectively. (b) Interactions of domain and process effects as revealed by serial subtractions: The two contrasts 1. [all expected emotional pictures > all unexpected emotional pictures] > [all expected neutral pictures> all unexpected neutral pictures] (Runs 1–4) and 2. [all expected erotic pictures > all unexpected erotic pictures] > [all expected emotional pictures > all unexpected emotional pictures] (Runs 5–8), were conjoined to show the interaction effects between process (expected > unexpected) and domain (erotic > emotional > neutral) common to both Runs 1–4 and Runs 5–8. Resulting voxels were further restricted to those showing expectancy effects in all domains by inclusively masking this conjunction with the four separate contrasts of expected > unexpected neutral, erotic and emotional pictures from Runs 1–4 and Runs 5–8, respectively. The level of significance was set to P < 0.005, uncorrected and a mask P‐value of P < 0.05 uncorrected was applied. Note that the interaction effects between process and domain were located in the anterior part of the dorsomedial prefrontal cortex (b) while the main effects of expectancy on subsequent pictures revealed a region in the posterior part of the dorsomedial prefrontal cortex (a). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Interaction of domain and process
To test for an interaction of process and domain that was common in both types of runs consisting of different picture types, we sought for common interaction effects as revealed by serial subtractions that compared contrasts reflecting the effects of expected versus unexpected pictures between different picture types. Resulting voxels of this conjunction analysis combining two serial subtractions thus show significantly greater effects of expected as compared to unexpected pictures for emotional than for neutral pictures in Runs 1–4 and significantly stronger effects of expected as compared to unexpected pictures for erotic than for emotional pictures in Runs 5–8. To limit resulting interaction effects to interactions for positive effects of expected pictures, additionally an inclusive mask was applied for this analysis consisting of the contrasts of [expected pictures > unexpected picture] for our four picture types (see also methods). This analysis revealed one significant cluster (x, y, z = 0, 57, 36; Z = 2.93, P < 0.005, uncorrected) in an anterior subregion of DMPFC (Fig. 5b).
Rostro‐caudal differences for maineffects of process and domain
To investigate if above described differences in peak localizations with posterior main effects of process and anterior effects of interaction underlie a systematic distribution of different DMPFC subregions, main effects for process and domain were plotted for bilateral ROI's (spheres of 5 mm) oriented along a rostro‐caudal covering DMPFC (Fig. 6a).
Figure 6.
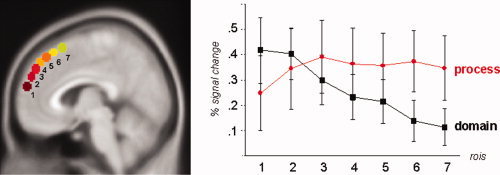
Regional differences in main effects of process and domain within DMPFC. (a) Seven spherical ROI's (diameter: 5 mm) were placed bilaterally (x = ±5 mm) in a rostro‐caudal direction covering most parts of the dorsomedial PFC that showed effects in our various analyses. (b) Process effects of preceding expectancy on subsequent pictures are plotted for each ROI contrasting percentage signal changes for the contrast all expected pictures > all unexpected pictures (red circles). Domain effects were calculated contrasting percentage signal changes during emotional > neutral conditions (Runs 1–4) or erotic > emotional conditions (Runs 5–8), respectively (error bars indicating SEM). Except for most rostral parts of DMPFC (BA 10) comparable process effects were found throughout DMPFC subregions while effects constantly declined the further posterior the ROI was placed. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Effects of process were estimated for distinct ROI's as differences in percentage signal changes during expected and unexpected pictures. Except for the most rostral ROI, comparable effects of process were found in all DMPFC ROI's (Fig. 6b).
Effects of domain were estimated as differences between emotional and neutral pictures or erotic and emotional pictures respectively. Mean effects of domain, that were strongest in the most rostral ROI's declined the further caudally ROI's were located (Fig. 6b).
This pattern was also found when left and right ROI's (x = ± 5 mm) were analyzed separately for runs consisting of emotional and neutral or erotic and emotional pictures (see supplementary Fig. 2 in online material).
Exclusion of expectancy effects in the control study
Neural effects during expectancy periods were compared between our study and the initially described control study of 14 healthy subjects taken from Walter et al. [in press] (see above). On an exploratory level of P < 0.05 uncorrected, the contrast [expectancy of pictures > fixation] was calculated and significant effects were overlaid on a standard MNI template brain and were for sagital, coronal and transversal sections at x = 0, y = 30 and z = 57 to explore effects in DMPFC region of interest for the effects of expected picture viewing. In both the control study (supplementary Fig. 3a) and our study (supplementary Fig. 3b) effects during expectancy periods were present in a broad set of cortical regions encompassing the dorsal attention network including premotor and supplementary motor areas, caudal anterior and posterior cingulate cortex, DLPFC and anterior insula/frontal operculum and occipitoparietal cortex. In contrast, DMPFC did not show any effects during preceding expectancy periods even on this considerably low statistical level and even when applying another experimental design focussing more on differentiation of expectancy and picture periods as described above.
DISCUSSION
We used two markers, fMRI and behavioral parameters (subjective ratings, reaction times), to test the alternative hypotheses of process‐specificity versus domain dependence in the DMPFC.
More specifically, we asked (1) whether a specific process, here the processing of attended, i.e., expected stimuli, is associated with neural activity in the DMPFC, and (2) whether this process and associated DMPFC activation occur only in a particular domain like emotion or also in other domains. First, we show specific DMPFC activation during processing of expected stimulus perception whereas no such involvement was observed in the other processes, perception of unexpected pictures and the preceding expectancy period itself. Second, DMPFC activation during perception of expected stimuli occurred in all of the three stimuli domains, sexual, emotional, and neutral, even when emotional stimuli were presented in different experimental context. This is further supported by the fact that clusters showing this task modulated behavior did not show activations during unexpected perception of either stimulus domain by itself, independently of the process of preceding attention. Third, our behavioral data including both reaction times and subjective ratings show significant modulation of stimulus perception by preceding attention in all three domains. Fourth subregions within DMPFC showing either a process specific, domian independent or rather an interaction of process and domain could be specified along a rostro‐caudal axis.
Taken together, our data provide strong evidence for the hypothesis of process‐specificity in posterior DMPFC and process by domain interaction in the anterior DMPFC.
Process‐Specificity and the DMPFC
We investigated the modulation of stimulus perception by preceding attention, i.e., expectancy, with regard to neural activity in the DMPFC. Comparing all expected pictures to unexpected ones, we observed significant signal changes in the DMPFC. Exclusive masking analysis revealed that DMPFC signal changes were associated only with the expected stimulus perception but neither with unexpected stimulus perception nor with the preceding expectancy period itself. This exclusion could be supported by an additional dataset, which thanks to reduced colinearity of predictors of pictures and expectancy cues was more specific for this distinction.
The association of the DMPFC with attentional processes, i.e., expectancy is in accordance with recent studies showing analogous DMPFC involvement in expected picture viewing when compared with unexpected picture viewing [Bermpohl et al.,2006a; Ueda et al.,2003]. Involvement of attention has also been suspected to account for DMPFC activation in tasks requiring evaluation as for example emotional judgment [Gusnard et al.,2001; Lane et al.,1997; Northoff et al.,2004; Phan et al.,2002], moral judgment [Greene and Haidt,2002; Moll et al.,2005], and reappraisal [Kalisch et al.,2006; Ochsner et al.,2002,2004; Ochsner and Gross,2005]. Finally, attentional processes may also be involved in other tasks associate with DMPFC activation like theory of mind, memory retrieval, verbal decision, sexual arousal, face recognition, and mentalizing [Beauregard et al.,2001; Ferretti et al.,2005; Fossati et al.,2003; Frith,2002; Frith and Frith,1999,2003; Gallagher and Frith,2003; Johnson et al.,2002; Kampe et al.,2003; Kjaer et al.,2002; Lou et al.,2004; Macrae et al.,2004; Maddock et al.,2003; Mitchell et al.,2005; Platek et al.,2004; Stoléru et al.,1999].
Our study complements and extends these findings. First, our results clearly distinguish perception of stimuli after expectancy periods from mere stimulus perception without preceding attention only the former but not the latter inducing DMPFC activation. Psychologically, this suggests that mere stimulus perception itself does not recruit the posterior DMPFC. Instead, our findings indicate that it is the attention to that stimulus that seems to induce DMPFC activation. Accordingly, the DMPFC seems to be crucial in attended stimulus perception and the associated mental states rather than in mere stimulus perception itself. Second, our results show that the DMPFC activation is related to the modulation of stimulus perception by attention, i.e., expectancy, rather than being involved in the expectancy period itself. This is suggested by our observation that we did not observe any DMPFC activation during the preceding expectancy period. This is further supported by analogous findings in recent studies who also observed DMPFC involvement only during attention to pictures but not in the preceding period [Bermpohl et al.,2006a,b] as well as by studies on simultaneous attention [Fichtenholtz et al.,2004; Keightley et al.,2003; Lane et al.,1999; Liberzon et al.,2000; Pessoa et al.,2002; Winston et al.,2003]. This suggests that the DMPFC is specifically involved in attending stimulus perception and its associated mental states rather than being recruited by attention, i.e., expectancy, itself. One should, however, be careful in interpreting these results. In addition to the temporal difference between preceding attention, i.e., expectancy without any stimulus perception and modulation of the latter by the former, there is another psychological difference involved. The expectancy period and its assocciated mental states are generated internally by the person itself independent (more or less because of the instruction) of the external context, whereas mental states associated with stimulus perception are induced externally, i.e., driven by the presented picture, its contents and the psychological context. Our observation of DMPFC involvement during expected stimulus perception but not during the expectancy period itself, thus, may suggest specific recruitment of this region by attention to externally generated mental states rather than to internally generated mental states (see also Mitchell et al. [2005] for empirical support of this assumption). Acordingly, the DMPFC seems to be recruited when we attend those mental states that are induced externally by our environment.
Domain‐Independence and DMPFC
We investigated if the domains by themselves, independent of preceding attention, recruited the DMPFC. To obtain all possible regional signal changes, we compared stimulus perception in each domain against baseline to cover potential domain dependent effects. No significant activation was observed in baseline comparisons for posterior DMPFC. Thus, activations observed here during expected pictures cannot be traced back to domain dependent effects of stimulus perception itself, therefore leaving modulation in surviving voxels of the applied masking analysis up to process specific effects.
In further support, the domain independence of expected picture viewing in posterior DMPFC was revealed for each domain separately in an additional conjunction. This inclines us to suggest that neural activity in the posterior DMPFC can be characterized by process‐specificity, i.e., attention to externally induced mental states, while at the same time remaining independent of a particular type or domain of stimuli thus showing domain‐independence.
It can, however, be stated that this domain independence holds true only for posterior part of DMPFC while in anterior DMPFC, an impact of the domain can be observed. In the anterior part, significant interactions of domain and process could be found for both emotional and erotic pictures. For a comparable peak localization Bermpohl et al. [2006] reported an interaction of emotional content of pictures and the effect of preceding expectancy in the DMPFC. We could not only replicate their finding of stronger expectancy effects in emotional as compared to neutral pictures but further extend and generalize it to other domains like erotic stimulation.
Taken together, our results suggest that neural activity in the posterior DMPFC as distinguished from anterior DMPFC is not predominantly determined by perception of a particular stimulus type or domain but rather by attention to stimulus perception.
Our conclusion of DMPFC domain‐independence seems to contradict recent studies in the domain of emotions where emotional stimuli induced activation in the DMPFC when compared with nonemotional or less emotional stimuli [Murphy et al.,2003; Northoff et al.,2004; Phan et al.,2002; Zysset et al.,2002]. Rather do our recent findings support a rostro‐caudal differentiation of DMPFC subregions as discussed by Steele et al. [2004] or Amodio and Frith [2006]. Neural activity in the anterior DMPFC seems to be strongly impacted by interaction between process and domain, which is well in accordance with the involvement of this region in emotional stimulation when compared with neutral stimuli. We could show that this impact declines the more posterior DMPFC subregions are located. This in turn is well compatible with the recently assumed involvement of the posterior DMPFC in more cognitive tasks [Amodio and Frith,2006].
The observed distinction in a rostral process‐ and domain‐, i.e., emotion related part, as identified by the interaction analysis and a caudal process‐, i.e., cognitive related division, as identified by both exclusive masking and trans‐domain conjunction analysis, followed an anatomical delineation proposed by Steele et al. [2004] on the basis of 330 imaging studies. Such distinction has previously been postulated for other medial prefrontal regions including anterior cingulate cortex [Bush et al.,2000; Devinsky et al.,1995]. However, further studies are necessary in the future to exactly delineate the functional role of anterior and posterior DMPFC subregions.
Methodological Limitations
One could argue that the distinction between our three domains is rather problematic. For example, in addition to motivational, cognitive, and vegetative components, sexual arousal, for example, is supposed to contain an emotional component [Ferretti et al.,2005; Karama et al.,2002; Stoléru et al.,1999]. The sexual domain is thus not clearly separable from the emotional domain so that one could argue that both do not represent separate domains. We included the sexual stimuli because we could match them in emotional valence and intensity with emotional stimuli while they were distinguished with respect to sexual intensity. This was also confirmed in subjective ratings of all three dimensions (emotional valence and intensity, sexual intensity). Based on different subjective ratings in sexual intensity (sexual vs. nonsexual), we therefore argue that emotional and sexual stimuli must be regarded as different domains while being matched in all other dimensions (emotinal valence and intensity) which makes their comparison perfectly suitable for an imaging paradigm. To exclude the emotional domain entirely, we also included nonemotional, i.e., neutral stimuli; this enabled us to compare three distinct contents, sexual, emotional nonsexual, and nonemotional nonsexual, reflecting three different domains. Further, an interaction of domain, process, and repetion of stimuli could be considered. However, as repetion was only the case for 25% of our stimuli with a maximum repetition of twice for the whole experiment, future studies will have to focus on this topic. For our main process specific effect, this potential influence could, however, be limited by finding significant effects for expected picturers when all domains were investigated separately.
One could also argue that we did not control for the preceding expectancy period [Sakai and Passingham,2003]. We did not include a preceding control period, e.g., an ambiguous expectancy period because we wanted to acocunt for mere stimulus percpetion which would have been confounded by such a preceding control period [Bermpohl et al.,2006a]. Another point in this direction is that we did not include an isolated expectancy period, i.e., without subsequent picture in our paradigm to exclude possible DMPFC activation in the preceding expectancy period itself. We therefore conducted another study with 13 subjects where we included such isolated expectancy period and could again not observe any DMPFC activation in this period. This suggests that the DMPFC activation observed during expected stimulus perception is not due to an overlap with DMPFC activation in the preceding expectancy period itself, as also stated by Bermpohl et al. [2006a].
CONCLUSION
In summary, we found neural activity in the posterior DMPFC to be associated with a specific process, i.e., (preceding) attention to externally induced stimulus perception, as distinguished from other processes like mere stimulus perception or the preceding expectancy period itself. Corresponding to fMRI effects, we observed significant modulation of behavioral parameters (reaction time, subjective ratings) of stimulus perception by preceding attention, i.e., expectancy. In addition to such process‐specificity, we observed this DMPFC involvement in three different stimulus‐domains, sexual, emotional, and neutral stimuli, which by themselves, independent of preceding attention, did not recruit the posterior DMPFC while they did induce differential neural activity in the anterior DMPFC. This suggests that process specific modulation of neural activity in parts of DMPFC remains independent of the respective stimulus domain thus showing domain‐independence. This, however, was true for only the posterior DMPFC while we observed stronger impact of the domain with process by domain interaction in more anterior parts of the DMPFC. Taken together, our findings indicate process‐specificity and domain‐independence in posterior DMPFC and domain by process interaction in more anterior parts of the DMPFC.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supportiong Information Figure 1
Supportiong Information Figure 2
Supportiong Information Figure 3
Acknowledgements
We are thankful to the skillful technicians in the Department of Neurology II.
REFERENCES
- Abler B,Erk S,Herwig U,Walter H ( 2007): Anticipation of aversive stimuli activates extended amygdala in unipolar depression. J Psychiatr Res 41: 511–522. [DOI] [PubMed] [Google Scholar]
- Amodio DM,Frith CD ( 2006): Meeting of minds: The medial frontal cortex and social cognition. Nat Rev Neurosci 7: 268–277. [DOI] [PubMed] [Google Scholar]
- Anderson MC,Ochsner KN,Kuhl B,Cooper J,Robertson E,Gabrieli SW,Glover GH,Gabrieli JD ( 2004): Neural systems underlying the suppression of unwanted memories. Science 303: 232–235. [DOI] [PubMed] [Google Scholar]
- Beauregard M,Levesque J,Bourgouin P ( 2001): Neural correlates of conscious self‐regulation of emotion. J Neurosci 21: RC165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermpohl F,Pascual‐Leone A,Amedi A,Merabet LB,Fregni F,Gaab N,Alsop D,Schlaug G,Northoff G ( 2006a): Attentional modulation of emotional stimulus processing: An fMRI study using emotional expectancy. Hum Brain Mapp 27: 662–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermpohl F,Pascual‐Leone A,Amedi A,Merabet LB,Fregni F,Gaab N,Alsop D,Schlaug G,Northoff G ( 2006b): Dissociable networks for the expectancy and perception of emotional stimuli in the human brain. Neuroimage 30: 588–600. [DOI] [PubMed] [Google Scholar]
- Blood AJ,Zatorre RJ ( 2001): Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proc Natl Acad Sci USA 98: 11818–11823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M,Anton JL,Valabregue R,Poline JB ( 2002): Region of interest analysis using an SPM toolbox. Neuroimage 16. Conference abstract—8th International Conference on Functional Mapping of the Human Brain, Sendai, Japan [supplement on CD‐ROM].11771970 [Google Scholar]
- Bush G,Luu P,Posner MI ( 2000): Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4: 215–222. [DOI] [PubMed] [Google Scholar]
- Chawla D,Rees G,Friston KJ ( 1999): The physiological basis of attentional modulation in extrastriate visual areas. Nat Neurosci 2: 671–676. [DOI] [PubMed] [Google Scholar]
- Courtney SM,Petit L,Haxby JV,Ungerleider LG ( 1998): The role of prefrontal cortex in working memory: Examining the contents of consciousness. Philos Trans R Soc Lond B Biol Sci 353: 1819–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Esposito M,Postle BR,Rypma B ( 2000): Prefrontal cortical contributions to working memory: Evidence from event‐related fMRI studies. Exp Brain Res 133: 3–11. [DOI] [PubMed] [Google Scholar]
- Devinsky O,Morrell MJ,Vogt BA ( 1995): Contributions of anterior cingulate cortex to behaviour. Brain 118 (Part 1): 279–306. [DOI] [PubMed] [Google Scholar]
- Ferretti A,Caulo M,Del Gratta C,Di Matteo R,Merla A,Montorsi F,Pizzella V,Pompa P,Rigatti P,Rossini PM,Salonia A,Tartaro A,Romani GL ( 2005): Dynamics of male sexual arousal: Distinct components of brain activation revealed by fMRI. Neuroimage 26: 1086–1096. [DOI] [PubMed] [Google Scholar]
- Fichtenholtz HM,Dean HL,Dillon DG,Yamasaki H,McCarthy G,LaBar KS ( 2004): Emotion‐attention network interactions during a visual oddball task. Brain Res Cogn Brain Res 20: 67–80. [DOI] [PubMed] [Google Scholar]
- Fossati P,Hevenor SJ,Graham SJ,Grady C,Keightley ML,Craik F,Mayberg H ( 2003): In search of the emotional self: An FMRI study using positive and negative emotional words. Am J Psychiatry 160: 1938–1945. [DOI] [PubMed] [Google Scholar]
- Friston KJ,Holmes AP,Worsley KJ,Poline JP,Frith CD,Frackowiak RS ( 1994): Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp 2: 189–210. [Google Scholar]
- Frith C ( 2002): Attention to action and awareness of other minds. Conscious Cogn 11: 481–487. [DOI] [PubMed] [Google Scholar]
- Frith CD,Frith U ( 1999): Interacting minds—A biological basis. Science 286: 1692–1695. [DOI] [PubMed] [Google Scholar]
- Frith U,Frith CD ( 2003): Development and neurophysiology of mentalizing. Philos Trans R Soc Lond B Biol Sci 358: 459–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM ( 2001): The prefrontal cortex—An update: Time is of the essence. Neuron 30: 319–333. [DOI] [PubMed] [Google Scholar]
- Gallagher HL,Frith CD ( 2003): Functional imaging of ‘theory of mind’. Trends Cogn Sci 7: 77–83. [DOI] [PubMed] [Google Scholar]
- Goldman‐Rakic P ( 2000): Localization of function all over again. Neuroimage 11(5 Part 1): 451–457. [DOI] [PubMed] [Google Scholar]
- Greene J,Haidt J ( 2002): How (and where) does moral judgment work? Trends Cogn Sci 6: 517–523. [DOI] [PubMed] [Google Scholar]
- Grimm S,Schmidt CF,Bermpohl F,Heinzel A,Dahlem Y,Wyss M,Hell D,Boesiger P,Boeker H,Northoff G ( 2006): Segregated neural representation of distinct emotion dimensions in the prefrontal cortex‐an fMRI study. Neuroimage 30: 325–340. [DOI] [PubMed] [Google Scholar]
- Gusnard DA,Akbudak E,Shulman GL,Raichle ME ( 2001): Medial prefrontal cortex and self‐referential mental activity: Relation to a default mode of brain function. Proc Natl Acad Sci USA 98: 4259–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel A,Walter M,Schneider F,Rotte M,Matthiae C,Tempelmann C,Heinze HJ,Bogerts B,Northoff G ( 2006): Self‐related processing in the sexual domain: Parametric event‐related fMRI study reveals neural activity in ventral cortical midline structures. Soc Neurosci 1: 41–51. [DOI] [PubMed] [Google Scholar]
- Herwig U,Abler B,Walter H,Erk S ( 2007a): Expecting unpleasant stimuli—An fMRI study. Psychiatry Res 154: 1–12. [DOI] [PubMed] [Google Scholar]
- Herwig U,Baumgartner T,Kaffenberger T,Bruhl A,Kottlow M,Schreiter‐Gasser U,Abler B,Jancke L,Rufer M ( 2007b): Modulation of anticipatory emotion and perception processing by cognitive control. Neuroimage 37: 652–662. [DOI] [PubMed] [Google Scholar]
- Herwig U,Kaffenberger T,Baumgartner T,Jancke L ( 2007c): Neural correlates of a ‘pessimistic’ attitude when anticipating events of unknown emotional valence. Neuroimage 34: 848–858. [DOI] [PubMed] [Google Scholar]
- Hornak J,O'Doherty J,Bramham J,Rolls ET,Morris RG,Bullock PR,Polkey CE ( 2004): Reward‐related reversal learning after surgical excisions in orbito‐frontal or dorsolateral prefrontal cortex in humans. J Cogn Neurosci 16: 463–478. [DOI] [PubMed] [Google Scholar]
- Ishai A,Pessoa L,Bikle PC,Ungerleider LG ( 2004): Repetition suppression of faces is modulated by emotion. Proc Natl Acad Sci USA 101: 9827–9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC,Baxter LC,Wilder LS,Pipe JG,Heiserman JE,Prigatano GP ( 2002): Neural correlates of self‐reflection. Brain 125 (Part 8): 1808–1814. [DOI] [PubMed] [Google Scholar]
- Kalisch R,Wiech K,Critchley HD,Dolan RJ ( 2006): Levels of appraisal: A medial prefrontal role in high‐level appraisal of emotional material. Neuroimage 30: 1458–1466. [DOI] [PubMed] [Google Scholar]
- Kampe KK,Frith CD,Frith U ( 2003): “Hey John”: Signals conveying communicative intention toward the self activate brain regions associated with “mentalizing,” regardless of modality. J Neurosci 23: 5258–5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karama S,Lecours AR,Leroux JM,Bourgouin P,Beaudoin G,Joubert S,Beauregard M ( 2002): Areas of brain activation in males and females during viewing of erotic film excerpts. Hum Brain Mapp 16: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner S,Pinsk MA,De Weerd P,Desimone R,Ungerleider LG ( 1999): Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron 22: 751–761. [DOI] [PubMed] [Google Scholar]
- Keightley ML,Winocur G,Graham SJ,Mayberg HS,Hevenor SJ,Grady CL ( 2003): An fMRI study investigating cognitive modulation of brain regions associated with emotional processing of visual stimuli. Neuropsychologia 41: 585–596. [DOI] [PubMed] [Google Scholar]
- Kelley WM,Macrae CN,Wyland CL,Caglar S,Inati S,Heatherton TF ( 2002): Finding the self? An event‐related fMRI study. J Cogn Neurosci 14: 785–794. [DOI] [PubMed] [Google Scholar]
- Kjaer TW,Nowak M,Lou HC ( 2002): Reflective self‐awareness and conscious states: PET evidence for a common midline parietofrontal core. Neuroimage 17: 1080–1086. [PubMed] [Google Scholar]
- Lane RD,Reiman EM,Bradley MM,Lang PJ,Ahern GL,Davidson RJ,Schwartz GE ( 1997): Neuroanatomical correlates of pleasant and unpleasant emotion. Neuropsychologia 35: 1437–1444. [DOI] [PubMed] [Google Scholar]
- Lane RD,Chua PM,Dolan RJ ( 1999): Common effects of emotional valence, arousal and attention on neural activation during visual processing of pictures. Neuropsychologia 37: 989–997. [DOI] [PubMed] [Google Scholar]
- Lang PJ,Bradley MM,Cuthbert BN. 2005. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical Report A‐6. Gainesville, FL: University of Florida. [Google Scholar]
- Levy R,Goldman‐Rakic PS ( 2000): Segregation of working memory functions within the dorsolateral prefrontal cortex. Exp Brain Res 133: 23–32. [DOI] [PubMed] [Google Scholar]
- Liberzon I,Taylor SF,Fig LM,Decker LR,Koeppe RA,Minoshima S ( 2000): Limbic activation and psychophysiologic responses to aversive visual stimuli. Interaction with cognitive task. Neuropsychopharmacology 23: 508–516. [DOI] [PubMed] [Google Scholar]
- Lou HC,Luber B,Crupain M,Keenan JP,Nowak M,Kjaer TW,Sackeim HA,Lisanby SH ( 2004): Parietal cortex and representation of the mental Self. Proc Natl Acad Sci USA 101: 6827–6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrae CN,Moran JM,Heatherton TF,Banfield JF,Kelley WM ( 2004): Medial prefrontal activity predicts memory for self. Cereb Cortex 14: 647–654. [DOI] [PubMed] [Google Scholar]
- Maddock RJ,Garrett AS,Buonocore MH ( 2003): Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Hum Brain Mapp 18: 30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK,Freedman DJ,Wallis JD ( 2002): The prefrontal cortex: Categories, concepts and cognition. Philos Trans R Soc Lond B Biol Sci 357: 1123–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP,Banaji MR,Macrae CN ( 2005): The link between social cognition and self‐referential thought in the medial prefrontal cortex. J Cogn Neurosci 17: 1306–1315. [DOI] [PubMed] [Google Scholar]
- Moll J,de Oliveira‐Souza R,Moll FT,Ignacio FA,Bramati IE,Caparelli‐Daquer EM,Eslinger PJ ( 2005): The moral affiliations of disgust: A functional MRI study. Cogn Behav Neurol 18: 68–78. [DOI] [PubMed] [Google Scholar]
- Mottaghy FM,Doring T,Muller‐Gartner HW,Topper R,Krause BJ ( 2002): Bilateral parieto‐frontal network for verbal working memory: An interference approach using repetitive transcranial magnetic stimulation (rTMS). Eur J Neurosci 16: 1627–1632. [DOI] [PubMed] [Google Scholar]
- Murphy FC,Nimmo‐Smith I,Lawrence AD ( 2003): Functional neuroanatomy of emotions: A meta‐analysis. Cogn Affect Behav Neurosci 3: 207–233. [DOI] [PubMed] [Google Scholar]
- Nieder A,Miller EK ( 2003): Coding of cognitive magnitude: Compressed scaling of numerical information in the primate prefrontal cortex. Neuron 37: 149–157. [DOI] [PubMed] [Google Scholar]
- Nitschke JB,Sarinopoulos I,Mackiewicz KL,Schaefer HS,Davidson RJ ( 2006): Functional neuroanatomy of aversion and its anticipation. Neuroimage 29: 106–116. [DOI] [PubMed] [Google Scholar]
- Northoff G,Bermpohl F ( 2004): Cortical midline structures and the self. Trends Cogn Sci 8: 102–107. [DOI] [PubMed] [Google Scholar]
- Northoff G,Heinzel A,Bermpohl F,Niese R,Pfennig A,Pascual‐Leone A,Schlaug G ( 2004): Reciprocal modulation and attenuation in the prefrontal cortex: An fMRI study on emotional‐cognitive interaction. Hum Brain Mapp 21: 202–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G,Heinzel A,de Greck M,Bermpohl F,Dobrowolny H,Panksepp J ( 2006): Self‐referential processing in our brain—A meta‐analysis of imaging studies on the self. Neuroimage 31: 440–457. [DOI] [PubMed] [Google Scholar]
- Nyberg L,Marklund P,Persson J,Cabeza R,Forkstam C,Petersson KM,Ingvar M ( 2003): Common prefrontal activations during working memory, episodic memory, and semantic memory. Neuropsychologia 41: 371–377. [DOI] [PubMed] [Google Scholar]
- Ochsner KN,Bunge SA,Gross JJ,Gabrieli JD ( 2002): Rethinking feelings: An FMRI study of the cognitive regulation of emotion. J Cogn Neurosci 14: 1215–1229. [DOI] [PubMed] [Google Scholar]
- Ochsner KN,Gross JJ ( 2005): The cognitive control of emotion. Trends Cogn Sci 9: 242–249. [DOI] [PubMed] [Google Scholar]
- Ochsner KN,Ray RD,Cooper JC,Robertson ER,Chopra S,Gabrieli JD,Gross JJ ( 2004): For better or for worse: Neural systems supporting the cognitive down‐ and up‐regulation of negative emotion. Neuroimage 23: 483–499. [DOI] [PubMed] [Google Scholar]
- Owen AM ( 2000): The role of the lateral frontal cortex in mnemonic processing: The contribution of functional neuroimaging. Exp Brain Res 133: 33–43. [DOI] [PubMed] [Google Scholar]
- Park K,Kang HK,Seo JJ,Kim HJ,Ryu SB,Jeong GW ( 2001): Blood‐oxygenation‐level‐dependent functional magnetic resonance imaging for evaluating cerebral regions of female sexual arousal response. Urology 57: 1189–1194. [DOI] [PubMed] [Google Scholar]
- Pessoa L,Kastner S,Ungerleider LG ( 2002): Attentional control of the processing of neural and emotional stimuli. Brain Res Cogn Brain Res 15: 31–45. [DOI] [PubMed] [Google Scholar]
- Phan KL,Wager T,Taylor SF,Liberzon I ( 2002): Functional neuroanatomy of emotion: A meta‐analysis of emotion activation studies in PET and fMRI. Neuroimage 16: 331–348. [DOI] [PubMed] [Google Scholar]
- Phillips ML,Drevets WC,Rauch SL,Lane R ( 2003): Neurobiology of emotion perception. I. THE neural basis of normal emotion perception. Biol Psychiatry 54: 504–514. [DOI] [PubMed] [Google Scholar]
- Platek SM,Keenan JP,Gallup GG Jr,Mohamed FB ( 2004): Where am I? The neurological correlates of self and other. Brain Res Cogn Brain Res 19: 114–122. [DOI] [PubMed] [Google Scholar]
- Price JL ( 1999): Prefrontal cortical networks related to visceral function and mood. Ann N Y Acad Sci 877: 383–396. [DOI] [PubMed] [Google Scholar]
- Sakai K,Passingham RE ( 2003): Prefrontal interactions reflect future task operations. Nat Neurosci 6: 75–81. [DOI] [PubMed] [Google Scholar]
- Steele JD,Lawrie SM ( 2004): Segregation of cognitive and emotional function in the prefrontal cortex: A stereotactic meta‐analysis. Neuroimage 21: 868–875. [DOI] [PubMed] [Google Scholar]
- Stoléru S,Grégoire MC,Gérard D,Decety J,Lafarge E,Cinotti L,Lavenne F,Le Bars D,Vernet‐Maury E,Rada H,Collet C,Mazoyer B,Forest MG,Magnin F,Spira A,Comar D ( 1999): Neuroanatomical correlates of visually evoked sexual arousal in human males. Arch Sex Behav 28: 1–21. [DOI] [PubMed] [Google Scholar]
- Ueda K,Okamoto Y,Okada G,Yamashita H,Hori T,Yamawaki S ( 2003): Brain activity during expectancy of emotional stimuli: An fMRI study. Neuroreport 14: 51–55. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG,Courtney SM,Haxby JV ( 1998): A neural system for human visual working memory. Proc Natl Acad Sci USA 95: 883–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M,Witzel J,Wiebking C,Gubka U,Rotte M,Schiltz K,Bermpohl F,Tempelmann C,Bogerts B,Heinze HJ,Northoff G ( 2007): Pedophilia is linked to reduced activation in hypothalamus and lateral prefrontal cortex during visual erotic stimulation. Biol Psychiatry 62: 698–701. [DOI] [PubMed] [Google Scholar]
- Wicker B,Ruby P,Royet JP,Fonlupt P ( 2003): A relation between rest and the self in the brain? Brain Res Brain Res Rev 43: 224–230. [DOI] [PubMed] [Google Scholar]
- Wig GS,Miller MB,Kingstone A,Kelley WM ( 2004): Separable routes to human memory formation: Dissociating task and material contributions in the prefrontal cortex. J Cogn Neurosci 16: 139–148. [DOI] [PubMed] [Google Scholar]
- Winston JS,O'Doherty J,Dolan RJ ( 2003): Common and distinct neural responses during direct and incidental processing of multiple facial emotions. Neuroimage 20: 84–97. [DOI] [PubMed] [Google Scholar]
- Yovel G,Kanwisher N ( 2004): Face perception: Domain specific, not process specific. Neuron 44: 889–898. [DOI] [PubMed] [Google Scholar]
- Zysset S,Huber O,Ferstl E,von Cramon DY ( 2002): The anterior frontomedian cortex and evaluative judgment: An fMRI study. Neuroimage 15: 983–991. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supportiong Information Figure 1
Supportiong Information Figure 2
Supportiong Information Figure 3


